Abstract
Objective
To observe the efficacy and safety of albumin-bound paclitaxel (ABP) monotherapy in treating recurrent advanced non-small-cell lung cancer (NSCLC).
Methods
We retrospectively analyzed the short-term efficacy and toxicities of ABP monotherapy in treating 21 patients who had previously undergone multiple cycles of therapy for their advanced NSCLC in our hospital since 2010. The treatment-related survival was also analyzed.
Results
Of these 21 patients, the best overall response was partial response (PR) in 6 patients (28.6%), stable disease (SD) in 10 patients (47.6%), and progressive disease (PD) in 5 patients (23.8%). The overall response rate (ORR) was 28.6% and the disease control rate (DCR) (PR + SD) was 76.2%. The median progression-free survival (PFS) was 4.0 months (95% CI, 5.0-7.0 months). The main grade 3/4 toxicities included neutropenia (11.1%), peripheral nerve toxicity (5.6%), muscle and joint aches (5.6%), and fatigue (5.6%).
Conclusions
The ABP monotherapy can achieve good objective response in advanced NSCLC patients who have previously received multiple cycles of treatment and be well tolerated.
Key Words: Albumin-bound paclitaxel, paclitaxel, advanced non-small cell lung cancer, chemotherapy
Introduction
Paclitaxel has a wide spectrum of anti-tumor activity, and its role in treating advanced non-small-cell lung cancer (NSCLC) has been confirmed (1,2). The albumin-bound paclitaxel (ABP) (brand name: Abraxane®) is a novel human albumin-bound nanoparticle form of paclitaxel; in addition to the milder side effects, the new preparation also has higher local concentration in the tumor microenvironment and stronger tumor-killing capability (3-5). Many clinical trials and related reports have suggested that ABP has good efficacy in treating advanced NSCLC (4,6,7), while few studies in China have explored this new drug. In our current study, we retrospectively analyzed the clinical data of 21 advanced NSCLC patients who had previously received multiple cycles of treatment and underwent ABP monotherapy in our department from January 2010 to March 2012.
Materials and methods
Clinical data
A total of 21 advanced NSCLC patients who had previously received multiple cycles of treatment and underwent ABP monotherapy in our department from January 2010 to March 2012 were enrolled in this study. Detailed records of smoking history, family history, pathological diagnosis, tumor stage, laboratory tests, imaging data and other clinical data were obtained for all patients (Table 1). Of these 21 patients, there were 13 males and 8 females aged 35- 81 years (mean: 56 years), with Karnofsky scores of ≥70. All patients were categorized as clinical stage I carcinoma; the pathological types included adenocarcinoma (n=13), squamous cell carcinoma (n=7), and “non-small cell lung cancer - not otherwise specified” (n=1). The patients had previously received 1-6 systemic therapies; 14 of them had received epidermal growth factor receptor - tyrosine kinase inhibitors (EGFR-TKIs). Of 10 patients who had received EGFR mutation testing, 3 were with mutant EGFR and 7 with wild-type EGFR. Eleven patients had previously treated with taxols (paclitaxel or docetaxel). Before ABP treatment, three patients were accompanied with central nervous system (CNS) metastases, two had brain metastases, one developed extracranial progression after chemotherapy plus brain radiotherapy, and two were assessed to be with staple tumors.
Table 1. Baseline data of all patients.
| Clinical features | N [21] | |
|---|---|---|
| Gender | ||
| Men | 13 | |
| Women | 8 | |
| Age | ||
| <65 years | 12 | |
| ≥65 years | 9 | |
| Pathologic type | ||
| Adenoma | 13 | |
| Squamous cell carcinoma | 7 | |
| Non-small cell lung cancer - not otherwise specified | 1 | |
| Previous chemotherapy | ||
| ≤2 lines | 10 | |
| >2 lines | 11 | |
| EGFR gene | ||
| Wild-type | 7 | |
| Mutant | 3 | |
| Central nervous system metastasis | ||
| Yes | 3 | |
| No | 18 | |
Methods
Treatment
The ABP (brand name: Abraxane®, Abraxis, USA; 100 mg/vial) was used as a monotherapy at a dose of 130 mg/m2 (injection for over 30 minutes). The drug was administered on a day 1 and day 8 schedule every three weeks. The efficacy was evaluated every two cycles. Hematologic and imaging examinations were routinely performed during the medication.
Efficacy and adverse reactions
The objective efficacy was evaluated using the Response Evaluation Criteria in Solid Tumors (RECIST) 1.1. The observation indicators included complete remission (CR), partial response (PR), stable disease (SD), disease progression (PD), objective response rate (ORR), and disease control rate (DCR). Progression-free survival (PFS) is defined as the time from first medication to the first objective progression of disease. The evaluation of adverse reactions was based on the NCI Common Toxicity Criteria (CTC) Version 3.0.
Statistical methods
All data were summarized descriptively, and 2-sided 95% confidence intervals (CIs) are presented. PFS was summarized using Kaplan-Meier methods.
Results
Short-term efficacy
From January 2010 to February 21, 2012, all these 21 patients received 2-10 cycles (median: 4 cycles) of ABP monotherapy. All patients were eligible for efficacy evaluation and they were followed up till August 31, 2012. The final observations included PR (n=6, 28.6%), SD (n=10, 47.6%), PD (n=5, 23.8%), ORR (CR+PR, n=6, 28.6%), and DCR (CR+PR+SD, n=16, 76.2%). Up to August 31, 2012, the median PFS was 6 months (95% CI: 5.0-7.0) (Figure 1).
Figure 1.
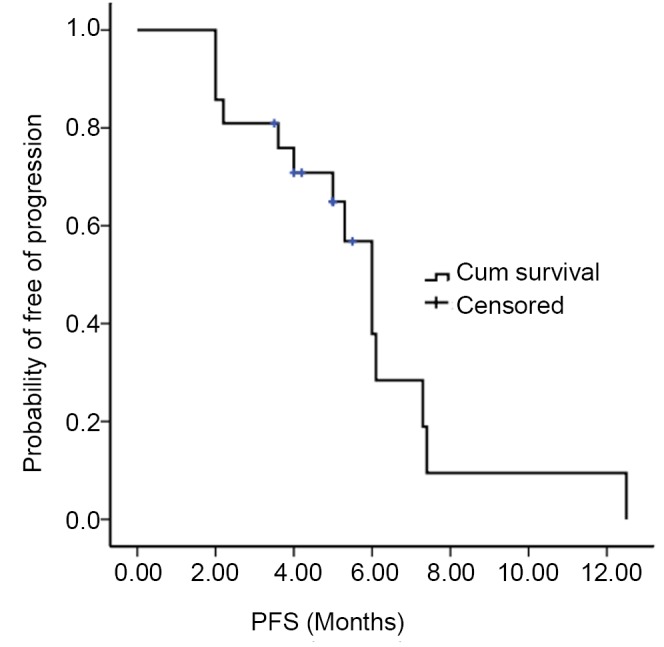
Progression-free survival curve of all patients
Adverse reactions
The main adverse reactions in our cohort included peripheral neurotoxicities, muscle and joint pain, and neutropenia (Table 2).
Table 2. Adverse reactions of albumin-bound paclitaxel in treating recurrent advanced non-small-cell lung cancer.
| Adverse reactions | All grades, n (%) | Grade 3/4, n (%) | ||
|---|---|---|---|---|
| Neutropenia | 10 | 55.6 | 2 | 11.1 |
| Peripheral neurotoxicities | 12 | 66.7 | 1 | 5.6 |
| Muscle and joint pain | 9 | 50.0 | 1 | 5.6 |
| Nausea/vomiting | 7 | 38.9 | 0 | 0 |
| Hair loss | 11 | 61.1 | 0 | 0 |
| Fatigue | 6 | 33.3 | 1 | 5.6 |
| Elevated ALT/AST | 3 | 16.7 | 0 | 0 |
| Mucositis | 2 | 11.1 | %0 | %0 |
Case analysis
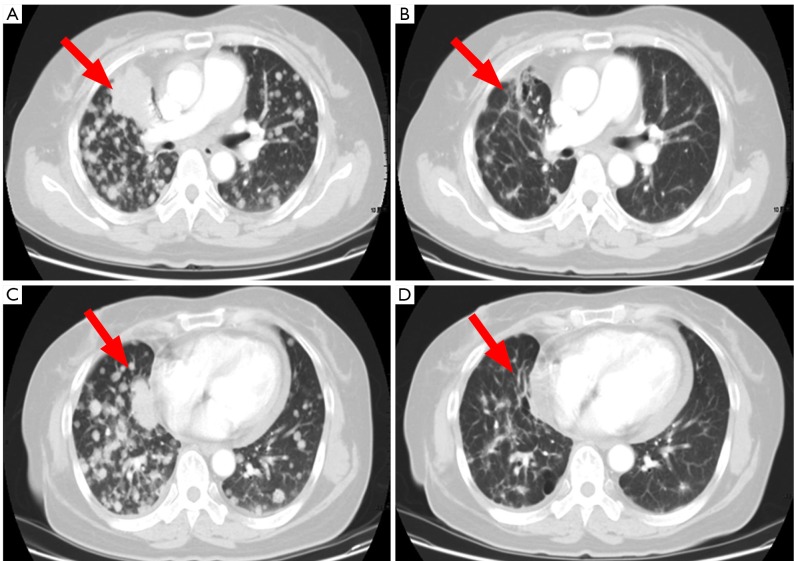
A 53-year-old non-smoking Asian woman was diagnosed with lung adenocarcinoma through surgery in May, 2006. She received four cycles of neoadjuvant chemotherapy with paclitaxel/cisplatin after surgery. A follow-up chest CT suggested intrapulmonary metastasis in November 2007. She was then successively treated with gemcitabine/cisplatin, docetaxel, EGFR-TKI, pemetrexed, and sunitinib malate capsules. The treatment was switched to albumin-bound paclitaxel therapy due to disease progression in June 2011. After a total of six cycles of chemotherapy, PR was achieved based on efficacy evaluation (see Figure 2). Maintenance therapy with tegafurgimeracil alone was prescribed until disease progression on July 4, 2012, with a PFS of 12.5 months. The patient tolerated ABP well, and presented with grade 1 neutropenia, grade I neurotoxicity, and muscle and joint pain.
Figure 2.
CT findings of the target lesions before (A,C) and after (B,D) treatment
Discussion
Traditional solvent-based paclitaxel is prepared with polyoxyethylene castor oil/ethanol as a cosolvent, which may cause allergic reaction and requires pretreatment with a large amount of corticosteroid. It may also extend extended axonal demyelinating neuropathy. The cosolvent creates large droplets that contain paclitaxel in the blood circulation, preventing it from entering into the target tissues from the blood flow, thereby reducing the dose-effect relationship of paclitaxel (8,9). Albumin-bound paclitaxel formulations are lyophilized powder of nanoparticles containing paclitaxel and human serum albumin generated by the high pressure vibration technology. Caveolae is a flask-shaped micro retraction structure in a diameter of 50-100 nm on the cytoplasmic membrane. It exists in many terminally differentiated cells such as fibroblasts, endothelial cells, and adipocytes. Caveolin-1 is an important structural protein of caveolae that plays a critical role in multiple processes like cell endocytosis, transcellular transport, signal transduction and tumorigenesis. The albumin in albumin-bound paclitaxel will bind to specific albumin cell surface receptors on the surface of the endothelial cell membrane, activating caveolae, the main component of plasma membrane vesicles, and leading the receptor-mediated albumin complex into the cave-like invaginations on the cell membrane. The subsequent transmembrane transport will eventually make the drug into the tumor cell (10). Some studies have shown that caveolin expression is 16.7% in adenocarcinoma, 38.4% in adenosquamous carcinoma, 67.1% in squamous cell carcinoma, and 66.7% in large cell carcinoma (11). Preclinical studies have also found that the secreted cysteine-rich acidic secretory protein (SPARC) in some malignant tissues acts similarly to albumin receptors by attracting and adhering to albumin. Therefore, SPARC can specifically adsorb albumin-bound cytotoxic drugs and have them accumulate in tumor cells, thereby increasing the local drug concentration and enhancing the ability of tumor destruction (3,12-14). A member of the stromal cell protein family, SPARC is a multifunctional calcium-binding glycoprotein widely distributed in the body, and is closely linked with the progression, development, invasion and metastasis of tumors. However, inconsistent findings have been associated with the expression and biological role of SPARC in tumor tissues. For example, high expression levels of SPARC are reported in breast cancer, esophageal cancer, and head and neck squamous cell carcinoma, which are related to poor prognosis. On the contrary, low expression is observed in non-small cell lung cancer, prostate cancer, and pancreatic cancer, where SPARC acts as a tumor suppressor. Hence, the role of SPARC in tumor prevention and treatment is yet to be determined. Further research will be needed particularly in non-small cell lung cancer (15).
Many clinical studies abroad have confirmed better efficacy and safety of albumin-bound paclitaxel single-agent treatment or combination with carboplatin in patients with advanced non-small cell lung cancer, compared with conventional solvent-based paclitaxel, and that weekly dosing regimens have better ORR and tolerability compared with administration once every three weeks (2-4,6,7). Socinski and colleagues conducted a Phase III clinical trial of albumin-bound paclitaxel in the treatment of advanced non-small cell lung cancer with the largest sample size to date. The study enrolled 1,050 patients with naive III B/IV non-small cell lung cancer, who were randomized at 1:1 to receive albumin-bound paclitaxel/carboplatin and solvent-based paclitaxel/carboplatin. Albumin-bound paclitaxel was given once weekly. The results showed an ORR of 33% vs. 25% in the two groups (P=0.005), median PFS of 6.3 vs. 5.8 months (P=0.214), median OS of 12.1 vs. 11.2 months (P=0.271), and ORR of 41% vs. 24% (P<0.001) in patients with squamous cell carcinoma and adenocarcinoma in subgroup analysis. Although superior ORR was also associated with albumin-bound paclitaxel in other subgroups, no significant difference was shown. It has also been observed that the incidence of grade 3/4 adverse events like neutropenia and muscle aches is significantly reduced in the albumin-bound paclitaxel group. In addition, several small-scale clinical trials have shown an ORR of 16.3-56% and PFS of 6-9.8 months for patients with non-small cell lung cancer receiving albumin-bound paclitaxel single-agent treatment or combination with carboplatin as the first- or second-line treatment. Grade 3/4 adverse reactions mainly include bone marrow suppression, sensory neuropathy, fatigue, and muscle and joint pain.
Many clinical trials have demonstrated that platinum-based first-line chemotherapy can improve the survive the advanced NSCLC patients; however, after a relatively short disease remission, most patients will need further salvage therapy due to disease progression. Recommended drugs for second-line therapy of advanced non-small cell lung cancer by the NCCN Guidelines include docetaxel, pemetrexed, erlotinib (tarceva), and gefitinib (Iressa) (16-19). However, there is only evidence for erlotinib as an option after the second-line treatment (20). The treatment with docetaxel alone yields an ORR of less than 20% and a DCR between 50% and 60%, and prolongs about 2 months of survival with a median PFS of 2.2-3.9 months compared with the optimal supportive care, making it the first standard second-line chemotherapy supported by clinical trial data. In contrast, the single-agent second-line treatment with pemetrexed has similar ORR, DCR, median PFS, and better tolerability. EGFR-TKIs, such as erlotinib and gefitinib, have an ORR of 23% and median PFS of 5.4 months. They have shown slightly better results as second-line treatment for the Asian population, with significantly improved quality of life compared with chemotherapy. In the BR.21 study, 49.4% patients were treated using erlotinib as the third-line drug with a total median survival of 6.7 months. The larger-scale TRUST study showed (21), an ORR of 22%, DCR of 75% and median PFS of 6.9 months when erlotinib was used alone in the second/third-line treatment. In the present study, all patients were prescribed with a single-agent weekly regimen using albumin-bound paclitaxel, and the resultant ORR was 28.6%, DCR 76.2%, and median PFS 6 months, a better outcome than the traditional second-line chemotherapy in the non-selective population. Although this could be due to the retrospective, single-center design of the present study, it also demonstrated the high therapeutic efficacy of albumin paclitaxel for advanced non-small cell lung cancer even after multiple treatment courses. All patients tolerated the chemotherapy well without any serious adverse event related to the study drug. Grade 3 adverse events included neutropenia, peripheral neurotoxicity, muscle and joint pain, and fatigue, and there was no grade 4 adverse reaction. Although most patients had undergone multiple chemotherapy courses, they were in good physical state at the time of the present treatment, which might have contributed to the satisfying short-term efficacy and tolerance of chemotherapy.
In summary, albumin-bound paclitaxel is a safe and effective option in the treatment of advanced non-small cell lung cancer, and is recommended for certain patients due to its good efficacy even in patients who have received multiple treatment courses. Meanwhile, a large-scale, prospective, randomized clinical study will be needed to provide supportive data for this method.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- 1.Hoang T, Dahlberg SE, Sandler AB, et al. Prognostic models to predict survival in non-small-cell lung cancer patients treated with first-line paclitaxel and carboplatin with or without bevacizumab. J Thorac Oncol 2012;7:1361-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pilz LR, Manegold C, Schmid-Bindert G. Statistical considerations and endpoints for clinical lung cancer studies: can progression free survival (PFS) substitute overall survival (OS) as a valid endpoint in clinical trials for advanced non-small-cell lung cancer? Transl Lung Cancer Res 2012;1:26-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Das M, Wakelee H.Anti-angiogenic agents in Non-Small-Cell Lung Cancer (NSCLC): a perspective on the MONET1 (Motesanib NSCLC Efficacy and Tolerability) study. J Thorac Dis 2012;4:558-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rizvi NA, Riely GJ, Azzoli CG, et al. Phase I/II trial of weekly intravenous 130-nm albumin-bound paclitaxel as initial chemotherapy in patients with stage IV non-small-cell lung cancer. J Clin Oncol 2008;26:639-43 [DOI] [PubMed] [Google Scholar]
- 5.Stinchcombe TE, Socinski MA, Lee CB, et al. Phase I trial of nanoparticle albumin-bound paclitaxel in combination with gemcitabine in patients with thoracic malignancies. J Thorac Oncol 2008;3:521-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reynolds C, Barrera D, Jotte R, et al. Phase II trial of nanoparticle albumin-bound paclitaxel, carboplatin, and bevacizumab in first-line patients with advanced nonsquamous non-small cell lung cancer. J Thorac Oncol 2009;4:1537-43 [DOI] [PubMed] [Google Scholar]
- 7.Socinski MA, Manikhas GM, Stroyakovsky DL, et al. A dose finding study of weekly and every-3-week nab-Paclitaxel followed by carboplatin as first-line therapy in patients with advanced non-small cell lung cancer. J Thorac Oncol 2010;5:852-61 [DOI] [PubMed] [Google Scholar]
- 8.Sparreboom A, van Zuylen L, Brouwer E, et al. Cremophor EL-mediated alteration of paclitaxel distribution in human blood: clinical pharmacokinetic implications. Cancer Res 1999;59:1454-7 [PubMed] [Google Scholar]
- 9.Winer EP, Berry DA, Woolf S, et al. Failure of higher-dose paclitaxel to improve outcome in patients with metastatic breast cancer: cancer and leukemia group B trial 9342. J Clin Oncol 2004;22:2061-8 [DOI] [PubMed] [Google Scholar]
- 10.Pelkmans L, Bürli T, Zerial M, et al. Caveolin-stabilized membrane domains as multifunctional transport and sorting devices in endocytic membrane traffic. Cell 2004;118:767-80 [DOI] [PubMed] [Google Scholar]
- 11.Chen HL, Fan LF, Gao J, et al. Differential expression and function of the caveolin-1 gene in non-small cell lung carcinoma. Oncol Rep 2011;25:359-66 [DOI] [PubMed] [Google Scholar]
- 12.Zhang XJ, Zhang Y. Advances in clinical research and development of new form of paclitaxel. Ai Zheng Jin Zhan 2007;5:66-72 [Google Scholar]
- 13.Desai N, Trieu V, Damascelli B, et al. SPARC Expression Correlates with Tumor Response to Albumin-Bound Paclitaxel in Head and Neck Cancer Patients. Transl Oncol 2009;2:59-64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Von Hoff DD, Ramanathan R, Borad M, et al. SPARC correlation with response to gemcitabine (G) plus nab-paclitaxel (nab-P) in patients with advanced metastatic pancreatic cancer: A phase I/II study. J Clin Oncol 2009;27:abstr 4525.
- 15.Chong HC, Tan CK, Huang RL, et al. Matricellular proteins: a sticky affair with cancers. J Oncol 2012;2012:351089. [DOI] [PMC free article] [PubMed]
- 16.Shepherd FA, Dancey J, Ramlau R, et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol 2000;18:2095-103 [DOI] [PubMed] [Google Scholar]
- 17.Hanna N, Shepherd FA, Fossella FV, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small cell lung cancer previously treated with chemotherapy. J Clin Oncol 2004;22:1589-97 [DOI] [PubMed] [Google Scholar]
- 18.Douillard JY, Shepherd FA, Hirsh V, et al. Molecular predictors of outcome with gefitinib and docetaxel in previously treated non-small-cell lung cancer: data from the randomized phase III INTEREST trial. J Clin Oncol 2010;28:744-52 [DOI] [PubMed] [Google Scholar]
- 19.Fossella FV, DeVore R, Kerr RN, et al. Randomized phase III trial of docetaxel versus vinorelbine or ifosfamide in patients with advanced non-small-cell lung cancer previously treated with platinum-containing chemotherapy regimens. The TAX 320 Non-Small Cell Lung Cancer Study Group. J Clin Oncol 2000;18:2354-62 [DOI] [PubMed] [Google Scholar]
- 20.Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med 2005;353:123-32 [DOI] [PubMed] [Google Scholar]
- 21.Heigener DF, Wu YL, van Zandwijk N, et al. Second-line erlotinib in patients with advanced non-small-cell lung cancer: subgroup analyses from the TRUST study. Lung Cancer 2011;74:274-9 [DOI] [PubMed] [Google Scholar]