Abstract
Background
Bendamustine is an alkylating agent with clinical activity against a variety of hematologic malignancies and solid tumors. To assess the roles of renal and hepatic drug elimination pathways in the excretion and metabolism of bendamustine, a mass balance study was performed in patients with relapsed or refractory malignancies.
Methods
A single 60-minute intravenous dose of 120 mg/m2, 80–95 μCi 14C-bendamustine hydrochloride was administered to six patients, followed by collection of blood, urine, and fecal samples at specified time points up to day 8 or until the radioactivity of the 24-hour urine and fecal collections was below 1% of the administered dose (whichever was longer). Total radioactivity (TRA) was measured in all samples, and concentrations of unchanged bendamustine and its metabolites γ-hydroxy-bendamustine (M3), N-desmethyl-bendamustine (M4), and dihydroxy bendamustine (HP2) were determined in plasma and urine, using validated liquid chromatography–tandem mass spectrometry methods.
Results
The mean recovery of TRA in excreta was 76% of the radiochemical dose. Approximately half of the administered dose was recovered in urine and a quarter in feces. Less than 5% of the administered dose was recovered in urine as unchanged bendamustine. Bendamustine clearance from plasma was rapid, with a half-life of ~40 minutes. Plasma concentrations of M3, M4, and HP2 were very low relative to bendamustine concentrations. Plasma levels of TRA were higher and more sustained as compared with plasma concentrations of bendamustine, M3, M4, and HP2, suggesting the presence of one or more longer-lived 14C-bendamustine–derived compounds. Fatigue (50%) and vomiting (50%) were the most frequent treatment-related adverse events. A grade 3/4 absolute lymphocyte count decrease occurred in all patients at some point during the study.
Conclusion
Bendamustine is extensively metabolized, with subsequent excretion in both urine and feces. Accumulation of bendamustine is not anticipated in cancer patients with renal or hepatic impairment, because of the dose administration schedule and short half-life.
Introduction
Bendamustine is a unique alkylating agent, which combines a nitrogen mustard moiety of mechlorethamine with a benzimidazole [1]. It has shown clinical activity against a variety of hematologic malignancies [2–5] and solid tumors [6, 7] as a single agent or in combination with other anticancer agents. Bendamustine is indicated in the USA for the treatment of chronic lymphocytic leukemia and for indolent B-cell non-Hodgkin’s lymphoma that has progressed during or within 6 months of treatment with rituximab or a rituximab-containing regimen.
Like other alkylating agents, bendamustine causes cross-links between DNA bases, resulting in DNA damage. However, in vitro studies with human ovarian and breast cancer cell lines showed that the double-strand breaks caused by bendamustine are more extensive and durable than those produced by the alkylating agents cyclophosphamide and carmustine [8]. This, combined with unique mechanistic features, including activation of DNA damage stress response and apoptosis, inhibition of mitotic checkpoints, and induction of mitotic catastrophe [1], may explain the activity of bendamustine in drug-resistant cells in vitro [8] and in patients with therapy-refractory lymphoma [3].
Bendamustine was generally well tolerated in patients with relapsed or refractory non-Hodgkin’s lymphoma or mantle cell lymphoma [3, 9–12]. The main toxicities observed were reversible myelosuppression, including leukocytopenia, neutropenia, thrombocytopenia, and anemia. Nonhematologic toxicities included mild gastrointestinal events and fatigue [3, 9].
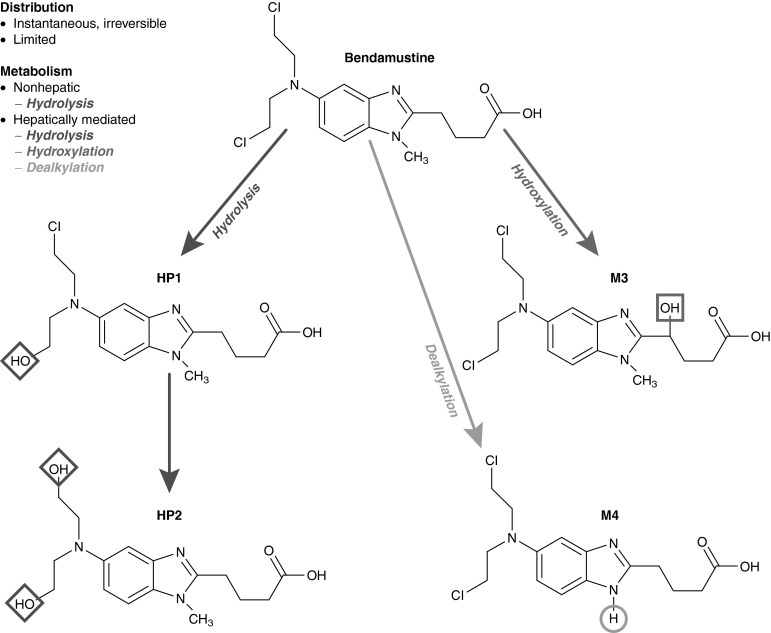
A major route of bendamustine metabolism is hydrolysis to the inactive products monohydroxy bendamustine (HP1) and dihydroxy bendamustine (HP2), which make little or no contribution to the anti-cancer effects of bendamustine (Fig. 1). Two phase I metabolites of bendamustine have been identified: γ-hydroxy-bendamustine (M3) and N-desmethyl-bendamustine (M4) [Fig. 1]. Both are formed via the cytochrome P450 (CYP) 1A2 oxidative pathway, and they have potency similar to that of bendamustine (M3) or 5- to 10-fold lower than that of bendamustine (M4) [13].
Fig. 1.
Metabolic conversion of bendamustine to the primary but inactive metabolites monohydroxy bendamustine and dihydroxy bendamustine via hydrolysis, as well as to the active metabolites γ-hydroxy-bendamustine and N-desmethyl-bendamustine via hepatic hydroxylation and dealkylation. HP1 monohydroxy bendamustine, HP2 dihydroxy bendamustine, M3 γ-hydroxy-bendamustine, M4 N-desmethyl-bendamustine
In a mass balance study of 14C-bendamustine performed in rats, approximately 90% of the dose was recovered in excreta after 7 days, and substantial radioactivity (49%) was recovered in feces [14]. Limited information, however, is available on the extent of renal and hepatic elimination of bendamustine in humans. Previously reported urinary pharmacokinetic data on bendamustine and its metabolites are characterized by high variability, suspected to be caused by varying degrees of hydrolysis of bendamustine during sample handling and preparation [15, 16].
Materials and Methods
Study Design
This was a phase I, open-label, single-center study, which enrolled six patients. The study was conducted in accordance with International Conference on Harmonization guidelines for Good Clinical Practice; the Code of Federal Regulations Title 21, Parts 50, 54, 56, 312, and 314; and the European Clinical Trials Directive (2001/20/EC). The protocol was approved by the Netherlands Cancer Institute Independent Ethics Committee.
The primary objective of this study was to determine the pharmacokinetics and excretion of 14C-bendamustine and its metabolites M3, M4, and HP2 in humans. To this end, the mass balance of a single dose of 120 mg/m2 (~80–95 μCi) 14C-bendamustine was investigated in cancer patients by comparing the administered radioactivity with the radioactivity recovered in urine and fecal samples. Concentrations of bendamustine, M3, M4, and HP2 in plasma and urine were determined using validated liquid chromatography–tandem mass spectrometry (LC-MS/MS) assays, and special procedures were followed to minimize the chemical degradation of bendamustine in the study samples. The secondary objective was to further assess the safety profile of bendamustine.
The study was divided into two assessment periods: period A, during which the mass balance and pharmacokinetics of 14C-bendamustine were investigated; and period B, an extended-use period of up to six 28-day cycles with nonlabeled bendamustine administration on days 1 and 2, during which safety continued to be assessed.
After giving written informed consent, patients received a 60-minute intravenous infusion containing a 120-mg/m2 dose of 14C-bendamustine HCl (~80–95 μCi) on day 1 and a 120-mg/m2 dose of nonlabeled bendamustine on day 2. During days 1–8 of cycle 1, blood samples and excreta were collected while the patients remained hospitalized. In this period, patients received a high-fiber diet and adequate fluid intake (≥2 L/day). After day 8, collection of excreta was continued on an outpatient basis until levels of radioactivity in the 24-hour urine and fecal collections were below 1% of the administered dose. Upon completion of period A, the patients were given the option to continue with period B.
Patients
Patients aged ≥18 years with a histologically or cytologically confirmed relapsed or refractory malignancy (hematologic or nonhematologic except for uveal melanoma, sarcoma, or primary brain tumors), considered unresponsive or poorly responsive to accepted treatment, were eligible for this study. Other eligibility criteria included World Health Organization (WHO) performance status ≤2; estimated life expectancy ≥3 months; adequate bone marrow function (absolute neutrophil count ≥1.0 × 103/mm3 and platelet count ≥1.0 × 106/mm3); adequate hepatic function (bilirubin ≤1.5 times the upper limit of normal [ULN] and alanine aminotransferase [ALT] and aspartate aminotransferase [AST] ≤2.5 × ULN or ≤5 × ULN in the case of liver metastases); adequate renal function (creatinine clearance [CLCR] >30 mL/min); and use of an approved method of birth control until ≥90 days after drug discontinuation. Patients were excluded if they smoked or used topical or oral nicotine preparations within 3 months; received mitomycin within 42 days; received CYP1A2 inducers, chemotherapy, radiotherapy, radioimmunotherapy, or immunotherapy within a month; received CYP1A2 inhibitors or hematopoietic growth factors within 14 days prior to the first study dose; required treatment with CYP1A2 inhibitors or inducers during days 1–8 of cycle 1; or had not recovered from adverse events (AEs) due to previously administered agents. Other reasons for exclusion included pregnancy or breastfeeding, known cerebral metastases, known positive human immunodeficiency virus status, serious infection or medical/psychiatric conditions, other treatments for hematologic or nonhematologic malignancy, previous treatment with bendamustine, or significant constipation or obstruction of the urinary tract.
Study Medication
Brown borosilicate glass vials containing 100 mg 14C-bendamustine HCl (90–95 μCi) were manufactured by Parenteral Medications Laboratories (Memphis, TN, USA), supplied by Teva Pharmaceutical Industries Ltd. (Frazer, PA, USA). They contained a mixture of 14C-bendamustine (chemical and radiochemical purity >99.6%) and nonlabeled bendamustine (chemical purity 99.6%) as a lyophilized powder. Vials with 100 mg nonlabeled bendamustine HCl (chemical purity 99%) were provided by Pharmachemie BV (Haarlem, The Netherlands).
Individual aseptic preparations of 14C-bendamustine infusions were prepared with one vial of 14C-bendamustine and one or more vials of nonlabeled bendamustine to obtain a final dose of 120 mg/m2. Each vial was reconstituted with 20 mL of Sterile Water for Injection. The complete volume of the vial with 14C-bendamustine and the required volume of nonlabeled bendamustine were transferred to a 500-mL infusion bag with 0.9% sodium chloride. The actual administered radioactivity was calculated by determination of the total radioactivity (TRA) in the infusion bag before and after the administration.
Sample Collection
Blood samples of 4 mL were collected in K2EDTA tubes prior to the start of the 14C-bendamustine infusion, at 15, 30, 45, 65, and 75 minutes, and at 1.5, 2, 2.5, 3, 4, 6, 8, 10, 12, 24, 36, 48, 72, 96, 120, 144, and 168 hours after the start of the infusion. Between collection and centrifugation (1,200 × g, 4 °C, 10 minutes), the tubes were placed on ice (maximally 30 minutes). An additional 1-mL whole-blood sample was collected at the end of the infusion, at 168 hours after the start of the infusion, and optionally once every week thereafter.
Urine samples were collected before the start of the 14C-bendamustine infusion, as voided during specified time intervals (0–2, 2–4, 4–6, 6–8, 8–10, 10–12, 12–18, 18–24, 24–30, 30–36, 36–42, 42–48, 48–72, 72–96, 96–120, 120–144, and 144–168 hours) through 168 hours after the start of the infusion, and over additional 24-hour periods if collection was continued. Each urine sample was measured for TRA, and several aliquots were prepared. For analysis of bendamustine, M3, M4, and HP2, 20-μL urine aliquots were mixed with 1,980 μL of prechilled control human K2EDTA plasma to stabilize the compounds during storage and processing [17].
Fecal samples were collected per portion, prior to the start of the 14C-bendamustine infusion, and then as voided through 168 hours following the start of the infusion, or for longer if TRA represented ≥1% of the radiochemical dose in the 144- to 168-hour collection of feces. The fecal portions were weighed, stored refrigerated, combined over 24-hour periods, and homogenized after addition of water (1:3 w/v).
Plasma aliquots, urine aliquots, and whole-blood samples were stored within the range of −70 °C to −90 °C.
Analysis of TRA
TRA in plasma, whole blood, urine, and fecal samples was determined by liquid scintillation counting (LSC). Plasma (0.2 mL) and urine (1 mL) samples were directly mixed with 10 mL liquid scintillation cocktail (Ultima Gold™; PerkinElmer Inc.; Waltham, MA, USA). Whole-blood samples (0.2 mL) and fecal homogenates (0.2 mL) were dissolved and decolorized first as described elsewhere [18], using Solvable™ (PerkinElmer Inc.), 30% hydrogen peroxide, and either aqueous 0.1 M EDTA or isopropanol, respectively.
Samples were counted on a Tri-Carb® 2800TR LSC (PerkinElmer Inc.). Quench correction was applied with a calibration curve of quenched radioactive reference standards. Samples were counted to a sigma 2 counting error of 1% or for maximally 60 minutes.
Analysis of Bendamustine, M3, M4, and HP2
Concentrations of bendamustine, M3, M4, and HP2 in plasma and urine samples obtained through 24 hours were determined with validated LC-MS/MS assays, as described elsewhere [17]. Briefly, 200 μL of plasma and 200 μL of urine fortified with control human plasma (1:99 v/v) were processed identically using solid-phase extraction after addition of an internal standard mixture. The assay for bendamustine, M3, and M4 used a Synergi™ Hydro-RP column, and the assay for HP2 used a Synergi™ Polar-RP column (Phenomenex, Inc.; Torrance, CA, USA). On both columns, gradient elution was performed with 5 mM ammonium formate with 0.1% formic acid in water and methanol. The quantifiable ranges for bendamustine, M3, and M4 were 0.5–500 ng/mL in plasma and 0.5–50 μg/mL in urine, and for HP2 were 1–500 ng/mL in plasma and 0.1–50 μg/mL in urine. Quality control samples were prepared and analyzed together with the study samples, and acceptance criteria for bioanalytic data during routine drug analysis, as described in the US Food and Drug Administration (FDA) guidelines [19], were applied.
Pharmacokinetic Analysis
Pharmacokinetic parameters for bendamustine, M3, M4, HP2, and TRA were estimated by noncompartmental analysis using WinNonlin™ software (version 4.1.a; Pharsight Corporation; Mountain View, CA, USA). Parameters that were determined for all analytes included the maximum observed plasma concentration (Cmax), the elimination half-life (t½), and the area under the plasma concentration–time curve from time zero to infinity (AUC∞). Additionally, the plasma clearance (CL) and the apparent volume of distribution at steady state (Vss) were determined for bendamustine and estimated for TRA, and the renal clearance (CLR) was determined for bendamustine.
Safety Assessments
The safety of bendamustine was assessed by evaluating AEs according to Common Terminology Criteria for AEs v3.0; serum chemistry, hematology, and urinalysis test results; vital signs; 12-lead electrocardiograms (ECGs); body weight; physical examinations; and concomitant medication. ECGs were performed prior to study drug administration and at multiple time points on day 1 of cycle 1.
No formal statistical analysis was applied in this study; descriptive statistics were used when appropriate.
Results
Patients
Six patients with confirmed relapsed or refractory malignancy were enrolled (Table 1). They had a median age of 66 years (range 48–75), a mean weight of 72.7 kg (range 59–94), a mean height of 173.2 cm (range 155–181), and a mean body surface area of 1.9 m2 (range 1.6–2.2). All patients had a history of cancer drug therapy and anticancer surgery. At the time of enrollment, four patients (67%) had a WHO performance status of 0 and two (33%) had a status of 1.
Table 1.
Patient characteristics
| Characteristic | Value |
|---|---|
| Median age (years [range]) | 66 [48–75] |
| Sex (n [%]) | |
| Male | 3 [50] |
| Female | 3 [50] |
| Race (n [%]) | |
| White | 6 [100] |
| Ethnicity (n [%]) | |
| Non-Hispanic and non-Latino | 6 [100] |
| Mean weight (kg [range]) | 72.7 [59–94] |
| Mean height (cm [range]) | 173.2 [155–181] |
| Mean body surface area (m2 [range]) | 1.9 [1.6–2.2] |
| Mean time since cancer diagnosis (years [range]) | 4.7 [2–13] |
| Primary cancer types (n [%]) | |
| Breast | 1 [17] |
| Colorectal | 2 [33] |
| Melanoma | 2 [33] |
| Renal | 1 [17] |
| History of cancer drug therapy (n [%]) | 6 [100] |
| History of cancer surgery (n [%]) | 6 [100] |
| History of radiotherapy (n [%]) | 4 [67] |
| WHO performance status (n [%]) | |
| 0 | 4 [67] |
| 1 | 2 [33] |
| 2 | 0 |
WHO World Health Organization
Pharmacokinetics
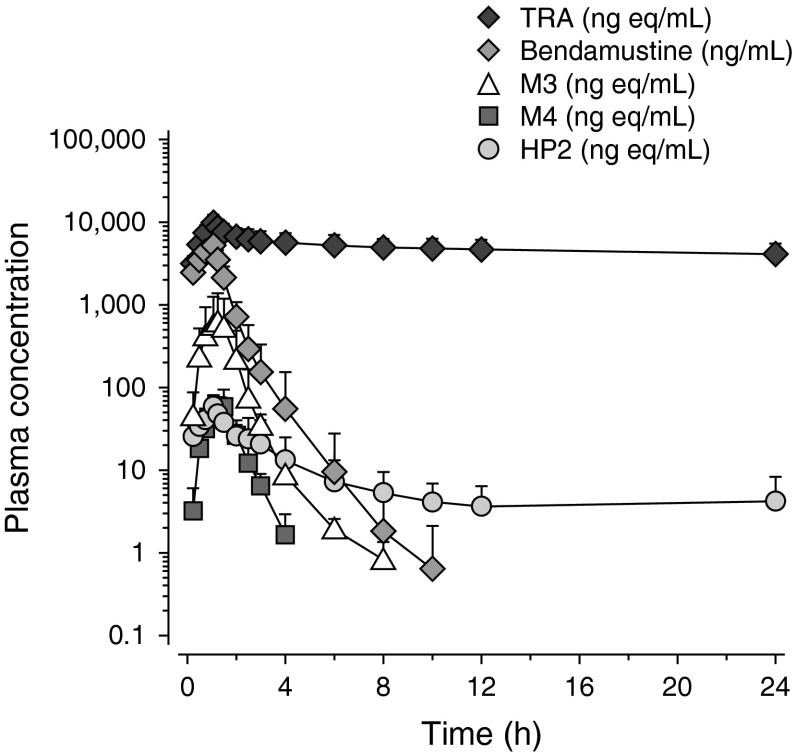
Plasma concentration–time curves of TRA, bendamustine, M3, M4, and HP2 during 24 hours after the start of the 14C-bendamustine infusion are presented in Fig. 2. Table 2 summarizes the individual and mean pharmacokinetic parameters.
Fig. 2.
Mean (±standard deviation) [n = 6] log-linear plasma concentration–time curves of total radioactivity; unchanged bendamustine; and the metabolites γ-hydroxy-bendamustine, N-desmethyl-bendamustine, and dihydroxy bendamustine up to 24 hours after the start of a 60-minute (120 mg/m2, 80–95 μCi) 14C-bendamustine hydrochloride infusion. HP2 dihydroxy bendamustine, M3 γ-hydroxy-bendamustine, M4 N-desmethyl-bendamustine, TRA total radioactivity
Table 2.
Plasma pharmacokinetic parameters for total radioactivity, bendamustine, and the metabolites γ-hydroxy-bendamustine, N-desmethyl-bendamustine, and dihydroxy bendamustine following an intravenous 60-minute infusion of 120 mg/m2 of 14C-bendamustine hydrochloride
| Parameter | Patient | Mean [SD] | ||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |||
| BSA (m2) | 2.17 | 1.84 | 1.85 | 1.6 | 2.05 | 1.7 | NC | |
| Dose (mg)a | 233 | 198 | 197 | 172 | 215 | 182 | NC | |
| TRA (bendamustine equivalents) | Cmax (μg/mL) | 6.88 | 12.4 | 9.31 | 12.1 | 8.54 | 12 | 10.2 [2.29] |
| AUC∞ (μg·h/mL) | 904 | 1,147 | 1,504 | 695 | 1,403 | 1,571 | 1,204 [351] | |
| t½ (h) | 225 | 110 | 261 | 171 | 222 | 193 | 197 [52.5] | |
| Vss (L) | 81.2 | 27.4 | 48.3 | 59.2 | 49.6 | 31.3 | 49.5 [19.6] | |
| CL (mL/min) | 4.27 | 2.89 | 2.16 | 4.13 | 2.56 | 1.92 | 2.99 [1] | |
| Bendamustine | Cmax (μg/mL) | 3.25 | 7.48 | 4.2 | 8.19 | 3.6 | 5.2 | 5.32 [2.07] |
| AUC∞ (ng·h/mL) | 3,963 | 10,619 | 4,906 | 8,041 | 4,487 | 6,371 | 6,398 [2,543] | |
| t½ (h) | 0.57 | 0.96 | 0.58 | 0.86 | 0.45 | 0.46 | 0.65 [0.21] | |
| Vss (L) | 27.1 | 15.3 | 24.4 | 10.7 | 27.5 | 15.5 | 20.1 [7.1] | |
| CL (mL/min) | 977 | 313 | 666 | 358 | 800 | 476 | 598 [262] | |
| CLR (mL/min) | 14.3 | 16.1 | 11 | 6.6 | 29.9 | 28.5 | 17.7 [9.5] | |
| M3 | Cmax (ng/mL) | 644 | 264 | 714 | 1,125 | 550 | 816 | 685 [286] |
| AUC∞ (ng·h/mL) | 829 | 389 | 975 | 1,428 | 792 | 1,137 | 925 [351] | |
| t½ (h) | 3.58 | 0.82 | 1.41 | 2.14 | 1.09 | 1.12 | 1.69 [1.03] | |
| M4 | Cmax (ng/mL) | 38.7 | 29.8 | 50.1 | 87.9 | 28.5 | 117 | 58.7 [36.1] |
| AUC∞ (ng·h/mL) | 59 | 61 | 81 | 119 | 43 | 135 | 83 [37] | |
| t½ (h) | 0.48 | 0.8 | 0.48 | 0.44 | 0.45 | 0.45 | 0.52 [0.14] | |
| HP2 | Cmax (ng/mL) | 35 | 73.3 | 43.2 | 53.1 | 40.8 | 81.4 | 54.5 [18.8] |
| AUC∞ (ng·h/mL) | NC | NC | 188 | 153 | 215 | NC | 185 [31] | |
| t½ (h) | NC | NC | 15.4 | 14.1 | 23.8 | NC | 17.8 [5.3] | |
AUC ∞ area under the plasma concentration–time curve from time zero to infinity, BSA body surface area, C max maximum observed plasma concentration, CL clearance, CL R renal clearance, HP2 dihydroxy bendamustine, M3 γ-hydroxy-bendamustine, M4 N-desmethyl-bendamustine, NC not calculable, SD standard deviation, TRA total radioactivity, t ½ elimination half-life, V ss apparent volume of distribution at steady state
aBendamustine free base (mg)
The Cmax values of TRA, bendamustine, and HP2 were typically observed in the first sample after completion of the infusion (median time to reach Cmax [tmax] 1.10 hours), and the median tmax durations of M3 (1.26 hours) and M4 (1.28 hours) were slightly longer. After reaching Cmax values, the concentrations of bendamustine decreased in a multiphasic manner, which was characterized by an initial rapid phase of decline, followed by a somewhat slower secondary phase, with a mean apparent t½ of 0.65 hours. The mean Vss was 20.1 L, and the CL was 598.3 mL/min.
The active metabolite M3 showed a biphasic decline in concentration after reaching Cmax values (mean t½ 1.69 hours), whereas the decline of M4 appeared monophasic (mean t½ 0.52 hours). The concentrations of these metabolites were substantially lower than those of bendamustine. The concentrations of the dihydrolysis product HP2 were initially also much lower than the concentrations of bendamustine but, unlike the other analytes, small but measurable levels of HP2 were still present at 24 hours after the start of the infusion, with a mean concentration at 24 hours of 3.75 ng/mL.
The TRA concentrations were characterized by a very slow decrease after reaching Cmax values. After 168 hours, the mean TRA concentration was still 2.29 μg Eq/mL, and the mean t½ of the apparent terminal phase was estimated at 197 hours (Table 2). Bendamustine, M3, M4, and HP2 composed the bulk of the TRA early in the profile (almost 80%); however, their contribution to the TRA quickly declined to approximately 1% at 4 hours after the start of the infusion.
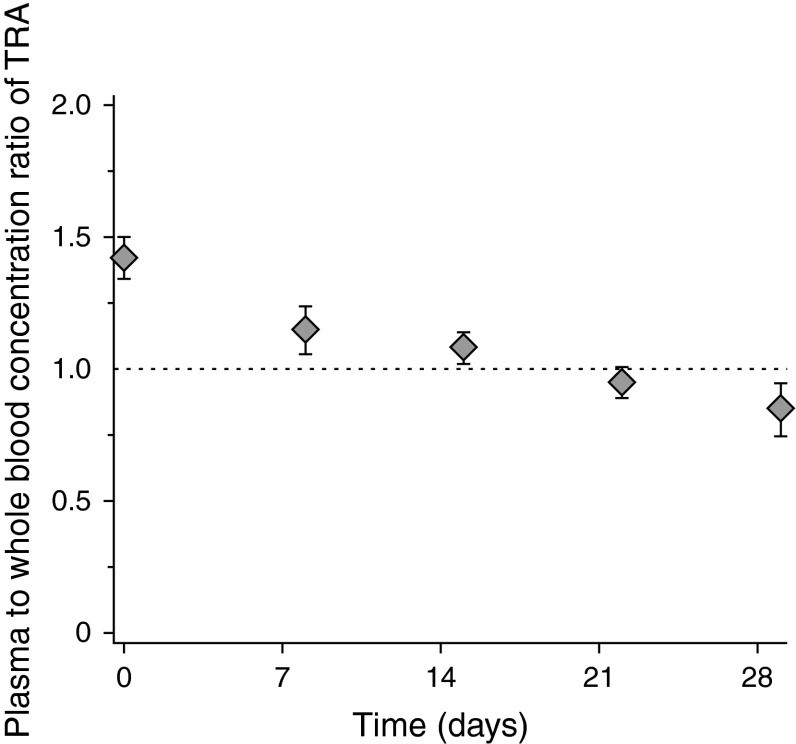
The mean concentration ratio of TRA in plasma and in whole blood (Fig. 3) was ~1.4 immediately after the end of the infusion and approximately 1 at later time points.
Fig. 3.
Mean (±standard deviation) [n = 4–6] plasma to whole-blood concentration ratio of total radioactivity immediately after the end of a 60-minute (120 mg/m2, 80–95 μCi) 14C-bendamustine hydrochloride infusion and at weekly time points thereafter. TRA total radioactivity
Excretion Balance
For all six patients, urine and fecal samples were collected as planned during the first 168 hours after administration of 14C-bendamustine. Thereafter, urine and feces continued to be collected for longer periods in five and three patients, respectively, for up to 3 weeks.
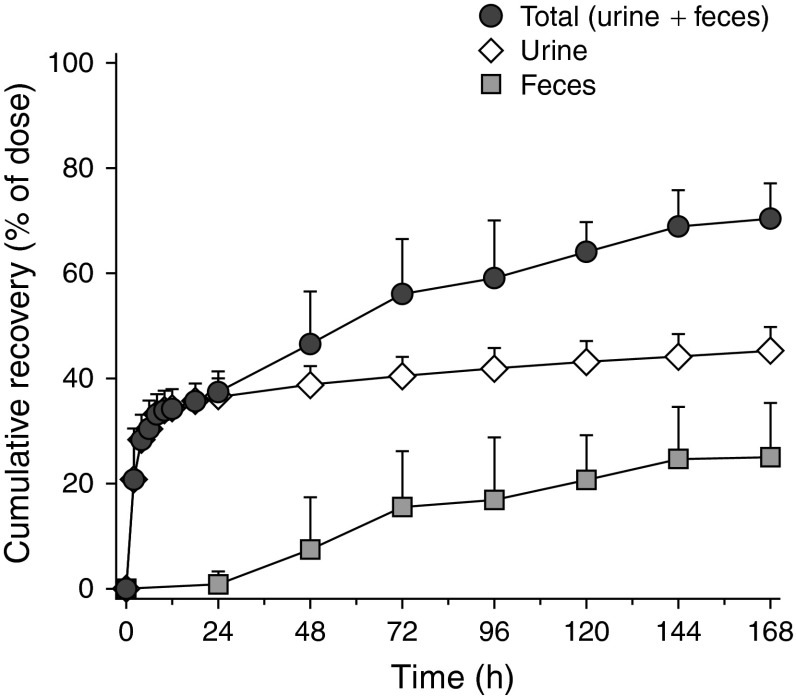
Figure 4 shows the mean cumulative urinary, fecal, and total recovery of TRA during 168 hours after 14C-bendamustine administration. At this point, approximately half (45.5%) of the administered radioactivity was recovered in urine and a quarter (25.2%) in feces, resulting in total recovery of 70.6% after 168 hours. After the extended collection period, the total recovery was increased to 76.0%. Individual excretion values are tabulated in Table 3.
Fig. 4.
Mean (±standard deviation) [n = 6] cumulative recovery of 14C-bendamustine-derived total radioactivity in urine, in feces, and in total during 7 days after a 60-minute (120 mg/m2, 80–95 μCi) 14C-bendamustine hydrochloride infusion
Table 3.
Cumulative percentage excretion of bendamustine, the metabolites γ-hydroxy-bendamustine, N-desmethyl-bendamustine, and dihydroxy bendamustine, and total radioactivity in urine over 24 hours and total radioactivity in urine and feces over 168 hours and until the end of the collection period following an intravenous 60-minute infusion of 120 mg/m2 of 14C-bendamustine hydrochloride
| Collection | Patient | Mean [SD] | ||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |||
| Urine (% excretion) | ||||||||
| 0–24 hours | Bendamustine | 1.46 | 5.17 | 1.65 | 1.85 | 3.74 | 5.98 | 3.31 [1.95] |
| M3 | 0.64 | 0.36 | 0.5 | 0.7 | 0.76 | 1.42 | 0.73 [0.37] | |
| M4 | 0 | 0.12 | 0.08 | 0 | 0 | 0.27 | 0.08 [0.11] | |
| HP2 | 8.65 | 4.09 | 4.18 | 8.25 | 2.12 | 2.04 | 4.89 [2.9] | |
| Suma | 10.75 | 9.74 | 6.41 | 10.8 | 6.62 | 9.71 | 9.01 [1.99] | |
| TRA | 36.36 | 36.08 | 36.53 | 34.77 | 32.83 | 43.1 | 36.61 [3.47] | |
| 0–168 hours | TRA | 42.16 | 50.69 | 45.26 | 40.44 | 43.11 | 51.11 | 45.46 [4.49] |
| 0–EoCb | TRA | 47.6 | 57.93 | 48.26 | 40.44 | 47.86 | 51.93 | 49 [5.75] |
| Feces (% excretion) | ||||||||
| 0–168 hours | TRA | 30.3 | 8.92 | 34.44 | 31.88 | 29.45 | 16.1 | 25.18 [10.22] |
| 0–EoCb | TRA | 32.64 | 8.92 | 34.44 | 31.88 | 35.08 | 18.89 | 26.98 [10.67] |
| Total (% excretion) | ||||||||
| 0–168 hours | TRA | 72.46 | 59.61 | 79.7 | 72.32 | 72.56 | 67.21 | 70.64 [6.71] |
| 0–EoCb | TRA | 80.24 | 66.85 | 82.7 | 72.32 | 82.94 | 70.82 | 75.98 [6.86] |
EoC end of collection period, HP2 dihydroxy bendamustine, M3 γ-hydroxy-bendamustine, M4 N-desmethyl-bendamustine, SD standard deviation, TRA total radioactivity
aThese values represent the sum of bendamustine, M3, M4, and HP2
bThe time of the EoC varied among patients and ranged from 168 to 504 hours
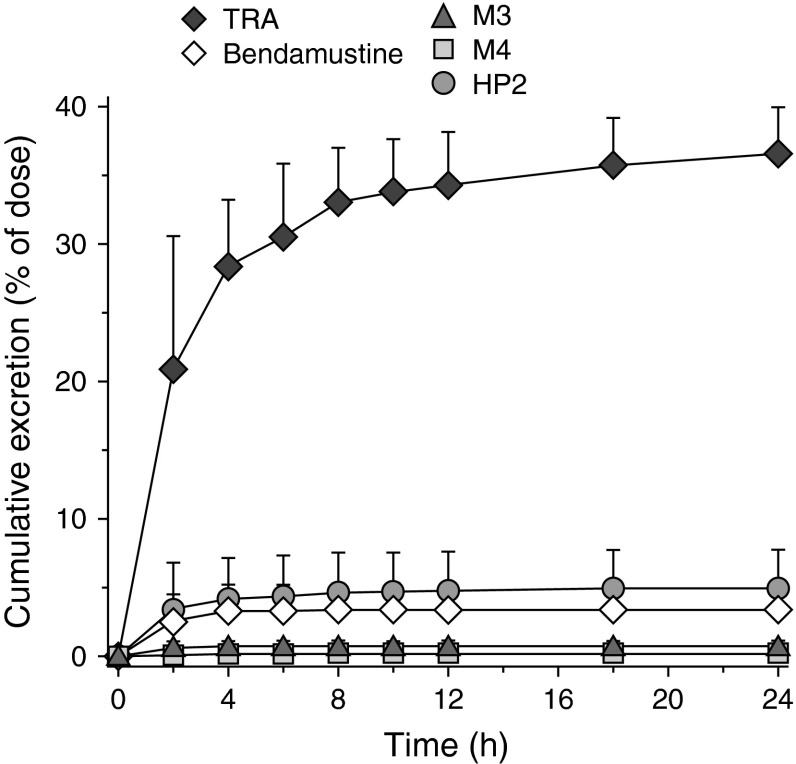
The mean cumulative urinary excretion of TRA and unchanged bendamustine, M3, M4, and HP2 during the first 24 hours is shown in Fig. 5 and is summarized per patient in Table 3. At 24 hours, approximately 3.3% of the dose was recovered in urine as bendamustine, <1% as M3 and M4, and <5% as HP2. Urinary recovery of bendamustine, M3, and M4 was predominantly in collections during the first 4 hours after the start of the infusion. After 8 hours, there were no measurable levels of these compounds in urine. The excretion of HP2 continued slowly, and low but quantifiable levels were still present in the urine samples of 16–24 hours.
Fig. 5.
Mean (±standard deviation) [n = 6] cumulative urinary excretion of total radioactivity; unchanged bendamustine; and the metabolites γ-hydroxy-bendamustine, N-desmethyl-bendamustine, and dihydroxy bendamustine up to 24 hours after the start of a 60-minute (120 mg/m2, 80–95 μCi) 14C-bendamustine hydrochloride infusion. HP2 dihydroxy bendamustine, M3 γ-hydroxy-bendamustine, M4 N-desmethyl-bendamustine, TRA total radioactivity
Safety
All patients completed assessment period A, receiving a mean of 4 (range 2–6) doses of bendamustine. All were withdrawn during assessment period B: four because of disease progression, one because of an AE (dyspnea), and one because of election to discontinue from the study.
During the treatment period, all six patients experienced at least one AE that was considered treatment related. The numbers of patients experiencing worst-value hematologic toxicities occurring during the study are shown in Table 4. A grade 3 or 4 absolute lymphocyte count decrease was observed in all six patients at some point during the study. No other grade 3 or 4 hematologic abnormalities were seen. The numbers of patients experiencing nonhematologic AEs are shown in Table 5. The most frequently occurring treatment-related nonhematologic AEs were grade 1 and 2 fatigue (n = 3; 50%) and vomiting (n = 3; 50%).
Table 4.
Numbers of patients experiencing worst-value hematologic toxicities during the study
| Parameter | Patients (n [%]) | |
|---|---|---|
| Grade 1/2 abnormality | Grade 3/4 abnormality | |
| Lymphocyte count | 0 | 6 [100] |
| Hemoglobin level | 6 [100] | 0 |
| Neutrophil count | 2 [33] | 0 |
| White blood cell count | 2 [33] | 0 |
| Platelet count | 1 [17] | 0 |
Table 5.
Nonhematologic adverse events
| System organ class: MedDRA Preferred Term | Patients (n [%])a | |||
|---|---|---|---|---|
| Grade 1 AE | Grade 2 AE | Grade 3 AE | Grade 4 AE | |
| Gastrointestinal disorders | ||||
| Abdominal pain | 1 [17] | 1 [17] | 0 | 0 |
| Constipation | 1 [17] | 3 [50] | 0 | 0 |
| Diarrhea | 1 [17] | 0 | 1 [17] | 0 |
| Dry mouth | 2 [33] | 0 | 0 | 0 |
| Nausea | 3 [50] | 1 [17] | 0 | 0 |
| Vomiting | 1 [17] | 2 [33] | 0 | 0 |
| Salivary hypersecretion | 1 [17] | 0 | 0 | 0 |
| General disorders and administration site conditions | ||||
| Chills | 2 [33] | 0 | 0 | 0 |
| Fatigue | 1 [17] | 2 [33] | 2 [33] | 0 |
| Pyrexia | 3 [50] | 1 [17] | 0 | 0 |
| Asthenia | 1 [17] | 0 | 0 | 0 |
| Chest pain | 1 [17] | 0 | 0 | 0 |
| Localized edema | 1 [17] | 0 | 0 | 0 |
| Peripheral edema | 1 [17] | 0 | 0 | 0 |
| Peripheral coldness | 1 [17] | 0 | 0 | 0 |
| Investigations | ||||
| Weight decreased | 1 [17] | 0 | 0 | 0 |
| Metabolism and nutrition disorders | ||||
| Anorexia | 2 [33] | 0 | 0 | 0 |
| Musculoskeletal and connective tissue disorders | ||||
| Back pain | 1 [17] | 1 [17] | 0 | 0 |
| Flank pain | 1 [17] | 0 | 0 | 0 |
| Musculoskeletal pain | 1 [17] | 0 | 0 | 0 |
| Neoplasms | ||||
| Metastases to skin | 0 | 1 [17] | 0 | 0 |
| Nervous system disorders | ||||
| Dizziness | 3 [50] | 0 | 0 | 0 |
| Headache | 2 [33] | 0 | 0 | 0 |
| Dysgeusia | 1 [17] | 0 | 0 | 0 |
| Migraine | 1 [17] | 0 | 0 | 0 |
| Peripheral sensory neuropathy | 0 | 1 [17] | 0 | 0 |
| Psychiatric disorders | ||||
| Insomnia | 1 [17] | 0 | 0 | 0 |
| Renal and urinary disorders | ||||
| Renal pain | 0 | 0 | 1 [17] | 0 |
| Respiratory, thoracic, and mediastinal disorders | ||||
| Dyspnea | 1 [17] | 1 [17] | 0 | 0 |
| Cough | 1 [17] | 0 | 0 | 0 |
| Skin and subcutaneous tissue disorders | ||||
| Dry skin | 1 [17] | 0 | 0 | 0 |
| Hyperhidrosis | 1 [17] | 0 | 0 | 0 |
If a patient reported an AE more than once, the highest grade was presented for that AE. Patients were counted only once in each preferred term category and only once in each system organ class category, at the highest grade for each
AE adverse event, MedDRA Medical Dictionary for Regulatory Activities
aPatients (n = 6) may have reported more than one AE
No deaths or treatment-related serious AEs occurred. One patient experienced a serious AE (constipation). Both this event and the AE of dyspnea in one patient, resulting in withdrawal of that patient from the study, appeared to be manifestations of the underlying medical condition and were considered unlikely to be related to bendamustine treatment.
There was no evidence of any drug-related trends in the values of serum chemistry, urinalysis, or vital signs, and no AEs were related to findings of physical examinations or ECG findings. The mean change (±standard deviation [SD]) from baseline in creatinine was −0.08 μmol (6.79), and the mean change in total bilirubin was −0.05 μmol (1.87).
Discussion
This study investigated the pharmacokinetics and excretion of 14C-bendamustine in adult cancer patients. It was found that bendamustine is extensively metabolized, with subsequent excretion in urine and feces.
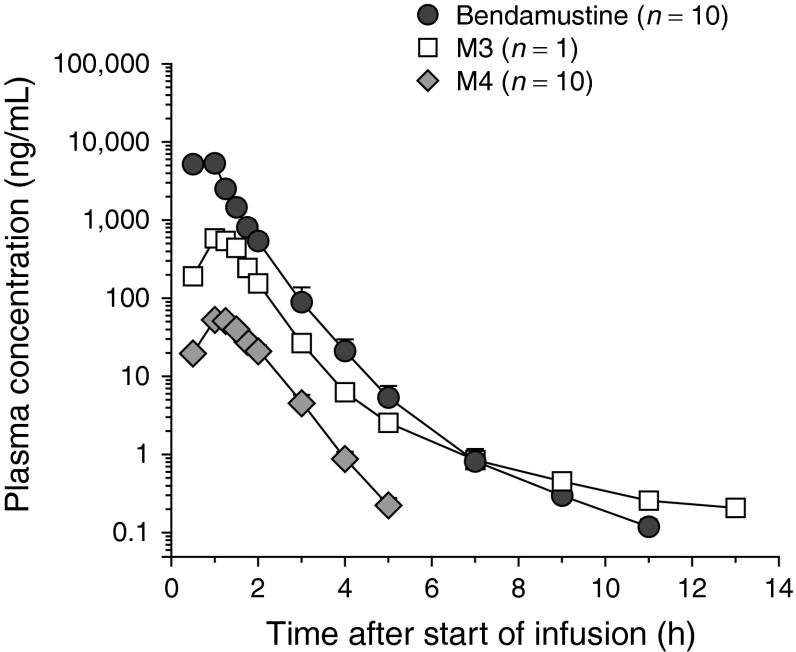
The short pharmacologically relevant t½ (0.65 hours), limited Vss (20.1 L), and rapid CL (598 mL/min) of bendamustine are in line with results of previous studies [4, 15, 16, 20]. However, a third, much slower elimination phase of bendamustine plasma concentrations (Fig. 6), as reported by Owen and colleagues [20], was not observed in this study. The higher LLQ (lower limit of quantification) of the bendamustine assay used in the present study (0.5 vs. 0.1 ng/mL) probably explains why the third phase was not detected. Nevertheless, the influence on the pharmacokinetic results is expected to be minimal because the AUC of the third (terminal) phase accounted for less than 1% of the total AUC, the ratio of observed plasma concentrations at 12 hours and tmax had a mean value of 1:25,000, and the t½ of the intermediate phase was considered to be the most pharmacologically relevant [20].
Fig. 6.
Mean (+standard error) plasma concentration–time profiles of bendamustine, γ-hydroxy-bendamustine, and N-desmethyl-bendamustine following administration of a single dose of intravenous bendamustine 120 mg/m2 on day 1 of cycle 1 from a phase III, multicenter, open-label study of patients with indolent B-cell non-Hodgkin’s lymphoma refractory to rituximab [20]. M3 γ-hydroxy-bendamustine, M4 N-desmethyl-bendamustine
Consistent with the population pharmacokinetic models for the active metabolites M3 and M4 (Fig. 6) [20], the plasma elimination profiles of M3 and M4 were biphasic and monophasic, respectively. The exposures to M3 and M4 were almost one and two orders of magnitude lower than those to bendamustine, respectively. This was also found in previous studies (Fig. 6) [4, 13, 16, 20] and suggests a limited contribution of these active metabolites to the therapeutic activity of bendamustine. Additionally, the low plasma concentrations of M3 and M4 relative to the bendamustine concentration suggest a minor role of the CYP1A2 pathway, responsible for the formation of M3 and M4 [13], in the elimination of bendamustine. Consequently, the effect of concomitant treatment that influences CYP1A2 activity on the safety and efficacy of bendamustine is expected to be minimal.
The high and persistent plasma levels of TRA compared with the concentrations of bendamustine, M3, M4, and HP2 combined indicate the presence of one or more long-lived bendamustine-related compounds and emphasize the importance of metabolism in the elimination of bendamustine. The Vss of bendamustine (20.1 L) implied that the drug is not extensively distributed into tissues. The Vss of TRA (49.5 L) seemed slightly larger but was overestimated, since more than a third of the radiochemical dose was eliminated during the first 24 hours postdose, a period that represented only approximately 10% of the AUC for TRA (Fig. 4). In addition, the plasma to whole-blood concentration ratios of TRA approximated unity at the later time points, suggesting no preferential association of bendamustine-related compounds with any specific blood components.
The incomplete recovery of TRA (~76%) is probably a result of the long t½ of TRA (197 hours) and is not uncommon for an alkylating agent [21]. Measurable levels of TRA were still present in the last urine and fecal samples, even in those collected 3 weeks after the 14C-bendamustine infusion, suggesting that higher recovery could have been obtained if the collection time had been further extended. However, the added value of additional excretion data was, in this case, considered limited and did not outweigh the accompanying additional burden for the patients.
Urinary excretion of 14C-bendamustine–derived radioactivity (49% of the administered dose) was more predominant than fecal excretion (27%). The urinary to fecal excretion ratio differed slightly from the ratio in rats, where ~49% of the administered dose was recovered in feces, with total recovery of ~90% [14]. Consistent with the rapid CL of bendamustine, M3, and M4 from plasma, these compounds were predominantly found in the 0- to 2-hour urine samples. Additionally, their relative amounts in urine were qualitatively the same as in plasma (i.e., amount of bendamustine > amount of M3 > amount of M4). In contrast, although HP2 concentrations in plasma were substantially lower than the bendamustine concentrations, the amount of HP2 recovered in urine was comparable to the recovered amount of bendamustine, indicating that hydrolysis of bendamustine facilitates renal excretion. The continuing recovery of small amounts of HP2 in urine correlates with the continuing low levels of HP2 that were measured in plasma.
The first 24-hour urine recovery values of unchanged bendamustine (3.31 ± 1.95%), M3 (0.73 ± 0.37%), M4 (0.08 ± 0.11%), and HP2 (4.89 ± 2.91%), adding up to a total of 9.01 ± 1.99%, are comparable to values seen in previous studies. Teichert and colleagues [13] recovered 3.23 ± 3.69%, 0.30 ± 0.31%, 0.05 ± 0.03%, and 0.94 ± 0.13% of the administered dose as bendamustine, M3, M4, and HP2, respectively, in the 0- to 24-hour urine samples after bendamustine infusion. In two studies, Rasschaert and colleagues recovered 8.3% (range 2.7–26.0%) [15] and 9.8% [16] of the administered dose in the first micturition after a bendamustine infusion as bendamustine, M3, M4, HP1, and HP2 combined.
In the present study, extensive measures were applied to minimize degradation of bendamustine. Each urine void was processed individually and immediately; urine was diluted in prechilled control human plasma for stabilization and immediately stored at −70 °C pending bioanalysis, when samples were thawed in ice water and kept in ice water whenever possible during sample preparation. The stability of bendamustine was confirmed under these conditions [17]. Still, considerable variation was present in the urinary recovery of bendamustine. One cause may be the intrinsic variation of bendamustine elimination (Table 2), which was also observed in plasma [20]. Additionally, even though patients were asked to void their bladder every 2 hours during the first 12 hours, variable intravesical conversion of bendamustine may have contributed to variations in recovery and possibly to an underprediction of unchanged bendamustine excretion.
The relatively low recovery of bendamustine, M3, M4, and HP2 (combined 9.01% ± 1.99%) compared with the recovery of TRA (36.61% ± 3.47% after 24 hours) indicates the presence of additional metabolites. This finding is consistent with the metabolite profile in rat urine. Sixteen metabolites of bendamustine were detected in rat urine collected 0–4 hours after administration of 14C-bendamustine to rats, and a major portion of the radioactivity in urine was accounted for by products of N-deethylation and N-acetylcysteine conjugates [14].
Bendamustine was well tolerated when administered at a dose of 120 mg/m2. Bendamustine has been associated with myelosuppression, mild gastrointestinal events, and fatigue [3, 9, 22]. Although bendamustine has a short t½, prolonged myelosuppression [3, 9, 22] has been observed, which may be related to the DNA cross-linking properties of bendamustine [8, 23].
This dosage (120 mg/m2) is the same as that used for treatment of indolent B-cell non-Hodgkin’s lymphoma that has progressed during or within 6 months of treatment with rituximab or a rituximab-containing regimen [3]; however, 90 mg/m2 is used in combination with rituximab [10–12, 24], and bendamustine in chronic lymphocytic leukemia was studied at a 100-mg/m2 dose [22]. Higher-dose bendamustine (160 to 200 mg/m2) has also been investigated [25]; because of the rapid hydrolysis of bendamustine, accumulation of bendamustine at these doses is not expected.
Despite the small sample size of the present study, the treatment-related AEs in the present study, with vomiting (50%) and fatigue (50%) as those most frequently reported, and lymphocytopenia, were generally consistent with the known safety profile of bendamustine.
The short intermediate t½ and dosing schedule of bendamustine of two consecutive days in 21- or 28-day cycles, in addition to the fact that bendamustine is extensively metabolized via multiple pathways, suggest that accumulation is unlikely in patients with hepatic insufficiency. A recent study of metabolite profiling in cancer patients [26], as well as findings of small amounts of unchanged bendamustine in urine in this and previous studies [13, 15, 16], suggest that bendamustine is primarily metabolized by hydrolysis via extrahepatic pathways, with more limited hepatic metabolism. However, in another study in humans [27], a longer intermediate t½ (47 vs. 33 minutes) and slower CL (304 vs. 639 mL/min) were demonstrated in patients with hepatic impairment, leading to current labeling recommendations to use bendamustine with caution in mildly hepatically impaired patients and a contraindication for use in patients with moderate to severe impairment.
Renal excretion of unchanged bendamustine is minor, representing only ~3% of the administered dose. Even though bendamustine excretion might be underestimated because of intravesical degradation, these results combined with the short t½ of bendamustine and the dosing schedule suggest that renal impairment is also unlikely to have a substantial impact on systemic exposure to bendamustine. This is in line with a small myeloma study, which showed that moderate to severe renal insufficiency or renal failure requiring dialysis did not significantly affect the plasma kinetics of bendamustine and its metabolites M3 and M4 [28].
Conclusion
Metabolism—in particular, hydrolysis via extrahepatic and hepatic pathways—plays a major role in the elimination of bendamustine. AEs and hematologic changes in this study were consistent with the known safety profile of bendamustine. Additional research is being conducted to further elucidate the metabolic profile of bendamustine in humans.
Acknowledgments
The authors acknowledge Matthijs Tibben and Lianda Nan for their bioanalytic support for the study and Dr. Ly Tran for preparation of the radiolabeled patient dosing solutions. Additionally, we gratefully thank the patients who participated for giving their valuable time to the study.
Disclosures
Mona Darwish, Denise D’Andrea, Mary Bond, Edward Hellriegel, and Philmore Robertson, Jr., are employees of Teva Pharmaceutical Industries Ltd. The other authors have no relevant conflicts of interest to declare.
Funding Sources
This study was sponsored by Teva Pharmaceutical Industries Ltd. Funding for editorial support was provided by Teva Pharmaceutical Industries Ltd. to The Curry Rockefeller Group, LLC (Tarrytown, NY, USA).
References
- 1.Leoni LM, Bailey B, Reifert J, et al. Bendamustine (Treanda) displays a distinct pattern of cytotoxicity and unique mechanistic features compared with other alkylating agents. Clin Cancer Res. 2008;14(1):309–317. doi: 10.1158/1078-0432.CCR-07-1061. [DOI] [PubMed] [Google Scholar]
- 2.Fowler N, Kahl BS, Lee P, et al. Bortezomib, bendamustine, and rituximab in patients with relapsed or refractory follicular lymphoma: the phase II VERTICAL study. J Clin Oncol. 2011;29(25):3389–3395. doi: 10.1200/JCO.2010.32.1844. [DOI] [PubMed] [Google Scholar]
- 3.Friedberg JW, Cohen P, Chen L, et al. Bendamustine in patients with rituximab-refractory indolent and transformed non-Hodgkin’s lymphoma: results from a phase II multicenter, single-agent study [published erratum appears in J Clin Oncol. 2008 Apr; 26(11):1911] J Clin Oncol. 2008;26(2):204–210. doi: 10.1200/JCO.2007.12.5070. [DOI] [PubMed] [Google Scholar]
- 4.Ogura M, Uchida T, Taniwaki M, et al. Phase I and pharmacokinetic study of bendamustine hydrochloride in relapsed or refractory indolent B-cell non-Hodgkin lymphoma and mantle cell lymphoma. Cancer Sci. 2010;101(9):2054–2058. doi: 10.1111/j.1349-7006.2010.01633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ponisch W, Rozanski M, Goldschmidt H, et al. Combined bendamustine, prednisolone and thalidomide for refractory or relapsed multiple myeloma after autologous stem-cell transplantation or conventional chemotherapy: results of a phase I clinical trial. Br J Haematol. 2008;143(2):191–200. doi: 10.1111/j.1365-2141.2008.07076.x. [DOI] [PubMed] [Google Scholar]
- 6.von Minckwitz G, Chernozemsky I, Sirakova L, et al. Bendamustine prolongs progression-free survival in metastatic breast cancer (MBC): a phase III prospective, randomized, multicenter trial of bendamustine hydrochloride, methotrexate and 5-fluorouracil (BMF) versus cyclophosphamide, methotrexate and 5-fluorouracil (CMF) as first-line treatment of MBC. Anticancer Drugs. 2005;16(8):871–877. doi: 10.1097/01.cad.0000175587.31940.19. [DOI] [PubMed] [Google Scholar]
- 7.Eichbaum MH, Schuetz F, Khbeis T, et al. Weekly administration of bendamustine as salvage therapy in metastatic breast cancer: final results of a phase II study. Anticancer Drugs. 2007;18(8):963–968. doi: 10.1097/CAD.0b013e328165d11a. [DOI] [PubMed] [Google Scholar]
- 8.Strumberg D, Harstrick A, Doll K, et al. Bendamustine hydrochloride activity against doxorubicin-resistant human breast carcinoma cell lines. Anticancer Drugs. 1996;7(4):415–421. doi: 10.1097/00001813-199606000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Ohmachi K, Ando K, Ogura M, et al. Multicenter phase II study of bendamustine for relapsed or refractory indolent B-cell non-Hodgkin lymphoma and mantle cell lymphoma. Cancer Sci. 2010;101(9):2059–2064. doi: 10.1111/j.1349-7006.2010.01635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Friedberg JW, Vose JM, Kelly JL, et al. The combination of bendamustine, bortezomib, and rituximab for patients with relapsed/refractory indolent and mantle cell non-Hodgkin lymphoma. Blood. 2011;117(10):2807–2812. doi: 10.1182/blood-2010-11-314708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robinson KS, Williams ME, van der Jagt RH, et al. Phase II multicenter study of bendamustine plus rituximab in patients with relapsed indolent B-cell and mantle cell non-Hodgkin’s lymphoma. J Clin Oncol. 2008;26(27):4473–4479. doi: 10.1200/JCO.2008.17.0001. [DOI] [PubMed] [Google Scholar]
- 12.Rummel MJ, Al-Batran SE, Kim SZ, et al. Bendamustine plus rituximab is effective and has a favorable toxicity profile in the treatment of mantle cell and low-grade non-Hodgkin’s lymphoma. J Clin Oncol. 2005;23(15):3383–3389. doi: 10.1200/JCO.2005.08.100. [DOI] [PubMed] [Google Scholar]
- 13.Teichert J, Baumann F, Chao Q, et al. Characterization of two phase I metabolites of bendamustine in human liver microsomes and in cancer patients treated with bendamustine hydrochloride. Cancer Chemother Pharmacol. 2007;59(6):759–770. doi: 10.1007/s00280-006-0331-5. [DOI] [PubMed] [Google Scholar]
- 14.Chovan JP, Li F, Yu E, et al. Metabolic profile of [(14)C]bendamustine in rat urine and bile: preliminary structural identification of metabolites. Drug Metab Dispos. 2007;35(10):1744–1753. doi: 10.1124/dmd.107.015958. [DOI] [PubMed] [Google Scholar]
- 15.Rasschaert M, Schrijvers D, Van den BJ, et al. A phase I study of bendamustine hydrochloride administered day 1 + 2 every 3 weeks in patients with solid tumours. Br J Cancer. 2007;96(11):1692–1698. doi: 10.1038/sj.bjc.6603776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rasschaert M, Schrijvers D, Van den BJ, et al. A phase I study of bendamustine hydrochloride administered once every 3 weeks in patients with solid tumors. Anticancer Drugs. 2007;18(5):587–595. doi: 10.1097/CAD.0b013e3280149eb1. [DOI] [PubMed] [Google Scholar]
- 17.Dubbelman AC, Tibben M, Rosing H, et al. Development and validation of LC-MS/MS assays for the quantification of bendamustine and its metabolites in human plasma and urine. J Chromatogr B Analyt Technol Biomed Life Sci. 2012;893–894:92–100. doi: 10.1016/j.jchromb.2012.02.039. [DOI] [PubMed] [Google Scholar]
- 18.Dubbelman AC, Rosing H, Jansen RS, et al. Mass balance study of 14C-eribulin in patients with advanced solid tumours. Drug Metab Dispos. 2012;40(2):313–321. doi: 10.1124/dmd.111.042762. [DOI] [PubMed] [Google Scholar]
- 19.US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Veterinary Medicine (CVM). Guidance for industry: bioanalytical method validation. Rockville: CDER, 2001 May. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm070107.pdf. Accessed 4 Oct 2012.
- 20.Owen JS, Melhem M, Passarell JA, et al. Bendamustine pharmacokinetic profile and exposure-response relationships in patients with indolent non-Hodgkin’s lymphoma. Cancer Chemother Pharmacol. 2010;66(6):1039–1049. doi: 10.1007/s00280-010-1254-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beumer JH, Beijnen JH, Schellens JH. Mass balance studies, with a focus on anticancer drugs. Clin Pharmacokinet. 2006;45(1):33–58. doi: 10.2165/00003088-200645010-00003. [DOI] [PubMed] [Google Scholar]
- 22.Knauf WU, Lissichkov T, Aldaoud A, et al. Phase III randomized study of bendamustine compared with chlorambucil in previously untreated patients with chronic lymphocytic leukemia. J Clin Oncol. 2009;27(26):4378–4384. doi: 10.1200/JCO.2008.20.8389. [DOI] [PubMed] [Google Scholar]
- 23.Bagnobianchi A, Spanswick VJ, Bingham JP, et al. Persistence of drug-induced DNA interstrand cross-links distinguishes bendamustine from conventional DNA cross-linking agents [abstract no. 1766]. 103rd Annual Meeting of the American Association for Cancer Research; 2012 Mar 31–Apr 4; Chicago.
- 24.Cheson BD, Wendtner C-M, Pieper A, et al. Optimal use of bendamustine in chronic lymphocytic leukemia, non-Hodgkin lymphomas, and multiple myeloma: treatment recommendations from an international consensus panel. Clin Lymphoma Myeloma Leuk. 2010;10(1):21–27. doi: 10.3816/CLML.2010.n.002. [DOI] [PubMed] [Google Scholar]
- 25.Visani G, Malerba L, Stefani PM, et al. BeEAM (bendamustine, etoposide, cytarabine, melphalan) before autologous stem cell transplantation is safe and effective for resistant/relapsed lymphoma patients. Blood. 2011;118:3419–3425. doi: 10.1182/blood-2011-04-351924. [DOI] [PubMed] [Google Scholar]
- 26.Dubbelman AC, Jansen RS, Rosing H, et al. Metabolite profiling of bendamustine urine of cancer patients after administration of [14C]bendamustine. Drug Metab Dispos. 2012;40(7):1297–1307. doi: 10.1124/dmd.112.045229. [DOI] [PubMed] [Google Scholar]
- 27.Preiss R, Teichert J, Athmani A, et al. Pharmacokinetics and toxicity profile of bendamustine in patients with impaired liver function [poster]. 2nd International Conference on Drug Discovery and Therapy; 2010 Feb 1–4; Dubai.
- 28.Preiss R, Teichert J, Poenisch W, et al. Bendamustine pharmacokinetics and safety are not afflicted by impaired renal function in patients with multiple myeloma [abstract no. 5254]. Blood. 2003;102:381–2b.