Summary
Dietary restriction can extend lifespan in most organisms tested to date, suggesting that mechanisms sensing nutrient and energy availability might regulate longevity. The AMP-activated protein kinase (AMPK) has emerged as a key energy sensor with the ability to transcriptionally reprogram the cell and metabolically adapt to external cues. In this review we will discuss the possible role of AMPK in the beneficial effects of calorie restriction on health and lifespan.
Introduction
It is fascinating how a simple intervention like caloric restriction (CR) is the most consistent intervention increasing lifespan, protecting against the deterioration of biological functions and reducing the risk factor for the apparition of diabetes cardiovascular disease and cancer (27). CR is usually defined as a moderate reduction, generally around 20-40%, in caloric intake compared with ad libitum feeding, without compromising the basic nutritional needs. The beneficial effects of CR on lifespan stretch all along the evolutionary scale (27). In Rhesus monkeys CR lowered the incidence of aging-related death and reduced the incidence of diabetes, cancer, cardiovascular disease and brain atrophy (18). Despite that we only have preliminary evidence based on surrogate measures on how CR might impact on human longevity, most available data sets indicate that CR exerts similar adaptative responses in humans as in laboratory animals, and prevents the development of age-associated health complications (47). In light of these attractive properties, many efforts have been dedicated to finding out how CR acts. At first, it would be intuitive to think that the adaptive response to low nutrient availability should be triggered by a mechanism with the ability to respond to nutrient availability via either humoral or intracellular metabolites. Importantly, the longevity of unicellular organisms, such as yeast, is modulated by CR. This indicates that autocrine signals and intracellular nutrient sensors can fully account for such effects.
The AMP-activated protein kinase (AMPK) has emerged as a key nutrient sensor with the ability to regulate whole-body metabolism. AMPK is activated upon an increase in the AMP/ATP ratio, which reflects the energy status of the cell. A recent report combining structural and biochemical approaches revealed that, apart from AMP, also ADP binding can also crucially contribute to AMPK activation . Upon activation, AMPK turns on catabolic pathways to restore ATP levels, both in a short time frame, by promoting glycolysis and fatty acid oxidation, and in a long time frame, by increasing mitochondrial content and the use of mitochondrial substrates as an energy source (12). Precisely, mitochondrial fitness is emerging as a particularly interesting topic in the ageing field as many reports indicate that the effective control of mitochondrial biogenesis, metabolism and turnover is crucial for a healthy cellular and whole-body ageing (reviewed in (78)). The role of AMPK to sense energy stresses and act as a master regulator of mitochondrial biogenesis and metabolism, therefore, has led to the speculation that AMPK might mediate the beneficial effects of CR. While many reports in lower eukaryotic organism, like worms, clearly support such notion, the translation of such observations on other organisms remains largely unexplored. In this review we aim to discuss a number of observations that either direct or indirectly indicate that AMPK might act as an important agent on the regulation of lifespan and a mediator of the beneficial effects of CR, while also pointing out the diverse caveats present in such hypothesis.
AMPK enzymatic regulation and actions
AMPK is a heterotrimeric Ser/Thr kinase composed of an α, β and γ subunit (40). There are two different forms of α (α1 and α2) and β (β1 and β2) subunits, while three different γ isoforms (γ1, γ2 and γ3) exist (40). The α subunits are the catalytic subunits of the functional heterotrimer and contains the Thr172 residue, whose phosphorylation is required for full enzymatic activity (43). The β subunit contains an evolutionary conserved carbohydrate binding domain, which allows AMPK to interact with glycogen particles, resulting in inhibition of AMPK when glycogen stores are high (51). The γ subunits contains four tandem repeats known as cystathionine β-synthase (CBS) motifs, which form an interface for interaction with two AMP or ATP molecules in a mutually exclusive way and a third AMP molecule in a non-exchangeable fashion (110). In basal conditions, the binding of ATP keeps the activity of the enzyme low. AMPK is only fully active after phosphorylation of Thr172 within the activation loop of the α subunit catatytic domain. The main upstream kinase in most cell types is the LKB1/STRAD/MO25 complex. This complex seems to be constitutively active and phosphorylates AMPK continuously, but, in basal conditions, the phosphate is immediately removed by protein phosphatises. However, upon energy stress, AMP concentrations increase and bind to the AMPKγ subunits, promoting a conformational change that renders AMPK a poorer substrate for dephosphorylation. Then, the increased phosphorylation levels of the Thr172 residue results in the full activation of the enzyme (96).
AMPK activation is generally linked to the stimulation of metabolic responses in order to prevent metabolic and energetic crisis in situations where ATP synthesis is compromised, such as in hypoxia, ischemia, low nutrient availability, or ATP consumption is accelerated, such as during exercise or fasting. Consequently, AMPK activation stimulates catabolic processes to generate ATP and inhibits ATP-consuming anabolic processes that are not required for the immediate survival of the cell. For the purpose of this review it is important to point out that AMPK acts as a master controller of mitochondrial metabolism, by promoting mitochondrial oxidation of lipid substrates and mitochondrial biogenesis through transcriptional means (12). For a detailed review of the metabolic and transcriptional actions of AMPK, we refer the reader to other recently published reviews (12, 40)
AMPK as a mediator of CR effects (I): Evidence from genetic models
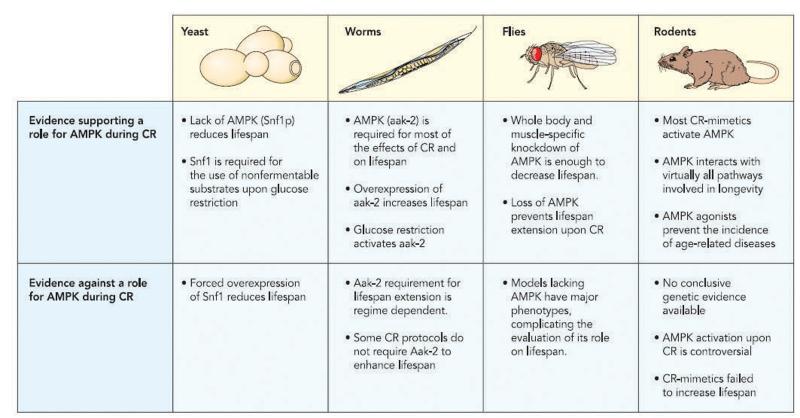
In the absence of glucose, the sucrose non-fermenting 1 kinase (SNF1), the yeast homolog of AMPK, translocates to the nucleus, where it transcriptionally potentiates the respiratory metabolism of non-fermentable carbon sources (107). Interestingly, the beneficial effects of glucose restriction in yeast are related to increases in respiratory rates that occur when glucose levels in the media are lower. Several models (76, 86), albeit not all (60), indentify the capability to promote respiration with the ability to increase replicative and/or chronological lifespan. Hence, it would logical to postulate that if Snf1 is responsible in majority for derepressing genes linked to the use of non-fermentable carbon sources upon low glucose availability, it should be key for the effects of CR. In line with this hypothesis, pioneer work by Ashrafi and colleagues demonstrated that Snf1 deletion results in loss of cellular fitness and decreased lifespan (5). The reasons for this decreased longevity, however, did not seem to be related to accelerated ageing, but, rather, to metabolic malfunctions (5). The role of Snf1 in yeast longevity seems, however, to be complex as both disruption and forced overactivation of Snf1 lead to reduced lifespan (5, 77, 79). It is likely that Snf1 activity must be tightly regulated upon CR in order to properly adapt to low nutrient availability without compromising lifespan.
While the evidence supporting a role for AMPK homologs in yeast for the adaptations to CR is somehow poor, it gains strength when moving to the C. Elegans model. Arguably, most of the genetic evidence supporting the role of AMPK as a mediator of the effects of CR on longevity has been raised in worms. In 2004, Apfeld and colleagues reported that the genetic deletion of the worm AMPKα homolog (aak-2) decreases worm lifespan by 12 % due to accelerated ageing (4). Conversely, worms overexpressing aak-2 lived 13% longer (4). Subsequent research elucidated that glucose restriction increases aak-2 activity in nemadotes and that aak-2 is required for the shift to respiratory metabolism in glucose-restricted conditions (97). Finally, extensive work (34, 35, 97) has determined that several CR regimes, albeit not all (34, 80), require aak-2 to promote lifespan extension.
Global deletion of the drosophila AMPK is lethal (70), forcing alternative strategies to study its influence on the responses to CR and impact on longevity. Using time- and tissue-specific RNAi systems in Drosophila, it was recently reported that inhibition of AMPK in muscle is enough to decrease the lifespan of flies (106). A parallel study indicates that reduced global AMPK activity decreases lifespan in drosophila and reduces lifespan extension by starvation (58). Interestingly, another recent report indicates that overexpression of LKB1, the upstream kinase for AMPK, promotes lifespan on Drosophila (31). However, whether AMPK participates in the lifespan promoting properties of CR in Drosophila or any mammalian organism, has not yet been tested.
AMPK as a mediator of CR effects (II): Evidence based molecular actions
Regulation of mitochondrial biogenesis
Initial clues linking AMPK with CR and the ageing process were provided by studies indicating that the capacity to pharmacologically or physiologically activate AMPK is lower in aged rodents than in young ones (92). While the mechanisms underlying such observation are still elusive, it might provide indications on why ageing is linked with defective mitochondrial and dysregulated lipid homeostasis, and why the effects of CR are generally linked to mitochondrial fitness.
Defective mitochondrial function seems to be the signature of mammalian ageing. Using NMR spectroscopy it was shown how ageing was associated with increased fat accumulation in muscle and liver, probably due a notable 40% reduction in mitochondrial oxidative phosphoryaltion activity (89). Mitochondria constitute a very attractive link to the physiological decay observed during ageing, as defective mitochondrial function and energy metabolism would explain the constellation of defects observed during ageing, such as oxidative stress, excessive inflammation, defective protein and organelle turnover and accumulation of covalent protein modifications emerging from metabolism side-products.
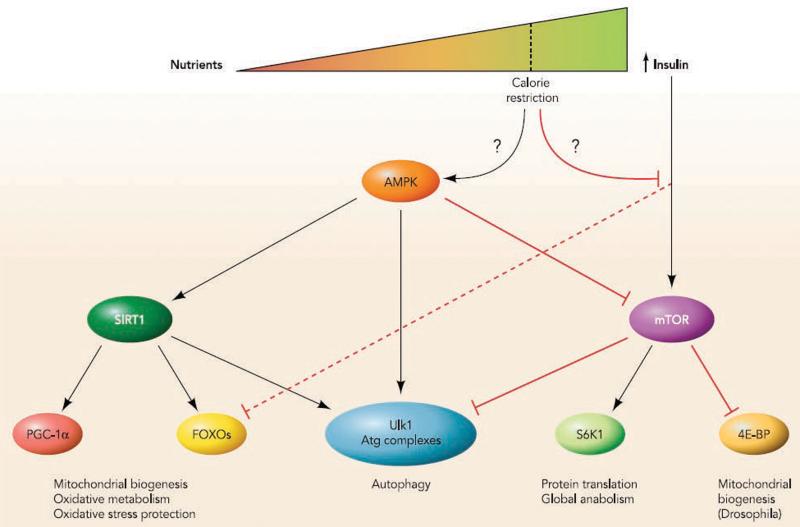
The fact that AMPK is a master regulator of mitochondrial biogenesis and lipid metabolism makes it a likely candidate to influence lifespan. The mechanisms by which AMPK regulates mitochondrial biogenesis are beginning to be elucidated. Transgenic animals have helped identifying PGC-1α as a crucial mediator of AMPK action on mitochondrial genes in mouse skeletal muscle (54, 73). PGC-1α is a transcriptional coregulator that orchestrates the mitochondrial biogenesis program by coactivating a number of nuclear transcription factors that control genes involved in mitochondrial function and lipid oxidation (14). The activity of PGC-1α is critically controlled by its acetylation status (56). Generally, the basal activity of PGC-1α is rather low, as it is highly acetylated (74). In order to fully achieve its coactivating potential, PGC-1α requires deacetylation by the NAD+-dependent deacetylase SIRT1 (93). The mechanism by which AMPK impacts on PGC-1α has been recently deciphered. Upon activation, AMPK directly phosphorylates PGC-1α at Thr177 and Ser538. The phosphorylation of these residues enables deacetylation by SIRT1 and, therefore, activation (15). In addition to PGC-1α, AMPK can also influence the activity of a number of transcriptional factors related to mitochondrial biogenesis and lipid oxidation, such as MEF2, PPARα and PPARδ [for extensive review, see (12)].
Regulation of known CR regulators of lifespan
The cooperative nature of AMPK and SIRT1 expands beyond PGC-1α. Work from Vittorio Sartorelli’s and our lab demonstrated that AMPK and SIRT1 activities are positively linked (15, 30). The mechanism by which AMPK impacts on SIRT1 activity does not rely on direct interaction or phosphorylation events, but AMPK rather promotes an intracellular increase in NAD+ levels, the rate-limiting substrate for the deacetylase activity of SIRT1 (15, 30). By increasing NAD+ levels, AMPK allows higher levels of SIRT1 activity. By phosphorylating PGC-1α, AMPK promotes specificity in SIRT1 action. This exemplifies how the cross-talk between different post-translational modifications (i.e: phosphorylation and acetylation) is of utmost importance to understand how similar transcriptional regulators can elicit specific actions depending on the context of its activation.
Furthermore, the link between AMPK and SIRT1 has important consequences on the CR and ageing fields. In lower eukaryotes manipulations of the SIRT1 gene or of its homologs has a strong influence in lifespan (13). In yeast, overexpression of Sir2, the SIRT1 homolog, is enough to enhance replicative lifespan (75). Furthermore, mimicking CR in yeast by reducing glucose in the medium from 2% to 0.5% was unable to extend lifespan in yeast lacking the Sir2 gene or models aimed to decrease Sir2 function (1, 75), suggesting that Sir2 is a crucial mediator of the effects of CR on lifespan in yeast. Further reinforcing such concept, overexpression of the SIRT1 homolog in worms and Drosophila also enhanced lifespan (104). Furthermore, the lack of Sir2 in Drosophila is enough to prevent the effects of CR on lifespan (94). While there are no reports so far supporting that SIRT1 enhances lifespan in mammals, the evidence obtained to date support that it improves healthy aging (6, 46, 90). Consequently, if AMPK promotes SIRT1 activity and expression, it would be likely that it could act as a lifespan/healthspan regulator.
The FOXO family of transcription factors provide a second molecular link between AMPK, CR and enhanced lifespan. Genetic studies in many organisms have provide substantial evidence that the FOXO transcription factors have conserved the ability to promote longevity (27). The activity of FOXOs is linked to the promotion of lipid metabolism, resistance to oxidative stress and pathogens, protection of protein structure and authophagy (for review, see (37)). These finding suggest that FOXOs enhance lifespan by protecting the cell from various stresses, including nutritional stress. The relation of AMPK with FOXOs was brought into the spotlight when FOXOs were reported as possible mediators of the effects of AMPK on autophagy (83). Furthermore, AMPK can directly phosphorylate different members of the FOXO family of transcription factors (36). Amongst them, FOXO3 is phosphorylated by AMPK in up to 6 residues (36). Mutation of these residues impaired the ability of AMPK to promote key transcriptional responses during glucose-deprivation, including the transcriptional activation of oxidative protection genes (36). FOXO phosphorylation by AMPK does not influence FOXO subcellular localization, but rather its activity (36). As for PGC-1α, FOXO activity is also critically controlled through acetylation/deacetylation, which is altered by SIRT1 (10, 29, 82). It is tempting to speculate that AMPK phosphorylation of FOXO could also serve as a signal for its deacetylation, which, in turn, seems to provide FOXO with specificity towards the regulation of oxidative stress genes (10), suggesting that, like for PGC-1α, the modifications of FOXO by AMPK and SIRT1 might be interconnected.
Regulation of mTOR signalling
The mammalian target of rapamycin (mTOR) kinase provides another riveting link between AMPK, CR and longevity. mTOR is as a central regulator of eukaryotic growth and cell division in response to nutrient and growth factor cues. TOR proteins are highly conserved from yeast to humans (100). The identification and name of mTOR derives from studies of the growth inhibiting properties of the anti-fungal compound rapamycin (45). mTOR is generally activated by growth hormones (e.g. insulin) and promotes anabolism, cell growth and division (100). The role of mTOR, or its homologs, as an important longevity pathway has been firmly established by studies on many different eukaryotic models, indicating that a reduction in the activity of the mTOR complex 1 (mTORC1) is sufficient to increase lifespan significantly [for review, see (48, 103)]. In mammals it has been shown that CR decreases mTOR signalling (103). Feeding mice with rapamycin extends median and maximal lifespan of different mouse strains (42). Further supporting the role of mTOR on longevity, deletion of ribosomal S6 protein kinase 1 (S6K1), a downstream component of the mTOR signaling pathway, led to increased life span and resistance to age-related pathologies, such as bone, immune, and motor dysfunction and loss of insulin sensitivity (98). Importantly, deletion of S6K1 induced gene expression patterns similar to those seen in CR (98). From a metabolic perspective, mice lacking S6K are prevented against obesity and metabolic disease, linked to a higher mitochondrial biogenesis (109). It is also interesting to note that in a genome-wide array for genes changed upon dietary restriction in Drosophila, it became evident that 4E-BP, another downstream target of mTOR, might be key for the effects of CR (115). 4E-BP (eukaryotic translation initiation factor 4E binding protein) is a translational repressor, which is inhibited upon mTOR activation (32). 4E-BP is induced upon CR in flies, and mediates the effects of CR on mitochondrial biogenesis and longevity (115). These findings further support that attenuation of mTOR signalling might be a key step by which CR impacts on lifespan extension. Interestingly, AMPK activation is the best-described intracellular trigger for mTOR inhibition. By phosphorylating both Raptor (a component of the mTORC1 complex) (38) and TSC2 (an upstream regulator of mTOR) (53), AMPK inhibits mTOR, and, therefore, of S6K1 and leads to the reduction of many anabolic processes. As the downregulation of mTOR has been shown to promote CR-like effects on lifespan in several organisms (64), the regulation of mTOR by AMPK provides a very likely mechanism by which AMPK could influence lifespan.
Regulation of autophagy
The possible influence of autophagy on longevity is raising major attention (66). Autophagy is an evolutionarily conserved process in which portions of the cytoplasm, including superfluous or damaged organelles and misfolded or aggregated proteins, are engulfed in double-membrane vesicles called autophagosomes for degradation and recycling (66). It has been repeatedly reported that the autophagic activity of living cells decreases with age, probably contributing to the accumulation of damaged macromolecules and organelles during aging. Moreover, autophagy modulation in different model organisms has yielded results suggesting that the maintenance of a proper autophagic activity is a key adaptation by which CR enhances longevity [for review, see (66)]. Autophagy is controlled through a tight molecular network which includes most of the above-described players: AMPK, SIRT1, FOXO and mTOR. While nutrient scarcity promotes autophagy, the mechanisms by which this happens are only beginning to be elucidated. Under low nutrient conditions, AMPK activation promotes authophagy by activating Ulk1 through direct phosphorylation (25, 65, 71). Ulk1 is a critical kinase that governs the cascade of events triggering autophagy (81). Elegant studies by two different groups identified multiple, yet different, key AMPK sites on Ulk1 (25, 65). Therefore, while phosphorylation of Ulk1 by AMPK is convincingly demonstrated, the mechanistic implications of the different phosphorylable residues are not yet clear. Upon nutrient and growth factor abundance, mTOR gets active and phosphorylates Ulk1 at Ser757, which disrupts the interaction between AMPK and Ulk1, therefore, inactivating Ulk1 and downregulating autophagy rates (65). AMPK can also impact on autophagy through the modulation of SIRT1 (69). SIRT1 forms molecular complexes with several components of the autophagy machinery, including Atg5, Atg7 and Atg8, which act as critical regulators of the autophagosome formation. SIRT1 deacetylates these proteins in an NAD+-dependent manner, even though the substrate residues and the mechanistic consequences of this deacetylation have not yet been elucidated (69). The absence of SIRT1 considerably increased the acetylation level of these autophagy proteins. Consistent with these observations, autophagy during starvation is impeded in embryonic fibroblasts of SIRT1−/− mice (69). The lack of SIRT1 led to the accumulation of damaged organelles, especially mitochondria (69), which might explain why defective SIRT1 activity systematically correlates with deficiencies in energy metabolism. This way, the impact of AMPK on autophagy could be mediated not only by the direct activation of Ulk1, but also through the indirect modulation of the acetylation status of key autophagosome formation proteins by SIRT1.
Finally, the FOXO family of transcription factors has also been linked to autophagic processes, especially in cardiac and skeletal muscle. During nutrient scarcity, FOXO1 and FOXO3 move to the nucleus and occupy the promoters of genes related to autophagy (99, 112, 113). By blocking FOXO activity, the autophagic capacity of the tissues is greatly compromised. This is evidenced, for example, by the fact that cardiomyocyte cell size is reduced by autophagic procedures upon starvation, an effect that its blocked when FOXO activity is prevented (99). Interestingly, there is evidence indicating that the deacetylation of FOXO by SIRT1 is required for FOXO-induced autophagy-related gene expression (41). This further illustrates the networking interaction between AMPK, FOXO and SIRT1 in the regulation of many cellular processes involved in the adaptations to calorie restriction and promotion of longevity, opposing the action of mTOR (Figure 1).
Figure 1.
AMPK as a mediator of CR effects (III): Pharmacological evidence
The probable influence of AMPK on the adapations to CR and the promotion of healthy longevity is also supported by a number of observations derived from pharmacological approaches. Given the numerous beneficial effects of CR but the unlikeliness that people would adapt such a rather “severe” diet, there has been a strong interest in developing calorie restriction mimetic compounds that could be used pharmacologically. Probably, the first approach to achieve such a goal was the use of 2-deoxyglucose (2DG), a glycolysis inhibitor. This simple approach recapitulated many features of calorie restriction, like an increase in insulin sensitivity and a decrease in core body temperature (52). Of note, treatment with 2DG at therapeutic doses leads to energy stress and potently activates AMPK (44). The strong toxicity of 2DG use at the therapeutic doses, however, discouraged the use of such compound and strategy as a mimic for CR studies.
The compound that has probably epitomized the concept of “CR mimetic” might be resveratrol (Rsv). Rsv is a small polyphenol present in, amongst others, red grapes and was initially described to have cancer chemopreventive activity (55). A decade ago, SIRT1 emerged as candidate mediator of CR-induced replicative life extension in yeast. In an attempt to find small-molecule SIRT1 activators, Rsv was identified as a direct SIRT1 activator in an in vitro screen (49). Subsequently, Rsv was shown to extend lifespan in numerous studies in yeast, worms and flies in a sirtuin dependent manner (13). These observations indicated that Rsv treatment could by itself be enough to mimic many features of CR. This CR-mimetic effects were also observed in mammals from a metabolic perspective, as Rsv protects against insulin resistance, enhances mitochondrial biogenesis and recapitulates metabolic transcriptional profiles that resemble those of animals on CR (8, 67). While there are some differences between the effects of Rsv and CR in mammals [summarized in (7)], its characteristics as a CR mimetic are arguably the most extensively documented to date. However, a couple of studies in 2005 alerted about the possibility that Rsv may not be a direct SIRT1 activator and that the results on the in vitro screen were an artifact derived from the use of a fluorescent substrate (9, 62). The SIRT1-dependency of many Rsv effects clearly argued that SIRT1 was being activated upon Rsv treatment (67), but as an indirect downstream event of some mechanism that was not yet clear. Some light was brought into this issue when our lab identified SIRT1 as a downstream metabolic mediator of AMPK actions (15). Indeed, by early 2007 several labs reported that Rsv had the ability to activate AMPK (8, 20, 111). In our hands Rsv can activate AMPK in C2C12 myotubes in less than 5 minutes after treatment, while activation of SIRT1 takes hours (Canto C and Auwerx J, unpublished observations). To date, the activation of AMPK is the earliest signalling event described for Rsv. The mechanism by which Rsv activates AMPK has also been exquisitely unveiled recently. In early reports on Rsv, this polyphenol was described to compromise ATP synthesis by directly inhibiting ATP synthase in the mitochondrial respiratory chain (114). Using AMPK mutants that are insensitive to changes in AMP/ATP ratio, it was demonstrated that Rsv activates AMPK as a consequence of decreased ATP production (44). The question was then, do the metabolic effects of Rsv arise from AMPK activation? The answer so far might be notoriously affirmative. Using the AMPKγ3 knock-out mouse model we demonstrated that defective AMPK prevents the activation of SIRT1 upon Rsv treatment (16). Consequently, PGC-1α acetylation levels and, therefore, activity were unresponsive to Rsv treatment when AMPK activation is compromised (16). A parallel study by Um et al, using either AMPKα1 or AMPKα2 knock-out, showed that AMPK was required by resveratrol to induce CR-like effects, like increasing insulin sensitivity, glucose tolerance and enhancing mitochondrial biogenesis (108). A third study in worms indicates that resveratrol requires intact AMPK activity to enhance lifespan (34). In combination, these studies unequivocally prove that AMPK is a central target for the CR-like metabolic effects of resveratrol.
Other pharmacological evidence supporting the role of AMPK as a key effector of CR would come from the effects of metfomin on longevity. Metfomin is a biguanide commonly used in the treatment of type 2 diabetes, due to its ability to suppress glucose production in liver. Strikingly, metformin promotes CR-like transcriptional changes (22). Similarly, other CR effects are also observed upon metformin treatment, as for example, the prevention of tumor development (2, 3). In addition, reduced all-cause mortality has been associated with metformin treatment in both diabetic and cardiovascular disease patients (95). As with resveratrol, many effects of metformin derive from its capacity to inhibit complex I of the mitochondrial respiratory chain, decrease ATP production and activate AMPK (44). While the real burden of metformin effects that can be attributed to AMPK is still a matter of debate (28), this observation also contributes to the notion that AMPK might be a convergent point for these compounds to promote CR mimetic effects.
Evidence against AMPK as a mediator of calorie restriction effects
Despite the amount of evidence supporting that AMPK could be a good candidate mediator of the adaptations to CR, this hypothesis has also some caveats (summarized in Figure 2). The fact that AMPK triggers adaptations resembling those seen with CR does not imply that this is the mechanism by which CR acts. Indeed, this might be the case, as a major concern is whether the stress promoted by CR is robust enough to activate AMPK, at least in mammals. There are conflictive observations in this sense. Early studies indicated that heart, muscle and liver from mice that have been on CR for 4 months did not show any change in AMPK activity compared to ad libitum fed mice (33). Another report supports these observations by showing no changes or even decreases in AMPK activity upon CR (105). In some sense, this is not surprising, as it is also not evident to see AMPK activation in response to even more drastic nutritional challenges such as fasting (see (21) for review). These publications, however, are at odds with other studies indicating that CR leads to AMPK activation in rat heart and liver, as well as mouse skeletal muscle (24, 57, 87, 101). Therefore, whether AMPK is activated or not upon CR is still a largely unresolved issue. Similarly, whether AMPK activation in the latter studies constitutes the cause or the consequence of the beneficial effects of CR is also not clear. Genetic studies in yeast (5), indicate that constitutive activation of AMPK might actually be deleterious for lifespan. Similarly, higher basal AMPK activity can be deleterious in some mammalian tissues, as reported in the Wolf-Parkinson-White syndrome, where mutations that activate AMPK predispose to the development of cardiomyopathy (11). These observations cast some doubt about whether higher AMPK activity could be the way by which CR acts. While it is unlikely that AMPK is constitutively and continuously active upon CR or that CR per se is an energy stress intense enough to increase AMPK activity, this does not rule out, however, that AMPK might be activated in a more subtle temporally- or spatially-restricted fashion upon CR. Similarly, it is likely that CR prevents the decrease in AMPK sensitivity for activation observed upon ageing (92), allowing the maintenance of its proper function on metabolic adaptations and mitochondrial gene expression modulation. The question is, if AMPK activation upon CR is not clear, why is there such abundant genetic evidence indicating that AMPK is required for many responses to CR? One explanation might be that AMPK defective models are known to have lower basal mitochondrial gene expression (59). Hence, the abnormal response to CR in AMPK-deficient models might not stem from AMPK activation per se, but from the deficient mitochondrial function.
Figure 2.
A second argument that complicates the vision of AMPK as a crucial mediator of CR-induced effects is that the AMPK-dependency seems to be contingent on the particularities of the regime/diet and organism used for the experiment. It is evident that CR can promote, under certain conditions, lifespan extension in the absence of AMPK (34, 80). While it is generally assumed that AMPK might contribute to the lifespan extension effects of CR by promoting mitochondrial fitness, this hypothesis also raises some concerns. These concerns can be summarized as follows: 1./ the fact that CR promotes mitochondrial biogenesis and activity in mammals is still contested (17, 39, 85, 91, 102); 2./ CR can also promote longevity in respiratory-deficient yeast models (60); and 3./ there are numerous models indicating that, actually, decreased mitochondrial respiration can also provide CR-like effects on worms lifespan (23, 26, 72). A similar argument could be made for the hypothesis that AMPK might mediate CR-induced lifespan extension through SIRT1, as activation of SIRT1 upon CR has not been observed in some organisms (60, 63) or mammalian tissues (19), and there is also evidence indicating that SIRT1 can be dispensable in lower eukaryote models for the effects of CR on replicative lifespan (61, 80, 97).
A third weakness relates to the current pharmacological evidence obtained in mammals. A key feature of CR is that it can enhance healthspan and lifespan both in control and disease models. Rsv, which has been proposed as a CR mimetic, only extends mammalian lifespan when mice are fed a high-fat diet, but not in mice fed a regular chow diet, despite the clear metabolic effects that Rsv has on chow-fed mice (8, 88). Given that most of the metabolic effects of Rsv are attributed to AMPK activation (16, 108), this observation argues against the possibility that activation of AMPK per se could be enough to promote all CR-induced effects. Similarly, while metformin improves healthspan in situations of disease, there is no report to date indicating that metformin can promote CR-like effects in otherwise healthy mammalian models. In contrast, the effects of the mTORC1 inhibitor, rapamycin, can be observed in regular rodents, suggesting that many of the effects from AMPK activation might be a secondary consequence from mTOR inhibition.
Conclusions and future directions
Genetic evidence in lower eukaryotes indicated that AMPK is required for many of the adaptations triggered by CR, including lifespan extension. Similarly, AMPK activation impacts on mitochondrial metabolism and on the activity of the FOXO, the sirtuin and mTOR signalling pathways, all of which have been tightly linked to CR and the promotion of a healthy longevity. Combined these arguments indicate that AMPK might be an important link to sense and adapt to CR. However, a number of caveats also indicate that the fact that AMPK can mimic certain aspects of CR does not necessarily mean that AMPK is the natural effector of the effects of CR. The clarification of these controversies will require future attention. The available AMPK deficient mouse models and the standardization of mammalian CR protocols will contribute to shed light into this issue. In any of these cases, the evidence reviewed here argues that AMPK activation can be a useful pharmacological tool to achieve a major number of the beneficial effects of CR. Culminating evidence clearly indicates that chronic feeding of rodents with AMPK agonists improves muscle endurance (67, 84), prevents against metabolic disease (67), allows proper circadian regulation (68) and suppresses tumorigenesis (50). For these reasons, it is likely that the unwillingness of mankind to engage in drastic lifestyle interventions, such as CR, will further strengthen AMPK’s position as a main beacon of hope for the prevention and/or treatment of the current epidemic of metabolic and age-related diseases.
ACKNOWLEDGEMENTS
The work in the laboratory of the authors was supported by grants of the Ecole Polytechnique Fédérale de Lausanne, Swiss National Science Foundation, NIH (DK59820), and the European Research Council Ideas programme (Sirtuins; ERC-2008-AdG23118). The authors thank all the members of the Auwerx lab for inspiring discussions.
REFERENCES
- 1.Anderson RM, Bitterman KJ, Wood JG, Medvedik O, Sinclair DA. Nicotinamide and PNC1 govern lifespan extension by calorie restriction in Saccharomyces cerevisiae. Nature. 2003;423:181–185. doi: 10.1038/nature01578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anisimov VN, Berstein LM, Egormin PA, Piskunova TS, Popovich IG, Zabezhinski MA, Kovalenko IG, Poroshina TE, Semenchenko AV, Provinciali M, Re F, Franceschi C. Effect of metformin on life span and on the development of spontaneous mammary tumors in HER-2/neu transgenic mice. Exp Gerontol. 2005;40:685–693. doi: 10.1016/j.exger.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 3.Anisimov VN, Egormin PA, Bershtein LM, Zabezhinskii MA, Piskunova TS, Popovich IG, Semenchenko AV. Metformin decelerates aging and development of mammary tumors in HER-2/neu transgenic mice. Bull Exp Biol Med. 2005;139:721–723. doi: 10.1007/s10517-005-0389-9. [DOI] [PubMed] [Google Scholar]
- 4.Apfeld J, O’Connor G, McDonagh T, DiStefano PS, Curtis R. The AMP-activated protein kinase AAK-2 links energy levels and insulin-like signals to lifespan in C. elegans. Genes Dev. 2004;18:3004–3009. doi: 10.1101/gad.1255404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ashrafi K, Lin SS, Manchester JK, Gordon JI. Sip2p and its partner snf1p kinase affect aging in S. cerevisiae. Genes Dev. 2000;14:1872–1885. [PMC free article] [PubMed] [Google Scholar]
- 6.Banks AS, Kon N, Knight C, Matsumoto M, Gutierrez-Juarez R, Rossetti L, Gu W, Accili D. SirT1 gain of function increases energy efficiency and prevents diabetes in mice. Cell Metab. 2008;8:333–341. doi: 10.1016/j.cmet.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baur JA. Resveratrol, sirtuins, and the promise of a DR mimetic. Mech Ageing Dev. 2010;131:261–269. doi: 10.1016/j.mad.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borra MT, Smith BC, Denu JM. Mechanism of human SIRT1 activation by resveratrol. J Biol Chem. 2005;280:17187–17195. doi: 10.1074/jbc.M501250200. [DOI] [PubMed] [Google Scholar]
- 10.Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, Tran H, Ross SE, Mostoslavsky R, Cohen HY, Hu LS, Cheng HL, Jedrychowski MP, Gygi SP, Sinclair DA, Alt FW, Greenberg ME. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303:2011–2015. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- 11.Burwinkel B, Scott JW, Buhrer C, van Landeghem FK, Cox GF, Wilson CJ, Grahame Hardie D, Kilimann MW. Fatal congenital heart glycogenosis caused by a recurrent activating R531Q mutation in the gamma 2-subunit of AMP-activated protein kinase (PRKAG2), not by phosphorylase kinase deficiency. Am J Hum Genet. 2005;76:1034–1049. doi: 10.1086/430840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Canto C, Auwerx J. AMP-activated protein kinase and its downstream transcriptional pathways. Cell Mol Life Sci. 2010;67:3407–3423. doi: 10.1007/s00018-010-0454-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Canto C, Auwerx J. Caloric restriction, SIRT1 and longevity. Trends Endocrinol Metab. 2009;20:325–331. doi: 10.1016/j.tem.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Canto C, Auwerx J. PGC-1alpha, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr Opin Lipidol. 2009;20:98–105. doi: 10.1097/MOL.0b013e328328d0a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Canto C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, Elliott PJ, Puigserver P, Auwerx J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009;458:1056–1060. doi: 10.1038/nature07813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Canto C, Jiang LQ, Deshmukh AS, Mataki C, Coste A, Lagouge M, Zierath JR, Auwerx J. Interdependence of AMPK and SIRT1 for metabolic adaptation to fasting and exercise in skeletal muscle. Cell Metab. 2010;11:213–219. doi: 10.1016/j.cmet.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Civitarese AE, Carling S, Heilbronn LK, Hulver MH, Ukropcova B, Deutsch WA, Smith SR, Ravussin E. Calorie restriction increases muscle mitochondrial biogenesis in healthy humans. PLoS Med. 2007;4:e76. doi: 10.1371/journal.pmed.0040076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, Allison DB, Cruzen C, Simmons HA, Kemnitz JW, Weindruch R. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325:201–204. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen D, Bruno J, Easlon E, Lin SJ, Cheng HL, Alt FW, Guarente L. Tissue-specific regulation of SIRT1 by calorie restriction. Genes Dev. 2008;22:1753–1757. doi: 10.1101/gad.1650608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dasgupta B, Milbrandt J. Resveratrol stimulates AMP kinase activity in neurons. Proc Natl Acad Sci U S A. 2007;104:7217–7222. doi: 10.1073/pnas.0610068104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Lange P, Moreno M, Silvestri E, Lombardi A, Goglia F, Lanni A. Fuel economy in food-deprived skeletal muscle: signaling pathways and regulatory mechanisms. FASEB J. 2007;21:3431–3441. doi: 10.1096/fj.07-8527rev. [DOI] [PubMed] [Google Scholar]
- 22.Dhahbi JM, Mote PL, Fahy GM, Spindler SR. Identification of potential caloric restriction mimetics by microarray profiling. Physiol Genomics. 2005;23:343–350. doi: 10.1152/physiolgenomics.00069.2005. [DOI] [PubMed] [Google Scholar]
- 23.Dillin A, Hsu AL, Arantes-Oliveira N, Lehrer-Graiwer J, Hsin H, Fraser AG, Kamath RS, Ahringer J, Kenyon C. Rates of behavior and aging specified by mitochondrial function during development. Science. 2002;298:2398–2401. doi: 10.1126/science.1077780. [DOI] [PubMed] [Google Scholar]
- 24.Edwards AG, Donato AJ, Lesniewski LA, Gioscia RA, Seals DR, Moore RL. Life-long caloric restriction elicits pronounced protection of the aged myocardium: a role for AMPK. Mech Ageing Dev. 2010;131:739–742. doi: 10.1016/j.mad.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Egan DF, Shackelford DB, Mihaylova MM, Gelino S, Kohnz RA, Mair W, Vasquez DS, Joshi A, Gwinn DM, Taylor R, Asara JM, Fitzpatrick J, Dillin A, Viollet B, Kundu M, Hansen M, Shaw RJ. Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science. 2011;331:456–461. doi: 10.1126/science.1196371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feng J, Bussiere F, Hekimi S. Mitochondrial electron transport is a key determinant of life span in Caenorhabditis elegans. Dev Cell. 2001;1:633–644. doi: 10.1016/s1534-5807(01)00071-5. [DOI] [PubMed] [Google Scholar]
- 27.Fontana L, Partridge L, Longo VD. Extending healthy life span--from yeast to humans. Science. 2010;328:321–326. doi: 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Foretz M, Hebrard S, Leclerc J, Zarrinpashneh E, Soty M, Mithieux G, Sakamoto K, Andreelli F, Viollet B. Metformin inhibits hepatic gluconeogenesis in mice independently of the LKB1/AMPK pathway via a decrease in hepatic energy state. J Clin Invest. 2010;120:2355–2369. doi: 10.1172/JCI40671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frescas D, Valenti L, Accili D. Nuclear trapping of the forkhead transcription factor FoxO1 via Sirt-dependent deacetylation promotes expression of glucogenetic genes. J Biol Chem. 2005;280:20589–20595. doi: 10.1074/jbc.M412357200. [DOI] [PubMed] [Google Scholar]
- 30.Fulco M, Cen Y, Zhao P, Hoffman EP, McBurney MW, Sauve AA, Sartorelli V. Glucose restriction inhibits skeletal myoblast differentiation by activating SIRT1 through AMPK-mediated regulation of Nampt. Dev Cell. 2008;14:661–673. doi: 10.1016/j.devcel.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Funakoshi M, Tsuda M, Muramatsu K, Hatsuda H, Morishita S, Aigaki T. A gain-of-function screen identifies wdb and lkb1 as lifespan-extending genes in Drosophila. Biochem Biophys Res Commun. 2011;405:667–672. doi: 10.1016/j.bbrc.2011.01.090. [DOI] [PubMed] [Google Scholar]
- 32.Gingras AC, Gygi SP, Raught B, Polakiewicz RD, Abraham RT, Hoekstra MF, Aebersold R, Sonenberg N. Regulation of 4E-BP1 phosphorylation: a novel two-step mechanism. Genes Dev. 1999;13:1422–1437. doi: 10.1101/gad.13.11.1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gonzalez AA, Kumar R, Mulligan JD, Davis AJ, Weindruch R, Saupe KW. Metabolic adaptations to fasting and chronic caloric restriction in heart, muscle, and liver do not include changes in AMPK activity. Am J Physiol Endocrinol Metab. 2004;287:E1032–1037. doi: 10.1152/ajpendo.00172.2004. [DOI] [PubMed] [Google Scholar]
- 34.Greer EL, Brunet A. Different dietary restriction regimens extend lifespan by both independent and overlapping genetic pathways in C. elegans. Aging Cell. 2009;8:113–127. doi: 10.1111/j.1474-9726.2009.00459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Greer EL, Dowlatshahi D, Banko MR, Villen J, Hoang K, Blanchard D, Gygi SP, Brunet A. An AMPK-FOXO pathway mediates longevity induced by a novel method of dietary restriction in C. elegans. Curr Biol. 2007;17:1646–1656. doi: 10.1016/j.cub.2007.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Greer EL, Oskoui PR, Banko MR, Maniar JM, Gygi MP, Gygi SP, Brunet A. The energy sensor AMP-activated protein kinase directly regulates the mammalian FOXO3 transcription factor. J Biol Chem. 2007;282:30107–30119. doi: 10.1074/jbc.M705325200. [DOI] [PubMed] [Google Scholar]
- 37.Gross DN, van den Heuvel AP, Birnbaum MJ. The role of FoxO in the regulation of metabolism. Oncogene. 2008;27:2320–2336. doi: 10.1038/onc.2008.25. [DOI] [PubMed] [Google Scholar]
- 38.Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, Turk BE, Shaw RJ. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30:214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hancock CR, Han DH, Higashida K, Kim SH, Holloszy JO. Does calorie restriction induce mitochondrial biogenesis? A reevaluation. FASEB J. 2011;25:785–791. doi: 10.1096/fj.10-170415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hardie DG. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat Rev Mol Cell Biol. 2007;8:774–785. doi: 10.1038/nrm2249. [DOI] [PubMed] [Google Scholar]
- 41.Hariharan N, Maejima Y, Nakae J, Paik J, Depinho RA, Sadoshima J. Deacetylation of FoxO by Sirt1 Plays an Essential Role in Mediating Starvation-Induced Autophagy in Cardiac Myocytes. Circ Res. 2010;107:1470–1482. doi: 10.1161/CIRCRESAHA.110.227371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, Pahor M, Javors MA, Fernandez E, Miller RA. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hawley SA, Davison M, Woods A, Davies SP, Beri RK, Carling D, Hardie DG. Characterization of the AMP-activated protein kinase kinase from rat liver and identification of threonine 172 as the major site at which it phosphorylates AMP-activated protein kinase. J Biol Chem. 1996;271:27879–27887. doi: 10.1074/jbc.271.44.27879. [DOI] [PubMed] [Google Scholar]
- 44.Hawley SA, Ross FA, Chevtzoff C, Green KA, Evans A, Fogarty S, Towler MC, Brown LJ, Ogunbayo OA, Evans AM, Hardie DG. Use of cells expressing gamma subunit variants to identify diverse mechanisms of AMPK activation. Cell Metab. 2010;11:554–565. doi: 10.1016/j.cmet.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Heitman J, Movva NR, Hall MN. Targets for cell cycle arrest by the immunosuppressant rapamycin in yeast. Science. 1991;253:905–909. doi: 10.1126/science.1715094. [DOI] [PubMed] [Google Scholar]
- 46.Herranz D, Munoz-Martin M, Canamero M, Mulero F, Martinez-Pastor B, Fernandez-Capetillo O, Serrano M. Sirt1 improves healthy ageing and protects from metabolic syndrome-associated cancer. Nat Commun. 2010;1:1–8. doi: 10.1038/ncomms1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Holloszy JO, Fontana L. Caloric restriction in humans. Exp Gerontol. 2007;42:709–712. doi: 10.1016/j.exger.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Houtkooper RH, Williams RW, Auwerx J. Metabolic networks of longevity. Cell. 2010;142:9–14. doi: 10.1016/j.cell.2010.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, Scherer B, Sinclair DA. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- 50.Huang X, Wullschleger S, Shpiro N, McGuire VA, Sakamoto K, Woods YL, McBurnie W, Fleming S, Alessi DR. Important role of the LKB1-AMPK pathway in suppressing tumorigenesis in PTEN-deficient mice. Biochem J. 2008;412:211–221. doi: 10.1042/BJ20080557. [DOI] [PubMed] [Google Scholar]
- 51.Hudson ER, Pan DA, James J, Lucocq JM, Hawley SA, Green KA, Baba O, Terashima T, Hardie DG. A novel domain in AMP-activated protein kinase causes glycogen storage bodies similar to those seen in hereditary cardiac arrhythmias. Curr Biol. 2003;13:861–866. doi: 10.1016/s0960-9822(03)00249-5. [DOI] [PubMed] [Google Scholar]
- 52.Ingram DK, Roth GS. Glycolytic inhibition as a strategy for developing calorie restriction mimetics. Exp Gerontol. 2011;46:148–154. doi: 10.1016/j.exger.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 53.Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–590. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- 54.Jager S, Handschin C, St-Pierre J, Spiegelman BM. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc Natl Acad Sci U S A. 2007;104:12017–12022. doi: 10.1073/pnas.0705070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CW, Fong HH, Farnsworth NR, Kinghorn AD, Mehta RG, Moon RC, Pezzuto JM. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275:218–220. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- 56.Jeninga EH, Schoonjans K, Auwerx J. Reversible acetylation of PGC-1: connecting energy sensors and effectors to guarantee metabolic flexibility. Oncogene. 2010;29:4617–4624. doi: 10.1038/onc.2010.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jiang W, Zhu Z, Thompson HJ. Dietary energy restriction modulates the activity of AMP-activated protein kinase, Akt, and mammalian target of rapamycin in mammary carcinomas, mammary gland, and liver. Cancer Res. 2008;68:5492–5499. doi: 10.1158/0008-5472.CAN-07-6721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Johnson EC, Kazgan N, Bretz CA, Forsberg LJ, Hector CE, Worthen RJ, Onyenwoke R, Brenman JE. Altered metabolism and persistent starvation behaviors caused by reduced AMPK function in Drosophila. PLoS One. 2010;5 doi: 10.1371/journal.pone.0012799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jorgensen SB, Wojtaszewski JF, Viollet B, Andreelli F, Birk JB, Hellsten Y, Schjerling P, Vaulont S, Neufer PD, Richter EA, Pilegaard H. Effects of alpha-AMPK knockout on exercise-induced gene activation in mouse skeletal muscle. FASEB J. 2005;19:1146–1148. doi: 10.1096/fj.04-3144fje. [DOI] [PubMed] [Google Scholar]
- 60.Kaeberlein M, Hu D, Kerr EO, Tsuchiya M, Westman EA, Dang N, Fields S, Kennedy BK. Increased life span due to calorie restriction in respiratory-deficient yeast. PLoS Genet. 2005;1:e69. doi: 10.1371/journal.pgen.0010069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kaeberlein M, Kirkland KT, Fields S, Kennedy BK. Sir2-independent life span extension by calorie restriction in yeast. PLoS Biol. 2004;2:E296. doi: 10.1371/journal.pbio.0020296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kaeberlein M, McDonagh T, Heltweg B, Hixon J, Westman EA, Caldwell SD, Napper A, Curtis R, DiStefano PS, Fields S, Bedalov A, Kennedy BK. Substrate-specific activation of sirtuins by resveratrol. J Biol Chem. 2005;280:17038–17045. doi: 10.1074/jbc.M500655200. [DOI] [PubMed] [Google Scholar]
- 63.Kaeberlein M, Powers RW, 3rd, Steffen KK, Westman EA, Hu D, Dang N, Kerr EO, Kirkland KT, Fields S, Kennedy BK. Regulation of yeast replicative life span by TOR and Sch9 in response to nutrients. Science. 2005;310:1193–1196. doi: 10.1126/science.1115535. [DOI] [PubMed] [Google Scholar]
- 64.Kapahi P, Chen D, Rogers AN, Katewa SD, Li PW, Thomas EL, Kockel L. With TOR, less is more: a key role for the conserved nutrient-sensing TOR pathway in aging. Cell Metab. 2010;11:453–465. doi: 10.1016/j.cmet.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim J, Kundu M, Viollet B, Guan KL. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol. 2011;13:132–141. doi: 10.1038/ncb2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Koga H, Kaushik S, Cuervo AM. Protein homeostasis and aging: The importance of exquisite quality control. Ageing Res Rev. 2011;10:205–215. doi: 10.1016/j.arr.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, Geny B, Laakso M, Puigserver P, Auwerx J. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 68.Lamia KA, Sachdeva UM, DiTacchio L, Williams EC, Alvarez JG, Egan DF, Vasquez DS, Juguilon H, Panda S, Shaw RJ, Thompson CB, Evans RM. AMPK regulates the circadian clock by cryptochrome phosphorylation and degradation. Science. 2009;326:437–440. doi: 10.1126/science.1172156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lee IH, Cao L, Mostoslavsky R, Lombard DB, Liu J, Bruns NE, Tsokos M, Alt FW, Finkel T. A role for the NAD-dependent deacetylase Sirt1 in the regulation of autophagy. Proc Natl Acad Sci U S A. 2008;105:3374–3379. doi: 10.1073/pnas.0712145105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lee JH, Koh H, Kim M, Kim Y, Lee SY, Karess RE, Lee SH, Shong M, Kim JM, Kim J, Chung J. Energy-dependent regulation of cell structure by AMP-activated protein kinase. Nature. 2007;447:1017–1020. doi: 10.1038/nature05828. [DOI] [PubMed] [Google Scholar]
- 71.Lee JW, Park S, Takahashi Y, Wang HG. The association of AMPK with ULK1 regulates autophagy. PLoS One. 2010;5:e15394. doi: 10.1371/journal.pone.0015394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee SS, Lee RY, Fraser AG, Kamath RS, Ahringer J, Ruvkun G. A systematic RNAi screen identifies a critical role for mitochondria in C. elegans longevity. Nat Genet. 2003;33:40–48. doi: 10.1038/ng1056. [DOI] [PubMed] [Google Scholar]
- 73.Leick L, Fentz J, Bienso RS, Knudsen JG, Jeppesen J, Kiens B, Wojtaszewski JF, Pilegaard H. PGC-1{alpha} is required for AICAR-induced expression of GLUT4 and mitochondrial proteins in mouse skeletal muscle. Am J Physiol Endocrinol Metab. 2010;299:E456–465. doi: 10.1152/ajpendo.00648.2009. [DOI] [PubMed] [Google Scholar]
- 74.Lerin C, Rodgers JT, Kalume DE, Kim SH, Pandey A, Puigserver P. GCN5 acetyltransferase complex controls glucose metabolism through transcriptional repression of PGC-1alpha. Cell Metab. 2006;3:429–438. doi: 10.1016/j.cmet.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 75.Lin SJ, Defossez PA, Guarente L. Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae. Science. 2000;289:2126–2128. doi: 10.1126/science.289.5487.2126. [DOI] [PubMed] [Google Scholar]
- 76.Lin SJ, Kaeberlein M, Andalis AA, Sturtz LA, Defossez PA, Culotta VC, Fink GR, Guarente L. Calorie restriction extends Saccharomyces cerevisiae lifespan by increasing respiration. Nature. 2002;418:344–348. doi: 10.1038/nature00829. [DOI] [PubMed] [Google Scholar]
- 77.Lin SS, Manchester JK, Gordon JI. Sip2, an N-myristoylated beta subunit of Snf1 kinase, regulates aging in Saccharomyces cerevisiae by affecting cellular histone kinase activity, recombination at rDNA loci, and silencing. J Biol Chem. 2003;278:13390–13397. doi: 10.1074/jbc.M212818200. [DOI] [PubMed] [Google Scholar]
- 78.Lopez-Lluch G, Irusta PM, Navas P, de Cabo R. Mitochondrial biogenesis and healthy aging. Exp Gerontol. 2008;43:813–819. doi: 10.1016/j.exger.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lorenz DR, Cantor CR, Collins JJ. A network biology approach to aging in yeast. Proc Natl Acad Sci U S A. 2009;106:1145–1150. doi: 10.1073/pnas.0812551106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mair W, Panowski SH, Shaw RJ, Dillin A. Optimizing dietary restriction for genetic epistasis analysis and gene discovery in C. elegans. PLoS One. 2009;4:e4535. doi: 10.1371/journal.pone.0004535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mizushima N. The role of the Atg1/ULK1 complex in autophagy regulation. Curr Opin Cell Biol. 2010;22:132–139. doi: 10.1016/j.ceb.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 82.Motta MC, Divecha N, Lemieux M, Kamel C, Chen D, Gu W, Bultsma Y, McBurney M, Guarente L. Mammalian SIRT1 represses forkhead transcription factors. Cell. 2004;116:551–563. doi: 10.1016/s0092-8674(04)00126-6. [DOI] [PubMed] [Google Scholar]
- 83.Nakashima K, Yakabe Y. AMPK activation stimulates myofibrillar protein degradation and expression of atrophy-related ubiquitin ligases by increasing FOXO transcription factors in C2C12 myotubes. Biosci Biotechnol Biochem. 2007;71:1650–1656. doi: 10.1271/bbb.70057. [DOI] [PubMed] [Google Scholar]
- 84.Narkar VA, Downes M, Yu RT, Embler E, Wang YX, Banayo E, Mihaylova MM, Nelson MC, Zou Y, Juguilon H, Kang H, Shaw RJ, Evans RM. AMPK and PPARdelta agonists are exercise mimetics. Cell. 2008;134:405–415. doi: 10.1016/j.cell.2008.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nisoli E, Tonello C, Cardile A, Cozzi V, Bracale R, Tedesco L, Falcone S, Valerio A, Cantoni O, Clementi E, Moncada S, Carruba MO. Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science. 2005;310:314–317. doi: 10.1126/science.1117728. [DOI] [PubMed] [Google Scholar]
- 86.Oliveira GA, Tahara EB, Gombert AK, Barros MH, Kowaltowski AJ. Increased aerobic metabolism is essential for the beneficial effects of caloric restriction on yeast life span. J Bioenerg Biomembr. 2008;40:381–388. doi: 10.1007/s10863-008-9159-5. [DOI] [PubMed] [Google Scholar]
- 87.Palacios OM, Carmona JJ, Michan S, Chen KY, Manabe Y, Ward JL, 3rd, Goodyear LJ, Tong Q. Diet and exercise signals regulate SIRT3 and activate AMPK and PGC-1alpha in skeletal muscle. Aging (Albany NY) 2009;1:771–783. doi: 10.18632/aging.100075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pearson KJ, Baur JA, Lewis KN, Peshkin L, Price NL, Labinskyy N, Swindell WR, Kamara D, Minor RK, Perez E, Jamieson HA, Zhang Y, Dunn SR, Sharma K, Pleshko N, Woollett LA, Csiszar A, Ikeno Y, Le Couteur D, Elliott PJ, Becker KG, Navas P, Ingram DK, Wolf NS, Ungvari Z, Sinclair DA, de Cabo R. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab. 2008;8:157–168. doi: 10.1016/j.cmet.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Petersen KF, Befroy D, Dufour S, Dziura J, Ariyan C, Rothman DL, DiPietro L, Cline GW, Shulman GI. Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science. 2003;300:1140–1142. doi: 10.1126/science.1082889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pfluger PT, Herranz D, Velasco-Miguel S, Serrano M, Tschop MH. Sirt1 protects against high-fat diet-induced metabolic damage. Proc Natl Acad Sci U S A. 2008;105:9793–9798. doi: 10.1073/pnas.0802917105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rabol R, Svendsen PF, Skovbro M, Boushel R, Haugaard SB, Schjerling P, Schrauwen P, Hesselink MK, Nilas L, Madsbad S, Dela F. Reduced skeletal muscle mitochondrial respiration and improved glucose metabolism in nondiabetic obese women during a very low calorie dietary intervention leading to rapid weight loss. Metabolism. 2009;58:1145–1152. doi: 10.1016/j.metabol.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 92.Reznick RM, Zong H, Li J, Morino K, Moore IK, Yu HJ, Liu ZX, Dong J, Mustard KJ, Hawley SA, Befroy D, Pypaert M, Hardie DG, Young LH, Shulman GI. Aging-associated reductions in AMP-activated protein kinase activity and mitochondrial biogenesis. Cell Metab. 2007;5:151–156. doi: 10.1016/j.cmet.2007.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 2005;434:113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- 94.Rogina B, Helfand SL. Sir2 mediates longevity in the fly through a pathway related to calorie restriction. Proc Natl Acad Sci U S A. 2004;101:15998–16003. doi: 10.1073/pnas.0404184101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Roussel R, Travert F, Pasquet B, Wilson PW, Smith SC, Jr., Goto S, Ravaud P, Marre M, Porath A, Bhatt DL, Steg PG. Metformin use and mortality among patients with diabetes and atherothrombosis. Arch Intern Med. 2010;170:1892–1899. doi: 10.1001/archinternmed.2010.409. [DOI] [PubMed] [Google Scholar]
- 96.Sanders MJ, Grondin PO, Hegarty BD, Snowden MA, Carling D. Investigating the mechanism for AMP activation of the AMP-activated protein kinase cascade. Biochem J. 2007;403:139–148. doi: 10.1042/BJ20061520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schulz TJ, Zarse K, Voigt A, Urban N, Birringer M, Ristow M. Glucose restriction extends Caenorhabditis elegans life span by inducing mitochondrial respiration and increasing oxidative stress. Cell Metab. 2007;6:280–293. doi: 10.1016/j.cmet.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 98.Selman C, Tullet JM, Wieser D, Irvine E, Lingard SJ, Choudhury AI, Claret M, Al-Qassab H, Carmignac D, Ramadani F, Woods A, Robinson IC, Schuster E, Batterham RL, Kozma SC, Thomas G, Carling D, Okkenhaug K, Thornton JM, Partridge L, Gems D, Withers DJ. Ribosomal protein S6 kinase 1 signaling regulates mammalian life span. Science. 2009;326:140–144. doi: 10.1126/science.1177221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sengupta A, Molkentin JD, Yutzey KE. FoxO transcription factors promote autophagy in cardiomyocytes. J Biol Chem. 2009;284:28319–28331. doi: 10.1074/jbc.M109.024406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sengupta S, Peterson TR, Sabatini DM. Regulation of the mTOR complex 1 pathway by nutrients, growth factors, and stress. Mol Cell. 2010;40:310–322. doi: 10.1016/j.molcel.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shinmura K, Tamaki K, Bolli R. Short-term caloric restriction improves ischemic tolerance independent of opening of ATP-sensitive K+ channels in both young and aged hearts. J Mol Cell Cardiol. 2005;39:285–296. doi: 10.1016/j.yjmcc.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 102.Sreekumar R, Unnikrishnan J, Fu A, Nygren J, Short KR, Schimke J, Barazzoni R, Nair KS. Effects of caloric restriction on mitochondrial function and gene transcripts in rat muscle. Am J Physiol Endocrinol Metab. 2002;283:E38–43. doi: 10.1152/ajpendo.00387.2001. [DOI] [PubMed] [Google Scholar]
- 103.Stanfel MN, Shamieh LS, Kaeberlein M, Kennedy BK. The TOR pathway comes of age. Biochim Biophys Acta. 2009;1790:1067–1074. doi: 10.1016/j.bbagen.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tissenbaum HA, Guarente L. Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature. 2001;410:227–230. doi: 10.1038/35065638. [DOI] [PubMed] [Google Scholar]
- 105.To K, Yamaza H, Komatsu T, Hayashida T, Hayashi H, Toyama H, Chiba T, Higami Y, Shimokawa I. Down-regulation of AMP-activated protein kinase by calorie restriction in rat liver. Exp Gerontol. 2007;42:1063–1071. doi: 10.1016/j.exger.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 106.Tohyama D, Yamaguchi A. A critical role of SNF1A/dAMPKalpha (Drosophila AMP-activated protein kinase alpha) in muscle on longevity and stress resistance in Drosophila melanogaster. Biochem Biophys Res Commun. 2010;394:112–118. doi: 10.1016/j.bbrc.2010.02.126. [DOI] [PubMed] [Google Scholar]
- 107.Turcotte B, Liang XB, Robert F, Soontorngun N. Transcriptional regulation of nonfermentable carbon utilization in budding yeast. FEMS Yeast Res. 2010;10:2–13. doi: 10.1111/j.1567-1364.2009.00555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Um JH, Park SJ, Kang H, Yang S, Foretz M, McBurney MW, Kim MK, Viollet B, Chung JH. AMP-activated protein kinase-deficient mice are resistant to the metabolic effects of resveratrol. Diabetes. 2010;59:554–563. doi: 10.2337/db09-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Um SH, Frigerio F, Watanabe M, Picard F, Joaquin M, Sticker M, Fumagalli S, Allegrini PR, Kozma SC, Auwerx J, Thomas G. Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature. 2004;431:200–205. doi: 10.1038/nature02866. [DOI] [PubMed] [Google Scholar]
- 110.Xiao B, Heath R, Saiu P, Leiper FC, Leone P, Jing C, Walker PA, Haire L, Eccleston JF, Davis CT, Martin SR, Carling D, Gamblin SJ. Structural basis for AMP binding to mammalian AMP-activated protein kinase. Nature. 2007;449:496–500. doi: 10.1038/nature06161. [DOI] [PubMed] [Google Scholar]
- 111.Zang M, Xu S, Maitland-Toolan KA, Zuccollo A, Hou X, Jiang B, Wierzbicki M, Verbeuren TJ, Cohen RA. Polyphenols stimulate AMP-activated protein kinase, lower lipids, and inhibit accelerated atherosclerosis in diabetic LDL receptor-deficient mice. Diabetes. 2006;55:2180–2191. doi: 10.2337/db05-1188. [DOI] [PubMed] [Google Scholar]
- 112.Zhao J, Brault JJ, Schild A, Cao P, Sandri M, Schiaffino S, Lecker SH, Goldberg AL. FoxO3 coordinately activates protein degradation by the autophagic/lysosomal and proteasomal pathways in atrophying muscle cells. Cell Metab. 2007;6:472–483. doi: 10.1016/j.cmet.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 113.Zhao J, Brault JJ, Schild A, Goldberg AL. Coordinate activation of autophagy and the proteasome pathway by FoxO transcription factor. Autophagy. 2008;4:378–380. doi: 10.4161/auto.5633. [DOI] [PubMed] [Google Scholar]
- 114.Zheng J, Ramirez VD. Piceatannol, a stilbene phytochemical, inhibits mitochondrial F0F1-ATPase activity by targeting the F1 complex. Biochem Biophys Res Commun. 1999;261:499–503. doi: 10.1006/bbrc.1999.1063. [DOI] [PubMed] [Google Scholar]
- 115.Zid BM, Rogers AN, Katewa SD, Vargas MA, Kolipinski MC, Lu TA, Benzer S, Kapahi P. 4E-BP extends lifespan upon dietary restriction by enhancing mitochondrial activity in Drosophila. Cell. 2009;139:149–160. doi: 10.1016/j.cell.2009.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]