Abstract
Heart attack, or acute myocardial infarction (AMI), is a leading cause of death in the United States (US). The most effective therapy for AMI is rapid revascularization: the mechanical opening of the clogged artery in the heart. Forty-four percent of patients with AMI who are admitted to a non-revascularization hospital in the US are transferred to a hospital with that capacity. Yet, we know little about the process by which community hospitals complete these transfers, and why publicly available hospital quality data plays a small role in community hospitals’ choice of transfer destinations. Therefore, we investigated how community hospital staff implement patient transfers and select destinations. We conducted a mixed methods study involving: interviews with staff at three community hospitals (n = 25) in a Midwestern state and analysis of US national Medicare records for 1996–2006. Community hospitals in the US, including our field sites, typically had longstanding relationships with one key receiving hospital. Community hospitals addressed the need for rapid AMI patient transfers by routinizing the collective, interhospital work process. Routinization reduced staff uncertainty, coordinated their efforts and conserved their cognitive resources for patient care. While destination selection was nominally a physician role, the decision was routinized, such that staff immediately contacted a “usual” transfer destination upon AMI diagnosis. Transfer destination selection was primarily driven at an institutional level by organizational concerns and bed supply, rather than physician choice or patient preference. Transfer routinization emerged as a form of social order that invoked tradeoffs between process speed and efficiency and patient-centered, quality-driven decision making. We consider the implications of routinization and institutional imperatives for health policy, quality improvement and health informatics interventions.
Keywords: Interhospital patient transfers, Quality information, AMI, United States
Introduction
Heart attack, or acute myocardial infarction (AMI), is a leading cause of death in the United States (US) (Miniño, 2011). AMI is also a disease for which life-saving therapies are available. The most effective therapy for AMI is revascularization: the mechanical opening of the clogged artery in the heart, a treatment called “Primary Percutaneous Coronary Intervention” (PCI). Yet, almost two-thirds of community hospitals in the US lack the capacity to revascularize patients (Iwashyna, Kahn, Hayward, & Nallamothu, 2010). Despite the importance of rapid treatment, AMI patients who are transferred to PCI-capable hospitals seem to fare better than those who remain in a community hospital and receive thrombolysis treatment (Cannon et al., 2001; De Luca, Biondi-Zoccai, & Marino, 2008). Consequently, 44% of patients with AMI admitted to a non-PCI-capable hospital in the US are transferred to a hospital with that capacity (Iwashyna et al., 2010).
There is a direct relationship between time-to-treatment and survival of AMI patients with an ST-elevation type AMI (STEMI) (Nallamothu, Bradley, & Krumholz, 2007). STEMI is a severe type of heart attack easily detected through an electrocardiogram (EKG). An additional AMI classification, non-ST-elevation AMI (NSTEMI), is a heart attack with less severe coronary occlusion. NSTEMI is typically detected through a combination of EKG and blood tests. Since 2007, American guidelines have recommended that STEMI patients receive PCI within 90 min of their arrival at a hospital (Antman et al., 2008). Time-to-treatment, called “door-to-balloon-time”, became a focus of national quality improvement efforts after it became clear that few STEMI patients received treatment within the recommended time frame (Nallamothu et al., 2007). Professional organizations have developed performance measures and quality improvement campaigns to focus on timeliness in treatment (Krumholz, Anderson, et al., 2008). The Door-to-Balloon (D2B) Alliance advocates time-reduction strategies such as activation of the catherization laboratory (the facility that provides PCI treatment) with a single call (Krumholz, Bradley, et al., 2008). The broader-based Mission: Lifeline program promotes regionalization of STEMI care, including creating protocols for care pathways between referring hospitals and PCI-capable hospitals (Diercks, 2010). These campaigns have contributed to a significant reduction in door-to-balloon times in the US between 2005 and 2010 (Krumholz et al., 2011) with 63% of patients presenting at a PCI-capable hospital receiving treatment within the recommended time frame (Wang et al., 2011). In contrast only 10% of STEMI patients transferred from a community hospital to a PCI-capable hospital receive treatment within 90 min, suggesting that interhospital patient transfers may be a source of critical delays in high-stakes care.
Although patient transfer is a vital and problematic component of AMI care, it has received little scholarly attention with extant literature focusing on quality improvement efforts at PCI-capable hospitals, rather than their community hospital counterparts (e.g., Bradley et al., 2006). A lack of understanding about the processes by which community hospitals implement STEMI transfers impedes efforts to improve patient care and outcomes (e.g. pilot programs to regionalize STEMI care (Blankenship et al., 2011)).
Beyond overall consideration of how community hospitals complete interhospital patient transfers, we know little about the sub-activity of choosing a transfer destination. This is important because revascularization hospitals vary widely in their quality of care, and community hospitals typically have several nearby options (Chen et al., 2003; Krumholz, Normand, Spertus, Shahian, & Bradley, 2007). Centers for Medicare and Medicaid Services, part of the US Department of Health and Human Services, have recently made statistically rigorous hospital outcomes data for AMI readily available to the public over the Internet (U.S. Department of Health and Human Services, 2011). These rigorous, clinically relevant quality metrics (e.g., death and readmission rates for AMI patients) are based on Medicare claims data, and are made possible by recent methodological advances in multi-level modeling, comorbidity adjustment, and reliability adjustment (e.g., Dimick, Staiger, Baser, & Birkmeyer, 2009).
However, for quality data to improve patient care (Dudley, Johansen, Brand, Rennie, & Milstein, 2000), they must be used. Empirically, such data had little influence on hospitals’ improvements in cardiac care quality (Tu et al., 2009) or on patient choices of cardiac surgery providers (Schneider & Epstein, 1998). Cross-sectionally, AMI patients are somewhat more likely to be referred to hospitals with better publicly reported outcomes, but quality data play a small role relative to other factors (Iwashyna et al., 2010). If hospital quality information is valid and freely available, why would it not govern the choice of a transfer destination for AMI patients? Existing literature suggests that factors other than quality information might drive transfer decisions. Diffusion of innovations research stresses the importance of colleagues in the choice to use a new technology (Rogers, 2003) — such as, perhaps, a web portal of hospital quality information. Similarly, information seeking research points to the importance of colleagues as information sources (e.g., Bennett, Casebeer, Kristofco, & Collins, 2005). Patient preferences and insurance rules could also drive referral choices (Anthony, 2003; Dy, Rubin, & Lehmann, 2005). Such organizational decisions may also manifest organizational political dynamics (Heimer & Stinchcombe, 1999).
Most prior research on patient transfers has adopted a health management perspective that—while indispensable for clinical care—may miss insights facilitated by social scientific study. Social scientific approaches can clarify how social and organizational relationships both influence care and challenge assumptions about how care is delivered. Anthony (2003) demonstrated that managed care shifted referral patterns among physicians as referrals were increasingly made to unknown colleagues rather than to other physicians with a high social status (Shortell, 1973). Similarly, Dy et al. (2005) identified factors, such as perceived quality, that may stimulate patient requests for transfers from community hospitals to tertiary care centers. Our study broadens the research lens to encompass interorganizational relationships, and contributes to the growing sociological interest in health care arrangements that extend beyond single organizations (Davies, 2003).
Theoretical framework: organizational routines
Linking individual behavior with collective action (Becker, 2008), organizational routine theory offers theoretical traction for understanding recurrent “problems” in health care organizations. Organizational routines are “recurrent interaction patterns” involving multiple actors — a “typical pattern of accomplishing” a task within an organization (Becker, 2008). Organizational routines are a collective phenomenon, and may be distributed across, time, space, individual actors and organizations (Becker, 2004). They operate by triggering individual habits (Hodgson, 2008), which are stored as procedural memory (Cohen & Bacdayan, 1994). Thus routines may be tacit in character, and they may or may not be codified. Routines tend to be locally and historically specific since they are linked to local learning processes and selective environmental pressures (Becker, 2004). Routines are the basis of organizational capabilities (Becker, 2004) because of their contributions to: storing and transmitting knowledge (Nelson & Winter, 1982), facilitating coordination and simultaneity of activity (Grant, 1996), reducing uncertainty (Becker & Knudsen, 2005) and conserving cognitive resources (Becker, 2004).
There has been relatively little development of organizational routine theory in health care. Exceptions include Greenhalgh’s et al. (2007) study that showed that process routinization explained differential adoption of new care practices by physician practices. Heimer’s (1999) study of a neonatal intensive care unit also demonstrated that new rules emanating from outside organizations are “domesticated” within them through their incorporation into routines. The current focus on protocols as tools for quality improvement suggests the power of organizational routines for directing behavior; however organizational routines may exist without formal protocols, but for protocols to be effective, they must create new organizational routines (Dixon-Woods, Bosk, Aveling, Goeschel, & Pronovost, 2011; Gawande, 2010).
Given that time pressured, repetitive activities are more likely to be routinized (Cohen & Bacdayan, 1994), we hypothesized that the theory would explain the work of urgent patient transfers. Additionally, because we would expect routinized transfer behavior to be unresponsive to new information about hospital quality, we hypothesized that organizational routines theory would help explain the fact that hospital quality does not appear to drive destination selection. Therefore, we posed the following research questions:
RQ1. Do organizational routines play a central role in the actual practice of community hospitals in the interhospital transfer of AMI patients?
RQ3. Do qualitative data regarding transfer destination choices indicate a routinized decision making pattern? If so, why are these decisions routinized?
RQ3. Is quantitative, nation-wide data on the interhospital transfer patterns consistent with a routinized destination selection procedure?
Research methods
We conducted an exploratory, mixed methods study involving 1) semi-structured, in-depth interviews with health care providers at three community hospitals in a Midwestern state; and 2) quantitative analysis of nationwide data for all patients who received critical care in fee-for-service Medicare.
Qualitative interviews
We conducted interviews at emergency departments (EDs) and intensive care units (ICUs) in three hospitals intentionally sampled to represent the geographic and administrative diversity of community hospitals — non-teaching hospitals that regularly transferred patients for common conditions. One hospital was urban, one suburban, and one rural. One was independent, one under longstanding ownership by a tertiary care center, and one had recently been purchased by a tertiary care center — although their care was not clinically integrated. None of these hospitals had a cardiac catherization laboratory, and routinely transferred STEMI patients for PCI rather than treat with thrombolytics. Further details about the sites are presented in Table 1.
Table 1.
Characteristics of the three hospitals.
| Setting | Site One | Site Two | Site Three |
|---|---|---|---|
| Suburban | Urban | Rural | |
| Relationship to tertiary care center | Recently purchased, not clinically integrated | Longstanding ownership by tertiary care center | Independent |
| Teaching hospital | No | No | No |
| Cardiac catheterization facilities | No | No | No |
| Hospital beds (not critical care) | 69 | 74 | 57 |
| Critical care beds | 6 | 16 | 3 |
| Payer mix: % Medicare (of inpatient days) | 28% | 62% | 38% |
| % of Medicare transfers to most common transfer hospital | 71% | 68% | 87% |
Data Sources: 2008 Centers for Medicare and Medicaid Services (CMS) Healthcare Provider Cost Reporting Information System, the 2008 MedPAR files for inpatient admissions, and Hospital Compare data for 2010.
Using a semi-structured interview guide, face-to-face interviews were conducted between fall 2009 and winter 2010. Questions addressed patient transfer processes, including factors determining where a patient was transferred. Interviews ranged from 30 to 120 min. Within each hospital, we used a snowball sampling method to recruit participants. We arranged initial interviews with nurses and physicians working in the ED and the ICU, as identified by the medical or nursing director at each site. Initial contacts then referred their colleagues who also participated in AMI patient transfers. Typically we interviewed all staff in a unit during the two consecutive shifts. One exception to this sampling strategy occurred at Site Two, where there was only one attending physician in the ICU; this person did not consent to be interviewed. Accordingly, at this site, interviews began with non-attending clinical staff, and excluded the only physician on duty at the time. Interviews were audio-taped and transcribed.
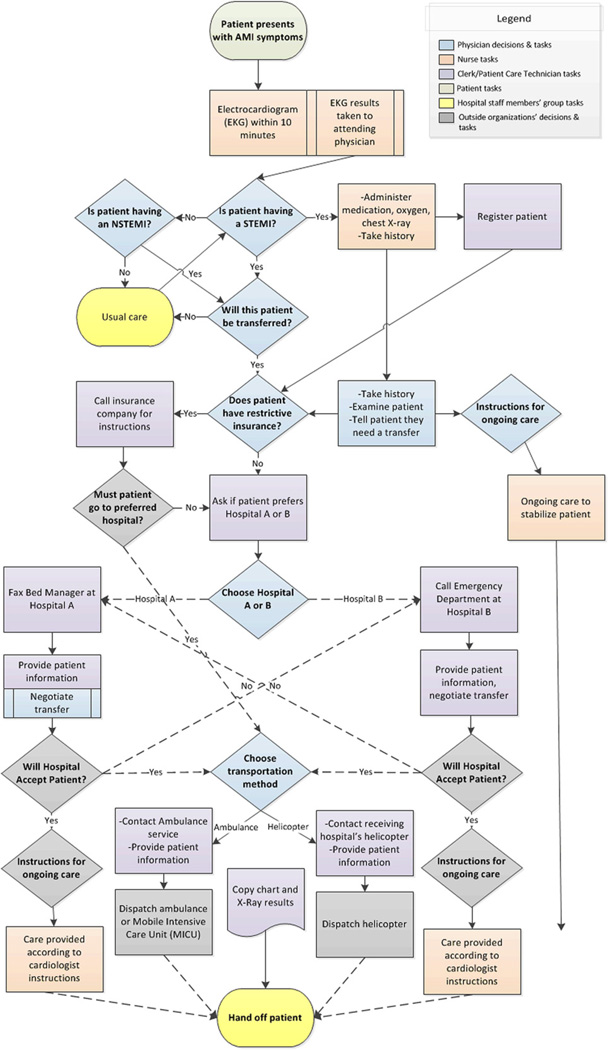
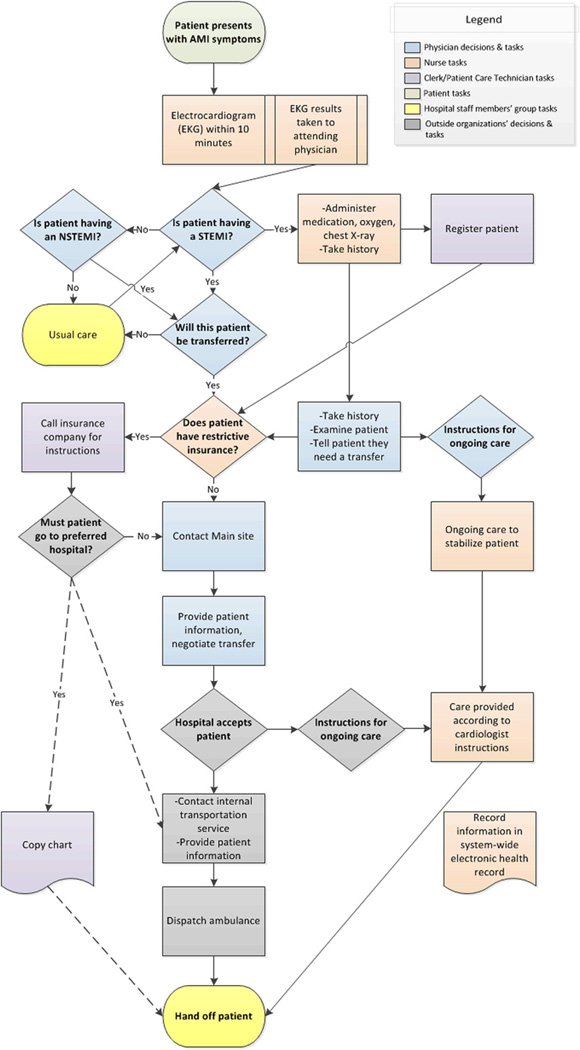
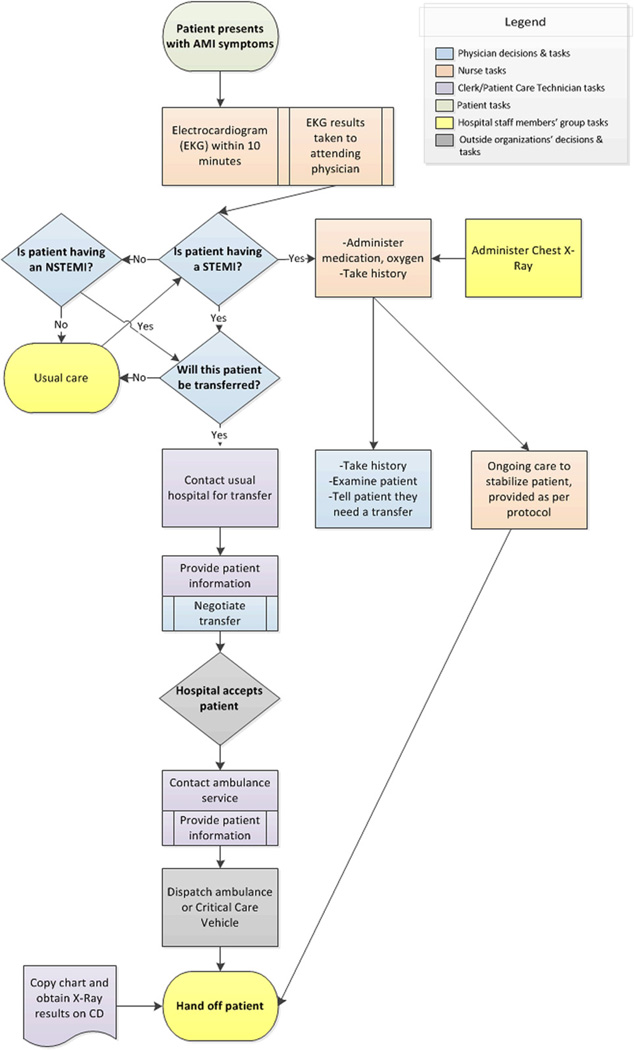
Data were analyzed by content analysis (Krippendorff, 2004) in NVivo software, with a codebook derived from the literature on patient transfers, organizational routines and organizational decision-making. After initial coding by one author (T.C.V.), the second author (E.A.B.) recoded a random sample of 20% of the interviews to assess interrater reliability, which was substantial (interrater agreement: 96.9%; Cohen’s κ = 0.61) (Landis and Koch, 1977). After coding, transcripts were clustered by hospital to search for hospital-specific patterns. Each site’s transfer process was then represented in a cross-case, time-ordered display (Miles & Huberman, 1994). Each display was refined iteratively through constant comparison with individual transcripts. These displays were drawn with Visio diagramming software, as Figs. 1, 2, and 3, which correspond to Sites One, Two and Three, respectively. This study was declared exempt from ongoing ethical review by the Institutional Review Boards of the University of Michigan Medical School (IRBMED).
Fig. 1.
Site One AMI patient transfer routine.
Fig. 2.
Site Two AMI patient transfer routine.
Fig. 3.
Site Three AMI patient transfer routine.
Quantitative analysis of Medicare records
We used Medicare claims data to examine whether each hospital had a primary transfer partner, along with the stability of transfer relationships over time. Medicare records have advantages for this purpose: Medicare provides hospital insurance for nearly all Americans aged 65 and above, most of whom are in a fee-for-service program. Medicare is accepted at nearly all hospitals, and for AMI care, it provides attractive reimbursement (Iwashyna et al., 2010). Medicare places few restrictions or incentives on interhospital patient transfer decisions.
We examined all hospitalizations for short-stay hospitals in the MedPAR (Medicare Provider Analysis and Review) files for 1996–2006. These data include claims for inpatient hospital stays for Medicare beneficiaries, along with relevant diagnoses. Each hospital stay begins with an admission date, and ends with a discharge date, and may include one or more service claims. We identified interhospital critical care transfers using a previously published methodology (Iwashyna, Christie, Moody, Kahn, & Asch, 2009; Unnikrishnan, Patnaik, & Iwashyna, 2011). Briefly, we identified all hospitalizations during which a patient used an ICU or coronary care unit. Because there are no separate Medicare claims for the interhospital transfer, a transfer was defined as two critical care hospitalizations for AMI, where the admission date for the second hospitalization was the same or one day more than the discharge date for the first hospitalization.
To assess the stability of interhospital transfer patterns, we defined each hospital’s primary referral destination as the hospital to which it sent the most patients. We divided the data into six month periods (n = 20). For each period, we examined the fraction of hospitals for which 50% or more of interhospital critical care transfers were sent to a single primary referral destination. We further examined the number of distinct primary referral hospitals that each hospital used over the decade-long period.
Results
Study participants
Interviews were conducted with 25 key actors in the transfer process including ED and ICU nurses, physician, patient technicians and clerks (See Table 2).
Table 2.
Roles of respondents at each site.
| Site One | Site Two | Site Three | |
|---|---|---|---|
| Nurses | 6 | 8 | 4 |
| Physicians | 1 | 0 | 2 |
| Patient Technicians and Clerks | 2 | 0 | 2 |
RQ1. Do organizational routines play a central role in the actual practice of community hospitals in the interhospital transfer of AMI patients?
Features of organizational routines
Routines are collective, repeated and stable activities. To rapidly complete AMI patient transfers, each field site had significantly restructured its transfer process for STEMI patients toward greater routinization. These changes emerged from deliberate planning of new care pathways in response to the aforementioned care guidelines. Consistent with an organizational routine, such care pathways included multiple staff members. For example, a nurse at Site One explained that, “…at a leadership meeting…we…agreed…when we do have an MI…everyone comes to that room and helps…” Also in line with routinization, staff established defined roles in the process — such as the decision at Site One that a charge nurse would gather all staff to complete the transfer.
Routines are also characterized by recurrence: without repetition, a process cannot become routine. Participants at all sites indicated that STEMI patients frequently presented at their hospitals, although not at predictable intervals. As this Site Three technician said, sometimes, “it’ll be, ‘We’ve had three STEMIs this week’…” Similarly, this Site Two nurse said, “…it’s typical because we do it so often…”
Organizational routines are initiated by cues to action in a stable environment, which automatically trigger coordinated, individual habits. Similarly, at each site, the routine was initially cued by the arrival of a patient with potential symptoms of an AMI, particularly chest pain (see the first box in Figs. 1–3). Such patients were identified by a check-in clerk, who notified nursing. A Site One nurse explained the routinized response,
“…if…they’re complaining of chest pain, we immediately take them by wheelchair back to an appropriate room…and …two to three staff…will come in and we immediately get them set up for an EKG monitor.”
The second cue to action was a clear diagnosis of an AMI, the first decision box in Figs. 1–3. The most straightforward diagnosis was a clear STEMI identified by a physician’s reading of the EKG results. Such identification was coterminous with the decision to transfer, as this Site Three nurse explained, “…there really isn’t a discussion, it’s automatic. If it’s a STEMI, it goes.” While the physician was technically responsible for the transfer decision, the fit between a STEMI diagnosis and clinical guidelines assured the results of this “decision”. These transfers were not pursued only if the patient refused treatment through a do-not-resuscitate (DNR) order. The diagnosis of a STEMI was also a signal for hospital staff to begin their parts in the transfer process. As this Site Three nurse described, “[The] physician… walked in and he said, “It’s a STEMI” which is like signal to us…”
If the patient did not show immediate signs of a STEMI on EKG, patients underwent monitoring and continued testing to determine if they were having an NSTEMI, or to determine another diagnosis (see “Is patient having an NSTEMI?” in Figs. 1–3). Blood tests were evaluated for a non-ST-elevation AMI (NSTEMI) — a condition with different treatment guidelines, albeit ones that also favor transfer. Figs. 1–3 show a process whereby STEMI patients receive an automatic transfer and NSTEMI patients do not – although many NSTEMI patients were ultimately transferred to a PCI-capable hospital. Additionally, some patients evolve clear signs of a STEMI over time, and thus were transferred only after usual care (see Figs. 1–3). Notably, participants described the AMI transfer process inclusively — including both STEMI and NSTEMI transfers. Accordingly, we focus on AMI transfer processes as a whole in this paper.
After a STEMI diagnosis or an assessment that PCI treatment was indicated for an NSTEMI patient, there were two cues to action at the interface with other organizations: contact with receiving hospital and contact with transporter (ambulance or helicopter). These processes were initiated with a phone call or fax, depending on the external organization’s policy. These processes, however, varied in complexity and substance across sites (see decision boxes and ensuing steps in Figs. 1–3).
A critical feature of organization routines is stability of interaction patterns — evident at all three hospitals. The AMI transfer process as a whole was described by nurses at each site with adjectives such as “methodical”, “streamlined”, “generic” or “smooth”. Physicians characterized the STEMI process in particular as an established series of decisions, with a Site Three physician describing it as a positive manifestation of “cookbook medicine”. Clerks and technicians also described the STEMI transfer process as a predictable series of tasks, repeated in an expected order. As this Site Three technician explained, “…with a STEMI, you do everything the same just about within reason.” As a result, a Site Three physician described interhospital transfers of STEMI patients as “second nature”.
The AMI transfer process had several sources of stability. One stabilizing factor concerned the existence of a narrow roster of receiving hospitals (see RQ2 and RQ3). Several receiving hospitals also designated consistent contact points for negotiating the transfer — such as fax lines or clearinghouses staffed by bed managers. As a technician explained, “That one call does it all…a clearinghouse for getting transfers done.”
Standardization also stabilized the process — particularly at Site Three. In collaboration with its preferred hospital — which had spearheaded the development of a regional STEMI care network — the site adopted a standard care pathway, forms, checklists and standing orders to guide the process. This standardization involved a reduction in decision points, as evidenced by this site’s simpler patient transfer process (see Fig. 3). This Site Three physician explained the effects of creating a standard set of medications for use with each STEMI patient,
“…Some wanted Plavix, some didn’t want Plavix. Some wanted Integrilin, some didn’t want Integrilin, some didn’t want… anything… but transfer. Some wanted heparin, some wanted Lovenox you know…who knew what you were going to end up with. So now…with the protocol in place, you don’t have to ask anything, you just give the stuff and so it’s much easier…”
In contrast, as Figs. 1 and 2 show, staff members at Sites One and Two awaited instructions from a receiving cardiologist before administering medications, since their preferences often differed. For example, this nurse at Site One explained that, “… he’ll talk to the doc at the cath lab as far as what orders they want…heparin or nitroglycerin or Lopressor…we go by what they want…”
Figs. 1–3 show process variations at each site based on: differential diagnosis between STEMI and NSTEMI patients; the presence of two ‘usual’ destinations at Site One; intermittent insurer-driven hospital selection at Sites One and Two; variable medications based on receiving cardiologist instructions and Sites One and Two; and the choice between transporters at Site One. Nevertheless, participants conceptualized AMI patient transfers, including both STEMI and NSTEMI patients, at their own sites as similar enough from performance to performance to be labeled a coherent, repetitive work process.
Effects of organizational routines
The STEMI patient transfers at each site demonstrated many of the posited effects of organizational routines. One effect is uncertainty reduction among people participating in work routines. This was partially achieved by standardizing key decisions in advance, such as the aforementioned medications for AMI patients. Such standardization of decisions had the effect of increasing staff members’ certainty as to the steps involved in completing a transfer. Additionally, all staff conveyed a firm understanding of their own roles in the transfer process, which could even led to an ability to anticipate what would be needed of them, and this Site Two nurse explained, “…we know the patient’s going to need…we’re getting the nitro, we’re getting the heparin, we’re getting the stuff that we know they’re about to order.” The resulting certainty regarding the process decreased staff members’ earlier uneasiness, as this Site Three nurse said, “…it’s made us much more confident…because…it’s always the same…”
According to hospital staff, current approaches to patient transfers conserved cognitive resources — another theorized effect of organizational routines. Because unanticipated changes in the condition of AMI patients occur frequently, providers need to be constantly alert — at the same time as completing an urgent transfer. These complex and concurrent demands are challenging in the face of human cognitive limitations. Staff noted that the often-predictable nature of the AMI transfer process thus helped them manage the attentional demands of the transfer, allowing them to focus on direct patient care. For example, a structured transfer routine made it possible to delay administrative procedures associated with the transfer until after the patient was gone. The importance of conserving one’s cognitive resources for patient care was evidenced by staff frustration when other aspects of the transfer process competed for their attention, as this Site One nurse explained,
“…for me to stop caring for a patient that I’m acutely concerned about… to re-focus on a new piece of equipment is a difficult transition.”
The coordination benefits of organizational routines were also evident. Simultaneity of activity was facilitated by the clear roles that each person assumed. The routine aligned people’s goals and incentives. For example, Site Three staff had embraced “time to transfer completion” as a collective performance goal for STEMI transfers, as this nurse noted, “I think that pulls people together as a team…we have this goal, we want them out in 30 min. It makes it more cohesive…” The coordinative benefits of routinization may be particularly clarified by examining where they are absent. In a site with comparatively greater process variability (Site One), several staff members complained that they were not always aware of their collective progress in the transfer process. Staff at this also site noted that communication during the transfer process was rushed and disjointed, and clerks complained that they did not always have needed information. This clerk suggested that the transfer process would be improved if,
“…when they initially come out and ask us to start the calling…they could provide us information that we need, versus, sometimes they’ll go by and kind of shout it at us and then they’ll come back and shout something else at us…”
RQ2. Do qualitative data regarding transfer destination choices indicate a routinized decision making pattern? If so, why are these decisions routinized?
Routine choices and path dependence
Participants relied upon a usual pattern of activity when selecting a transfer destination. As this Site Three nurse explained, “It’s extremely rare for us to go anywhere but [Hospital A]. A Site Two nurse said, “…an AMI usually goes to [Main site].” At Site One, however, the availability of two proximate, high-quality revascularization hospitals made it more common to choose between options (see Fig. 1).
The presence of one usual destination at Sites Two and Three meant that it was rare for there to be an explicit choice of transfer destination at the point of care (see Figs. 2 and 3). Rather, the decision to transfer was immediately followed by contacting the “usual” hospital. As this Site Three technician explained, the phone call to the preferred destination was an automatic part of the work routine, “…we generally transfer to [Hospital A] so we get [Hospital A] on the phone…” And at Site Two, they called the main site as soon as a decision to transfer was made, as this nurse explained, “…once they let them know [that the patient will be transferred], we call over to [Main site] bed control…”
Yet even at Site One, the explicit choice was between two established destinations —suggesting some path dependency, as there were other hospitals within a feasible transfer radius. Nevertheless, decisions were often constrained by bed availability. While refusal of AMI patients was infrequent, its occurrence transformed decision making into a process of elimination, as this Site One clerk explained,
“…the receiving doctor [will] refuse the patient…then the doctor will come out and say, “Okay get the [Hospital A] on the line, [Hospital B] is full…” so then I call [Hospital A] and then we go through the…whole thing all over again.”
How was this narrow choice field constructed?
Espoused ethical norms would suggest that patient preferences should influence the decision of where to transfer a patient. But in the interviews, patients and families were described as exerting little overt influence on transfer decisions, unless the patient had a DNR. (One insurance company also had highly restrictive transfer policies, so patients with that plan were never given a choice of hospital.) For patients with other insurance, it was only at Site One that physicians regularly consulted patients regarding their preferred destination, and even then, this involved providing them with a narrow range of choices. As this physician explained,
“Frequently we just tell the patients they have to go to cath lab and we usually tell them, it’s usually people either go to [Hospital A] or [Hospital B] because that’s the two closest and quickest hospitals to get them to and they’ll give us their preference.”
Even so, physicians did not always elicit patient preferences, as this nurse observed, “…if [the doctors] are not exactly committed to one or the other, I’ve heard them ask patients.”
Although eliciting preferences was not a part of every hospital’s transfer process, staff at all three sites asserted that they would respect a patient’s request for a hospital other than their preferred destination. However, they did not necessarily give patients a structured opportunity to make a choice, instead moving forward with their default destination unless a patient or family member requested something different, as this Site Three nurse related, “If the patient says, ‘I’d like to go to [Hospital B]’ we’ll go to [Hospital B]. If they don’t specify, we go to [Hospital A]…” Such a request, particularly at Sites Two and Three, would require a patient or family member to intervene in an ongoing, time-sensitive work process conducted by staff members dispersed throughout the emergency room. Not all patients or families might feel comfortable doing so.
Principles of beneficence might assert that patients should be steered toward the best destination hospitals. Despite the US investment in making hospital quality metrics publicly available, consideration of quality data was virtually absent from discussions of the transfer process. “Quality” was mentioned broadly by only one staff member at Sites Two and Three, respectively. When mentioned, quality was assessed heuristically, with reference to the marketing efforts of their chosen receiving hospitals. Thus, a nurse said that Site Two’s preferred destination hospital “report[s] themselves to be one of the top 50 cardiac hospitals in the nation…” However, an alternative hospital with a 1.1-percentage point lower AMI patient mortality rate was only ten miles further from the sending hospital — suggesting that this other hospital might be a more quality-driven choice.
Instead, the routine nature of transfer destination selection could be traced to decisions made at the institutional level, which helps to explain the observed stability of transfer relationships. Notably, these institutions narrowed the “choice field” beyond straightforward questions of patient well-being, introducing their own priorities into transfer relationships.
Most prominently, the financial interests of hospitals and insurers appeared to drive the establishment of ongoing, preferential transfer relationships. According to participants, because patients were viewed as sources of revenue in a profit-driven health care market, both sending and receiving hospitals channeled patients to preferred facilities. At Site Two, this seemed to be reinforced by the community hospital’s membership in a hospital system that included a PCI-capable main branch. Joint ownership meant that the main branch was regarded as the destination for AMI patients. As this nurse explained, “They don’t want anybody transferred out of the system, that’s revenue that’s going to be missed.”
At Site Three, financial considerations were also critical. This independent hospital’s preferred destination had marketed itself extensively to community hospitals while establishing a regional STEMI care network. As this Site Three physician noted, this tertiary hospital monitored transfer requests and reprimanded staff who refused AMI patients because the hospital “…doesn’t want them refusing patients”. Furthermore, a more proximate hospital that was the non-preferred destination was perceived as “competition” for the community hospital studied, as this Site Three physician said,
“…we’ll transfer them to [Hospital A] instead because we have a competition to [Hospital B]. You know, there’s kind of some bad blood between the two hospitals.”
Financial imperatives also drove the participation of insurers in transfer destination selection at Sites One and Two. This was true even for Medicare patients, since many had secondary insurance providers. At these sites, one insurance company diverted patients to a hospital under its ownership. Although this hospital had better-than-average AMI care quality, it was more distant than either site’s preferred destination(s).
It was only at Site One that institutional revenue considerations receded often enough to permit direct choice between two nearby, PCI-capable hospitals. Yet, physicians at Site One were intermittently unable to transfer patients to one of these hospitals due to bed shortages, “…it seems like [Hospital B] is full a lot less than [Hospital A] is…” Accordingly, transfer decisions were again constrained by institutional factors — in this case, the limited supply of beds and skilled cardiologists and surgeons at area hospitals. Importantly, the hospital that most frequently turned patients away had the best AMI mortality rates in the region.
RQ3. Is quantitiative, nation-wide data on the interhospital transfer patterns consistent with a routinized destination selection procedure?
To assess the generalizability of our qualitative results, we analyzed Medicare claim records, after finding that each of our field sites, initially selected for geographic and administrative diversity, had a primary transfer destination that received 68–87% of their AMI patients (see Table 1). To determine whether this pattern was also characteristic of other hospitals in the US, we then examined 765,171 interhospital AMI transfers between 5083 distinct hospitals between 1996 and 2006. Within these data, 2200 hospitals accounted for more than 75% of all transfers. Most hospitals had at least three potential revascularization hospital choices nearby (Iwashyna et al., 2010). We designated the hospital that received most transfers a target hospital’s “primary referral partner” if that hospital received 50% or more of the patients in a given target hospital’s transfers. Across all six-month periods, 64.6% of hospitals had a primary referral partner, meaning they sent 50% or more of their AMI patients to a single other hospital. Furthermore, hospitals are very stable in their choice of primary referral partner. As shown in Table 3, 72.0% of hospitals had the same primary referral partner across all the six-month periods included in the data. Table 3 also shows that 97.0% of hospitals had only one or two primary referral partners in the 11 years of data. That is, AMI patient transfer destinations were typified by relatively monogamous, longstanding referral relationships. Accordingly, the routine destination selection pattern observed at field sites appears to be typical of the US.
Table 3.
Primary referral hospitals for AMI patient transfers, for all US hospitals included in Medicare claims data.
| Number of primary referral hospitals, 1996–2006 |
Number (%) of hospitals |
|---|---|
| 1 | 1583 (72.0%) |
| 2 | 550 (25.0%) |
| 3 | 60 (2.7%) |
| 4 | 7 (0.3%) |
Discussion
Community hospitals accomplished the time-sensitive transfer of AMI patients through routinization. The transfer routine involved cueing of a recurrent, stable and collective work process. This was true of the overall approach to the work of transferring STEMI patients, and for the micro-decision of destination selection. Nationwide quantitative data reinforced that the processes seen at these three sites may be generalizable to the US system as a whole. Although physicians were nominally the decision makers in patient transfers, participants at our field sites believed that revenue-maximization efforts by hospitals and insurance companies channeled patients to preferred destinations. Due to the primacy afforded to financial considerations, routinization was achieved alongside the exclusion of some information from the decision-making process, notably patient preferences and published hospital quality data.
This study is among the first to describe work routinization as a key organizing factor in patient transfers, pointing to a scarcely documented form of social order in health care. As described, a routine approach to care organization offered many benefits, including speed, uncertainty reduction and coordination. Our research, however, points to important tradeoffs that result as routinization increases, regardless of whether written protocols are used (e.g., Gawande, 2010). A focus on speed may mean that community hospitals do not use new information about hospital quality, and they may thus send patients to lower quality hospitals. Differences in rigorous death rate quality metrics suggest that this may decrease their likelihood of survival.
Moreover, such a routine process gives patients little opportunity for patients to state a preferred destination. Although involvement of acute AMI patients in decisions is challenging because they may be in pain, distressed, or sedated, there is an ethical and legal obligation to obtain informed consent while balancing the need for speedy treatment (Levine et al., 2011). Patient or proxy consent is routinely obtained before interhospital transfer or PCI is performed in clinical practice; many recent STEMI trials obtain full written informed consent as a part of their time-sensitive enrollment practices (Foex, 2004). We argue that, given its clinical gravity, transfer destination quality information should be included in these mandated informed consent discussions. This appears feasible since some AMI patients wish to participate in decisions about their care, even in emergency situations (Decker et al., 2007). Because informed consent to participate in a clinical trial is more demanding and time consuming than choosing a transfer destination based on quality, we believe that precedent shows the plausibility of patient participation in this important decision.
Our findings suggest that there is a need to revisit the assumptions of US federal policies mandating the provision of hospital quality information. These policies assume that by providing quality information and aligning incentives, patients and purchasers will be empowered to select better care, thus stimulating hospitals to compete on care quality. Our findings, however, suggest that this agenda is based on an unrealistic model of health care work in two ways. First, the patient transfer process provides an example of the institutional embeddedness of the health care market (e.g., Scott, Ruef, Mendel, & Caronna, 2000). The set of hospitals to which a patient may be transferred is not simply the set of capable hospitals within a given geographic locale; previous, established relationships determine which destinations are considered possibilities, and that limitation of the choice set does not occur primarily on the basis of quality (Lomi & Pallotti, 2012). With greater routinization, destinations are not so much selected as they are automatically triggered. Therefore, there is little opportunity for a countervailing personal push for quality (based on publicly reported outcomes) to balance institutional structures focused on goals other than patient outcomes. Effective competition between health care organizations cannot be assumed nor achieved through simple provision of information.
Our research suggests that patient transfer destination selection is more an issue of priorities than information. Competition for revenue results in strategic moves to channel patients to preferred destinations — a key form of market advantage. Community hospitals may also undermine perceived competitors by refusing to transfer to them, as at Site Three. Hence, a shift toward more quality-focused patient transfers would necessitate hospital redefinition of the problem as one of clinical (rather than simply financial) import. One informatics-based strategy to do so might be to incorporate patient transfers into clinical decision support systems (CDSS), rather than simply making information available on a public website. One benefit of such an approach would be greater integration of patient transfers into the care process. However, disadvantages to this approach include distance from the manual processes in some hospitals (such as our Sites One and Three) and difficulties surrounding information sharing across organizations. Accordingly, it is unlikely to be enough to simply design an informatics solution and hope for the best. Indeed, changes that field sites had already made to their STEMI patient care processes (such as rapid EKGs for chest pain patients) resulted from both Medicare reimbursement pressures and national quality campaigns. As Heimer (1999) argues, national guidelines were “domesticated” through incorporation into organizational routines. Thus, organizational change would require proactive strategies to undo established routines and create new ones — routines that incorporate current hospital quality information, as well as new information that is available annually. Such establishment of new routines would be optimally pursued through training and job design, while establishing controls for critical steps in the process (Pentland & Feldman, 2008). From a policy perspective, such controls might be provided by incentives for quality-driven patient transfers — perhaps through new Medicare reimbursement strategies. Broadly, then, there is a need to develop a socio-technical system (Coiera, 2007) for patient transfers — a system in which organizational processes support the use of technologies containing care quality information, and vice versa.
Several limitations of this paper should be noted. We conducted interviews at three purposively selected community hospitals in the US; additional perspectives may be present in other settings. Additionally, aside from data gathered from Medicare records, we cannot ascertain the generalizability of observed phenomena to the population of community hospitals. We also heavily interviewed nurses, given the prominent role they play in patient care at community hospitals and their central perspective on the work of hospitals; other providers may offer different views. Complementary data from direct ethnographic observation could determine whether actual behavior varies from the self-reported behavior reported here.
In sum, this study documented the revenue and relationships at stake in AMI patient transfer decisions, as well as the routine nature of the work process. Observed characteristics of the patient transfer process provide insight into why community hospital staff may little value objective hospital quality information. Our research therefore provides a portrait of the barriers that would be faced in attempting to shift transfers to a more quality-driven and patient-centered process. Accordingly, we should anticipate that such changes would be controversial and meet with institutional resistance. Consequently, efforts to promote quality-driven interhospital patient transfers should be re-conceptualized as a complex process that will require sustained health policy and organizational change efforts, as well as informatics.
Acknowledgments
We gratefully acknowledge funding from the US National Institutes of Health – K08 HL091249 and the University of Michigan Office of the Vice President for Research Pilot Grants. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the US government.
References
- Anthony D. Changing the nature of physician referral relationships in the US: the impact of managed care. Social Science & Medicine. 2003;56(10):2033–2044. doi: 10.1016/s0277-9536(02)00198-3. [DOI] [PubMed] [Google Scholar]
- Antman EM, Hand M, Armstrong PW, Bates ER, Green LA, Halasyamani LK, et al. 2007 Focused update of the ACC/AHA 2004 guidelines for the management of patients with ST-elevation myocardial infarction. Circulation. 2008;117(2):296–329. doi: 10.1161/CIRCULATIONAHA.107.188209. [DOI] [PubMed] [Google Scholar]
- Becker MC. Organizational routines: a review of the literature. Industrial and Corporate Change. 2004;13(4):643–678. [Google Scholar]
- Becker MC. The past, present and future of organizational routines. In: Becker MC, editor. Handbook of organizational routines. Northhampton, MA: Edward Elgar; 2008. pp. 3–14. [Google Scholar]
- Becker MC, Knudsen T. The role of routines in reducing pervasive uncertainty. Journal of Business Research. 2005;58(6):746–757. [Google Scholar]
- Bennett NL, Casebeer LL, Kristofco R, Collins BC. Family physicians’ information seeking behaviors: a survey comparison with other specialties. BMC Medical Informatics and Decision Making. 2005;5(1):9. doi: 10.1186/1472-6947-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankenship JC, Scott TD, Skelding KA, Haldis TA, Tompkins-Weber K, Sledgen MY, et al. Door-to-Balloon times under 90 min can be routinely achieved for patients transferred for ST-segment elevation myocardial infarction. Journal of the American College of Cardiology. 2011;57(3):272–279. doi: 10.1016/j.jacc.2010.06.056. [DOI] [PubMed] [Google Scholar]
- Bradley EH, Herrin J, Wang Y, Barton BA, Webster TR, Mattera JA, et al. Strategies for reducing the door-to-balloon time in acute myocardial infarction. New England Journal of Medicine. 2006;355(22):2308–2320. doi: 10.1056/NEJMsa063117. [DOI] [PubMed] [Google Scholar]
- Cannon CP, Weintraub WS, Demopoulos LA, Vicari R, Frey MJ, Lakkis N, et al. Comparison of early invasive and conservative strategies in patients with unstable coronary syndromes treated with the glycoprotein IIb/IIIa inhibitor tirofiban. New England Journal of Medicine. 2001;344(25):1879–1887. doi: 10.1056/NEJM200106213442501. [DOI] [PubMed] [Google Scholar]
- Chen EW, Canto JG, Parsons LS, Peterson ED, Littrell KA, Every NR, et al. Relation between hospital intra-aortic balloon counterpulsation volume and mortality in acute myocardial infarction complicated by cardiogenic shock. Circulation. 2003;108(8):951–957. doi: 10.1161/01.CIR.0000085068.59734.E4. [DOI] [PubMed] [Google Scholar]
- Cohen MD, Bacdayan P. Organizational routines are stored as procedural memory: evidence from a laboratory study. Organization Science. 1994;5(4):554–568. [Google Scholar]
- Coiera E. Putting the technical back into socio-technical systems research. International Journal of Medical Informatics. 2007;76(S1):S98–S103. doi: 10.1016/j.ijmedinf.2006.05.026. [DOI] [PubMed] [Google Scholar]
- Davies C. Some of our concepts are missing: reflections on the absence of a sociology of organisations in Sociology of Health and Illness. Sociology of Health & Illness. 2003;25(3):172–190. [PubMed] [Google Scholar]
- De Luca G, Biondi-Zoccai G, Marino P. Transferring patients with ST-segment elevation myocardial infarction for mechanical reperfusion: a meta-regression analysis of randomized trials. Annals of Emergency Medicine. 2008;52(6):665–676. doi: 10.1016/j.annemergmed.2008.08.033. [DOI] [PubMed] [Google Scholar]
- Decker C, Garavalia L, Chen C, Buchanan DM, Nugent K, Shipman A, et al. Acute myocardial infarction patients’ information needs over the course of treatment and recovery. Journal of Cardiovascular Nursing. 2007;22(6):459–465. doi: 10.1097/01.JCN.0000297391.11324.0f. [DOI] [PubMed] [Google Scholar]
- Diercks DB. American Heart Association Mission Lifeline: Developing a STEMI regional care system. Cincinnati, OH: Emergency Medicine Cardiac Research and Education Group; 2010. [Google Scholar]
- Dimick JB, Staiger DO, Baser O, Birkmeyer JD. Composite measures for predicting surgical mortality in the hospital. Health Affairs. 2009;28(4):1189–1198. doi: 10.1377/hlthaff.28.4.1189. [DOI] [PubMed] [Google Scholar]
- Dixon-Woods M, Bosk CL, Aveling EL, Goeschel CA, Pronovost PJ. Explaining Michigan: developing an ex post theory of a quality improvement program. Milbank Quarterly. 2011;89(2):167–205. doi: 10.1111/j.1468-0009.2011.00625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley RA, Johansen KL, Brand R, Rennie DJ, Milstein A. Selective referral to high-volume hospitals. Journal of the American Medical Association. 2000;283(9):1159–1166. doi: 10.1001/jama.283.9.1159. [DOI] [PubMed] [Google Scholar]
- Dy SM, Rubin HR, Lehmann HP. Why do patients and families request transfers to tertiary care? A qualitative study. Social Science & Medicine. 2005;61(8):1846–1853. doi: 10.1016/j.socscimed.2005.03.037. [DOI] [PubMed] [Google Scholar]
- Foex BA. Is informed consent possible in acute myocardial infarction? Heart. 2004;90(11):1237–1238. doi: 10.1136/hrt.2003.020255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawande A. The checklist manifesto: How to get things right. New York, N.Y: Metropolitan; 2010. [Google Scholar]
- Grant RM. Toward a knowledge-based theory of the firm. Strategic Management Journal. 1996 Winter;17:109–122. [Google Scholar]
- Greenhalgh T, Voisey C, Robb N. Interpreted consultations as ‘business as usual’? An analysis of organisational routines in general practices. Sociology of Health & Illness. 2007;29(6):931–954. doi: 10.1111/j.1467-9566.2007.01047.x. [DOI] [PubMed] [Google Scholar]
- Heimer CA. Competing institutions: law, medicine, and family in neonatal intensive care. Law & Society Review. 1999;33(1):17–66. [Google Scholar]
- Heimer CA, Stinchcombe AL. Remodeling the garbage can: implications of the origins of items in decision streams. In: Egeberg M, Lægreid P, editors. Organizing political institutions. Oslo: Scandinavian University Press; 1999. [Google Scholar]
- Hodgson GM. The concept of a routine. In: Becker MC, editor. Handbook of organizational routines. Northhampton, MA: Edward Elgar; 2008. pp. 15–28. [Google Scholar]
- Iwashyna TJ, Christie JD, Moody J, Kahn JM, Asch DA. The structure of critical care transfer networks. Medical Care. 2009;47(7):787–793. doi: 10.1097/MLR.0b013e318197b1f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwashyna TJ, Kahn JM, Hayward RA, Nallamothu BK. Interhospital transfers among Medicare beneficiaries admitted for acute myocardial infarction at non-revascularization hospitals. Circulation: Cardiovascular Quality & Outcomes. 2010;3(5):468–475. doi: 10.1161/CIRCOUTCOMES.110.957993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krippendorff K. Content analysis: An introduction to its methodology. Thousand Oaks, CA: Sage; 2004. [Google Scholar]
- Krumholz HM, Anderson JL, Bachelder BL, Fesmire FM, Fihn SD, Foody JM, et al. ACC/AHA 2008 performance measures for Adults with ST-Elevation and Non-ST-elevation myocardial infarction. Circulation. 2008;118(4):2596–2648. doi: 10.1161/CIRCULATIONAHA.108.191099. [DOI] [PubMed] [Google Scholar]
- Krumholz HM, Bradley EH, Nallamothu BK, Ting HH, Batchelor WB, Kline-Rogers E, et al. A campaign to improve the timeliness of primary percutaneous coronary intervention: door-to-balloon: an alliance for quality. Journal of the American College of Cardiology Interventions. 2008;1(1):97–104. doi: 10.1016/j.jcin.2007.10.006. [DOI] [PubMed] [Google Scholar]
- Krumholz HM, Herrin J, Miller LE, Drye EE, Ling SM, Han LF, et al. Improvements in door-to-balloon time in the United States, 2005 to 2010. Circulation. 2011;124(9):1038–1045. doi: 10.1161/CIRCULATIONAHA.111.044107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krumholz HM, Normand S-LT, Spertus JA, Shahian DM, Bradley EH. Measuring performance for treating heart attacks and heart failure: the case for outcomes measurement. Health Affairs. 2007;26(1):75–85. doi: 10.1377/hlthaff.26.1.75. [DOI] [PubMed] [Google Scholar]
- Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–174. [PubMed] [Google Scholar]
- Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention. Journal of the American College of Cardiology. 2011;58(24):e44–e122. doi: 10.1016/j.jacc.2011.08.007. [DOI] [PubMed] [Google Scholar]
- Lomi A, Pallotti F. Relational collaboration among spatial multipoint competitors. Social Networks. 2012;34(1):101–111. [Google Scholar]
- Miles MB, Huberman AM. Qualitative data analysis: An expanded sourcebook. Thousand Oaks: Sage; 1994. [Google Scholar]
- Miniño AM. Death in the United States, 2009. Hyattsville, MD: National Center for Health Statistics; 2011. [Google Scholar]
- Nallamothu BK, Bradley EH, Krumholz HM. Time to treatment in primary percutaneous coronary intervention. New England Journal of Medicine. 2007;357(16):1631–1638. doi: 10.1056/NEJMra065985. [DOI] [PubMed] [Google Scholar]
- Nelson RR, Winter SG. An evolutionary theory of economic change. Boston, MA: Harvard; 1982. [Google Scholar]
- Pentland BT, Feldman MS. Designing routines: on the folly of designing artifacts, while hoping for patterns of action. Information and Organization. 2008;18(4):235–250. [Google Scholar]
- Rogers EM. Diffusion of innovations. New York: Free Press; 2003. [Google Scholar]
- Schneider EC, Epstein AM. Use of public performance reports: a survey of patients undergoing cardiac surgery. Journal of the American Medical Association. 1998;279(20):1638–1642. doi: 10.1001/jama.279.20.1638. [DOI] [PubMed] [Google Scholar]
- Scott WR, Ruef M, Mendel PJ, Caronna CA. Institutional change and healthcare organizations: From professional dominance to managed care. Chicago, IL: University of Chicago Press; 2000. [Google Scholar]
- Shortell SM. Patterns of referral among internists in private practice: a social exchange model. Journal of Health and Social Behavior. 1973;14(4):335–348. [PubMed] [Google Scholar]
- Tu JV, Donovan LR, Lee DS, Wang JT, Austin PC, Alter DA, et al. Effectiveness of public report cards for improving the quality of cardiac care. Journal of the American Medical Association. 2009;302(21):2330–2337. doi: 10.1001/jama.2009.1731. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. Hospital Compare. Centers for Medicare and Medicaid Services; 2011. [Google Scholar]
- Unnikrishnan KP, Patnaik D, Iwashyna TJ. Spatio-temporal structure of US critical care transfer network. Proceedings of the 2011 AMIA Clinical Research Informatics Summit. 2011:74–78. [PMC free article] [PubMed] [Google Scholar]
- Wang TY, Peterson ED, Ou F-S, Nallamothu BK, Rumsfeld JS, Roe MT. Door-to-balloon times for patients with ST-segment elevation myocardial infarction requiring interhospital transfer for primary percutaneous coronary intervention. American Heart Journal. 2011;161(1):76–86. doi: 10.1016/j.ahj.2010.10.001. [DOI] [PubMed] [Google Scholar]