Abstract
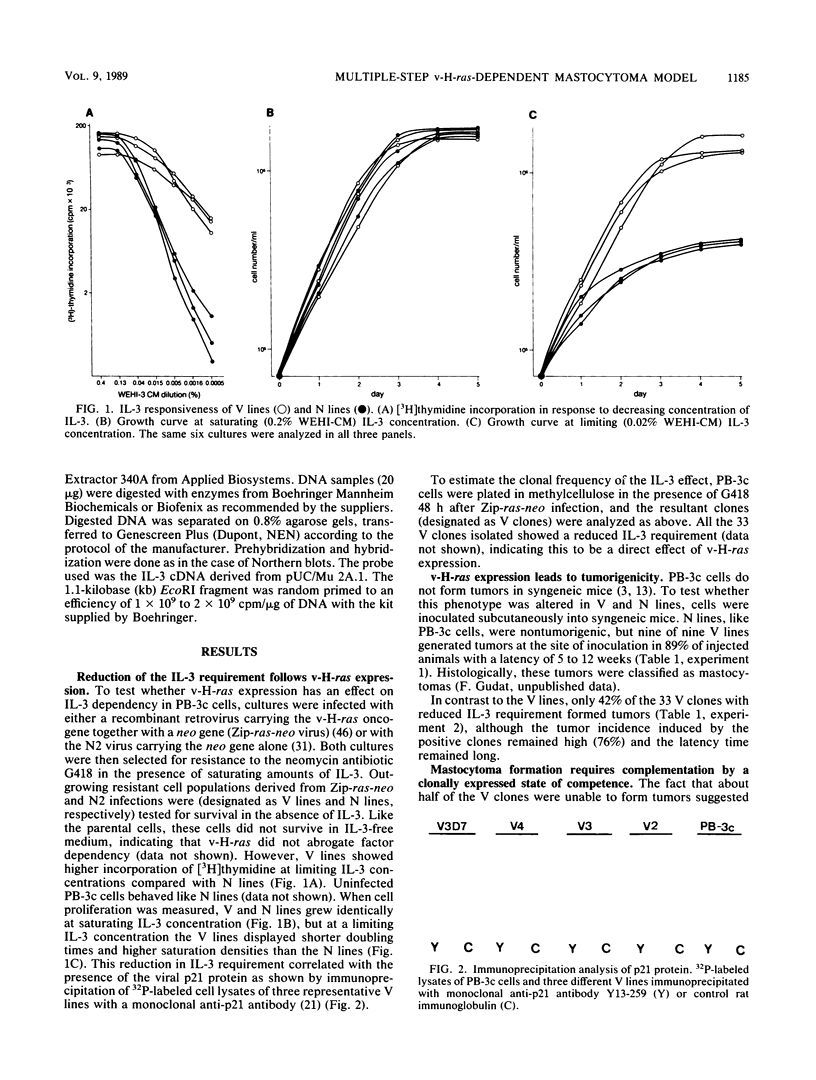
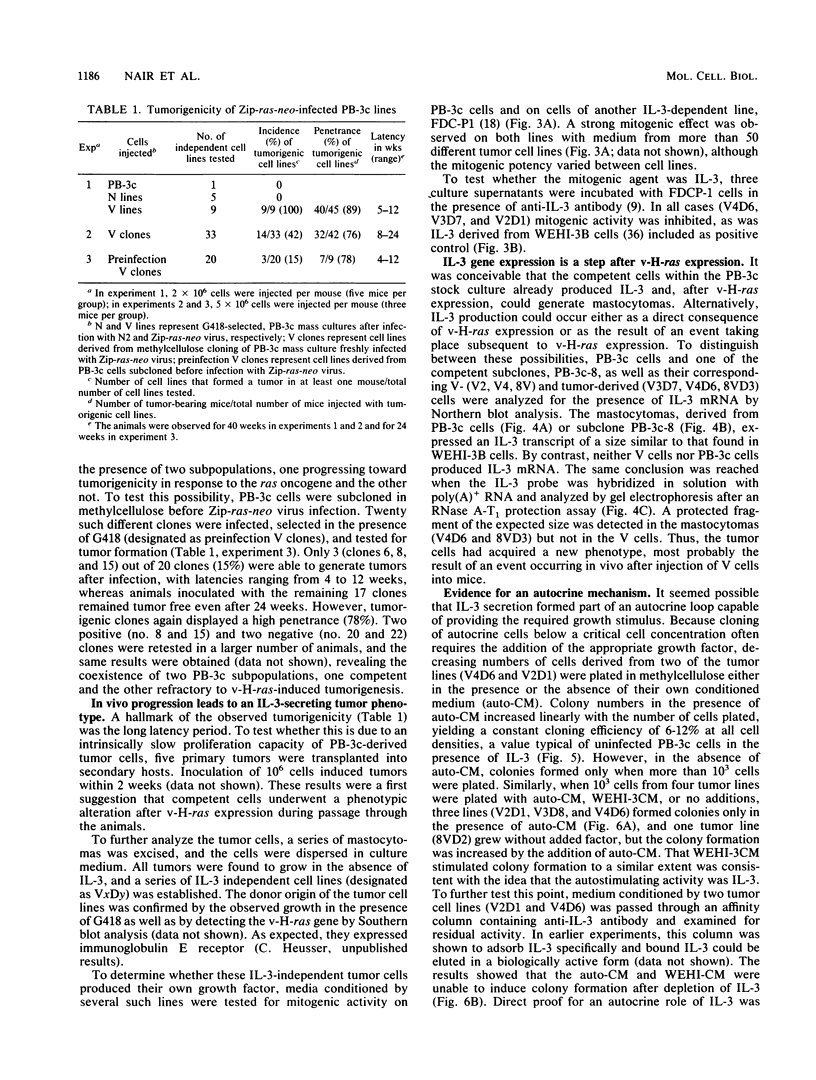
Autocrine interleukin 3 (IL-3)-secreting tumors were generated from an IL-3-dependent mouse mast cell line (PB-3c) after introduction of the v-H-ras oncogene. Tumor progression was characterized by four distinct phenotypes. The first corresponded to immortalized mast cells unresponsive to the oncogenic effect of v-H-ras. The second was expressed in a clonable subpopulation of PB-3c cells and was marked by the competence to form v-H-ras-dependent tumors (immortalized transformation competence). The third was a direct effect of v-H-ras expression on all PB-3c cells and was characterized in vitro by a reduced IL-3 requirement. Upon injection of v-H-ras-expressing, transformation-competent cells into mice, the final, fully malignant phenotype developed with a long latency period and was marked in vitro by independence of exogenous IL-3 and by autocrine IL-3 stimulation. Northern (RNA) blot analysis and an RNase A-T1 protection assay showed that IL-3 production was strictly associated with the tumor phenotype. Two of six tumors showed an alteration at the 5' region of the IL-3 gene. We conclude that v-H-ras required complementation by IL-3 gene rearrangement or an alternate event to generate autocrine mastocytomas.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adkins B., Leutz A., Graf T. Autocrine growth induced by src-related oncogenes in transformed chicken myeloid cells. Cell. 1984 Dec;39(3 Pt 2):439–445. doi: 10.1016/0092-8674(84)90451-3. [DOI] [PubMed] [Google Scholar]
- Amasino R. M. Acceleration of nucleic acid hybridization rate by polyethylene glycol. Anal Biochem. 1986 Feb 1;152(2):304–307. doi: 10.1016/0003-2697(86)90413-6. [DOI] [PubMed] [Google Scholar]
- Ball P. E., Conroy M. C., Heusser C. H., Davis J. M., Conscience J. F. Spontaneous, in vitro, malignant transformation of a basophil/mast cell line. Differentiation. 1983;24(1):74–78. doi: 10.1111/j.1432-0436.1983.tb01305.x. [DOI] [PubMed] [Google Scholar]
- Balmain A. Transforming ras oncogenes and multistage carcinogenesis. Br J Cancer. 1985 Jan;51(1):1–7. doi: 10.1038/bjc.1985.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumbach W. R., Stanley E. R., Cole M. D. Induction of clonal monocyte-macrophage tumors in vivo by a mouse c-myc retrovirus: rearrangement of the CSF-1 gene as a secondary transforming event. Mol Cell Biol. 1987 Feb;7(2):664–671. doi: 10.1128/mcb.7.2.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop J. M. The molecular genetics of cancer. Science. 1987 Jan 16;235(4786):305–311. doi: 10.1126/science.3541204. [DOI] [PubMed] [Google Scholar]
- Bos J. L., Toksoz D., Marshall C. J., Verlaan-de Vries M., Veeneman G. H., van der Eb A. J., van Boom J. H., Janssen J. W., Steenvoorden A. C. Amino-acid substitutions at codon 13 of the N-ras oncogene in human acute myeloid leukaemia. 1985 Jun 27-Jul 3Nature. 315(6022):726–730. doi: 10.1038/315726a0. [DOI] [PubMed] [Google Scholar]
- Bowlin T. L., Scott A. N., Ihle J. N. Biologic properties of interleukin 3. II. Serologic comparison of 20-alpha-SDH-inducing activity, colony-stimulating activity, and WEHI-3 growth factor activity by using an antiserum against IL 3. J Immunol. 1984 Oct;133(4):2001–2006. [PubMed] [Google Scholar]
- Brown M. A., Pierce J. H., Watson C. J., Falco J., Ihle J. N., Paul W. E. B cell stimulatory factor-1/interleukin-4 mRNA is expressed by normal and transformed mast cells. Cell. 1987 Aug 28;50(5):809–818. doi: 10.1016/0092-8674(87)90339-4. [DOI] [PubMed] [Google Scholar]
- Cavenee W. K., Dryja T. P., Phillips R. A., Benedict W. F., Godbout R., Gallie B. L., Murphree A. L., Strong L. C., White R. L. Expression of recessive alleles by chromosomal mechanisms in retinoblastoma. 1983 Oct 27-Nov 2Nature. 305(5937):779–784. doi: 10.1038/305779a0. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Conscience J. F., Fischer F. Growth-factor-independent proliferation in vitro and tumorigenicity in vivo are associated in basophil/mast-cell lines and their somatic hybrids. Differentiation. 1985;28(3):291–295. doi: 10.1111/j.1432-0436.1985.tb00838.x. [DOI] [PubMed] [Google Scholar]
- Conscience J. F., Verrier B., Martin G. Interleukin-3-dependent expression of the c-myc and c-fos proto-oncogenes in hemopoietic cell lines. EMBO J. 1986 Feb;5(2):317–323. doi: 10.1002/j.1460-2075.1986.tb04215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook W. D., Metcalf D., Nicola N. A., Burgess A. W., Walker F. Malignant transformation of a growth factor-dependent myeloid cell line by Abelson virus without evidence of an autocrine mechanism. Cell. 1985 Jul;41(3):677–683. doi: 10.1016/s0092-8674(85)80048-9. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P., Parikh I. Adsorbents for affinity chromatography. Use of N-hydroxysuccinimide esters of agarose. Biochemistry. 1972 Jun 6;11(12):2291–2299. doi: 10.1021/bi00762a013. [DOI] [PubMed] [Google Scholar]
- Davis J. M., Pennington J. E., Kubler A. M., Conscience J. F. A simple, single-step technique for selecting and cloning hybridomas for the production of monoclonal antibodies. J Immunol Methods. 1982;50(2):161–171. doi: 10.1016/0022-1759(82)90222-8. [DOI] [PubMed] [Google Scholar]
- Dexter T. M., Garland J., Scott D., Scolnick E., Metcalf D. Growth of factor-dependent hemopoietic precursor cell lines. J Exp Med. 1980 Oct 1;152(4):1036–1047. doi: 10.1084/jem.152.4.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eva A., Tronick S. R., Gol R. A., Pierce J. H., Aaronson S. A. Transforming genes of human hematopoietic tumors: frequent detection of ras-related oncogenes whose activation appears to be independent of tumor phenotype. Proc Natl Acad Sci U S A. 1983 Aug;80(16):4926–4930. doi: 10.1073/pnas.80.16.4926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farr C. J., Saiki R. K., Erlich H. A., McCormick F., Marshall C. J. Analysis of RAS gene mutations in acute myeloid leukemia by polymerase chain reaction and oligonucleotide probes. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1629–1633. doi: 10.1073/pnas.85.5.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furth M. E., Davis L. J., Fleurdelys B., Scolnick E. M. Monoclonal antibodies to the p21 products of the transforming gene of Harvey murine sarcoma virus and of the cellular ras gene family. J Virol. 1982 Jul;43(1):294–304. doi: 10.1128/jvi.43.1.294-304.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambke C., Hall A., Moroni C. Activation of an N-ras gene in acute myeloblastic leukemia through somatic mutation in the first exon. Proc Natl Acad Sci U S A. 1985 Feb;82(3):879–882. doi: 10.1073/pnas.82.3.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambke C., Signer E., Moroni C. Activation of N-ras gene in bone marrow cells from a patient with acute myeloblastic leukaemia. Nature. 1984 Feb 2;307(5950):476–478. doi: 10.1038/307476a0. [DOI] [PubMed] [Google Scholar]
- Graf T., von Weizsaecker F., Grieser S., Coll J., Stehelin D., Patschinsky T., Bister K., Bechade C., Calothy G., Leutz A. v-mil induces autocrine growth and enhanced tumorigenicity in v-myc-transformed avian macrophages. Cell. 1986 May 9;45(3):357–364. doi: 10.1016/0092-8674(86)90321-1. [DOI] [PubMed] [Google Scholar]
- Hankins W. D., Scolnick E. M. Harvey and Kirsten sarcoma viruses promote the growth and differentiation of erythroid precursor cells in vitro. Cell. 1981 Oct;26(1 Pt 1):91–97. doi: 10.1016/0092-8674(81)90036-2. [DOI] [PubMed] [Google Scholar]
- Hapel A. J., Vande Woude G., Campbell H. D., Young I. G., Robins T. Generation of an autocrine leukaemia using a retroviral expression vector carrying the interleukin-3 gene. Lymphokine Res. 1986 Fall;5(4):249–254. [PubMed] [Google Scholar]
- Hapel A. J., Warren H. S., Hume D. A. Different colony-stimulating factors are detected by the "interleukin-3"-dependent cell lines FDC-Pl and 32D cl-23. Blood. 1984 Oct;64(4):786–790. [PubMed] [Google Scholar]
- Hirai H., Kobayashi Y., Mano H., Hagiwara K., Maru Y., Omine M., Mizoguchi H., Nishida J., Takaku F. A point mutation at codon 13 of the N-ras oncogene in myelodysplastic syndrome. Nature. 1987 Jun 4;327(6121):430–432. doi: 10.1038/327430a0. [DOI] [PubMed] [Google Scholar]
- Kahn P., Frykberg L., Brady C., Stanley I., Beug H., Vennström B., Graf T. v-erbA cooperates with sarcoma oncogenes in leukemic cell transformation. Cell. 1986 May 9;45(3):349–356. doi: 10.1016/0092-8674(86)90320-x. [DOI] [PubMed] [Google Scholar]
- Kawano M., Hirano T., Matsuda T., Taga T., Horii Y., Iwato K., Asaoku H., Tang B., Tanabe O., Tanaka H. Autocrine generation and requirement of BSF-2/IL-6 for human multiple myelomas. Nature. 1988 Mar 3;332(6159):83–85. doi: 10.1038/332083a0. [DOI] [PubMed] [Google Scholar]
- Keller G., Paige C., Gilboa E., Wagner E. F. Expression of a foreign gene in myeloid and lymphoid cells derived from multipotent haematopoietic precursors. Nature. 1985 Nov 14;318(6042):149–154. doi: 10.1038/318149a0. [DOI] [PubMed] [Google Scholar]
- Klein G., Klein E. Conditioned tumorigenicity of activated oncogenes. Cancer Res. 1986 Jul;46(7):3211–3224. [PubMed] [Google Scholar]
- Land H., Parada L. F., Weinberg R. A. Cellular oncogenes and multistep carcinogenesis. Science. 1983 Nov 18;222(4625):771–778. doi: 10.1126/science.6356358. [DOI] [PubMed] [Google Scholar]
- Land H., Parada L. F., Weinberg R. A. Tumorigenic conversion of primary embryo fibroblasts requires at least two cooperating oncogenes. Nature. 1983 Aug 18;304(5927):596–602. doi: 10.1038/304596a0. [DOI] [PubMed] [Google Scholar]
- Lang R. A., Metcalf D., Gough N. M., Dunn A. R., Gonda T. J. Expression of a hemopoietic growth factor cDNA in a factor-dependent cell line results in autonomous growth and tumorigenicity. Cell. 1985 Dec;43(2 Pt 1):531–542. doi: 10.1016/0092-8674(85)90182-5. [DOI] [PubMed] [Google Scholar]
- Lee J. C., Hapel A. J., Ihle J. N. Constitutive production of a unique lymphokine (IL 3) by the WEHI-3 cell line. J Immunol. 1982 Jun;128(6):2393–2398. [PubMed] [Google Scholar]
- Lichtman A. H., Reynolds D. S., Faller D. V., Abbas A. K. Mature murine B lymphocytes immortalized by Kirsten sarcoma virus. Nature. 1986 Dec 4;324(6096):489–491. doi: 10.1038/324489a0. [DOI] [PubMed] [Google Scholar]
- Mougneau E., Lemieux L., Rassoulzadegan M., Cuzin F. Biological activities of v-myc and rearranged c-myc oncogenes in rat fibroblast cells in culture. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5758–5762. doi: 10.1073/pnas.81.18.5758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needleman S. W., Kraus M. H., Srivastava S. K., Levine P. H., Aaronson S. A. High frequency of N-ras activation in acute myelogenous leukemia. Blood. 1986 Mar;67(3):753–757. [PubMed] [Google Scholar]
- Pierce J. H., Aaronson S. A. Myeloid cell transformation by ras-containing murine sarcoma viruses. Mol Cell Biol. 1985 Apr;5(4):667–674. doi: 10.1128/mcb.5.4.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce J. H., Di Fiore P. P., Aaronson S. A., Potter M., Pumphrey J., Scott A., Ihle J. N. Neoplastic transformation of mast cells by Abelson-MuLV: abrogation of IL-3 dependence by a nonautocrine mechanism. Cell. 1985 Jul;41(3):685–693. doi: 10.1016/s0092-8674(85)80049-0. [DOI] [PubMed] [Google Scholar]
- Pierce J. H., Ruggiero M., Fleming T. P., Di Fiore P. P., Greenberger J. S., Varticovski L., Schlessinger J., Rovera G., Aaronson S. A. Signal transduction through the EGF receptor transfected in IL-3-dependent hematopoietic cells. Science. 1988 Feb 5;239(4840):628–631. doi: 10.1126/science.3257584. [DOI] [PubMed] [Google Scholar]
- Rapp U. R., Cleveland J. L., Brightman K., Scott A., Ihle J. N. Abrogation of IL-3 and IL-2 dependence by recombinant murine retroviruses expressing v-myc oncogenes. Nature. 1985 Oct 3;317(6036):434–438. doi: 10.1038/317434a0. [DOI] [PubMed] [Google Scholar]
- Rein A., Keller J., Schultz A. M., Holmes K. L., Medicus R., Ihle J. N. Infection of immune mast cells by Harvey sarcoma virus: immortalization without loss of requirement for interleukin-3. Mol Cell Biol. 1985 Sep;5(9):2257–2264. doi: 10.1128/mcb.5.9.2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenborn E. T., Mierendorf R. C., Jr A novel transcription property of SP6 and T7 RNA polymerases: dependence on template structure. Nucleic Acids Res. 1985 Sep 11;13(17):6223–6236. doi: 10.1093/nar/13.17.6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz R. C., Stanton L. W., Riley S. C., Marcu K. B., Witte O. N. Synergism of v-myc and v-Ha-ras in the in vitro neoplastic progression of murine lymphoid cells. Mol Cell Biol. 1986 Sep;6(9):3221–3231. doi: 10.1128/mcb.6.9.3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senn H. P., Jiricny J., Fopp M., Schmid L., Moroni C. Relapse cell population differs from acute onset clone as shown by absence of the initially activated N-ras oncogene in a patient with acute myelomonocytic leukemia. Blood. 1988 Sep;72(3):931–935. [PubMed] [Google Scholar]
- Senn H. P., Trân-Thang C., Wodnar-Filipowicz A., Jiricny J., Fopp M., Gratwohl A., Signer E., Weber W., Moroni C. Mutation analysis of the N-ras proto-oncogene in active and remission phase of human acute leukemias. Int J Cancer. 1988 Jan 15;41(1):59–64. doi: 10.1002/ijc.2910410112. [DOI] [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B. Autocrine growth factors and cancer. 1985 Feb 28-Mar 6Nature. 313(6005):745–747. doi: 10.1038/313745a0. [DOI] [PubMed] [Google Scholar]
- Stocking C., Löliger C., Kawai M., Suciu S., Gough N., Ostertag W. Identification of genes involved in growth autonomy of hematopoietic cells by analysis of factor-independent mutants. Cell. 1988 Jun 17;53(6):869–879. doi: 10.1016/s0092-8674(88)90329-7. [DOI] [PubMed] [Google Scholar]
- Toksoz D., Farr C. J., Marshall C. J. ras gene activation in a minor proportion of the blast population in acute myeloid leukemia. Oncogene. 1987;1(4):409–413. [PubMed] [Google Scholar]
- Vogt M., Lesley J., Bogenberger J., Volkman S., Haas M. Coinfection with viruses carrying the v-Ha-ras and v-myc oncogenes leads to growth factor independence by an indirect mechanism. Mol Cell Biol. 1986 Oct;6(10):3545–3549. doi: 10.1128/mcb.6.10.3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson J. D., Eszes M., Overell R., Conlon P., Widmer M., Gillis S. Effect of infection with murine recombinant retroviruses containing the v-src oncogene on interleukin 2- and interleukin 3-dependent growth states. J Immunol. 1987 Jul 1;139(1):123–129. [PubMed] [Google Scholar]
- Wodnar-Filipowicz A., Senn H. P., Jiricny J., Signer E., Moroni C. Glycine-cysteine substitution at codon 13 of the N-ras proto-oncogene in a human T cell non-Hodgkin's lymphoma. Oncogene. 1987;1(4):457–461. [PubMed] [Google Scholar]
- Ymer S., Tucker W. Q., Sanderson C. J., Hapel A. J., Campbell H. D., Young I. G. Constitutive synthesis of interleukin-3 by leukaemia cell line WEHI-3B is due to retroviral insertion near the gene. Nature. 1985 Sep 19;317(6034):255–258. doi: 10.1038/317255a0. [DOI] [PubMed] [Google Scholar]