Abstract
Objective
We retrospectively studied the efficacy of bevacizumab as salvage therapy for recurrent malignant glioma with a focus on the overall survival (OS).
Methods
Patients who received a therapy other than surgery for recurrent malignant glioma were included. Efficacy was evaluated using MRI. Neurological function was evaluated using the Response Assessment in Neuro-Oncology (RANO). The survival rate was calculated using the Kaplan-Meier method.
Results
Fifty-one patients with recurrent glioma (31 grade III, 20 grade IV) were included. Among them, 22 subjects (43.1%) received bevacizumab. The median OS was 10.2 months (range, 1 to 27 months). Patients receiving bevacizumab had comparable OS (a median of 9.9 vs. 10.0 months) and similar 6-month survival rate (43% vs. 34%) to those who did not receive bevacizumab. A subgroup analysis failed to notice any significant difference in grade III glioma patients receiving bevacizumab vs. those who did not. The median survival was significantly longer at 8.9 months (range, 4 to 13 months) in grade IV glioma patients receiving bevacizumab than in those who did not (5.6 months, range, 2 to 7 months, P=0.042). The 6-month survival rate was higher (83%) in those who received bevacizumab than in those who did not (47%, P=0.046). No grade 3/4 adverse events were observed in any patient.
Conclusions
Bevacizumab, as a rescue therapy, provides a survival benefit for recurrent grade IV glioma.
Key Words: Bevacizumab, recurrent malignant glioma
Introduction
Gliomas are the most common form of primary brain tumors, accounting for approximately 45% of all brain tumors (1). Two thirds of gliomas are high grade. Anaplastic glioma (grade III) and glioblastoma multiforme (grade IV) patients have an overall survival (OS) of 2 to 5 years and 12 to 15 months, respectively (2). Glioblastoma is characterized by rapid, aggressive, and infiltrative growth, rendering most tumors unresectable at the time of diagnosis. Current standard therapy includes surgical resection, radiotherapy, and temozolomide adjuvant chemotherapy (3). However, patients typically succumb to glioblastoma despite the best available therapeutic regimen due to the chemoresistance to anticancer drugs (4). Salvage therapy for recurrent glioma also has a dismal outcome with a 6-month progression free survival (PFS) rate of 28% to 31% for anaplastic glioma and 15% to 16% for glioblastoma multiforme (2,5).
Bevacizumab is a monoclonal antibody that inhibits vascular endothelial growth factor (VEGF) and is approved for metastatic colorectal cancer, non-small-cell lung cancer, breast cancer, ovarian cancer and renal cancers (6). Bevacizumab suppresses the progression of glioma as a salvage therapy (6,7), but the effects on the survival remain unclear. Bevacizumab has been used as a salvage agent for patients with recurrent glioma at our center since 2010. Here, we retrospectively compared the OS in recurrent glioma patients who received bevacizumab as a salvage therapy vs. those who did not.
Patients and methods
Patients
We retrospectively reviewed the clinicopathologic data of patients with recurrent glioma who sought treatment at our center between July 2010 and May 2012. Patients were eligible for inclusion in this analysis if: (I) Surgical treatment was not indicated or refused by the patients upon relapse; (II) the diagnosis of glioma was verified with biopsy, secondary surgery, or imaging (CT or MRI) findings of edema in tissues surrounding the lesions or deteriorating space-occupying lesions during follow-up visits. Patients were excluded if they had (I) coagulation disorder; (II) grade 3/4 leukopenia or thrombocytopenia; or (III) a previous history of intracranial or internal hemorrhage.
Therapy
Cranial conformal radiotherapy was initiated 2 to 4 weeks after surgery at a dose of 2 Gy/f and 5 f/W (total dose: 60 Gy over a 6-week period). Concurrent chemotherapy with temozolomide was conducted at a dose of 75 mg/m2/day from day 1 to the end of radiotherapy, followed by the following regimen one month later: 150 mg/m2/day for 5 days, repeated at an interval of 28 days. A dose-dense chemotherapy (75 mg/m2/day for 21 days, repeated at an interval of 28 days) with temozolomide was used for patients with first recurrence or progressive disease with the following regimen. Twenty-two patients with second recurrence or poor response to dense-dose chemotherapy received salvage bevacizumab therapy at a dose of 10 mg/kg. The remaining 29 patients received best care support, with or without other chemotherapeutic regimens including temozolomide plus cisplatin, nimustine and teniposide, or nimustine, cisplatin and VP-16, and nimustine monotherapy. Bevacizumab was terminated upon disease progression or treatment-related toxicity (grade 4 or above).
Follow-up
Patients were followed up at scheduled hospital visits. Efficacy was evaluated using MRI every two cycles of chemotherapy and neurological function using the Response Assessment in Neuro-Oncology (RANO) (8) and recorded as complete remission (CR), partial remission (PR), stable disease (SD) and progressive disease (PD). Chemotherapy-related toxicity was evaluated according to the Common Terminology Criteria for Adverse Events (CTCAE, version 3.0).
Statistical analysis
The primary endpoint of this retrospective analysis was OS. Secondary endpoints included PFS and safety outcomes. The survival rate was calculated using the Kaplan-Meier method and univariate prognostic analysis was performed using a log rank test. Statistical significance was set at P<0.05. Stratification and multivariate analysis was not performed due to the small sample size.
Results
Demographic, clinicopathologic and treatment characteristics
Fifty-one patients (37 men) were included in the current retrospective analysis. Patient demographic, clinicopathologic and treatment characteristics are shown in Table 1. Their median age was 38 (range, 12 to 71) years. Thirty-one (60.8%) patients had grade III glioma and the remaining 20 (39.2%) had grade IV glioma. The majority of the subjects (62.7%, 32/51) had a pretreatment Karnofsky performance status (KPS) score between 90 and 100. About half of the patients (54.9%, 28/51) underwent subtotal resection and 29.4% (15/51) of the patients received curative resection. Most patients (84.3%, 43/51) received radiotherapy at a dose of 60 Gy. Approximately three fourths of the patients (74.5%, 38/51) received more than six cycles of temozolomide-based chemotherapy. Twenty-two patients (43.1%) of the patients received bevacizumab. No significant difference in demographic, clinicopathologic and treatment characteristics was noted between the patients who received bevacizumab vs. those who did not.
Table 1. Patient demographic, clinicopathologic and treatment characteristics.
| Total (n=51) | Bevacizumab (n=22) | Controls (n=29) | |
|---|---|---|---|
| Age, years, median [range] | 40 [12-71] | 34 [12-58] | 42 [15-71] |
| ≤40, n (%) | 28 (54.9) | 13 (59.1) | 15 (51.7) |
| >40, n (%) | 23 (45.1) | 9 (40.9) | 14 (48.3) |
| Gender, n (%) | |||
| Male | 37 (72.5) | 18 (81.2) | 19 (65.5) |
| Pathological grade, n (%) | |||
| III | 31 (60.8) | 10 (45.4) | 21 (72.4%) |
| IV | 21 (39.2) | 12 (54.6) | 8 (27.6%) |
| Corticosteroid use, n (%) | |||
| Yes | 22 (43.1) | 10 (45.4) | 12 (41.4) |
| No | 29 (56.9) | 12 (54.6) | 17 (58.6) |
| Pretreatment KPS score, n (%) | |||
| 90-100 | 32 (62.7) | 12 (54.6) | 20 (69.0) |
| 70-80 | 19 (37.3) | 10 (45.4) | 9 (31.0) |
| Primary surgery | |||
| Curative resection | 15 (29.4) | 8 (36.4) | 7 (24.1) |
| Subtotal resection | 28 (54.9) | 12 (54.6) | 16 (55.2) |
| Biopsy | 8 (15.7) | 2 (9.1) | 6 (20.7) |
| Adjuvant radiotherapy | |||
| 60 Gy | 43 (84.3) | 18 (81.8) | 25 (86.2) |
| <60 Gy | 8 (15.7) | 4 (8.2) | 4 (13.8) |
| Adjuvant chemotherapy with temozolomide | |||
| >6 cycles | 38 (74.5) | 17 (77.3) | 21 (72.4) |
| 4-6 cycles | 8 (15.7) | 3 (13.6) | 5 (17.2) |
| <4 cycles | 5 (9.8) | 2 (9.1) | 3 (10.3) |
Therapeutic outcomes
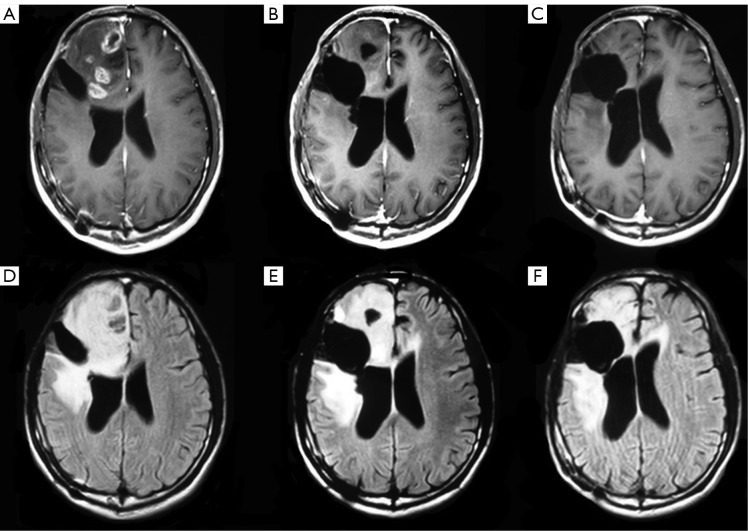
The median follow-up was 6 (range, 1 to 27) months in the entire sample, 6 (range, 1 to 23) months in those who received bevacizumab 6 (range, 1 to 27) months for those who did not receive bevacizumab. Two patients who did not receive bevacizumab were lost to the follow-up at 2 and 6 months, respectively. Therapeutic outcomes, OS and PFS are shown in Table 2. Seven (31.8%, 7/22) patients receiving bevacizumab achieved PR and 50% (11/22) had SD. Figure 1 shows the MRI results of a representative patient who achieved PR after 4 cycles of bevacizumab therapy. The overall effective rate was 81.8% for patients receiving bevacizumab. Three (10.3%, 3/29) patients not receiving bevacizumab achieved PR and 31.0% (9/29) of the patients had SD. The overall effective rate was 41.3% for patients who did not receive bevacizumab. Statistically significant difference was noted in the overall effectiveness rate between the patients receiving bevacizumab vs. those who did not (P=0.005).
Table 2. Therapeutic outcomes.
| Bevacizumab | Controls | P | |
|---|---|---|---|
| Overall effectiveness rate | 81.8% | 41.3% | 0.005 |
| Median survival (months) | 9.9 | 10.0 | 0.781 |
| Median survival (months) of grade III | 14 | 19 | 0.592 |
| Median survival (months) of grade IV | 8.9 | 5.6 | 0.042 |
| 6-month survival rate | 43% | 34% | 0.752 |
| 6-month survival rate of grade III | 64% | 57% | 0.581 |
| 6-month survival rate of grade IV | 83% | 47% | 0.046 |
| Progression free survival | 5.9 | 5.0 | 0.786 |
| PFS of grade III | 15.4 | 8.3 | 0.386 |
| PFS of grade IV | 3 | 1 | 0.575 |
Figure 1.
T1 +Gd (upper) and T2-Flair (lower) weighted MRI image in a representative a patient with recurrent GBM who achieved PR after 4 cycles of bevacizumab therapy. A. prior to bevacizumab; B. one month after 4 cycles of bnevacizumab therapy; C. eighteen months after 4 cycles of bevacizumab therapy
Survival
The median OS was 10.2 (range, 1 to 27) months for the entire study sample. Patients receiving bevacizumab rescue therapy had a median survival (9.9 months; range, 2 to 27 months) comparable to those who did not receive bevacizumab (10.0 months; range, 1 to 27 months). The 6-month survival rate was 34% in the entire study sample, and did not differ between those who received bevacizumab and those who did not (bevacizumab, 43% vs. non-bevacizumab, 34%, P=0.752). The median survival in grade III glioma patients was longer than in grade IV glioma patients (16.1 vs. 6.6 months, P=0.014). When stratified by tumor grade, grade III glioma patients receiving bevacizumab showed a similar median survival (19 months; range, 2 to 27 months) to those who did not receive bevacizumab (14 months; range, 2 to 27 months). The 6-month survival rate was comparable between patients who received bevacizumab (64%) and those who did not (57%, P=0.581). The median survival was 8.9 (range, 4 to 13) months for grade IV glioma patients who received bevacizumab and 5.6 (range, 2 to 7) months for those who did not (P=0.042). There was a significant difference in the 6-month survival rate between those who received bevacizumab and those who did not (bevacizumab, 83% vs. non-bevacizumab, 47%, P=0.046).
PFS
The PFS was 5.5 (range, 1 to 27) months and the 6-month PFS rate was 31%. Patients receiving bevacizumab rescue therapy exhibited a median PFS (5.9 months; range, 1 to 27 months) comparable to those who did not receive bevacizumab (5.0 months; range, 1 to 26 months). The median PFS was 15.4 (range, 1 to 27) months for grade III glioma patients who received bevacizumab and 8.3 (range, 1 to 26) months for those who did not with a statistical difference (P=0.386). There was no significant difference in the 6-month PFS rate (bevacizumab, 33% vs. non-bevacizumab, 28%, P=0.750) between those who received bevacizumab and those who did not. The PFS was 3 (range, 1 to 18) months for grade IV glioma patients who received bevacizumab and 1 (range, 1 to 6) month for those who did not without statistical difference (P=0.575).
Safety
Nine of the 12 patients who received corticosteroids prior to the study treatment experienced dose reduction or ceased taking corticosteroids. No grade 3/4 adverse event was observed in all patients. Reversible hypertension (91.1%, 2/22) and proteinuria (4.6%, 1/22) were reported in patients receiving bevacizumab.
Discussion
Surgery and temozolomide-based adjuvant chemotherapy and radiotherapy are associated with a 6-month PFS rate of 9-21% (9). Patients responsive to temozolomide at initial treatment may not respond to the agent during recurrence or progression of the disease (10). Multiple mechanisms may underlie recalcitrance of recurrent gliomas to temozolomide, including impaired DNA damage response (4), aberrant microRNA expression (11) and neoangiogenesis (12). Glioma, particularly malignant glioma, may contain numerous blood vessels and abundant VEGF (13). Bevacizumab was first used to treat recurrent malignant glioma in 2005 (6). Vredenburgh et al. (7) further reported superiority of bevacizumab plus CPT-11 over monotherapy with CPT-11 for glioma patients. Bevacizumab has been used as a rescue therapy in the treatment of recurrent malignant glioma since 2010. We showed here that patients receiving bevacizumab rescue therapy exhibited a median survival comparable to those who did not receive bevacizumab, and we failed to observe any significant difference in the 6-month survival rate between the two groups. Stratification of survival by tumor grade indicated no survival advantage with bevacizumab recue therapy for grade III glioma patients. By contrast, bevacizumab confers a significant survival benefit for patients with grade IV glioma (8.9 vs. 5.6 months). This finding is consistent with those reported by other investigators (7,14,15), indicating a survival benefit of bevacizumab for patients with grade IV glioma. It remains likely that, compared to less aggressive gliomas, glioblastoma may possess greater angiogenic activities, which makes it vulnerable to anti-angiogenic agents like bevacizumab.
The Macdonald criteria were used for the evaluation of patient response to bevacizumab during early studies on glioma (16). The Macdonald criteria, without MRI evaluation of the malignancy at T2 phase, may contribute to the occurrence of “pseudo progression” or “pseudo reaction” (17). A more reliable diagnosis can be obtained using the RANO criteria by incorporating T2 MRI so that pseudo progression could be favorably diagnosed (8). The remission rate in our study (31.8%, 7/22) is similar to that (27.6%, 8/31) reported by Nagane et al. (17) on Japanese patients with glioblastoma who were treated with bevacizumab, but is lower than that reported by Vredenbugh et al. (7,15), probably due to discrepancy between the two criteria used in the evaluation of patient response.
Due to the relatively short life expectancy, an important goal of palliative treatment for recurrent glioma is to relieve the symptom and improve the quality of life. Besides the survival benefit reported previously, reduction or withdrawal of corticosteroids was also found in 75% (9/12) of the patients in this series who were administered with corticosteroids prior to the study treatment. Corticosteroids are commonly used in patients with recurrent glioma as a result of cerebral edema and they may elevate the risk of infection, glycometabolic disorder and/or water-sodium retention in these patients. Dose reduction of corticosteroids without worsening clinical manifestations is another advantage of rescue therapy with bevacizumab. In the present study, the median PFS (2 months) and 6-month PFS rate (0%) was dismal in patients with recurrent grade IV glioma, consistent with the results reported by Iwamoto et al. (18) and Quant et al. (19) (6-month PFS rate: 0-2%). Whether temozolomide, other targeting agents or ketogenic diet should be used if disease progression is found following treatment with bevacizumab should be investigated more extensively.
In conclusion, salvage therapy with bevacizumab is effective and safe for patients with grade IV glioma, conferring a survival benefit without incurring additional side effects.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- 1.Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med 2008;359:492-507 [DOI] [PubMed] [Google Scholar]
- 2.Lamborn KR, Yung WK, Chang SM, et al. Progression-free survival: an important end point in evaluating therapy for recurrent high-grade gliomas. Neuro Oncol 2008;10:162-70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 2009;10:459-66 [DOI] [PubMed] [Google Scholar]
- 4.Cui B, Johnson SP, Bullock N, et al. Decoupling of DNA damage response signaling from DNA damages underlies temozolomide resistance in glioblastoma cells. J Biomed Res 2010;24:424-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ballman KV, Buckner JC, Brown PD, et al. The relationship between six-month progression-free survival and 12-month overall survival end points for phase II trials in patients with glioblastoma multiforme. Neuro Oncol 2007;9:29-38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol 2009;27:4733-40 [DOI] [PubMed] [Google Scholar]
- 7.Vredenburgh JJ, Desjardins A, Herndon JE, 2nd, et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol 2007;25:4722-9 [DOI] [PubMed] [Google Scholar]
- 8.Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol 2010;28:1963-72 [DOI] [PubMed] [Google Scholar]
- 9.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005;352:987-96 [DOI] [PubMed] [Google Scholar]
- 10.Kyritsis AP, Levin VA. An algorithm for chemotherapy treatment of recurrent glioma patients after temozolomide failure in the general oncology setting. Cancer Chemother Pharmacol 2011;67:971-83 [DOI] [PubMed] [Google Scholar]
- 11.Li P, Lu X, Wang Y, et al. MiR-181b suppresses proliferation of and reduces chemoresistance to temozolomide in U87 glioma stem cells. J Biomed Res 2010;24:436-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liang C, Guo S, Yang L.All-trans retinoic acid upregulates VEGF expression in glioma cells in vitro. J Biomed Res 2013; 27:51-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reardon DA, Desjardins A, Rich JN, et al. The emerging role of anti-angiogenic therapy for malignant glioma. Curr Treat Options Oncol 2008;9:1-22 [DOI] [PubMed] [Google Scholar]
- 14.Kreisl TN, Kim L, Moore K, et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol 2009;27:740-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khasraw M, Lassman AB. Advances in the treatment of malignant gliomas. Curr Oncol Rep 2010;12:26-33 [DOI] [PubMed] [Google Scholar]
- 16.Macdonald DR, Cascino TL, Schold SC, Jr, et al. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol 1990;8:1277-80 [DOI] [PubMed] [Google Scholar]
- 17.Nagane M, Nishikawa R, Narita Y, et al. Phase II study of single-agent bevacizumab in Japanese patients with recurrent malignant glioma. Jpn J Clin Oncol 2012;42:887-95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iwamoto FM, Abrey LE, Beal K, et al. Patterns of relapse and prognosis after bevacizumab failure in recurrent glioblastoma. Neurology 2009;73:1200-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quant EC, Norden AD, Drappatz J, et al. Role of a second chemotherapy in recurrent malignant glioma patients who progress on bevacizumab. Neuro Oncol 2009;11:550-5 [DOI] [PMC free article] [PubMed] [Google Scholar]