Abstract
Demand for energy is projected to increase at least twofold by mid-century relative to the present global consumption because of predicted population and economic growth. This demand could be met, in principle, from fossil energy resources, particularly coal. However, the cumulative nature of carbon dioxide (CO2) emissions demands that stabilizing the atmospheric CO2 levels to just twice their pre-anthropogenic values by mid-century will be extremely challenging, requiring invention, development and deployment of schemes for carbon-neutral energy production on a scale commensurate with, or larger than, the entire present-day energy supply from all sources combined. Among renewable and exploitable energy resources, nuclear fusion energy or solar energy are by far the largest. However, in both cases, technological breakthroughs are required with nuclear fusion being very difficult, if not impossible on the scale required. On the other hand, 1 h of sunlight falling on our planet is equivalent to all the energy consumed by humans in an entire year. If solar energy is to be a major primary energy source, then it must be stored and despatched on demand to the end user. An especially attractive approach is to store solar energy in the form of chemical bonds as occurs in natural photosynthesis. However, a technology is needed which has a year-round average conversion efficiency significantly higher than currently available by natural photosynthesis so as to reduce land-area requirements and to be independent of food production. Therefore, the scientific challenge is to construct an ‘artificial leaf’ able to efficiently capture and convert solar energy and then store it in the form of chemical bonds of a high-energy density fuel such as hydrogen while at the same time producing oxygen from water. Realistically, the efficiency target for such a technology must be 10 per cent or better. Here, we review the molecular details of the energy capturing reactions of natural photosynthesis, particularly the water-splitting reaction of photosystem II and the hydrogen-generating reaction of hydrogenases. We then follow on to describe how these two reactions are being mimicked in physico-chemical-based catalytic or electrocatalytic systems with the challenge of creating a large-scale robust and efficient artificial leaf technology.
Keywords: artificial leaf, hydrogenases, photosystem II, solar energy, solar fuels, water splitting
1. Introduction
Natural photosynthesis is the process by which sunlight is captured and converted into the energy of chemical bonds of organic molecules that are the building blocks of all living organisms and also of oil, gas and coal. These fossil fuels are the products of photosynthetic activity millions of years ago and provide us with most of the energy needed to power our technologies, heat our homes and produce the wide range of chemicals and materials that support everyday life. Sooner or later, the readily available reserves of fossil fuels will become scarce and then what? Even before then, as a consequence of our ever-growing use of oil, gas and coal, we are faced with the problem of increasing levels of CO2 and other greenhouse gases in the atmosphere with implications for global climate change.
The success of photosynthesis as an energy-generating and -storage system stems from the fact that the raw materials and power needed for the synthesis of biomass are available in almost unlimited amounts; sunlight, water and CO2. At the heart of the photosynthetic process is the splitting of water by sunlight into oxygen and hydrogen equivalents. The oxygen is released into the atmosphere where it is available for living organisms to breathe and for burning fuels to drive our technologies. The hydrogen equivalents are used to reduce CO2 to sugars and other organic molecules of various types. When we burn fuels (fossil, biomass and other biofuels) to release energy, we are simply combining the ‘hydrogen’ stored in these organic molecules with atmospheric oxygen to form water, so completing a cycle started millions of years ago. Similarly, energy is also released from the organic molecules which constitute our food, when they are metabolized within our bodies by the process of respiration. Thus, in the biological world, photosynthesis brings about the splitting of water into oxygen and ‘hydrogen’, whereas respiration is the reverse, combining oxygen and hydrogen in a carefully controlled and highly efficient way so as to create metabolic energy. Therefore, from an energetic view, the synthesis of organic molecules represents a way of storing hydrogen and therefore storing solar energy in the form of chemical bonds (figure 1).
Figure 1.
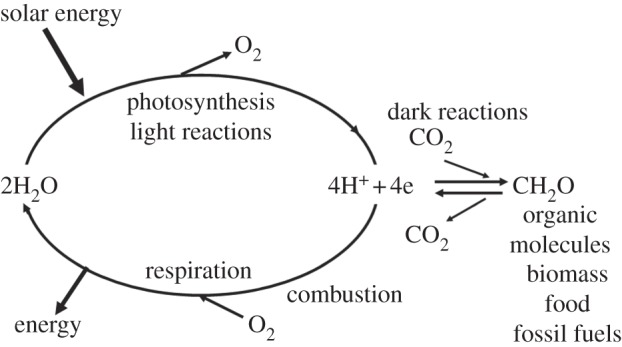
A diagrammatic representation of energy flow in biology. The light reactions of photosynthesis (light absorption, charge separation, water splitting, electron/proton transfer) provides the reducing equivalents in the form of energized electrons (e) and protons (H+) to convert carbon dioxide (CO2) into carbohydrates (CH2O) and other organic molecules which make up living organisms (biomass), including those that provide humankind with food. The same photosynthetic reactions gave rise to the fossil fuels formed millions of years ago. The burning of these organic molecules either by respiration (controlled oxidation within our bodies) or by combustion of biomass and fossil fuels to power our technologies, is the reverse to photosynthesis, releasing CO2 and combining the stored ‘hydrogen’ back with oxygen to form water. In so doing energy, which is originated from sunlight, is released.
In this study, we will briefly emphasize the enormity of the energy/CO2 problem that we face within the coming decades and discuss the contributions that could be made by fuels derived directly from developing new technologies based on the successful principles of natural photosynthesis. We will particularly emphasize the possibility of exploiting the vast amounts of solar energy available to split water to produce dioxygen and the hydrogen equivalents required to produce fuels such as alcohols and methane, and particularly the simplest of all solar fuels, hydrogen gas. This technology we will refer to as artificial photosynthesis.
2. Global energy consumption and the enormity of the problem
At the present time, the rate of global energy consumption is in the region of 16.3 TW [1], with the USA and the extended EU each representing about 40 per cent of this. In future, this global value will rise owing to industrialization in underdeveloped and developing countries coupled with increasing world population. Based on current projections, the global annual energy consumption rate will reach 20 TW or more by 2030, doubled by 2050 and tripled by the end of the century [2–4]. About 85 per cent of the total global energy consumed at present comes from burning fossil fuels with the proportion approaching 90 per cent for developed countries. Oil, gas and coal contribute approximately equally to this demand. The remaining sources of energy are hydroelectric, nuclear, biomass and renewable, such as solar, wind, tide and wave. At present, the use of biomass is a major player and is mainly localized in underdeveloped regions such as Africa and India where wood and other organic matter is used as a fuel. Much of this is not strictly renewable, because there is no planned regeneration and the trend is towards more use of fossil fuels.
The low level of contribution of non-fossil energy sources to present-day global energy demand reflects the readily available resources of oil, gas and coal. Even when oil reserves become limiting, there will remain large reservoirs of gas (including from shale) and, particularly, coal to exploit [5]. Therefore, in the global arena, the problem for the immediate future is not a limitation of fossil fuel reserves but the consequences of its combustion. If the total fossil fuel reserve is burnt, then the CO2 level in the atmosphere and oceans would rise to values equivalent to those that existed on our planet long before humankind evolved [6]. Despite this consideration, it is certain that fossil fuels will continue to be a major source of energy for humankind for some years to come but it is vital that they should be used in such a way as to minimize CO2 release into the atmosphere. Technologies for sequestration of CO2 must be developed [7]. Hand in hand with this, there will almost certainly be an improvement in the efficiency of energy use and supplementation whenever possible from non-fossil fuel sources. Against this background, we must also strive to develop new technologies based on principles that have yet to be revealed from basic studies and in particular those that focus on using the enormous amount of energy available to us as solar radiation [8]. The sun provides solar energy to our planet on an annual basis at a rate of 100 000 TW. Therefore, the energy from 1 h of sunlight is equivalent to all the energy humankind currently uses in a year. We do have existing technologies to capture sunlight and produce electricity and the efficiency and robustness of these photovoltaic systems is improving daily [9,10]. Compared with the present-day price of fossil fuels, photovoltaic systems represent an expensive way to generate electricity because of the high cost of their construction. In time, these costs will decrease relative to the cost of fossil fuel. Moreover, a blending of the principles of photovoltaic systems, especially those using cheap organic or inorganic materials, with concepts derived from natural photosynthetic systems may ultimately provide a long-term solution in the form of artificial photosynthesis technology [4,8]. In considering this long-term solution, let us take a look at the efficiency of the natural photosynthetic process.
3. Natural photosynthesis
3.1. Efficiency
As emphasized in figure 1, photosynthesis is a process that converts light energy into the organic molecules of biomass which is composed of mainly carbohydrates symbolized as CH2O. To estimate the efficiency of this process, two main factors must be appreciated.
(i) Although photosynthetic organisms can efficiently trap light energy at all wavelengths of visible solar radiation, the energy used for splitting water and reducing CO2 is only equivalent to the red region of the spectrum. Higher energy photons are degraded to heat by internal conversion within the light-harvesting pigments to the energy level of ‘red’ photons at about 1.8 eV.
(ii) For every electron/proton extracted from water and used to reduce CO2, the energy of two ‘red’ photons is required. This is accomplished by linking together, in series, two different photosystems, photosystem II (PSII), which uses light to power the extraction of electrons/protons from water, and photosystem I (PSI), which uses light to provide additional energy to the ‘PSII-energized’ electrons/protons so as to drive the CO2-fixation process (figure 2). Therefore, in photosynthesis, the energy of at least eight ‘red’ photons is required per O2 molecule released or CO2 molecule fixed. A typical product of carbon fixation is glucose (C6H12O6) whose energy content is 673 kcal per quantum mole (2805 kJ mole−1) when burnt in a calorimeter. To make a glucose molecule, the energy of 48 ‘red’ photons is required and assuming a wavelength of 680 nm, corresponding to 42 kcal per quantum mole (175 kJ mole−1), gives the efficiency of conversion at about 30 per cent. Although this is an impressive number, in reality, the overall conversion of solar energy to the glucose and the very large variety of other organic molecules that constitutes biomass, is much lower. Energy is lost through degrading shorter wavelength light (e.g. blue light) to the energy of ‘red’ photons, by saturation processes and more significantly, in driving the enormous number of reactions that occur in photosynthetic organisms to maintain their organization, metabolism, reproduction and survival. Taking these various factors into account, the estimated maximum efficiency of photosynthesis is about 4.5 per cent [11–15].
Figure 2.
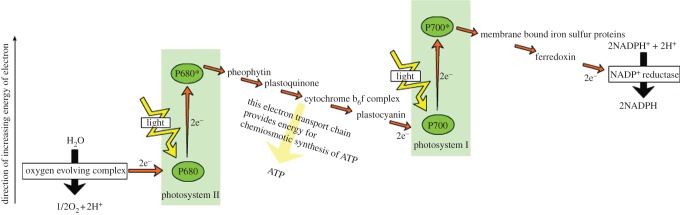
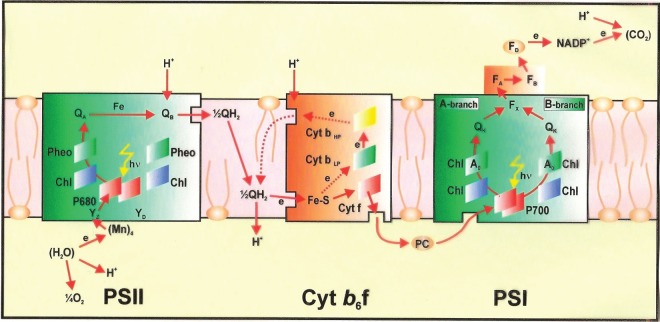
A simplified Z-scheme of the light reactions of photosynthesis taken from http://en.wikipedia.org/wiki/photosynthesis. For every electron extracted from water and transferred to CO2, the energy of two photons of light is required. One is absorbed by photosystem II (PSII) that generates a strong oxidizing species (P680+), able to drive the water-splitting reaction and a reduction of pheophytin (Pheo) and then plastoquionel (Q) to plastoquinol (QH2). The other photosystem, photosystem I (PSI) generates a strong reducing species, NADPH, which donates reducing equivalents to CO2 to produce sugars and other organic molecules, and a weak oxidant P700+. Electron and proton flow from QH2 to P700+ is aided by the cytochrome b6f (Cyt b6f) complex and plastocyanin (PC) and results in the release of energy to convert ADP to ATP. The ATP produced is required, along with NADPH, to convert CO2 to sugars. Because the production of O2 requires the splitting of two water molecules, the overall process involves the removal of two electrons per water molecule as shown and therefore four photons per PSII and PSI reaction centre. The reduction of oxidized nicotinamide adenine dinucleotide phosphate (NADP+) by PSI is facilitated by membrane bound iron sulfur proteins (Fx, FA and FB) and soluble ferredoxin (FD).
In fact, an efficiency of 4.5 per cent is rarely reached. Only in exceptional cases will dry matter yield exceed 1 or 2 per cent, such as with the intense growing of sugar cane in tropical climates or with optimized culturing of algae. Normally, agricultural crops produce yields of biomass at efficiencies less than 1 per cent, even when pampered with ample supplies of fertilizer and water. Environmental conditions, degree of light interception, nutrient and water supply are key factors in reducing the efficiency below the maximum, whereas specific genetic characteristics of particular plant species also dictate growth rates and maximum yields of biomass.
On a global basis, the efficiency of photosynthesis is significantly lower than for agricultural and energy crops or algal cultures growing under optimal conditions because of seasonal changes and the existence of large portions of land and oceans on our planet that do not sustain significant levels of photosynthetic activity [13]. Thus, the rate of energy storage averaged over a year by photosynthesis is 100 TW, representing just 0.1 per cent conversion given that solar energy arriving at our planet is at a rate of100 000 TW over the same time period. This energy is mainly stored in wood and fibres of terrestrial trees and plants. A similar amount of photosynthetic activity occurs in the oceans, but there the fixed carbon is rapidly recycled into the food chain [16]. Therefore, an approximate efficiency of global photosynthesis is 0.2 per cent but with only half being stored in biomass. Of course, it was terrestrial biomass that was the major source of energy for humankind prior to the exploitation of fossil fuels. It is not surprising, therefore, that there is now a growing interest in returning to the use of biomass and biofuels as an alternative to fossil fuels, because their production and use is CO2 neutral. However, the scale required for satisfying the current global energy requirement is far from attainable because of competing with large-scale food production and general land use needs to sustain a global population of seven billion.
Although it may be possible to engineer plants and other types of photosynthetic organisms (algae) as energy-converting ‘machines’ and ‘chemical factories’, the overall efficiency of solar energy conversion will rarely exceed 1 per cent and will usually be much less, so much so that this approach can make only a minor contribution to our future energy requirements. However, the efficiencies of the early photochemical and chemical reactions of photosynthesis, which are not directly involved in biomass production, are significantly higher. Because of this, there are alternative and complementary approaches for using solar energy. For example, it may be possible to develop a highly efficient, artificial, molecular-based, solar-energy-converting technology that exploits the principles of the ‘front-end’ of natural photosynthesis. Indeed, our knowledge of the natural process is such as to provide a blueprint for the design and assembly of such ‘artificial photosynthetic’ devices as described in the following sections.
3.2. Molecular processes
As emphasized earlier, photosynthesis has produced most of the energy that fuels human society and sustains life on our planet. The process is underpinned by the light-driven water-splitting reaction that occurs in PSII of plants, algae and cyanobacteria (figure 2). Solar energy is absorbed by chlorophyll and other pigments, and is transferred efficiently to the PSII reaction centre where charge separation takes place. This initial conversion of light energy into electrochemical potential occurs in the reaction centre of PSII with a maximum thermodynamic efficiency of about 70 per cent and generates a radical pair state P680•+Pheo•−, where P680 is a chlorophyll a molecule, and Pheo is a pheophytin a molecule (chlorophyll molecule without a Mg ion ligated into its tetrapyrrole head group). The redox potential of P680•+ is highly oxidizing, estimated to be about +1.2 V, while that of Pheo•− is about −0.5 V. The latter is sufficiently negative such that, in principle, it could drive the formation of hydrogen. Instead, the reducing equivalent is passed along an electron transport chain to PSI (figure 2), where it is excited by the energy of a second ‘red’ photon absorbed by a chlorophyll molecule, known as P700, to lift it to a reducing potential of −1 V or more. In this way, sufficient energy is accumulated to drive the fixation of CO2, which not only requires the generation of the reduced ‘hydrogen carrier’, nicotinamide adenine dinucleotide phosphate (NADPH), but also the energy-rich molecule adenosine triphosphate (ATP) formed by the release of some energy during electron transfer from PSII to PSI (in the form of an electrochemical potential gradient of protons; figures 2 and 3). The P680•+ species generated in PSII drives the splitting of water at the ‘water oxidizing centre’ (WOC). It does so by extracting electrons from a catalytic centre composed of a cluster of four manganese (Mn) ions and a calcium ion (Ca2+). The splitting of water into dioxygen and reducing equivalents is a four-electron process and therefore PSII must absorb four photons (4 hν) to drive this half-reaction with PSI also using 4 hν to give sufficient potential for subsequent reductive reactions. 2H2O + 4hv → O2+4H++ 4e−.
Figure 3.
Schematic of the electron–proton transport chain of oxygenic photosynthesis in the thylakoid membrane, showing how photosystem I (PSI) and photosystem II (PSII) work together to use absorbed light to oxidize water and reduce NADP+, in an alternative representation to the Z-scheme shown in figure 2. The diagram also shows how the vectorial flow of electrons across the membrane generates a proton gradient which is used to power the conversion of ADP to ATP at the ATP synthase complex (CFoCF1) which is also embedded in the thylakoid membrane (not shown). In both PSI and PSII, the redox-active cofactors are arranged around a pseudo-twofold axis. In PSII, primary charge separation and subsequent electron flow occurs along one branch of the reaction centre. However, in the case of PSI, electron flow occurs up both branches as shown. Electron flow through the cytochrome b6 f complex also involves a cyclic process known as the Q cycle. YZ = tyrosine; P680 = primary electron donor of PSII composed of chlorophyll (Chl); Pheo = pheophytin; QA and QB = plastoquinone; Cyt b6f = cytochrome b6f complex, consisting of an Fe–S Rieske centre, cytochrome f (Cyt f), cytochrome b low- and high-potential forms (Cyt bLP and Cyt bHP), plastoquinone binding sites, Q1 and Q0; PC = plastocyanin; P700 = primary electron Chl donor of PSI; A0 = Chl; A1[Q] = phylloquinone; Fx, FA and FB = Fe–S centres, FD = ferredoxin; FNR = ferredoxin NADP reductase; NADP+ = oxidized nicotinamide adenine dinucleotide phosphate. YD = symmetrically related tyrosine to Yz but not directly involved in water oxidation, and QH2 = reduced plastoquinone (plastoquinol), which acts as a mobile electron/proton carrier from PSII to the cytochrome b6f complex. With the exception of the mobile electron carriers Q/QH2, PC and FD, the remaining redox-active cofactors are bound to multisubunit protein complexes that span the membrane depicted as coloured boxes.
The reducing equivalents leave PSII in the form of plastoquinol (QH2), whereas the dioxygen is released into the atmosphere. 4H++ 4e−+ 2Q → 2QH2.
The efficiency of this reaction is high being almost 55 per cent when driven by the energy of ‘red’ photons but decreases to about 20 per cent when taking into account the fact that light is absorbed across the whole solar spectrum. Of course, in photosynthetic organisms, the reaction can proceed only continuously when the QH2 molecules are oxidized by the light absorbed by PSI, thus allowing reducing equivalents to be transferred to NADH+ and then to CO2. Because of this saturation effect, the quantum efficiency of photosynthetic water splitting is further decreased.
The light-driven transfer of electrons and protons from H2O to CO2 involves a number of redox-active cofactors located in the PSII and PSI protein complexes (see figure 3 and its legend for specific details). The transfer of reducing equivalents between PSII and PSI is aided by a third membrane protein complex known as cytochrome b6f (Cyt b6f) as detailed in figure 3. The three complexes, PSI, PSII and Cyt b6f, are located in the photosynthetic membrane such that electron flow from water to NADP+ is vectorial, leading to the generation of a proton gradient across the membrane (figure 3). This gradient is used chemiosmotically by a fourth complex, CF0–CF1, to drive its ATP synthase activity to convert ADP to ATP and thus provide chemical energy for the CO2-reduction process (for details, see reference [17]).
In many ways, the photosystems of photosynthesis, including those of anoxygenic photosynthetic bacteria (organisms that do not split water) are highly efficient molecular photovoltaic nanomachines in that they use light energy to bring about electrical charge separation across a membrane of high dielectric strength [18]. The organization of the electron carriers and other cofactors in these nanomolecular devices are optimized to facilitate forward energy-storing reactions and minimizing backward and wasteful-energy-releasing reactions. There is considerable information about these photosystems which indicates that they are structurally and functionally very similar [19,20]. Indeed, there are aspects of their design that provide a blue print for constructing ‘artificial photosynthetic’ systems.
Similarly, the light-harvesting systems associated with the photosystems of different types of photosynthetic organisms have common principles for capturing solar energy across the whole of the visible spectrum and facilitating efficient energy transfer to the associated reaction centres with minimum loss of energy. Again, detailed spectroscopic and structural studies have revealed the molecular basis of these systems, details that could also be adopted for designing light-concentrating systems for a new generation of solar-energy-converting technologies [21].
However, it is the water-splitting reaction of PSII that holds the greatest promise for the development new technologies for converting solar radiation into usable energy, particularly, in generating hydrogen equivalents for reducing CO2. In this way, PSII is unique when compared with all other types of photosystems that are far more limited in the redox chemistry they catalyse.
3.3. Photosystem II
The photosynthetic water-splitting reaction appeared on our planet about 2.5 billion years ago and was the ‘big bang of evolution’ since for the first time living organisms had available an inexhaustible supply of ‘hydrogen’ (in the form of hydrogen equivalents) to convert CO2 into organic molecules. From that moment, living organisms on the Earth could prosper and diversify on an enormous scale; biology had solved its energy problem and PSII established itself as the ‘engine of life’ [22].
Clearly, using solar energy to split water to produce hydrogen equivalents or ‘high energy’ electrons is also the perfect solution for humankind. In principle, the technology exists today to do this. Electricity can be generated by photovoltaic solar cells and used to carry out the electrolysis of water. With a solar cell efficiency of 10 per cent and 65 per cent efficiency for the electrolytic system, the overall efficiency would be 6.5 per cent. Electrolysis relies on platinum or other catalysts for gas evolution, which are in limiting supply and therefore expensive. At present very little hydrogen is produced by electrolysis because it is more economical to generate from fossil fuels. Similarly, the cost of photovoltaic solar cells marginalizes this route for using solar energy to produce hydrogen directly from water. The challenge is to devise a water-splitting catalyst that is robust and composed of abundant non-toxic materials that work along similar chemical principles to those used by the WOC of PSII.
Because of the importance of understanding the chemistry of the water-splitting reaction of PSII, there has been a wide range of techniques applied to probe the molecular mechanisms involved and to investigate the structure of the catalytic centre (see various articles in references [23,24]), being particularly spurred by the recent structural analyses of PSII by X-ray absorption spectroscopy [25–27] and X-ray crystallography [28–32]. These studies, coupled with quantum mechanical analyses, have provided a refinement of the structure of the WOC [33–35] and given detailed schemes for the water-splitting chemistry leading to O–O bond formation [36–43].
It is now clear that the water-splitting reaction takes place at a catalytic centre consisting of three Mn ions and a Ca ion forming a cubane-like structure with the four metal ions linked by oxo-bridges. A fourth Mn ion is linked to the cubane by two oxo-bridges and is adjacent to the Ca ion. This general arrangement was first suggested by Ferreira et al. [30] and recently confirmed, refined and improved in a 1.9 Å X-ray derived model [32] (figure 4a). Surrounding the Mn4Ca-cluster are a number of amino acid residues that either provide ligands to the metal ions or act to facilitate hydrogen bonding networks that almost certainly play a key role in the deprotonation of the substrate water molecules (figure 4b).
Figure 4.
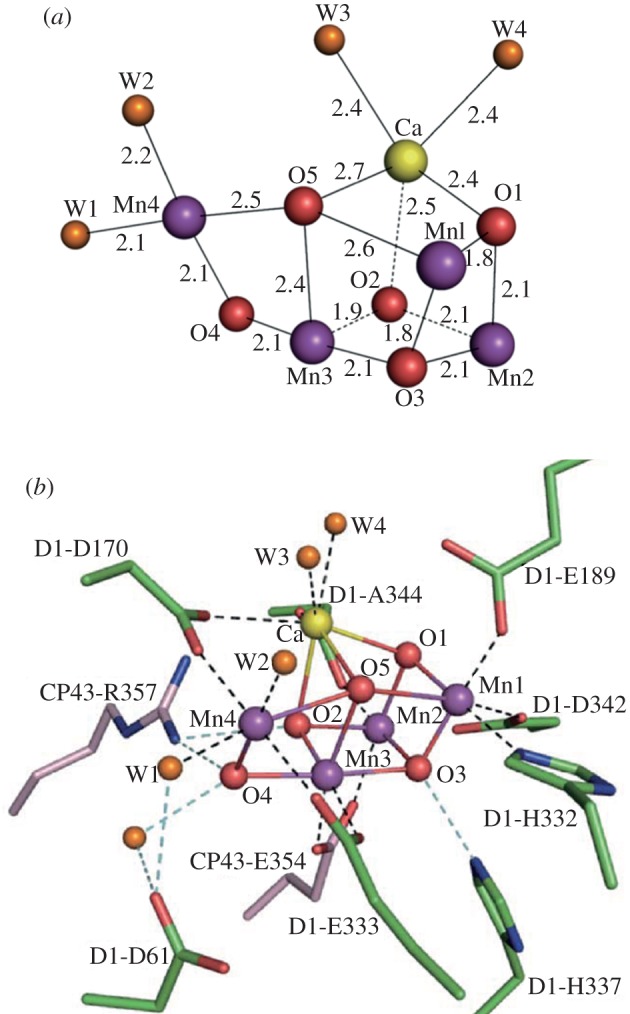
(a) Structure of the Mn4CaO5 cluster and (b) its ligand environment as determined at a resolution of 1.9 Å by Umena et al. [32].
With structural information available, realistic chemical schemes are now being formulated for the water-splitting reaction and the formation of molecular oxygen. It has been known, for some time, that there are at least five intermediate states leading to the formation of dioxygen, known as S-states [44].
The sequential advancement from S0 to S4 is driven by each photochemical turnover. The progression through the S-states to S4 results in the storing of four oxidizing equivalents, which are reduced in the final step (S4–S0) by four electrons derived from two substrate water molecules with the concomitant formation of dioxygen.
Although the geometry of the Mn4Ca-cluster and its exact ligand field characteristics are now known at a high resolution, there has been concern that it represents a more reduced form of the cluster than that of S0 because free electrons are generated during the collection of the X-ray diffraction data. This concern has been countered by recent studies [45–48]. Despite some uncertainties about the exact S-state represented by the high-resolution model, it is providing a basis for developing chemical mechanisms for the water oxidation and dioxygen formation. The Mn ion linked to the cubane structure (Mn4) is immediately adjacent to the Ca2+ and their positioning towards the side chains of several key amino acids, including the redox-active YZ, suggests that they provide the ‘catalytic’ surface for binding the substrate water molecules and their subsequent oxidation. Indeed, the 1.9 Å structure has revealed two water molecules bound to each of the ions. In fact, they are the only water molecules directly ligated to the metal cluster. One well-championed mechanism suggests that the substrate water, associated with Mn4, is deprotonated during the S-state cycle and converted into a highly electrophilic oxo (figure 5a) [36–40]. This mechanism is dependent on Mn4 being converted to a high oxidation state (possible Mn(V)) during progression to the S4-state just prior to O–O bond formation. The other three Mn ions are progressively driven into high valency states (Mn(IV)) and act as a further ‘oxidizing battery’ for the Mn(V)-oxo species in the S4-state. In this way, the reactive oxo is electron deficient, so much so that it makes an ideal target for a nucleophilic attack by the oxygen of the second substrate water bound within the coordination sphere of the Ca2+ (figure 5a).
Figure 5.
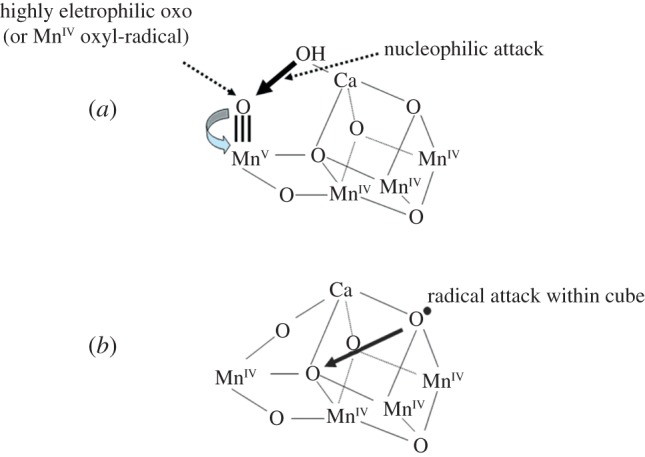
Two different mechanisms for the final step of the S-state cycle when the dioxygen bond of O2 is formed. (a) Mechanism 1. The very high oxidation state of the Mn-cluster, particularly the Mn ion outside the Mn3CaO4-cubane, leads to a high electron deficient oxo (after deprotonation of water molecules during the S-state cycle). Nucleophilic attack by the hydroxide of the second substrate water within the coordination sphere of Ca2+ leads to O2 formation. (b) Mechanism 2. The formation of an oxo-radical within the Mn3CaO4-cubane attacks a bridging oxo species to form the O–O bond. (Online version in colour.)
An alternative mechanism proposed by Siegbahn, which is based on in depth DFT calculations, suggests that an oxo-radical forms within the Mn3CaO4-cubane and attacks a bridging oxo species to form the O–O bond (figure 5b) [41–43]. The DFT calculations did not support the alternative mechanism shown in figure 5a, and experimental support has recently emerged in favour of the Siegbahn mechanism [49,50].
3.4. Hydrogenases
Many microorganisms, including some which have photosynthetic activity, have the ability to either extract electrons from hydrogen molecules to power their metabolism or remove excess low-potential electrons by reducing protons and releasing hydrogen. This microbial inter-conversion between hydrogen consumption and release is efficiently mediated by metalloenzymes, named hydrogenases. These hydrogenases are divided into two main classes: [NiFe]- and [FeFe]-hydrogenases, based on the chemical composition of their catalytic sites [51–53]. A third class, [Fe]-hydrogenases, catalyses the reversible reduction of methenyltetrahydromethanopterin with hydrogen to methylenetetrahydromethanopterin and protons [54].
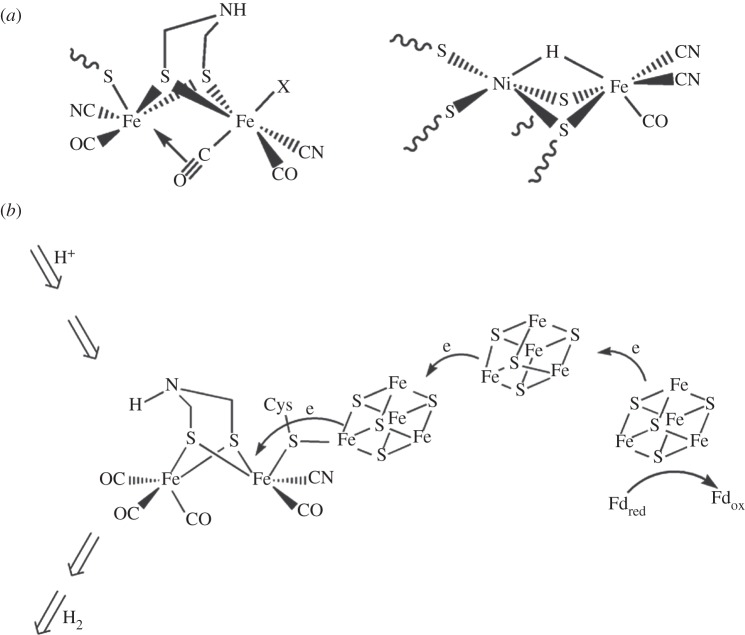
The catalytic active sites of the [NiFe]- and [FeFe]-hydrogenases consist of a bimetallic centre whose coordination is provided by residual cysteine, dithiolate and surprisingly, CO and CN− ligands where the latter pair are unusual in biology (figure 6a) [51–53]. These active sites are buried within the specific protein environments that function to tune the catalytic activity. The transfer of protons and molecular hydrogen is facilitated by a specific proton transfer pathway and by gas channels, whereas the [FeS] clusters ensure the transfer of electrons between ferredoxin, the redox mediator bound to the protein surface and the catalytic site (figure 6b) [55]. As shown by electrochemical studies, hydrogenases are as electrocatalytically active as platinum nanoparticles are for hydrogen evolution and uptake [56].
Figure 6.
Catalytic active sites of [FeFe]- and [NiFe]-hydrogenases (a); schematized structure and function of [FeFe]-hydrogenase for hydrogen evolution reaction (b). Schematized electron, proton and hydrogen transfer pathways are included.
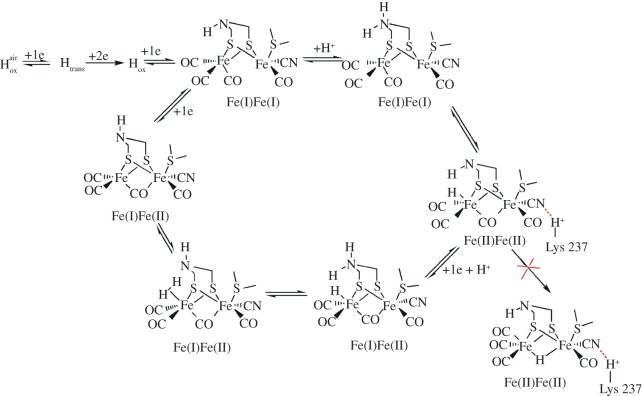
A simplified mechanism for the proton-reduction reaction in [FeFe]-hydrogenase is given in figure 7 [57–59]. The first step is thought to be the protonation of the azapropanedithiolate ligand that lowers the redox potential of the catalytic centre, commonly referred to as H-cluster. Reduction of this protonated system results in the formation of a highly active terminal hydride that rapidly generates hydrogen gas via a heterolysis mechanism once in contact with a proton. The specific protein environment is believed to place constraints on the H-cluster to adopt the CO-bridged conformation and avoid the tautomerization of terminal hydride into the more thermodynamically stable bridged hydride isomer [60]. This could well be the main reason for the impressive catalytic activities of [FeFe]-hydrogenase for hydrogen evolution with TOF up to 9000 s−1 [61].
Figure 7.
Proposed activation of [FeFe]-hydrogenase and catalytic cycle for hydrogen evolution reaction [57–59].
In the case of [NiFe]-hydrogenases, the protonation site could be sulfur atoms of cysteine ligands, either bridged or terminal, and the redox platform was proposed to be located on the NiII centre rather than on FeII. Details of the proposed mechanistic cycle for proton reduction in this enzyme can be found in recent reviews [55,57,58,62].
The hydrogenases have been investigated as an alternative to platinum in an electrolyser and in a proton exchange membrane fuel cell [63]. However, scaling up the application of hydrogenases for catalysis technology is not practical given its oxygen sensitivity, the requirement for large-scale cultivation of organisms and difficulties in isolation of active enzyme and the overall lack of robustness for long-term operation. Nevertheless, because hydrogenases are highly catalytically active and use metals that are abundant in the Earth's crust (Fe and Ni), there are considerable efforts to mimic these enzymes by the synthesis of molecular catalysts. Indeed, considerable progress has been made with this challenge and several functional organometallic catalysts containing Ni, Fe and/or Co have been reported that show structural and/or functional features similar to those of the hydrogenases catalytic sites [64–67]. Some of these have been integrated within photo-activated systems for hydrogen generation [65–67]. We will discuss these successes in more detail in §4.
4. Artificial photosynthesis
While some progress has been made in mimicking photosynthesis in artificial systems, researchers have not yet developed components that are both efficient and robust for incorporation into a working system for capturing and storing solar energy in chemical bonds on a large scale as does natural photosynthesis. To date, the main focus has been to design and synthesize electrocatalysts that can be linked to a light-driven charge separation system [68]. Dyes have been used for the latter, but inorganic semiconductors offer a more realistic and robust approach for providing the oxidizing and/or reducing potentials necessary to split water and power reductive chemistry. Indeed, rational engineering of semiconductors to efficiently capture and stabilize the energy of solar radiation for driving multi-electron chemistry is currently a great challenge of material sciences.
In the following sections, we first discuss the design and use of semiconductors as a simple photocatalyst for water-splitting and hydrogen-generation processes. Hydrogen can be used directly as a fuel but also used to reduce CO2 to formic acid or carbon monoxide as precursors for higher molecular weight carbon compounds as discussed by Benson et al. [69]. Although the generation of high-energy carbon containing fuels, such as methanol is desirable, the multi-electron nature of the CO2-reduction process adds additional complexity compared with that for hydrogen production [68] and in this study, we restrict ourselves to latter fuel. In so doing, we discuss how an electrocatalyst could be linked to a semiconductor to enhance or promote the chemical reactions on the semiconductor surface. We end by considering the possibilities of integrating separated components (photocatalyst/photoelectrode) into a complete device that can split water into oxygen and hydrogen using only photon energy. Even though there have been demonstration systems, there remains the challenge of scaling up to a meaningful levels and reaching conversion efficiencies of at least 10 per cent.
4.1. Semiconductor: simple artificial photocatalyst
Semiconductors with the appropriate electronic properties can capture solar photons by charge separation between their valence and conduction bands, thus generating the power required for driving chemical reactions at their surfaces. In this way, a semiconductor functions in a similar manner to the reaction centre of natural photosynthesis (figure 2 and figure 8a). There are some semiconductors that on illumination can provide sufficient electrochemical potential to drive the water oxidation and/or proton-reduction reactions without the requirement of an electrocatalyst. Moreover, being inorganic materials means that they are usually photochemically stable that make them good candidates for developing robust technology for large-scale solar fuel production.
Figure 8.
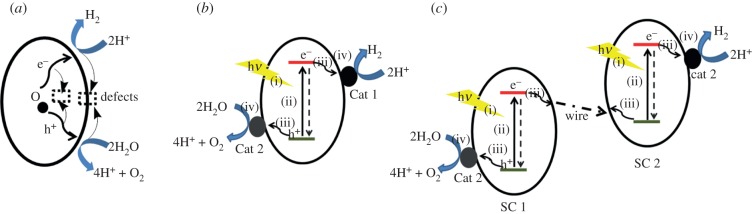
Schematic of how semiconducting materials can be used as photocatalysts for water oxidation and hydrogen generation. Large band gap semiconductors can be used without (a) or with electrocatalysts Cat1 and Cat2 (b). Two narrow band gap semiconductors could be wired in a Z-scheme tandem configuration (c).
Since the pioneering work reported by Fujishima & Honda [70] using a TiO2 photoanode for water splitting, several families of semiconductor have been investigated. They include metal oxides (Cu2O, TiO2, Fe2O3, WO3, BiVO4), metal sulfides (CdS, CdZnS) and chalcopyrites (CuInS, CuGaS) [71,72]. Large band gap semiconductors (more than 3 eV) such as TiO2 and graphitic carbon nitride g-C3N4 can be suitable for driving the overall water-splitting process. However, these materials absorb only UV and near-to-UV light, which is a small portion of the solar spectrum at the Earth's surface. Considerable efforts are currently underway to extend the absorption of these materials to the visible light region. Elemental doping, rational nanostructuring and surface functionalization are among the strategies to induce appropriate absorption shifts to longer wavelengths [73–75].
Narrow band gap materials such as Fe2O3 (2.2 eV) and Cu2O (2.0–2.2 eV) match well with the absorption of visible light. However, the energies of their valence and conduction bands are usually not appropriate for driving the overall water-splitting process to generate both molecular O2 and H2 at the same time. Some of them, for example α-Fe2O3, have their conduction band energies lying at a more positive potential than the reduction potential required to convert protons to hydrogen, whereas others, e.g. Cu2O, have the energy of their valence bands positioned at more negative potentials than required for water oxidation. Consequently, these materials can be used only for the half-reaction: either photo-driven water oxidation or hydrogen evolution. To construct a complete system for the overall water-splitting process, the two matching narrow band gap materials must be wired in a tandem configuration (figure 8c) akin to PSII and PSI in the Z-scheme (figure 2). In this case, as with natural photosynthesis, the energy of two photons is required for each overall electron transfer, thus decreasing the efficiency of the charge separation reaction by 50 per cent. Another issue concerning narrow band gap semiconductors being studied at the moment is their photochemical instability. Nevertheless, rational nanostructuring is proving to be a valuable approach to overcome the stability problem [76,77].
It is worth mentioning that charge recombination within, and on the surface, of semiconductors can often be a key factor controlling the photo-to-energy conversion efficiency. In the reaction centres of natural photosynthesis, the recombination reactions are minimized by subsequent rapid secondary electron transfer steps. This concept of optimization of charge separation has also been successfully applied in semiconductor engineering. Charge separation within a semiconductor can be enhanced by combining with another appropriate semiconductor in a pn junction system [76] or interfacing with an electron acceptor, e.g. graphene, carbon nanotubes [77,78]. Enhancing charge separation efficiency can be addressed by dealing with the intrinsic conductivity of a semiconductor by elemental doping [79,80] or by effacing the semiconductor surface defects by applying a thin oxide layer [81–83].
To accelerate chemical reactions on the semiconductor surface, loading of an oxygen-evolving catalyst (OEC) and a catalyst for hydrogen evolution reaction (HER) is often beneficial (figure 8b,c). In these hybrid systems, the OEC and HER catalysts extract generated holes (oxidizing equivalent) and electrons (reducing equivalents) from the semiconductor and subsequently drive the water oxidation and the HER on its surface. In §4.2, we will discuss how catalysts such as enzymes and their biomimetic synthetic equivalents have been linked to semiconductors for engineering hybrid photocatalysts.
4.2. Hybrid photocatalysts for water oxidation and oxygen evolution
A dinuclear ruthenium complex (the blue dimer) was the first example of a molecular catalyst that could electrochemically split water into O2, protons and electrons [84]. Since then, considerable efforts have been focused on designing appropriate organic ligands to improve the activity and stability of related Ru-based catalysts. The most striking success was recently reported by Sun and co-workers [85], who synthesized a super Ru-complex that catalyses the oxygen evolution reaction at a rate comparable with that of PSII.
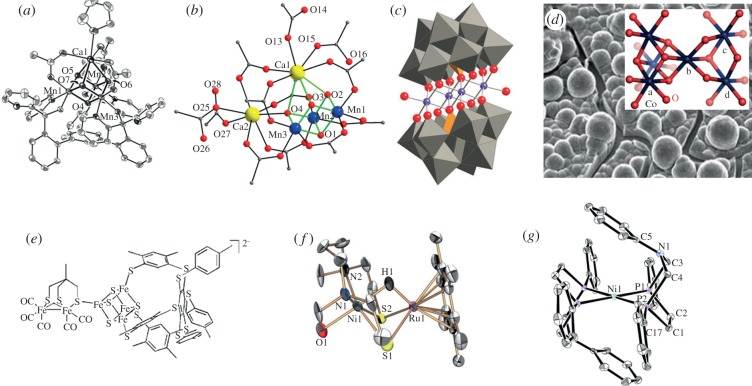
Given that ruthenium is not an abundant metal, attention has been focused on the design and synthesis of water-splitting catalysts composed of readily available elements such as Mn, Co, Fe [66,67,86]. Remarkable advances were recently achieved by Agapie and co-worker [87] and Christou and co-worker [88] in the synthesis of Mn3CaO4-clusters (figure 9a,b) geometrically very closely resembling the Mn4Ca-cluster of PSII. These PSII-mimics have yet to be investigated for any catalytic activity and almost certainly will require further modification of their coordination spheres to give stability and facilitate efficient water-splitting properties. Indeed, stability under catalytic functional conditions, at high oxidative potential, is a critical issue for all known organometallic water oxidation catalysts. To address this issue, one possible solution is to use all-inorganic catalysts that do not contain any fragile organic ligands in their structures. For example, Hill and co-workers [89] demonstrated the possibility of using polyoxometallate ligands to stabilize a O–O bond forming catalytic centre composed of a cobalt oxide core (Co4O4; figure 9c). Cobalt- and nickel-based systems, together with inorganic anionic ligands acting as good proton transfer agents, have been developed by Nocera and co-workers [90,94] and demonstrated to be very impressive catalysts for water oxidation. The cobalt-oxide-phosphate catalyst they discovered (CoPi) functions in a neutral pH solution with an overpotential of only ca 400 mV (figure 9d). Interestingly, this solid-state catalyst is made up of cubic structures with oxo-bridging, therefore showing structural similarities to the geometry of the Mn4Ca-cluster of PSII [95,96]. A very important property of this CoPi catalyst is its ability to self-repair which is reminiscent of the property of PSII to do the same [97].
Figure 9.
Selected synthetic electrocatalysts that mimic the [Mn4Ca]-cluster and the active sites of hydrogenases. Synthetic [Mn3CaO4]-clusters designed by Agapie and co-workers (a) [87] and Christou and co-workers (b) [88]; Co4O16 core stabilized within [PW9O34] ligand synthesized by Hill and co-workers (c) [89]; Nocera CoPi solid catalyst and its proposed atomic structure (d) [90]; synthetic [FeFe] and [NiFe] models designed by Pickett and co-workers [91] and Ogo and co-workers [92] (e) and (f); bioinspired model designed by Dubois and co-workers (g) [93].
Taking a different approach, Frei & Jiao [98,99] have demonstrated that a silica scaffold is a good support for stabilizing and activating cobalt-oxide or manganese-oxide nanoparticles for water-splitting activity.
Immobilizing an OEC onto an n-type semiconductor surface to engineer a photoanode or a photocatalyst for water splitting can be accomplished by adopting several different strategies. Organometallic molecular catalysts can be simply absorbed via a physical process [100,101] or covalently grafted via a robust chemical linker (figure 10a) [102,103]. The latter approach usually requires a complicated and expensive synthesis to introduce appropriate grafting anchors to the catalyst [108]. However, such systems are likely to be more robust compared with those relying on physical adsorption, thanks to the stabilizing effect of having a linker present.
Figure 10.
Selected hybrid photocatalysts/photoelectrodes engineered by assembling an OEC or a HER catalyst with a semiconductor. Ir-molecular OEC catalyst covalently grafted onto a dye-sensitized TiO2 electrode (a) [102,103]; Co3O4 OEC within α-Fe2O3 nanowires photoanode (b) [104]; immobilization of a [NiFeSe]-hydrogenase onto dye-sensitized TiO2 nanoparticles (c) [105]; immobilization of a synthetic FeFe-molecular HER catalyst within a mesoporous InP electrode (d) [106]; Si/MoS2 photocathode (e) [107].
In the case of inorganic-based OEC catalysts, physical adsorption onto the semiconductor surface can be very satisfactory. For example, by taking this approach, it was reported that spinel Co3O4 nanoparticles were homogeneously incorporated within a mesoporous g-C3N4 matrix resulting in an efficient Co3O4/g-C3N4 photocatalyst [109]. Alternatively, spinel Co3O4 catalyst can be directly co-grown with the α-Fe2O3 nanowires via a hydrothermal process (figure 10b) [104]. However, electrodeposition and photo-assisted electrodeposition seem to be a better approach to introduce cobalt-oxide-based catalysts onto the surface of photoanodes such as Fe2O3, ZnO, WO3 [110–112]. There is evidence that the photo-assisted method provides a well-controlled deposition of CoPi for the construction of a photoanode. For example, a better performance was achieved for a α-Fe2O3/CoPi when using photo-assisted electrodeposition compared with a α-Fe2O3/CoPi electrodeposition [110].
Despite successes with cobalt-oxide-based catalysts deposited in nanoparticles form, they suffer from not having a robust linker to the semiconductor surface. Thus, during catalytic turnover with the production of O2 bubbles at the surface, detachment of these catalysts can occur over time. Introducing a covalent linker, as in the case of molecular organo-metallic catalysts, is unlikely to be a credible solution because chemical functionalization changes the surface properties of nanoparticles and therefore likely to change their catalytic activities. Therefore, a system that is self-repairing under functional conditions is ideal as in the case of the Nocera CoPi OEC catalyst.
4.3. Hybrid photocatalysts for proton reduction and hydrogen evolution
An example of integrating isolated natural hydrogenase within a photocatalytic system was demonstrated by Armstrong and co-workers [105] when they immobilized a [NiFeSe]-hydrogenase from Desulfomicrobium baculatum on to the surface of Ru-dye-sensitized TiO2 nanoparticles (figure 10c). This photocatalyst produced H2 when illuminated with visible light in the presence of a sacrificial electron donor. However, owing to the large geometric size of hydrogenases, the level of loading onto the semiconductor surface was low, thus limiting the efficiency of this approach. Moreover, as mentioned earlier, the instability of this class of isolated enzymes and their sensitivity to oxygen, makes this biological approach unlikely for technological advancement. In addition, the requirement for large quantities of isolated enzyme is not conducive for a technology to produce solar fuel at worthwhile levels.
A possible solution is to use small and more robust molecular catalysts (figure 9e–g) [91–93] that mimic the catalytic active site of the hydrogenases and hybridize them with a p-type semiconductor. As in the case of molecular OEC, the hybridization could be accomplished by either a non-covalent or a covalent grafting. The first demonstration of this approach was reported by Nann et al. [106] when they adsorbed a Fe2S2(CO)6, the simplest mimicking equivalent of the [FeFe]-hydrogenase, with a mesoporous p-type InP photoelectrode (figure 10d). Cobaloxime, a bioinspired equivalent of hydrogenases, was also successfully integrated with an organic dye-sensitized-NiO or a Ru-dye-sensitized TiO2 electrode via either non-covalent [113] or covalent grafting [114]. However, these photocathodes showed moderate efficiency; typical photocurrents of only a few microamperes were obtained. Several reasons can explain this low photon-to-energy conversion efficiency, including moderate photo-to-current conversion of a dye-sensitized electrode interfacing with an aqueous solution,1 low overpotential provided by the conduction band of TiO2 and low electrocatalytic activities of FeFe or cobaloxime catalyst in aqueous solution, especially in weak acidic conditions.
Therefore, further efforts are required to address the low activities of synthetic molecular HER catalysts in aqueous, near-to-neutral pH solution. Covalently grafting these molecular catalysts onto a visible light-absorbing p-type semiconductor, such as Cu2O or CuInS(Se) could be of interest, because these semiconductors provide large reductive potential (thanks to their favourable conduction band energies).
While using hydrogenases and their mimics are still in the very early stages of proof-of-concept, all-inorganic HER electrocatalysts seem to be better candidates for interfacing with appropriate semiconductors. Nanoclusters of Ni, Co and their alloys with molybdenum were demonstrated to be potential electrocatalysts for HER [116,117]. Interfacing NiMo alloy nanoparticles with Si microarrays resulted in an effective photocathode that produced impressive photocurrents in the region of 15 mA cm−2 at 0 V versus reversible hydrogen electrode (RHE) powered by one Sun illumination [118]. Recently, NiMoZn was used for construction of the Si triple junction artificial leaf (see §4.4 and figure 11c) [121]. The chemical dissolution of Mo in an alkaline solution resulted in production of highly porous and thus, highly active NiMoZn catalyst.
Figure 11.
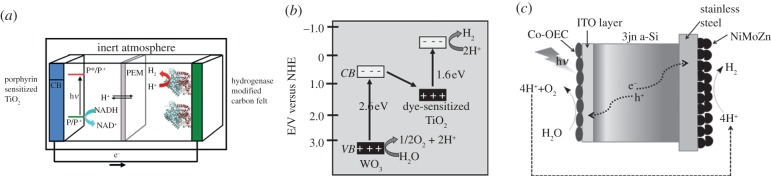
Selected complete devices for the overall water-splitting process. A PEC consisted of a dye-sensitized TiO2 photoanode and a hydrogenase cathode (a) [119]; a PEC with two photoelectrodes in the tandem configuration (b) [120]; the artificial leaf constructed from an amorphous Si triple junction solar cell and appropriate OEC and HER catalysts (c) [121,122].
Recently, MoS2 has emerged as one of the most promising noble-metal-free electrocatalysts for HER. This catalyst functions over a wide range of pH solutions (pH 0–13) with low over-potential requirement (ca 100–200 mV) [123,124]. It can be introduced to a semiconductor surface via a thermal deposition [125], electrodeposition [126] or a photo-assisted deposition process [107]. On a Si nanowire electrode or on CdS nanopowder surface, MoS2 is as efficient as Pt nanoparticles for hydrogen photogeneration (figure 10e) [107,125]. Moreover, MoS2 is chemically and photochemically stable, thus making the engineering of a robust photocatalyst/photoelectrode feasible.
4.4. Constructing a complete photocatalytic system for oxygen and hydrogen generation from water
The ultimate goal of artificial photosynthesis research is to construct a viable photocatalyst or photocatalytic system that can convert solar irradiation into hydrogen via the water-splitting process without the requirement of any external bias. To date, only a few examples of such systems have been reported and all still function with low solar-to-fuel conversion efficiency. These systems can be divided into three main classes: (i) suspended nanopowder photocatalysts, (ii) photoelectrochemical cells (PECs), and (iii) photovoltaic cell-driven electrolysers.
(i) As mentioned above, some large band gap semiconductors such as TiO2 or g-C3N4 can split water into oxygen and hydrogen under UV or near-to-UV illumination without requirement of any electrocatalyst. Introducing an OEC and/or HER catalyst on the surface of these semiconductors enhances their photocatalytic activities. These electrocatalysts act as traps for electrons and holes as well as enhancing the rates of the chemical reactions. However, co-loading of two electrocatalysts on the same semiconductor is technically challenging. Moreover, almost all types of HER catalysts, e.g. hydrogenase, synthetic molecular organo-metallic and inorganic catalysts, are not usually stable under high oxidative potentials. By contrast, almost all OECs, such as CoPi, are not stable under high reductive potential. This stability issue can be addressed by using noble metal oxide catalysts that are robust under both reductive and oxidative conditions such as Rh2−yCryO3 and RuO2 [127,128]. However, the noble metal oxides are not abundant and therefore are expensive. An alternative approach has recently emerged involving the use of single metal oxide catalysts. Domen and co-workers [129] reported that a NiOx-loaded-SrTiO3 acted as an artificial photocatalyst for the overall water-splitting process producing both oxygen and hydrogen on illumination. Recent investigations revealed that some of NiOx is reduced by electrons generated in the conduction band of SrTiO2 resulting in Ni0 clusters [117]. Therefore, the NiOx/SrTiO3 system was converted in situ into NiOx/SrTiO3/Ni nanoscale ‘artificial leaf’ in which Ni acts as a HER catalyst, whereas NiOx acts as an OEC. A similar inter-conversion between OEC and HER activities governed by oxidizing or reducing potential could also allow a cobalt-oxide-phosphate system to function in a single device [130].
Another approach is to use oxygen- and hydrogen-producing electrocatalysts deposited onto two different semiconductors and ‘wired’ into a complete photocatalytic system for the overall water-splitting process (figure 11a). By doing this, two low-energy semiconductors can be used in a tandem configuration (figure 8c). The two nanopowder photocatalysts would be electrically connected by either a hard wire (a solid wire such as graphene sheet) [131] or a soft-wire (a soluble redox couple such as IO3−/I−) [132–134]. In principle, by using a soluble electron shuttle, it is possible to separate water oxidation photocatalysts and hydrogen evolution photocatalysts into two compartments. Such an arrangement allows the separating of H2 and O2 products that is not possible when using a single large band gap semiconductor with HER and OEC catalysts co-loaded. Indeed, production of a H2/O2 mixture is not ideal for large-scale application for safety reasons.
(ii) PEC system. A PEC cell for water-splitting application should contain a photoanode for extracting electrons from water using solar irradiation as the energy source and a photocathode or a cathode to use these reductive electrons for the hydrogen-generation reaction. The ideal cathode material is platinum and it was used by Fujishima & Honda [70] in their classic work with TiO2. As stated earlier, hydrogenase is an electrocatalyst as active as Pt [56]. Indeed, an effective PEC was constructed by Moore et al. [119] using a hydrogenase-decorated carbon fibre electrode with a dye-sensitized TiO2 photoanode (figure 11a). Both Pt and hydrogenase function with near-to-zero over-potential requirement. As a result, electrons in the TiO2 conduction band possess enough reductive potential for the HER with these catalysts. However, to replace the Pt or hydrogenase by a robust electrocatalyst engineered from abundant chemicals such as NiP4 [135], MoS2 [124] or Cu2MoS4 [136], a photoanode material that possesses a more negative conduction band is required. Examples on such designs have not yet been reported.
Alternatively, low-energy conduction band photoanodes can be wired to a photocathode in a tandem configuration (figure 11b) [120]. By doing so, electrons generated by the photoanode can be further energized by the photocathode so as to provide enough reductive potential to power platinum-free or hydrogenase-free HER electrocatalysis leading to hydrogen production. To make full use of the visible spectrum, a blue-light-absorbing material should ideally be used for the photoanode while the photocathode should be made of a red-light-absorbing material. The two electrodes can be on opposite sides of a proton transfer membrane, e.g. Nafion, and thus allow separation of H2 and O2 products as well as managing proton transportation from the photoanode to the (photo)cathode. In principle, with a proton exchange membrane, it should be possible to engineer a PEC cell with two compartments at different pHs. Indeed, in general, an alkaline solution is preferred for anodic function, whereas acidic solution is preferred for cathodic reactions. However, with the perspective of a large-scale solar hydrogen production, a PEC with two electrodes functioning in neutral or near-to-neutral pH solutions, and if possible with sea water, is desirable. To this end, further efforts are needed to improve the activities of current photoelectrode materials as well as electrocatalysts. At the current stage of development, several potential photoanodes and photocathodes are being investigated as separate entities. The ultimate goal is to screen these systems for the possibility of combining them into a fully assembled PEC device along the lines described below.
(iii) Photovoltaic-electrolyser combination. Coupling a Si solar cell or a dye-sensitized solar cell to an electrolyser could be a possible technological solution to initially convert solar radiation into electricity and then use this electrical energy to split water into hydrogen and oxygen via classical electrolysis. It is still not clear whether this configuration is more efficient than a photocatalyst or a PEC type system and whether cost and practical considerations will identify the most effective route for solar fuel production on a large scale.
Rocheleau et al. [122] and more recently Nocera and co-workers [121] reported a relatively simple PEC system that effectively used sunlight to split water into oxygen and hydrogen. This ‘artificial leaf’ was constructed by direct deposition of two electrocatalysts onto the sides of an amorphous triple junction Si solar cell (figure 11c). The OEC, consisting of either a NiFexOy or CoPi catalyst, was deposited onto a ITO layer on one surface of Si wafer, whereas the HER catalyst, a CoMo alloy or NiMoZn, was assembled on steel plate in contact with the other side of the Si wafer. On illuminating the Si solar cell, charge separation occurred within the triple junction cell. The positive holes in the valence band extracted electrons from water with the aid of the OEC. The negative charges present in the conduction band were transferred into the HER catalyst to mediate the reduction of protons into hydrogen. Although not fully optimized, these two examples of a working ‘artificial leaf’ system had an overall photon-to-hydrogen conversion efficiency of ca 7.8 per cent [122]. These two examples were also demonstrated to be robust under outdoor test conditions for thousands of hours. Although it is still early days, these devices represent a significant advance in the ultimate goal to engineer a robust artificial photosynthetic system composed of Earth's abundant elements to harvest and convert sunlight into hydrogen with a desired efficiency of 10 per cent or more.
5. Conclusions
It is anticipated that the global demand for energy will more than double by the mid-century and perhaps more than triple by the end of the century. Satisfying this demand will be necessary in order to achieve vibrant technological progress, economic growth and most importantly, political stability over the coming decades. Already we are faced with the prospect of catastrophic climate change owing to the release of CO2 into the atmosphere brought about by the burning of fossil fuels. In the short-term, we must exploit all technologies known to us to produce energy while at the same time reduce CO2 emission. The nature of mix of the approaches adopted will vary between different countries depending on their resources and populations with some dominating factors (e.g. geothermal in Iceland, biomass in Brazil, etc.). Coupled with this, challenge is to use energy more efficiently. Here again, we can learn from nature. In biology, the ‘combustion’ of fuel (food) is accomplished isothermally by highly efficient and subtle biological reactions involving a host of clever enzymes. For example, when the ‘hydrogen’ of glucose is combined with oxygen during the process of respiration to produce water and CO2, about 30 ATP molecules are made. ATP is the energy currency of cells. Because ATP stores 12 kcal per mole (50 kJ mol−1) of usable energy and the energy content of glucose is 673 kcal per quantum mole (2805 kJ mole−1), the efficiency of energy conversion is in the region of 54 per cent. It therefore seems to us that mankind should learn from biology's example and strive to develop new technologies that are as energy efficient as natural enzymes.
For the long term, we will have very few options to replace fossil fuels and satisfy the increased energy demands of a global population of 10 billion or more. Renewables such as hydropower, wind, wave, geothermal and biomass will not be able to supply energy equivalent to 20 TW even when taken together [4]. Nuclear fission is a short-term solution but in the long-run will probably not be a realistic option. Nuclear fusion is a possibility but the construction of a working reactor is proving problematic. Nevertheless, we must continue to explore this potential technology with the hope that it will come on stream sooner or later. However, there is another nuclear reaction that is already up and running, namely the Sun. Our sun is the champion of energy sources: delivering more energy to the Earth in an hour than we currently use in a year from fossil, nuclear and all renewable sources combined. Its energy supply is inexhaustible in human terms, more or less evenly distributed globally and its use is harmless to our environment and climate.
The enormous untapped potential of solar energy is an opportunity that should be addressed with urgency. Biology chose this energy source, and there is no reason why the chemical reactions devised by photosynthetic organisms cannot be mimicked by the ingenuity of humans. We already have a considerable knowledge base and the emerging nanotechnologies to exploit. With a concerted input of the talents of scientists trained in different disciplines, it should be possible to move the technologies of solar energy capture and storage forward. The recognition of Manhattan- or Apollo-like initiatives to develop new sustainable energy technologies in response to the CO2 problem as suggested by Hoffert et al. [2] should be the driver for encouraging basic and applied research in this area. As outlined in this study, significant progress is being made although the overall challenge to go from micro- to macroscale should not be underestimated.
Endnote
In a Graetzel solar cell, the dye-sensitized TiO2 electrode is interfaced with acetonitrile or an ionic liquid solution and the holes generated are efficiently quenched by I− [115].
References
- 1.International Energy Agency. 2012. Key World Energy Statistics 2012. Paris, France: International Energy Agency. (http://www.iea.org. )
- 2.Hoffert MT, et al. 1998. Energy implications of future stabilization of atmospheric CO2 content. Nature 395, 881–884 10.1038/27638 (doi:10.1038/27638) [DOI] [Google Scholar]
- 3.Nakicenovic N, Swart R. 2000. Special report on emissions scenarios, pp. 48–55 Washington, DC: Intergovernmental Panel on Climate Change [Google Scholar]
- 4.Lewis NS, Nocera DG. 2006. Powering the planet: chemical challenges in solar energy utilization. Proc. Natl Acad. Sci. USA 103, 15 729–15 735 10.1073/pnas.0603395103 (doi:10.1073/pnas.0603395103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.United Nations Development Program 2003. World energy assessment report: energy and the challenge of sustainability. New York, NY: United Nations [Google Scholar]
- 6.Pachauri R, Reisinger A. 2007. Contribution of working groups I, II, and III to the fourth assessment. Report of the Intergovernmental Panel on Climate Change. In Climate change 2007, synthesis report (eds Core Writing Team, Bernstein, et al.), Geneva, Switzerland: IPCC; See http://www.ipcc.ch/pdf/assessment-report/ar4/syr/ar4_syr.pdf. [Google Scholar]
- 7.Metz B, Davidson O, de Coninck H, Loos M, Meyer L. 2005. Carbon dioxide capture and storage. Cambridge, UK: Intergovernmental Panel on Climate Change, Cambridge University Press [Google Scholar]
- 8.Solar Energy Utilization Workshop 2005. Basic science needs for solar energy utilization. Washington, DC: US Department of Energy [Google Scholar]
- 9.Shaheen SE, Ginley DS, Jabbour GE. 2005. Organic-based photovoltaics toward low-cost power generation. MRS Bull. 30, 10–19 10.1557/mrs2005.2 (doi:10.1557/mrs2005.2) [DOI] [Google Scholar]
- 10.Hagberg DP, et al. 2008. Molecular engineering of organic sensitizers for dye-sensitized solar cell applications. J. Am. Chem. Soc. 130, 6259–6266 10.1021/ja800066y (doi:10.1021/ja800066y) [DOI] [PubMed] [Google Scholar]
- 11.Thorndike EH. 1996. Energy and the environment. Reading, MA: Addison-Wesley [Google Scholar]
- 12.Walker DA. 1977. Energy, plants and man. Chichester, UK: Packard Publishing Ltd [Google Scholar]
- 13.Archer MD, Barber J. 2004. Photosynthesis and photoconversion. In Molecular to global photosynthesis (eds Archer MD, Barber J.), pp. 1–41 London, UK: Imperial College Press [Google Scholar]
- 14.Bolton JR, Hall DO. 1991. The maximum efficiency of photosynthesis. Photochem. Photobiol. 53, 545–548 10.1111/j.1751-1097.1991.tb03668.x (doi:10.1111/j.1751-1097.1991.tb03668.x) [DOI] [Google Scholar]
- 15.Blankenship RE, et al. 2011. Comparing photosynthetic and photovoltaic efficiencies and recognizing the potential for improvement. Science 332, 805–809 10.1126/science.1200165 (doi:10.1126/science.1200165) [DOI] [PubMed] [Google Scholar]
- 16.Falkowsky PG, Raven JA. 1997. Aquatic photosynthesis. Oxford, UK: Blackwell [Google Scholar]
- 17.Blankenship RE. 2002. Molecular mechanisms of photosynthesis. Oxford, UK: Blackwell Science [Google Scholar]
- 18.Barber J, Andersson B. 1994. Revealing the blueprint of photosynthesis. Nature 370, 31–34 10.1038/370031a0 (doi:10.1038/370031a0) [DOI] [Google Scholar]
- 19.Schubert W-D, Klukas O, Saenger W, Witt HT, Fromme P, Krauß N. 1998. A common ancestor for oxygenic and anoxygenic photosynthetic systems: a comparison based on the structural model of photosystem I. J. Mol. Biol. 280, 297–314 10.1006/jmbi.1998.1824 (doi:10.1006/jmbi.1998.1824) [DOI] [PubMed] [Google Scholar]
- 20.Rhee K-H, Morris EP, Barber J, Kuhlbrandt W. 1998. Three-dimensional structure of the photosystem II reaction center at 8 Å resolution. Nature 396, 283–286 10.1038/24421 (doi:10.1038/24421) [DOI] [PubMed] [Google Scholar]
- 21.van Amerongen H, Valkunas L, van Grondelle R. 2000. Photosynthetic excitons. Singapore: World Scientific [Google Scholar]
- 22.Barber J. 2003. Photosystem II: the engine of life. Q. Rev. Biophys. 36, 71–89 10.1017/S0033583502003839 (doi:10.1017/S0033583502003839) [DOI] [PubMed] [Google Scholar]
- 23.Wydrzynski TJ, Satoh K. 2005. Photosystem II: the light-driven water: plastoquinone oxidoreductase. In Advances in photosynthesis and respiration, vol. 22, pp. 1–786 Dordrecht, The Netherlands: Springer [Google Scholar]
- 24.Barber J, Rutherford AW. 2008. Revealing how nature uses sunlight to split water. Phil. Trans. R. Soc. B 363, 1125–1128 10.1098/rstb.2007.2227 (doi:10.1098/rstb.2007.2227) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yano J, et al. 2006. Where water is oxidized to dioxygen: structure of the photosynthetic Mn4Ca cluster. Science 314, 821–825 10.1126/science.1128186 (doi:10.1126/science.1128186) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sauer K, Yano J, Yachandra VK. 2008. X-ray spectroscopy of the photosynthetic oxygen-evolving complex. Coord. Chem. Rev. 252, 318–335 10.1016/j.ccr.2007.08.009 (doi:10.1016/j.ccr.2007.08.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yano J, Yachandra VK. 2008. Where water is oxidized to dioxygen: structure of the photosynthetic Mn4Ca cluster from X-ray spectroscopy. Inorg. Chem. 47, 1711–1726 10.1021/ic7016837 (doi:10.1021/ic7016837) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zouni A, Witt HT, Kern J, Fromme P, Krauss N, Saenger W, Orth P. 2001. Crystal structure of photosystem II from Synechococcus elongatus at 3.8 Å resolution. Nature 409, 739–743 10.1038/35055589 (doi:10.1038/35055589) [DOI] [PubMed] [Google Scholar]
- 29.Kamiya N, Shen J-R. 2003. Crystal structure of oxygen-evolving photosystem II from Thermosynechococcus vulcanus at 3.7 Å resolution. Proc. Natl Acad. Sci. USA 100, 98–103 10.1073/pnas.0135651100 (doi:10.1073/pnas.0135651100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferreira KN, Iverson TM, Maghlaoui K, Barber J, Iwata S. 2004. Architecture of the photosynthetic oxygen-evolving center. Science 303, 1831–1838 10.1126/science.1093087 (doi:10.1126/science.1093087) [DOI] [PubMed] [Google Scholar]
- 31.Loll B, Kern J, Saenger W, Zouni A, Biesiadka J. 2005. Towards complete cofactor arrangement in the 3.0 Å resolution structure of photosystem II. Nature 438, 1040–1044 10.1038/nature04224 (doi:10.1038/nature04224) [DOI] [PubMed] [Google Scholar]
- 32.Umena Y, Kawakami K, Shen J-R, Kamiya N. 2011. Crystal structure of oxygen-evolving photosystem II at a resolution of 1.9 Å. Nature 473, 55–60 10.1038/nature09913 (doi:10.1038/nature09913) [DOI] [PubMed] [Google Scholar]
- 33.Sproviero EM, Gascon JA, McEvoy JP, Brudvig GW, Batista VS. 2006. QM/MM models of the O2-evolving complex of photosystem II. J. Chem. Theory Comput. 2, 1119–1134 10.1021/ct060018l (doi:10.1021/ct060018l) [DOI] [PubMed] [Google Scholar]
- 34.Sproviero EM, Gascon JA, McEvoy JP, Brudvig GW, Batista VS. 2007. Quantum mechanics/molecular mechanics structure models of the oxygen-evolving complex of photosystem II. Curr. Opin. Struct. Biol. 17, 173–180 10.1016/j.sbi.2007.03.015 (doi:10.1016/j.sbi.2007.03.015) [DOI] [PubMed] [Google Scholar]
- 35.Sproviero EM, Gascón JA, McEvoy JP, Brudvig GW, Batista VS. 2008. Quantum mechanics/molecular mechanics study of the catalytic cycle of water splitting in photosystem II. J. Am. Chem. Soc. 130, 3428–3442 10.1021/ja076130q (doi:10.1021/ja076130q) [DOI] [PubMed] [Google Scholar]
- 36.Messinger J, Badger M, Wydrzynski T. 1995. Detection of one slowly exchanging substrate water molecule in the S3 state of photosystem II. Proc. Natl Acad. Sci. USA 92, 3209–3213 10.1073/pnas.92.8.3209 (doi:10.1073/pnas.92.8.3209) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pecoraro VL, Baldwin MJ, Caudle MT, Hsieh WY, Law NA. 1998. A proposal for water oxidation in photosystem II. Pure Appl. Chem . 70, 925 [Google Scholar]
- 38.McEvoy JP, Brudvig GW. 2004. Structure-based mechanism of photosynthetic water oxidation. Phys. Chem. Chem. Phys. 6, 4754–4763 10.1039/b407500e (doi:10.1039/b407500e) [DOI] [Google Scholar]
- 39.McEvoy JP, Brudvig GW. 2006. Water-splitting chemistry of photosystem II. Chem. Rev. 106, 4455–4483 10.1021/cr0204294 (doi:10.1021/cr0204294) [DOI] [PubMed] [Google Scholar]
- 40.Sproviero EM, Gascón JA, McEvoy JP, Brudvig GW, Batista VS. 2008. Computational studies of the O2-evolving complex of photosystem II and biomimetic oxomanganese complexes. Coord. Chem. Rev. 252, 395–415 10.1016/j.ccr.2007.09.006 (doi:10.1016/j.ccr.2007.09.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Siegbahn PEM. 2006. O–O bond formation in the S4 state of the oxygen-evolving complex in photosystem II. Chem. Eur. J. 12, 9217–9227 10.1002/chem.200600774 (doi:10.1002/chem.200600774) [DOI] [PubMed] [Google Scholar]
- 42.Siegbahn PEM. 2009. Structures and energetics for O2 formation in photosystem II. Accounts Chem. Res. 42, 1871–1880 10.1021/ar900117k (doi:10.1021/ar900117k) [DOI] [PubMed] [Google Scholar]
- 43.Siegbahn PEM. 2012. Mechanisms for proton release during water oxidation in the S2 to S3 and S3 to S4 transitions in photosystem II. Phys. Chem. Chem. Phys. 14, 4849–4856 10.1039/c2cp00034b (doi:10.1039/c2cp00034b) [DOI] [PubMed] [Google Scholar]
- 44.Kok B, Forbush B, McGloin M. 1970. Cooperation of charges in photosynthetic O2 evolution-I. A linear four step mechanism. Photochem. Photobiol. 11, 457–475 10.1111/j.1751-1097.1970.tb06017.x (doi:10.1111/j.1751-1097.1970.tb06017.x) [DOI] [PubMed] [Google Scholar]
- 45.Gatt P, Petrie S, Stranger R, Pace RJ. 2012. Rationalizing the 1.9 Å crystal structure of photosystem II. A remarkable Jahn–Teller balancing act induced by a single proton transfer. Angew. Chem. Int. Ed. 51, 12 025–12 028 10.1002/anie.201206316 (doi:10.1002/anie.201206316) [DOI] [PubMed] [Google Scholar]
- 46.Kolling DRJ, Cox N, Ananyev GM, Pace RJ, Dismukes GC. 2012. What are the oxidation states of manganese required to catalyze photosynthetic water oxidation? Biophys. J. 103, 313–322 10.1016/j.bpj.2012.05.031 (doi:10.1016/j.bpj.2012.05.031) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Isobe H, Shoji M, Yamanaka S, Umena Y, Kawakami K, Kamiya N, Shen J-R, Yamaguchi K. 2012. Theoretical illumination of water-inserted structures of the CaMn4O5 cluster in the S2 and S3 states of oxygen-evolving complex of photosystem II: full geometry optimizations by B3LYP hybrid density functional. Dalton Trans. 41, 13 727–13 740 10.1039/c2dt31420g (doi:10.1039/c2dt31420g) [DOI] [PubMed] [Google Scholar]
- 48.Kusunoki M. 2011. S1-state Mn4Ca complex of photosystem II exists in equilibrium between the two most-stable isomeric substates: XRD and EXAFS evidence. J. Photochem. Photobiol. B Biol. 104, 100–110 10.1016/j.jphotobiol.2011.03.002 (doi:10.1016/j.jphotobiol.2011.03.002) [DOI] [PubMed] [Google Scholar]
- 49.Rapatskiy L, et al. 2012. Detection of the water-binding sites of the oxygen-evolving complex of photosystem II using W-Band 17O electron–electron double resonance-detected NMR spectroscopy. J. Am. Chem. Soc. 134, 16 619–16 634 10.1021/ja3053267 (doi:10.1021/ja3053267) [DOI] [PubMed] [Google Scholar]
- 50.Ames W, Pantazis DA, Krewald V, Cox N, Messinger J, Lubitz W, Neese F. 2011. Theoretical evaluation of structural models of the S2 state in the oxygen evolving complex of photosystem II: protonation states and magnetic interactions. J. Am. Chem. Soc. 133, 19 743–19 757 10.1021/ja2041805 (doi:10.1021/ja2041805) [DOI] [PubMed] [Google Scholar]
- 51.Volbeda A, Charon M-H, Piras C, Hatchikian EC, Frey M, Fontecilla-Camps JC. 1995. Crystal structure of the nickel-iron hydrogenase from Desulfovibrio gigas. Nature 373, 580–587 10.1038/373580a0 (doi:10.1038/373580a0) [DOI] [PubMed] [Google Scholar]
- 52.Peters JW, Lanzilotta WN, Lemon BJ, Seefeldt LC. 1998. X-ray crystal structure of the Fe-only hydrogenase (CpI) from Clostridium pasteurianum to 1.8 Å resolution. Science 282, 1853–1858 10.1126/science.282.5395.1853 (doi:10.1126/science.282.5395.1853) [DOI] [PubMed] [Google Scholar]
- 53.Nicolet Y, Piras C, Legrand P, Hatchikian CE, Fontecilla-Camps JC. 1999. Desulfovibrio desulfuricans iron hydrogenase: the structure shows unusual coordination to an active site Fe binuclear center. Structure 7, 13–23 10.1016/S0969-2126(99)80005-7 (doi:10.1016/S0969-2126(99)80005-7) [DOI] [PubMed] [Google Scholar]
- 54.Zirngibl C, Van Dongen W, Schworer B, Von Bunau R, Richter M, Klein A, Thauer RK. 1992. H2-forming methylenetetrahydromethanopterin dehydrogenase, a novel type of hydrogenase without iron-sulfur clusters in methanogenic archaea. Eur. J. Biochem. 208, 511–520 10.1111/j.1432-1033.1992.tb17215.x (doi:10.1111/j.1432-1033.1992.tb17215.x) [DOI] [PubMed] [Google Scholar]
- 55.Fontecilla-Camps JC, Volbeda A, Cavazza C, Nicolet Y. 2007. Structure/function relationships of [NiFe]- and [FeFe]-hydrogenases. Chem. Rev. 107, 4273–4303 10.1021/cr050195z (doi:10.1021/cr050195z) [DOI] [PubMed] [Google Scholar]
- 56.Jones AK, Sillery E, Albracht SPJ, Armstrong FA. 2002. Direct comparison of the electrocatalytic oxidation of hydrogen by an enzyme and a platinum catalyst. Chem. Commun. 2002, 866–867 10.1039/B201337A (doi:10.1039/B201337A) [DOI] [PubMed] [Google Scholar]
- 57.De Lacey AL, Fernández VM, Rousset M, Cammack R. 2007. Activation and inactivation of hydrogenase function and the catalytic cycle: spectroelectrochemical studies. Chem. Rev. 107, 4304–4330 10.1021/cr0501947 (doi:10.1021/cr0501947) [DOI] [PubMed] [Google Scholar]
- 58.Siegbahn PEM, Tye JW, Hall MB. 2007. Computational studies of [NiFe] and [FeFe] hydrogenases. Chem. Rev. 107, 4414–4435 10.1021/cr050185y (doi:10.1021/cr050185y) [DOI] [PubMed] [Google Scholar]
- 59.Greco C, Bruschi M, De Gioia L, Ryde U. 2007. A QM/MM investigation of the activation and catalytic mechanism of Fe-only hydrogenases. Inorg. Chem. 46, 5911–5921 10.1021/ic062320a (doi:10.1021/ic062320a) [DOI] [PubMed] [Google Scholar]
- 60.Bruschi M, Greco C, Kaukonen M, Fantucci P, Ryde U, De Gioia L. 2009. Influence of the [2Fe]H subcluster environment on the properties of key intermediates in the catalytic cysubcluster environment on the properties of key intermediates in the catalytic cycle of [FeFe] hydrogenases: hints for the rational design of synthetic catalysts. Angew. Chem. Int. Ed. 48, 3503–3506 10.1002/ange.200900494 (doi:10.1002/ange.200900494) [DOI] [PubMed] [Google Scholar]
- 61.Frey M. 2002. Hydrogenases: hydrogen-activating enzymes. ChemBioChem 3, 153–160 (doi:10.1002/1439-7633(20020301)3:2/3<153::AID-CBIC153>3.0.CO;2-B) [DOI] [PubMed] [Google Scholar]
- 62.Volbeda A, Fontecilla-Camps JC. 2003. The active site and catalytic mechanism of NiFe hydrogenases. Dalton Trans. 2003, 4030–4038 10.1039/B304316A (doi:10.1039/B304316A) [DOI] [Google Scholar]
- 63.Wait AF, Parkin A, Morley GM, dos Santos L, Armstrong FA. 2010. Characteristics of enzyme-based hydrogen fuel cells using an oxygen-tolerant hydrogenase as the anodic catalyst. J. Phys. Chem. C 114, 12 003–12 009 10.1021/jp102616m (doi:10.1021/jp102616m) [DOI] [Google Scholar]
- 64.Tard Cd, Pickett CJ. 2009. Structural and functional analogues of the active sites of the [Fe]-, [NiFe]-, and [FeFe]-Hydrogenases. Chem. Rev. 109, 2245–2274 10.1021/cr800542q (doi:10.1021/cr800542q) [DOI] [PubMed] [Google Scholar]
- 65.Wang M, Chen L, Sun L. 2012. Recent progress in electrochemical hydrogen production with earth-abundant metal complexes as catalysts. Energy Environ. Sci. 5, 6763–6778 10.1039/c2ee03309g (doi:10.1039/c2ee03309g) [DOI] [Google Scholar]
- 66.Du P, Eisenberg R. 2012. Catalysts made of earth-abundant elements (Co, Ni, Fe) for water splitting: recent progress and future challenges. Energy Environ. Sci. 5, 6012–6021 10.1039/c2ee03250c (doi:10.1039/c2ee03250c) [DOI] [Google Scholar]
- 67.Artero V, Chavarot-Kerlidou M, Fontecave M. 2011. Splitting water with cobalt. Angew. Chem. Int. Ed. 50, 7238–7266 10.1002/anie.201007987 (doi:10.1002/anie.201007987) [DOI] [PubMed] [Google Scholar]
- 68.Tran PD, Wong LH, Barber J, Loo JSC. 2012. Recent advances in hybrid photocatalysts for solar fuel production. Energy Environ. Sci. 5, 5902–5918 10.1039/c2ee02849b (doi:10.1039/c2ee02849b) [DOI] [Google Scholar]
- 69.Benson EE, Kubiak CP, Sathrum AJ, Smieja JM. 2009. Electrocatalytic and homogeneous approaches to conversion of CO2 to liquid fuels. Chem. Soc. Rev. 38, 89–99 10.1039/b804323j (doi:10.1039/b804323j) [DOI] [PubMed] [Google Scholar]
- 70.Fujishima A, Honda K. 1972. Electrochemical photolysis of water at a semiconductor electrode. Nature 238, 37–38 10.1038/238037a0 (doi:10.1038/238037a0) [DOI] [PubMed] [Google Scholar]
- 71.Chen X, Shen S, Guo L, Mao SS. 2010. Semiconductor-based photocatalytic hydrogen generation. Chem. Rev. 110, 6503–6570 10.1021/cr1001645 (doi:10.1021/cr1001645) [DOI] [PubMed] [Google Scholar]
- 72.Kudo A, Miseki Y. 2009. Heterogeneous photocatalyst materials for water splitting. Chem. Soc. Rev. 38, 253–278 10.1039/b800489g (doi:10.1039/b800489g) [DOI] [PubMed] [Google Scholar]
- 73.Wu M-C, et al. 2011. Nitrogen-doped anatase nanofibers decorated with noble metal nanoparticles for photocatalytic production of hydrogen. ACS Nano 5, 5025–5030 10.1021/nn201111j (doi:10.1021/nn201111j) [DOI] [PubMed] [Google Scholar]
- 74.Tao J, Luttrell T, Batzill M. 2011. A two-dimensional phase of TiO2 with a reduced bandgap. Nat. Chem. 3, 296–300 10.1038/nchem.1006 (doi:10.1038/nchem.1006) [DOI] [PubMed] [Google Scholar]
- 75.Chen X, Liu L, Yu PY, Mao SS. 2011. Increasing solar absorption for photocatalysis with black hydrogenated titanium dioxide nanocrystals. Science 331, 746–750 10.1126/science.1200448 (doi:10.1126/science.1200448) [DOI] [PubMed] [Google Scholar]
- 76.Paracchino A, Laporte V, Sivula K, Grätzel M, Thimsen E. 2011. Highly active oxide photocathode for photoelectrochemical water reduction. Nat. Mater. 10, 456–461 10.1038/nmat3017 (doi:10.1038/nmat3017) [DOI] [PubMed] [Google Scholar]
- 77.Tran PD, Batabyal SK, Pramana SS, Barber J, Wong LH, Loo SCJ. 2012. A cuprous oxide-reduced graphene oxide (Cu2O–rGO) composite photocatalyst for hydrogen generation: employing rGO as an electron acceptor to enhance the photocatalytic activity and stability of Cu2O. Nanoscale 4, 3875–3878 10.1039/c2nr30881a (doi:10.1039/c2nr30881a) [DOI] [PubMed] [Google Scholar]
- 78.Du A, Ng YH, Bell NJ, Zhu Z, Amal R, Smith SC. 2011. Hybrid graphene/titania nanocomposite: interface charge transfer, hole doping, and sensitization for visible light response. J. Phys. Chem. Lett. 2, 894–899 10.1021/jz2002698 (doi:10.1021/jz2002698) [DOI] [PubMed] [Google Scholar]
- 79.Kay A, Cesar I, Grätzel M. 2006. New benchmark for water photooxidation by nanostructured α-Fe2O3 films. J. Am. Chem. Soc. 128, 15 714–15 721 10.1021/ja064380l (doi:10.1021/ja064380l) [DOI] [PubMed] [Google Scholar]
- 80.Ling Y, Wang G, Wheeler DA, Zhang JZ, Li Y. 2011. Sn-doped hematite nanostructures for photoelectrochemical water splitting. Nano Lett. 11, 2119–2125 10.1021/nl200708y (doi:10.1021/nl200708y) [DOI] [PubMed] [Google Scholar]
- 81.Xi L, Bassi PS, Chiam SY, Mak WF, Tran PD, Barber J, Loo JSC, Wong LH. 2012. Surface treatment of hematite photoanodes with zinc acetate for water oxidation. Nanoscale 4, 4430–4433 10.1039/c2nr30862b (doi:10.1039/c2nr30862b) [DOI] [PubMed] [Google Scholar]
- 82.Xi L, Chiam SY, Mak WF, Tran PD, Barber J, Loo SCJ, Wong LH. 2013. A novel strategy for surface treatment on hematite photoanode for efficient water oxidation. Chem. Sci. 4, 164–169 10.1039/c2sc20881d (doi:10.1039/c2sc20881d) [DOI] [Google Scholar]
- 83.Hisatomi T, Dotan H, Stefik M, Sivula K, Rothschild A, Grätzel M, Mathews N. 2012. Enhancement in the performance of ultrathin hematite photoanode for water splitting by an oxide underlayer. Adv. Mater. 24, 2699–2702 10.1002/adma.201104868 (doi:10.1002/adma.201104868) [DOI] [PubMed] [Google Scholar]
- 84.Gersten SW, Samuels GJ, Meyer TJ. 1982. Catalytic oxidation of water by an oxo-bridged ruthenium dimer. J. Am. Chem. Soc. 104, 4029–4030 10.1021/ja00378a053 (doi:10.1021/ja00378a053) [DOI] [Google Scholar]
- 85.Duan L, Bozoglian F, Mandal S, Stewart B, Privalov T, Llobet A, Sun L. 2012. A molecular ruthenium catalyst with water-oxidation activity comparable to that of photosystem II. Nat. Chem. 4, 418–423 10.1038/nchem.1301 (doi:10.1038/nchem.1301) [DOI] [PubMed] [Google Scholar]
- 86.Yamazaki H, Shouji A, Kajita M, Yagi M. 2010. Electrocatalytic and photocatalytic water oxidation to dioxygen based on metal complexes. Coord. Chem. Rev. 254, 2483–2491 10.1016/j.ccr.2010.02.008 (doi:10.1016/j.ccr.2010.02.008) [DOI] [Google Scholar]
- 87.Kanady JS, Tsui EY, Day MW, Agapie T. 2011. A synthetic model of the Mn3Ca subsite of the oxygen-evolving complex in photosystem II. Science 333, 733–736 10.1126/science.1206036 (doi:10.1126/science.1206036) [DOI] [PubMed] [Google Scholar]
- 88.Mukherjee S, et al. 2012. Synthetic model of the asymmetric [Mn3CaO4] cubane core of the oxygen-evolving complex of photosystem II. Proc. Natl Acad. Sci. USA 109, 2257–2262 10.1073/pnas.1115290109 (doi:10.1073/pnas.1115290109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yin Q, Tan JM, Besson C, Geletii YV, Musaev DG, Kuznetsov AE, Luo Z, Hardcastle KI, Hill CL. 2010. A fast soluble carbon-free molecular water oxidation catalyst based on abundant metals. Science 328, 342–345 10.1126/science.1185372 (doi:10.1126/science.1185372) [DOI] [PubMed] [Google Scholar]
- 90.Kanan MW, Nocera DG. 2008. In situ formation of an oxygen-evolving catalyst in neutral water containing phosphate and Co2+. Science 321, 1072–1075 10.1126/science.1162018 (doi:10.1126/science.1162018) [DOI] [PubMed] [Google Scholar]
- 91.Tard C, et al. 2005. Synthesis of the H-cluster framework of iron-only hydrogenase. Nature 433, 610–613 10.1038/nature03298 (doi:10.1038/nature03298) [DOI] [PubMed] [Google Scholar]
- 92.Ogo S, et al. 2007. A dinuclear Ni(µ-H)Ru complex derived from H2. Science 316, 585–587 10.1126/science.1138751 (doi:10.1126/science.1138751) [DOI] [PubMed] [Google Scholar]
- 93.Helm ML, Stewart MP, Bullock RM, DuBois MR, DuBois DL. 2011. A synthetic nickel electrocatalyst with a turnover frequency above 100 000 s−1 for H2 production. Science 333, 863–866 10.1126/science.1205864 (doi:10.1126/science.1205864) [DOI] [PubMed] [Google Scholar]
- 94.Dinca M, Surendranath Y, Nocera DG. 2010. Nickel-borate oxygen-evolving catalyst that functions under benign conditions. Proc. Natl Acad. Sci. USA 107, 10 337–10 341 10.1073/pnas.1001859107 (doi:10.1073/pnas.1001859107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Risch M, Khare V, Zaharieva I, Gerencser L, Chernev P, Dau H. 2009. Cobalt-oxo core of a water-oxidizing catalyst film. J. Am. Chem. Soc. 131, 6936–6937 10.1021/ja902121f (doi:10.1021/ja902121f) [DOI] [PubMed] [Google Scholar]
- 96.Kanan MW, Yano J, Surendranath Y, Dinca M, Yachandra VK, Nocera DG. 2010. Structure and valency of a cobalt-phosphate water oxidation catalyst determined by in situ X-ray spectroscopy. J. Am. Chem. Soc. 132, 13 692–13 701 10.1021/ja1023767 (doi:10.1021/ja1023767) [DOI] [PubMed] [Google Scholar]
- 97.Lutterman DA, Surendranath Y, Nocera DG. 2009. A self-healing oxygen-evolving catalyst. J. Am. Chem. Soc. 131, 3838–3839 10.1021/ja900023k (doi:10.1021/ja900023k) [DOI] [PubMed] [Google Scholar]
- 98.Jiao F, Frei H. 2009. Nanostructured cobalt oxide clusters in mesoporous silica as efficient oxygen-evolving catalysts. Angew. Chem. Int. Ed. 48, 1841–1844 10.1002/anie.200805534 (doi:10.1002/anie.200805534) [DOI] [PubMed] [Google Scholar]
- 99.Jiao F, Frei H. 2010. Nanostructured manganese oxide clusters supported on mesoporous silica as efficient oxygen-evolving catalysts. Chem. Commun. 46, 2920–2922 10.1039/b921820c (doi:10.1039/b921820c) [DOI] [PubMed] [Google Scholar]
- 100.Brimblecombe R, Koo A, Dismukes GC, Swiegers GF, Spiccia L. 2010. Solar driven water oxidation by a bioinspired manganese molecular catalyst. J. Am. Chem. Soc. 132, 2892–2894 10.1021/ja910055a (doi:10.1021/ja910055a) [DOI] [PubMed] [Google Scholar]
- 101.Li L, Duan L, Xu Y, Gorlov M, Hagfeldt A, Sun L. 2010. A photoelectrochemical device for visible light driven water splitting by a molecular ruthenium catalyst assembled on dye-sensitized nanostructured TiO2. Chem. Commun. 46, 7307–7309 10.1039/c0cc01828g (doi:10.1039/c0cc01828g) [DOI] [PubMed] [Google Scholar]
- 102.Li G, Sproviero EM, McNamara WR, Snoeberger RC, Crabtree RH, Brudvig GW, Batista VS. 2009. Reversible visible-light photooxidation of an oxomanganese water-oxidation catalyst covalently anchored to TiO2 nanoparticles. J. Phys. Chem. B 114, 14 214–14 222 10.1021/jp908925z (doi:10.1021/jp908925z) [DOI] [PubMed] [Google Scholar]
- 103.Moore GF, Blakemore JD, Milot RL, Hull JF, Song H-E, Cai L, Schmuttenmaer CA, Crabtree RH, Brudvig GW. 2011. A visible light water-splitting cell with a photoanode formed by codeposition of a high-potential porphyrin and an iridium water-oxidation catalyst. Energy Environ. Sci. 4, 2389–2392 10.1039/c1ee01037a (doi:10.1039/c1ee01037a) [DOI] [Google Scholar]
- 104.Xi L, et al. 2012. Co3O4-decorated hematite nanorods as an effective photoanode for solar water oxidation. J. Phys. Chem. C 116, 13 884–13 889 10.1021/jp304285r (doi:10.1021/jp304285r) [DOI] [Google Scholar]
- 105.Reisner E, Powell DJ, Cavazza C, Fontecilla-Camps JC, Armstrong FA. 2009. Visible light-driven H2 production by hydrogenases attached to dye-sensitized TiO2 nanoparticles. J. Am. Chem. Soc. 131, 18 457–18 466 10.1021/ja907923r (doi:10.1021/ja907923r) [DOI] [PubMed] [Google Scholar]
- 106.Nann T, Ibrahim SK, Woi P-M, Xu S, Ziegler J, Pickett CJ. 2010. Water splitting by visible light: a nanophotocathode for hydrogen production. Angew. Chem. Int. Ed. 49, 1574–1577 [DOI] [PubMed] [Google Scholar]
- 107.Tran PD, Pramana SS, Kale VS, Nguyen M, Chiam SY, Batabyal SK, Wong LH, Barber J, Loo J. 2012. Novel assembly of an MOS2 electrocatalyst onto a silicon nanowire array electrode to construct a photocathode composed of elements abundant on the Earth for hydrogen generation. Chem. Eur. J. 18, 13 994–13 999 10.1002/chem.201202214 (doi:10.1002/chem.201202214) [DOI] [PubMed] [Google Scholar]
- 108.Tran PD, Artero V, Fontecave M. 2010. Water electrolysis and photoelectrolysis on electrodes engineered using biological and bio-inspired molecular systems. Energy Environ. Sci. 3, 727–747 10.1039/b926749b (doi:10.1039/b926749b) [DOI] [Google Scholar]
- 109.Zhang J, Grzelczak M, Hou Y, Maeda K, Domen K, Fu X, Antonietti M, Wang X. 2012. Photocatalytic oxidation of water by polymeric carbon nitride nanohybrids made of sustainable elements. Chem. Sci. 3, 443–446 10.1039/c1sc00644d (doi:10.1039/c1sc00644d) [DOI] [Google Scholar]
- 110.Zhong DK, Cornuz M, Sivula K, Gratzel M, Gamelin DR. 2011. Photo-assisted electrodeposition of cobalt-phosphate (Co-Pi) catalyst on hematite photoanodes for solar water oxidation. Energy Environ. Sci. 4, 1759–1764 10.1039/c1ee01034d (doi:10.1039/c1ee01034d) [DOI] [Google Scholar]
- 111.Steinmiller EMP, Choi K-S. 2009. Photochemical deposition of cobalt-based oxygen evolving catalyst on a semiconductor photoanode for solar oxygen production. Proc. Natl Acad. Sci. USA 106, 20 633–20 636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Seabold JA, Choi K-S. 2011. Effect of a cobalt-based oxygen evolution catalyst on the stability and the selectivity of photo-oxidation reactions of a WO3 photoanode. Chem. Mater. 23, 1105–1112 10.1021/cm1019469 (doi:10.1021/cm1019469) [DOI] [Google Scholar]
- 113.Li L, Duan L, Wen F, Li C, Wang M, Hagfeldt A, Sun L. 2012. Visible light driven hydrogen production from a photo-active cathode based on a molecular catalyst and organic dye-sensitized p-type nanostructured NiO. Chem. Commun. 48, 988–990 10.1039/c2cc16101j (doi:10.1039/c2cc16101j) [DOI] [PubMed] [Google Scholar]
- 114.Lakadamyali F, Reisner E. 2011. Photocatalytic H2 evolution from neutral water with a molecular cobalt catalyst on a dye-sensitised TiO2 nanoparticle. Chem. Commun. 47, 1695–1697 10.1039/c0cc04658b (doi:10.1039/c0cc04658b) [DOI] [PubMed] [Google Scholar]
- 115.Hagfeldt A, Boschloo G, Sun L, Kloo L, Pettersson H. 2010. Dye-sensitized solar cells. Chem. Rev. 110, 6595–6663 10.1021/cr900356p (doi:10.1021/cr900356p) [DOI] [PubMed] [Google Scholar]
- 116.Tran PD, Xi L, Batabyal SK, Wong LH, Barber J, Chye Loo JS. 2012. Enhancing the photocatalytic efficiency of TiO2 nanopowders for H2 production by using non-noble transition metal co-catalysts. Phys. Chem. Chem. Phys. 14, 11 596–11 599 10.1039/c2cp41450c (doi:10.1039/c2cp41450c) [DOI] [PubMed] [Google Scholar]
- 117.Townsend TK, Browning ND, Osterloh F. 2012. Overall photocatalytic water splitting with NiOx–SrTiO3: a revised mechanism. Energy Environ. Sci. 5, 9543–9550 10.1039/c2ee22665k (doi:10.1039/c2ee22665k) [DOI] [Google Scholar]
- 118.McKone JR, Warren EL, Bierman MJ, Boettcher SW, Brunschwig BS, Lewis NS, Gray HB. 2011. Evaluation of Pt, Ni, and Ni-Mo electrocatalysts for hydrogen evolution on crystalline Si electrodes. Energy Environ. Sci. 4, 3573–3583 10.1039/c1ee01488a (doi:10.1039/c1ee01488a) [DOI] [Google Scholar]
- 119.Hambourger M, Gervaldo M, Svedruzic D, King PW, Gust D, Ghirardi M, Moore AL, Moore TA. 2008. [FeFe]-hydrogenase-catalyzed H2 production in a photoelectrochemical biofuel cell. J. Am. Chem. Soc. 130, 2015–2022 10.1021/ja077691k (doi:10.1021/ja077691k) [DOI] [PubMed] [Google Scholar]
- 120.Gratzel M. 2001. Photoelectrochemical cells. Nature 414, 338–344 10.1038/35104607 (doi:10.1038/35104607) [DOI] [PubMed] [Google Scholar]
- 121.Reece SY, Hamel JA, Sung K, Jarvi TD, Esswein AJ, Pijpers JJH, Nocera DG. 2011. Wireless solar water splitting using silicon-based semiconductors and earth-abundant catalysts. Science 334, 645–648 10.1126/science.1209816 (doi:10.1126/science.1209816) [DOI] [PubMed] [Google Scholar]
- 122.Rocheleau RE, Miller EL, Misra A. 1998. High-efficiency photoelectrochemical hydrogen production using multijunction amorphous silicon photoelectrodes. Energy Fuels 12, 3–10 10.1021/ef9701347 (doi:10.1021/ef9701347) [DOI] [Google Scholar]
- 123.Jaramillo TF, Jørgensen KP, Bonde J, Nielsen JH, Horch S, Chorkendorff I. 2007. Identification of active edge sites for electrochemical H2 evolution from MoS2 nanocatalysts. Science 317, 100–102 10.1126/science.1141483 (doi:10.1126/science.1141483) [DOI] [PubMed] [Google Scholar]
- 124.Merki D, Fierro S, Vrubel H, Hu X. 2011. Amorphous molybdenum sulfide films as catalysts for electrochemical hydrogen production in water. Chem. Sci. 2, 1262–1267 10.1039/c1sc00117e (doi:10.1039/c1sc00117e) [DOI] [Google Scholar]
- 125.Zong X, Yan H, Wu G, Ma G, Wen F, Wang L, Li C. 2008. Enhancement of photocatalytic H2 evolution on CdS by loading MoS2 as cocatalyst under visible light irradiation. J. Am. Chem. Soc. 130, 7176–7177 10.1021/ja8007825 (doi:10.1021/ja8007825) [DOI] [PubMed] [Google Scholar]
- 126.Seger B, Laursen AB, Vesborg PCK, Pedersen T, Hansen O, Dahl S, Chorkendorff IB. 2012. Hydrogen production using a molybdenum sulfide catalyst on a titanium-protected n+p-silicon photocathode. Angew. Chem. Int. Ed. 51, 9128–9131 10.1002/anie.201203585 (doi:10.1002/anie.201203585) [DOI] [PubMed] [Google Scholar]
- 127.Ohno T, Bai L, Hisatomi T, Maeda K, Domen K. 2012. Photocatalytic water splitting using modified GaN:ZnO solid solution under visible light: long-time operation and regeneration of activity. J. Am. Chem. Soc. 134, 8254–8259 10.1021/ja302479f (doi:10.1021/ja302479f) [DOI] [PubMed] [Google Scholar]
- 128.Ma SK, Maeda K, Abe R, Domen K. 2012. Visible-light-driven nonsacrificial water oxidation over tungsten trioxide powder modified with two different cocatalysts. Energy Environ. Sci. 5, 8390–8397 10.1039/c2ee21801a (doi:10.1039/c2ee21801a) [DOI] [Google Scholar]
- 129.Domen K, Naito S, Soma M, Onishi T, Tamaru K. 1980. Photocatalytic decomposition of water vapour on an NiO–SrTiO3 catalyst. J. Chem. Soc. Chem. Commun. 1980, 543–544 10.1039/C39800000543 (doi:10.1039/C39800000543) [DOI] [Google Scholar]
- 130.Cobo S, et al. 2012. A Janus cobalt-based catalytic material for electro-splitting of water. Nat. Mater. 11, 802–807 10.1038/nmat3385 (doi:10.1038/nmat3385) [DOI] [PubMed] [Google Scholar]
- 131.Iwase A, Ng YH, Ishiguro Y, Kudo A, Amal R. 2011. Reduced graphene oxide as a solid-state electron mediator in Z-scheme photocatalytic water splitting under visible light. J. Am. Chem. Soc. 133, 11 054–11 057 10.1021/ja203296z (doi:10.1021/ja203296z) [DOI] [PubMed] [Google Scholar]
- 132.Abe R, Takata T, Sugihara H, Domen K. 2005. Photocatalytic overall water splitting under visible light by TaON and WO3 with an IO3−/I− shuttle redox mediator. Chem. Commun. 3829–3831 10.1039/b505646b (doi:10.1039/b505646b) [DOI] [PubMed] [Google Scholar]
- 133.Maeda K, Abe R, Domen K. 2011. Role and function of ruthenium species as promoters with TaON-based photocatalysts for oxygen evolution in two-step water splitting under visible light. J. Phys. Chem. C 115, 3057–3064 10.1021/jp110025x (doi:10.1021/jp110025x) [DOI] [Google Scholar]
- 134.Higashi M, Abe R, Takata T, Domen K. 2009. Photocatalytic overall water splitting under visible light using ATaO2N (A = Ca, Sr, Ba) and WO3 in a IO3−/I− shuttle redox mediated system. Chem. Mater. 21, 1543–1549 10.1021/cm803145n (doi:10.1021/cm803145n) [DOI] [Google Scholar]
- 135.Le Goff A, Artero V, Jousselme B, Tran PD, Guillet N, Metaye R, Fihri A, Palacin S, Fontecave M. 2009. From hydrogenases to noble metal-free catalytic nanomaterials for H-2 production and uptake. Science 326, 1384–1387 10.1126/science.1179773 (doi:10.1126/science.1179773) [DOI] [PubMed] [Google Scholar]
- 136.Tran PD, et al. 2012. Copper molybdenum sulfide: a new efficient electrocatalyst for hydrogen production from water. Energy Environ. Sci. 5, 8912–8916 10.1039/c2ee22611a (doi:10.1039/c2ee22611a) [DOI] [Google Scholar]