Abstract
Background
The percentage of preterm births in Germany is high at 9%, but stable. 77% of cases of perinatal death are in prematurely born infants. Intensive research efforts are being directed toward the development of new means of primary and secondary prevention, diagnostic assessment, and pharmacotherapy of premature labor.
Methods
We review pertinent publications that were retrieved by a selective search of the literature from 1966 to 2012, including current meta-analyses from the Cochrane database and the guidelines of German and foreign obstetric societies.
Results
Preterm labor is a multifactorial problem. The current treatment options are symptomatic, rather than causally directed. Preventive treatment with progesterone can lower the rate of preterm birth in high-risk groups by more than 30%. Transporting the pregnant women to an appropriately qualified perinatal care center and induction of fetal lung maturation lowers perinatal mortality. A variety of tocolytic drugs with different mechanisms of action (betamimetics, oxytocin antagonists, calcium-channel blockers, NO donors, and inhibitors of prostaglandin synthesis) can be used for individualized tocolytic treatment. Premature rupture of the membranes is an indication for antibiotics.
Conclusion
The goal of all attempts to prevent and treat preterm labor is to improve preterm infants’ chances of surviving with as few complications as possible. The methods discussed here can be used to prolong pregnancies at risk for preterm labor and so to reduce perinatal morbidity and mortality.
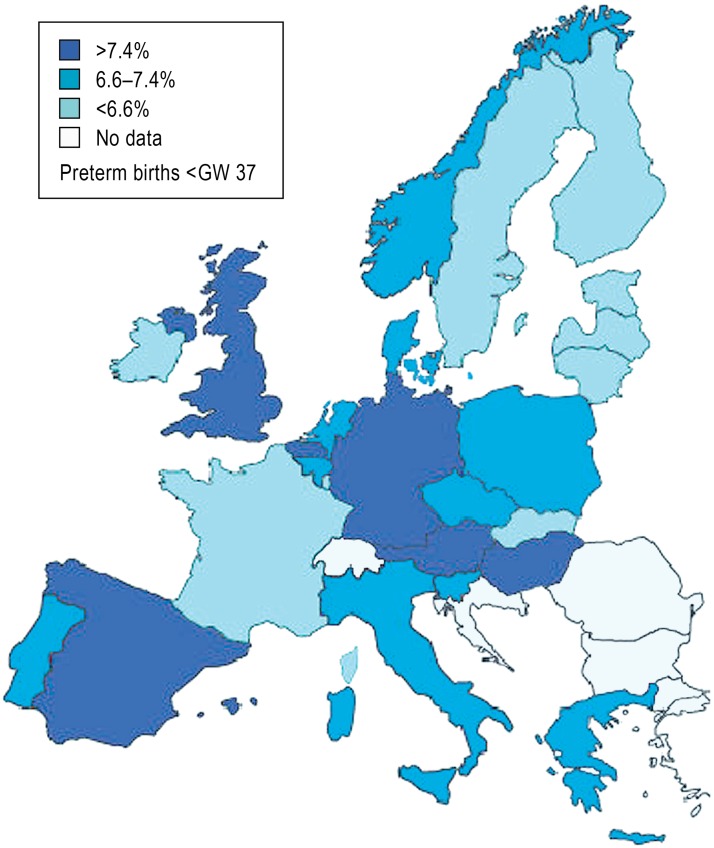
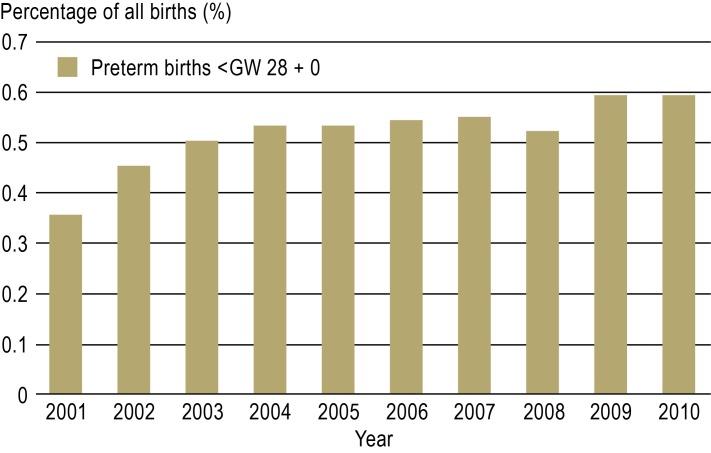
Preterm birth, defined as birth before gestational week (GW) 37 + 0, is a central problem in obstetrics and the single most important risk factor for perinatal morbidity and mortality (1). In 2011, 9% of all children born in Germany were born before the end of GW 37 (2). This rate is high compared to that of most other European countries (3) (Figure 1); it has remained stable over the last 10 years, yet the rate of extremely premature birth, i.e., birth before GW 28, has risen by 65% (Figure 2). Although the reasons for this development are not yet fully clear, it is attributed in large part to known demographic factors such as the trend toward higher maternal age in pregnancy and the rising prevalence of diabetes mellitus (4).
Figure 1.
The frequency of preterm birth before the end of the 37th week of gestation
(GW 37) in Europe, modified from the European Perinatal Health Report 2008 (3)
Figure 2.
The percentage of very early preterm births (before GW 28) in Germany, 2001–2010
In 2010, 77% of perinatal deaths were of prematurely born infants (2). Mortality was especially high (32%) for infants born before GW 28, while late preterm infants, i.e., those born after GW 32, still had 1.3% perinatal mortality (more than ten times that of non-premature infants). In addition to high mortality, very small preterm infants are at high risk for serious long-term complications (2).
The goal of all attempts to prevent and treat premature labor is to improve newborn infants’ chances of surviving with as few complications as possible.
Learning objectives
In this paper, we discuss the following topics:
the pathophysiology of premature labor
the primary and secondary prevention of premature labor
the diagnostic evaluation of premature labor
the pharmacotherapy of premature labor (tocolysis).
Premature neonates.
The goal of all attempts to prevent and treat premature labor is to improve newborn infants’ chances of surviving with as few complications as possible.
Methods
We selectively searched the PubMed database for articles published from 1966 to 2012 containing the key words “preterm delivery,” “preterm birth,” “tocolysis,” and “tocolytic therapy” in order to identify all relevant randomized controlled trials, systematic reviews, and meta-analyses. The search was limited to studies in human beings and to publications in English or German. The current guidelines of the European, British, and American obstetric societies were also considered in the analysis (5– 7).
The commonest causes of preterm birth.
Ascending infection
Hypoxic-ischemic damage to the uteroplacental unit
Chronic stress
Fetal and uterine developmental malformations
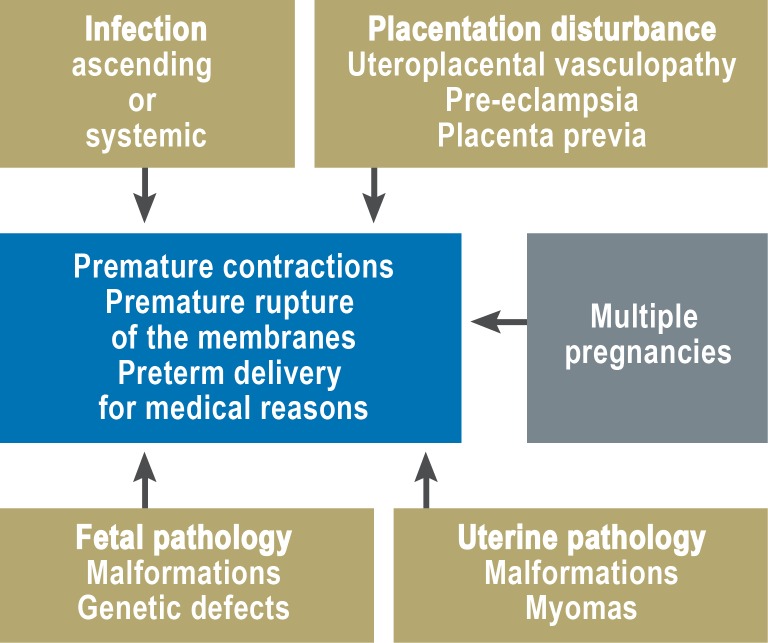
Preterm birth as a multifactorial problem
Premature labor can be thought of as the final common pathway of a variety of pathophysiological processes (Figure 3).
Figure 3.
The pathophysiology of premature labor (after 24)
Its causes include ascending infection, hypoxic-ischemic damage of the uteroplacental unit, chronic stress, and fetal and uterine developmental malformations (8).
The main risk factors for preterm birth are:
a history of obstetrical problems (previous preterm births or late miscarriages) (odds ratio [OR] 3.412, 95% confidence interval [CI] 1.342–8.676)
unfavorable socioeconomic status (low educational attainment: OR 1.75, 95% CI 1.65–1.86)
single mother (OR 1.61, 95% CI 1.26–2.07)
unhealthful lifestyle (smoking [OR 1.7, 95% CI 1.3–2.2], poor nutrition or malnutrition)
multiple pregnancy (ca. 10% of all preterm births)
maternal age under 18 years (OR 1.70, 95% CI 1.02–3.08) or over 35 years (9).
The prevention of premature labor
There have been many studies on the prevention of premature labor, and the Cochrane database alone contains 17 meta-analyses on the subject (10).
The goal of primary prevention is to lower the overall prevalence of premature labor by improving maternal health in general and by avoiding risk factors before or during pregnancy (8).
Smoking cessation alone lowers the risk of preterm birth significantly (OR 0.84, 95% CI 0.72–0.98) (8). On the other hand, mothers who are either underweight or obese, with a body-mass index (BMI) above 35, have a significantly higher risk of preterm birth. Mothers should make use of the nutritional counseling that is included in Germany as a regular component of preventive care in pregnancy. For women with stressful jobs, physicians may recommend a lower workload or even a temporary cessation of work to lower the risk of preterm birth.
The goal of secondary prevention is the early identification of pregnant women at an elevated risk of going into labor prematurely, so that these women can be helped to carry their pregnancies to term.
The main risk factors.
Poor nutrition and malnutrition
Multiple pregnancy
Maternal age
Unfavorable living situation
Prior preterm births or miscarriages
Secondary prevention measures
Self-measurement of the vaginal pH
As originally described by E. Saling, the vaginal pH value can be used as a marker for bacterial vaginosis, which, in turn, raises the risk of premature labor by a factor of 2.4 (95% CI 1.63–3.54) (11). If the pH is found to be elevated, antibiotics are given. A local interventional study (the Thuringia Preterm birth Prevention Project, Thüringer Frühgeburtenvermeidungsaktion) has yielded promising results. In a subsequent nation-wide pilot project sponsored by German statutory health-insurance carriers, a significantly reduced frequency of birth weight under 1500 g was demonstrated (OR 0.79, 95% CI 0.66–0.95), but due to considerable methodological deficiencies, these results cannot be generalized (11).
Cervix length measurement by transvaginal ultrasonography
The utility of transvaginal cervix length measurement for assessing the risk of preterm birth has been well documented in a structured analysis of 14 trials that included a total of 2258 pregnant women (12). The accepted cutoff value for cervix length is ≤ 25 before GW 24 (OR 2.76, 95% CI 2.41–3.17). The predictive value of a negative test is high (92%); this implies that pregnant women who are found not to have a shortened cervix can be reassured, and unnecessary therapeutic measures can be avoided.
Cerclage and complete closure of the birth canal
Cervical cerclage is a commonly performed operation for stabilizing and mechanically closing the cervical canal, as if with a purse-string suture. Prophylactic early complete closure of the birth canal, as described by Saling, is intended to prevent ascending infection, but its benefit has not been documented in prospective randomized trials. The German and foreign obstetric societies have not issued any binding recommendations for the indications and/or technique of either of these two interventions. A meta-analysis has revealed that, at least for a defined high-risk group of pregnant women who have had a preterm birth in the past and who now have a shortened cervix, perinatal morbidity and mortality can be significantly lowered (OR 0.64, 95% CI 0.45–0.91) (13).
The goal of secondary prevention.
... is the early identification of pregnant women at an elevated risk of going into labor prematurely, so that these women can be helped to carry their pregnancies to term.
Progesterone supplementation
The most important single advance of the past decade has been the introduction of progesterone supplementation for the prevention of premature labor. The likelihood of preterm birth can be lowered by more than 30%, both in women with a prior history of preterm birth (OR 0.65, 95% CI 0.54–0.79) (14) and in those whose cervix is currently shortened (OR 0.69, 95% CI 0.55–0.88) (15).
Progesterone can also be used beneficially for secondary prevention after tocolysis, although no benefit has been demonstrated in twin pregnancies (15). The available evidence supports the recommendation that all pregnant women who have either a prior history indicating increased risk or current, asymptomatic cervical insufficiency should receive progesterone supplementation until the end of GW 34.
The diagnosis of premature labor
The goals of diagnostic evaluation are to detect the conditions that predispose to premature labor (ascending infection, placental insufficiency, amniotic fluid changes, and others) and to provide an objective measure of the extent to which premature labor has already begun (characteristics of contractions, effect of contractions on the cervix, premature rupture of the membranes). Moreover, the condition of the fetus must be assessed, so that it can be determined whether there is a need to deliver the baby. The components of a rationally based diagnostic evaluation are listed in Table 1.
Table 1. Diagnostic assessment of the pregnant patient with premature labor.
| Diagnostic test | Purpose |
| Cardiotocography | Objectification of uterine contractions and their frequency; assessment of the condition of the fetus |
| Vaginal examination | |
|
Diagnosis of infection |
|
Diagnosis of infection |
|
Biochemical test of amniotic fluid proteins |
|
Biochemical marker of cervical stage |
|
Subjective assessment of cervical stage |
| Transvaginal ultrasonography for measurement of cervix length | Objectification of cervical stage |
| Abdominal ultrasonography of the fetus | |
|
Oligo- /polyhydramnios |
|
Growth impairment/macrosomia |
|
Discordant growth |
| Feto-fetal transfusion syndrome | |
| Doppler ultrasonography of the uteroplacental and fetoplacental vessels | Assessment of placental insufficiency and/or inadequate blood supply to fetus |
Progesterone supplementation.
All pregnant women who have either a prior history indicating increased risk or current, asymptomatic cervical insufficiency should receive progesterone supplementation until the end of GW 34.
The treatment of premature labor
The goal of all interventions is not just to prolong pregnancy per se, but rather to give the newborn infant the best chance of surviving with as few complications as possible. Thus, depending on the particular clinical situation, the treatment of choice might be either to prolong the pregnancy or to deliver the baby.
As a rule, however, prolongation of pregnancy by at least 48 hours is an important objective, so that the pregnant woman can be transferred to a high-level perinatal care center, and fetal lung maturation can be induced with glucocorticoids. These two measures have been demonstrated to improve survival in babies born before GW 34.
Premature labor is treated with the following measures:
inhibition of uterine contractions with drugs— tocolysis (for its indications and contraindications, see Box)
glucocorticoid administration to induce fetal lung maturation
treatment of local or systemic infection with antibiotics
avoidance of physical exertion—bed rest and hospitalization.
Box. Indications and contraindications for tocolysis.
Indications
generally, from GW 24 + 0 onward
until GW 34 + 0 at the latest
spontaneous premature contractions
painful, palpable contractions that last longer than 30 seconds each and occur more than 3 times in 30 minutes
and
functional cervix length (transvaginal measurement) <25 mm and/or cervical dilatation
Contraindications
fetal indication for delivery
maternal indication for delivery
amniotic infection syndrome
developmentally malformed, non-viable fetus
Therapeutic measures.
Inhibition of uterine contraction with drugs
Glucocorticoid administration to promote fetal lung maturation
Antibiotics to treat infections, if present
Avoidance of physical exertion
Inhibition of uterine contractions with drugs—tocolysis
Any decision to inhibit uterine contractions when fetal viability is considered borderline (before GW 24) should be taken only after the pregnant woman has been thoroughly informed of the high risk of neonatal morbidity and after informed consent has been documented in writing. According to the German guidelines, the decision to intervene or not must always serve the interests of the child, while taking the parents’ interests into account as well.
After GW 34, careful weighing of the benefits and risks generally leads to the conclusion that prolonging pregnancy with drugs is not indicated.
Tocolytic therapy should be given for as short a time as possible and promptly terminated once contractions have ceased. There is no indication in routine clinical practice for continuing tocolytic therapy for more than 48 hours. Tocolysis for more than 48 hours and after the cessation of contractions is indicated only in exceptional cases (e.g., placenta previa hemorrhage, amniotic sac prolapse).
Individualized therapy consists of the selection of the tocolytic agent that is most effective for each patient, and that has the least side effects, from among the agents discussed in the following paragraphs, which are also listed in Table 2. There is no single tocolytic agent of first choice.
Table 2. Tocolytic drugs that are used in clinical practice.
| Substance class | Active substances |
| Calcium antagonists* | Nifedipine |
| Oxytocin-receptor antagonists | Atosiban |
| Inhibitors of prostaglandin synthesis* | Indomethacin |
| NO donors* | Nitroglycerin |
| Betamimetics | Fenoterol, terbutaline, ritodrine |
| Magnesium* |
*can only be used off-label in Germany
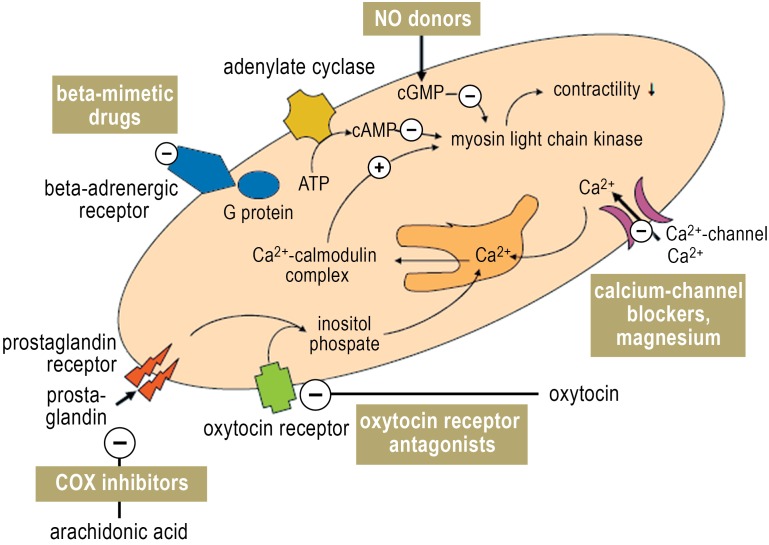
Betamimetics are the best-studied tocolytic drugs; they inhibit myometrial contractions by raising the intracellular concentration of cAMP (Figure 4). Fenoterol has been approved for this purpose only in Germany and Austria, where it is used in 95% of hospitals. In other countries, ritodrine and terbutaline are used. According to a recent Cochrane meta-analysis of eleven placebo-controlled trials of ritodrine and terbutaline, these drugs prolong pregnancy by two and seven days, respectively, but do not lower perinatal mortality (16).
Figure 4.
Mechanisms of action of tocolytic drugs
Tocolysis.
There is no drug of first choice. The tocolytic agent that is most effective and has the least side effects is selected individually for each patient.
Because these drugs activate the sympathetic nervous system, nearly all patients who take them suffer from tachycardia, sweating, tremulousness, nausea, or headaches in the first few hours of use (17). Betamimetics have the highest side-effect rates of all tocolytic drugs. Their maternal side effects can be severe, including cardiac arrhythmia and pulmonary edema, and fatalities have been reported (18). Even though fenoterol has been used as a tocolytic for decades in 95% of all German hospitals, often as the sole tocolytic agent in the hospital, the foreign guidelines no longer recommend the use of betamimetics (5). If they are used at all, then preferably as bolus tocolysis, which has fewer side effects (19).
Oxytocin antagonists (atosiban) bind competitively to the oxytocin receptor, thereby inhibiting the oxytocin-mediated rise of the intracellular calcium concentration that induces muscle contraction (Figure 4). According to a current meta-analysis of nine randomized trials, atosiban is as effective as betamimetics with respect to the prolongation of pregnancy and neonatal development (20), and its side-effect rate is less than 1% (18). No fetal side effects have been reported; the maternal side effects are mild (headache, nausea, vomiting). A follow-up study of infants born after tocolysis with atosiban revealed no ill effect on their psychosocial and motor development up to the age of two years (21).
Calcium antagonists are preferred above all other tocolytic agents in the Royal College guidelines because of their effectiveness and tolerability (5), and they are being used increasingly often in Germany as well. They inhibit both the direct influx of calcium into myocytes and the release of intracellular calcium (Figure 4). A Cochrane meta-analysis of twelve randomized and controlled trials revealed that nifedipine, the most commonly used calcium antagonist, is superior to betamimetics with respect to the prolongation of pregnancy by seven days and past the 34th week of gestation (22). The administration of nifedipine lowers the frequency of neonatal intraventricular hemorrhage (OR 0.53, 95% CI 0.34–0.84), respiratory distress syndrome (OR 0.63, 95% CI 0.46–0.86), and necrotizing enterocolitis (OR 0.21, 95% CI 0.05–0.94) (23). Its side effects, including nausea, flushing, headache, palpitations, and (often) reflex tachycardia, are less severe than those of betamimetics (18).
Calcium antagonists.
Nifedipine is more effective than betamimetics and lowers neonatal morbidity.
Contraindications.
Maternal or fetal indications for delivery
Amniotic infection syndrome
Fetal non-viability due to developmental malformation
NO donors—Nitric oxide (NO) is the most important mediator of smooth-muscle relaxation. During pregnancy, contractions of the myometrium are inhibited by an NO-mediated rise in intracellular cGMP synthesis and a resulting efflux of calcium from the myocytes (Figure 4). In eleven randomized trials, the transdermal application of an NO donor was found to be at least as effective as betamimetics for tocolysis lasting 48 hours or seven days, with a significantly better maternal side-effect profile (24). A placebo-controlled trial demonstrated a significant reduction of severe neonatal complications (OR 0.29, 95% CI 0.09–1.00) (25). Women with known migraine or recurrent headaches should not take NO, as it causes headache in as many as two-thirds of all patients taking it (17). Other potential side effects include myalgia, contact dermatitis from the adhesive in the patch, and hypotension and/or orthostatic dysregulation at the start of treatment. No fetal side effects or teratological effects have been described. In a follow-up study, children born after nitroglycerin tocolysis were found to be neurologically normal 18 months later (26).
Inhibitors of prostaglandin synthesis block the inducible cyclo-oxygenase COX-2 and thereby affect the number of myometrial gap junctions and the release of intracellular calcium (Figure 4). Indomethacin is the best-tested agent in this class, but selective COX-2 inhibitors are also used. A recent meta-analysis concluded that prostaglandin inhibitors are superior to all other tocolytic agents with respect to both efficacy and safety and are thus the drugs of first choice for premature labor before the 32nd week of gestation (27).
Inhibitors of prostaglandin synthesis.
These drugs are effective tocolytic agents before the 32nd week of gestation, but their prolonged use can cause serious fetal complications.
Maternal side effects are few as long as these agents are used for a short time only, and as long as no contraindicating conditions are present (gastrointestinal ulcers, bronchial asthma, coronary heart disease). Indomethacin crosses the placenta and can cause serious fetal complications if it is used for more than 48 hours or after GW 32; these range from a reduction of the volume of amniotic fluid to persistent fetal anuria and, in up to 50% of fetuses, constriction of the ductus arteriosus (28). A meta-analysis of neonatal complications after indomethacin tocolysis revealed no association with neonatal respiratory distress syndrome or with intraventricular hemorrhage, but it did reveal an elevated risk of periventricular leukomalacia (OR 2.0, 95% CI 1.3–3.1) and early necrotizing enterocolitis (OR 2.2, 95% CI 1.1–4.2) (29).
Magnesium sulfate non-specifically and competitively displaces calcium from the voltage-dependent calcium channels of myometrial cell membranes (Figure 4). A Cochrane meta-analysis of 23 trials on a total of 2036 patients failed to document efficacy for the prolongation of pregnancy by 48 hours, to the end of GW 34, or to the end of GW 37 (30). The meta-analysis did, however, reveal a 2.82-fold elevation of perinatal mortality when high-dose magnesium sulfate was given for more than 24 hours. It was concluded that magnesium sulfate cannot be recommended as a treatment for premature labor because of its lack of tocolytic efficacy, increased perinatal mortality, and considerable maternal side effects (1, 5).
Despite this, another recent meta-analysis documented a 31% reduction in the frequency of severe cerebral hemorrhage through the use of magnesium sulfate (31). This finding conflicts, however, with the findings of an evaluation by the German Neonatology Network (GNN) of 1965 preterm neonates weighing less than 1500 grams: In this cohort, the combination of fenoterol and magnesium sulfate was found to be associated with the highest rate of cerebral hemorrhage of all the tocolytic drugs and drug combinations that were analyzed (32).
Off-label use of drugs for tocolysis.
Special information forms explaining the effects of off-label drugs and the related medicolegal considerations have been found useful. It is also wise for obstetrical services to have internal guidelines about tocolytic drugs.
Off-label use—Despite their well-documented efficacy and therapeutic safety, most of the tocolytic drugs discussed here have not been approved for this indication in Germany, with the exception of betamimetics and the oxytocin antagonist atosiban. Lack of approval of effective medications is common in many branches of pediatrics. All of these medications, however, are commercially available in Germany, and physicians are free to use them for tocolysis as long as they obtain the patient’s explicit informed consent. Special patient information forms explaining the tocolytic effect of these drugs, their side effects, and the medicolegal situation have been found useful in routine clinical practice. It is also wise for each obstetrical service to have its own internal guidelines regarding the drugs that are to be used to treat premature labor; such guidelines enable individual physicians to take decisions more confidently and with less concern about the potential legal repercussions, even in difficult situations.
The induction of lung maturation with glucocorticoids
The prenatal administration of glucocorticoids in premature labor before the end of GW 34 is the most effective treatment known for the prevention of serious complications in the neonate. The treatment consists of two 12 mg doses of betamethasone given intramuscularly 24 hours apart, or four 6 mg doses of dexamathasone given intramuscularly 12 hours apart. Antenatal corticosteroid treatment has been found to lower neonatal mortality (OR 0.69, 95% CI 0.58–0.81; 18 trials including 3956 children), the risk of neonatal respiratory distress syndrome (OR 0.66, 95% CI 0.59–0.73; 21 trials in 4083 children), the frequency of cerebral intraventricular hemorrhage (OR 0.54, 95% CI 0.43–0.69; 13 trials in 2872 children), and the frequency of necrotizing enterocolitis (OR 0.46, 95% CI 0.29–0.74; eight trials in 1675 children) (33). This clear benefit for the neonates is also present if there is an incipient amniotic fluid infection syndrome; thus, prolongation of pregnancy is also sensible in this group of patients in order to permit the induction of lung maturation. The induction of pulmonary maturation with glucocorticoids should be offered to every pregnant woman with acute risk of preterm birth.
Glucocorticoids to induce lung maturation.
The prenatal administration of glucocorticoids in premature labor before the end of GW 34 is the most effective treatment known for the prevention of serious complications in the neonate.
Antibiotic treatment
Vaginal infections are considered to be the main cause of premature labor and premature rupture of the membranes. It thus seems reasonable to treat vaginal infections with antibiotics in order to prevent preterm birth. For women with premature rupture of the membranes, a meta-analysis of 22 studies with a total of 6800 women demonstrated the benefit of antibiotics both for lowering the frequency of chorioamnionitis (OR 0.66, 95% CI 0.46–0.96) and for preventing preterm birth within 48 hours (OR 0.71, 95% CI 0.58–0.87) or seven days (OR 0.79, 95% CI 0.71–0.89) (35). When antibiotics are given for preterm labor without premature rupture of the membranes, the rate of maternal infection is lower (OR 0.74, 95% CI 0.64–0.87), but pregnancy is not prolonged, nor is there any reduction of the rate of neonatal complications (36). For these reasons, the routine administration of antibiotics in premature labor is currently not recommended (37).
Bed rest
Although clinical experience suggests that restricting physical exertion may help women at high risk of premature labor, or for women who are already in premature labor, there is no evidence that this actually lowers the rate of preterm birth (38). There has not been any randomized trial of bed rest in the prevention or treatment of premature labor in single pregnancy, and a trial of bed rest in twin pregnancies revealed no benefit (39). The greater the degree of immobilization, the higher the risk of maternal complications such as thrombosis and muscle atrophy (38).
Bed rest.
Clinical experience suggests that restricting physical exertion may help women at high risk of preterm labor, or for women who are already in preterm labor, but there is no evidence that this actually lowers the rate of preterm birth.
Overview
Advances in the prevention and treatment of premature labor have improved the chances that a neonate will develop normally but have not lowered the preterm birth rate. Although many clinical trials have been performed, there is little evidence to support most of the diagnostic and therapeutic measures currently in use. There is a pressing need for high-quality research in this area of obstetrics.
Further information on CME.
This article has been certified by the North Rhine Academy for Postgraduate and Continuing Medical Education. Deutsches Ärzteblatt provides certified continuing medical education (CME) in accordance with the requirements of the Medical Associations of the German federal states (Länder). CME points of the Medical Associations can be acquired only through the Internet, not by mail or fax, by the use of the German version of the CME questionnaire within 6 weeks of publication of the article. See the following website: cme.aerzteblatt.de.
Participants in the CME program can manage their CME points with their 15-digit “uniform CME number” (einheitliche Fortbildungs nummer, EFN). The EFN must be entered in the appropriate field in the cme.aerzteblatt.de website under “meine Daten” (“my data”), or upon registration. The EFN appears on each participant’s CME certificate.
The CME unit “Specific Immunotherapy“ (issue 9/2013) can be accessed until 2 June 2013. For issue 17/2013, we plan to offer the topic “The diagnosis and treatment of generalized anxiety disorder.”
Solutions to the CME questions in Issue 5/2013:
Rassaf T et al.: Postoperative Care and Follow-up After Coronary Stenting. Solutions: 1a, 2b, 3e, 4a, 5e, 6c, 7e, 8d, 9a, 10
Please answer the following questions to participate in our certified Continuing Medical Education program.Only one answer is possible per question. Please select the answer that is most appropriate.
Question 1
What is the perinatal mortality rate of infants born before term but after the 32nd week of gestation?
77%
10 times higher than that of infants born at term
13%
32%
10 times lower than that of infants born very early
Question 2
Which of the following is a common cause of premature labor?
Ascending infection
Prior miscarriage
Hyperemesis gravidarum
Multiparity
Vaginal bleeding
Question 3
Which of the following is a major risk factor for preterm birth?
Gestational diabetes
Endurance sports during pregnancy
Maternal occupational activity
Prior preterm birth(s)
Prior caesarean section
Question 4
What would you advise for a pregnant woman who is found to have a closed cervix length of 29 mm on a routine check by vaginal ultrasonography in the 25th week of gestation?
The cervix is shortened; recheck cervix length regularly.
The cervix is shortened; tocolytic treatment is indicated.
The cervix is shortened; hospitalization is indicated.
The cervix is of normal length and premature labor is unlikely. The patient can be reassured.
Cervix length measurement is not a good way to assess the risk of impending premature labor; other tests must be performed.
Question 5
Which of the following situations is an indication for tocolytic therapy indicated for more than 48 hours in the absence of contractions?
Maternal endocarditis
Ascending infection
Placenta previa hemorrhage
Fetal malformation
Hypertension
Question 6
In what situation is treatment with tocolytic drugs indicated?
Cervix shorter than 25 mm on transvaginal ultrasound after GW 34
Cervix shorter than 30 mm on transvaginal ultrasound
Contractions with fewer than 2 subjectively painful and palpable contractions per hour, lasting less than 20 seconds each, or cervix shorter than 25 mm on transvaginal ultrasound
Contractions with more than 3 subjectively painful and palpable contractions every 30 minutes, lasting more than 30 seconds each, and cervix shorter than 25 mm on transvaginal ultrasound
Contractions with more than 3 subjectively painful and palpable contractions every 30 minutes, lasting more than 30 seconds each
Question 7
What drugs are approved for tocolysis in Germany?
Indomethacin and nifedipine
Fenoterol and atosiban
Nifedipine and nitroglycerin
Atosiban and magnesium sulfate
Fenoterol and indomethacin
Question 8
Which of the following is a demonstrated effect of nifedipine?
It has more side effects than betamimetics.
It does not cross the placenta.
It is more effective for tocolysis than betamimetics.
It lowers perinatal mortality.
It works by promoting calcium influx into myocytes.
Question 9
Which of the following is a contraindication for tocolysis?
Crohn’s disease
Gestational diabetes
Amniotic infection syndrome
Multiple pregnancy
Hypertension
Question 10
What do you recommend for a woman who has already had one preterm birth and plans to get pregnant again?
Prophylactic cervical cerclage
Avoidance of physical exertion during the entire pregnancy
Cervix length measurement by ultrasound every 4 weeks
Progesterone supplementation from the start of pregancy onward
Prophylactic tocolysis
Acknowledgments
Translated from the original German by Ethan Taub, M.D.
Footnotes
Conflict of interest statement
Prof. Schleußner declares that no conflict of interest exists.
References
- 1.Deutsche Gesellschaft für Gynäkologie und Geburtshilfe Leitlinie 015/025 Medikamentöse Wehenhemmung bei drohender Frühgeburt. www.agmfm.de/_download/unprotected/g_04_03_01_medikamentoese_wehenhemmung.pdf.
- 2.AQUA - Institut für angewandte Qualitätsförderung und Forschung im Gesundheitswesen GmbH. Bundesauswertung zum Verfahrensjahr 2010 16/1 - Geburtshilfe. www.sqg.de/ergebnisse/leistungsbereiche/geburtshilfe.html.
- 3.EURO-PERISTAT Project. European Perinatal Health Report 2008. www.europeristat.com/our-publications/european-perinatal-health-report.html.
- 4.Dudenhausen JW, Friese K, Kirschner W. Präkonzeptionelle Gesundheitsberatung und Beratung zur Wahl der Geburtsklinik als weitere Instrumente zur Verringerung von Frühgeburten. Z Geburtsh Neonatol. 2007;211:142–146. doi: 10.1055/s-2007-960658. [DOI] [PubMed] [Google Scholar]
- 5.RCOG Green-top Guideline No. 1b, February 2011. Tocolysis for women in preterm labour. www.rcog.org.uk/files/rcog-corp/GTG1b26072011.pdf.
- 6.Di Renzo GC for the European Association of Perinatal Medicine-study group preterm birth. Guidelines for the management of spontaneous preterm labor: identification of spontaneous preterm labor, diagnosis of preterm premature rupture of membranes, and preventive tools for preterm birth. J Mat Fetal Neonat Med. 2011;24:659–667. doi: 10.3109/14767058.2011.553694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Management of preterm labour. Practice Bulletin 127. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2012;119:1308–1317. doi: 10.1097/AOG.0b013e31825af2f0. [DOI] [PubMed] [Google Scholar]
- 8.Flood K, Malone FD. Prevention of preterm birth. Seminars Fetal Neonat Med. 2012;17:58e–e63. doi: 10.1016/j.siny.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 9.Murphy DJ. Epidemiology and environmental factors in preterm labour. Best Practice & Research Clinical Obstetrics and Gynaecology. 2007;21:773–789. doi: 10.1016/j.bpobgyn.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 10.Cochrane search “prevention and preterm labour”. http://summaries.cochrane.org/search/site?f[0]=im_field_terms_cochrane_library%3A51374&f[1]=im_field_stage%3A3&f[2]=im_field_terms_cochrane_library%3A51378. Last accessed on 26 Februrary 2013. [Google Scholar]
- 11.Bitzer E, Schneider A, Wenzlaff P, Hoyme UB, Siegmund-Schultze E. Self-testing of vaginal pH to prevent preterm delivery: A controlled trial. Dtsch Arztebl Int. 2011;108(6):81–86. doi: 10.3238/arztebl.2011.0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crane JM, Hutchens D. Transvaginal sonographic measurement of cervical length to predict preterm birth in asymptomatic women at increased risk: a systematic review. Ultrasound Obstet Gynecol. 2008;31:579–587. doi: 10.1002/uog.5323. [DOI] [PubMed] [Google Scholar]
- 13.Berghella V, Rafael T, Szychowski JM, Rust OA, Owen J. Cerclage for short cervix on ultrasonography in women with singleton gestations and previous preterm birth. Obstet Gynecol. 2011;117:663–671. doi: 10.1097/AOG.0b013e31820ca847. [DOI] [PubMed] [Google Scholar]
- 14.Dodd JM, Flenady V, Cincotta R, Crowther CA. Prenatal administration of progesterone for preventing preterm birth. Cochrane Database of Systematic Reviews. 2006;(Issue 1) doi: 10.1002/14651858.CD004947.pub2. Art No.: CD004947. DOI: 10.1002/14651858.CD004947.pub2. [DOI] [PubMed] [Google Scholar]
- 15.Romero R, Nicolaides K, Conde-Agudelo A, et al. Vaginal progesterone in women with an asymptomatic sonographic short cervix in the midtrimester decreases preterm delivery and neonatal morbidity: a systematic review and meta-analysis of individual patient data. Am J Obstet Gynecol. 2012;206 124:e1–e19. doi: 10.1016/j.ajog.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anotayanonth S, Subhedar NV, Garner P, Neilson JP, Harigopal S. Betamimetics for inhibiting preterm labour. The Cochrane Database of Systematic Reviews. 2010;2 doi: 10.1002/14651858.CD004352.pub2. CD004352. DOI: 10.1002/14651858.CD004352.pub2. [DOI] [PubMed] [Google Scholar]
- 17.Schleussner E, Möller A, Groß W, et al. Maternal and fetal side effects of tocolysis using transdermal nitroglycerin or intravenous fenoterol combined with magnesium sulfate. Eur J Obstet Gynecol Reprod Biol. 2003;106:14–19. doi: 10.1016/s0301-2115(02)00197-5. [DOI] [PubMed] [Google Scholar]
- 18.de Heus R, Mol BW, Erwich JJ, et al. Adverse drug reactions to tocolytic treatment for preterm labour: prospective cohort study. BMJ. 2009;338 doi: 10.1136/bmj.b744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spätling L, Fallenstein F, Schneider H, Dancis J. Bolus tocolysis: treatment of preterm labor with pulsatile administration of a beta-adrenergic agonist. Am J Obstet Gynecol. 1989;160:713–717. doi: 10.1016/s0002-9378(89)80066-3. [DOI] [PubMed] [Google Scholar]
- 20.Papatsonis D, Flenady V, Cole S, Liley H. Oxytocin receptor antagonists for inhibiting preterm labour. Cochrane Database of Systematic Reviews. 2005 doi: 10.1002/14651858.CD004452.pub2. 3 CD004452. DOI: 10.1002/14651858.CD004452.pub2. [DOI] [PubMed] [Google Scholar]
- 21.The Worldwide Atosiban Versus Beta-agonists Study group. Effectiveness and safety of the oxytocin antagonist atosiban versus beta-adrenergic agonists in the treatment of preterm labour. Br J Obstet Gynaecol. 2001;108:133–142. [PubMed] [Google Scholar]
- 22.King JF, Flenady V, Papatsonis D, Dekker G, Carbonne B. Calcium channel blockers for inhibiting preterm labour. Cochrane Database of Systematic Reviews. 2003;(Issue 1) doi: 10.1002/14651858.CD002255. Art. No.: CD002255. DOI: 10.1002/14651858.CD002255. [DOI] [PubMed] [Google Scholar]
- 23.Conde-Agudelo A, Romero R, Kusanovic JP. Nifedipine in the management of preterm labor: a systematic review and metaanalysis. Am J Obstet Gynecol. 2011;204 134:e1–e20. doi: 10.1016/j.ajog.2010.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schleußner E. Medikamentöse Behandlung bei drohender Frühgeburt. Gynäkol Prax. 2010;34:231–241. [Google Scholar]
- 25.Smith GN, Walker MC, Ohlsson A, et al. Randomized double-blind placebo-controlled trial of transdermal nitroglycerin for preterm labor. Am J Obstet Gynecol. 2007;196(37):e1–e8. doi: 10.1016/j.ajog.2006.10.868. [DOI] [PubMed] [Google Scholar]
- 26.Gill A, Madsen G, Knox M, et al. Neonatal neurodevelopmental outcomes following tocolysis with glycerol trinitrate patches. Am J Obstet Gynecol. 2006;195:484–487. doi: 10.1016/j.ajog.2006.01.103. [DOI] [PubMed] [Google Scholar]
- 27.Haas DM, Imperiale TF, Kirkpatrick PR, Klein RW, Zollinger TW, Golichowski AM. Tocolytic therapy: a meta-analysis and decision analysis. Obstet Gynecol. 2009;113:585–594. doi: 10.1097/AOG.0b013e318199924a. [DOI] [PubMed] [Google Scholar]
- 28.Norton ME, Merill J, Cooper BA, et al. Neonatal complications after the administration of indomethacin for preterm labor. N Engl J Med. 1993;329:1602–1607. doi: 10.1056/NEJM199311253292202. [DOI] [PubMed] [Google Scholar]
- 29.Amin SB, Sinkin RA, Glantz JC. Meta-analysis of the effect of antenatal indomethacin on neonatal outcomes. Am J Obstet Gynecol. 2007;197:486e1–48610. doi: 10.1016/j.ajog.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crowther CA, Hiller JE, Doyle LW. Magnesium sulphate for preventing preterm birth in threatened preterm labour. Cochrane Database of Systematic Reviews. 2002 doi: 10.1002/14651858.CD001060. 4 CD001060. DOI: 10.1002/14651858.CD001060. [DOI] [PubMed] [Google Scholar]
- 31.Doyle LW, Crowther CA, Middleton P, Marret S, Rouse D. Magnesium suphate for women at risk of preterm birth for neuroprotection of the fetus. Cochrane Database of Systematic Reviews. 2009 doi: 10.1002/14651858.CD004661.pub3. 1 Art. No.: CD004661. DOI: 10.1002/14651858.CD004661.pub3. [DOI] [PubMed] [Google Scholar]
- 32.Schleußner E, Göpel W. Magnesiumsulfat zur Neuroprotektion? Frauenarzt. 2011;52:570–571. [Google Scholar]
- 33.Roberts D, Dalziel SR. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database of Systematic Reviews. 2006;(Issue 3. Art. No.) doi: 10.1002/14651858.CD004454.pub2. CD004454. DOI: 10.1002/14651858.CD004454.pub2. [DOI] [PubMed] [Google Scholar]
- 34.Been J, Degraeuwe P, Kramer B, Zimmermann L. Antenatal steroids and neonatal outcome after chorioamnionitis: a meta-analysis. BJOG. 2011;118:113–122. doi: 10.1111/j.1471-0528.2010.02751.x. [DOI] [PubMed] [Google Scholar]
- 35.Kenyon S, Boulvain M, Neilson JP. Antibiotics for preterm rupture of membranes. Cochrane Database Syst Rev. 2010 doi: 10.1002/14651858.CD001058.pub2. Aug 4;(8): CD001058. Doi: 10.1002/14651858.CD001058.pub2. [DOI] [PubMed] [Google Scholar]
- 36.King JF, Flenady V. Prophylactic antibiotics for inhibiting preterm labour with intact membranes. Cochrane Database of Systematic Reviews. 2002;4 doi: 10.1002/14651858.CD000246. CD000246. [DOI] [PubMed] [Google Scholar]
- 37.Subramaniam A, Abramovici A, Andrews A, Tita AT. Antimicrobials for preterm birth prevention: an overview. Infect Dis Obstet Gynecol. 2012 doi: 10.1155/2012/157159. 57159. Doi: 10.1155/2012/157159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maloni JA. Lack of evidence for prescription of antepartum bed rest. Expert Rev Obstet Gynecol. 2011;6:385–393. doi: 10.1586/eog.11.28. Doi: 10.1586/eog.11.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Crowther CA, Han S. Hospitalisation and bed rest for multiple pregnancy. Cochrane Database Syst. Rev. 2010 doi: 10.1002/14651858.CD000110.pub2. 7 CD000110. [DOI] [PMC free article] [PubMed] [Google Scholar]