Abstract
Replication defective Adenovirus vectors based on the human serotype 5 (Ad5) have been shown to induce protective immune responses against diverse pathogens and cancer in animal models and to elicit robust and sustained cellular immunity in humans. However, most humans have anti-Ad5 neutralising antibodies that can impair the immunological potency of such vaccines. Here we show that most other human Adenoviruses from rare serotypes are far less potent as vaccine vectors than Ad5 in mice and non-human primates, casting doubt on their potential efficacy in humans. To identify novel vaccine carriers suitable for vaccine delivery in humans we isolated and sequenced over a thousand Adenovirus strains from chimpanzees (ChAd). Replication-defective vectors were generated from different ChAd serotypes and were screened for neutralization by human sera and for ability to grow in human cell lines already approved for clinical studies. Most importantly, we devised a screening strategy to rank the ChAd vectors by immunological potency in mice which predicts their immunogenicity in non-human primates and humans. The vectors studied varied by up to a thousand-fold in potency for CD8 T cell induction in mice. Two of the most potent ChAd vectors were selected for clinical studies as carriers for Malaria and Hepatitis C virus (HCV) genetic vaccines. These ChAd vectors were found to be safe and immunologically potent in Phase I clinical trials thereby validating our screening approach. The ChAd vectors that we have developed represent a large collection of non cross-reactive, potent vectors that can be exploited for diverse vaccine strategies.
Introduction
Novel vaccines for the prevention or treatment of diseases such as HIV, HCV, malaria, TB and cancers are a major unmet need. Pre-clinical and clinical evidence supports the role of T cell immunity and in particular CD8+ T cells in the clearance of intracellular pathogens and tumour cells (1). The most efficient way to induce a CD8+ T cell response against a given antigen is to deliver the gene encoding for that antigen intracellularly, along with suitable pathogen-derived innate activators, thereby recapitulating the physiological pathway of antigen processing and MHC class I presentation. This concept has led to the development of a large number of gene delivery approaches to elicit adaptive immunity, which are collectively defined as gene-based vaccines or genetic vaccines.
Replication-defective Adenovirus 5 (Ad5) vectors have been extensively used for genetic vaccine delivery because Ad5 infects replicating and non-replicating cells, has a broad tissue tropism, propagates very efficiently in the available packaging cell lines and its production process is scalable and affordable. Most importantly, head-to-head comparisons with other genetic vaccine vectors (ie.: poxviruses, lentiviruses, alpha virus-based vectors and naked DNA) in animal models and the results obtained in human clinical trials, clearly showed that Ad5-based vectors represent the most potent currently available delivery system for eliciting a CD8+ T cell response against the encoded antigen(s) (2-7). However, the majority of the human population is exposed to Ad5 in first years of life and develops high titers of anti-Ad5 neutralizing antibodies (nAb). These pre-existing Ad5 nAb impair the immunogenicity of Ad5-based vaccines in animal models and in humans (3, 8-10) and may also potentially compromise their safety (11). For these reasons, other human Adenovirus (Ad) vectors based on rare serotypes such as Ad11, Ad24, Ad26, Ad34, Ad35, Ad48, Ad49, and Ad50 have been proposed as potential alternatives to Ad5 because they are rarely neutralised by antibodies present in humans, and are currently being evaluated in a number of pre-clinical and clinical studies (12-16). However, successful development of Ad vectors as genetic vaccine carriers will eventually depend not only on the low frequency of nAb present in the target population (seroprevalence), but also on their immunological potency as well as other characteristics including good biological tractability and availability of cell substrates approved by regulators for efficient manufacturing and scalable and reproducible production processes. We find here that Ad vectors from rare human serotypes have lower immunological potency than Ad5 in mice and non-human primates indicating that different Ad strains are not equivalent with respect to the above properties.
Lacking a reliable way to predict the immunological potency of alternative Ad strains as genetic vaccine carriers, we generated a large collection of replication defective vectors based on Ad isolated from chimpanzees. This collection was screened for i) susceptibility to neutralising antibodies present in humans, ii) ability to grow in human embryonic kidney 293 (HEK293) and PER.C6 cell lines, and iii) immunological potency in rodents and non-human primates. The best ranking vectors were further developed and tested in human clinical trials where they have shown excellent immunogenicity, thereby confirming the predictive value of our screening approach.
Results
Ad vectors from rare human serotypes are weak immunogens in rodents and non-human primates
There are 52 human Ad serotypes that have been classified into six subgroups (A to F) based on hemagglutination properties, oncogenicity in rodents, DNA homology, and genomic organization (17). Ad clusters obtained by sequence homology in the hexon capsid protein are consistent with this classification into species. To date, very limited comparative data on the immunological potency of Ad vectors from different species or serotypes are available. Therefore, we tested the immunological potency of human Ad vectors from different serotypes in a head-to-head comparison with Ad5. We chose representative Ad serotypes from species B (Ad34 and Ad35), C (Ad5 and Ad6) and D (Ad24), and constructed replication defective (E1-deleted) Ad vectors encoding the HIV-1 gag gene (Ad-gag). Since very high doses (on a per Kg basis) can be reached in mice we reasoned that a dose/response analysis of the vectors’ immunogenicity would have a higher predictive value in view of their potential use in humans. Therefore, we immunised groups of BALB/c mice (five/group) intramuscularly with escalating doses of each Ad vector and determined the relative immunological potency by establishing the minimal dose of vector capable of inducing a HIV-1 gag-specific T-cell response in at least 2 out of 5 animals within the same immunization group. T-cell responses were measured three weeks after the immunization by ex-vivo IFNγ-ELISpot using an immune-dominant gag CD8+ T cell epitope.
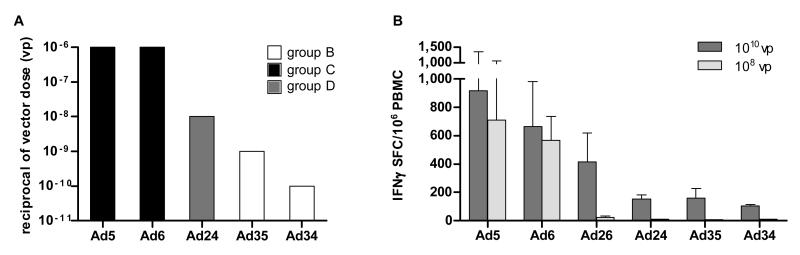
All vectors were immunogenic at the highest tested dose: 1010 viral particles (vp) for Ad34, Ad35 and Ad24, and 108 vp for Ad5 and Ad6, with similar levels of gag-specific IFNγ secreting T lymphocytes in all groups (geometric mean values of 430, 549, 1790, 1241 and 718, respectively). However, while Ad5 and Ad6 were still immunogenic at lower doses down to 106 vp, the other three vectors progressively lost the ability to induce a response by decreasing the dose, with the minimal immunogenic dose being 1010 vp for Ad34, 109 vp for Ad35 and 108 vp for Ad24 (Figure 1A). Thus, Ad5 and Ad6 showed a 10-100-fold higher immunological potency than Ad24 and at least 1000-fold higher than Ad34 and Ad35 vectors. A parallel ranking among vectors deriving from different serotypes was observed by measuring the anti-HIV 1 gag antibody titers by anti-P24 Elisa in mice three weeks post-immunization. Animals immunized with Ad5-gag at the doses of 107 and 108 vp/mouse elicited anti-P24 titers of 4500 and 20000, respectively while 100-fold higher doses of Ad24-gag were necessary (109 and 1010 vp/mouse) to measure comparable anti-P24 titers of 4100 and 9300 in the serum of immunized animals (average values from groups of 5 mice, data not shown).
Fig. 1.
Dose-response immunogenicity of human adenovirus in mice and macaques. Immunological potency of human Ad vectors encoding for HIV-1 gag in BALB/c mice (A). Five animals per group were immunized intramuscularly with escalating doses of each Ad vector. IFN-γ ELISpot was performed on splenocytes collected three weeks later using as antigen a 9mer peptide encoding the HIV gag major H-2 Kd CD8 epitope (AMQMLKETI). Each bar represents the relative potency defined as the minimal Ad vectors dose capable of inducing a HIV-1 gag-specific T-cell response in at least 2 out of 5 animals. Data are shown as the reciprocal of minimal dose. The adenovirus serogroups are shown with different bar colours (white=group B, black=group C, dark grey=group D).
Immunological potency of human Ad vectors in macaques (B). Three animals per group were vaccinated intramuscularly with 1010 and 108 vp of each Ad vector encoding for HIV-1 gag. Four weeks after vaccination, T cell responses to a 15mer peptide pool covering gag were measured by IFN-γ ELISpot on PBMC. Data are expressed as IFNγ Spot Forming Cells (SFC) per million PBMC. The mean responses + SEM are shown for each immunization group.
We performed similar dose/response experiments in non-human primates by immunising groups of three animals with 1010 and 108 vp of the Ad-gag vectors. Ad26 (another group D serotype) was also included in this study. Four weeks after the intramuscular immunization, T-cell responses were measured by ex-vivo IFNγ-ELISpot using a pool of overlapping peptides (15-mer) representing the HIV-1 gag sequence. The results of this experiment confirmed the ranking observed in mice, with Ad5 and Ad6 being the only vectors capable of inducing a T-cell response at both tested doses, and Ad24, Ad34 and Ad35 showing a lower immunogenicity also at 1010 vp (Figure 1B). Ad26 had an intermediate phenotype as it did induce a cellular immunity comparable to that of Ad5 and Ad6 at the higher dose, but it failed to elicit a response at the lower dose.
Isolation of Ad vectors from chimpanzees
Lacking a reliable predictor of in vivo immunogenicity, we decided to isolate a large number of Adenovirus strains and to screen them for their immunological potency to identify candidates for vaccine vector development. To this end, we focused on chimpanzee Adenoviruses (ChAd) for a number of reasons: i) previous experience in the isolation of infectious agents from chimpanzees led to the identification of ChAd strains(18-21); ii) the large biodiversity within the genus Pan would predict that there are several different ChAd strains circulating in chimpanzees; iii) the evolutionary relatedness between humans and chimpanzees should allow for functional complementation and growth of E1-deleted replication-defective ChAd in the available packaging human cell lines (HEK293 and PER.C6); iv) the available data on the few simian-derived Ad known indicate that these viruses are rarely neutralised by human sera (22-24).
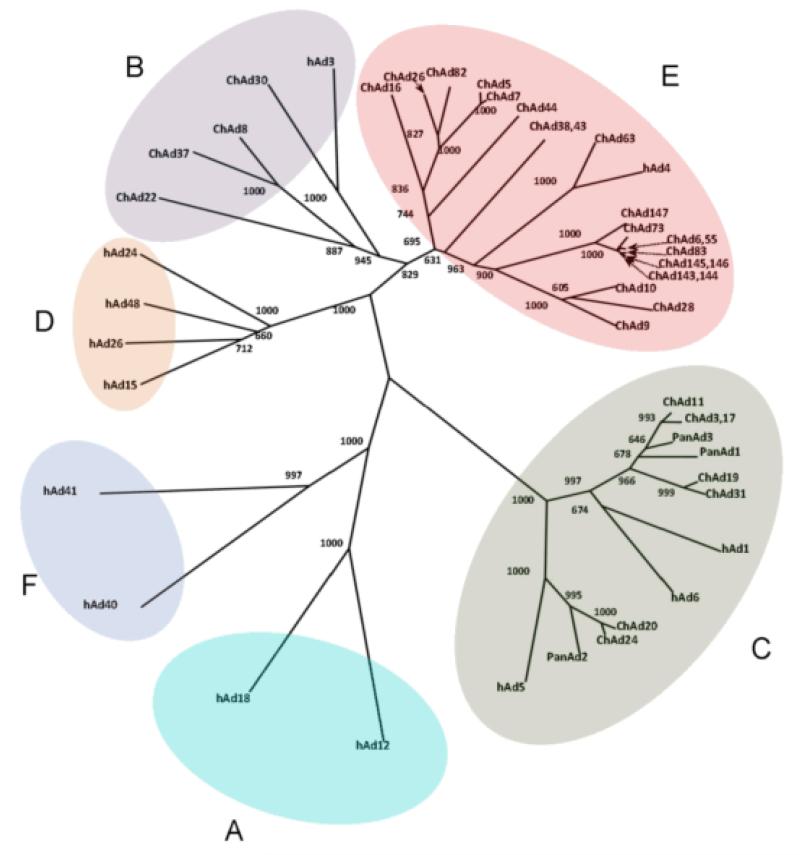
We collected more than one thousand stool specimens from common chimpanzees (Pan troglodytes) and bonobos (Pan paniscus or pygmy chimpanzee) housed in animal facilities and zoos in Europe and in the US, and were able to isolate Ads from about 50% of these samples by infecting human A549 and HEK293 cells. To identify different strains, we developed a rapid screening method based on amplification and sequencing of the hypervariable regions of the capsid hexon protein. Sequencing of the hypervariable hexon regions of about 500 isolates, allowed the classification of the different ChAd isolates according to the existing B, C and E species of human Adenoviruses. Interestingly, most of the isolates belonged to species C and E (46% and 45%, respectively), while species B ChAd viruses were less frequently found (9 %) and no isolate could be classified as species A, D or F. A complete sequence was obtained for selected members of each species and a phylogenetic tree was obtained by hexon protein sequence alignment (Figure 2). A similar high frequency of isolation of B, C and E Adenoviruses was previously reported from non-human primates (25).
Fig. 2.
Phylogenetic analysis of chimpanzee adenovirus. The phylogenetic tree showing the different human adenovirus species (A-F) was obtained by aligning the adenovirus hexon sequences. Human adenovirus (hAd) representative of each species and chimpanzee adenoviruses (ChAd) were included in the analysis. The phylogenetic tree was calculated using the neighbor-joining method as implemented in ClustalX and displayed using drawtree from PHYLIP version 3.69. Alignment positions containing gaps were excluded from the analysis. The alignment of hexon proteins was manually optimized taking into account structural restraints from the Ad5 hexon Xray structure. Bootstrap confidence values are reported at branch points (1000 bootstrap cycles).
Replication-defective ChAd vectors grow efficiently in human packaging cell lines and do not form replication competent virus
Twenty five ChAd isolates each one belonging to a different genotype were selected for further analysis (1 from group B, 8 from group C and 16 from group E). We constructed replication-defective (E1-deleted) vectors encoding HIV-1 gag (ChAd-gag) and secreted alkaline phosphatase (ChAd-SEAP) and produced them in HEK293 and PER.C6 cells. The growth efficiency of ChAd vectors was evaluated by qPCR of the viral genome from lysates of infected HEK293 or PER.C6 cells. Although we found some variability among the different isolates, the range of productivity of the ChAd (from 103 to over 105 vp/cell) was comparable to that of the human Ad vectors (ie.: the productivity of Ad5-gag was 104 vp/cell).
We tested two representative ChAd isolates, one from group C (ChAd3) and the second from group E (ChAd63) for their ability to generate replication-competent adenovirus (RCA) in HEK293 cells. After six cycles of amplification in HEK293 mimicking a large scale production process, no plaques could be observed by infecting A549 cells with 3×1010 ChAd3 or ChAd63 of HEK293 derived virus, while human Ad5 grown in HEK293 scored positive in this assay (data not shown), indicating that propagation of E1-deleted ChAd vectors in HEK293 cells does not give rise to RCA.
ChAds are potent genetic vaccine carriers
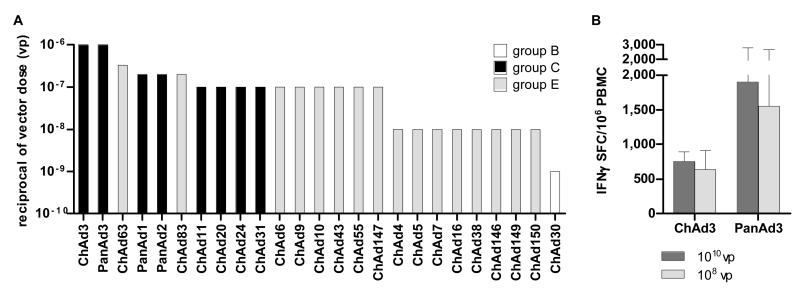
ChAd-gag vectors from the twenty-five different isolates were screened for immunological potency by dose/response in BALB/c mice (Supplementary Figure 1). Similarly to the human Adenoviruses, ChAds displayed a wide range of immunogenicity. Also in this case the group C vectors were the most potent while the group B vectors induced a T-cell response only at very high doses (Figure 3A). The group E vectors can be divided in two categories: those with high immunological potency such as ChAd63, ChAd83, ChAd6, ChAd9, ChAd10, ChAd43, ChAd55 and ChAd147, and those characterised by a lower immunogenicity (ie. ChAd4, ChAd5, ChAd7, ChAd16, ChAd38, ChAd146, ChAd149 and ChAd150). Some of the ChAd vectors were able to induce a T cell response at very low doses (1-3×106 vp), thus ranking in the same category of the clinically validated human Ad5 and Ad6 (4).
Fig. 3.
Dose-response immunogenicity of chimpanzee adenovirus in mice and macaques. Immunological potency of chimpanzee Ad vectors encoding for HIV-1 gag in BALB/c mice (A) was measured as described in Fig. 1. Data are shown as the reciprocal of minimal dose. The adenovirus serogroups are shown with different bar colours (white=group B, black=group C, light gray=group E).
Immunological potency of human Ad vectors in macaques (B). Three animals per group were vaccinated intramuscularly with 1010 and 108 vp of each Ad vector encoding for HIV-1 gag. Data are expressed as IFNγ SFC per million PBMC. The mean responses + SEM are shown for each immunization group.
Two of the most potent vectors, ChAd3-gag and PanAd3-gag, were tested in macaques at 1010 and 108 vp. Similarly to the human Ad5 and Ad6, the two ChAd vectors were immunogenic at both tested doses confirming the ranking observed in mice, and indicating that the high level of immunological potency of the ChAd vectors is not a species-specific phenomenon (Figure 3B).
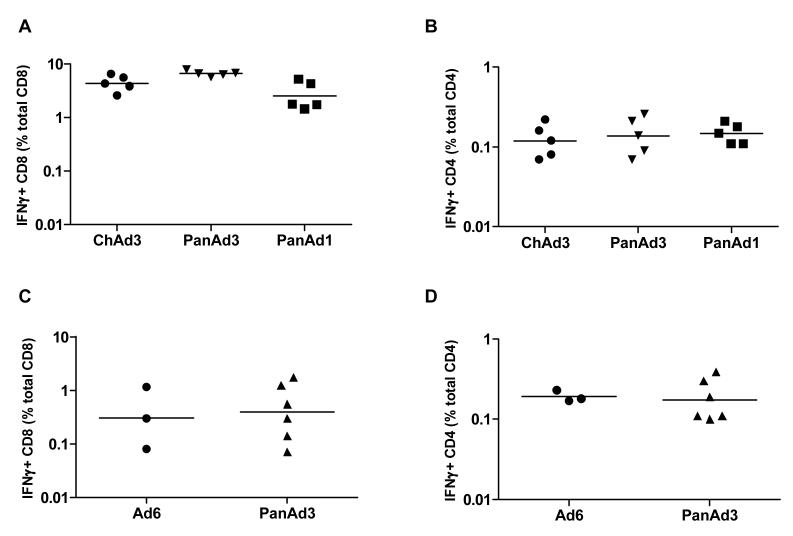
ChAd vectors readily induced very high levels of antigen-specific IFNγ+ CD8+ T cells both in mice (range of peak responses: 1.44-7.86% of total CD8, Figure 4A) and in non-human primates (range of peak responses: 0.08-1.72% of total CD8, Figure 4C). CD4+ T cells were also induced in mice and in non-human primates, albeit at a lower level (range: 0.07-0.26% and 0.1-0.39% of total CD4, respectively; Figure 4B and 4D). Importantly, the level of IFNγ secreting anti-gag CD4+ and CD8+ T-lymphocytes induced by the PanAd3 vector in non-human primates was comparable to that induced by the clinically validated human Ad6.
Fig. 4.
Frequency of IFNγ-secreting CD8 and CD4 T cells in mice and macaques. Frequency of CD8 and CD4 T cells secreting IFNγ in response to stimulation with a gag peptide pool was assessed using Intracellular Staining (ICS) and FACS analysis in mice (A and B, CD8 and CD4, respectively) and macaques (C and D, CD8 and CD4, respectively). Each symbol corresponds to IFNγ+ CD8 or CD4 frequency in individual animals, expressed as the percentage of total CD8 or CD4. Horizontal line represents geometric mean.
ChAds are rarely neutralised by human sera
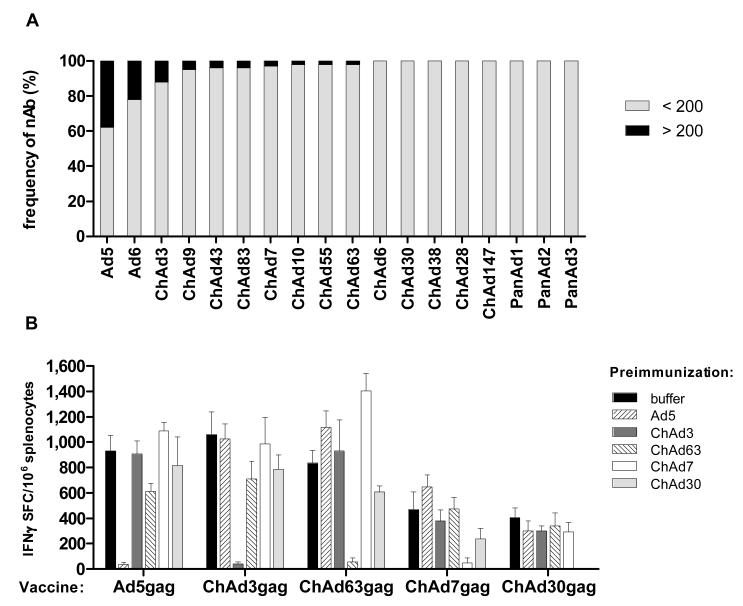
The ChAd-SEAP vectors were used to measure the presence of nAbs against ChAds in the humans (26). Human Ad5 and Ad6 were also included in this analysis. 193 sera from healthy individuals belonging to different geographical areas in Europe and US were tested for their ability to neutralise the infectivity of ChAd-SEAP vectors in HEK293 cells. It was previously shown that anti-Ad nAb titers above 200 measured by this same neutralization assay can dampen the immunogenicity of the vector in humans (11). The results of this screening indicated that ChAds are much less frequently neutralised by antibodies present in the tested human population than Ad5 and Ad6. Both Ad5 and Ad6 were neutralised by human sera with a frequency of samples displaying titers over 200 of 50% and 20%, respectively. In contrast, only half of the ChAd vectors were neutralised by some sera, and even in these cases the frequency of positive samples was below 10% (Figure 5A).
Fig. 5.
Neutralizing antibody response and lack of cross neutralization among different chimp adenovirus strains. Neutralizing antibody titers to a panel of human and chimpanzee adenoviruses measured in sera collected from a large cohort of Caucasian human healthy volunteers (A). The graph reports the percentage of individuals that show neutralizing titers above (black bar segment) or below (grey bar segment) 200 against each individual adenovirus tested. Lack of cross-neutralization among different chimp adenovirus strains (B). Chimp Ad belonging to different Ad serological groups were selected according to the classification reported in Fig. 2. Mice were pre-immunized twice with 1010 vp of EGFP-expressing vectors (identified by different bar color and pattern) and then vaccinated with 109 vp of HIV-1 gag expressing vectors as reported in the x axis. Three weeks later mice were tested for T-cell response against gag by IFNγ ELISpot. Bars represent the mean+SEM in each immunization group, expressed as IFNγSFC per million splenocytes.
We used Ad5 and ChAd vectors belonging to different species (subgroup C: ChAd3; subgroup E: ChAd7 and ChAd63, subgroup B: ChAd30) to assess their in vivo cross-neutralization potential.
Mice (N=5) were pre-immunized twice with high doses (1010 vp) of Ad5 or ChAd vectors expressing EFGP, and developed titers of neutralizing antibodies against the injected virus above 1000 in all animals. These mouse hyperimmune sera were not able to neutralize heterologous Adenoviruses (data not shown). Pre-immunised mice were then injected with 109 vp of ChAd-gag vectors, and gag-specific T-cell responses were measured by IFNγ ELISpot three weeks post-injection. Only pre-injection with the homologous virus prevented the induction of a T-cell response by Ad5-gag or ChAd-gag vectors (Figure 5B). These results confirmed that the sequence analysis of hypervariable regions in the hexon protein can predict antibody cross-neutralization, and that the different ChAd genotypes that we have identified represent individual serotypes.
ChAd3-gag induces a long lasting T and B cell memory responses in non-human primates
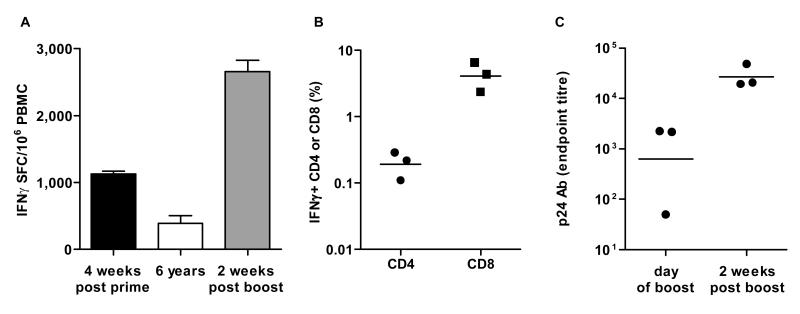
To verify the longevity of the T-cell response induced by ChAd vectors, we immunised a group of macaques (N=3) with ChAd3-gag (1010 vp) and then rested the animals without additional vaccinations. After more than five years (274 weeks), the animals were bled again and their anti-gag T-cell response was measured by ex vivo IFNγ ELISpot. Potent cellular immunity was induced upon priming (group mean average of 1128 IFNγ SFC/million PBMC) which then contracted, but lasted for several years as witnessed by good levels of gag-specific IFNγ T-cells still present at week 274 (group mean average of 392 IFNγ SFC/million PBMC; Figure 6A).
Fig. 6.
Longevity of memory T and B cell response in macaques. Three macaques were vaccinated in early 2003 with 1010 vp of ChAd3-gag and boosted 6 years later with the same dosage of PanAd3-gag. IFNγ ELISpot response is shown at peak post prime (4 weeks after vaccination), at the time of long term boost (6 years) and 2 weeks post boost (A). Data are shown as the group mean+SEM response at each time point. Quality of the IFNγ secreting T cell subset, assessed ICS and FACS analysis 2 weeks after PanAd3 long term boost, is shown in (B). Each symbol corresponds to IFNγ + CD4 or CD8 frequency in individual animals, expressed as the percentage of total CD4 or CD8. Horizontal line represents geometric mean. Antibody titers to p24 gag protein, assessed by ELISA and expressed as endpoint titers, are shown in (C) for the three individual animals at the time of PanAd3-gag boost and two weeks later. Horizontal line represents geometric mean
We then tested if the long-lasting T cell pool induced by ChAd3-gag could be expanded in vivo upon re-encounter of the same antigen. Therefore, we boosted the ChAd3-gag primed animals with 1010 vp of the heterologous PanAd3-gag vector at week 299 and measured the gag-specific T-cell response two weeks later. All animals experienced a massive expansion of gag-specific IFNγ secreting T-cells with a peak about three-fold higher than that observed post-priming (mean of 2661 IFNγ SFC/million PBMC; Figure 6A). Both CD4+ and CD8+ T cells were detected post-boost, reaching a geometric mean value of gag-specific IFNγ+ CD4+ and CD8+ cells of 0.19% and 4.08%, respectively (Figure 6B). Antibodies to HIV-1 gag were still detectable at the time of boost (week 299) in two out of three animals with titers of about 2000, and boosting with PanAd3-gag increased their levels by more than ten-fold in all three animals already two weeks after boost, reaching titers of about 20.000 (Figure 6C), indicating that ChAd3-gag is able to induce long-lived T- and B-cell memory responses in non-human primates.
ChAd vaccine vectors are highly immunogenic in humans
Two of the most potent ChAd vectors, the group C ChAd3, and the group E ChAd63, were selected for clinical development.
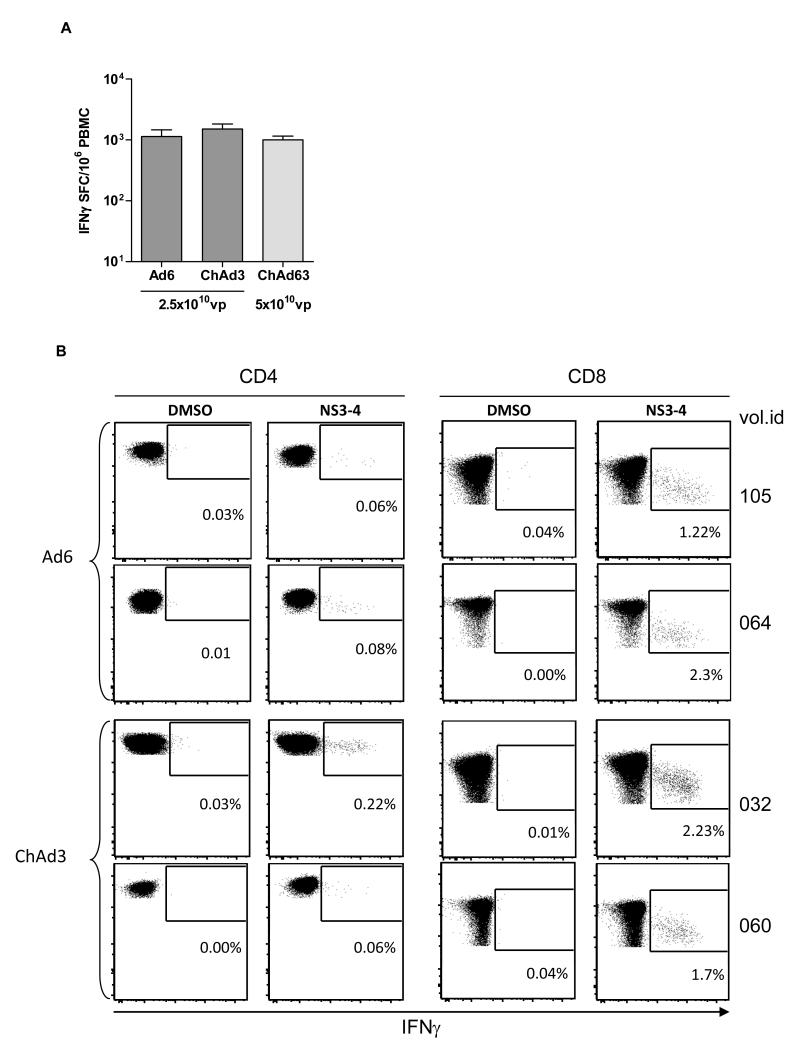
ChAd3 was chosen to deliver a genetic vaccine against hepatitis C virus (HCV). We previously showed that a genetic T cell-based vaccine based on the Non Structural region (NS) of HCV induces high level of T-cell responses in non-human primates and protects chimpanzees from acute and chronic infection after challenge with a high dose of heterologous HCV infectious inoculum (27-29). We generated a ChAd3 vector encoding for this HCV antigen (ChAd3-NSmut) and tested its safety and immunogenicity in a Phase I clinical trial in healthy volunteers. A similar vector based on the human Ad6 (Ad6-NSmut) was also tested in the same trial. The trial and results are reported in detail elsewhere (30). ChAd3-NSmut was highly immunogenic with group mean T-cell response as measured by IFNγ ELISpot of 1863 SFC/million PBMC (Figure 7A) and 100% frequency of responders at a dose of 2.5×1010 vp. Notably, the human vector Ad6-NSmut showed comparable immunogenicity (mean of 2185 SFC/million PBMC and 100% response rate).
Fig. 7.
Immunogenicity of human and chimpanzee adenovirus in humans. IFNγ ELISpot data from human healthy volunteers vaccinated once with the indicated dosage of HCV adenovirus vaccines Ad6-NSmut (n=9, (30)) and ChAd3-NSmut (n=10, (30)) or malaria adenovirus vaccine ChAd63-METRAP (n=39, (31, 32)) are shown as mean+SEM of individual responses to the vaccine inserts (A). Individual responses are obtained by summing each volunteer’s reactivity to peptide pools (six pools for the HCV NS region and six pools for TRAP plus one pool for ME regions of the Malaria vaccine insert). Peak response was measured two or four weeks after vaccination. Dot plots showing IFNγ secretion by CD4 and CD8 T cells from two representative volunteers vaccinated with Ad6 or ChAd3 HCV vaccine vectors are shown in (B). PBMC were stimulated with either a mixture of the three HCV NS pools covering NS3 and NS4 region of the HCV vaccine insert (NS3-4), or with DMSO, the peptide pool diluent, as negative control. Numbers in each plot correspond to frequency of IFNγ + cells over total CD4 and CD8.
Intracellular staining assays showed that ChAd3-NSmut and Ad6-NSmut primed a large number of IFNγ producing CD8+ T cells (range 0.025% to 4% HCV-specific/total CD8. Antigen-specific CD4 producing IFNγ were also detected albeit at a lower level (range 0.01% to 0.28% HCV-specific/total CD4) which is consistent with the data obtained in mice and non-human primates (Figure 7B).
The second vector, ChAd63, was used for the development of a genetic vaccine against Plasmodium falciparum Malaria. For this candidate vaccine we generated a ChAd63 replication defective vector encoding the liver-stage TRAP antigen fused to a string of Plasmodium falciparum CD4 and CD8 epitopes mapped in humans (ChAd63-METRAP). This vector was shown to induce high level of T-cell responses in rodents and non-human primates and to protect against mouse malaria (ref). The group E ChAd63-METRAP was tested in human healthy volunteers (31, 32), showing potent T cell responses as detected by IFNγ ELISpot with a mean response of 978 SFC/million PBMC at a dose of 5×1010 vp (Figure 7A).
Discussion
For reasons that are not clear, there are large differences in the ability to induce CD8+ T-cells by diverse viral and non viral genetic vaccine carriers. This is particularly true in humans where many different approaches have failed to induce CD8+ T cell immunity even in the face of positive results obtained in pre-clinical animal studies.Ad vectors derived from strains that have not circulated widely in humans (“rare serotypes”) are under investigation as vaccine vectors, based on the expectation that they would be as potent as Ad5 while being insensitive to neutralisation by anti-Ad5 antibodies.
To help predicting the immunological potency in humans of the different Ad vectors, we screened them for their ability to stimulate an immune response over a wide range of doses using the clinically validated human Ad5 as a benchmark. By dose/response comparative studies in mice and non-human primates, we found that Ad vectors from the rare human serotypes Ad24, Ad26, Ad34 and Ad35 are substantially less immunogenic (100-1000 fold) than the clinically validated Ad5. Interestingly, Ad6 was comparable to Ad5 in these experiments, a finding consistent with previous immunological data obtained in mice, non-human primates and humans (4, 27), and an important validation of the dose/response approach to establish the relative immunological potency of Ad vectors across species.
The association between low seroprevalence and weak immunological potency may not be a mere coincidence, but rather reflect a reduced fitness of the virus in the human population possibly leading to a reduced immunological potency. Thus, while potentially overcoming the problem of the pre-existing immunity to Ad5, the use of Ad vectors from rare human serotypes appears not to address other issues such as the immunological potency, thus running a high risk of failure in humans, as suggested by recent Phase I clinical data with an Ad35-based HIV candidate vaccine (33). Preliminary flow cytometry data from this trial show rather low frequency of responders with 8% and 14% responding subjects over total examined for CD8 and CD4, respectively.
Lacking a useful biomarker of Ad vector immunogenicity in vivo, our strategy to identify alternative Ad vectors with all the features necessary for vaccine development was to generate a large collection of such vectors and to screen them to find suitable candidates for vaccine development. This ‘reverse vectorology’ approach required a “library” of different Ads to be available for the screening. To generate such a library we decided to isolate novel Ad strains from chimpanzees. This choice was based on the prediction that the substantial genetic diversity between individuals of the Pan species would extend to their pathogens (34) making it possible to isolate a large repertoire of different Ads. This turned out to be the case as we isolated a large number of different Ads from chimpanzees and bonobos which were classified into the same groups as human Ads based on the amino acid sequence of their hexon proteins. Among these isolates we could identify at least twenty-five different strains based on hexon homology and we confirmed that they represent individual serotypes by in vitro cross-neutralization assays and in vivo interference studies.
Sequencing the whole genome of some of the ChAd isolates (ChAd3, ChAd6, ChAd9, ChAd19, ChAd43, ChAd63, ChAd83, PanAd1, PanAd2, PanAd3) revealed that they are strongly related to human Ads, showing a high degree of DNA homology with human Ads belonging to the same species (80-95%) and similar genomic structure. The latter feature was particularly relevant to map the ChAd E1 regions to be deleted for the generation of replication incompetent vectors. We could not identify a feature in the Chimpanzee adenovirus DNA sequence that would allow to distinguish between viruses of human or chimpanzee origin.
The ChAd collection was used as a source of vectors for functional screening to assess: i) growth capability in HEK293 and PER.C6 cells, ii) immunological potency in mice and non-human primates, iii) sensitivity to neutralizing antibodies present in humans.
Replication defective E1-deleted ChAd vectors were efficiently propagated in PER.C6 and HEK293 cells, confirming the functional equivalence of human Ad5 and chimpanzee Ad E1. Importantly, we did not observe the formation of RCA during propagation of ChAd in HEK293, indicating that there is insufficient sequence homology between human and chimpanzee Ads to allow for homologous recombination between the two genomes, and opening the possibility to use HEK293 for large scale manufacturing of ChAds.
All ChAds revealed significant diversity in the hypervariable regions of the hexon protein from the highly seroprevalent Ad5 and were not neutralized by anti-Ad5 antibodies in vitro and in vivo. Consequently, they were all found to be very rarely neutralized by antibodies present in humans, and for about half of them none of the tested sera displayed any neutralizing activity. Conversely, most ChAds were very often neutralized by chimpanzee sera (data not shown), further supporting the notion that serologically distinct strains of Adenoviruses circulate in the two species and that Adenovirus infection across human and chimpanzees is a very rare event.
Screening for immunological potency by dose/response in mice confirmed that there is a high degree of heterogeneity between ChAds as already observed for human Ads. Nevertheless, given the large number of screened candidates we were able to identify some ChAds with immunological potency equivalent to Ad5 (ChAd3, ChAd63, ChAd83, PanAd1, PanAd2 and PanAd3), and several others with a slightly lower immunogenicity within a factor of five to ten-fold. Importantly, the high level of immunogenicity of the top ranking ChAd3 and PanAd3 was confirmed in non-human primates, where they induced a level of T-cell response comparable to that of Ad5 even at low dose (108 vp). Similarly, we recently showed that another high scoring ChAd vector (ChAd63) encoding for the Malaria TRAP antigen induced a very potent T-cell response in Rhesus macaques (35). Notably, the ability of ChAds to induce strong cellular immunity is not an antigen-dependent phenomenon as we could induce substantial T-cell response by immunising rodents and non human primates with ChAd vectors encoding different antigens from infectious agents such as HIV, HCV, Malaria, HSV2, Ebola, RSV and Influenza as well as cancer.
Despite their overall similarity in structure and genome organization, human and chimpanzee Adenoviruses display a wide range of immunological potency when used as replication defective vectors in vivo. We were unable to identify a mechanism for such a large difference in the immunological potency between Adenovirus vectors. Nevertheless we could establish a correlation between the efficiency of stimulating the adaptive immune system and the phylogenetic classification into the different species according to the homology of the hexon protein sequence. In fact, all tested group C viruses (both human and chimpanzee) were very potent with a breakpoint of 107 vp or lower, while human or chimpanzee group B Ad were immunogenic only when used at the dose of 109 vp or higher. Human group D and some of the group E ChAd viruses were also rather weak carriers as they were able to induce an immune response only at the dose of 108 vp. Within the group E ChAd we could distinguish two clusters on the basis of their hexon sequence homology: ChAd6, 9, 10, 43, 47, 63, 83 and 147 belonged to one family (E1) and all the others clustered into a separate family (E2). Interestingly, most of the E1 viruses were potent vectors, while members of the E2 family displayed lower immunogenicity.
One of the possible explanations for the different immunological potency displayed by human and chimpanzee Ad strains could be their receptor usage and/or cell tropism (36). Indeed, the most potent group C and group E Adenoviruses use the CAR receptor to infect a variety of different cell types, while Group B Adenoviruses (ie.: the human Ad11, Ad34 and Ad35 and the chimpanzee ChAd30) recognise the CD46 surface protein and infect DC cells more efficiently than group C isolates in vitro (37). This finding originally led to the hypothesis that the latter viruses would be a more effective class of genetic vaccine carrier. In contrast, we showed that they are the least potent among all human and chimpanzee derived Ad vectors. To explain this apparent contradiction one might speculate that highly efficient infection of professional antigen presenting cells by the group B Ad would lead to a more rapid elimination of the vector thereby reducing the efficiency and longevity of expression of the encoded antigen. This hypothesis is consistent with the low prevalence of group B Adenoviruses in both humans and chimpanzees which may result from a more efficient immunological clearance as compared to other Adenoviruses such as those from the group C or E.
Predicting the immunological potency in humans is a key factor for vaccine vector development. Final validation of our dose/response immunological screening in mice came from the observation that two of the most potent ChAd vectors, ChAd3 and ChAd63, induced T-cell immunity in 100% of immunised human volunteers with average peak responses exceeding 1500 IFNγ SFC/106 PBMCs for ChAd3 and about 1000 IFNγ SFC/106 PBMCs for ChAd63. These are by far the highest levels of T-cell responses ever observed in humans with a single non-replicating genetic vaccine vector. Of note, the ChAd-induced cellular response was characterised by extremely potent IFNγ+ CD8 T-cells in all tested species from rodents to humans, with high values of over 1% antigen-specific IFNγ+ CD8 T-cells in ChAd3 vaccinated healthy humans.
The success of vaccine-induced T-cell immunity in clearing infected cells before the onset of an acute disease or the establishment of a chronic infection is likely to depend on two factors: i) the level of CD8+ T cell effectors that are present at the time of the infection and can rapidly recognise and eliminate infected cells, and ii) the presence of a pool of memory population of T cells that are capable of rapid re-expansion upon encountering of the pathogen antigens. Our finding that non-human primates immunised with ChAd3 developed HIV gag-specific IFNγ+ T-cells which persisted for more than five years as detected by ex-vivo ELISpot indicates that ChAd have the potential to induce high frequencies of long lasting antigen-specific T-cells with effector function. Furthermore, these long lived T-cell pools also displayed the typical feature of a memory population as they underwent rapid expansion in vivo upon boosting with a second non cross-reacting ChAd vector encoding the same HIV gag antigen.
We could demonstrate that pre-immunization with high doses of a heterologous ChAd did not affect the efficiency of immunization of a subsequently injected ChAd from a different serotype. Thus, our collection of potent, non cross-reacting ChAd vectors can be exploited for several vaccine applications following the strategy of ‘one vector-one disease’ to avoid potential interference between different vaccines. Additional evidence in favour of this strategy comes from experiments in non-human primates where we have successfully immunised animals with ChAd3-gag or ChAd3-NSmut and subsequently with ChAd63 encoding the AMA-1 Malaria antigen without any detectable interference between the different vaccine vectors. In addition, the availability of several different ChAd vectors allows the design of a vaccination strategy based on heterologous prime-boost modality with serologically distinct ChAd to maximize immunization strength.
Materials and Methods
Chimpanzee Adenovirus Isolation and amplification
Stool specimens were collected in viral transport medium (VTM; Microtest M4-R Multi-Microbe Transport Medium, Remel Inc.) then frozen or frozen directly at −70°C. The specimens were kept frozen at < −70°C until they were processed for inoculation into cell cultures. At that time, the specimens were thawed and then vortexed in excess of chilled viral transport medium. After the specimens had dissociated into suspensions, they were centrifuged for 10 min at 1500-1800 rpm. The supernatants were filtered through 0.8 and 0.2 mm syringe filters in series and then the filtered material was inoculated into cell cultures. Each processed specimen was inoculated into tube cultures and shell vial cultures seeded with HEK293 cells or A549 cells cultivated in DMEM, 10% fetal bovine serum (FBS), 1% Pen-Strep. Cultures were visually monitored for cytopathic effect (CPE) for at least 21 days after inoculation. Cell monolayers showing clear sign of CPE were detached and suspended in the culture supernatant then stored at −70°C. Adenoviruses were cloned by infecting HEK293 cells seeded in 96-well plates. The virus cloning was performed by limiting dilution of the cell lysate obtained at the first passage of the virus amplification. 5 isolated clones were picked up and serially propagated. After 2-3 serial passages on HEK293 cells, a large-scale preparation of adenovirus was performed on cells planted on 1-2 two-layer cell-factories (NUNC) (2×106 cells/cell factory). Purified viral particles were obtained from cell lysate by two ultra-centrifugation steps on cesium chloride density gradients following a standard procedure.
Classification of the isolates
An initial classification of the new isolates was obtained by sequence analysis of the hypervariable region 7 (HVR7) of the hexon gene. To this end two set of primers were designed on the highly conserved regions flanking HVR7: TGTCCTACCARCTCTTGCTTGA and GTGGAARGGCACGTAGCG; TGTCCTACCAGCTCTTGCTTGA and GTTCATGTAATCGTAGGTGTTG. These set of primers pair annealed on all isolates we have obtained so far. In addition a pair of primers was designed to amplify hexon hypervariable region 1-6, CAYGATGTGACCACCGACCG and GTGTTYCTGTCYTGCAAGTC.
The HVR 7 and/or HVR 1-6 were amplified by PCR using purified viral DNA or crude HEK293 lysate as template. The PCR reaction was performed following this protocol: 4 μL of crude lysate or 100 ng of purified viral DNA in 45 μL of reaction mixture containing 2X Master Mix (GoTaq Colorless Master Mix, Promega Corporation) and 10 pmol of each primers. Then, 5 μL of the reaction mixture was analyzed on 1% agarose gel containing ethidium bromide to detect the expected amplicon.
The PCR product was then purified to remove excess nucleotides and primers (using Wizard® SV Gel and PCR Clean-Up System, Promega Corporation) and subjected to sequencing reaction by using the same primers of the PCR reaction. Based on HVR7 and HVR 1-6 sequence alignment we classified the new isolated viruses into the subgroups (B, C and E) proposed for human Ad viruses (17).
Vector Construction
Genomic viral DNAs were cloned into a standard plasmid vectors by homologous recombination in E.coli BJ5183 using a shuttle vector containing viral DNA sequences derived from both left and right end of viral genome. As described more in detail in the supplementary Materials and Methods, the high degree of sequence homology within each species was exploited to clone the entire genome of different isolates using shuttle vectors specific for species B, C and E adenoviruses. The shuttle vectors were constructed by cloning right and left genome ends plus pIX gene deleting at the same time the E1 region that was substituted with the expression cassette. The shuttle was linearized by restriction digestion between pIX and the right ITR and BJ5183 cells were co-transformed with the purified virus genome. The homologous recombination between right ITR and pIX allowed for the insertion of the chimp Ad genome in the shuttle plasmid with the deletion of the E1 region.
All different expression cassettes inserted in ChAd and PanAd vectors were based on HCMV promoter and BGH pA. HIV-1 gag, HCV NS, plasmodium falciparum METRAP and SEAP expression vectors were first cloned under HCMV and BGH pA control then transferred in the different ChAd vectors containing HCMV/BGH pA cassette by homologous recombination in BJ5183 cells.
Cloning of human Ad and vector construction
Human adenovirus vectors were constructed following the same strategy described for chimpanzee adenovirus. Human Ad5, Ad6, Ad24, Ad26, Ad34 and Ad35 were obtained from ATCC.
Adenoviral vector rescue, amplification and purification
Chimpanzee preAd plasmid were first digested with PmeI to release the viral ITRs then 3-5×106 HEK293/PER.C6 cells grown in DMEM, 10% fetal bovine serum (FBS), 1% Penn-Strept in 6 cm cell culture dishes, were transfected with 10 micrograms of cloned viral vector. DNA transfection was performed using Lipofectamine (Invitrogen). Vectors were then expanded up to a production scale of 2×109 cells. Purification was performed by two step Cesium Chloride gradient.
Neutralizing Antibody Assay
Neutralizing antibody (nAb) titers in human and mouse sera were assayed as previously described (26). Briefly, 3.5×104 HEK293 cells were seeded per well in a 96 well plate for 2 days. Each adenoviral vector encoding for secreted alkaline phosphatase (SEAP) was pre-incubated for 1h at 37°C alone or with serial dilutions of tested serum and then added to the 95-100% confluent HEK293 cells and incubated for 1h at 37°C. Supernatant was then removed and replaced with 10% FCS in DMEM. SEAP expression was measured 24 hours later with the chemiluminescent substrate from the Phospha-Light™ kit (Applied Biosystems). Neutralization titers were defined as the dilution at which a 50% reduction of SEAP activity from serum sample was observed relative to SEAP activity from virus alone.
Animals and Immunisation
Female, six weeks old BALB/c mice were purchased by Charles River (Charles River, Como, Italy). Male Rhesus macaques (Macaca mulatta) of Chinese origin were housed at the Italian National Research Council primate facility (Rome, Italy) or at IRBM (Rome, Italy). The animal care routine and experimental procedures were in compliance with national and international laws and policies (EEC Council Directive 86/609; Italian Legislative Decree 116/92; Gazzetta Ufficiale della Repubblica Italiana n. 40, Feb. 18, 1992). The ethical committee of the Italian Ministry of Health approved this research. During handling, the animals were anesthetized.
To determine immunological potency, five mice per group were immunized with escalating doses of each adenoviral vector suspended in 100μl of adenovirus stabilization buffers A195 or A438 (see Supplementary Material and Methods) and injected in the quadriceps muscles (50μl/site). Three weeks after vaccination mice were euthanized to measure T cell responses in splenocytes. In a second set of experiments, mice were pre-immunized twice every two weeks with 1010 vp of Ad5, ChAd3, ChAd63, ChAd7 or ChAd30 expressing EGFP, or mock vaccinated with buffer. Pre-immunized mice were then immunized once with 109 vp of the above vectors encoding HIVgag in all possible vector combinations. Immune response was tested on splenocytes two weeks after immunization.
Macaques were immunized intramuscularly in the deltoid with adenovirus diluted in 0.5 ml of adenovirus stabilization buffer. At serial time points EDTA-treated blood was drawn, PBMC were prepared by standard technique using Accuspin™ tubes containing Histopaque-1077 (Sigma, UK), and used for immunological assays.
Human clinical trials
All volunteers gave written informed consent prior to participation and the studies were conducted according to the principles of the Declaration of Helsinki and in accordance with Good Clinical Practice (GCP). The HCV vaccine phase I trial (HCV001) was registered with the European Clinical Trial database (EudraCT Number: 2007-004259-12) and with the ClinicalTrial.gov database (ID: NCT01070407). The Malaria vaccine clinical trials (VAC 033 and MAL 034) were registered with the European Clinical Trial database (EudraCT Numbers: 2006-005966-37 and 2008-006804-46, respectively).
Antigens for immunological assays
For BALB/c mouse experiments, a 9mer peptide encoding the HIVgag major H-2 Kd CD8 epitope (AMQMLKETI) was used as antigen in ELISpot assay, at a final concentration of 0.5μg/ml. For mouse ICS and macaque ELISpot and ICS assays, a pool of 122 15mer peptides overlapping by 11 aminoacids dissolved in DMSO, covering the entire HIVgag protein was used as antigen at a final concentration of 4μg/ml of each peptide. DMSO and ConA were used respectively as negative and positive controls. For HCV vaccine clinical trial a set of 494 15mer peptides overlapping by 11 amino acids encompassing NS3 to NS5B proteins of the Non Structural (NS) region from HCV genotype 1b, BK isolate, were arranged in six pools covering NS3 protease (NS3p), NS3 helicase (NS3h), NS4, NS5A and NS5B (split in two pools NS5B-I and NS5B-II). Pools were used at a final concentration of 3μg/ml of each single peptide in the ELISpot assay. For the Malaria vaccine clinical trial, 20mer peptides overlapping by 10 amino acids, spanning ME and TRAP protein were used for ex vivo ELISpot as described (38).
IFNγ ex vivo ELISpot
Antigen specific IFNγ production by splenocytes of immunized mice and by macaques PBMC were determined by a standard ELISpot assay described in details elsewhere (27, 39). Ex vivo ELISpot with human PBMC for the HCV vaccine trial was performed as described (40) with the following minor modifications: MSIP S4510 plates (Millipore) were coated with 100μl/well of anti-human IFNγ monoclonal antibody (clone 1-D1K, MAbTech) at 5 μg/ml; PBMC were plated in triplicate at 200.000 per well; detecting biotinylated anti human IFNγ antibody (MAbTech clone 7-B6-1) was added in 50μl volume at 0.5 μg/ml, and 50μl volume was kept in all subsequent steps of plate development. Ex vivo ELISpot with human PBMC for the malaria vaccine trial was performed as previously described (38).
ELISpot data are expressed as IFNγ SFC (spot forming cells) per million splenocytes or PBMC. The ELISpot response was considered positive when all of the following conditions were met: IFNγ production present in Con-A stimulated wells; at least 50 specific spots/million splenocytes or PBMC; the number of spots seen in positive wells was three times the number detected in the mock control wells (DMSO).
Intracellular staining and FACS analysis
IFNγ intracellular staining and FACS analysis on mouse splenocytes was performed by stimulating four millions cells for 16 hours with HIVgag 15mer peptide pool in the presence of GolgiPlug (BD Biosciences) according to the manufacturer instructions. After stimulation the cells were first incubated with 1 μg/million cells of Fc Block (BD), than stained superficially with the following antibodies: CD3e APC (clone 145-2C11), CD4 PE (clone RM4-5) and CD8a P (clone 53-6.7). Cells were then permeabilized with Cytofix/Cytoperm (BD) and stained in Perm/Wash (BD) with anti IFNγ FITC (clone XMG1.2). All antibodies were purchased from BD Biosciences.
IFNγ ICS with human and macaque PBMC was performed as previously described (40) using the following antibodies: CD3 APC (clone SP34-2), CD4 PerCP Cy5.5 (clone L-200), CD8 PE (clone RPA-T8), all from BD, and IFNγ FITC (clone MD-1) from U-CyTech. Acquisition was performed on the day of staining on a FACS Calibur; at least 30,000 CD8+ events were collected per sample. Data was prepared and analysis performed using CellQuest. Cells were gated on small lymphocytes, CD3+, then CD4+ or CD8+ (excluding double positives), and finally IFNγ.
Antibody responses
Antibody responses were assessed by ELISA after coating plates (NUNC Immuno plates) with 1 μg/ml of recombinant HIV-1 gag p24 antigen (Austral Biologicals) in PBS, 100 ng protein/well. Plates were blocked 1 hour at 37°C with Milk buffer (5% non-fat dry milk in PBS, 0.05% Tween-20). Macaque serum samples were serially diluted 1:3 in Milk buffer, starting at a dilution of 1:100, added to the blocked plate and incubated overnight at 4°C. After wash, bound antibodies were detected using an anti-monkey IgG alkaline phosphatase conjugated (Sigma A1929) diluted 1:5000 in Milk buffer and incubated 1 hour at room temperature. After wash the plates were developed using SigmaFast AP substrate (Sigma A1891) and optical density (OD) was read at dual wave lenght (405 and 620nm). Endpoint titers were taken as the x-axis intercept of the dilution curve at an absorbance value 3x standard deviations greater than the OD405 for pre-immune serum in each individual macaque (typical cut-off OD405 for positive sera = 0.15).
Supplementary Material
Supplementary Fig.1 legend. Representative dose/response experiments with PanAdgag vectors. Groups of five BALB/c mice were immunized with escalating doses (indicated at the bottom of the graph) of PanAd1, PanAd2 or PanAd3 encoding HIV-1 gag and sacrificed three weeks later. Splenocyte IFNγ ELISpot responses to the CD8+ peptide AMQMLKETI are reported on the vertical axis and are expressed as IFNγ SFC per 106 splenocytes. Symbols correspond to individual mice responses, subtracted of the DMSO background (tipically not higher than 10 SFC/million spenocytes). Horizontal lines represent geometric mean of each group. A dashed line set at 50 SFC shows cut off to define a positive response. The immunological potency of each vector is defined as the minimal dose at which at least two out of five mice show a positive response, resulting in 106 vp for PanAd3 and 5×106 vp for PanAd1 and PanAd2, as reported in Figure 1 panel C.
Acknowledgments
We thank G. Perretta and A. Taglioni from Italian National Research Council primate facility for the excellent animal care.
Funding: This work was supported in part by Hepacivac (LSH-2005-1.2.4-2 project 037435) and the Wellcome Trust. AVSH was supported by a Wellcome Trust Principal Research Fellow.
Footnotes
List of Supplementary Material
- Supplementary material and methods
- Supplementary Fig. 1 and legend
Author contributions: Conceived and designed the experiments: SCo, AF, AVHS, EB, PK, RC, AN; performed the experiments: VA, SCa, AC, LS, MN, FG, MLE, MA, AS, MB, AM, KS, AK, GAOH, KJE; wrote the manuscript: AN, SCo, AF, SCa; project manager: CT; principal investigator: SCo.
Competing Interests: S. Colloca, A. Folgori, V. Ammendola, A. Cirillo, M. Ambrosio, A. Meola, R. Cortese and A. Nicosia are named inventors on patent applications covering HCV vectored vaccines and chimpanzee adenovirus vectors. G. O’Hara and A. Hill are named inventors on patent applications covering malaria vectored vaccines and immunisation regimes. Authors from Okairos are employees of and / or shareholders in Okairos which is developing vectored HCV and malaria vaccines.
References
- 1.Kim PS, Ahmed R. Features of responding T cells in cancer and chronic infection. Curr Opin Immunol. 2010;22:223–30. doi: 10.1016/j.coi.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barouch DH. Novel adenovirus vector-based vaccines for HIV-1. Curr Opin HIV AIDS. 2010;5:386–90. doi: 10.1097/COH.0b013e32833cfe4c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Casimiro DR, Chen L, Fu TM, Evans RK, Caulfield MJ, Davies ME, Tang A, Chen M, Huang L, Harris V, Freed DC, Wilson KA, Dubey S, Zhu DM, Nawrocki D, Mach H, Troutman R, Isopi L, Williams D, Hurni W, Xu Z, Smith JG, Wang S, Liu X, Guan L, Long R, Trigona W, Heidecker GJ, Perry HC, Persaud N, Toner TJ, Su Q, Liang X, Youil R, Chastain M, Bett AJ, Volkin DB, Emini EA, Shiver JW. Comparative immunogenicity in rhesus monkeys of DNA plasmid, recombinant vaccinia virus, and replication-defective adenovirus vectors expressing a human immunodeficiency virus type 1 gag gene. J Virol. 2003;77:6305–13. doi: 10.1128/JVI.77.11.6305-6313.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harro C, Sun X, Stek JE, Leavitt RY, Mehrotra DV, Wang F, Bett AJ, Casimiro DR, Shiver JW, DiNubile MJ, Quirk E. Safety and immunogenicity of the Merck adenovirus serotype 5 (MRKAd5) and MRKAd6 human immunodeficiency virus type 1 trigene vaccines alone and in combination in healthy adults. Clin Vaccine Immunol. 2009;16:1285–92. doi: 10.1128/CVI.00144-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ledgerwood JE, Costner P, Desai N, Holman L, Enama ME, Yamshchikov G, Mulangu S, Hu Z, Andrews CA, Sheets RA, Koup RA, Roederer M, Bailer R, Mascola JR, Pau MG, Sullivan NJ, Goudsmit J, Nabel GJ, Graham BS. A replication defective recombinant Ad5 vaccine expressing Ebola virus GP is safe and immunogenic in healthy adults. Vaccine. 2010;29:304–13. doi: 10.1016/j.vaccine.2010.10.037. [DOI] [PubMed] [Google Scholar]
- 6.Liu J, O’Brien KL, Lynch DM, Simmons NL, La Porte A, Riggs AM, Abbink P, Coffey RT, Grandpre LE, Seaman MS, Landucci G, Forthal DN, Montefiori DC, Carville A, Mansfield KG, Havenga MJ, Pau MG, Goudsmit J, Barouch DH. Immune control of an SIV challenge by a T-cell-based vaccine in rhesus monkeys. Nature. 2009;457:87–91. doi: 10.1038/nature07469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tatsis N, Ertl HC. Adenoviruses as vaccine vectors. Mol Ther. 2004;10:616–29. doi: 10.1016/j.ymthe.2004.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Catanzaro AT, Koup RA, Roederer M, Bailer RT, Enama ME, Moodie Z, Gu L, Martin JE, Novik L, Chakrabarti BK, Butman BT, Gall JG, King CR, Andrews CA, Sheets R, Gomez PL, Mascola JR, Nabel GJ, Graham BS. Phase 1 safety and immunogenicity evaluation of a multiclade HIV-1 candidate vaccine delivered by a replication-defective recombinant adenovirus vector. J Infect Dis. 2006;194:1638–49. doi: 10.1086/509258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Priddy FH, Brown D, Kublin J, Monahan K, Wright DP, Lalezari J, Santiago S, Marmor M, Lally M, Novak RM, Brown SJ, Kulkarni P, Dubey SA, Kierstead LS, Casimiro DR, Mogg R, DiNubile MJ, Shiver JW, Leavitt RY, Robertson MN, Mehrotra DV, Quirk E. Safety and immunogenicity of a replication-incompetent adenovirus type 5 HIV-1 clade B gag/pol/nef vaccine in healthy adults. Clin Infect Dis. 2008;46:1769–81. doi: 10.1086/587993. [DOI] [PubMed] [Google Scholar]
- 10.McElrath M, De Rosa S, Moodie Z, Dubey S, Kierstead L, Janes H, Defawe O, Carter D, Hural J, Akondy R, Buchbinder S, Robertson M, Mehrotra D, Self S, Corey L, Shiver J, Casimiro D, Team. SSP HIV-1 vaccine-induced immunity in the test-of-concept Step Study: a case-cohort analysis. Lancet. 2008;372(9653):1894–905. doi: 10.1016/S0140-6736(08)61592-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buchbinder SP, Mehrotra DV, Duerr A, Fitzgerald DW, Mogg R, Li D, Gilbert PB, Lama JR, Marmor M, Del Rio C, McElrath MJ, Casimiro DR, Gottesdiener KM, Chodakewitz JA, Corey L, Robertson MN. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet. 2008;372:1881–93. doi: 10.1016/S0140-6736(08)61591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abbink P, Lemckert AA, Ewald BA, Lynch DM, Denholtz M, Smits S, Holterman L, Damen I, Vogels R, Thorner AR, O’Brien KL, Carville A, Mansfield KG, Goudsmit J, Havenga MJ, Barouch DH. Comparative seroprevalence and immunogenicity of six rare serotype recombinant adenovirus vaccine vectors from subgroups B and D. J Virol. 2007;81:4654–63. doi: 10.1128/JVI.02696-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lemckert AA, Sumida SM, Holterman L, Vogels R, Truitt DM, Lynch DM, Nanda A, Ewald BA, Gorgone DA, Lifton MA, Goudsmit J, Havenga MJ, Barouch DH. Immunogenicity of heterologous prime-boost regimens involving recombinant adenovirus serotype 11 (Ad11) and Ad35 vaccine vectors in the presence of anti-ad5 immunity. J Virol. 2005;79:9694–701. doi: 10.1128/JVI.79.15.9694-9701.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu J, Ewald BA, Lynch DM, Denholtz M, Abbink P, Lemckert AA, Carville A, Mansfield KG, Havenga MJ, Goudsmit J, Barouch DH. Magnitude and phenotype of cellular immune responses elicited by recombinant adenovirus vectors and heterologous prime-boost regimens in rhesus monkeys. J Virol. 2008;82:4844–52. doi: 10.1128/JVI.02616-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Radosevic K, Rodriguez A, Lemckert AA, van der Meer M, Gillissen G, Warnar C, von Eyben R, Pau MG, Goudsmit J. The Th1 immune response to Plasmodium falciparum circumsporozoite protein is boosted by adenovirus vectors 35 and 26 with a homologous insert. Clin Vaccine Immunol. 2010;17:1687–94. doi: 10.1128/CVI.00311-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geisbert TW, Bailey M, Hensley L, Asiedu C, Geisbert J, Stanley D, Honko A, Johnson J, Mulangu S, Grazia Pau M, Custers J, Vellinga J, Hendriks J, Jahrling P, Roederer M, Goudsmit J, Koup R, Sullivan NJ. Recombinant Adenovirus Serotypes 26 and 35 Vaccine Vectors Bypass Immunity to Ad5 and Protect Nonhuman Primates Against Ebolavirus Challenge. J Virol. 2011 doi: 10.1128/JVI.02407-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horowitz M, editor. Adenoviridae and their replication. Raven Press; New York: 1990. pp. 1679–740. [Google Scholar]
- 18.Rowe WP, Hartley JW, Huebner RJ. Additional serotypes of the APC virus group. Proc Soc Exp Biol Med. 1956;91:260–2. doi: 10.3181/00379727-91-22231. [DOI] [PubMed] [Google Scholar]
- 19.Hillis WD, Garner AC, Hillis AI. A new simian adenovirus serologically related to human adenovirus type 2 and a chimpanzee with “viral hepatitis”. Am J Epidemiol. 1969;90:344–53. doi: 10.1093/oxfordjournals.aje.a121079. [DOI] [PubMed] [Google Scholar]
- 20.Rogers NG, Basnight M, Gibbs CJ, Gajdusek DC. Latent viruses in chimpanzees with experimental kuru. Nature. 1967;216:446–9. doi: 10.1038/216446a0. [DOI] [PubMed] [Google Scholar]
- 21.Basnight M, Jr., Rogers NG, Gibbs CJ, Jr., Gajdusek DC. Characterization of four new adenovirus serotypes isolated from chimpanzee tissue explants. Am J Epidemiol. 1971;94:166–71. doi: 10.1093/oxfordjournals.aje.a121308. [DOI] [PubMed] [Google Scholar]
- 22.Xiang Z, Li Y, Cun A, Yang W, Ellenberg S, Switzer WM, Kalish ML, Ertl HC. Chimpanzee adenovirus antibodies in humans, sub-Saharan Africa. Emerg Infect Dis. 2006;12:1596–9. doi: 10.3201/eid1210.060078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dudareva M, Andrews L, Gilbert SC, Bejon P, Marsh K, Mwacharo J, Kai O, Nicosia A, Hill AV. Prevalence of serum neutralizing antibodies against chimpanzee adenovirus 63 and human adenovirus 5 in Kenyan children, in the context of vaccine vector efficacy. Vaccine. 2009;27:3501–4. doi: 10.1016/j.vaccine.2009.03.080. [DOI] [PubMed] [Google Scholar]
- 24.Chen H, Xiang ZQ, Li Y, Kurupati RK, Jia B, Bian A, Zhou DM, Hutnick N, Yuan S, Gray C, Serwanga J, Auma B, Kaleebu P, Zhou X, Betts MR, Ertl HC. Adenovirus-based vaccines: comparison of vectors from three species of adenoviridae. J Virol. 2010;84:10522–32. doi: 10.1128/JVI.00450-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roy S, Vandenberghe LH, Kryazhimskiy S, Grant R, Calcedo R, Yuan X, Keough M, Sandhu A, Wang Q, Medina-Jaszek CA, Plotkin JB, Wilson JM. Isolation and characterization of adenoviruses persistently shed from the gastrointestinal tract of non-human primates. PLoS Pathog. 2009;5:e1000503. doi: 10.1371/journal.ppat.1000503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aste-Amezaga M, Bett AJ, Wang F, Casimiro DR, Antonello JM, Patel DK, Dell EC, Franlin LL, Dougherty NM, Bennett PS, Perry HC, Davies ME, Shiver JW, Keller PM, Yeager MD. Quantitative adenovirus neutralization assays based on the secreted alkaline phosphatase reporter gene: application in epidemiologic studies and in the design of adenovector vaccines. Hum Gene Ther. 2004;15:293–304. doi: 10.1089/104303404322886147. [DOI] [PubMed] [Google Scholar]
- 27.Capone S, Meola A, Ercole BB, Vitelli A, Pezzanera M, Ruggeri L, Davies ME, Tafi R, Santini C, Luzzago A, Fu TM, Bett A, Colloca S, Cortese R, Nicosia A, Folgori A. A novel adenovirus type 6 (Ad6)-based hepatitis C virus vector that overcomes preexisting anti-ad5 immunity and induces potent and broad cellular immune responses in rhesus macaques. J Virol. 2006;80:1688–99. doi: 10.1128/JVI.80.4.1688-1699.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Capone S, Zampaglione I, Vitelli A, Pezzanera M, Kierstead L, Burns J, Ruggeri L, Arcuri M, Cappelletti M, Meola A, Ercole BB, Tafi R, Santini C, Luzzago A, Fu TM, Colloca S, Ciliberto G, Cortese R, Nicosia A, Fattori E, Folgori A. Modulation of the immune response induced by gene electrotransfer of a hepatitis C virus DNA vaccine in nonhuman primates. J Immunol. 2006;177:7462–71. doi: 10.4049/jimmunol.177.10.7462. [DOI] [PubMed] [Google Scholar]
- 29.Folgori A, Capone S, Ruggeri L, Meola A, Sporeno E, Ercole BB, Pezzanera M, Tafi R, Arcuri M, Fattori E, Lahm A, Luzzago A, Vitelli A, Colloca S, Cortese R, Nicosia A. A T-cell HCV vaccine eliciting effective immunity against heterologous virus challenge in chimpanzees. Nat Med. 2006;12:190–7. doi: 10.1038/nm1353. [DOI] [PubMed] [Google Scholar]
- 30.Barnes EFA, Capone S, Huddart R, Smith K, Swadling L, Aston S, Brown A, Townsend R, Antrobus R, Ambrosio M, O’Hara G, Ammendola V, Bartiromo M, Naddeo M, Sparacino A, Siani L, Traboni C, Adams D, Hill A, Colloca S, Nicosia A, Cortese R, Klenerman P. Broad and sustained T cell responses to HCV induced by novel adenovirus-based vaccines in man. Submitted for publication. [DOI] [PMC free article] [PubMed]
- 31.Ewer KJOHGA, Duncan CJA, Collins KA, Sheehy S, Reyes-Sandoval A, Goodman AL, Edwards NJ, Elias SC, Halstead FD, Rowland R, Poulton ID, Draper SJ, Blagborough A, Berrie E, Moyle S, Alder N, Siani L, Folgori A, Colloca S, Sinden RE, Lawrie AM, Cortese R, Gilbert SC, Nicosia A, Hill AVS. Protective CD8+ T cell immunity to human malaria induced by chimpanzee adenovirus-MVA immunization. Submitted for publication. [DOI] [PMC free article] [PubMed]
- 32.O’Hara GADCJA, Ewer KJ, Collins KA, Elias SC, Halstead FD, Goodman AL, Edwwards NL, Reyes-Sandoval A, Bird P, Rowland R, Sheehy, Poulton ID, Hutchings C, Todryk S, Andrews L, Folgori A, Berrie E, Moyle S, Nicosia A, Colloca S, Cortese R, Siani L, Lawrie AM, Gilbert SC, Hill AVS. Clinical assessment of a recombinant simian adenovirus AdCh63: a potent new vaccine vector. Submitted for publication. [DOI] [PMC free article] [PubMed]
- 33. http://www.hvtn.org/meeting/ppt/may10/2/P3/DeRosa_HVTN077.pdf.
- 34.Liu W, Li Y, Learn GH, Rudicell RS, Robertson JD, Keele BF, Ndjango JB, Sanz CM, Morgan DB, Locatelli S, Gonder MK, Kranzusch PJ, Walsh PD, Delaporte E, Mpoudi-Ngole E, Georgiev AV, Muller MN, Shaw GM, Peeters M, Sharp PM, Rayner JC, Hahn BH. Origin of the human malaria parasite Plasmodium falciparum in gorillas. Nature. 2010;467:420–5. doi: 10.1038/nature09442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Capone S, Reyes-Sandoval A, Naddeo M, Siani L, Ammendola V, Rollier CS, Nicosia A, Colloca S, Cortese R, Folgori A, Hill AV. Immune responses against a liver-stage malaria antigen induced by simian adenoviral vector AdCh63 and MVA prime-boost immunisation in non-human primates. Vaccine. 2010;29:256–65. doi: 10.1016/j.vaccine.2010.10.041. [DOI] [PubMed] [Google Scholar]
- 36.Lindsay RW, Darrah PA, Quinn KM, Wille-Reece U, Mattei LM, Iwasaki A, Kasturi SP, Pulendran B, Gall JG, Spies AG, Seder RA. CD8+ T cell responses following replication-defective adenovirus serotype 5 immunization are dependent on CD11c+ dendritic cells but show redundancy in their requirement of TLR and nucleotide-binding oligomerization domain-like receptor signaling. J Immunol. 2010;185:1513–21. doi: 10.4049/jimmunol.1000338. [DOI] [PubMed] [Google Scholar]
- 37.Lore K, Adams WC, Havenga MJ, Precopio ML, Holterman L, Goudsmit J, Koup RA. Myeloid and plasmacytoid dendritic cells are susceptible to recombinant adenovirus vectors and stimulate polyfunctional memory T cell responses. J Immunol. 2007;179:1721–9. doi: 10.4049/jimmunol.179.3.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Todryk SM, Walther M, Bejon P, Hutchings C, Thompson FM, Urban BC, Porter DW, Hill AV. Multiple functions of human T cells generated by experimental malaria challenge. Eur J Immunol. 2009;39:3042–51. doi: 10.1002/eji.200939434. [DOI] [PubMed] [Google Scholar]
- 39.Zucchelli S, Capone S, Fattori E, Folgori A, Di Marco A, Casimiro D, Simon AJ, Laufer R, La Monica N, Cortese R, Nicosia A. Enhancing B- and T-cell immune response to a hepatitis C virus E2 DNA vaccine by intramuscular electrical gene transfer. J Virol. 2000;74:11598–607. doi: 10.1128/jvi.74.24.11598-11607.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dubey S, Clair J, Fu TM, Guan L, Long R, Mogg R, Anderson K, Collins KB, Gaunt C, Fernandez VR, Zhu L, Kierstead L, Thaler S, Gupta SB, Straus W, Mehrotra D, Tobery TW, Casimiro DR, Shiver JW. Detection of HIV vaccine-induced cell-mediated immunity in HIV-seronegative clinical trial participants using an optimized and validated enzyme-linked immunospot assay. J Acquir Immune Defic Syndr. 2007;45:20–7. doi: 10.1097/QAI.0b013e3180377b5b. [DOI] [PubMed] [Google Scholar]
- 41.Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–8. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 42.Felsenstein J. Inferring phylogenies from protein sequences by parsimony, distance, and likelihood methods. Methods Enzymol. 1996;266:418–27. doi: 10.1016/s0076-6879(96)66026-1. [DOI] [PubMed] [Google Scholar]
- 43.Rux AH, Moore WT, Lambris JD, Abrams WR, Peng C, Friedman HM, Cohen GH, Eisenberg RJ. Disulfide bond structure determination and biochemical analysis of glycoprotein C from herpes simplex virus. J Virol. 1996;70:5455–65. doi: 10.1128/jvi.70.8.5455-5465.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Evans RK, Zhu DM, Casimiro DR, Nawrocki DK, Mach H, Troutman RD, Tang A, Wu S, Chin S, Ahn C, Isopi LA, Williams DM, Xu Z, Shiver JW, Volkin DB. Characterization and biological evaluation of a microparticle adjuvant formulation for plasmid DNA vaccines. J Pharm Sci. 2004;93:1924–39. doi: 10.1002/jps.20112. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig.1 legend. Representative dose/response experiments with PanAdgag vectors. Groups of five BALB/c mice were immunized with escalating doses (indicated at the bottom of the graph) of PanAd1, PanAd2 or PanAd3 encoding HIV-1 gag and sacrificed three weeks later. Splenocyte IFNγ ELISpot responses to the CD8+ peptide AMQMLKETI are reported on the vertical axis and are expressed as IFNγ SFC per 106 splenocytes. Symbols correspond to individual mice responses, subtracted of the DMSO background (tipically not higher than 10 SFC/million spenocytes). Horizontal lines represent geometric mean of each group. A dashed line set at 50 SFC shows cut off to define a positive response. The immunological potency of each vector is defined as the minimal dose at which at least two out of five mice show a positive response, resulting in 106 vp for PanAd3 and 5×106 vp for PanAd1 and PanAd2, as reported in Figure 1 panel C.