Abstract
On a treadmill, humans switch from walking to running beyond a characteristic transition speed. Here, we study human choice between walking and running in a more ecological (non-treadmill) setting. We asked subjects to travel a given distance overground in a given allowed time duration. During this task, the subjects carried, and could look at, a stopwatch that counted down to zero. As expected, if the total time available were large, humans walk the whole distance. If the time available were small, humans mostly run. For an intermediate total time, humans often use a mixture of walking at a slow speed and running at a higher speed. With analytical and computational optimization, we show that using a walk–run mixture at intermediate speeds and a walk–rest mixture at the lowest average speeds is predicted by metabolic energy minimization, even with costs for transients—a consequence of non-convex energy curves. Thus, sometimes, steady locomotion may not be energy optimal, and not preferred, even in the absence of fatigue. Assuming similar non-convex energy curves, we conjecture that similar walk–run mixtures may be energetically beneficial to children following a parent and animals on long leashes. Humans and other animals might also benefit energetically from alternating between moving forward and standing still on a slow and sufficiently long treadmill.
Keywords: legged locomotion, walking and running, optimization, energy minimization, gait transition
1. Introduction
Imagine you wish to go from your house to the bus stop and have very little time to do so. You would likely run the whole distance. If you had a lot of time, you would likely walk the whole distance. If there was an intermediate amount of time, perhaps you would walk for a while and run for a while. While it is not immediately obvious that using a walk–run mixture is advantageous here, it seems consistent with common experience. In this article, we make this anecdotal experience precise by performing human subject experiments. Most significantly, we then interpret the experimental observations using metabolic energy minimization, without appealing to fatigue or poor time-estimation as mechanisms. We review and extend various mathematical results related to metabolic energy minimization and locomotor choice, deriving, for the first time, predictions for travelling finite distances and for travelling on treadmills of finite lengths, in the presence of costs for the transients, using analytical arguments and numerical optimization. In these models, the key mathematical criterion for obtaining walk–run (and walk–rest) mixtures is non-convexity of the energy cost curves. Assuming similar energy curves, we conjecture that similar walk–run–rest mixture strategies may be energetically beneficial in superficially diverse situations: children walking with parents, animals on long leashes or long slow treadmills, non-elite marathon runners, etc.
2. An overground gait transition experiment
2.1. Background
Humans have two qualitatively distinct ‘gaits’, walking and running. Some treadmill gait transition experiments have shown that a person walking on a slow treadmill switches to running when the treadmill speed is slowly increased [1–3]. When the speed is decreased, the person switches from running to walking. Historically, most such gait transition experiments have been performed on a treadmill. Because an ideal treadmill (with perfect speed regulation) is an inertial frame, treadmill locomotion has the potential to be mechanically identical to overground locomotion [4]. However, treadmill locomotion is different from overground locomotion in two key respects. First, a treadmill with a short length limits voluntary speed fluctuations by the subject. No such strict speed constraint exists in real life. Second, a typical treadmill provides no visual flow, the feeling of objects moving past one's eye (but see [5]). Thus, in part to make these experiments more ecological, we introduce a simple non-treadmill overground gait transition experiment.
2.2. Experimental protocol
In the ‘basic protocol’ of our experiment, the subjects had to go from a starting point S to an endpoint E, separated by a distance Drequired, in a given time duration Tallowed. The subjects carried a stopwatch that counted down to zero from the total time duration Tallowed, so they could see, when necessary, the time remaining. The subjects had to reach the endpoint exactly when the stopwatch ran out, rather than arrive early or late. The subjects received no further instructions.
By constraining the total distance and the total time, we are prescribing an average speed constraint Vavg = Drequired /Tallowed, without constraining the speed at any moment. Each subject had 15 trials, with different Tallowed resulting in prescribed average speeds Vavg from 1 to 3.8 m s−1. All subjects were video-taped and instrumented for stride frequency. Some subjects were instrumented with a global positioning system (GPS) device for speed measurements. Some subjects performed indoor trials, and others, outdoors.
Variants of this ‘basic protocol’ were performed for small subject numbers, using longer and different distances, allowing some trial repetition, and allowing arriving early, to check if the results change substantially with protocol details. See §7 at the end of this article and electronic supplementary material, §S5 for more details.
2.3. Experimental results: walking and running fractions
The subjects were able to travel the distance on time, with the mean and standard deviations of late arrival just over a second. If the subjects approached the destination too early, they slowed down to arrive on time. As expected, for low prescribed average speed Vavg, the subjects walked the entire distance. For high Vavg, the subjects ran almost the entire distance. For intermediate Vavg, a majority of the subjects used a mixture of walking and running (a walk–run mixture).
Figure 1a shows the fraction of running as a function of the prescribed average speed Vavg, for all the subjects involved in the basic protocol. A single data point (red dot) in figure 1a is the fraction of time spent running by one subject in one trial, obtained from the video by counting the seconds spent running; also shown are the median running fraction as a function of average speed and a (pink) band containing 50 per cent of the data points. Here, running is defined as any gait with a flight phase, in which the hip goes down and then up when one leg is in contact with the ground, as if the body bounces on a springy leg [6]. In walking, the hip vaults over on the leg more like an inverted pendulum and at least one foot always contacts the ground [7].
Figure 1.
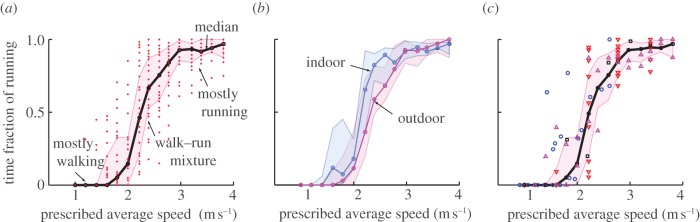
(a) All subjects with the basic protocol (n= 28 subjects, indoor and outdoor, Drequired = 122 m). The time fraction of running is shown: the red dots are the raw data (each dot is a trial, all data points shown), the solid black line is the population median, and the pink band denotes 50% of the data centred around the median (25th–75th percentile). Pure walking dominates low prescribed speeds and pure running dominates high prescribed speeds, and most subjects use a walk–run mixture for intermediate speeds. (b) The data in the previous panel, decomposed into indoor and outdoor data. Median fractions are blue and pink solid lines, surrounded by the respective 50% bands in corresponding lighter shades. (c) Variants of the basic protocol overlaid on the 50% band from the first panel: experiments in which subjects had three trials per prescribed average speed for five different prescribed average speeds (red triangles), in which the trials had different random distances ranging between 70 and 250 m and different time allowed (blue circles), in which the subject travelled twice the distance, 244 m (black squares), and in which the subjects were allowed to arrive earlier than Tallowed (magenta triangles).
Unlike on a treadmill, there is no sharp gait transition speed here. Instead, there is a ‘transition region’ between average speeds of 2 and 3 m s−1 (roughly), in which a majority of the subjects use a walk–run mixture, transitioning from walking most of the time to running most of the time. The average speed with equal amounts of walking and running (running fraction = 0.5) is about 2.2 m s−1 in figure 1a, which is close to, but slightly higher than the treadmill gait transition speeds in the literature [1,3]. At the highest average speeds, the running fraction is not quite 100 per cent, but this might be because the subjects walked one or two steps before running the rest of the way; longer Drequired may find higher running fractions.
The indoor and outdoor trials (figure 1b), while yielding qualitatively similar trends appear to be slightly different; outdoor data's median is close to the indoor data's 25th percentile. These differences could be due to small differences in protocol (having to turn around indoors), and increased visual flow indoors due to nearby walls, resulting in higher perceived speed, shifting the actual gait transition region to lower speeds [5,8]. The data from the protocol variants, taken as a whole, are not qualitatively different from the basic protocol (figure 1c); 75 per cent of the protocol variants' data points fell within the centred 50 per cent band of the data from the basic protocol.
2.4. Experimental results: speed variations within a trial
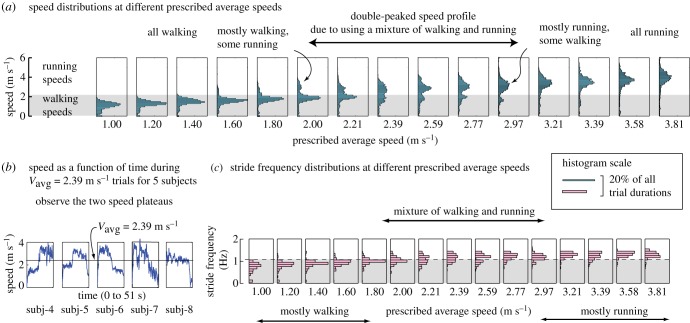
When the subjects used a walk–run mixture, the walking speed was low and the running speed was high, so that the average speed is as prescribed. Figure 2a shows the trial-by-trial distribution of speeds pooled over all the outdoor subjects with GPS speed measurements. As we would expect, with increasing prescribed average speed, the speed distribution slowly shifts upward to higher speeds.
Figure 2.
(a) Histograms of the speeds at which the subjects travelled during each of the 15 trials with different prescribed average speeds Vavg; pooled over the 10 subjects with GPS speed measurements, with histogram bin width = 0.1 m s−1. The histograms have a single peak for low and high prescribed average speed, but have two peaks for intermediate average speeds, suggesting a walk–run mixture. (b) The unfiltered GPS-derived speed as a function of time during the trial corresponding to Vavg = 2.39 m s−1 and Tallowed = 51 s, for five subjects. Notice the two speed plateaus in subjects 4–6. (c) Histograms of stride frequencies used during various trials, pooled over outdoor subjects with this pedometer-based data. Histogram bin width = 0.1 Hz. The horizontal scale of the histograms are such that the area within the histogram are the same across different prescribed speeds (proportional to total number of trials). The horizontal grey band across the histograms (a and c) indicating normal walking and running speeds or frequencies is simply to guide the eye, not meant to be a strict separator.
Significantly, the speed distribution is double-peaked for Vavg between 2.0 and 3 m s−1. Such double-peaked distributions indicate (many) subjects walking slow and running fast in a walk–run mixture. At Vavg = 2 m s−1, the lower speed walking peak has a higher enclosed area (in the histogram) than the higher speed running peak, suggesting that subjects walked for most of the time. The relative sizes of the peaks are reversed at Vavg = 2.97 m s−1, when subjects ran most of the time. For Vavg below 2 m s−1 and above 3 m s−1, we observe only a single peak in the speed distributions, indicative of walking or running the whole distance.
Figure 2b shows the raw GPS-derived speed as a function of time for five subjects, for the Vavg = 2.39 m s−1 trial, which is in the walk–run transition region. For three subjects shown (subjects 4–6), we see two distinct speed plateaus, corresponding to running and walking. Not all subjects had such distinct speed plateaus in the transition regime. To illustrate subject-to-subject variability (also seen in figure 1), we show one subject (subject 7) for whom the plateaus are less distinct, and another subject (subject 8) who used roughly the same speed over the whole trial.
The stride frequency histograms shown in figure 2c also show a speed region (about 2–3 m s−1) in which the stride frequency distribution is broader, overlapping both walking and running stride frequencies. In this region, however, the histograms are not as distinctly double-peaked, perhaps both on account of larger subject-to-subject variability and higher measurement errors in the stride frequencies (see the electronic supplementary material).
3. Energy minimization as a candidate theory of gait choice
3.1. On a treadmill, switch to save energy
Margaria [2] and others found that walking requires less energy at low speeds and running requires less energy at higher speeds, as also suggested by mathematical models of bipedal walking and running [7,9–11]. Example walking and running metabolic rates as functions of speed, based on published data [12–15], are shown in figure 3a. On a treadmill, humans switch between walking and running close to the intersection of the cost curves (Q). While some researchers [1,16,17] found a small difference (∼ 0.1 m s−1) between the energy-optimal and measured treadmill transition speeds (and also a slight difference between the walk-to-run and run-to-walk transition speeds [1]), others [18,19] did not; see [9,20] for possible explanations.
Figure 3.
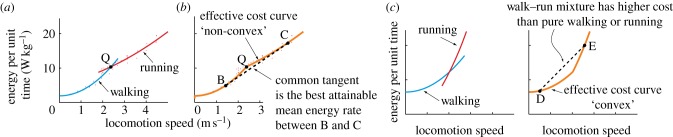
(a) Metabolic energy rate for walking and running as a function of speed, intersecting at speed v = VQ. (b) The combined ‘effective cost curve’ is shown in orange, by picking the gait that has the lower cost at every relevant speed: resting at v = 0, walking below VQ, and running above VQ. Walking at speed VB and running at VC for different fractions of time results in an average metabolic rate as given by the line BC. When BC is the unique common tangent to the two curves, switching between B and C results in a lower average metabolic rate than is possible by exclusively walking or running (in fact the lowest possible for this model). This lowering of cost is possible because of the ‘non-convexity’ of the effective cost curve. (c) Hypothetical metabolic rate curves for walking and running that result in a ‘convex’ effective cost curve, implying no direct energetic benefit from walk–run mixtures.
We now describe what metabolic energy minimization predicts in the context of our non-treadmill experiment, first ignoring, then considering the cost for transitions between walking and running.
3.2. Human locomotion energetics preliminaries
For the rest of this article, we will simply use the curves in figure 3a. The qualitative predictions are preserved as long as the curves have approximately the same shapes. We use the notation 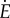 and ‘rate’ to refer to energy per unit time. The walking metabolic rate data
and ‘rate’ to refer to energy per unit time. The walking metabolic rate data 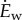 is adequately fit by a quadratic function of speed [12,15]:
is adequately fit by a quadratic function of speed [12,15]: 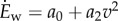 ; we used a0 = 1.91 W kg−1 and a2 = 1.49 W (ms−1)−2. For running, while it is hard to statistically distinguish between linear [2,21] and quadratic models [14] using metabolic data, we used a quadratic model
; we used a0 = 1.91 W kg−1 and a2 = 1.49 W (ms−1)−2. For running, while it is hard to statistically distinguish between linear [2,21] and quadratic models [14] using metabolic data, we used a quadratic model 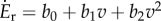 , with b0 = 5.17 W kg−1, b1 = 1.38 W (ms−1)−1 and b2 = 0.34 W (ms−1)−2 [13,14]. Finally, humans consume energy while resting (that is, not moving), modelled here as a constant rate erest = 1.22 W kg−1 [21].
, with b0 = 5.17 W kg−1, b1 = 1.38 W (ms−1)−1 and b2 = 0.34 W (ms−1)−2 [13,14]. Finally, humans consume energy while resting (that is, not moving), modelled here as a constant rate erest = 1.22 W kg−1 [21].
We combine these three metabolic rates into an ‘effective cost curve’, shown in figure 3b, by picking the lower of the three rates at every speed; the resting rate is relevant only at v = 0.
3.3. Two choices: walk or run
In our experiment, the subjects had to cover a distance Drequired in time Tallowed. For simplicity, say a subject runs for time duration λrTallowed at constant speed Vr and walks for a time duration 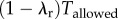 at constant speed Vw, such that she satisfies the distance, and, therefore, the average speed constraint of the experiment:
at constant speed Vw, such that she satisfies the distance, and, therefore, the average speed constraint of the experiment: 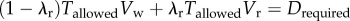 . Here, the fraction of time spent running is λr, with
. Here, the fraction of time spent running is λr, with 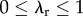 . Rearranging, we have the average speed constraint
. Rearranging, we have the average speed constraint
Subject to this constraint, we wish to find Vw, Vr and λr so as to minimize the total energy expenditure Etotal over the whole journey, first ignoring any costs for transients
Minimizing Etotal is equivalent to minimizing the average energy rate 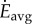 over the total time duration:
over the total time duration: 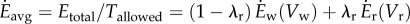 .
.
3.4. Optimal solution through the common tangent construction
Alexander [9] states the correct optimal strategies for the above problem, without a complete solution. Drummond [22] presents an elegant solution via the so-called ‘common tangent construction,’ as described below, while analysing a closely related exercise called ‘scout's pace’ that mixes walking and running.
As in figure 3b, average speeds Vavg between speeds VB and VC can be obtained by walking at speed VB and running at speed VC. The average energy rate 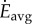 for this walk–run mixture is given by the straight line between B and C, which is also the common tangent here. Because this straight line is below both cost curves, the mixture strategy has lower average energy rate compared with pure walking or running at such Vavg. Thus, a walk–run mixture is predicted by metabolic energy minimization for a range of intermediate speeds (between VB and VC). For
for this walk–run mixture is given by the straight line between B and C, which is also the common tangent here. Because this straight line is below both cost curves, the mixture strategy has lower average energy rate compared with pure walking or running at such Vavg. Thus, a walk–run mixture is predicted by metabolic energy minimization for a range of intermediate speeds (between VB and VC). For 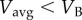 , it is optimal to walk the entire distance. For
, it is optimal to walk the entire distance. For 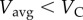 , it is optimal to run the entire distance.
, it is optimal to run the entire distance.
The necessary and sufficient condition for a walk–run mixture to be optimal for this model is that the effective cost curve (figure 3b) is non-convex near the intersection point Q. Non-convexity, by definition [23], means that we can draw a lower tangent to this effective cost curve that touches it at two points B and C as in figure 3b. To contrast with such non-convexity, we devised the hypothetical walking and running cost curves of figure 3c, which give rise to a convex effective cost curve, therefore implying no direct energy benefits from walk-run mixtures; the chord DE (corresponding to a walk–run mixture) is strictly above walking and running costs between D and E, and any lower tangent will touch the curve at only one point. See the electronic supplementary material, §S1 for a discussion of convexity and related mathematics.
3.5. Three choices: walk, run or rest
In the above mathematical analysis and in our basic protocol experiments, the choice was between only walking and running. Arriving early and resting was not an option. If we allow resting, for low average speeds Vavg, a mixture of walking and resting becomes optimal.
The necessary and sufficient condition for the optimality of a walk–rest mixture is that the resting rate erest is strictly less than a0, the walking metabolic rate at infinitesimally small speeds (as is true for human walking [12]). This condition allows us to construct a lower tangent touching the walking cost curve at A, starting from the resting state (0, erest), as shown in figure 4a. Then, for 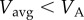 , a mixture of resting and walking at VA is optimal; note,
, a mixture of resting and walking at VA is optimal; note, 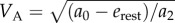 . This VA is also the constant walking speed that minimizes the ‘net’ energy cost per unit distance
. This VA is also the constant walking speed that minimizes the ‘net’ energy cost per unit distance
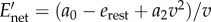 without time constraints [8].
without time constraints [8].
Figure 4.
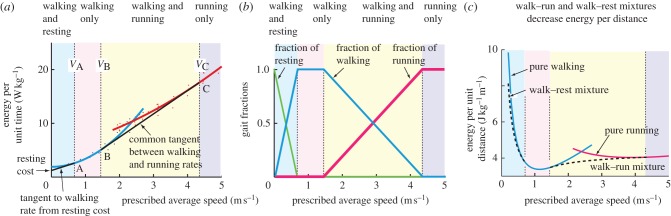
(a) Total metabolic rate as a function of walking and running speed, normalized by body mass, showing common tangent constructions for optimal walk–rest and walk–run mixtures. (b) Walk–run–rest fractions shown. As the average speed increases, the energy optimal strategy changes from a mixture of resting and walking to pure walking to a walk–run mixture to pure running. (c) Energy per unit distance for walking, running, walk–run mixtures and walk–rest mixtures. The mixture strategies (dotted lines) reduce the average energy per unit distance in the respective speed regimes, as shown. Note, the cost per distance of the mixtures is not a linear function of average speed, which is why the ‘common tangent construction’ is performed on the cost per time curves.
Figure 4a consolidates the predictions of metabolic energy minimization, showing the four average speed regimes with their respective optimal behaviours: walk–rest mixtures (with walking at VA), constant speed pure walking (walk at Vavg), walk–run mixtures (walking at VB, running at VC), and constant speed pure running (run at Vavg). Figure 4b shows the predicted optimal fractions of resting, walking and running. Figure 4c shows how the walk–rest and walk–run mixtures reduce the energy per unit distance
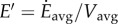 over pure walking and running. Note that the walking speeds VA and VB are not equal to, but are, respectively, less than and greater than the maximum range speed, which minimizes the cost per unit distance for walking [8].
over pure walking and running. Note that the walking speeds VA and VB are not equal to, but are, respectively, less than and greater than the maximum range speed, which minimizes the cost per unit distance for walking [8].
3.6. Costs for transients, the number of switches and finite distances
In the analysis thus far, we have assumed that the energy rate is purely a function of speed and gait, assuming that neither changing speeds nor switching gaits entail an energy cost. Such transients do have a cost, associated with a change in kinetic energy or limb movement patterns [24]. Without these costs for transients, the above model implies that one can switch between gaits arbitrarily often without affecting the optimality of the walk–run mixture, as long as the walking and running fractions are unchanged.
As soon as a cost for transients is added to the mathematical model, say, proportional to change in kinetic energy, having exactly one switch between walking and running, or exactly one switch between walking and resting, becomes optimal. In the electronic supplementary material, §S2, we describe numerical optimization to obtain the energy optimal gait strategies in the presence of a cost for transients, shown in figure 5a. Thus, we find that despite transient costs, the qualitative picture of figure 4a,b is preserved, but the specific transition speeds separating the various regimes are slightly changed and depend on Drequired (see the electronic supplementary material, figure S3 expands on figure 5a). The effect of transient costs become negligible over much larger distances, again giving figures 4a,b.
Figure 5.
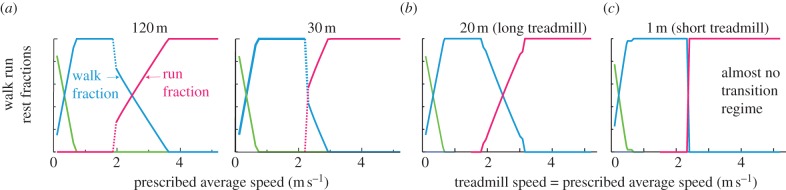
Effect of adding energy cost for transients: model predictions from numerical optimizations. (a) When travelling a finite distance overground, the extent of the walk–run transition regime depends on the distance travelled (Drequired = 120 and 30 m shown). Smaller distances have smaller transition regimes. (b) When having to remain on a constant-speed treadmill of finite length, the extent of the walk–run transition regime depends on the treadmill length. For long treadmills (e.g. 20 m), there is a substantial walk–run mixture regime. On very short treadmills (e.g. 1 m), essentially no walk–run mixture regime exists, and the transition from all walking to all running is sharp.
3.7. Locomotion on long treadmills with transient costs
Unlike short treadmills, long treadmills allow larger voluntary speed fluctuations while imposing an average speed constraint over the long term. For treadmill locomotion, there is no explicit time constraint or distance constraint, but only a constraint that the person remains on the treadmill. In the absence of transient costs, the energy optimal strategies for remaining on the treadmill are exactly as in figure 4. However, with transient costs, substantial walk–run mixtures become optimal only when the treadmill is long enough. This treadmill-length-dependence of transition speeds is shown in figure 5b (also in electronic supplementary material, figure S4 for greater detail). These figures were obtained using numerically computed optimal walk–run–rest mixtures, subject to the constraint that the person remains within the treadmill; the numerical computation is documented in more detail in the electronic supplementary material, §S3. Thus, if the goal is to simply stay on a long treadmill (e.g. an airport moving walkway), instead of traversing it, energy minimization predicts that humans will walk or run in place for some speeds, but use walk–stand or walk–run mixtures at other speeds, moving against the belt some of the time.
4. Discussion
4.1. Numerical predictions from our model
We are able to explain the qualitative features of walk–run mixtures using energy minimization. Using cost curves from different authors [2,12–15,21,21] will result in slightly different numerical predictions, by as much as 0.2 m s−1 for each of the predicted transition speeds. Also, averaging cost curves across subjects can move transition points. Ideally, one should use subject-specific energy rate curves and test if a subject's gait choice is consistent with her specific energetics. For our assumed cost curves and no transient costs (equivalent to travelling a very large distance), we obtained VA = 0.7 m s−1, VB = 1.45 m s−1, and VC = 4.35 m s−1 (figure 4). With transient costs and Drequired = 120 m, we obtained VA = 0.7 m s−1, VB = 1.9 m s−1, and VC = 3.6 m s−1 (figure 5a); this predicted walk–run transition region overlaps with those in figure 1.
The speed VC in figures 4 and 5 is sensitive to the curvature of the quadratic running cost model, approaching the maximum running speed for a linear model (which still predicts walk–run mixtures). Because humans never use maximum running speeds, perhaps the running rate is indeed slightly curved (assuming energy minimization, not fatigue, determines speeds). The slight curvature used here is consistent with metabolic data [13,14]. We ignored other complexities in the energy models, e.g. post-exercise increases in energy and oxygen consumption [25].
4.2. Higher variability near transition
Behavioural variability in the running fractions of figure 1a–c is higher near the gait transition region than away from it (figure 6). A few plausible mechanisms could contribute to such variability: (i) within-subject variability, perhaps due to sensory or computational noise in the human motor system in deciding which gait to use; because the walking–running costs are most similar near the transition, the errors could be higher; (ii) subjects using the correct optimal fraction for an incorrect average speed (with a given speed error) will produce variability proportional to the absolute slope of the running fraction curve λr(v), maximum at the transition; (iii) pooling different subjects with slightly different running fraction curves shifted sideways will also produce variability proportional to the absolute slope of the running fraction curve. See the electronic supplementary material, §S5 for a related mathematical note.
Figure 6.
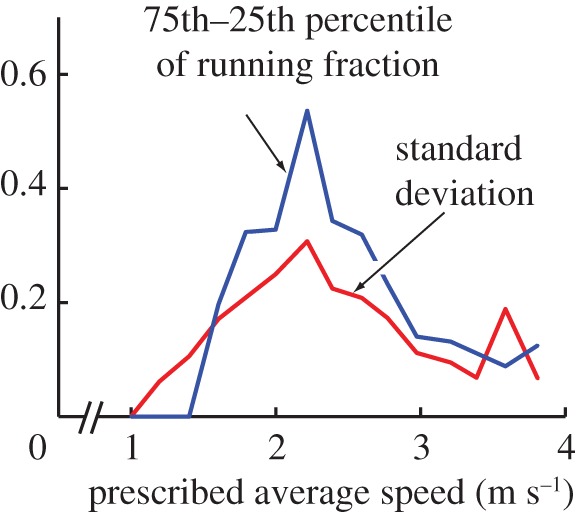
Two measures of variability in running fractions from figure 1a. Variability is maximal in the transition region, where the running fraction also has the greatest slope.
4.3. Other overground experiments
Recently, some non-treadmill overground gait transition experiments were performed, in which subjects were asked to walk with increasing speed and then start running when they found it natural (e.g. [26] found a transition speed of 2.85 m s−1, with 0.5 m s−2 natural acceleration). In contrast, in our more ecological experiment, we did not explicitly suggest increasing speeds or gait change, allowing more choice as in daily legged travel.
Also, Hoyt & Taylor [27] and Pennycuick [28] observed double-peaked speed distributions in free-ranging animals, but there were no explicit average speed constraints in such contexts.
4.4. Alternative non-energy-based explanations
Partly to address the slight discrepancy between energy optimality and the treadmill gait transition speed, a number of non-energy-based kinematic and kinetic factors have been posited as triggering the transition [6,29–35]—usually suggesting that humans switch to running because some force, strain, velocity or stability threshold is violated while walking. None of these intra-stride threshold-based hypotheses, as stated, can explain why humans use a walk–run mixture when there is no strict speed constraint. Further, unlike energy minimization, which predicts many phenomena related to human locomotion at least qualitatively [9,11], these other hypotheses have been not systematically tested for phenomena other than gait transitions.
We mention two further alternate hypotheses. First, perhaps subjects have poor time-to-destination estimation, so that the subjects initially start to walk and then run when they realize they have very little time left (or the reverse), but it is unclear how this could explain various systematic trends in our experiments without additional assumptions. Second, we can rule out fatigue as a reason for switching to walking after running for a while, as subjects were able to run the whole way at higher speeds in other trials. Also, our trials were short enough that fatigue is unlikely to be an issue. On the other hand, in the presence of fatigue, multiple switches between walking and running may well become favourable [22]. For instance, some animals use intermittent locomotion to extend their endurance [36] at high average speeds, while briefly exceeding their maximum aerobic speed.
Finally, while we have focused on energy minimization to explain trends, we recognize that animals must trade-off energy minimization with other constraints and goals [9]. Our implicit assumption is that the cost curves used here are for movements that have already taken such trade-offs into account.
4.5. What energy should we minimize?
We have assumed that subjects minimize the total energy cost over the experimentally allowed task duration (equivalently, the whole day [8]). Instead, if humans minimize the energy to arrive at the destination, not counting resting after arriving, the predictions for large distances are identical to figure 4a,b except the walking speed VA in a walk–rest mixture is replaced by the so-called maximum range speed, the minimum in figure 4c, given by 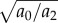 , typically 1.2–1.4 m s−1 [8].
, typically 1.2–1.4 m s−1 [8].
Also, in their daily life, instead of minimizing energy subject to a time constraint, it may be that humans trade-off a cost for time and energy, resulting in slightly higher speeds than predicted by pure energy optimality [37].
4.6. When should we minimize energy?
In future work, we suggest repeating our experiments over a range of distances, including much shorter (25 m, say) and much longer distances (3 km, say), to test if the results in figures 1 and 2, and other behaviour, change systematically. Over much longer distances, without practise and visual landmarks, perhaps humans will have greater difficulty judging time and distance to destination, and as a consequence, perhaps there will be greater behavioural variability.
It is sometimes argued that over short distances, humans and other animals may not move in a manner that minimizes energy because this energy would be a small fraction of the daily energy budget [38]. While plausible, this hypothesis has not been tested systematically. Below, we provide a few inter-related reasons for why humans may minimize energy even at short travel distances considered here.
First, we have shown empirically that over relatively short distances of 100 m, human behaviour seems qualitatively consistent with energy minimization. At least for steady walking, there are no major observed differences in how people walk a short distance versus a much longer distance (in the absence of fatigue); so the same principles may underlie walking both short and longer distances. Further, the walk–run strategy for minimizing energy over many thousand metres is the same as the walk–run strategy for minimizing the energy for a few hundred metres (with minor quantitative differences). Thus, one does not have to evolve or learn qualitatively different strategies for different distances.
Finally, we note that energy is a ‘fungible’ quantity. That is, metabolic energy saved in one task is available for use in any other task. (Fungibility is an economics concept, used most commonly as a descriptive property of money.) In the absence of other trade-offs and constraints, evolutionary processes could not differentiate between energy saved in one big task versus a hundred small tasks. Thus, energy reductions in the hundred small tasks might be as likely as in the one big task, if we controlled for the complexity of the two sets of tasks. To be sure, it is possible for human motor behaviour to be inconsistent with the fungibility of energy, just as there is evidence from behavioural economics that spending behaviour is sometimes inconsistent with the fungibility of money [39]. This is an open question for future empirical study.
4.7. Cognitive and motor mechanisms governing gait choice.
While we do not know how humans adjusted their gait strategy in our experiments, we see evidence of both feedback and feed-forward mechanisms [40]. In the short Tallowed trials, the subjects realize immediately that they have to run relatively fast to arrive on time, suggesting feed-forward mechanisms that convert the cognitively provided distance–time constraints into gait. And later in each trial, the subjects typically speed up or slow down to correct for earlier misestimations of the speed required, suggesting feedback mechanisms.
5. Related phenomena, conjectures and other applications
In this section, we discuss related situations that might benefit from similar models, make conjectures partly informed by available energy data, suggesting future experiments.
5.1. Moving walkways
Say the goal is to go from one end of a very long treadmill (e.g. an airport moving walkway) to the other, instead of just staying on the treadmill. If the goal speed is 1.2 m s−1 (say) relative to the ground, a walkway speed of 0.7 m s−1 requires 0.5 m s−1 relative to the walkway, at which average speed a walk–rest mixture may be optimal. Thus, we conjecture that faster walkways may promote more standing, possibly resulting in congestion and reduced people transport (see [8] for a similar conjecture).
5.2. Keeping up with someone else: human children, animals on a leash, etc.
Human children have a lower walk–run transition speed than adults. Extrapolating children's gait transition data in Tseh et al. [17] suggests that children with leg length of 50–55 cm will have a transition speed of about 1.4 m s−1, close to adult preferred walking speeds. Thus, while travelling with a parent at 1.4 m s−1, a small child might benefit energetically from a walk–run mixture; the walking–running cost curves for slightly older children [41] continue to show the necessary non-convexity, but such data are not available for very small children. We speculate that this energy benefit may get reflected in the child walking slowly and then running to catch up or overtake the parent, sometimes leading or lagging. While children's activity is often burst-like [42], we do not know of parent–child co-locomotion studies.
Similarly, dogs transition from walking to trotting between 0.9 and 1.3 m s−1 depending on size [43]. While we could not find walking–trotting energy data for dogs, we see evidence of the energy non-convexity necessary for walk–trot mixtures in horses and goats [27,44]. Extrapolating from these quadrupeds, we conjecture that dogs on a long leash, accompanying a walking human, might benefit from walk–trot mixtures (as might goats and horses at other speeds).
Finally, when animals (whose juveniles benefit from adult protection) migrate, juveniles and adults often travel long distances at the same average speed. For such animals, the adult's maximal range speed may (if the speeds so conspire and given the necessary non-convexity) correspond to a mixture of gaits for a juvenile, or vice versa. Such behaviour is not necessary, of course. For instance, both adult and juvenile gnus apparently use a mixture of walking and cantering [28].
5.3. Animals on very slow treadmills
Various untrained animals, especially small animals, when placed on a slow treadmill, sometimes perform a mixture of standing still, coasting to the back of the treadmill, and then scooting forward, giving the impression of being hard to train; researchers often have to use some stimulation to get the animal to move steadily [45]. We conjecture that some of this behaviour could be due to the energy optimality of walk–rest mixtures. Recall that the necessary condition for such optimality is that the resting cost be less than the extrapolated locomotion cost at zero speed, documented for white rats by Schmidt-Nielsen [46].
5.4. Walk faster if you can lie down at the end
So far, we have used a single resting energy rate erest. Given that standing, sitting and lying down have progressively lower energy costs [47], energy minimization over the whole time duration implies, surprisingly, a higher walking speed in walk–rest mixtures if the subject is allowed to lie down on arrival than if the subject is allowed to only stand on arrival. See the electronic supplementary material, figure S5. We have not extensively explored experimental protocols involving rest, except the arriving-early variant in figure 1c, in which subjects did arrive early when Tallowed was large. Also, the experiment needed to test the above prediction may not be very ecological.
5.5. Altered body or environment
The walking and running cost curves, in humans and in other animals, are affected by changes either to the body or to the environment (e.g. wearing a loaded backpack, going uphill or downhill, altered gravity). The predicted speed regime corresponding to optimality of a walk–run mixture will be affected by such changes, which can be tested by repeating the experiments herein in those altered circumstances.
5.6. Marathoners, hunters and soccer players
During marathons and ultra-marathons, runners sometimes use a walk–run mixture [48], partly explained by fatigue. But note that the average US marathon finish times including non-elite runners are 4.5–5 h, with average speeds of 2.3–2.6 m s−1, when a walk–run mixture is energetically beneficial, as pointed out earlier by Alexander [9] and Drummond [22]. Analogously, humans that run 10-min miles (2.7 m s−1) might need less energy using walk–run mixtures (extrapolating figure 4a).
Human ‘persistence hunters’ pursue prey for many hours, mixing running and tracking the animal. Hunts witnessed by Liebenberg [49] in the Kalahari averaged 1.75 m s−1. While energy-minimizing humans might mostly walk with a small running fraction at these average speeds (using figure 1a or 4b), it may be worthwhile to study the gait fractions used by the hunters, likely different from energy optimal owing to hunting constraints (like keeping up with the animal), resulting in extra energetic cost.
Analogously, over a 90-min game, soccer players average about 2.1 m s−1 [50]. Given various game-play constraints (defending, intercepting, etc.), which clearly supersede energy conservation, one might quantify the extra energy such constraints impose, over and above the minimum energy required at the observed average speed; also of interest may be the speed and gait distributions.
5.7. Other transport modes: flying, swimming and driving
Finally, we remark that the optimality of mixture strategies may not be exclusive to legged locomotion. For instance, bounding flight in small birds, the intermittent swimming of mammals, and extreme accelerate–coast mixtures in some cars (also called burn and coast, or pulse and glide), especially those that participate in ‘eco-marathon’ races, have been argued to reduce energy consumption [51–53]. It may also be of interest to see whether legged robot locomotion cost curves have the necessary non-convexity, implying energetic benefits for using gait mixtures.
6. Conclusion
We observed that humans use a walk–run mixture at intermediate average locomotion speeds and argued that this behaviour is consistent with energy optimality. We have commented on possible application of the ideas to superficially diverse phenomena in humans and other animals. Exploring these related phenomena (and the underlying assumptions) quantitatively with controlled experiments and using subject-specific energy measurements for the mathematical models would enable us to better understand the limitations of energy minimization as a predictor of animal movement behaviour.
7. Material and methods
The protocols were approved by the Ohio State University's Institutional Review Board. Subjects participated with informed consent. The subjects' (n = 36, aged 20–32, eight female) had mean body mass 74.2 kg (12 kg s.d.) and mean height 1.79 m (0.078 m s.d.).
7.1. Basic experimental protocol
The outdoor subjects (n = 19) used a relatively straight and horizontal sidewalk. The indoor subjects (n = 9) performed them in a 2.5 m wide building corridor. Total distance was Drequired = 122 m. We used 15 different total times Tallowed, giving average speeds of 1 to 3.8 ms−1: 32, 34, 36, 38, 41, 44, 47, 51, 55, 61, 68, 76, 87, 102 and 122 s, presented in a random sequence. The subjects had no practice trials specific to these time durations except for a single initial practice trial to ensure protocol understanding. All subjects wore a watch (Garmin Forerunner 305) that recorded stride frequency from a pedometer (Garmin footpod). Some outdoor subjects (n = 10) carried a high-end GPS unit (VBOX Mini, Racelogic UK) to measure speed accurately. See the electronic supplementary material for more details about the subject population, protocol and instrumentation. The outdoor experiment required the subject to travel in only one direction from S to E. In the indoor experiment, S and E coincided and the subject turned back midway.
7.2. Variants of the basic protocol
We performed minor variants of the basic protocol for a total of n = 8 subjects. For n = 3 subjects, we repeated each speed three times, for a total of five speeds in random sequence. For n = 2 subjects, each of 10 average speed trials had a different distance, ranging from 70 to 250 m. For n = 2 subjects, subjects could arrive earlier than Tallowed. For one subject, we used a fixed longer distance 244 m for all trials.
Acknowledgements
We thank Andy Ruina from whom M.S. learnt about the possible optimality of walk–run mixtures, credited here to earlier work by Alexander [9] and Drummond [22]. Thanks to Alison Sheets, Carlos Castro, Yang Wang, the rest of the Movement Laboratory, and four anonymous reviewers for insightful comments.
References
- 1.Hreljac A. 1993. Preferred and energetically optimal gait transition speeds in human locomotion. Med. Sci. Sports Exerc. 25, 1158–1162 10.1249/00005768-199310000-00012 (doi:10.1249/00005768-199310000-00012) [DOI] [PubMed] [Google Scholar]
- 2.Margaria R. 1976. Biomechanics and energetics of muscular exercise. Oxford, UK: Clarendon Press [Google Scholar]
- 3.Thorstensson A, Robertson H. 1987. Adaptations to changing speed in human locomotion: speed of transition between walking and running. Acta Physiol. Scand. 131, 211–214 10.1111/j.1748-1716.1987.tb08228.x (doi:10.1111/j.1748-1716.1987.tb08228.x) [DOI] [PubMed] [Google Scholar]
- 4.van Ingen Schenau GJ. 1980. Some fundamental aspects of the biomechanics of overground versus treadmill locomotion. Med. Sci. Sports Exer. 12, 257–261 10.1249/00005768-198024000-00005 (doi:10.1249/00005768-198024000-00005) [DOI] [PubMed] [Google Scholar]
- 5.Mohler BJ, Thompson WB, Creem-Regehr SH, Pick HL, Warren WH. 2007. Visual flow influences gait transition speed and preferred walking speed. Exp. Brain Res. 181, 221–228 10.1007/s00221-007-0917-0 (doi:10.1007/s00221-007-0917-0) [DOI] [PubMed] [Google Scholar]
- 6.Geyer H, Seyfarth A, Blickhan R. 2006. Compliant leg behaviour explains basic dynamics of walking and running. Proc. R. Soc. B 273, 2861–2867 10.1098/rspb.2006.3637 (doi:10.1098/rspb.2006.3637) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Srinivasan M, Ruina A. 2006. Computer optimization of a minimal biped model discovers walking and running. Nature 439, 72–75 10.1038/nature04113 (doi:10.1038/nature04113) [DOI] [PubMed] [Google Scholar]
- 8.Srinivasan M. 2009. Optimal speeds for walking and running, and walking on a moving walkway. CHAOS 19, 026112. 10.1063/1.3141428 (doi:10.1063/1.3141428) [DOI] [PubMed] [Google Scholar]
- 9.Alexander RM. 1989. Optimization and gaits in the locomotion of vertebrates. Physiol. Rev. 69, 1199–1227 [DOI] [PubMed] [Google Scholar]
- 10.Minetti A, Alexander RM. 1997. A theory of metabolic costs for bipedal gaits. J. Theor. Biol. 186, 467–476 10.1006/jtbi.1997.0407 (doi:10.1006/jtbi.1997.0407) [DOI] [PubMed] [Google Scholar]
- 11.Srinivasan M. 2011. Fifteen observations on the structure of energy minimizing gaits in many simple biped models. J. R. Soc. Interface 8, 74–98 10.1098/rsif.2009.0544 (doi:10.1098/rsif.2009.0544) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bobbert AC. 1960. Energy expenditure in level and grade walking. J. Appl. Physiol. 15, 1015–1021 [Google Scholar]
- 13.Minetti AE, Ardigo LP, Saibene F. 1994. The transition between walking and running in humans: metabolic and mechanical aspects at different gradients. Acta Physiol. Scand. 150, 315–323 10.1111/j.1748-1716.1994.tb09692.x (doi:10.1111/j.1748-1716.1994.tb09692.x) [DOI] [PubMed] [Google Scholar]
- 14.Steudel-Numbers KL, Wall-Scheffler CM. 2009. Optimal running speed and the evolution of hominin hunting strategies. J. Human Evol. 56, 355–360 10.1016/j.jhevol.2008.11.002 (doi:10.1016/j.jhevol.2008.11.002) [DOI] [PubMed] [Google Scholar]
- 15.Walt WHVD, Wyndham CH. 1973. An equation for prediction of energy expenditure of walking and running. J. Appl. Physiol. 34 559–563 [DOI] [PubMed] [Google Scholar]
- 16.Terblanche E, Cloete WA, du Plessis PAL, Sadie JN, Strauss A, Unger M. 2003. The metabolic transition speed between backward walking and running. Eur. J. Appl. Physiol. 90, 520–525 10.1007/s00421-003-0890-7 (doi:10.1007/s00421-003-0890-7) [DOI] [PubMed] [Google Scholar]
- 17.Tseh W, Bennett J, Caputo JL, Morgan DW. 2002. Comparison between preferred and energetically optimal transition speeds in adolescents. Eur. J. Appl. Physiol. 88, 117–121 10.1007/s00421-002-0698-x (doi:10.1007/s00421-002-0698-x) [DOI] [PubMed] [Google Scholar]
- 18.Mercier J, Gallais DL, Durand M, Goudal C, Micallef JP, Préfaut C. 1994. Energy expenditure and cardiorespiratory responses at the transition between walking and running. Eur. J Appl. Physiol. Occup. Physiol. 69, 525–529 10.1007/BF00239870 (doi:10.1007/BF00239870) [DOI] [PubMed] [Google Scholar]
- 19.Noble B, Metz K, Pandolf KB, Bell CW, Cafarelli E, Sime WE. 1973. Perceived exertion during walking and running. II. Med. Sci. Sports 5, 116–120 [PubMed] [Google Scholar]
- 20.Diedrich FJ, Warren WH., Jr 1995. Why change gaits? dynamics of the walk–run transition. J. Exp. Psychol. Hum. Percept. Perform. 21, 183–202 10.1037/0096-1523.21.1.183 (doi:10.1037/0096-1523.21.1.183) [DOI] [PubMed] [Google Scholar]
- 21.Glass S, Dwyer GB. 2006. ACSM'S metabolic calculations handbook. Indianapolis, IN: American College of Sports Medicine [Google Scholar]
- 22.Drummond JE. 1986. The mathematics of scout's pace. Math. Gazette 70, 185–190 10.2307/3615673 (doi:10.2307/3615673) [DOI] [Google Scholar]
- 23.Rudin W. 1987. Real and complex analysis. New York, NY: McGraw-Hill [Google Scholar]
- 24.Usherwood JR, Bertram JEA. 2003. Gait transition cost in humans. Eur J. Appl. Physiol. 90, 647–650 10.1007/s00421-003-0980-6 (doi:10.1007/s00421-003-0980-6) [DOI] [PubMed] [Google Scholar]
- 25.Melby C, Scholl C, Edwards G, Bullough R. 1993. Effect of acute resistance exercise on postexercise energy expenditure and resting metabolic rate. J. Appl. Physiol. 75, 1847–1853 [DOI] [PubMed] [Google Scholar]
- 26.Caekenberghe IV, Smet KD, Segers V, Clercq DD. 2010. Overground vs. treadmill walk-to-run transition. Gait Posture 31, 420–428 10.1016/j.gaitpost.2010.01.011 (doi:10.1016/j.gaitpost.2010.01.011) [DOI] [PubMed] [Google Scholar]
- 27.Hoyt DF, Taylor CR. 1981. Gait and the energetics of locomotion in horses. Nature 292, 239–240 10.1038/292239a0 (doi:10.1038/292239a0) [DOI] [Google Scholar]
- 28.Pennycuick CJ. 1975. On the running of the gnu Connochaetes taurinus and other animals. J. Exp. Biol. 63, 775–799 [Google Scholar]
- 29.Farris DJ, Sawicki GS. 2012. Human medial gastrocnemius force–velocity behavior shifts with locomotion speed and gait. Proc. Natl Acad. Sci. USA 109, 977–982 10.1073/pnas.1107972109 (doi:10.1073/pnas.1107972109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hanna A, Abernethy B, Neal RJ, Burgess-Limerick R. 2000. Triggers for the transition between human walking and running. In Energetics of human activity (ed. Sparrow WA.), pp. 124–164 Champaign, IL: Human Kinetics [Google Scholar]
- 31.Hreljac A. 2008. The relationship between joint kinetic factors and the walk–run gait transition speed during human locomotion. J. Appl. Biomech. 24, 149–157 [DOI] [PubMed] [Google Scholar]
- 32.Neptune RR, Sasaki K. 2005. Ankle plantar flexor force production is an important determinant of the preferred walk-to-run transition speed. J. Exp. Biol. 208, 799–808 10.1242/jeb.01435 (doi:10.1242/jeb.01435) [DOI] [PubMed] [Google Scholar]
- 33.Prilutsky BI, Gregor RJ. 2001. Swing- and support-related muscle actions differentially trigger human walk–run and run–walk transitions. J. Exp. Biol. 204, 2277–2287 [DOI] [PubMed] [Google Scholar]
- 34.Raynor AJ, Yi CJ, Abernethy B, Jong QJ. 2002. Are transitions in human gait determined by mechanical, kinetic or energetic factors? Hum. Mov. Sci. 21, 785–805 10.1016/S0167-9457(02)00180-X (doi:10.1016/S0167-9457(02)00180-X) [DOI] [PubMed] [Google Scholar]
- 35.Usherwood JR. 2005. Why not walk faster? Biol. Lett. 1, 338–341 10.1098/rsbl.2005.0312 (doi:10.1098/rsbl.2005.0312) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weinstein RB, Full RJ. 1999. Intermittent locomotion increases endurance in a gecko. Physiol. Biochem. Zool. 72, 732–739 10.1086/316710 (doi:10.1086/316710) [DOI] [PubMed] [Google Scholar]
- 37.Bornstein MH, Bornstein HG. 1976. The pace of life. Nature 259, 557–558 10.1038/259557a0 (doi:10.1038/259557a0) [DOI] [Google Scholar]
- 38.Garland T. 1983. Scaling the ecological cost of transport to body mass in terrestrial mammals. Am. Nat. 571–587 10.1086/284084 (doi:10.1086/284084) [DOI] [Google Scholar]
- 39.Hastings J, Shapiro JM. 2012. Mental accounting and consumer choice: evidence from commodity price shocks. NBER Working Paper No. 18248. Cambridge, MA: National Bureau of Economic Research (http://www.nber.org/papers/w18248). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kuo AD. 2002. The relative roles of feedforward and feedback in the control of rhythmic movements. Motor Control 6, 129–145 [DOI] [PubMed] [Google Scholar]
- 41.Maffels C, Schutz YY, Schena F, Zaffanello M, Pinelli L. 1993. Energy expenditure during walking and running in obese and nonobese prepubertal children. J. Pediatr. 123, 193–199 10.1016/S0022-3476(05)81688-9 (doi:10.1016/S0022-3476(05)81688-9) [DOI] [PubMed] [Google Scholar]
- 42.Bailey RC, Olson J, Pepper SL, Porszasz J, Barstow TJ, Cooper DM. 1995. The level and tempo of children's physical activities: an observational study. Med. Sci. Sports Exerc. 27, 1033–1041 [DOI] [PubMed] [Google Scholar]
- 43.Blaszczyk J. 2001. Gait transitions during unrestrained locomotion in dogs. Equine Vet. J. 33, S33, 112–115 10.1111/j.2042-3306.2001.tb05372.x (doi:10.1111/j.2042-3306.2001.tb05372.x) [DOI] [PubMed] [Google Scholar]
- 44.Pontzer H. 2007. Predicting the energy cost of terrestrial locomotion: a test of the limb model in humans and quadrupeds. J. Exp. Biol. 210, 484–494 10.1242/jeb.02662 (doi:10.1242/jeb.02662) [DOI] [PubMed] [Google Scholar]
- 45.Dishman RK, Armstrong RB, Delp MD, Graham RE, Dunn AL. 1988. Open-field behavior is not related to treadmill performance in exercising rats. Physiol. Behav. 43, 541–546 10.1016/0031-9384(88)90206-5 (doi:10.1016/0031-9384(88)90206-5) [DOI] [PubMed] [Google Scholar]
- 46.Schmidt-Nielsen K. 1972. Locomotion: energy cost of swimming, flying, and running. Science 177, 222–228 10.1126/science.177.4045.222 (doi:10.1126/science.177.4045.222) [DOI] [PubMed] [Google Scholar]
- 47.Ainsworth BE, et al. 2000. Compendium of physical activities: an update of activity codes and met intensities. Med. Sci. Sports Exerc. 32(Suppl), S498–S516 [DOI] [PubMed] [Google Scholar]
- 48.Galloway J. 2010. Marathon: you can do it! Bolinas, CA: Shelter Publications [Google Scholar]
- 49.Liebenberg L. 2006. Persistence hunting by modern hunter–gatherers. Curr. Anthrop. 47, 1017–1025 10.1086/508695 (doi:10.1086/508695) [DOI] [Google Scholar]
- 50.Salvo VD, Baron R, Tschan H, Montero FJC, Bachl N, Pigozzi F. 2007. Performance characteristics according to playing position in elite soccer. Int. J. Sports Med. 28, 222–227 10.1055/s-2006-924294 (doi:10.1055/s-2006-924294) [DOI] [PubMed] [Google Scholar]
- 51.Lee J. 2009. Vehicle inertia impact on fuel consumption of conventional and hybrid electric vehicles using acceleration and coast driving strategy. PhD thesis, Virginia Polytechnic Institute and State University, Blacksburg [Google Scholar]
- 52.Ward-Smith AJ. 1984. Aerodynamic and energetic considerations relating to undulating and bounding flight in birds. J. Theor. Biol. 111, 407–417 10.1016/S0022-5193(84)80219-2 (doi:10.1016/S0022-5193(84)80219-2) [DOI] [Google Scholar]
- 53.Williams TM. 2001. Intermittent swimming by mammals: a strategy for increasing energetic efficiency during diving. Am. Zool. 41, 166–176 10.1668/0003-1569(2001)041[0166:ISBMAS]2.0.CO;2 (doi:10.1668/0003-1569(2001)041[0166:ISBMAS]2.0.CO;2) [DOI] [Google Scholar]