Abstract
Objective
To examine the chemical variability in inflorescences of wild populations of Stachys lavandulifolia Vahl (S. lavandulifolia) collected throughout two provinces (Isfahan and Chaharmahal va Bakhtiary), Southwest Iran.
Methods
The essential oils of S. lavandulifolia Vahl from seven locations were obtained by hydro-distillation and analysed by gas chromatography and gas chromatography-mass spectrometry.
Results
The results revealed that distinct differences in the content of compounds depending on region of sample collection. The main constituents of the essential oils were α-thujone (0.3%-32.3%), α-pinene (trace to 37.3%), myrcene (0.5%-15.9%), β-phellandrene (1.1%-37.9%), germacrene D (0.4%-11.3%), Δ-cadinene (trace to 11.6%) and 1, 4-methano-1 H-indene (trace to 10.1%).
Conclusions
The results of the present study indicated that essential oil components of S. lavandulifolia Vahl can be varied with genetic (ecotype), environmental conditions and geographic origin. In general, the essential oils of various populations of S. lavandulifolia Vahl were rich in monoterpenoids and sesquiterpenoids.
Keywords: Stachys lavandulifolia Vahl, Lamiaceae, Essential oil, α-thujene, α-pinene, β-phellandrene, Chemotype
1. Introduction
The genus Stachys, which belongs to the Lamiaceae family, consists of about 300 species, and is justifiably considered as one of the largest genera of Lamiaceae that widespread throughout the world[1]. The genus is distributed mainly in warm temperate regions of the Mediterranean and Southwest Asia, Southern Africa, North and South America[2],[3]. Assessed by the number and distribution of the species, two main centers of diversity for the genus in the old world area exist. One is confined to South and East Anatolia, Caucasia, North and West Iran and North Iraq, and the other to the Balkan Peninsula. The majority of species prefers alpine and subalpine habitats and grows under various ecological conditions in habitats like rocky places, mountain steppes, and stream banks or sometimes in forests[4]. In Iran, 34 species of this genus are present, among which, 13 are endemic[5]. Stachys lavandulifolia Vahl (S. lavandulifolia) is a native plant, which is known as Chay-e-kohi in Persian and Betony in English[6]. In Iranian traditional medicine, it is used as the herbal tea in gastrointestinal disorders, inflammatory diseases, anxiolytic, cough, sedative, antispasmodic, diuretic, ulcers, fevers and diarrhoea[6]. The decoction of the leaves and flowers is being used by the tribal people of Chaharmahal va Bakhtiari for treatment of skin infection, menorrhagia and anti-bacterial[7]. Also, the aerial part of S. lavandulifolia Vahl has been used by tribal people of Ilam Province West Iran, as carminative, sedative and cardio tonic, and for treatment of rheumatism, indigestion and headache. The aqueous extract of the aerial parts of S. lavandulifolia Vahl shows potent anti-inflammatory and wound healing activities in rat[8]. The previous experimental studies have demonstrated the anxiolytic and anti-diarrheal effects in mice[9],[10]. Anti-inflammatory, antioxidant, antibacterial and anti-leishmania activities and effect reduced primary dysmenorrheal of S. lavandulifolia Vahl extract and essential oil have been shown in several pharmacological studies[11]–[13]. Previous investigations revealed that the main iridoid components of Stachys species, such as aucubin, harpagide and harpagoside, exert anti-inflammatory activities, as demonstrated in cellular systems of arachidonic acid metabolism[14],[15]. Even though Stachys is one of the largest members of the Lamiaceae, the essential oil chemistry of the genus has not been studied in much detail. The composition of the volatiles is known only for a few species; for example, studies proved that the volatile oil of members of S. lavandulifolia Vahl (sect. Zietenia) consists mainly of monoterpene hydrocarbons, oxygenated monoterpenes, sesquiterpene hydrocarbons, oxygenated sesquiterpenes, diterpenoids and terpene esters[16]–[23]. The result of new study showed that the essential oil of Stachys mialhesi de Noé has antioxidant, antinociceptive and anti-inflammatory effects in laboratory animals. It can also be a natural source of isoscutellarein 7-O-(2″-O-6″′-O-acetyl-β-D-allopyranosyl-β-D-glucopyranoside. Germacrene-D, the dominant compound of the essential oil of Stachys inflate Benth, was reported as the major components of the volatile oils of S. lavandulifolia Vahl, Stachys laxa Boiss and Stachys obtusicrena Boiss[17],[20],[24]. In a different report, the major components in Stachys tibetica essential oil were aciphyllene (66.415%), fenchyl alcohol (8.897%), α-pinene (8.188%) and caryophyllene oxide[25]. In a study, lavandulifolioside B and 5-O-β-allopyranosyloxy-aucubin in S. lavandulifolia Vahl were identified comprehensively by extensive 1D and 2D NMR analyses[26]. There are some differences amongst Stachys taxa, indicating the existence of a chemical polymorphism. Chemical polymorphisms or chemotypes have been reported for many medicinal plants[27],[28]. To our knowledge, there are no published reports on the chemical composition of the essential oils of the populations of S. lavandulifolia Vahl in Iran. For this reason, the chemical composition of these oils was analysed.
2. Materials and methods
2.1. Locations
Seven specimens of wild S. lavandulifolia Vahl were collected in different localities of two provinces in Iran. The monthly precipitation was obtained from variation weather stations located within the study area and the surrounding zones. The number of years registered at the weather stations ranged from 10-15. Soil physical and chemical characteristics such as pH, electricity conductivity, texture, organic carbon (%), contents of N, P, K, etc. were taken from a soil-sampling from different locations of Isfahan and Chaharmahal va Bakhtiari provinces. The slope and elevation information were obtained from the Digital Elevation Model using two well-known GIS software packages ILWIS(3.0 Academic). This array was geo-referenced using a metric UTM coordinate system and the geometric correction was carried out in the GIS ILWIS.
2.2. Plants
The inflorescences (0.05-0.10 kg) of wild populations of S. lavandulifolia Vahl were collected at the early flowering stage on 1-20 June 2010 (During 20 day's period). The samples of the plants were identified by regional floras and authors with floristic and taxonomic references[3], and voucher specimens were deposited at the Herbarium of Agriculture and Natural Resources Researches Centre of Chaharhal va Bakhtiari province, Iran (No.: SHK 2164) (Figure 1). Harvested inflorescence was shade dried at room temperature for one week and ground into a powder. Air-dried and ground (50 mesh) plant material was subjected to hydro-distillation (1 000 mL distillated water) for 2 h using a Clevenger-type apparatus according to the method recommended in British pharmacopoeia[29]. Samples were dried with anhydrous sodium sulphate and kept in amber vials at 4 °C until chromatographic analysis.
Figure 1. Aerial plant of S. lavandulifolia Vahl (Lamiaceae).

2.3. Identification of the oil components
Ghromatography (GC) and gas ghromatography-mass spectrometry (GC-MS) analysis methods were carried out to determine the composition of the essential oils. GC analysis was carried out on a Younglin Acme 6000 gas chromatograph equipped with Flame Ionization Detector (FID) and a HP-5MS 5% capillary column (30.00 m×0.25 mm, 0.25 µm film thicknesses). Carrier gas was helium at flow of 0.8 mL/min. GC oven temperature was kept at 50 °C for 5 min and programmed to 240 °C at a rate of 3 °C/min, and then programmed to 300 °C at a rate of 15 °C/min. Split ratio was adjusted at 40:1. The injector temperature was set at 290 °C. The purity of Helium gas was 99.999% and 0.5 µL samples were injected manually in the split mode. GC-MS analysis was performed on above mentioned Agilent Technologies 5973 Mass system. Mass spectra were recorded at 70 eV. Mass range was from m/z 50-550. Retention indices were calculated for all components using a homologous series of n-alkanes (C5-C24) injected in conditions equal to samples ones. Identification of oil components was accomplished based on comparison of their retention times with those of authentic standards and by comparison of their mass spectral fragmentation patterns (WILLEY /ChemStation data system)[30]. Area percent was obtained electronically from the GC-FID response without the use of an internal standard or correction factors.
2.4. Chemical variability
Analytical data for cluster analysis were treated by means of the statistical package Minitab release 13.
3. Results
The seven specimens of S. lavandulifolia Vahl collected in different localities in Iran are as shown in Table 1.
Table 1. Environment condition of natural habitats of seven populations belonging to S. lavandulifolia (Lamiaceae).
| Region | Province | Altitude (m ASL*) | Latitude | Longitude | P | pH | EC | OC | TN | N | K | TNV | Clay | Silt | Sand |
| Sheyda | Chaharmahal va Bakhtiari | 2 822 | 32°37′ 22″ N | 50°34′ 47″ E | 382 | 7.67 | 0.48 | 0.36 | 9.9 | 0.041 | 103 | 2.5 | 30 | 35 | 35 |
| Broujen | Chaharmahal va Bakhtiari | 2 300 | 33°00′ 40″ N | 52°18′ 44″ E | 251 | 7.69 | 0.62 | 0.71 | 12.9 | 0.078 | 556 | 49.1 | 14 | 52 | 34 |
| Naghan | Chaharmahal va Bakhtiari | 1 951 | 31°56′ 11″ N | 50°43′ 27″ E | 593 | 7.78 | 0.56 | 0.94 | 29.9 | 0.087 | 485 | 36.0 | 50 | 26 | 24 |
| Frasan | Chaharmahal va Bakhtiari | 2 200 | 32°19′ 01″ N | 50°32′ 11″ E | 507 | 7.60 | 0.63 | 1.07 | 44.2 | 0.098 | 846 | 5.5 | 34 | 52 | 14 |
| Gal-e-sang | Chaharmahal va Bakhtiari | 2 544 | 32°27′ 59″ N | 50°17′ 18″ E | 1 415 | 7.46 | 0.61 | 2.32 | 40.2 | 0.206 | 819 | 9.0 | 32 | 56 | 12 |
| Kelishadrokh | Isfahan | 2 090 | 32°21′ 33″ N | 51°04′ 03″ E | 324 | 7.76 | 0.74 | 0.16 | 17.5 | 0.026 | 503 | 24.0 | 26 | 38 | 36 |
| Faraydan | Isfahan | 2 300 | 32°58′ 59″ N | 50°24′ 22″ E | 348 | 7.78 | 0.48 | 0.82 | 18.1 | 0.085 | 538 | 17.0 | 45 | 30 | 25 |
ASL: Above sea level, P: Annual precipitation (mm), EC: Electrical conductivity (dS/m), OC: Organic carbon (%), TN: Total nitrogen (%), N: Available P (mg/kg), K: Available K (mg/kg), TNV: Total neutralising value (%), Clay (%), Silt (%) and sand (%).
The chemical constituents identified by GC and GC-MS and the results concerning the qualitative and quantitative analysis of the essential oils are presented in Table 2. The twenty seven compounds consisting up to 80% of the essential oil were identified by GC and GC-MS analysis, the major components of S. lavandulifolia Vahl oil were: α-thujone (0.3%-32.3%), α-pinene (trace to 37.3%), myrcene (0.5%-15.9%),β-phellandrene (1.1%-37.9%), germacrene D (0.4%-11.3%), Δ-cadinene (trace to 11.6%) and 1,4-methano-1 H-indene (trace to 10.1%).
Table 2. Essential oil composition (%) of seven populations of S. lavandulifolia.
| Compound | RI | Broujen (%) | Gal-e-sang (%) | Naghan (%) | Sheyda (%) | Frasan (%) | Faraydan (%) | Kelishadrokh (%) |
| α-thujene | 930 | 16.29 | 23.52 | 13.39 | 23.76 | 0.28 | 26.17 | 32.34 |
| α-pinene | 935 | 0.19 | 0.71 | 0.23 | 0.70 | 37.29 | 0.68 | tr |
| Benzaldehyde | 959 | - | - | - | 6.28 | 2.91 | 0.18 | - |
| Sabinene | 974 | - | 3.13 | 4.10 | - | - | 4.71 | 2.22 |
| β-pinene | 978 | 0.78 | 1.11 | 3.47 | - | 1.60 | 2.01 | 1.95 |
| Myrcene | 990 | 3.86 | 0.91 | 15.87 | 4.41 | 6.14 | 6.72 | 0.52 |
| α-phellandrene | 1 003 | 0.03 | - | 7.26 | - | 0.32 | 2.81 | 13.99 |
| β-phellandrene | 1 027 | 14.44 | 28.27 | 1.10 | 37.93 | 12.37 | 17.89 | 2.11 |
| β-Ocimene <(Z)> | 1 037 | 2.26 | 0.67 | 0.11 | 2.16 | 2.23 | 0.36 | 0.21 |
| γ-terpinene | 1 061 | 0.29 | 0.18 | 0.21 | 0.15 | 0.35 | 0.16 | 0.34 |
| Trans-sabinene hydrate | 1 092 | 0.03 | 0.12 | 0.03 | 0.08 | 0.09 | 0.51 | 0.08 |
| Linalool | 1 099 | 0.09 | tr | 0.19 | 0.02 | 0.05 | 0.49 | 0.06 |
| Allo ocimene | 1 121 | tr | tr | 0.11 | 0.05 | 0.18 | 0.01 | 0.17 |
| α-Copaene | 1 372 | 1.10 | 0.05 | 1.03 | 0.04 | - | tr | - |
| β-bourbonene | 1 383 | 1.18 | 0.13 | - | - | - | 1.64 | 0.54 |
| β-cubebene | 1 393 | - | 0.16 | - | 0.09 | 0.21 | 0.36 | - |
| β-elemene | 1 396 | - | 0.11 | - | - | - | 0.07 | 0.21 |
| Naphthalene (1-methoxy) | 1 440 | 0.45 | 0.68 | 0.34 | 0.34 | - | 0.22 | 0.14 |
| β- farnesene <(Z)> | 1 447 | 0.21 | 0.09 | 0.25 | 0.06 | 0.09 | 0.11 | 2.31 |
| α-amorphene | 1 475 | - | 2.96 | 5.57 | - | 0.03 | - | 0.04 |
| Germacrene D | 1 479 | 11.26 | 2.66 | 5.85 | 2.51 | 6.58 | 6.75 | 0.37 |
| Bicyclogermacrene | 1 495 | - | - | 4.02 | 3.98 | - | - | 10.67 |
| Δ-cadinene | 1 524 | 11.61 | 7.67 | 0.51 | 0.17 | 1.28 | 0.18 | 0.12 |
| Cis-α-bisabolene | 1 536 | 0.08 | 0.04 | 0.23 | 0.15 | 0.09 | 0.04 | 0.76 |
| Spathulenol | 1 570 | 0.07 | - | - | 0.07 | 0.13 | 0.07 | 0.09 |
| 1,4-methano-1 H-indene | 1 959 | 8.43 | 3.22 | 10.07 | 0.17 | - | 3.62 | - |
| Isospathulenol | 2 259 | 0.70 | 0.07 | 0.47 | 0.03 | 0.31 | 0.21 | - |
| Oil yield (%) | - | 0.32 | 0.35 | 0.33 | 0.31 | 0.32 | 0.34 | 0.35 |
RI: Retention index, tr: trace (<0.01%).
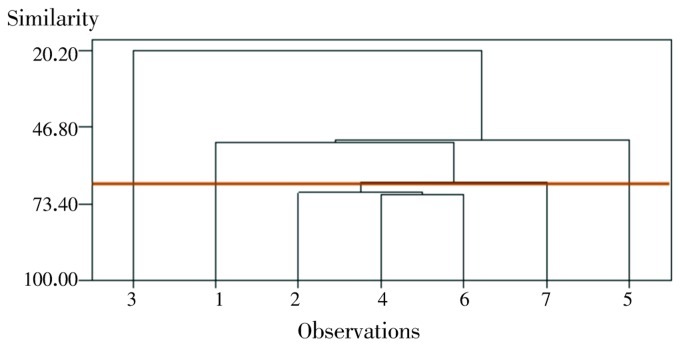
The dendrogram (Figure 2) represents graphically the relationships among the populations and the groups, based on their essential oil composition. Four groups were formed by the Average Linkage cluster analysis. The first cluster encompassed S. lavandulifolia Vahl accessions of Feryadan, Ghalehsang, Sheyda and Broujen, and exhibited high β-phellandrene (28.3%, 37.9%, 17.9% and 14.4%, respectively) and α-thujene (23.5%, 23.8%, 26.2% and 16.3%, respectively) contents. The second cluster enclosed accession of Kelishadrokh, and displayed high α-thujene and α-phellandrene contents (32.3% and 14.0%, respectively). The third cluster enclosed accession of Naghan, and displayed high myrcene and α-thujene contents (15.9% and 13.4%, respectively). The fourth cluster enclosed accession of Farsan, and displayed high α-pinene and β-phellandrene contents (37.3% and 12.4%, respectively).
Figure 2. A dendrogram of seven populations of S. lavandulifolia using cluster analysis.
1: Kelishadrokh, 2: Faraydan, 3: Farsan, 4: Sheyda, 5: Naghan, 6: Gal-e-sang, 7: Broujen.
4. Discussion
The yellow oil of samples of seven populations of S. lavandulifolia Vahl was obtained by hydro-distillation in the yield of 0.30%-0.35% (w/w). As previously reported[16]–[18], the yield (w/w) of the obtained essential oil of S. lavandulifolia Vahl ranged from 0.25% to 0.8%, based on dry weight. Also, the yield of the oils extracted from other species was: 0.18% from Stachys setifera ssp. Iranica[31], 0.18% from Stachy chrysantha[15], and 0.12% from Stachy candida[14]. In other study, Radulovic et al. reported that the yield of the obtained essential oils from Balkan Peninsula endemics species (Stachys germanica ssp. heldreichii, Stachys iva, Stachys plumosa and Stachys scardica) ranged from 0.024% to 0.037%, based on dry weight[32]. The essential oils yield obtained from twenty-two different Stachys species from turkey ranged from 0.1% to 0.25%[33].
The results of this study showed that monoterpenes and sesquiterpenes were the main constituent groups. Our results were in agreement with report by Ebrahimabad et al. on chemical components of Stachys inflata Benth essential oil, linalool and terpinol were major components[21]. The result of chemical analysis of Stachys serotina (Host) Fritsch essential oil by Jerkovic et al. showed that sesquiterpene hydrocarbons were the most abundant class of isolated volatiles of β-caryophyllene, δ-cadinene and α-humulene, germacrene D[34]. In previous study[33], the essential oils from twenty-two different Stachys species have been identified thirty-nine compounds. Germacrene-D (2.9%-45.3%), β-caryophyllene (2.3%-62.3%), caryophyllene oxide (trace to 7.8%), spathulenol (trace to 7.8%) and α-cadinene (1.4%-8.5%) have been identified as the main components of the essential oils[33]. Mirza and Baher reported that the oil of Stachys lanata Jacq collected from the National Botanical Garden in Tehran, Iran, was rich in α-thujone (25.9%), α-humulene (24.9%), β-caryophyllene (12.6%) and viridiflorol (10.5%)[35]. In previous studies, the main components of S. lavandulifolia Vahl oil were reported to be germacrene-D (13.2%), β-phellandrene (12.7%), β-pinene (10.2%), myrcene (9.4%), α-pinene (8.4%) and Z-β-ocimene (5.8%) for Tehran population in Central Iran[17], myrcene (20.9%), α-pinene (16.3%), α-terpinene (20%) and bicyclogermacrene (8.7%) for Lorestan population in West Iran[16], α-pinene (7.9%), 4-hydroxy-4-methyl-2-pentanone (9.3%) and hexadecanoic acid (5.9%) for Mazandaran population in North Iran[18] and β-caryophyllene and 1,8-cineole for S. lavandulisolia in Turkey[36]. In 2004, Sajjadi and Somae reported germacrene D, bicyclogermacrene, α-pinene, β-phellandrene, bicycloelemene, β-pinene and spathulenol as the major components of the oil of Stachys inflata Benth growing in Iran[37]. Feizbaksh et al. found α-pinene (20.1%), β-pinene (12.1%), spathulenol (7.2%) and germacrene D (5.3%) as the major components of oil of S. lavandulifolia Vahl collected from Ab-ali (Tehran province, Iran)[19]; this oil was rich in monoterpenoids (51.8%). In present study, a high α-thujene (32.34%) chemotype in Kelishadrokh from the Southwest of Isfahan province, Iran has previously been proposed in the oil (25.9%) of Stachys lanata collected from the National Botanical Garden in Tehran, Iran[35], and we now confirm this chemotype in S. lavandulifolia Vahl and report that it is also present in an area study. Also, we confirm high β-phellandrene (37.9%) in Sheyda population with high altitude (2 822 meter above sea level) in an area study. Javidnia et al. reported that β-phellandrene is as a main component of the oil of S. lavandulifolia Vahl collected from Fasham area, Tehran[17]. A high α-pinene (37.3%) in Farsan population has previously been proposed in the oil (20.1%) of S. lavandulifolia Vahl collected from Tehran, Iran[19]. In the essential oil of Naghah population, a main constituent was found to be myrcene (15.87%), which we now confirm this chemotype in S. lavandulifolia Vahl. Polymorphism chemical or chemotypes have been reported for many medicinal plants. The composition of the essential oil of S. lavandulifolia Vahl depends on many factors of genetic, environmental and their interaction effects, such as plant part, harvest-time, extraction-method, ecotype and geographic origin (climate, edaphic, elevation and topography)[36]. Genetic differences cannot be directly deduced from the varying amounts of a secondary plant product[37]. Plants growing in different environments grow ordinarily at different rates; they differ in size and developmental stage[38]. The result of study by Sadrmomtaz et al. found microwave-assisted hydrodistillation method can achieve comparable results with those by hydrodistillation for determination of essential oils in fresh materials[39]. They reported that the major components by two methods were carvacrol (1.43%, 2.63%) and thymol (10.80%, 8.14%).
In conclusion, S. lavandulifolia Vahl with different chemical compositions have been reported. It is known that many factors influence the chemical constitution of S. lavandulifolia Vahl oils. We have shown that the volatile oil composition of S. lavandulifolia Vahl, in Iran is extremely variable. We propose four chemotypes with intriguing patterns in their geographic distribution. These chemotypes are distinguished by different levels of classes of natural products: monoterpenes and sesquiterpenes. The differences in the quantity or quality of the oils composition of the present and previous studies may be because of the chemotypes, phenological stage, drying conditions, mode of distillation and geographic and climatic factors[16]–[19],[22],[36].
Acknowledgments
We are grateful to Mr. Shirmardi for the identification of the plant accessions and thank Mr. Taghipoor Farid (Institute of Medicinal Plants Researches, Karaj, Iran) for assistance with essential oil analysis. This work was supported by deputy of Researches and Technology, Islamic Azad University of Shahrekord Branch, Iran (Grant no. IAUSHK: 6121).
Comments
Background
Monoterpenes are the most representative metabolites constituting 90% of the essential oils in medicinal plants. Some researchers, however, have reported that levels of sesquiterpenes were higher in vegetative organs in most species compared with monoterpenes. Moreover, some sesquiterpenes have different medicinal functions as well as monoterpenes. It is interesting to note that there is significant diversity of chemical compositions of the essential oil for wild populations of S. lavandulifolia in different geographic regions and altitudes.
Research frontiers
The cutting-edge in the field of the research in this paper Human intervention in plant breeding has always aimed to increase production, improve quality, and protect plants against pests. Negative ecological impacts resulting from the use of chemicals and cultivation limited the number of genotypes. Knowledge of diversity in crop species and their wild relatives is of critical importance for crop improvement. For traditional Iranian medicinal plants, the major constraints in achieving higher yield and consistency are lack of genetic variability, absence of suitable genotypes for different planting systems, poor harvest index, and susceptibility to diseases. Research on most endemic medicinal plants has lagged behind that of crops; therefore, improvements depend on the utilization of the available genetic diversity.
Related reports
Results and discussion are based on related and topics researches about this study.
Innovations and breakthroughs
The innovations in the paper, according to my knowledge, there are no published articles on the diversity in phytochemical of essential oils of wild various populations of S, lavandulifolia flowers collected from Iran.
Applications
This study suggested that populations have the highest in α-pinene can selective as natural products for food and pharmaceutical industries.
Peer review
This is a good study in which the authors evaluated the diversity in phytochemical composition of essential oils of wild various populations of S. lavandulifolia flowers. This paper showed secondary metabolites in the essential oil of S. lavandulifolia like α-pinene that is really an important natural compound. In finally, this study suggests that populations having α-pinene can selective as natural products for food and pharmaceutical industries.
Footnotes
Foundation Project: Supported by deputy of Researches and Technology, Islamic Azad University of Shahrekord Branch, Iran (Grant no. IAUSHK: 6121).
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Mabberley DJ. The plant-book. 3rd ed. New York: Cambridge University Press; 2008. [Google Scholar]
- 2.Ghasemi Pirbalouti A. Medicinal and aromatic plants (introduction and application) 3rd ed. Iran: I.A.U. Shahrekord Branch Press; 2011. (in Persian) [Google Scholar]
- 3.Rechinger KH. Stachys. In: Rechinger KH, editor. Flora Iranica. Iran: NHBS; 1982. pp. 354–396. [Google Scholar]
- 4.Bhattacharjee R. Taxonomic studies in Stachys: II. A new infra generic classification of Stachys L. Notes Roy Bot Gard Edinburgh. 1980;38:65–96. [Google Scholar]
- 5.Mozaffarian V. A pictorial dictionary of botany botanical taxonomy Latin-English-French-Germany-Persian. Germany: Koeltz Scientific Books; 2008. p. 522. [Google Scholar]
- 6.Zargari A. Iranian medicinal plants. 1-6 Tehran: University Publication; 1982-1992. [Google Scholar]
- 7.Pirbalouti AG. Medicinal plants used in Chaharmahal and Bakhtyari districts, Iran. Herba Pol. 2009;55:69–75. [Google Scholar]
- 8.Pirbalouti AG, Karimi I, Koohpayh A. Wound healing activity of extracts of Malva sylvestris and Stachys lavandulifolia. Int J Biol. 2011;3:174–179. [Google Scholar]
- 9.Rabbani M, Sajjadi SE, Jalali A. Hydroalcoholic extract and fractions of Stachys lavandulifolia Vahl: effects on spontaneous motor activity and elevated plus-maze behavior. Phytother Res. 2005;19:854–858. doi: 10.1002/ptr.1701. [DOI] [PubMed] [Google Scholar]
- 10.Gharib Naseri MK, Adibpour N, Namjooyan F, Rezaee S, Shahbazi Z. Spasmolytic effect of Stachys lavandulifolia vahl crude methanolic extract and fractions on rat ileum. Iran J Pharm Res. 2011;10(2):307–312. [PMC free article] [PubMed] [Google Scholar]
- 11.Jenabi E, Asltoghiri M, Hajiloomohajeran M, Torkamani M. Effect of Stachys lavandulifolia on fatigue, nausea and vomiting associated with primary dysmenorrheal. Procedia Soc Behav Sci. 2012;31:124–128. [Google Scholar]
- 12.Khanavi M, Sharifzadeh M, Hadji Akhoondi A, Shafiee A. Phytochemical investigation and anti-inflammatory activity of aerial parts of Stachys byzanthina C. Koch. J Ethnopharmacol. 2005;97:463–468. doi: 10.1016/j.jep.2004.11.037. [DOI] [PubMed] [Google Scholar]
- 13.Asadi M, Bahrami S, Ansari Samani R, Pakniat N. Effect of hydroalcoholic extracts of Stachys lavandulifolia Vahl and Mespilus germanica leaves on leishmania major. Med J Hormozgan Univ. 2012;15(4):279–284. [Google Scholar]
- 14.Gyurkovska V, Alipieva K, Maciuk A, Dimitrova P, Ivanovska N, Haas C, et al. Anti-inflammatory activity of Devil's claw in vitro systems and their active constituents. Food Chem. 2011;125:171–178. [Google Scholar]
- 15.Háznagy-Radnai E, Balogh Á, Czigle S, Máthé I, Hohmann J, Blazsó G. Antiinflammatory activities of Hungarian Stachys species and their Iridoids. Phytother Res. 2012;26:505–509. doi: 10.1002/ptr.3582. [DOI] [PubMed] [Google Scholar]
- 16.Amiri H, Rustaiyan A, Lari Yazdi H, Haghir Chehregani AK. Antibacterial activity and composition of the essential oil of Stachys lavandulifolia Vahl. Basic Sci J. 2008;18:43–50. [Google Scholar]
- 17.Javidnia K, Mojab F, Mojahedi SA. Chemical constituents of the essential oil of Stachys lavandulifolia Vahl from Iran. Iran J Pharm Res. 2004;3:61–63. [Google Scholar]
- 18.Morteza-Semnani K, Akbarzadeh M, Changizi S. Essential oils composition of Stachys byzantina, S. inflata, S. lavandulifolia and S. laxa from Iran. Flavour Frag J. 2006;21:300–303. [Google Scholar]
- 19.Feizbaksh A, Saber Tehrani A, Rustaiyan A, Masoudi S. Composition of the essential oil of Stachys lavandulifolia Vahl from Iran. J Essent Oil Res. 2003;15:72–73. [Google Scholar]
- 20.Sajjadi SE, Mehregan I. Composition of the essential oil of Stachys laxa Boiss. & Buhse. Iran J Pharm Res. 2004;2:57–58. [Google Scholar]
- 21.Ebrahimabadia AH, Ebrahimabadia EH, Djafari-Bidgolia Z, Jookar Kashia F, Mazoochia A, Batoolib H. Composition and antioxidant and antimicrobial activity of the essential oil and extracts of Stachys inflata Benth from Iran. Food Chem. 2010;119(2):452–458. [Google Scholar]
- 22.Meshkatalsadat MH, Sajjadi SE, Amiri H. Chemical constituents of the essential oils of different stages of the growth of Stachys lavandulifolia Vahl. from Iran. Pak J Biol Sci. 2007;10:2784–2786. doi: 10.3923/pjbs.2007.2784.2786. [DOI] [PubMed] [Google Scholar]
- 23.Confortia F, Menichinia F, Formisanob C, Riganob D, Senatoreb F, Apostolides AN, et al. Comparative chemical composition, free radical-scavenging and cytotoxic properties of essential oils of six Stachys species from different regions of the Mediterranean Area. Food Chem. 2009;116(4):898–905. [Google Scholar]
- 24.Masoudi S, Rustaiyan A, Mohebat R, Mosslemin MH. Composition of the essential oils and antibacterial activities of Hymenocrater yazdianus, Stachys obtusicrena and Nepeta asterotricha three Labiatae herbs growing wild in Iran. Nat Prod Commun. 2012;7(1):117–120. [PubMed] [Google Scholar]
- 25.Kumar D, Bhat ZA, Kumar V, Khan NA, Chashoo IA, Zargar MI, et al. Effects of Stachys tibetica essential oil in anxiety. Eur J Integr Med. 2012;4(2):169–176. [Google Scholar]
- 26.Delazar A, Delnavazi MR, Nahar L, Moghadam SB, Mojarab M, Gupta A, et al. Lavandulifolioside B: a new phenylethanoid glycoside from the aerial parts of Stachys lavandulifolia Vahl. Nat Prod Rep. 2011;25(1):8–16. doi: 10.1080/14786411003754330. [DOI] [PubMed] [Google Scholar]
- 27.Curado MA, Oliveira CBA, Jesus JG, Santos SC, Searphin JC, Ferri PH. Environmental factors influence on chemical polymorphism of the essential oils of Lychnophora ericoides. Phytochem. 2006;67:2363–2369. doi: 10.1016/j.phytochem.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 28.Evans WC. Trease and Evans' pharmacognosy. 16th ed. Edinburg: W.B. Saunders; 2009. p. 83. [Google Scholar]
- 29.British Pharmacopoeia . British pharmacopoeia. London: HMSO; 1988. pp. 137–138. [Google Scholar]
- 30.Adams RP. Identification of essential oil components by gas chromatography/mass spectroscopy. Carol Stream, IL: Allured Pub Corp; 2001. [Google Scholar]
- 31.Javidnia K, Miri R, Azarpira A, Tabaei SMH. Composition of the essential oil of Stachys setifera C. A. Mey ssp. iranica growing in Iran. Flavour Frag J. 2003;18(4):299–300. [Google Scholar]
- 32.Radulovic N, Lazarevic J, Ristic N, Palic R. Chemotaxonomic significance of the volatiles in the genus Stachys (Lamiaceae): essential oil composition of four Balkan Stachys species. Biochem Syst Ecol. 2007;35:196–208. [Google Scholar]
- 33.Gorena AC, Piozzib F, Akcicekc E, Kılıçd T, Çarıkçıd S, Mozioğlua E, et al. Essential oil composition of twenty-two Stachys species (mountain tea) and their biological activities. Phytochem Lett. 2011;4(4):448–453. [Google Scholar]
- 34.Jerkovic I, Gugic M, Males J, Pilepic KH. Chemical composition of the essential oil from Stachys serotina. Nat Prod Commun. 2012;48(2):508–509. [Google Scholar]
- 35.Mirza M, Baher ZF. Essential oil of Stachys lanata Jacq from Iran. J Essent Oil Res. 2003;15:46–47. [Google Scholar]
- 36.Sezik E, Basaran A. Phytochemical investigation on the plants used as folk medicine and herbal tea in Turkey; essential oil of Stachys lavandulifolia Vahl. var. lavandulifolia. J Faculty Pharm Istanbul. 1985;21:98. [Google Scholar]
- 37.Sajjadi SE, Somae M. Chemical composition of the essential oil of Stachys inflata Benth. from Iran. Chem Nat Compd. 2004;40:378–380. [Google Scholar]
- 38.Pala-Paul J, Perez-Alonso MJ, Velasco-Negueruela A, Palá-Paú R, Sanz J, Conejero F. Seasonal variation in the chemical constituents of Santolina rosmarinifolia L. ssp. rosmarinifolia. Biochem Syst Ecol. 2001;29:663–672. doi: 10.1016/s0305-1978(01)00032-1. [DOI] [PubMed] [Google Scholar]
- 39.Sadrmomtaz A, Meshkatalsadatb MH, Taherparvara P. Comparison of volatile components of Stachys lavandulifolia vahl obtained by MWHD and HD techniques. Dig J Nanomater Biostruct. 2011;6(3):1343–1348. [Google Scholar]