SUMMARY
Presynaptic inhibition onto axons regulates neuronal output, but how such inhibitory synapses develop and are maintained in vivo remains unclear. Axon terminals of glutamatergic retinal rod bipolar cells (RBCs) receive GABAA and GABAC receptor-mediated synaptic inhibition. We found that perturbing GABAergic or glutamatergic neurotransmission does not prevent GABAergic synaptogenesis onto RBC axons. But, GABA release is necessary for maintaining axonal GABA receptors. This activity-dependent process is receptor subtype specific: GABAC receptors are maintained, whereas GABAA receptors containing α1, but not α3 subunits, decrease over time in mice with deficient GABA synthesis. GABAA receptor distribution on RBC axons is unaffected in GABAC receptor knockout mice. Thus, GABAA and GABAC receptor maintenance are regulated separately. Although immature RBCs elevate their glutamate release when GABA synthesis is impaired, homeostatic mechanisms ensure that the RBC output operates within its normal range after eye-opening, perhaps to regain proper visual processing within the scotopic pathway.
INTRODUCTION
Presynaptic inhibition onto axonal terminals, commonly provided by GABAergic transmission, regulates neurotransmitter release. GABA receptors on the axon hillock of pyramidal cells (Nusser et al., 1996; Szabadics et al., 2006), on mossy fiber terminals of hippocampal granule cells (Ruiz et al., 2003), on cerebellar parallel fibers (Stell et al., 2007), as well as on retinal bipolar cell axon terminals (Shields et al., 2000; Vardi and Sterling, 1994) serve to modulate action potential firing and neurotransmitter release (Kullmann et al., 2005; Luscher et al., 2011). Although much is known about how presynaptic GABAergic inhibition shapes neuronal output, mechanisms that regulate the development and maintenance of such inhibition onto axon terminals are not yet well understood. Here we addressed the role of neurotransmission in the development, maturation and maintenance of GABAergic synapses onto axonal terminals involved in modulation of neurotransmitter release. To do so, we took advantage of a well-characterized circuit in the mammalian retina where glutamate release from axons is regulated by robust presynaptic GABAergic inhibition.
At dim light levels, visual information from rod photoreceptors is conveyed to rod bipolar cells (RBCs), which relay the signal to amacrine cells in the inner retina (reviewed by Wässle, 2004). Specifically, RBCs contact GABAergic A17 amacrine cells (A17s) (Nelson and Kolb, 1985), which provide local feedback inhibition onto the rod bipolar cell axon terminals to modulate their release of glutamate (Chavez et al., 2006; Chun et al., 1993; Hartveit, 1999). In addition, RBC axon terminals also receive GABAergic drive from other widefield amacrine cells (Chavez et al., 2010; McGuire et al., 1984). Unlike other parts of the brain where GABAergic inhibition is mediated mainly by GABAA receptors, GABAergic input onto RBC axon terminals involves both GABAA and GABAC receptor types (Enz et al., 1996; Koulen et al., 1998). GABAA and GABAC receptors do not co-cluster at the same synaptic site but are expressed at different postsynaptic sites on RBC axon terminals (Fletcher et al., 1998; Koulen et al., 1998). There are also functional differences between the two receptor types: GABAA receptors are less sensitive to GABA but have faster kinetics compared to GABAC receptors, and thus the two receptor types regulate different aspects of glutamate release from RBCs (Eggers and Lukasiewicz, 2006b; Sagdullaev et al., 2006). The diversity of GABA receptors on RBCs presents an ideal opportunity to investigate how neurotransmission regulates the development and maintenance of not only GABA receptors, but also different GABA receptor types on the same axon terminal.
To examine the role of neurotransmission on the formation and maintenance of axonal GABA receptors, we utilized transgenic mice in which GABAergic or glutamatergic transmission is perturbed throughout the period of circuit assembly. Using electron microscopy, immunohistochemistry and electrophysiology, we assessed whether perturbed neurotransmission affects the structural formation and maintenance of GABA receptor subtypes on RBC axon terminals. Because RBC terminals also receive some glycinergic inhibition (Eggers et al., 2007, Wässle et al., 2009), we further analyzed whether an upregulation of glycine receptors occurred to compensate for the reduction in GABAergic transmission. Furthermore, we asked whether the loss of GABAergic inhibition causes alterations to RBC output beyond that expected solely from disinhibition in the GABA deficient circuit. Because GABA release from A17 amacrine cells normally requires RBC drive due to the reciprocal synaptic arrangement between these two cell types, we determined whether lack of GABAergic transmission also caused developmental changes in the glutamatergic synapses of A17 amacrine cells.
RESULTS
Development of GABAergic synapses on RBC axon terminals
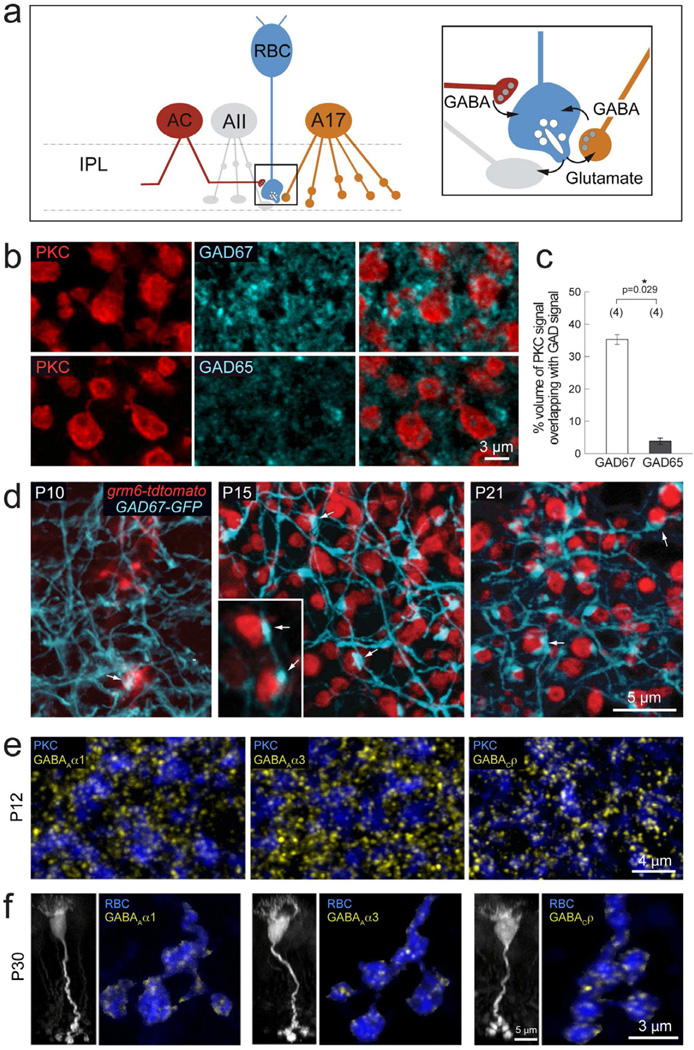
GABAergic inhibition onto axon terminals of RBCs is mediated by a variety of amacrine cells, including A17 cells that provide reciprocal feedback inhibition (Figure 1a). GABA is synthesized in amacrine cells by two isoforms of glutamate decarboxylase, GAD67 and GAD65 (Haverkamp and Wässle, 2000; Vardi and Auerbach, 1995). We found that in the sublamina of the inner plexiform layer (IPL) where RBC axonal terminals stratify (sublamina 5, ON-layer), GAD67 immunoreactivity was more abundant compared to GAD65 (Figure 1b). Quantification of the immunolabeling (see Methods) showed that dendritic processes positive for GAD67 exhibited greater volume overlap with PKC immunoreactive RBC axonal boutons, compared to GAD65 containing processes (Figure 1c), suggesting that GAD67 positive amacrine cell processes provide the majority of GABAergic inhibition onto RBC axon terminals.
Figure 1. Development of GABAergic synapses onto RBC axon terminals.
(a) Schematic showing RBC connections with amacrine cells in retina IPL. RBCs (blue) provide excitatory glutamatergic drive (glu) onto glycinergic AII (grey) and GABAergic A17 (orange) amacrine cells. In turn, they receive feedback inhibition from A17 cells and additional GABAergic input from other as yet unidentified amacrine cells (AC, red). (b) PKC labeled RBC terminals (red) colabeled with GAD67 or GAD65 (cyan), reveal more abundant immunoreactivity of GAD67 as compared to GAD65 in the IPL lamina where RBC axons stratify. (c) Quantification of % volume of GAD signal overlapping with PKC immunoreactivity revealed a significantly higher % overlap with GAD67 positive processes as compared to GAD65. Asterisk marks significant difference. (d) Development of contacts (arrows) between RBCs (red) and GAD67-GFP positive amacrine processes (cyan) in the grm6-tdtomato×GAD67-GFP double transgenic line. By P15, large varicosities can be seen at sites of contact between RBC axon terminals and GAD67-GFP amacrine processes. (e) Axonal terminals of P12 PKC-labeled RBCs (blue) express GABAAα1, GABAAα3, and GABACρ receptor clusters (yellow), indicating the presence of these three GABAergic postsynapses on RBC terminals before eye-opening. (f) P30 RBCs in the grm6-tdtomato transgenic line (left, vertical view, grayscale). Axonal terminals of individual P30 RBCs (horizontal view, blue) express all three GABA receptor cluster types: GABAAα1, GABAAα3, and GABACρ (yellow),
To visualize amacrine cell contacts onto RBC terminals during development, we utilized the GAD67-GFP transgenic line (Chattopadhyaya et al., 2004), where a fraction of GAD67 positive-amacrine cells (Figure S1a) express GFP. Amacrine cells typically synapse onto bipolar cell axons at enlarged varicosities (Dowling and Boycott, 1966). Using the GAD67-GFP transgenic line we visualized large varicosities of GFP labeled amacrine processes contacting RBC boutons at sites immunopositive for GAD67 but not GAD65 (Figure S1b). We further determined when GABAergic contacts are formed onto RBCs that are labeled in the GAD67-GFP line by expression of the fluorescent protein tdtomato under the grm6 promoter (Figure 1d). Appositions between GAD67 positive processes and RBC axonal boutons were already apparent at postnatal day (P) 10 (Figure 1d), several days after axonal differentiation in the bipolar cells (Morgan et al., 2006). At P15, large varicosities were clearly present at sites of contact between amacrine cell dendrites and RBC boutons, which remained evident at P21. We previously found that spontaneous GABAergic inhibitory postsynaptic currents (sIPSCs) can be recorded from RBCs from P10 (Schubert et al., 2008). Thus, the appearance of structural contacts between GABAergic amacrine cells and RBC axon terminals coincides with the emergence of sIPSCs in RBCs. This suggests that functional postsynaptic GABA receptor clusters are already present on RBC terminals before eye-opening (P14).
Previous studies demonstrated that GABAA and GABAC receptors are present on axon terminals of RBCs across species (Fletcher et al., 1998; Koulen et al., 1998; Koulen et al., 1997; Lukasiewicz, 1996; Enz et al., 1996; Lukasiewicz et al., 2004; McCall et al., 2002). To monitor the appearance of GABA receptor clusters on RBC axonal boutons during circuit assembly, we labeled for GABAA and GABAC receptors at two developmental time points, before eye-opening (P12) and at maturity (P30). We found abundant GABAC receptor clusters on PKC-labeled axon terminals of RBCs as early as P12 and at P30 (Figure 1e, f). To determine the subunit composition of the GABAA receptor clusters located at these terminals, we performed immunostaining for GABAA α1–3 subunits together with PKC. α2-containing GABAA receptors were not present on RBC axon terminals (Figure S2a), supporting past findings (Fletcher et al., 1998). In contrast, both α1 and α3-containing GABAA receptor clusters were localized on RBC axonal boutons at both ages examined (Figure 1e, f). GABAA synapses containing α1 or α3 subunits did not co-localize with GABAC receptors (Figure S2b) as found previously in adult retina (Koulen et al., 1996; Wässle et al., 1998). Furthermore, these three GABA receptor cluster types (α1-GABAA, α3-GABAA and GABAC) on RBC boutons were each apposed to large GAD67-GFP amacrine cell varicosities (Figure S1c). Co-Immunolabeling for GAD67 and GABA receptors revealed that 93.27± 0.48% of GABAAα1 (n=2 animals), 93.89 ± 0.66% of GABAC (n=2) and 79.30 ± 2.04% of GABAAα3 (n=3) receptor clusters on RBC terminals were apposed to GAD67 positive terminals. Thus, all three GABA receptor types are present on RBC terminals before eye-opening, suggesting that synapses comprising these receptor types develop concurrently.
Synaptic contacts between RBCs and amacrine cells form despite reduction of GABAergic or glutamatergic neurotransmission
To determine the role of neurotransmission in the structural development of inhibitory synapses onto RBC axon terminals, we used two transgenic mouse lines (Figure 2a). First, we asked whether suppressing glutamatergic transmission onto amacrine cells that likely contact RBCs affects the development of amacrine cell synapses onto RBC axons. This was achieved using the grm6-TeNT line in which neurotransmission from all ON-bipolar cells including RBCs, is suppressed by expression of the light chain of tetanus toxin (Kerschensteiner et al., 2009). Next, we assessed the outcome when GABA release from amacrine cells is diminished from development onwards. To do so, we crossed GAD1 conditional knockout mice (Chattopadhyaya et al., 2004) with αPax6-Cre mice in which cre-recombinase expression is limited to the retina, to generate GAD1KO mice (Schubert et al., 2010) (Figure S3). In the GAD1KO retina, the level of GAD67 expression is reduced (Figure S3a), and immunolabeling for GAD67 reveals that loss of GAD67 (in the knockout region) occurs outside a dorsal-ventral strip (the WT-region) in which cre-recombinase is absent (Figure S3b, c; see Marquardt et al., 2001). We use ‘GAD1KO’ from here on to refer to the knockout regions.
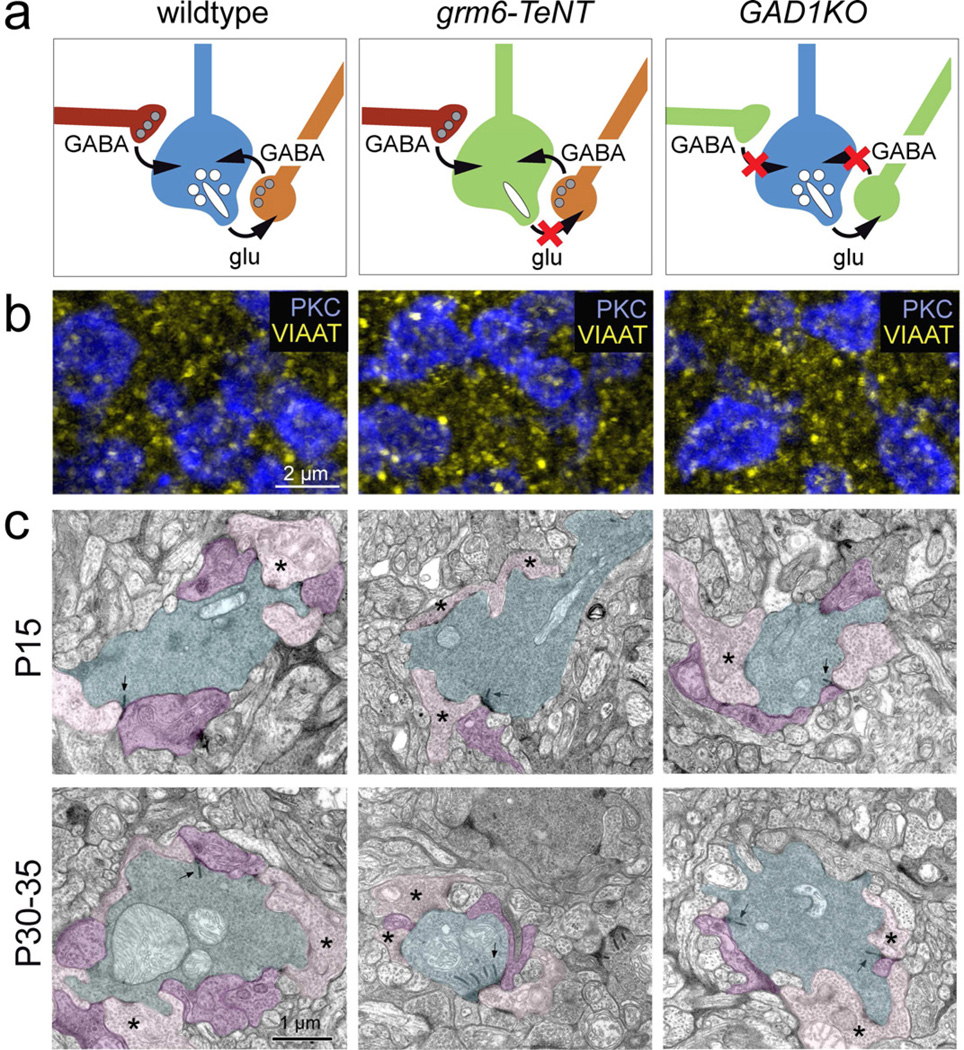
Figure 2. Inhibitory synapses onto RBC axons in the grm6-TeNT and GAD1KO retina.
(a) Schematic showing RBC axon terminal circuitry in wildtype and transgenic lines with suppressed glutamatergic transmission from ON-bipolar cells (grm6-TeNT) and reduced GABAergic transmission from amacrine cells (GAD1KO). (b) Immunolabeling for VIAAT (yellow) at the level of PKC positive RBC boutons (blue) in P30 wildtype, grm6-TeNT and GAD1KO retina. (c) Electron micrographs showing dyad synapses between RBC boutons (cyan) and AII (purple) and A17 (pink) amacrine cell processes in the wildtype (WT), grm6-TeNT and GAD1KO retinae at P15 and P30–35. Presynaptic ribbons are indicated by arrows. Asterisks mark examples of A17 contact. Note that RBCs in the P30 grm6-TeNT retina have multiple ribbons at a single dyad synapse.
We compared VIAAT (vesicular inhibitory amino acid transporter) immunolabeling across wildtype, grm6-TeNT and GAD1KO retinas to assess whether amacrine cell terminals surrounding RBC axons failed to differentiate when neurotransmission is perturbed. We found no gross changes in the density of VIAAT labeling surrounding P30 RBC axonal terminals in either transgenic line, suggesting that amacrine cell-RBC synapses still formed (Figure 2b). We confirmed that amacrine cell-RBC synapses still formed in the mutant mice, by examining the ultrastructural arrangement of RBC axonal boutons in both grm6-TeNT and GAD1KO retina. In both lines, amacrine cells still synapsed onto RBC boutons, and these synapses were apparent at eye-opening (P15) (n=14 GAD1KO; n=26 TeNT RBC boutons examined) as in wildtype animals (Figure 2c). However, some aspects of the synaptic arrangements differed between grm6-TeNT and GAD1KO retinas. P30 RBC boutons formed dyad synapses at sites containing a single ribbon in the GAD1KO (n= 20 RBC synapses per genotype) but such arrangements were disrupted in grm6-TeNT retinas where sometimes multiple ribbons were apposed to a single postsynaptic density (n= 16 of 26 at P15, and 13 of 23 synaptic sites had >1 ribbon at P30; e.g. Figure 2c), as shown previously (Kerschensteiner et al., 2009). Thus, although the fine arrangement of the RBC presynapse is differentially affected in the grm6-TeNT and GAD1KO retinas, the amacrine cell-RBC synapse forms and is maintained irrespective of whether bipolar or amacrine cell transmission is perturbed.
Development and maintenance of GABAergic synapses on RBC axon terminals does not rely on glutamatergic transmission
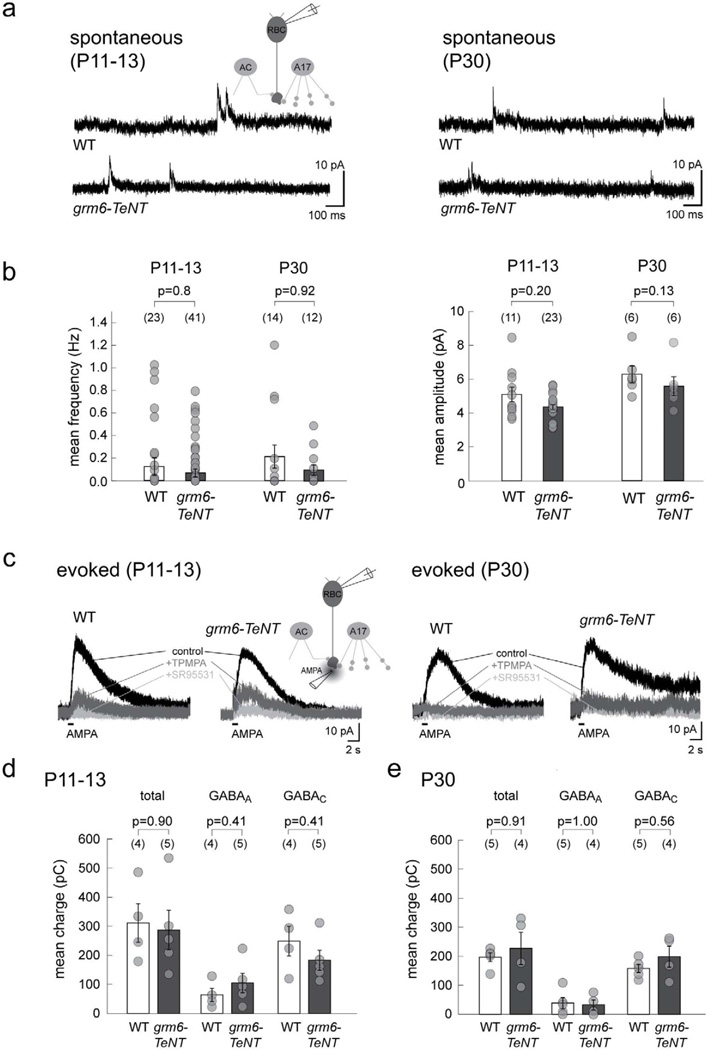
Although amacrine cell synapses are present structurally on RBC terminals in grm6-TeNT retinas, it is possible that suppression of glutamatergic drive from bipolar cells leads to alterations in GABA mediated transmission onto RBC axon terminals. We thus performed whole-cell recordings on wildtype and grm6-TeNT RBCs and analyzed spontaneous GABAergic inhibitory postsynaptic currents (sIPSCs) (Figure 3a). Spontaneous IPSCs in both genotypes were comparable in mean frequency and amplitude at both P11–13 and P30 indicating that the number of GABAergic synaptic contacts and the size of postsynaptic GABA receptor clusters were not significantly affected by a reduction of glutamatergic input to amacrine cells (Figure 3b). To evoke release of GABA from presynaptic amacrine cells, we puffed AMPA at the axon terminal of RBCs (Chavez et al., 2006) and recorded the evoked sPSCs at 0 mV holding potential (Figure 3c). The mean charge of AMPA-evoked currents was not different in wildtype and grm6-TeNT RBCs at both P11–13 and P30 (Figure S4). To distinguish GABAA and GABAC components of the evoked response, AMPA puffs were repeated in the presence of TPMPA (GABAC receptor antagonist) or SR95531 (GABAA receptor antagonist) (Figure 3c). Quantification of mean charge and amplitude (data not shown) of the evoked response (total, GABAA or GABAC) showed no significant differences between RBCs in wildtype and grm6-TeNT retinas at P11–13 (Figure 3d) and at P30 (Figure 3e). Together, these results suggest that functional GABAergic synapses are formed normally and maintained even when the bipolar cells fail to transmit effectively to amacrine cells.
Figure 3. Spontaneous and GABA-evoked currents of RBCs in the grm6-TeNT retina.
(a) Spontaneous GABAergic currents recorded in grm6-TeNT and wildtype (WT) RBCs at P11–13 and P30. (b) Quantification revealed no significant differences in either the mean frequency or mean amplitude of GABAergic sIPSCs from grm6-TeNT RBCs as compared to WT. (c) Example traces showing chloride-mediated outward currents in RBCs evoked by AMPA puffs in grm6-TeNT and WT RBCs at both ages. The GABAC component was isolated by application of TPMPA, and residual GABAA-mediated current blocked by SR95531. Scatter plots show that the mean charge transfer of the total, GABAA-mediated and GABAC-mediated currents are comparable in grm6-TeNT RBCs and WT at P11–13 (d) and P30 (e). Numbers in brackets in b, d, and e represents number of cells. Error bars represent S.E.M.
RBC responses to GABA application decline with maturation in the GAD1 mutant
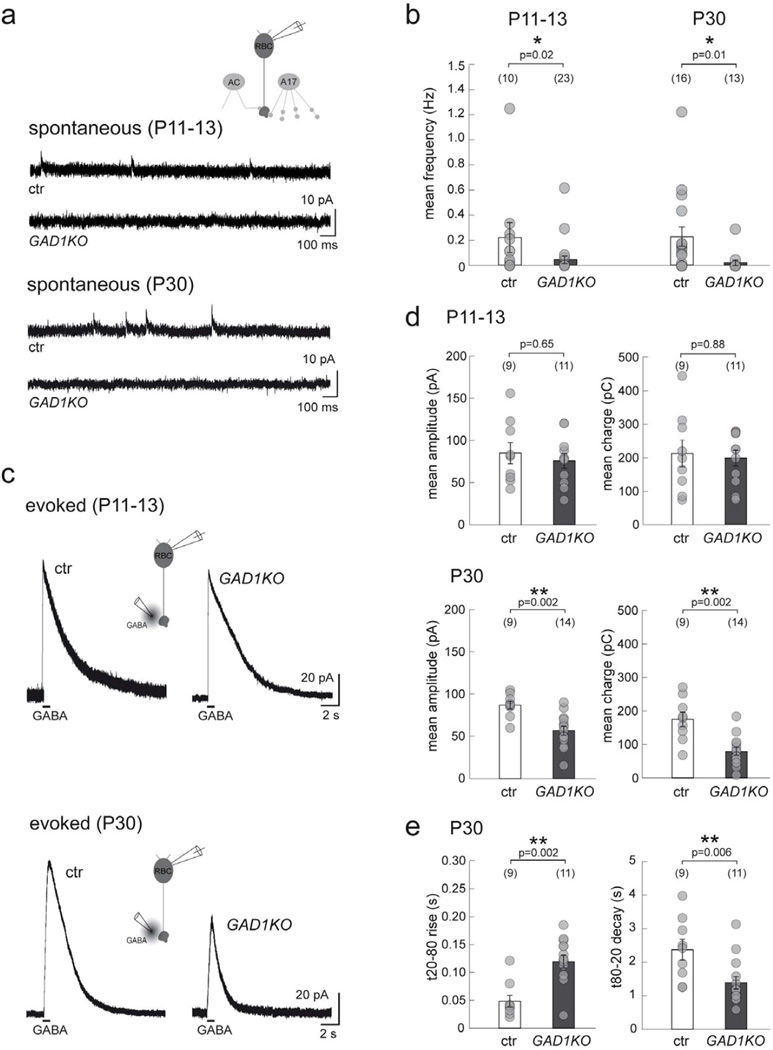
To assess the functional consequences of reduced GABA synthesis on the formation and maintenance of inhibitory synapses onto RBC axon terminals, we performed whole-cell recordings of these neurons in the GAD1KO retina. As expected, spontaneous sIPSCs were rare in the GAD1KO at both ages examined (Figure 4a, b). This indicates that deletion of GAD67 was not accompanied by a compensatory upregulation of the other GABA synthesizing enzyme, GAD65. Because synaptic release of GABA in these mutants is greatly impaired, we puffed GABA onto GAD1KO RBC axon terminals and recorded the evoked chloride currents in order to assess whether any postsynaptic changes occurred in the GAD1KO RBCs. Interestingly, RBC responses to GABA application in the GAD1KO were unchanged at P11–13 but were dramatically reduced at P30 (example recordings in Figure 4c). Both mean amplitude and charge of the evoked responses decreased by P30 (Figure 4d). Moreover, the rise time for evoked responses in P30 GAD1KO RBCs was slower, whereas their decay time was faster, as compared to control (Figure 4e). Thus, GABAergic transmission plays an important role in the maintenance, although not in the initial formation, of functional GABAergic synapses on RBC axon terminals.
Figure 4. Spontaneous and GABA-evoked currents of RBCs in GAD1KO retina.
(a) Spontaneous GABAergic IPSCs at RBC axon terminals recorded from GAD1KO and littermate control (ctr) at P11–13 and P30. (b) Scatter plots show that the mean frequency of these sIPSCs are reduced in GAD1KO as compared to ctr. (c) Puffs of GABA evoke similar chloride-mediated outward currents in RBCs from ctr and GAD1KO at P11–13, which are markedly reduced for GAD1KO RBCs at P30. (d) Quantification reveals a significant reduction for both the mean peak amplitude and charge of GABA-evoked currents from GAD1KO RBCs at P30. (e) In addition, the rise time for the P30 evoked response from GAD1KO RBCs is longer while the decay is faster as compared to control. Numbers in brackets in b, d, and e represents number of cells. Asterisks mark significant difference. Error bars represent S.E.M.
In wildtype animals, RBCs receive little glycinergic input (Eggers et al., 2007). Indeed, immunolabeling for glycine receptors (α1- and α2-subunit containing receptor clusters)(Ivanova and Muller, 2006; Wässle et al., 2009) showed little glycine receptor expression on RBC axon terminals (Figure S5a, b). However, a severe reduction of GABAergic transmission in the GAD1KO may be compensated for by an upregulation of glycine receptor-driven input onto RBC axon terminals. We investigated this possibility by quantifying the expression of glycine receptors containing α1 or α2 subunits on RBC axon terminals in the GAD1KO retina. But, we did not find any upregulation of these glycine receptor subtypes in the RBC terminals of the GAD1KO (Figure S6).
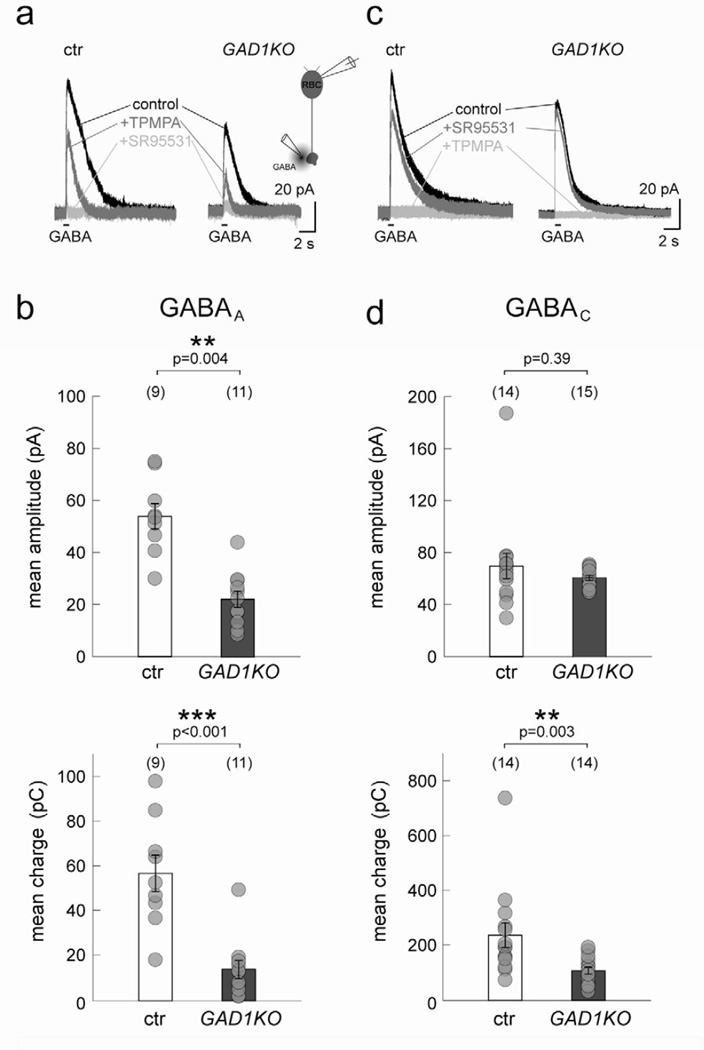
GABAA and GABAC receptors on RBC axon terminals are differentially altered in the GAD1KO
Because GABAA and GABAC receptor clusters are found at distinct postsynaptic sites on RBC axon terminals, we next asked whether loss of GABAergic transmission affected both receptor types. Figure 5a shows examples of the GABA-evoked responses of P30 RBCs from a littermate control and a GAD1KO animal in which the GABAA component is revealed upon blocking the GABAC receptor-mediated current. Quantification of the mean amplitude and charge of the evoked GABAA responses in RBCs revealed significant reduction in the KO animal (Figure 5b). Similarly, the GABAC-evoked responses were isolated for RBCs in the GAD1KO and control upon blocking GABAA currents (Figure 5c). In contrast to GABAA-mediated responses, the mean amplitude of the GABAC-mediated response was unchanged (Figure 5d). However, the net charge carried by the GABAC currents was significantly reduced in the GAD1KO (Figure 5d). This may reflect faster GABAC-mediated response kinetics in RBCs from GAD1KO compared to littermate control (see Figure 4e).
Figure 5. GABAA and GABAC receptor-mediated currents from P30 RBCs in GAD1KO retina.
(a) Example traces of P30 GAD1KO and littermate control (ctr) RBC responses to GABA puffs. TPMPA was applied to isolate the GABAA-mediated component. Further addition of SR95531 blocked all evoked response. (b) Scatter plots show that mean peak amplitude and charge of GABAA-mediated currents in GAD1KO RBCs are significantly reduced as compared to ctr. (c) Application of SR95531 alone isolates the GABAC-mediated component of evoked responses in RBCs. (d) Quantification of GABAC-mediated currents indicates a significant reduction of the mean charge in GAD1KO RBCs as compared to ctr. However, the mean amplitude remained unchanged. Numbers in brackets in b and d represents number of cells. Asterisks mark significant difference. Error bars represent S.E.M.
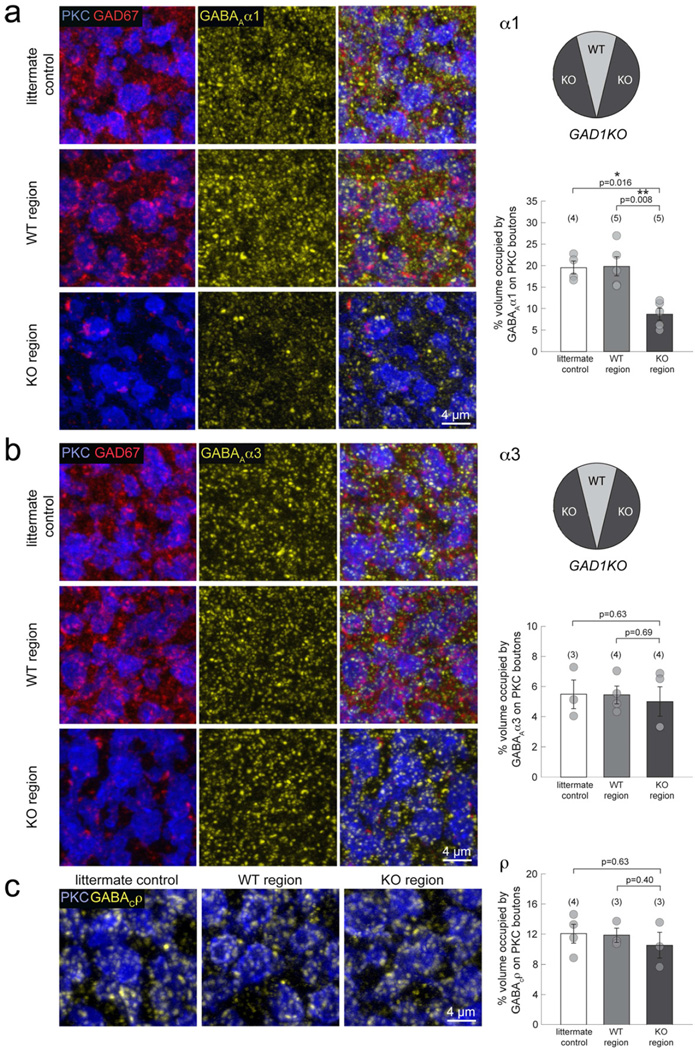
To correlate these functional changes at P30 with the expression of GABAA and GABAC receptor types, we immunostained for the α1 and α3 subunits of the GABAA receptors, and the ρ subunits of the GABAC receptors. We compared the immunolabeling of P30 knockout regions in the GAD1KO mutant with corresponding wildtype regions (which provides an ideal control because these regions are from within the same retina) as well as with littermate control retinas. For GABAA receptors, GAD67 immunostaining was used to distinguish knockout regions from wildtype regions in the GAD1KO (Figure 6a,b). However, we could not co-label GAD67 and GABAC receptors due to species specificity of the antibodies. Instead, we used the GFP signal to identify the knockout regions because GFP is expressed specifically in cells where the GAD1 exon is excised (Marquardt et al., 2001). Overall, immunoreactivity for α1-containing GABAA receptors was significantly reduced in the knockout region compared to the wildtype region and the littermate control (Figure 6a). In contrast, α3-containing GABAA receptor labeling did not appear to have changed in the knockout regions (Figure 6b). Similarly, GABAC receptor staining was comparable across regions and genotypes (Figure 6c). Because of the high density of GABA receptor clusters on RBC boutons, it was not always possible to separate individual clusters. Thus, instead of determining the number of receptor puncta, we quantified the percent volume occupied by each receptor subtype on PKC positive RBC boutons (see Methods). We found that the percent volume occupied by α1-containing GABAA receptors, but not α3-containing GABAA receptors or GABAC receptors, was significantly reduced in the knockout regions (Figure 6). This reduction of GABAAα1 clusters in GAD67 deficient regions was corroborated by using another GABAAα1 antibody raised in a different species (Figure S6a). To assess whether GABAAα1 synthesis levels in GAD1KO retina was diminished overall, we performed western blot analysis using P30 retina homogenates from which the dorsal-ventral wedge was removed. The total GABAAα1 synthesis levels in the GAD1KO mutant was comparable to littermate control samples (Figure S6b, n=2 GAD1KO and 2 littermate controls), suggesting that overall synthesis of GABAAα1 receptors in GAD67 deficient retina was unchanged. The reduction in immunostaining for GABAAα1 in the GAD1KO is thus unlikely to be caused by an alteration in receptor synthesis, and is more likely the result of a deficit in the maintenance of GABAAα1 clusters on RBC terminals. Because we found little expression of the known inhibitory postsynaptic scaffolding proteins, Neuroligin2 and Gephyrin, at GABAA receptors in RBC axon terminals (Figure S5b, c), we decided against analyzing the expression levels of these proteins in GAD1KO RBCs. Taken together, our findings demonstrate that GABAA and GABAC receptors on RBC axon terminals are differentially maintained by GABAergic transmission.
Figure 6. GABAA receptor subsets on mature RBC boutons in GAD1KO retina.
(a) Triple immunolabeling of PKC immunopositive RBCs (blue), GAD67 (red) and GABAAα1 subunits (yellow), revealed a visible reduction of α1-containing GABAA receptor clusters in the KO region of GAD1KO retina, compared to the WT region or littermate control. Quantification of the % volume occupied by GABAAα1 clusters on PKC positive boutons confirmed a significant reduction for the KO region. (b) Immunostaining for the GABAAα3 receptor subunit revealed no significant differences in the % volume occupied by these receptor clusters on KO region RBC boutons. (c) Immunolabeling and % volume occupancy of GABACρ receptor subunits were comparable across littermate control, WT region and KO region. Numbers in brackets represent number of animals. Asterisks mark significant difference. Error bars represent S.E.M.
The specific reduction of GABAA and not GABAC receptor clusters in GAD1KO RBCs suggests an independent regulation of the maintenance of these two ionotropic GABA receptor types that coexist on the same axonal terminal. To further test this hypothesis, we immunolabeled α1-containing GABAA receptor clusters in GABACKO mice (McCall et al., 2002)(Figure S7). We found no alteration in the percent volume occupied by α1-containing GABAA receptors in P30 GABACKO RBC terminals (Figure S7). Thus, we conclude that there exists independent regulatory mechanisms for maintaining GABAA and GABAC receptor clusters on RBC axon terminals.
Reduced GABAergic drive transiently increases glutamate release from developing RBCs
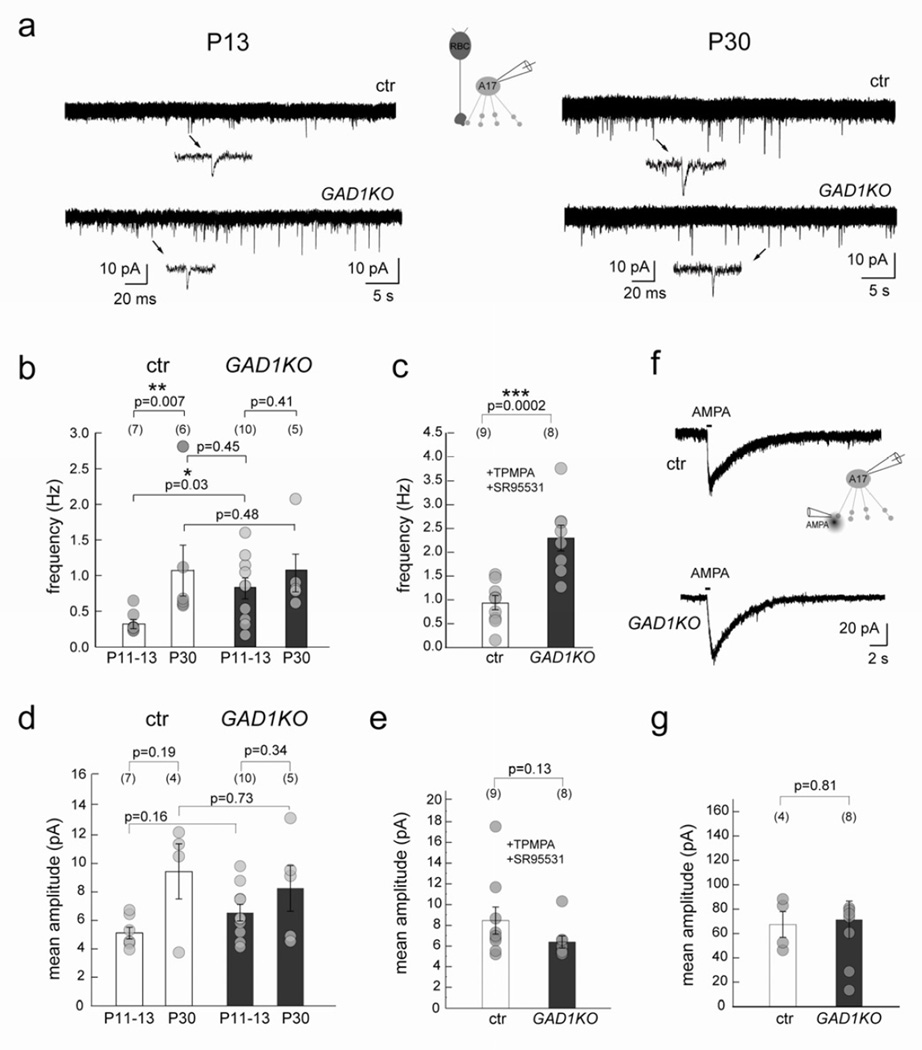
GABAergic A17 amacrine cells form reciprocal synapses with glutamatergic RBCs. We thus wondered whether there were corresponding changes in the RBC input onto the A17 cells in the GAD1KO. We compared RBC input onto A17 cells in GAD1KO with wildtype littermate controls at two developmental time-points, P11-P13 and P30, by recording spontaneous excitatory postsynaptic currents (sEPSCs) from A17 amacrine cells. In littermate controls, the mean frequency of sEPSCs in these amacrine cells normally increased with age (Figure 7a,b). In the GAD1KO, the mean frequency of sEPSCs from A17 amacrine cells at P11–13 was significantly higher compared to their control littermates (Figure 7a, b). In the KO, there was no further increase in sEPSCs frequency in the A17 cells with maturation (Figure 7a, b), and in fact, the P11–13 sEPSCs were already comparable to that of P30 controls. These observations together suggest that although the RBC output is initially elevated during development when inhibition is reduced, homeostatic mechanisms ensure that the bipolar cell output operates within its normal range at maturity (Figure 7b).
Figure 7. RBC output is transiently increased in GAD1KO retina during circuit development.
(a) sEPSCs recorded from A17 amacrine cells at P13 and P30 in GAD1KO and littermate control retina shows an increase during normal development (ctr). (b) The sEPSC frequency was increased for A17 amacrine cells in GAD1KO as compared to ctr early in development at P13, but was comparable to control at P30. (c) Mean frequency of sEPSCs from P11–13 A17 amacrine cells in GAD1KO and ctr in the presence of GABA receptor antagonists TPMPA and SR95531, also showed increased frequency in the GAD1KO. (d) Mean sEPSC amplitudes recorded in A17s from littermate control and GAD1KO at P11–13 and P30 revealed no differences. (e) Mean amplitude of sEPSCs recorded from P11-P13 A17 amacrine cells in GAD1KO and ctr in the presence of GABA receptor antagonists TPMPA and SR95531 showed no difference. (f) Example traces showing AMPA puff evoked cation-mediated inward currents from A17 cells clamped at −60 mV evoked by AMPA puffs in ctr and GAD1KO at P11–13. Scatter plots show that the mean amplitude (g) is comparable between genotypes. Numbers in brackets represents number of cells. Asterisk marks significant difference. Error bars represent S.E.M.
Is the transient increase in frequency of the sEPSCs from developing A17 cells in the KO simply a result of reduced inhibition onto the RBC axonal terminals? To answer this, we recorded sEPSCs from A17s in P11-P13 GAD1KO and littermate control animals in the presence of GABAA and GABAC receptor blockers, SR95531 and TPMPA respectively (Figure 7c). Even in the presence of GABA receptor blockers, P11-P13 A17 cells showed an increased frequency of sEPSCs in the GAD1KO as compared to control (Figure 7c), suggesting that there is an upregulation of glutamatergic drive onto A17 cells in the GAD1KO. This upregulation, however, is not the consequence of a loss of GABAC-receptor mediated transmission alone, despite GABAC receptors carrying the majority of the total charge transfer (Figure 5b,d; Eggers and Lukasiewicz, 2006a; McCall et al., 2002). In the presence of a GABAA receptor antagonist, the mean sEPSC frequency of P11–13 A17 cells in the GABAC receptor KO is not significantly different from that of littermate controls (Figure S8).
Could the upregulation of glutamatergic drive onto developing A17 cells be due to changes in receptor density on A17 cells? We found that at P11–13, the mean amplitude of the sEPSCs was unchanged for A17s in the GAD1KO (Figure 7d,e), indicating that the glutamate receptor density at individual postsynaptic sites is not altered. Also, A17 cell responses to AMPA puffs revealed no differences between GAD1KO and control (Figure 7f, g). Thus, the total density (or number) of glutamatergic synapses on A17s is unperturbed in GAD1KO animals (Figure 7d-g). This suggests that the increase in A17 sEPSC mean frequency in the GAD1KO is not the result of changes in glutamate receptor density on the A17 cell, but more likely due to presynaptic changes, such as the probability of release, in the RBC terminal.
DISCUSSION
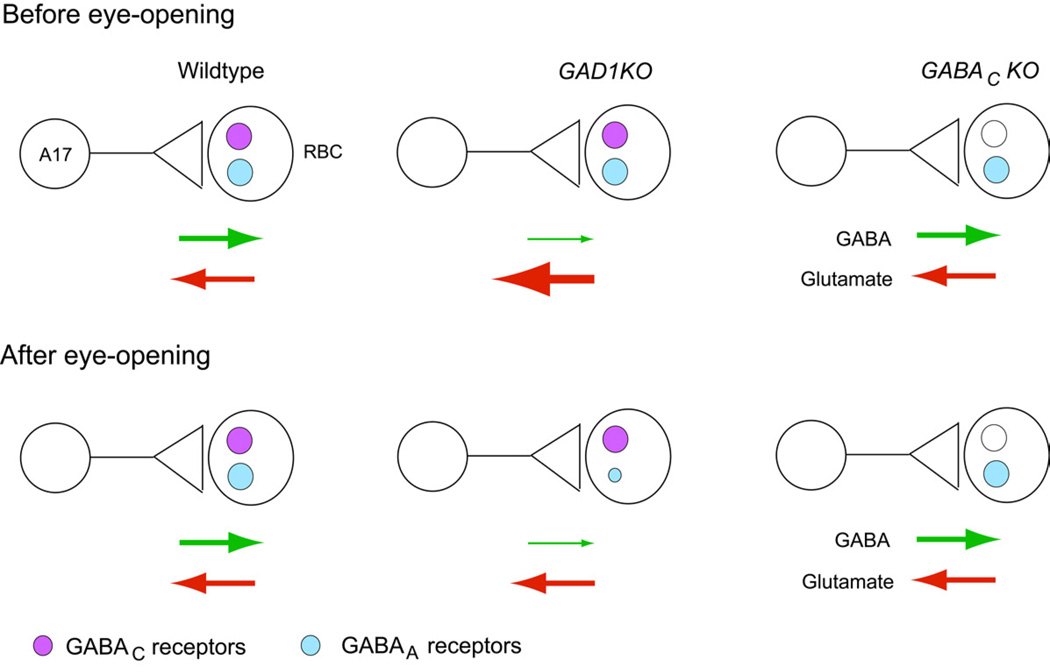
We found that RBC axon terminals receive GABAergic inhibition from GAD67-positive amacrine cells via three distinct GABA receptor subtypes (GABAAα1, GABAAα3 and GABAC). Using mutant mice, we found that GABAergic synapses are still established on RBC axonal terminals when either glutamatergic or GABAergic transmission is perturbed. However, the maintenance of GABAAα1 receptor clusters on RBC axonal terminals is selectively disturbed when GABA synthesis is much reduced in the presynaptic amacrine cells (Figure 8). Further, the maintenance of GABAAα1 receptor clusters is not dependent on the presence or synaptic drive via GABAC receptors. We also discovered that glutamate release from developing RBCs increased in the GAD1KO mutant, but not in the GABACKO mutant (Figure 8).
Figure 8. Summary of the alterations in RBC-A17 synapses in GABAergic transmission-defective mutants before and after eye-opening.
Schematic illustrating the A17-RBC synapse before and after eye-opening in the wildtype, GAD1KO and GABACKO retina. GABA and glutamate release is similar between wildtype and GABACKO (see also Eggers and Lukasiewicz, 2006a), but is altered in the GAD1KO. There is a transient upregulation of glutamate release from developing RBCs in the GAD1KO before eye-opening. However, after eye-opening, glutamate release from RBCs in the GAD1KO becomes comparable to wildtype. GABAA and not GABAC receptors are selectively reduced at maturity in the GAD1KO. This reduction of GABAA receptors on RBCs does not occur in GABAC receptor mutants.
GABAergic synapse maintenance but not formation on axon terminals is influenced by GABAergic transmission
How neurotransmission modulates the formation of inhibitory synapses has primarily been addressed for inhibitory synapses onto somata and dendrites of neurons (Chattopadhyaya et al., 2007; Harms and Craig, 2005; Kilman et al., 2002). Blocking global activity in developing pyramidal neurons in culture leads to a decrease in the number and size of inhibitory synapses (Kilman et al., 2002). Here, we assessed the importance of neurotransmission in the development of GABAergic synapses on axon terminals, focusing on amacrine cell-RBC connectivity. Our results demonstrate that presynaptic GABAergic amacrine cells do not require glutamatergic drive from bipolar cells to establish their inhibitory synapses onto the axons. GABA release onto RBCs as well as GABA receptor density of RBCs are unchanged in the grm6:TeNT retinas. This is in contrast to a reduced number of inhibitory synapses and decrease in synaptic vesicle density of terminals contacting the dendrites of spinal cord neurons cultured in the presence of glutamate (non-NMDA) receptor antagonists (Rosato-Siri et al., 2002).
Prior work has demonstrated that GABAergic transmission is not essential for inhibitory synapse formation on dendrites and somas per se (Chattopadhyaya et al., 2007; Wojcik et al., 2006; Wu et al., 2012). We found this to also be true for inhibitory synapse formation onto axon terminals as amacrine cell-RBC synapses are evident in the retinal-specific GAD1KO. Deletion of GAD67 in basket cells of the visual cortex, however, results in fewer perisomatic inhibitory synapses on pyramidal neurons (Chattopadhyaya et al., 2007). This reduction in inhibitory synapse number onto the cell bodies appears to be due to the lack of GABAergic transmission during synaptogenesis, rather than a failure to maintain established synapses. In the retina, we found that reducing GABAergic transmission during development affects the maintenance of GABA receptors on the RBC axons but not the initial formation of these synapses. From previous work (Burrone and Murthy, 2003; Pozo and Goda, 2010; Turrigiano, 2007), we had expected that RBCs in the GAD1KO might undergo homeostatic adjustment and recruit more GABA receptors to their axons to compensate for reduced GABAergic transmission. Instead, we observed that RBC axon terminals lose GABA receptors at maturity when presynaptic GABA release is reduced chronically.
Activity dependent maintenance of axonal GABA receptors is receptor subtype specific
To date, most studies focusing on the activity-dependent maintenance of GABAA receptors in neurons have assessed the distribution of the entire GABAA receptor population, irrespective of their subunit composition. This is because in most parts of the nervous system, GABAA receptors can comprise mixed α-subunits together with β–and γ- subunits (Fritschy and Mohler, 1995; Kasugai et al., 2010). However, in the mammalian retina, three distinct subtypes of GABAA receptors can be distinguished by the presence of specific α-subunits (α1-α3) localized at non-overlapping synapses (Koulen et al., 1996; Wässle et al., 1998). On mouse RBC axon terminals, we identified two types of GABAA receptor synapses, containing either the α1-subunit or the α3-subunit. Both these GABAA receptor types were apposed to GAD67-positive processes but, functionally, they could provide GABAA receptor-mediated inhibition with different time courses, because α1-containing GABAA receptors exhibit faster response kinetics compared to α3-containing receptors (Gingrich et al., 1995; Ortinski et al., 2004; Vicini et al., 2001). Surprisingly, we found that reduced GABAergic neurotransmission selectively regulated the maintenance of GABAAα1 but not GABAAα3 receptor clusters.
Reducing GABAergic transmission also differentially affected the maintenance of the two ionotropic GABA receptor types (GABAA and GABAC) on RBC axons. While the amplitude of the GABAA receptor component of the GABA-evoked response was decreased at P30 in the GAD1KO, there was no change in amplitude of the GABAC component. Our immunostaining for GABAA and GABAC receptors supported this differential reduction in GABAA versus GABAC receptors on RBC axons. What might be the differences in GABAA and GABAC receptors that could account for this differential outcome in the GAD1KO? GABAC receptors have a higher affinity for GABA compared to GABAA receptors (Feigenspan and Bormann, 1994). Perhaps these receptors require only low levels of GABA for their maintenance. GABAA and GABAC receptors do not colocalize at the same postsynapse (Fletcher et al., 1998; Koulen et al., 1998). Thus, it could also be that these two GABA receptor types are differentially positioned on the RBC terminal relative to the GABA release site, such that GABAA receptors normally ‘sense’ higher GABA levels compared to GABAC receptors, and thus need a substantial amount of GABA in the synaptic cleft for their maintenance. We further showed that loss of GABAC receptors per se does not affect the maintenance of GABAAα1 receptors in the RBC terminals. We found no down-regulation of GABAA receptors in the GABACKO retina. Furthermore, in the GABACKO retina, the function of glycine receptors (Eggers and Lukasiewicz, 2006a) on RBCs axon terminals is not affected. Accordingly, in the GAD1KO we found no upregulation of glycine receptor clusters on RBC axon terminals. Taken together, our observations highlight independent mechanisms for regulating the distribution and density of distinct inhibitory receptor types (GABA receptors versus glycine receptors, GABAA versus GABAC receptors, GABAAα1 versus GABAAα3 receptor types) on the same RBC axon terminal.
What underlies the eventual reduction of α1-containing GABAA receptors in the GAD1KO? The role of activity in inhibitory receptor accumulation has been addressed in spinal cord cultures, where blocking neuronal activity by TTX application prevented glycine receptor accumulation (Kirsch and Betz, 1998). However, our physiological recordings suggest that GABA receptors accrue normally on RBC axons initially (P11–13), and total GABAAα1 synthesis in adult (P30) GAD1KO retina also appeared unimpaired. Another possibility is a failure to stabilize GABAAα1 receptor clusters after they have formed at synapses. Tracking movements of the inhibitory postsynaptic scaffold protein gephyrin in dissociated spinal cord neurons previously demonstrated activity-dependent stabilizations of the gephyrin-fluorescent protein conjugates at synaptic vs extrasynaptic locations (Hanus et al., 2006). The phenotypic alterations we observed in density of α1-containing GABAA receptors on RBC axons in the GAD1KO could be a result of failed stabilizations of GABAA receptors at this synapse. This could lead to greater receptor internalization and subsequent degradation detectable as reduced receptor volume occupancy. Indeed, blocking neuronal activity has been shown previously to induce ubiquitination and degradation of GABAA receptors (Luscher et al., 2011). However, because Neuroligin2 and Gephyrin do not appear to be at all GABAAα1 receptor clusters on the RBCs, future work is necessary to identify the postsynaptic scaffold proteins at the RBC axonal GABA receptors before we can systematically investigate the molecular mechanisms underlying the loss of GABAAα1 receptor clusters on RBC axon terminals in the GAD1 mutant.
RBCs adjust their output homeostatically in response to reduced GABAergic drive
We found that RBCs increase their output onto A17 amacrine cells with maturation. Our study shows that this output is structurally and functionally modified by alterations in neurotransmission during development and circuit maturation. When glutamate release from RBCs is suppressed by TeNT expression in these cells, more ribbons are often recruited to RBC output sites. When GABAergic transmission is impaired, glutamate release from developing RBC terminals is increased beyond that expected solely from disinhibition in the GABA deficient circuit, perhaps as an attempt to facilitate GABA release from the ‘silenced’ A17 amacrine cells with which it shares a reciprocal synapse. Interestingly, this increase in glutamate release in developing RBCs in GAD1KO retinas does not appear to evoke a change in glutamate receptor density on the A17 amacrine cell. The lack of a complementary change in the A17 cell is unexpected for two reasons: (i) the RBC-A17 synapse is bidirectional, and (ii) in other systems, down-regulation of postsynaptic glutamate receptors occurs when there is enhanced presynaptic glutamate release due to homeostatic mechanisms coming into play (Burrone and Murthy, 2003; Pozo and Goda, 2010; Turrigiano, 2007). In the GAD1KO, RBC output further undergoes homeostatic regulation with maturation to ‘cap’ or limit its output with circuit maturation, perhaps to maintain normal processing in the scotopic pathway involving the other postsynaptic partner, the AII amacrine cell (Bloomfield and Dacheux, 2001). This later homeostatic regulation of the RBC output parallels the loss of GABAA receptors on the RBCs, but these events are unlikely to be related. This is because the progressive loss of GABA receptors does not affect inhibition on the RBCs as there is little GABA release in the GAD1KO. Our results highlight divergent mechanisms behind the regulation of output from RBC axon terminals and GABA receptor maintenance on these terminals. Our current findings also underscore the importance of assessing changes in GABA receptors on axons in addition to the somata and dendrites of neurons for understanding neurodevelopmental disorders such as schizophrenia where GABAergic transmission is perturbed (Lewis et al., 2005; Wassef et al., 2003).
METHODS
Transgenic mouse lines
Several transgenic mouse lines were used in this study. In the GAD67-GFP (G42) line, the GAD67 promoter drives GFP expression in a fraction of GABAergic amacrine cells (Chattopadhyaya et al., 2004). In the grm6-tdtomato mouse line, the red fluorescent protein tdtomato is expressed specifically by ON-bipolar cell under the mGluR6 promoter (Kerschensteiner et al., 2009). In GAD1/lox/lox mice (Chattopadhyaya et al., 2007), exon 2 of GAD1, the gene encoding GAD67 (Bu et al., 1992), is flanked by loxP sites. To obtain retina-specific excision of exon 2, this line was bred to the α-Cre line, in which Cre-recombinase expression is regulated by the alpha enhancer of the Pax6 promoter (Marquardt et al., 2001). The excision of exon 2 in cells expressing Cre-recombinase results in a frameshift mutation of GAD1 (Chattopadhyaya et al., 2007). We refer to this double transgenic line as GAD1KO. To abolish glutamatergic transmission in RBCs, we used grm6-TeNT transgenic in which the light chain of tetanus toxin is expressed specifically in ON bipolar cells (Kerschensteiner et al., 2009). To eliminate GABAC receptors from the retina, the gene encoding GABACρ1 subunit was inactivated (McCall et al., 2002); we refer to these mice as GABACKO.
Immunohistochemistry
Animal protocols were approved by the Institutional Animal Care and Use Committee at the University of Washington. All procedures were in compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Mice deeply anaesthetized with Isoflurane were decapitated and enucleated. The cornea was punctured with a 30 gauge needle and the retinas removed in cold oxygenated mouse artificial cerebrospinal fluid (mACSF, pH 7.4) containing (in mM): 119 NaCl, 2.5 KCl, 2.5 CaCl2, 1.3 MgCl2, 1 NaH2PO4, 11 glucose, and 20 HEPES. For vibratome sectioning, the retina was fixed for 20 min in 4% paraformaldehyde in mACSF (pH 7.4). For fixed flat-mount preparations, retinas were isolated and mounted retinal ganglion cell side up on black membrane filters (Millipore, HABP013). The retina and filter paper were then immersed in 4% paraformaldehyde in mACSF (pH 7.4) for either 15 mins (for GABAAα1 and GABAAα3 immunolabeling) or 30 mins (for GABAC labeling). After fixation, the tissue was washed in 0.1 M phosphate-buffered saline (PBS, pH 7.4), preincubated in PBS containing 5% normal donkey serum (NDS) and 0.5% Triton X-100 and incubated with primary antibodies in the same solution. For retinal whole-mounts, primary antibody incubation was performed over 3 nights. Secondary antibody incubation was carried out in PBS and retinas were subsequently mounted in Vectashield (Vector Labs). Immunolabeling was performed using antibodies directed against PKC (rabbit polyclonal, 1:1000, Chemicon or mouse monoclonal, 1:1000, Sigma), GAD67 (mouse monoclonal, 1:1000, Chemicon), GAD65 (rabbit polyclonal, 1:1000, Chemicon), VIAAT (guinea pig polyclonal, 1:1000, Synaptic Systems), GFP (chicken polyclonal, 1:1000, Abcam), Glycine receptor α1-subunit (mouse monoclonal mAb2b, 1:500, Synaptic systems, Glycine receptor α2-subunit (goat polyclonal, 1:300, Santa Cruz), Gephyrin (mouse monoclonal 3B11, 1:1000, Synaptic Systems), Neuroligin2 (rabbit polyclonal, 1:4000, kindly provided by F. Varoqueaux and N. Brose), GABACρ receptor subunit (rabbit polyclonal, 1:500, kindly provided by R. Enz), GABAAα1 receptor subunit (polyclonal guineapig, 1:5000, kindly provided by J.M. Fritschy), GABAAα1 receptor subunit (polyclonal rabbit, 1:2000, kindly provided by J.M. Fritschy), GABAAα2 receptor subunit (polyclonal guinea-pig, 1:2000, kindly provided by J.M. Fritschy), and GABAAα3 receptor subunit (polyclonal guinea-pig, 1:3000, kindly provided by J.M. Fritschy). Secondary antibodies utilized were either anti-isotypic Alexa Fluor conjugates (1:1000, Invitrogen) or DyLight conjugates (1:1000, Jackson ImmunoResearch).
Imaging and Analysis
Image stacks were acquired on a Olympus FV1000 laser scanning confocal microscope. Fixed tissue was imaged using a 1.35 NA 60X oil immersion objective at a voxel size of 0.069–0.069–0.3 μm or 0.051–0.051–0.3 μm (x-y-z) for images used in quantification. To visualize RBC – amacrine cell contacts at different developmental time points, we crossed the GAD67-GFP and grm6-tdtomato mouse lines and imaged the retinas using a custom-built 2-photon microscope and an Olympus 60X water objective. Each optical plane was averaged 3–4 times. Raw image stacks were processed using MetaMorph (Universal Imaging) and Amira (Mercury Computer Systems). For volume estimation, the PKC signal of RBC boutons was masked in 3 dimensions using the ‘label field’ function of Amira. Subsequently this PKC mask was multiplied with the GAD or GABA receptor channel to isolate signal specifically within the RBC boutons. Next, a constant threshold for image stacks from the same retina (including WT-KO region comparison across the retina), was applied to detect the volume occupied by the signal using the ‘label voxel’ function of Amira. The % of signal volume as compared to PKC mask volume was estimated by the ‘tissue statistics’ function of Amira. The specificity of GABA receptor antibodies utilized in this study was tested by performing colocalization analysis and assessing the extent of random associations (Soto et al., 2011). In vertical retinal sections, we found an 87% apposition of GABAAα3 receptor clusters with the presynaptic marker VIAAT.
Electron Microscopy
Retinas were fixed by eye-cup immersion in 2% paraformaldehye/2% glutaraldehyde in 0.1 M sodium cacodylate buffer, pH 7.4 for 3 hours. The tissue was then washed in buffer, fixed further in 1% osmium tetroxide in cacodylate buffer for an hour prior to enbloc staining with 1% uranyl acetate. The tissue was then dehydrated in graded ethanol series, embedded in araldite resin, sectioned and stained with 5% lead citrate.
Western Blot
Retinas from GAD1KO and littermate control mice (2 retinas pooled per animal) were prepared on ice in homogenizing buffer (50 mM TRIS, 150 mM NaCl, 1% Triton X-100, 1% Na-deoxycholate, 0.1% SDS) containing protease inhibitor cocktail. Samples (30 μg/ml per lane) were run by SDS-PAGE (10% resolving gel), blotted onto nitrocellulose membranes, incubated with antibodies, and visualized with ECL. Primary antibodies utilized: GAD67 (mouse monoclonal, 1:2000, Chemicon), GABAAα1 receptor subunit (polyclonal rabbit, 1:2000, kindly provided by J.M. Fritschy) and Actin (mouse monoclonal, 1:3000, Chemicon). For preparation of samples to compare GABAAα1 levels from retinal homogenates, the dorsal-ventral wedge of the retina was not included for both GAD1KO and littermate control samples.
Preparation of mouse retinal slices for whole-cell recording
The preparation of retinal slices has been described in detail by Eggers and Lukasiewicz (Eggers and Lukasiewicz, 2006a). In brief, the eyecup was incubated for 20 min in mACSF containing 0.5 mg/ml hyaluronidase (Sigma) to remove any remaining vitreous. The retinas were then put onto filter paper (Millipore, HABP013) with the photoreceptor layer facing up. Vertical retinal slices of 200 μm thickness were cut using a standard technique (Werblin, 1978) and subsequently stored in carbogenated mACSF at room temperature.
Whole-cell recordings and data analysis
Whole-cell recordings were made from RBCs (in GAD1KO and grm6-TeNT) and A17s (in GAD1KO and GABACKO) in retinal slices as previously described (Eggers and Lukasiewicz, 2006a; Schubert et al., 2008). The resistance of the electrodes with standard solutions usually ranged between 6 and 8 MΩ. Liquid junction potentials of 15 mV were corrected before measurements with the pipette offset function. Series resistance (mean: 67.6 ± 3.7 MΩ, n=18) and capacitance of pipettes as well as cell capacitance were not compensated. Seal resistances > 1 GΩ were routinely obtained. RBCs were voltage-clamped at 0 mV, the reversal potential for cation-mediated currents through ionotropic glutamate receptors to record GABA-evoked currents in RBCs, and GABAergic spontaneous postsynaptic currents (sIPSCs). For all RBC recordings, glycinergic input and network activity was blocked by strychnine (0.5 μM) and TTX (1 μM), respectively. For A17 amacrine cells, excitatory postsynaptic currents through ionotropic glutamate receptors (sEPSCs) were recorded at a holding potential of −60 mV, the reversal potential for chloride-mediated currents set by the internal solution (Schubert et al., 2008). Action potentials in A17s were blocked by including QX-314 (2 mM) in the intracellular solution. For recording sEPSCs from P11-P13 A17s in the presence of GABA receptor blockers, TPMPA (50 μM) and SR95531 (5 μM) was included in the extracellular mACSF solution. The holding current was monitored during the experiment, and recordings with shifting holding currents were aborted. All experiments were carried out at room temperature (20–22°C) and under room light conditions.
Data acquisition was performed using an Axopatch 200B amplifier with an acquisition frequency of 10 kHz using the Clampex software (Axon Instruments, Foster City, CA). Signals were 2 kHz Bessel filtered. Data were further processed using a routine written in Igor Pro 6 (Wavemetrics, Portland, OR) and visualized with Origin software (Microcal, Northampton, MA). To quantify parameters of spontaneous synaptic events, the mean frequency ± S.E.M and the mean peak amplitude ± S.E.M of the events were determined. To quantify evoked currents, peak amplitude and total charge transfer (as complete area under the curve), both relative to the pre-application baseline current, were determined and averaged across cells. In addition, we characterized the kinetics of the evoked current by determining the time points from the exponential fits where the traces reached 20% and 80% of the peak amplitude, and calculated the differences, t20–80 rise and t80–20 decay.
Solution and drugs
The extracellular solution used for recording (mACSF) contained (in mM): 122.5 NaCl, 5 KCl, 1 MgCl2, 1.25 NaH2PO4, 26 NaHCO3, 2 CaCl2 and 20 glucose, adjusted to pH 7.4 with 95% O2 and 5% CO2. The intracellular solution contained (in mM): 120 cesium gluconate, 1 CaCl2, 1 MgCl2, 10 Na-Hepes, 11 EGTA, 10 TEA-Cl and was adjusted to pH 7.2 with CsOH. Under our experimental conditions, the calculated chloride equilibrium potential was −59.3 mV. To block GABAC receptors and GABAA receptors, (1,2,5,6-tetrahydropyridine-4yl) methyphospinic acid (TPMPA, 50 μM) and SR-95531 (5 μM) were used, respectively. All drugs were purchased from Sigma, St.Louis, MO. Solutions in the recording chamber were exchanged using a gravity-driven superfusion system previously described (Lukasiewicz and Roeder, 1995). GABA receptor and glutamate receptor agonists, GABA (200 μM) and AMPA (100 μM), were dissolved in mACSF with 0.005% sulforhodamine B and applied with a puff pipette (5–7 MΩ) using a Picospritzer system. The puffing direction as well as the duration of the 300 ms puff were chosen such that the axonal terminal of the patched RBC was completely covered by the puff, visualized by including sulforhodamine B in the puffer pipette.
Statistics
For comparisons across data sets, the Wilcoxon rank-sum test was used in all cases. *p<0.05; **p<0.01; ***p<0.001.
Supplementary Material
Highlights.
GABAergic but not glutamatergic transmission maintains axonal GABA receptors.
GABA controls maintenance of axonal GABAAα1 but not GABAAα3 or GABAC receptors.
GABAC receptor loss does not perturb RBC GABAA receptor distribution.
RBCs homeostatically regain normal output despite loss of presynaptic inhibition.
ACKNOWLEDGMENTS
Supported by NIH grant EY10699 to R.O.W., Research to Prevent Blindness and NIH EY08922 to P.D.L., EY02687 to the Dept. of Ophthalmology, Washington University, Vision Core grant, EY01730 to the Dept. of Ophthalmology, University of Washington, DFG SCHU2243/1-1 to T.S. and DFG, CIN, EXC307 to T.E. We thank A. Barria and W. Cerpa for help with western blots, and E. Parker for assistance with electron microscopy. We are grateful to R. Sinha, H. Okawa and L. Della Santina for helpful comments on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing financial interests: The authors declare no competing financial interests.
REFERENCES
- Bloomfield SA, Dacheux RF. Rod vision: pathways and processing in the mammalian retina. Prog Retin Eye Res. 2001;20:351–384. doi: 10.1016/s1350-9462(00)00031-8. [DOI] [PubMed] [Google Scholar]
- Bu DF, Erlander MG, Hitz BC, Tillakaratne NJ, Kaufman DL, Wagner-McPherson CB, Evans GA, Tobin AJ. Two human glutamate decarboxylases, 65-kDa GAD and 67-kDa GAD are each encoded by a single gene. Proc Natl Acad Sci U S A. 1992;89:2115–2119. doi: 10.1073/pnas.89.6.2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrone J, Murthy VN. Synaptic gain control and homeostasis. Curr Opin Neurobiol. 2003;13:560–567. doi: 10.1016/j.conb.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Chattopadhyaya B, Di Cristo G, Higashiyama H, Knott GW, Kuhlman SJ, Welker E, Huang ZJ. Experience and activity-dependent maturation of perisomatic GABAergic innervation in primary visual cortex during a postnatal critical period. J Neurosci. 2004;24:9598–9611. doi: 10.1523/JNEUROSCI.1851-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyaya B, Di Cristo G, Wu CZ, Knott G, Kuhlman S, Fu Y, Palmiter RD, Huang ZJ. GAD67-mediated GABA synthesis and signaling regulate inhibitory synaptic innervation in the visual cortex. Neuron. 2007;54:889–903. doi: 10.1016/j.neuron.2007.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez AE, Grimes WN, Diamond JS. Mechanisms underlying lateral GABAergic feedback onto rod bipolar cells in rat retina. J Neurosci. 2010;30:2330–2339. doi: 10.1523/JNEUROSCI.5574-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez AE, Singer JH, Diamond JS. Fast neurotransmitter release triggered by Ca influx through AMPA-type glutamate receptors. Nature. 2006;443:705–708. doi: 10.1038/nature05123. [DOI] [PubMed] [Google Scholar]
- Chun MH, Han SH, Chung JW, Wässle H. Electron microscopic analysis of the rod pathway of the rat retina. J Comp Neurol. 1993;332:421–432. doi: 10.1002/cne.903320404. [DOI] [PubMed] [Google Scholar]
- Dowling JE, Boycott BB. Organization of the primate retina: electron microscopy. Proc R Soc Lond B Biol Sci. 1966;166:80–111. doi: 10.1098/rspb.1966.0086. [DOI] [PubMed] [Google Scholar]
- Eggers ED, Lukasiewicz PD. GABA(A), GABA(C) and glycine receptor-mediated inhibition differentially affects light-evoked signalling from mouse retinal rod bipolar cells. J Physiol. 2006a;572:215–225. doi: 10.1113/jphysiol.2005.103648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggers ED, Lukasiewicz PD. Receptor and transmitter release properties set the time course of retinal inhibition. J Neurosci. 2006b;26:9413–9425. doi: 10.1523/JNEUROSCI.2591-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggers ED, McCall MA, Lukasiewicz PD. Presynaptic inhibition differentially shapes transmission in distinct circuits in the mouse retina. J Physiol. 2007;582:569–582. doi: 10.1113/jphysiol.2007.131763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enz R, Brandstatter JH, Wässle H, Bormann J. Immunocytochemical localization of the GABAc receptor rho subunits in the mammalian retina. J Neurosci. 1996;16:4479–4490. doi: 10.1523/JNEUROSCI.16-14-04479.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feigenspan A, Bormann J. Differential pharmacology of GABAA and GABAC receptors on rat retinal bipolar cells. Eur J Pharmacol. 1994;288:97–104. doi: 10.1016/0922-4106(94)90014-0. [DOI] [PubMed] [Google Scholar]
- Fletcher EL, Koulen P, Wässle H. GABAA and GABAC receptors on mammalian rod bipolar cells. J Comp Neurol. 1998;396:351–365. doi: 10.1002/(sici)1096-9861(19980706)396:3<351::aid-cne6>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Fritschy JM, Mohler H. GABAA-receptor heterogeneity in the adult rat brain: differential regional and cellular distribution of seven major subunits. J Comp Neurol. 1995;359:154–194. doi: 10.1002/cne.903590111. [DOI] [PubMed] [Google Scholar]
- Gingrich KJ, Roberts WA, Kass RS. Dependence of the GABAA receptor gating kinetics on the alpha-subunit isoform: implications for structure-function relations and synaptic transmission. J Physiol. 1995;489(Pt 2):529–543. doi: 10.1113/jphysiol.1995.sp021070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanus C, Ehrensperger MV, Triller A. Activity-dependent movements of postsynaptic scaffolds at inhibitory synapses. J Neurosci. 2006;26:4586–4595. doi: 10.1523/JNEUROSCI.5123-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms KJ, Craig AM. Synapse composition and organization following chronic activity blockade in cultured hippocampal neurons. J Comp Neurol. 2005;490:72–84. doi: 10.1002/cne.20635. [DOI] [PubMed] [Google Scholar]
- Hartveit E. Reciprocal synaptic interactions between rod bipolar cells and amacrine cells in the rat retina. J Neurophysiol. 1999;81:2923–2936. doi: 10.1152/jn.1999.81.6.2923. [DOI] [PubMed] [Google Scholar]
- Haverkamp S, Wässle H. Immunocytochemical analysis of the mouse retina. J Comp Neurol. 2000;424:1–23. [PubMed] [Google Scholar]
- Ivanova E, Muller F. Retinal bipolar cell types differ in their inventory of ion channels. Vis Neurosci. 2006;23:143–154. doi: 10.1017/S0952523806232048. [DOI] [PubMed] [Google Scholar]
- Kasugai Y, Swinny JD, Roberts JD, Dalezios Y, Fukazawa Y, Sieghart W, Shigemoto R, Somogyi P. Quantitative localisation of synaptic and extrasynaptic GABAA receptor subunits on hippocampal pyramidal cells by freeze-fracture replica immunolabelling. Eur J Neurosci. 2010;32:1868–1888. doi: 10.1111/j.1460-9568.2010.07473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerschensteiner D, Morgan JL, Parker ED, Lewis RM, Wong RO. Neurotransmission selectively regulates synapse formation in parallel circuits in vivo. Nature. 2009;460:1016–1020. doi: 10.1038/nature08236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilman V, van Rossum MC, Turrigiano GG. Activity deprivation reduces miniature IPSC amplitude by decreasing the number of postsynaptic GABA(A) receptors clustered at neocortical synapses. J Neurosci. 2002;22:1328–1337. doi: 10.1523/JNEUROSCI.22-04-01328.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch J, Betz H. Glycine-receptor activation is required for receptor clustering in spinal neurons. Nature. 1998;392:717–720. doi: 10.1038/33694. [DOI] [PubMed] [Google Scholar]
- Koulen P, Brandstatter JH, Enz R, Bormann J, Wässle H. Synaptic clustering of GABA(C) receptor rho-subunits in the rat retina. Eur J Neurosci. 1998;10:115–127. doi: 10.1046/j.1460-9568.1998.00005.x. [DOI] [PubMed] [Google Scholar]
- Koulen P, Brandstatter JH, Kroger S, Enz R, Bormann J, Wässle H. Immunocytochemical localization of the GABA(C) receptor rho subunits in the cat, goldfish, and chicken retina. J Comp Neurol. 1997;380:520–532. doi: 10.1002/(sici)1096-9861(19970421)380:4<520::aid-cne8>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Koulen P, Sassoe-Pognetto M, Grunert U, Wässle H. Selective clustering of GABA(A) and glycine receptors in the mammalian retina. J Neurosci. 1996;16:2127–2140. doi: 10.1523/JNEUROSCI.16-06-02127.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullmann DM, Ruiz A, Rusakov DM, Scott R, Semyanov A, Walker MC. Presynaptic, extrasynaptic and axonal GABAA receptors in the CNS: where and why? Prog Biophys Mol Biol. 2005;87:33–46. doi: 10.1016/j.pbiomolbio.2004.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- Lukasiewicz PD. GABAC receptors in the vertebrate retina. Mol Neurobiol. 1996;12:181–194. doi: 10.1007/BF02755587. [DOI] [PubMed] [Google Scholar]
- Lukasiewicz PD, Eggers ED, Sagdullaev BT, McCall MA. GABAC receptor-mediated inhibition in the retina. Vision Res. 2004;44:3289–3296. doi: 10.1016/j.visres.2004.07.023. [DOI] [PubMed] [Google Scholar]
- Lukasiewicz PD, Roeder RC. Evidence for glycine modulation of excitatory synaptic inputs to retinal ganglion cells. J Neurosci. 1995;15:4592–4601. doi: 10.1523/JNEUROSCI.15-06-04592.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher B, Fuchs T, Kilpatrick CL. GABAA receptor trafficking-mediated plasticity of inhibitory synapses. Neuron. 2011;70:385–409. doi: 10.1016/j.neuron.2011.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquardt T, Ashery-Padan R, Andrejewski N, Scardigli R, Guillemot F, Gruss P. Pax6 is required for the multipotent state of retinal progenitor cells. Cell. 2001;105:43–55. doi: 10.1016/s0092-8674(01)00295-1. [DOI] [PubMed] [Google Scholar]
- McCall MA, Lukasiewicz PD, Gregg RG, Peachey NS. Elimination of the rho1 subunit abolishes GABA(C) receptor expression and alters visual processing in the mouse retina. J Neurosci. 2002;22:4163–4174. doi: 10.1523/JNEUROSCI.22-10-04163.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire BA, Stevens JK, Sterling P. Microcircuitry of bipolar cells in cat retina. J Neurosci. 1984;4:2920–2938. doi: 10.1523/JNEUROSCI.04-12-02920.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan JL, Dhingra A, Vardi N, Wong RO. Axons and dendrites originate from neuroepithelial-like processes of retinal bipolar cells. Nat Neurosci. 2006;9:85–92. doi: 10.1038/nn1615. [DOI] [PubMed] [Google Scholar]
- Nelson R, Kolb H. A17: a broad-field amacrine cell in the rod system of the cat retina. J Neurophysiol. 1985;54:592–614. doi: 10.1152/jn.1985.54.3.592. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Sieghart W, Benke D, Fritschy JM, Somogyi P. Differential synaptic localization of two major gamma-aminobutyric acid type A receptor alpha subunits on hippocampal pyramidal cells. Proc Natl Acad Sci U S A. 1996;93:11939–11944. doi: 10.1073/pnas.93.21.11939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortinski PI, Lu C, Takagaki K, Fu Z, Vicini S. Expression of distinct alpha subunits of GABAA receptor regulates inhibitory synaptic strength. J Neurophysiol. 2004;92:1718–1727. doi: 10.1152/jn.00243.2004. [DOI] [PubMed] [Google Scholar]
- Pozo K, Goda Y. Unraveling mechanisms of homeostatic synaptic plasticity. Neuron. 2010;66:337–351. doi: 10.1016/j.neuron.2010.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosato-Siri M, Grandolfo M, Ballerini L. Activity-dependent modulation of GABAergic synapses in developing rat spinal networks in vitro. Eur J Neurosci. 2002;16:2123–2135. doi: 10.1046/j.1460-9568.2002.02291.x. [DOI] [PubMed] [Google Scholar]
- Ruiz A, Fabian-Fine R, Scott R, Walker MC, Rusakov DA, Kullmann DM. GABAA receptors at hippocampal mossy fibers. Neuron. 2003;39:961–973. doi: 10.1016/s0896-6273(03)00559-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagdullaev BT, McCall MA, Lukasiewicz PD. Presynaptic inhibition modulates spillover, creating distinct dynamic response ranges of sensory output. Neuron. 2006;50:923–935. doi: 10.1016/j.neuron.2006.05.015. [DOI] [PubMed] [Google Scholar]
- Schubert T, Huckfeldt RM, Parker E, Campbell JE, Wong RO. Assembly of the outer retina in the absence of GABA synthesis in horizontal cells. Neural Dev. 2010;5:15. doi: 10.1186/1749-8104-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert T, Kerschensteiner D, Eggers ED, Misgeld T, Kerschensteiner M, Lichtman JW, Lukasiewicz PD, Wong RO. Development of presynaptic inhibition onto retinal bipolar cell axon terminals is subclass-specific. J Neurophysiol. 2008;100:304–316. doi: 10.1152/jn.90202.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields CR, Tran MN, Wong RO, Lukasiewicz PD. Distinct ionotropic GABA receptors mediate presynaptic and postsynaptic inhibition in retinal bipolar cells. J Neurosci. 2000;20:2673–2682. doi: 10.1523/JNEUROSCI.20-07-02673.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto F, Bleckert A, Lewis R, Kang Y, Kerschensteiner D, Craig AM, Wong RO. Coordinated increase in inhibitory and excitatory synapses onto retinal ganglion cells during development. Neural Dev. 2011;6:31. doi: 10.1186/1749-8104-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stell BM, Rostaing P, Triller A, Marty A. Activation of presynaptic GABA(A) receptors induces glutamate release from parallel fiber synapses. J Neurosci. 2007;27:9022–9031. doi: 10.1523/JNEUROSCI.1954-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabadics J, Varga C, Molnar G, Olah S, Barzo P, Tamas G. Excitatory effect of GABAergic axo-axonic cells in cortical microcircuits. Science. 2006;311:233–235. doi: 10.1126/science.1121325. [DOI] [PubMed] [Google Scholar]
- Turrigiano G. Homeostatic signaling: the positive side of negative feedback. Curr Opin Neurobiol. 2007;17:318–324. doi: 10.1016/j.conb.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Vardi N, Auerbach P. Specific cell types in cat retina express different forms of glutamic acid decarboxylase. J Comp Neurol. 1995;351:374–384. doi: 10.1002/cne.903510305. [DOI] [PubMed] [Google Scholar]
- Vardi N, Sterling P. Subcellular localization of GABAA receptor on bipolar cells in macaque and human retina. Vision Res. 1994;34:1235–1246. doi: 10.1016/0042-6989(94)90198-8. [DOI] [PubMed] [Google Scholar]
- Vicini S, Ferguson C, Prybylowski K, Kralic J, Morrow AL, Homanics GE. GABA(A) receptor alpha1 subunit deletion prevents developmental changes of inhibitory synaptic currents in cerebellar neurons. J Neurosci. 2001;21:3009–3016. doi: 10.1523/JNEUROSCI.21-09-03009.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassef A, Baker J, Kochan LD. GABA and schizophrenia: a review of basic science and clinical studies. J Clin Psychopharmacol. 2003;23:601–640. doi: 10.1097/01.jcp.0000095349.32154.a5. [DOI] [PubMed] [Google Scholar]
- Wässle H. Parallel processing in the mammalian retina. Nat Rev Neurosci. 2004;5:747–757. doi: 10.1038/nrn1497. [DOI] [PubMed] [Google Scholar]
- Wässle H, Heinze L, Ivanova E, Majumdar S, Weiss J, Harvey RJ, Haverkamp S. Glycinergic transmission in the Mammalian retina. Front Mol Neurosci. 2009;2:6. doi: 10.3389/neuro.02.006.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wässle H, Koulen P, Brandstatter JH, Fletcher EL, Becker CM. Glycine and GABA receptors in the mammalian retina. Vision Res. 1998;38:1411–1430. doi: 10.1016/s0042-6989(97)00300-3. [DOI] [PubMed] [Google Scholar]
- Werblin FS. Transmission along and between rods in the tiger salamander retina. J Physiol. 1978;280:449–470. doi: 10.1113/jphysiol.1978.sp012394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojcik SM, Katsurabayashi S, Guillemin I, Friauf E, Rosenmund C, Brose N, Rhee JS. A shared vesicular carrier allows synaptic corelease of GABA and glycine. Neuron. 2006;50:575–587. doi: 10.1016/j.neuron.2006.04.016. [DOI] [PubMed] [Google Scholar]
- Wu X, Fu Y, Knott G, Lu J, Di Cristo G, Huang ZJ. GABA Signaling Promotes Synapse Elimination and Axon Pruning in Developing Cortical Inhibitory Interneurons. J Neurosci. 2012;32:331–343. doi: 10.1523/JNEUROSCI.3189-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.