Abstract
Introduction
Animal studies have shown that even a small temperature elevation of one degree Celsius can cause detrimental effects after brain injury. Since the skull acts as a potential thermal insulator, we hypothesized that decompressive hemicraniectomy facilitates surface cooling and lowers brain temperature.
Methods
Forty-eight patients with severe brain injury (TBI=38, ICH=10) with continuous brain temperature monitoring were retrospectively studied and grouped into “hemicraniectomy” (n=20) or “no hemicraniectomy” (n=28) group. The paired measurements of core body (TCore) and brain (TBr) temperature were recorded at 1-min intervals over 12±7 days. As a surrogate measure for the extent of surface heat loss from the brain, ΔTCore-Br was calculated as the difference between TCore and TBr with each recording. In order to accommodate within-patient temperature correlations, mixed-model regression was used to assess the differences in ΔTCore-Br between those with and without hemicraniectomy, adjusted for core body temperature and diagnosis.
Results
A total of 295,883 temperature data pairs were collected (median [IQR] per patient: 5047 [3125–8457]). Baseline characteristics were similar for age, sex, diagnosis, incidence of sepsis, Glasgow Coma Scale score, ICU mortality, and ICU length of stay between the two groups. The mean difference in ΔTCore-Br, was 1.29±0.87 °C for patients with and 0.80±0.86 °C for patients without hemicraniectomy (p<0.0001). In mixed-model regression, accounting for temperature correlations within patients, hemicraniectomy and higher TCore were associated with greater ΔTCore-Br (hemicraniectomy: estimated effect=0.60, p=0.003; TCore: estimated effect=0.21, p<0.0001).
Conclusion
Hemicraniectomy is associated with modestly but significantly lower brain temperature relative to core body temperature.
Keywords: Decompressive hemicraniectomy, brain temperature, brain tissue oxygen monitor
INTRODUCTION
Fever is a common condition after severe brain injury and is associated with worse clinical outcome.1–3 Experimental models of brain ischemia and trauma have shown that fever worsens secondary neuronal injury and is associated with elevation of levels of excitatory amino acids (i.e., dopamine and glutamate), oxygen radicals, ischemic abnormal depolarization, breakdown of blood-brain barrier, and cytoskeletal proteolysis.4–7 These detrimental effects are seen in histopathological studies even after a small temperature elevation of 1 degree Celsius.8, 9 Based on these findings, aggressive fever management with various cooling methods after severe brain injury is considered an accepted part of neurocritical care in an attempt to limit secondary brain injury.10 Currently available advanced neuromonitoring modalities include the ability to directly measure parenchymal brain temperature continuously.11, 12
Directly measured brain temperature often differs slightly from that of core body temperature. Earlier studies comparing the two temperature measurements found brain temperature as usually higher than the core body temperature.11, 13–15 This has generally been attributed to the high metabolic function of the brain after injury.15 However, a more recent study using a commercially available probe which measures both brain tissue oxygen tension and brain temperature found that the brain temperature was lower than the core body temperature by 0.5 – 0.8 °C.11 This was attributed to calibration differences between this probe and the prior methods of measuring brain temperature. Additionally, inter-individual differences may also contribute to variability between brain and core body temperature.
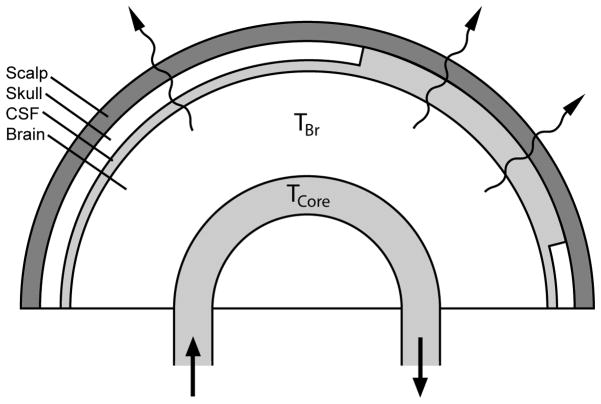
The presence of the skull as a physical barrier between the brain and the surrounding air likely serves to prevent some heat loss from the brain. Given that the density of the skull is estimated to be 1.357 kg/m3 with a modest heat conductivity of 1.16 × 10−2 W·cm−1· °C−1,16 the skull itself may function as a thermal insulator. Based on this, we hypothesized that decompressive hemicraniectomy lowers brain temperature by increasing the conductive surface heat loss from the brain to its surrounding air (Figure 1). We investigated this by conducting an observational study assessing the impact of hemicraniectomy on the difference between brain and core body temperature after controlling for variability across subjects and temperature measurement devices.
Figure 1.
Schematic drawing of the hemicraniectomy heat exchange model showing the outer layers consisting of scalp, skull, and CSF. Lack of the skull layer represents hemicraniectomy. Squiggle arrows represent conductive and convective surface heat loss. The difference between brain temperature (TBr) and core body temperature (TCore) is ΔTCore-Br. Arrows represent the direction of systemic blood circulation.
METHODS
This was a retrospective study of all patients presenting to San Francisco General Hospital (SFGH) with acute severe brain injury who underwent intensive care unit (ICU) admission and continuous brain tissue oxygen and brain temperature monitoring (Licox, Integra LifeSciences, Plainsboro, NJ) as part of routine care from January 1, 2007 to March 20, 2009. All aspects of this study were approved by the Committee on Human Research of the University of California, San Francisco (UCSF). Patients were treated in accordance with existing evidence-based guidelines for traumatic brain injury (TBI)17 and intracerebral hemorrhage (ICH).18
In order to control for potential variability between brain temperature measurement devices, we used the same type of brain temperature probe in all patients. The Licox device employed in this study utilizes a single probe that measures both brain tissue oxygen tension (PbtO2) and brain temperature (TBr) in conjunction with an automated card calibration system. Probes were inserted at Kocher’s point into the frontal lobe of the least injured hemisphere in all of the TBI patients, on the side contralateral to the hematoma in 8 of the 10 ICH patients (3 of whom underwent decompressive hemicraniectomy), and on the side ipsilateral to the hematoma in the other 2 ICH patients (neither of whom underwent decompressive hemicraniectomy). When the probe placement was done at the time of hemicraniectomy, it was inserted contralateral to the side of bone removal. Brain temperature and core body temperature (Tcore) as measured by bladder temperature (Lubri-Sil IC, C.R. Bard Inc., Covington, GA) were simultaneously recorded at 1-min intervals into the SFGH Neurotrauma and Critical Care Database via electronic data capture from the Licox computer and the Viridia bedside monitor (Philips Medical, Andover, MA). Demographic and other clinical data were recorded separately from the Carevue bedside clinical charting system (Philips Medical). Medical chart review was also performed as needed.
A total of 52 patients were identified, of whom 22 underwent decompressive hemicraniectomy. Since prophylactic hemicraniectomy is not routinely done at our institution, most of these were emergent cases performed to remove mass effect that was contributing to neurological deterioration or to treat refractory intracranial hypertension. Of these, all except 2 patients had the Licox probe placed at the time of hemicraniectomy, and thus all of their brain temperature measurements were recorded post-hemicraniectomy. Rather than attempt to account for within person correlation across conditions (pre- and post-hemicraniectomy) based on only 2 subjects with pre-and post-hemicraniectomy measurements, we excluded these two subjects, resulting in a slight loss of information, but yielding a more robust model. Because the majority of our patients primarily had a diagnosis of TBI or spontaneous ICH, 2 patients with subarachnoid hemorrhage were excluded since we would not have sufficient power to examine this subgroup. Since sepsis may affect the temperature gradient between the brain and core body, we identified 2 patients who were diagnosed with sepsis (1 patient with hemicraniectomy and 1 patient without hemicraniectomy) during their ICU stay who met the following criteria: (1) documented positive blood culture and (2) evidence of systemic inflammatory response syndrome (defined as presence of 2 or more of fever, tachycardia, tachypnea or leukocytosis),. In the final analysis, we compared the differences in ΔTCore-Br between 20 patients from the “hemicraniectomy” group and 28 patients from the “no hemicraniectomy” group.
Only paired data of Tcore and TBr measurements were used for analysis. Since none of the patients received induced hypothermia treatment, temperature data pairs with TBr or Tcore value less than 30 °C were considered artifactual and excluded from the final analysis. A total of 298,557 temperature data pairs were initially sampled. Out of those, 2,153 (0.72 %) TBr and 521 (0.17 %) TCore data pairs were excluded for temperature value less than 30 °C. As a surrogate measure for the degree of external heat loss from the brain, the “body to brain temperature difference” (ΔTCore-Br) was calculated as the difference between TCore and TBr (ΔTCore-Br= TCore − TBr) for each set of paired values. A higher ΔTCore-Br value is interpreted as greater conductive/convective heat loss from the brain at the surface, assuming that the rate of capillary-brain parenchyma convective heat exchange remains constant (Figure 1).
STATISTICAL ANALYSIS
Differences in baseline characteristics between groups with and without hemicraniectomy were compared using the X2 test or Fisher’s Exact test for categorical data and t-test for normally distributed continuous variables (age, ICU length of stay, TCore, TBr, and ΔTCore-Br). The non-parametric Wilcoxon test was used for comparison of data that were not normally distributed (GCS and number of paired data points).
Since multiple temperature measurements were taken from the same patients over a period of time, they are likely to be correlated. To accommodate this correlation, mixed model regression was used (SAS Proc MIXED, SAS v9.2, SAS Institute, Cary, NC). The mixed model allows calculation of patient-specific intercepts and slopes and not only models the mean structure (as in standard regression modeling) but the variance and covariance structure as well. This takes into account the temperature variability of each individual patient; coefficients from these models can be interpreted as the change in the response for each “cluster” (in this case, patient) in the population for a unit change in the predictor. This differs from the generalized estimating equations (GEE) approach which yields results that only hold averaged over the entire population of subjects, and in which coefficients are interpreted as the average change in the response over the entire population for a unit change in the predictor.19, 20 All mixed models were adjusted for core body temperature, diagnosis and sepsis.
RESULTS
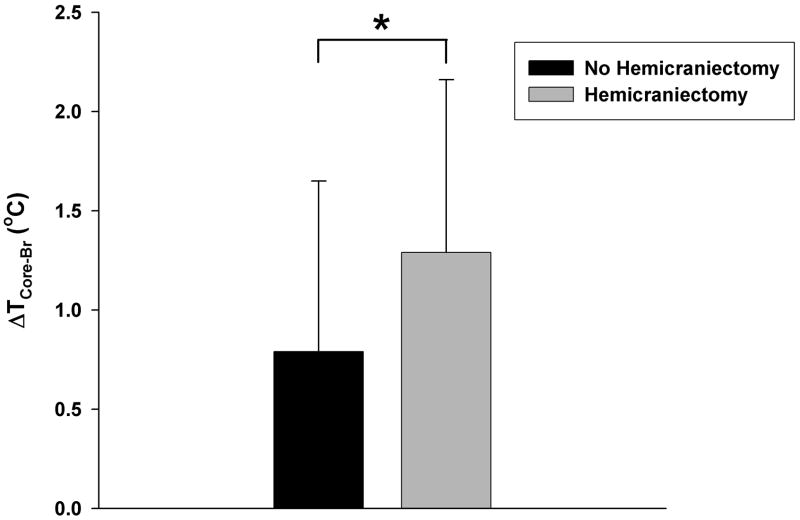
Clinical characteristics of the two groups are shown in Table 1. A total of 295,883 temperature data pairs were collected for analysis (median [IQR] data pairs per patient: 5047 [3125–8457]). At baseline, there were no significant differences in age, gender, diagnosis, GCS, incidence of sepsis, ICU mortality, and ICU length of stay (LOS) between those with and without hemicraniectomy. Without taking individual patient differences into account, hemicraniectomy patients, on average, had lower TBr (36.28 ± 1.06 °C vs. 36.63 ± 1.13 °C), higher Tcore (37.56 ± 0.61 °C vs. 37.42 ± 1.00 °C), and greater ΔTCore-Br (1.29 ± 0.87 °C vs. 0.80 ± 0.86 °C; Figure 2) than those without hemicraniectomy (p<0.0001 for all three comparisons). After adjusting for intra-individual variability using mixed-model regression, hemicraniectomy (estimated effect = 0.60, p=0.003) and higher TCore (estimated effect = 0.21, p<0.0001), but not the underlying diagnosis (estimated effect = −0.10, p=0.70) or sepsis (estimated effect = −0.58, p=0.25), were associated with higher ΔTCore-Br. Our results suggest that the differences between the core body and brain temperature are greater with hemicraniectomy and with higher core body temperature.
Table 1.
Patient characteristics
| Hemicraniectomy (N=20) | No Hemicraniectomy (N=28) | P | |
|---|---|---|---|
| Age in years | 43.7 ± 20.1 | 45.8 ±16.1 | 0.69 |
| Female, n (%) | 5 (25) | 3 (11) | 0.25 |
| Sepsis, n (%) | 1 (5) | 1 (4) | 0.06 |
| ICU Mortality, n (%) | 3 (15) | 7 (25) | 0.49 |
| ICU LOS, days | 13.3 ± 8.3 | 11.1 ± 5.9 | 0.30 |
| Arrival GCS* | 8.8 ± 4.4 [n=17] | 7.9 ± 4.6 [n=20] | 0.52 |
| ICP, mmHg** | 13.8 ± 5.2 [n=19] | 11.6 ± 4.5 [n=27] | 0.14 |
| Diagnosis, n (%) | 0.41 | ||
| TBI | 17 (85) | 21 (75) | |
| ICH | 3 (15) | 7 (25) | |
| Total No. of Data Pairs | 120,771 | 175,112 | NA |
| Paired data points/person, median [IQR] | 4896 [3340 – 7147] | 5166 [2791 – 9556] | 0.85 |
| Brain Temp (TBr).°C | 36.28 ± 1.06 | 36.63 ± 1.13 | <0.0001 |
| Core Body Temp (TCore),°C | 37.56 ± 0.61 | 37.42 ± 1.00 | <0.0001 |
| ΔTCore-Br,°C | 1.29 ± 0.87 | 0.80 ± 0.86 | <0.0001 |
Data are mean ± SD unless otherwise indicated. ICU, Intensive Care Unit; LOS, Length of Stay; GCS, Glasgow Coma Scale; ICP, intracranial pressure; TBI, traumatic brain injury; ICH, intracerebral hemorrhage.
Arrival GCS data were available in 17 patients with hemicraniectomy and 20 patients without hemicraniectomy.
ICP measurements were obtained in 19 patients with hemicraniectomy and 27 patients without hemicraniectomy. Brain temperature, core body temperature, and ΔTCore-Br, are expressed as mean ± SD of all measurements.
Figure 2.
Bar graphs showing the “body to brain temperature difference”, ΔTCore-Br, (mean ± SD) from ”Hemicraniectomy” and “No Hemicraniectomy” groups. *p<0.0001 (t-test, unadjusted).
DISCUSSION
In this analysis of a large temperature dataset utilizing mixed model regression, we found that measured brain temperature is lower than core body temperature, and that these temperature differences are greater in patients who have undergone decompressive hemicraniectomy. This suggests that the absence of skull may increase the rate of surface cooling by facilitating the rate of external heat loss, leading to modest lowering of brain temperature. Our hypothesis was generated based on the assumption that removal of skull would facilitate surface cooling by reducing the thickness of the outer layers, which normally consist of 3 layers (i.e. CSF, skull, and scalp). Since the rate of heat conduction is highly dependent on the thickness and thermal conductivity of the media, we expect the brain to dissipate more heat at the surface when the skull is absent. Assuming that the rate of heat exchange between the capillary and brain parenchyma remains constant, our findings support the hypothesis that a greater amount of heat is lost at the brain surface with hemicraniectomy, as shown in the schematic diagram (Figure 1).
The cooling effect of hemicraniectomy was modest (about 0.6 °C) and consistent with prior observation which showed that an attempt to lower brain temperature by isolated surface cooling of the head had a very limited effect.21 This could be explained by the large volume of human brain relative to its surface area (low surface-to-volume ratio) and relatively low thermal conductivity at the surface, which limits the effect of surface cooling. In fact, most of the previously described mathematical models of brain temperature using the heat transfer equations22–24 suggest that majority of heat exchange occurs at the capillary-brain interface through a convective cooling process that is proportional to the blood flow and inflow temperature rather than at the brain-surface interface. Therefore, cooling the intravascular core body temperature remains the most effective method of lowering the brain temperature.25, 26 However, because an elevation of brain temperature as small as one degree Celsius could potentially cause detrimental effect after severe brain injury, the modest brain temperature reduction by hemicraniectomy may provide further neuroprotection in addition to its well-known effect of reducing the intracranial pressure and increasing the intracranial compliance.
In our study, the Licox probe was placed contralateral to the side of hemicraniectomy, and thus the recorded brain temperature measurements were limited to that of the least injured hemisphere in most of our patients. Therefore, it is possible that hemicraniectomy may have a more substantial local cooling effect on the brain parenchyma directly under the site of skull removal where the injury is often more severe. Based on the principle of conductive heat transfer, we expect the surface cooling effect of hemicraniectomy to be at least equal to if not greater on the hemicraniectomy side.
Also, our group found previously that the Licox probe used in our study has a tendency to under-read brain temperature across a broad range of physiologic temperatures in both groups.11 However, in our current study we used the difference between brain and core body temperature to investigate the impact of hemicraniectomy. Thus, it is unlikely that differential measurement bias due to Licox probe characteristics explains the effect of hemicraniectomy which we found.
Notably, our analysis of 295,883 temperature data pairs from 48 patients with severe brain injury showed a substantial variability in the brain-to-body temperature gradient both within and across patients. We were unable to account for other potential factors that could have affected the observed variability. These include 1) calibration error of individual Licox probes, 2) measurement error of the bladder catheter thermistor, 3) inter-individual variability in sweat production and evaporation rate at the scalp, and 4) inter-individual variability in cortical metabolic heat production, which could theoretically have been influenced by the use of sedatives or severity of coma.
CONCLUSIONS
Brain temperature measured with the Licox probe is lower than core body temperature across a broad physiologic temperature range, and these differences between the core body and brain temperature are greater in patients who undergo decompressive hemicraniectomy. This suggests that hemicraniectomy lowers brain temperature, possibly by increasing conductive heat loss.
Table 2.
Random Effects Linear Regression Model for ΔTCore-Br
| Estimate | SE | 95% CI | P | |
|---|---|---|---|---|
| Hemicraniectomy | 0.60 | 0.20 | 0.20 – 1.00 | 0.003 |
| Core Body Temp | 0.21 | 0.001 | 0.206 – 0.212 | <0.0001 |
| Diagnosis | −0.10 | 0.25 | −0.59 – 0.39 | 0.78 |
| Sepsis | −0.58 | 0.50 | −1.56 – 0.41 | 0.25 |
Mixed-model regression analysis showing association between ΔTCore-Br and hemicraniectomy, adjusted for core body temperature, diagnosis, and sepsis. Parameter estimates, standard error (SE), 95% confidence interval (CI), and p-values are shown.
Acknowledgments
The authors thank Charles McCulloch, PhD, for his consultative work on the statistical analysis. Dr. Manley is supported by R01 NS050173, RC2 NS069409 and the Brain and Spinal Injury Center. Dr. Hemphill is supported by U10 NS058931.
References
- 1.Greer DM, Funk SE, Reaven NL, Ouzounelli M, Uman GC. Impact of fever on outcome in patients with stroke and neurologic injury: a comprehensive meta-analysis. Stroke. 2008;39:3029–35. doi: 10.1161/STROKEAHA.108.521583. [DOI] [PubMed] [Google Scholar]
- 2.Geffroy A, Bronchard R, Merckx P, et al. Severe traumatic head injury in adults: which patients are at risk of early hyperthermia? Intensive Care Med. 2004;30:785–90. doi: 10.1007/s00134-004-2280-y. [DOI] [PubMed] [Google Scholar]
- 3.Naidech AM, Bendok BR, Bernstein RA, et al. Fever burden and functional recovery after subarachnoid hemorrhage. Neurosurgery. 2008;63:212–7. doi: 10.1227/01.NEU.0000320453.61270.0F. discussion 7–8. [DOI] [PubMed] [Google Scholar]
- 4.Ginsberg MD, Busto R. Combating hyperthermia in acute stroke: a significant clinical concern. Stroke. 1998;29:529–34. doi: 10.1161/01.str.29.2.529. [DOI] [PubMed] [Google Scholar]
- 5.Dietrich WD, Alonso O, Halley M, Busto R. Delayed posttraumatic brain hyperthermia worsens outcome after fluid percussion brain injury: a light and electron microscopic study in rats. Neurosurgery. 1996;38:533–41. doi: 10.1097/00006123-199603000-00023. discussion 41. [DOI] [PubMed] [Google Scholar]
- 6.Minamisawa H, Smith ML, Siesjo BK. The effect of mild hyperthermia and hypothermia on brain damage following 5, 10, and 15 minutes of forebrain ischemia. Ann Neurol. 1990;28:26–33. doi: 10.1002/ana.410280107. [DOI] [PubMed] [Google Scholar]
- 7.Busto R, Globus MY, Dietrich WD, Martinez E, Valdes I, Ginsberg MD. Effect of mild hypothermia on ischemia-induced release of neurotransmitters and free fatty acids in rat brain. Stroke. 1989;20:904–10. doi: 10.1161/01.str.20.7.904. [DOI] [PubMed] [Google Scholar]
- 8.Wass CT, Lanier WL, Hofer RE, Scheithauer BW, Andrews AG. Temperature changes of > or = 1 degree C alter functional neurologic outcome and histopathology in a canine model of complete cerebral ischemia. Anesthesiology. 1995;83:325–35. doi: 10.1097/00000542-199508000-00013. [DOI] [PubMed] [Google Scholar]
- 9.Busto R, Dietrich WD, Globus MY, Valdes I, Scheinberg P, Ginsberg MD. Small differences in intraischemic brain temperature critically determine the extent of ischemic neuronal injury. J Cereb Blood Flow Metab. 1987;7:729–38. doi: 10.1038/jcbfm.1987.127. [DOI] [PubMed] [Google Scholar]
- 10.Badjatia N, Fernandez L, Schmidt JM, et al. Impact of induced normothermia on outcome after subarachnoid hemorrhage: a case-control study. Neurosurgery. 66:696–700. doi: 10.1227/01.NEU.0000367618.42794.AA. discussion -1. [DOI] [PubMed] [Google Scholar]
- 11.Stewart C, Haitsma I, Zador Z, et al. The new Licox combined brain tissue oxygen and brain temperature monitor: assessment of in vitro accuracy and clinical experience in severe traumatic brain injury. Neurosurgery. 2008;63:1159–64. doi: 10.1227/01.NEU.0000333265.19131.7C. discussion 64–5. [DOI] [PubMed] [Google Scholar]
- 12.Bhatia A, Gupta AK. Neuromonitoring in the intensive care unit. II. Cerebral oxygenation monitoring and microdialysis. Intensive Care Med. 2007;33:1322–8. doi: 10.1007/s00134-007-0660-9. [DOI] [PubMed] [Google Scholar]
- 13.McIlvoy L. Comparison of brain temperature to core temperature: a review of the literature. J Neurosci Nurs. 2004;36:23–31. doi: 10.1097/01376517-200402000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Rumana CS, Gopinath SP, Uzura M, Valadka AB, Robertson CS. Brain temperature exceeds systemic temperature in head-injured patients. Crit Care Med. 1998;26:562–7. doi: 10.1097/00003246-199803000-00032. [DOI] [PubMed] [Google Scholar]
- 15.Henker RA, Brown SD, Marion DW. Comparison of brain temperature with bladder and rectal temperatures in adults with severe head injury. Neurosurgery. 1998;42:1071–5. doi: 10.1097/00006123-199805000-00071. [DOI] [PubMed] [Google Scholar]
- 16.Olsen RW, Hayes LJ, Wissler EH, Nikaidoh H, Eberhart RC. Influence of hypothermia and circulatory arrest on cerebral temperature distributions. J Biomech Eng. 1985;107:354–60. doi: 10.1115/1.3138569. [DOI] [PubMed] [Google Scholar]
- 17.Guidelines for the management of severe traumatic brain injury. J Neurotrauma. 2007;24 (Suppl 1):S1–106. doi: 10.1089/neu.2007.9999. [DOI] [PubMed] [Google Scholar]
- 18.Broderick J, Connolly S, Feldmann E, et al. Guidelines for the management of spontaneous intracerebral hemorrhage in adults: 2007 update: a guideline from the American Heart Association/American Stroke Association Stroke Council, High Blood Pressure Research Council, and the Quality of Care and Outcomes in Research Interdisciplinary Working Group. Circulation. 2007;116:e391–413. doi: 10.1161/CIRCULATIONAHA.107.183689. [DOI] [PubMed] [Google Scholar]
- 19.McCulloch CE, Searle SR, Neuhaus JM. Generalized, linear, and mixed models. 2. Hoboken, N.J: Wiley; 2008. [Google Scholar]
- 20.Vittinghoff E. Regression methods in biostatistics: linear, logistic, survival, and repeated measures models. New York: Springer; 2005. [Google Scholar]
- 21.Mellergard P. Changes in human intracerebral temperature in response to different methods of brain cooling. Neurosurgery. 1992;31:671–7. doi: 10.1227/00006123-199210000-00009. discussion 7. [DOI] [PubMed] [Google Scholar]
- 22.Nelson DA, Nunneley SA. Brain temperature and limits on transcranial cooling in humans: quantitative modeling results. Eur J Appl Physiol Occup Physiol. 1998;78:353–9. doi: 10.1007/s004210050431. [DOI] [PubMed] [Google Scholar]
- 23.Xu X, Tikuisis P, Giesbrecht G. A mathematical model for human brain cooling during cold-water near-drowning. J Appl Physiol. 1999;86:265–72. doi: 10.1152/jappl.1999.86.1.265. [DOI] [PubMed] [Google Scholar]
- 24.Dexter F, Hindman BJ. Computer simulation of brain cooling during cardiopulmonary bypass. Ann Thorac Surg. 1994;57:1171–8. doi: 10.1016/0003-4975(94)91350-1. discussion 8–9. [DOI] [PubMed] [Google Scholar]
- 25.Hinz J, Rosmus M, Popov A, Moerer O, Frerichs I, Quintel M. Effectiveness of an intravascular cooling method compared with a conventional cooling technique in neurologic patients. J Neurosurg Anesthesiol. 2007;19:130–5. doi: 10.1097/ANA.0b013e318032a208. [DOI] [PubMed] [Google Scholar]
- 26.Polderman KH, Herold I. Therapeutic hypothermia and controlled normothermia in the intensive care unit: practical considerations, side effects, and cooling methods. Crit Care Med. 2009;37:1101–20. doi: 10.1097/CCM.0b013e3181962ad5. [DOI] [PubMed] [Google Scholar]