Abstract
Crowding in human transport networks reduces efficiency. Efficiency can be increased by appropriate control mechanisms, which are often imposed externally. Ant colonies also have distribution networks to feeding sites outside the nest and can experience crowding. However, ants do not have external controllers or leaders. Here, we report a self-organized negative feedback mechanism, based on local information, which downregulates the production of recruitment signals in crowded parts of a network by Lasius niger ants. We controlled crowding by manipulating trail width and the number of ants on a trail, and observed a 5.6-fold reduction in the number of ants depositing trail pheromone from least to most crowded conditions. We also simulated crowding by placing glass beads covered in nest-mate cuticular hydrocarbons on the trail. After 10 bead encounters over 20 cm, forager ants were 45 per cent less likely to deposit pheromone. The mechanism of negative feedback reported here is unusual in that it acts by downregulating the production of a positive feedback signal, rather than by direct inhibition or the production of an inhibitory signal.
Keywords: crowding, pheromone trails, recruitment, negative feedback, foraging, traffic
1. Introduction
Both human and insect societies face the challenge of coordinating many individuals. Top-down hierarchical control is evident in human organizations such as government, business corporations and the military. However, many modern challenges, such as dynamic task allocation in factories and routing of data and goods, can be too complex for any one controller to manage or even to have a global view of events [1]. Insect societies face similar challenges and have evolved bottom-up self-organized mechanisms to regulate collective behaviours. In bottom-up organization, individuals implementing behavioural rules interact with each other and the environment, which results in higher-level patterns and organization emerging [2].
Collective behaviours in social insects, including foraging and nest-site selection, are mediated in part by positive feedback loops, in which a response, either directly or indirectly, intensifies itself. For example, foragers returning from a food source deposit a pheromone trail, which upregulates another factor: more workers leave the nest, follow the trail and feed. This, in turn, upregulates the first response. Successful individuals, such as a scout who has found a new nest or feeding site, recruit nest-mates by making an appropriate signal such as a waggle dance or by laying pheromone [3,4]. Such positive feedback loops tend to exaggerate small initial differences in signal strengths. For example, two food sources may be discovered by a colony simultaneously, and both simultaneously recruited to. If one food source is recruited to slightly more strongly, then the small initial difference will result in more individuals reaching that food source, leading to yet more individuals recruiting to the food source. Small initial differences in recruitment can arise by chance, or may be due to differential recruitment as a result of resource quality [2,4–8] or other factors [9–11]. Such positive feedback loops lead to rapid group-level decisions, but can result in colonies becoming ‘trapped’ in suboptimal decisions, as the signal to one food source becomes too strong to be overcome [6,12].
Positive feedback is often halted or modulated via negative effects. These negative effects may be passive and simple, such as by the decay of pheromone trails in ants [13] or ants on an overcrowded route ‘pushing’ other ants onto an alternative route [14]. The negative effects may be in the form of negative feedback, in which a response causes, either directly or indirectly, a weakening of itself. Negative feedback may be passive—not require any active behaviours. For example, overcrowding at a food source preventing more foragers from feeding, and thus disrupting the feeding > returning > recruiting > feeding cycle [15]. Negative feedback may also active, in the form of a signal. Examples of inhibitory signals include the stop signal used by honeybees to reduce recruitment to dangerous foraging location [16,17] or competing alternative nest sites [18], and the ‘no entry’ trail pheromone used by Pharaoh ants to deter foragers from taking the wrong branch at a trail bifurcation [19]. Negative feedback may also be implemented by an active decision to reduce a response, such as reduced trail pheromone deposition in response to strongly developed pheromone trails [20,21].
Overcrowding in a trail network leads to a decrease in traffic flow with a subsequent loss of efficiency. Here, efficiency refers to the number of individuals or loads that can use or be transported via a trail per unit trail per unit time. For example, net walking speeds of leaf-cutter ants affected by head-on collisions were reduced by ca 20 per cent, with a corresponding reduction in efficiency [22]. Colonies of Lasius niger ants can adjust their foraging in parts of a trail system in response to crowding. For example, longer routes are used more when shorter routes are overcrowded [14,23]. This adjustment is mediated at least in part by ants being ‘pushed’ onto the longer route [14]: when a forager arrives at a junction and attempts to walk down a heavily crowded branch, she may be ‘pushed’ by the ants already on that branch, and thus be forced onto the less crowded branch. In this way, direct environmental constraints, in this case crowding, can lead to the emergence of improved network use without any explicit adjustment of the information shared between individual foragers [24,25]. Similarly, when encountering a narrow point on a crowded trail, L. niger foragers self-organize, via a system of ants pushing through the bottleneck and other ants following close behind, to form alternating groups of ants crossing the bottleneck. This allows high traffic levels, and so high efficiency, to be maintained in spite of crowding at a particular point [26]. However, responses to crowding are not only passive. Leaf-cutter ants can respond to decreased travel efficiency by a concurrent increase in transport efficiency: when challenged with a narrow bridge to a food source, a larger proportion of Atta colombica workers return to the nest carrying loads, more than compensating for the reduced rate of traffic flow [27]. In this study, we test and support the hypothesis that L. niger foragers actively respond to crowding by depositing less trail pheromone. As such, crowding causes negative feedback by downregulating the production of a positive feedback signal—the deposition of trail pheromone.
2. Methods
2.1. Study species
Colonies of the black garden ant, L. niger, were collected at the University of Sussex campus and housed in plastic foraging boxes (40 × 30 × 20 cm). The bottom of each box was covered with plaster of Paris and contained a circular plaster nest (14 cm diameter, 2 cm height). Colonies were queenless with 500–1000 workers and small amounts of brood. Colonies were fed three times per week with Bhatkar jelly [28] and deprived of food for 4 days prior to each trial in order to give high and consistent motivation to forage and recruit to experimental sucrose syrup feeders. Water was provided ad libitum.
2.2. Part 1: effect of trail crowding
This experiment was designed to investigate the effect of trail crowding on pheromone deposition rates by foraging ants walking between the nest and feeder. Five colonies were used. A hungry colony was allowed access to a 20 cm long walkway covered with a printer paper overlay, leading to a 1 M sucrose syrup feeder at the end (figure 1). The walkway was either 0.5 cm (narrow) or 2 cm (wide) in width. Walking L. niger workers are about 2.5 mm wide across their antennae, so ants passing on the narrow trail almost invariably contact each other on the narrow walkway. Ants were either allowed freely onto the bridge (many ants—mean ant flow rate 30.34 ants per minute, s.d. 15.69) or restricted (few ants—mean ant flow rate 3.10 ants per minute, s.d. 1.67). In the restricted treatment, only the first seven to nine ants to reach the feeder allowed to continue foraging and moving freely between the feeder and nest; additional ants were excluded by only lowering the drawbridge when a marked ant approached it. In all trials, the first seven to nine ants to reach the feeder were individually marked with a dot of acrylic paint being painted on their abdomen as they fed. Foraging was then allowed to proceed for 30 min from the time the first ant found the feeder. The walkway was video-recorded from above, using a high-definition camera (Sony HDR-XR520). A mirror angled at 45° was placed beside the trail, allowing the video camera to capture views of walking ants both from above and side (figure 1). The side view allowed pheromone depositions to be clearly detected. Pheromone deposition in L. niger is a highly stereotyped behaviour in which the ant pauses for ca 0.2 s and presses the tip of her abdomen firmly on the substrate [29]. This behaviour is easily observed and counted. Pheromone deposition behaviour has been used by previous authors as a proxy of the amount of pheromone deposited on a trail [2,29–31], and was used as such in the following experiments as well.
Figure 1.
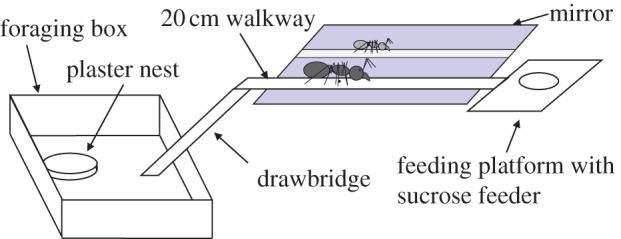
Experimental set-up. In experiment 1, either a free flow of ants was allowed to and from the feeder, or only the first seven to nine ants to find the feeder were allowed onto the walkway, the rest being excluded by only lowering the drawbridge as a marked ant approaches it. The walkway was either narrow (5 mm) or wide (20 mm). A mirror placed by the walkway at 45° allows the ants to be observed from above and the side simultaneously. In experiment 2, 10 glass beads, either black or clear, either coated in nest-mate cuticular hydrocarbons or not, were evenly spaced along the walkway, which was 5 mm wide. As a negative control in experiment 2, no beads were placed on the walkway. (Online version in colour.)
Individually marked ants were followed throughout a trial, recording both pheromone depositions on each trip to or from the feeder and head-on contacts with other ants. In addition, pheromone deposition behaviours made by all ants, and the number of head-on contacts between ants, were counted on the 4 cm section of trail nearest to the feeder as a proxy for the total number of depositions and head-on encounters performed on the trail as a whole. Five colonies were tested, and each colony was tested in all four treatment combinations (wide path/many ants, wide path/few ants, narrow path/many ants and narrow path/few ants). The paper overlay on the walkway was replaced, and the plastic walkway backing cleaned with ethanol after every trial.
2.3. Part 2: simulating crowding with glass beads
To further investigate how ants perceive crowding and to control for possible auto-correlation between the number of ants on the trail and the amount of trail pheromones on the trail (more ants on the trail result in more pheromone on the trail, and separating these effect statistically may not be possible), we ran an experiment with glass beads (artificial ants) coated in nest-mate cuticular hydrocarbons (CHCs). CHCs are used by ants to identify and discern nest-mates and non-nest-mates [32–34]. CHCs-coated glass beads have been used successfully to mimic both nest-mate [35–38] and non-nest-mate ants [33,36,38,39]. To prepare the beads, we collected 10 workers from the test colony, chilled them for 2 min at −20°C and then placed them in a glass vial with 500 μl pentane for 10 min to dissolve CHCs. Drops (2.5 μl) of solution per bead were then repeatedly dripped over 10 black glass beads (diameter 2.5 mm, height 1 mm—KnorrPrandell GmbH, Germany), allowing the pentane to evaporate and deposit the CHCs on the beads, until all the pentane had been dripped over the beads. CHCs were extracted and beads were prepared immediately prior to use. The 10 beads were then placed at 2 cm intervals on the 20 cm long walkway, and a single marked ant was allowed to make two return trips to the feeder. The walkway was 0.5 cm wide to ensure that marked ants contacted the beads. Trail pheromone deposition rates were recorded for each journey. The paper overlay on the walkway was replaced, and the plastic walkway cleaned with ethanol after every test. Four ants from each of 10 colonies were tested on each treatment. After each trial, the ant was removed from the colony.
Given that L. niger are black, we also tested the hypothesis that the colour of the glass beads, black versus clear, as well as the presence of CHCs (CHC+ versus CHC−) affects the perception of foragers. Four ants from each colony were also tested with the following treatments: clear glass beads coated with CHCs, uncoated (blank) black beads, uncoated (blank) clear beads and no beads. The same beads were used for each of the four ants tested per colony per treatment. Treatment orders were pseudo-randomized.
2.4. Statistical analyses
Data were analysed using generalized linear-mixed models in the statistical package R v. 2.9.2 [40]. Models were fitted using the lmer function [41]. Model selection followed Zuur et al. [42]. We first constructed a saturated model, including all predictor variables we had an a priori reason for testing, and all interactions between them. Only three-way interactions or lower were modelled. Random effect structures were explored, and competing models compared using their Akaike information criterion. Random effects included were colony (in all analyses) and ant (where individual ants' behaviour was followed over multiple visits). We removed non-significant effects and interactions, then explored the significance of fixed effects. Interaction effects were explored by making subsets. For example, if a significant interaction was found between trail width and collision rates, then the data would be split into wide and narrow trail treatments, and the effect of collision rates explored in both subsets. Binomial data, such as whether ants deposited pheromone or not, were analysed using a binomial distribution, and count data, such as number of pheromone depositions per ant, were modelled on a Poisson distribution. All p-values presented are adjusted using the Benjamini–Hochberg [43] correction to account for multiple testing.
3. Results
3.1. Part 1: effect of crowding
The number of ants on the trail (many or few), trail width (wide or narrow), visit number (e.g. first, second, nth visit to the feeder for an individually marked ant) and the number of head-on encounters were used as predictor variables.
3.2. Individual ants
As shown in figure 2, fewer trail pheromone depositions were made on both narrower and more crowded trails. There was a significant interaction between trail width and ant density (p = 0.0232, z = −2.55; figure 2): When many ants were present on a trail, focal ants performed fewer pheromone depositions on narrow than on wide trails (p = 0.00017, z = −3.928). However, when the number of ants on the trail was low, there was no effect of trail width on pheromone deposition (p = 0.507, z = −0.664). Focal ants made fewer pheromone depositions on later visits (both trail treatments p < 0.0001, z < 8), and this trend was more pronounced when many ants were on the trail (interaction: p < 0.0001, z = −14.483). This can be seen in figure 2 by the steeper decline in the curve in the many-ants treatments. This finding is mirrored in the trail width treatment, with ants on narrow trails initially making less pheromone depositions than ants on wide trails (z = −5.139, p < 0.0001). Notably, ants on narrow trails and under high crowding conditions deposit no pheromone at all after making a few visits (figure 2). In addition, we found that on narrow trails more collisions resulted in focal ants making fewer pheromone deposition behaviours (p = 0.00017, z = −3.931). There was no significant relationship between collision rates and pheromone deposition on wide trails (p = 0.507, z = −0.664, interaction between collisions and trail width: p = 0.000145, z = 3.888). The changes in total trail pheromone depositions were driven primarily by a reduction in the probability of ants depositing trail pheromone at all. Reduction in the number of pheromone-laying behaviours by individual ants played a smaller role (see electronic supplementary material, S1).
Figure 2.
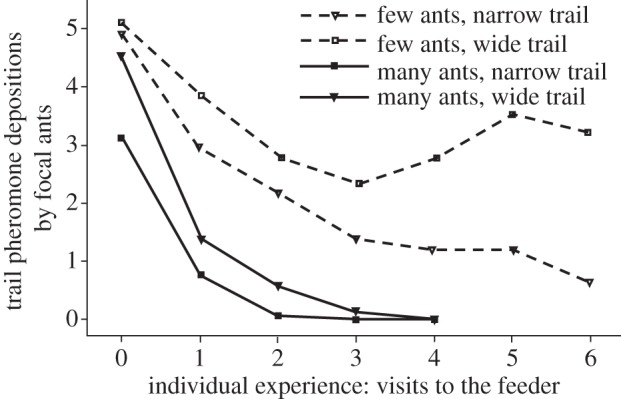
Effect of trail width (narrow, 5 mm, versus wide, 20 mm), crowding (few versus many ants) and individual experience, in terms of number of previous visits to the feeder, on pheromone-laying behaviour by individual foraging ants.
3.3. All ants
Total pheromone depositions on the first 4 cm of the trail nearest to the feeder gave a similar picture to the data obtained by observing individual ants (above). Less pheromone depositions in total were made on narrow than on wide trails at high ant numbers (p < 0.0001, z =−8.584) but not at low ant numbers (p = 0.894, z =−0.189, interaction between trail width and ant: p = 0.00159, z = −3.255; for details, see electronic supplementary material, figure S2a). Unexpectedly, we found that when many ants were on the trail there was a positive correlation between collision number and total pheromone depositions (p = 0.000171, z =−3.931), although we did not find this when few ants were on the trail (p = 0.507, z =−0.664; interaction between collision rate and ant number treatment: p < 0.0001, z =−4.429). This resulted in more pheromone depositions with higher ant density on the wide trail but not on the narrow trail (see the electronic supplementary material, figure S2a). However, if we consider pheromone depositions per ant (see the electronic supplementary material, figure S2b), then we find fewer depositions per ant when many ants are on the trail (p < 0.00191, z =−3.189) and when the trail is narrow (p = 0.0233, z =−2.267), mirroring the data from individual ants. We also find that more collisions result in less depositions per ant (p = 0.00142, z = −3.386). Similar to the results for individual ants, these effects are mainly driven by a reduction in the proportion of ants depositing pheromone at all, not by a reduction in the number of pheromone depositions per depositing ant (see the electronic supplementary material, S2). To illustrate this, we note that there was a 5.6-fold decrease in the proportion of ants depositing pheromone on the narrow, crowded treatment than on the wide trail with few ants (mean 0.055, s.d. 0.077 versus mean 0.315 s.d. 0.290; for graphs and statistical analysis, see electronic supplementary material, S2).
3.4. Part 2: crowding with glass beads
There were four bead treatments: black beads with CHC (black CHC+), clear beads with CHC (clear CHC+), black beads without CHC (black CHC−), clear beads without CHC (clear CHC−) and one control (no beads). As figure 3 shows, there were significant differences in pheromone deposition between treatments (see figure 3 and electronic supplementary material, S3 and table S1a). The number of visits an ant made to the feeder was not a significant predictor of its deposition probability (p = 0.3355, z = 1.081). Treatments were also compared in terms of pheromone depositions per journey by all ants (excluding the first journey to the food, when ants never deposit pheromone), in terms of whether ants deposited pheromone or not, and in terms of the number of depositions by depositing ants (see electronic supplementary material, table S3a–c).
Figure 3.
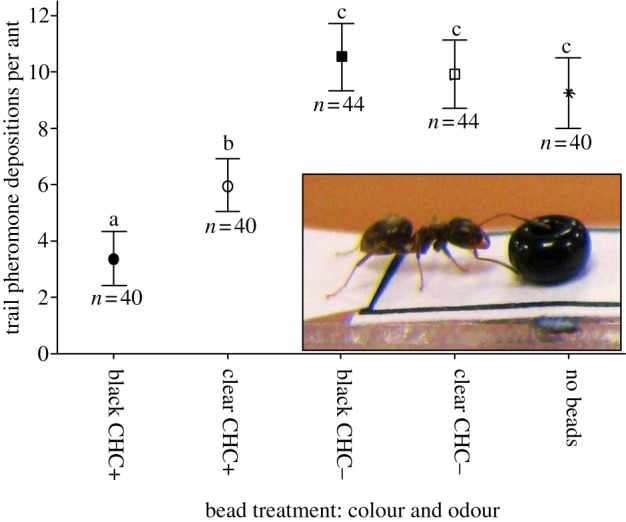
Effects of bead treatments on the number of pheromone depositions performed. Beads could either be black or clear, and either covered by CHCs (CHC+) or not (CHC−), or could be absent altogether, resulting in four treatments: black CHC+, black CHC−, clear CHC+, clear CHC− and a control (no beads). Dots represent means, whiskers 95% CI. Treatments headed by the same letter are not significantly different. n number of ants observed. Inset: an ant walking off the bridge (left) encountering and antennating a black bead coated in CHCs. (Online version in colour.)
The presence of black CHC+ beads reduced the average pheromone depositions of ants compared with all other treatments (see figure 3 and electronic supplementary material, S3). The presence of clear CHC+ beads also caused a reduction in the average number of pheromone depositions compared with the blank bead and control treatments (see figure 3 and electronic supplementary material, S3). The average number of pheromone depositions when blank beads (either black or clear) were placed on the path, or no beads were on the path, was not significantly different (see figure 3 and electronic supplementary material, S3). To determine whether the reduction in total pheromone depositions was driven by ants making fewer pheromone depositions, or ants choosing not to deposit pheromone at all, we also analysed the effect of the treatments in terms of proportion of ants depositing pheromone, and number of pheromone depositions per depositing ant (see the electronic supplementary material, S3). Both a reduction in depositions per ant and the proportion of ants depositing pheromone play a role in the reduction of total pheromone deposition in the CHC+ trials. To illustrate this, both the proportion of ants depositing pheromone and the number of pheromone depositions by depositing ants were significantly lower when comparing the black CHC+ treatment with the control (no beads) treatment (proportion of ants depositing pheromone mean 0.51, s.d. 0.5 versus mean 0.83 s.d. 0.27—a 45% reduction. Depositions per depositing ant mean 3.4 s.d. 5.3 versus mean 9.3 s.d. 6.9—a 64% reduction).
4. Discussion
Our results show clearly that crowding on trails reduces the number of pheromone depositions performed by each foraging ant. Ants on crowded trails are both less likely to deposit pheromone at all, and perform fewer deposition behaviours when they do deposit pheromone at least once. Foragers assess crowding on trails at least in part by noting the number of times they collide with nest-mates. An object is assessed as a nest-mate primarily by the presence of CHCs, but also by its colour. Black beads, which are the same colour as the ants, have a stronger effect than clear beads.
Although crowding reduced the number of pheromone depositions per ant, it is noteworthy that the total number of pheromone depositions on the wide trail is nevertheless higher when many ants are allowed onto a trail (see electronic supplementary material, figure S2a). This is reasonable, as the colony was hungry and had access to only one food source that was of high quality (1 M sucrose). However, the extent of the positive feedback signal, in terms of pheromone deposition to that food source, was reduced. In other words, crowding in the L. niger foraging system downregulates positive feedback. This dampening of positive feedback can indeed bring pheromone deposition to a halt, as can be seen in figure one for visit three onwards at the highest crowding treatment. Our interpretation is that foraging ants have determined that the level of foragers on the trail is sufficient, and no more foragers should be recruited. Recruitment to crowded trails is thus lower than what it would have been without this negative feedback. Reduction in the number of foraging ants on part of the trail system can also be caused via other negative influences, such as pheromone decay [15,30], feeder abandonment and cessation of foraging as a result of satiation [31].
We hypothesize that the modulation of positive feedback detected in this study probably plays several roles, including reducing unnecessary expenditure of metabolically expensive pheromone, adjusting and limiting the number of workers recruited to a food source in relation to the capacity of the trail to handle traffic, and preventing heavily used trails from becoming so strongly marked with trail pheromone that other trails cannot develop [6]. In addition, by possessing an active response to trail crowding, a colony may be able to react to crowding on straight sections of trail in addition to at trail bifurcations. This may not be possible if ants relied solely on U-turning owing to the passive effects of crowding [23].
Our results also show that L. niger use contact rates to estimate nest-mate abundance, as previously reported in other ant species for both nest-mates and non-nest-mates [44,45]. Contact rate is a simple cue to use, as the information is gathered at little or no cost [46] and does not require actual counting [47]. However, contact rates are not the only possible source of information for determining ant density or levels of trail use. Ants monitor trail pheromone levels in order to follow trails, and can thereby incidentally collect information on trail use by nest-mates. Thus, high levels of trail pheromone on a trail can also cause ants to reduce further pheromone deposition [21]. Ants can also estimate the number of ants that have visited an area by sensing levels of home-range markings, which are CHCs deposited passively as ants walk over a substrate [46], and regulate trail deposition accordingly [48,49]. By modulating trail deposition in reference to long-term, indirect cues (home-range markings), medium-term indirect cues (trail pheromones) and short-term direct cues (encounter rates) ants can respond to changes in colony needs and the environment over a wide range of time scales.
The modulation of positive feedback described here is a further example of a worker-level behavioural rule that may play an important role in the regulation of collective foraging behaviour in ants. A picture is emerging of complex organization in social insect foraging, with a combination of positive and negative feedback loops acting to adjust the amount of trail pheromone deposited as appropriate to both environmental and colony conditions. Social insect colonies use both passive processes and active responses to information integrated from multiple internal and external sources to organize themselves in an adaptive manner.
Acknowledgements
We thank the four anonymous referees for helpful comments on a previous version of this manuscript. T.C. was supported by a PhD studentship from the BBSRC. C.G. was supported by a postdoctoral fellowship from the Swiss National Science Foundation (SNSF grant no. PA00P3 129134).
References
- 1.Papadimitriou CH. 2003. Computational complexity. In Encyclopedia of computer science (eds Ralson A, Reilly ED, Hemmendinger D.), pp. 260–265 Chichester, UK: John Wiley and Sons Ltd [Google Scholar]
- 2.Portha S, Deneubourg J, Detrain C. 2002. Self-organized asymmetries in ant foraging: a functional response to food type and colony needs. Behav. Ecol. 13, 776–781 10.1093/beheco/13.6.776 (doi:10.1093/beheco/13.6.776) [DOI] [Google Scholar]
- 3.Jarau S, Hrncir M. 2009. Food exploitation by social insec ts, 1st edn Boca Raton, FL: CRC Press [Google Scholar]
- 4.Seeley TD. 1995. The wisdom of the hive: the social physiology of honey bee colonies. Cambridge, MA: Harvard University Press [Google Scholar]
- 5.Breton J, Fourcassie V. 2004. Information transfer during recruitment in the ant Lasius niger L. (Hymenoptera: Formicidae). Behav. Ecol. Sociobiol. 55, 242–250 10.1007/s00265-003-0704-2 (doi:10.1007/s00265-003-0704-2) [DOI] [Google Scholar]
- 6.Beckers R, Deneubourg JL, Goss S, Pasteels JM. 1990. Collective decision making through food recruitment. Insectes Sociaux 37, 258–267 10.1007/BF02224053 (doi:10.1007/BF02224053) [DOI] [Google Scholar]
- 7.Beckers R, Deneubourg L, Goss S. 1992. Trails and U-turns in the selection of a path by the ant Lasius niger. J. Theor. Biol. 159, 397–415 10.1016/S0022-5193(05)80686-1 (doi:10.1016/S0022-5193(05)80686-1) [DOI] [Google Scholar]
- 8.Franks NR, Mallon EB, Bray HE, Hamilton MJ, Mischler TC. 2003. Strategies for choosing between alternatives with different attributes: exemplified by house-hunting ants. Anim. Behav. 65, 215–223 10.1006/anbe.2002.2032 (doi:10.1006/anbe.2002.2032) [DOI] [Google Scholar]
- 9.Traniello JFA, Beshers SN. 1991. Maximization of foraging efficiency and resource defense by group retrieval in the ant Formica schaufussi. Behav. Ecol. Sociobiol. 29, 283–289 10.1007/BF00163986 (doi:10.1007/BF00163986) [DOI] [Google Scholar]
- 10.Mailleux A-C. 2006. Starvation drives a threshold triggering communication. J. Exp. Biol. 209, 4224–4229 10.1242/jeb.02461 (doi:10.1242/jeb.02461) [DOI] [PubMed] [Google Scholar]
- 11.Mailleux A-C, Detrain C, Deneubourg JL. 2005. Triggering and persistence of trail-laying in foragers of the ant Lasius niger. J. Insect Physiol. 51, 297–304 10.1016/j.jinsphys.2004.12.001 (doi:10.1016/j.jinsphys.2004.12.001) [DOI] [PubMed] [Google Scholar]
- 12.Sumpter DJT, Beekman M. 2003. From nonlinearity to optimality: pheromone trail foraging by ants. Anim. Behav. 66, 273–280 10.1006/anbe.2003.2224 (doi:10.1006/anbe.2003.2224) [DOI] [Google Scholar]
- 13.Wilson EO. 1972. The insect societies. Cambridge, MA: Harvard University Press [Google Scholar]
- 14.Dussutour A, Nicolis SC, Deneubourg J-L, Fourcassié V. 2006. Collective decisions in ants when foraging under crowded conditions. Behav. Ecol. Sociobiol. 61, 17–30 10.1007/s00265-006-0233-x (doi:10.1007/s00265-006-0233-x) [DOI] [Google Scholar]
- 15.Wilson EO. 1962. Chemical communication among workers of the fire ant Solenopsis saevissima (Fr. Smith) 1. The organization of mass-foraging. Anim. Behav. 10, 134–147 10.1016/0003-3472(62)90141-0 (doi:10.1016/0003-3472(62)90141-0) [DOI] [Google Scholar]
- 16.Nieh JC. 1993. The stop signal of honey bees: reconsidering its message. Behav. Ecol. Sociobiol. 33, 51–56 10.1007/BF00164346 (doi:10.1007/BF00164346) [DOI] [Google Scholar]
- 17.Nieh J. 2010. A negative feedback signal that is triggered by peril curbs honey bee recruitment. Curr. Biol. 20, 310–315 10.1016/j.cub.2009.12.060 (doi:10.1016/j.cub.2009.12.060) [DOI] [PubMed] [Google Scholar]
- 18.Seeley TD, Visscher PK, Schlegel T, Hogan PM, Franks NR, Marshall JAR. 2011. Stop signals provide cross inhibition in collective decision-making by honeybee swarms. Science 335, 108–111 10.1126/science.1210361 (doi:10.1126/science.1210361) [DOI] [PubMed] [Google Scholar]
- 19.Robinson EJH, Jackson DE, Holcombe M, Ratnieks FLW. 2005. Insect communication: ‘No entry’ signal in ant foraging. Nature 438, 442. 10.1038/438442a (doi:10.1038/438442a) [DOI] [PubMed] [Google Scholar]
- 20.Beckers R, Deneubourg J, Goss S. 1992. Trail laying behaviour during food recruitment in the ant Lasius niger (L.). Insectes Sociaux 39, 59–71 10.1007/BF01240531 (doi:10.1007/BF01240531) [DOI] [Google Scholar]
- 21.Czaczkes TJ, Grüter C, Ratnieks FLW. 2012. Ant foraging on complex trails: route learning and the role of trail pheromones in lasius niger. J. Exp. Biol. 216, 188–197 10.1242/jeb.076570 (doi:10.1242/jeb.076570) [DOI] [PubMed] [Google Scholar]
- 22.Burd M, Aranwela N. 2003. Head-on encounter rates and walking speed of foragers in leaf-cutting ant traffic. Insectes Sociaux 50, 3–8 10.1007/s000400300001 (doi:10.1007/s000400300001) [DOI] [Google Scholar]
- 23.Dussutour A, Fourcassie V, Helbing D, Deneubourg J-L. 2004. Optimal traffic organization in ants under crowded conditions. Nature 428, 70–73 10.1038/nature02345 (doi:10.1038/nature02345) [DOI] [PubMed] [Google Scholar]
- 24.Dussutour A, Deneubourg J-L, Fourcassié V. 2005. Temporal organization of bi-directional traffic in the ant Lasius niger (L.). J. Exp. Biol. 208, 2903–2912 10.1242/jeb.01711 (doi:10.1242/jeb.01711) [DOI] [PubMed] [Google Scholar]
- 25.Czaczkes TJ, Ratnieks FLW. 2011. Simple rules result in the adaptive turning of food items to reduce drag during cooperative food transport in the ant Pheidole oxyops. Insectes Sociaux 58, 91–96 10.1007/s00040-010-0121-2 (doi:10.1007/s00040-010-0121-2) [DOI] [Google Scholar]
- 26.Dussutour A, Beshers S, Deneubourg JL, Fourcassié V. 2009. Priority rules govern the organization of traffic on foraging trails under crowding conditions in the leaf-cutting ant Atta colombica. J. Exp. Biol. 212, 499–505 10.1242/jeb.022988 (doi:10.1242/jeb.022988) [DOI] [PubMed] [Google Scholar]
- 27.Dussutour A, Beshers S, Deneubourg J-L, Fourcassié V. 2007. Crowding increases foraging efficiency in the leaf-cutting ant Atta colombica. Insectes Sociaux 54, 158–165 10.1007/s00040-007-0926-9 (doi:10.1007/s00040-007-0926-9) [DOI] [Google Scholar]
- 28.Bhatkar A, Whitcomb WH. 1970. Artificial diet for rearing various species of ants. Florida Entomol. 53, 229–232 10.2307/3493193 (doi:10.2307/3493193) [DOI] [Google Scholar]
- 29.Beckers R, Deneubourg JL, Goss S. 1993. Modulation of trail laying in the ant Lasius niger (Hymenoptera: Formicidae) and its role in the collective selection of a food source. J. Insect Behav. 6, 751–759 10.1007/BF01201674 (doi:10.1007/BF01201674) [DOI] [Google Scholar]
- 30.Jaffe K, Howse PE. 1979. The mass recruitment system of the leaf cutting ant, Atta cephalotes (L.). Anim. Behav. 27, 930–939 10.1016/0003-3472(79)90031-9 (doi:10.1016/0003-3472(79)90031-9) [DOI] [Google Scholar]
- 31.Grüter C, Schürch R, Czaczkes TJ, Taylor K, Durance T, Jones SM, Ratnieks FLW. 2012. Negative feedback enables fast and flexible collective decision-making in ants. PLoS ONE 7, e44501. 10.1371/journal.pone.0044501 (doi:10.1371/journal.pone.0044501) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lahav S, Soroker V, Hefetz A, VanderMeer RK. 1999. Direct behavioral evidence for hydrocarbons as ant recognition discriminators. Naturwissenschaften 86, 246–249 10.1007/s001140050609 (doi:10.1007/s001140050609) [DOI] [Google Scholar]
- 33.Wagner D, Tissot M, Cuevas W, Gordon DM. 2000. Harvester ants utilize cuticular hydrocarbons in nestmate recognition. J. Chem. Ecol. 26, 2245–2257 10.1023/A:1005529224856 (doi:10.1023/A:1005529224856) [DOI] [Google Scholar]
- 34.van Zweden JS, d’ Ettorre P. 2010. Nestmate recognition in social insects and the role of hydrocarbons. In Insect hydrocarbons: biology, biochemistry and chemical ecology (eds Blomquist GJ, Bagnères A-G.), pp. 222–243 Cambridge, UK: Cambridge University Press [Google Scholar]
- 35.Greene MJ, Gordon DM. 2003. Social insects: cuticular hydrocarbons inform task decisions. Nature 423, 32. 10.1038/423032a (doi:10.1038/423032a) [DOI] [PubMed] [Google Scholar]
- 36.Ozaki M, Wada-Katsumata A, Fujikawa K, Iwasaki M, Yokohari F, Satoji Y, Nisimura T, Yamaoka R. 2005. Ant nestmate and non-nestmate discrimination by a chemosensory sensillum. Science 309, 311–314 10.1126/science.1105244 (doi:10.1126/science.1105244) [DOI] [PubMed] [Google Scholar]
- 37.Greene MJ, Gordon DM. 2007. Interaction rate informs harvester ant task decisions. Behav. Ecol. 18, 451–455 10.1093/beheco/arl105 (doi:10.1093/beheco/arl105) [DOI] [Google Scholar]
- 38.Akino T, Yamamura K, Wakamura S, Yamaoka R. 2004. Direct behavioral evidence for hydrocarbons as nestmate recognition cues in Formica japonica (Hymenoptera: Formicidae). Appl. Entomol. Zool. 39, 381–387 10.1303/aez.2004.381 (doi:10.1303/aez.2004.381) [DOI] [Google Scholar]
- 39.Martin SJ, Vitikainen E, Helanterä H, Drijfhout FP. 2008. Chemical basis of nest-mate discrimination in the ant Formica exsecta. Proc. R. Soc. B 275, 1271–1278 10.1098/rspb.2007.1708 (doi:10.1098/rspb.2007.1708) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.R Development Core Team 2009. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing [Google Scholar]
- 41.Bates D, Sarkar D, Bates MD, Matrix LT. 2007. The lme4 package. Linear Mixed-effects models using S 4. See http://212.219.56.142/sites/lib.stat.cmu.edu/R/CRAN/doc/packages/lme4.pdf. [Google Scholar]
- 42.Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM. 2009. Mixed effects models and extensions in ecology with R. Berlin, Germany: Springer [Google Scholar]
- 43.Benjamini Y, Hochberg Y. 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. B 57, 289–300 [Google Scholar]
- 44.Gordon DM, Paul RE, Thorpe K. 1993. What is the function of encounter patterns in ant colonies? Anim. Behav. 45, 1083–1100 10.1006/anbe.1993.1134 (doi:10.1006/anbe.1993.1134) [DOI] [Google Scholar]
- 45.Hölldobler B. 1981. Foraging and spatiotemporal territories in the honey ant Myrmecocystus mimicus wheeler (Hymenoptera: Formicidae). Behav. Ecol. Sociobiol. 9, 301–314 10.1007/BF00299887 (doi:10.1007/BF00299887) [DOI] [Google Scholar]
- 46.Detrain C, Deneubourg JL. 2009. Social cues and adaptive foraging strategies in ants. In Food exploitation by social insects (eds Jarau S, Hrncir M.), pp. 29–54. Boca Raton, FL: CRC Press. [Google Scholar]
- 47.Gordon DM. 1999. Interaction patterns and task allocation in ant colonies. In Information processing in social insects, pp. 51–67 Basel, Switzerland: Birkhäuser [Google Scholar]
- 48.Devigne C, Renon A, Detrain C. 2004. Out of sight but not out of mind: modulation of recruitment according to home range marking in ants. Anim. Behav. 67, 1023–1029 10.1016/j.anbehav.2003.09.012 (doi:10.1016/j.anbehav.2003.09.012) [DOI] [Google Scholar]
- 49.Czaczkes TJ, Grüter C, Jones SM, Ratnieks FLW. 2011. Synergy between social and private information increases foraging efficiency in ants. Biol. Lett. 7, 521–524 10.1098/rsbl.2011.0067 (doi:10.1098/rsbl.2011.0067) [DOI] [PMC free article] [PubMed] [Google Scholar]


