Abstract
Currently no vaccine exists for hepatitis C virus (HCV), a major pathogen thought to infect 170 million people globally. Many studies suggest that host T cell responses are critical for spontaneous resolution of disease, and preclinical studies have indicated a requirement for T cells in protection against challenge. We aimed to elicit HCV-specific T cells with the potential for protection using a recombinant adenoviral vector strategy in a Phase I study of healthy human volunteers. Two adenoviral vectors expressing NS proteins from HCV genotype 1B were constructed based on rare serotypes (Human Adenovirus 6 (Ad6) and Chimpanzee Adenovirus 3 (ChAd3)). Both vectors primed T cell responses against HCV proteins; these T cell responses targeted multiple proteins and were capable of recognizing heterologous strains (genotypes 1A and 3A). HCV-specific T cells consisted of both CD4+ and CD8+ T cells subsets, secreted IL-2, IFNγ, and TNFα, and could be sustained for at least a year after boosting with the heterologous adenoviral vector. Studies using MHC peptide tetramers revealed long-lived central and effector memory pools that retained polyfunctionality and proliferative capacity. These data indicate that an adenoviral vector strategy can induce sustained T cell responses of a magnitude and quality associated with protective immunity, and open the way for studies of prophylactic and therapeutic vaccines for HCV.
Introduction
Hepatitis C virus (HCV) is a major cause of liver disease globally. The virus is readily able to set up persistent infection in immunocompetent hosts, leading to chronic liver inflammation, cirrhosis, liver failure, and liver cancer1. Current treatments, although improving rapidly, are costly, imperfect, and associated with major side-effects. A vaccine to prevent chronic infection would be a major step forward.
HCV may be spontaneously controlled in a proportion of those infected. Many studies of the host genetics and immunology demonstrate an important role for T cells in protective immunity against HCV2-5. Although there is no single correlate of immune protection, many studies have indicated that CD4+ and CD8+ T cell responses that are broadly directed (for example target multiple viral antigens), functional (for example produce interferon-gamma (IFNγ) and maintain proliferative capacity) and sustained over time are linked to virologic control2,3,6. In chimpanzee models, depletion of either CD4+ or CD8+ T cell cells in vivo abrogates protective immunity induced by prior HCV exposure4,5. Although other mechanisms contribute to naturally induced host defence, including innate responses and neutralizing antibodies7,8, these data suggest that induction of robust T cell responses through vaccination could provide effective immune control of acute HCV infection.
This hypothesis has been tested in the chimpanzee challenge model by Folgori and colleagues using a target immunogen spanning the non-structural genes NS3-NS5B from HCV genotype 1B, a region that contains many well defined CD4+ and CD8+ epitopes, delivered in the form of recombinant, replication-deficient human adenovirus constructs9. In the vaccinated animals, strong CD8+ and CD4+ T cell responses were induced, and upon challenge, the brisk anamnestic response was linked with viral control in four out of five animals. Thus vaccination protocols that induce broad, sustained, and functional T cell responses may protect against persistent infection by limiting early viral replication upon challenge.
To test whether such an approach could be successful in man, we used two adenoviral vectors based on rare serotypes to induce T cell responses against HCV in healthy volunteers. Virally vectored vaccines have been used in many approaches to protect against infection. Amongst these, adenoviral vectors have shown superior capacity to prime immune responses compared to approaches such as modified vaccinia Ankara (MVA)10. Adenoviral vectors however suffer from the limitation that adenoviral infection in man is common and pre-existing high titre neutralizing antibodies may interfere with the vaccine efficacy: The use of rare serotypes may overcome this limitation. We therefore used vectors based on Ad6, a virus with a seroprevalence of about 22%, and chimpanzee adenovirus 3 (ChAd3), which is serologically distinct and has a seroprevalence around 12%11. We tested the safety and potency of such vaccines singly and in combination as a prime boost regimen.
Our data show good safety profiles of vaccination regimes associated with priming of CD8+ and CD4+ T cell responses by both vaccines, targeting multiple antigen regions and sustained up to 1 year. These responses mimic those associated with protection in natural infection. This approach shows promise for T cell-based vaccination against HCV.
Results
Safety
Overall we found the administration of vaccine to be safe and well tolerated. Mild local and systemic side effects, comparable to those previously reported with other adenoviral vectors, were observed. These increased with dose but were short-lived and did not differ significantly between the two vectors or between priming and boosting (Fig S1). No serious adverse events occurred. The overall study design is described in Table S1.
Ad6-NSmut- and ChAd3-NSmut-primed T cell responses in healthy volunteers
We first assessed the immunogenicity of the priming regimens using escalating doses of each vector in groups of 4-5 healthy donors. Each vaccine was administered twice at 0 and 4 weeks.
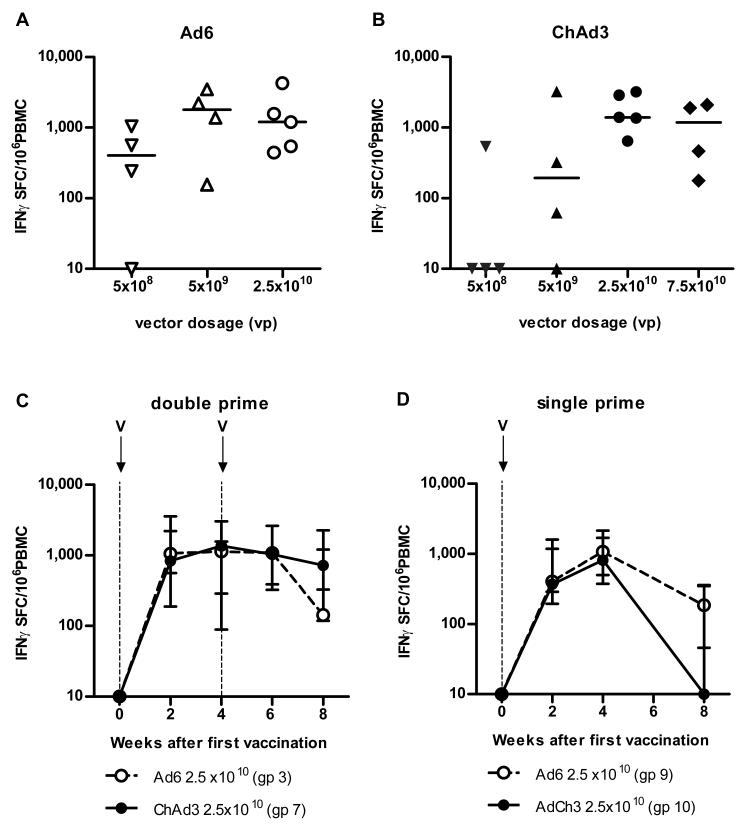
IFNγ ELISpot studies during dose escalation revealed responses detectable over the first 24 weeks in all groups, with 3/4, 4/4 and 5/5 responding in the Ad6-NSmut primed groups and 1/4, 3/4, and 5/5 individuals showing responses after low, medium, and high dose regimens using the comparable ChAd3-NSmut vector. Figs 1A and 1B shows the peak magnitude of these responses in the groups by individual volunteers. The peak response was seen at week 2-6 after prime, depending on the individual: In the high dose groups the peak responses were detectable at a median >1,000 IFNγ Spot Forming Cells (SFC)/106 PBMC (Ad6-NSmut median= 1202 range 443-4263; ChAd3-NSmut median =1400 range 642-3210).
Figure 1. Magnitude of T cell responses primed after vaccination with Ad6-NSmut or ChAd3-NSmut.
(A, B) Peak total IFNγ ELISpot response ex vivo: ELISpot data from individuals vaccinated with (A) Ad6-NSmut (open symbols) groups 1-3 (dose escalation 5 × 108-2.5 ×1010vp) and (B) ChAd3-NSmut (shaded symbols) groups 5-7 (dose escalation 5 × 108-7.5 ×1010vp). Vaccine dose is given as virus particles (vp). The responses shown are the total positive IFNγ ELISpot response across all pools (see methods) measured at peak response after prime (weeks 2-8) (Bars=median).
(C, D) Single vs double priming: The kinetics of the priming responses after (C) double prime (groups 3 and 7) and (D) single prime (groups 9 and 10) at high vaccine dose (2.5 ×1010 vp). Group medians and IQR over time are shown. Open circles indicate Ad6-NSmut priming and closed symbols ChAd3-NSmut priming.
We also enrolled a further arm in which ChAd3-NSmut was administered at a higher dose (ChAd3-NSmut 7.5 ×1010 viral particles, vp). However, this group did not show significantly enhanced priming than the previous highest dose (Fig 1B), with some modest increases in local reactions reactogenicity, so overall an optimized tolerated dose of 2.5 ×1010 vp was achieved.
We also compared the two-dose priming regimen, (dosing at weeks 0 and 4), with a single dose at 2.5 × 1010 vp (Fig 1C and D). The latter primed responses to similar levels as the two dose priming regimen for both vectors (peak Ad6-NSmut median 1173, range 245-3148, ChAd3-NSmut median 890, range 300-2488 (SFC)/106 PBMC between weeks 2-4). The levels of response at later timepoints in the single dose primed groups were lower than those in the double-primed groups. (p=0.02 at week 8), although kinetics were similar.
Overall these data indicate consistent priming using these vectors, optimized at a dose of 2.5 ×1010 vp in both cases. For comparison, although there is no defined cut-off for a protective response, observational studies of the responses seen in individuals who acutely clear virus are typically in the region of hundreds of SFC/million PBMC 2,3,6,9,12, remaining detectable for many years3.
Broad T cell responses from Ad6-NSmut and ChAd3-NSmut priming in healthy volunteers
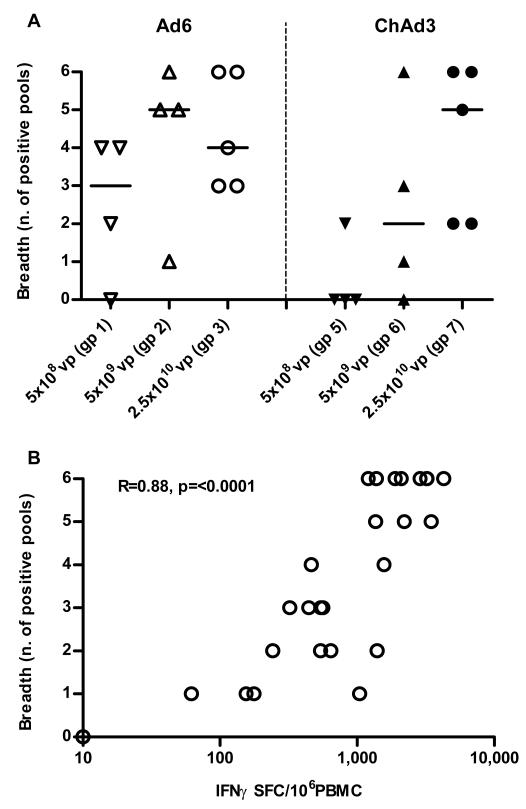
The breadth of responses is reproducibly associated with control of virus in human correlative studies2,3,12. Responses were assessed using peptides arranged into 6 pools corresponding to the viral gene products reproduced in the vaccine insert (pools F/G=NS3, pool H=NS4A/B, pool I=NS5A, pools L/M=NS5B). We assessed response breadth by analysing how many pools elicited significant IFNγ reactivity in the ELISpot. Across groups, response breadth increased with magnitude as vaccine dose was escalated (Fig 2A); indeed the two measures were correlated (r=0.88, p<0.0001; Fig 2B).
Figure 2. Breadth of T cell responses primed after vaccination with Ad6-NSmut or ChAd3-NSmut.
(A). Breadth of primed response ex vivo: ELISpot data from individuals vaccinated with Ad6-NSmut (open symbols) groups 1-3 (dose escalation 5 × 108-2.5 ×1010vp) and ChAd3-NSmut (shaded symbols) groups 5-7 (dose escalation 5 × 108-2.5 ×1010vp). The responses shown are the number of positive pools (see methods) measured at peak magnitude after prime (weeks 2-8) (Bars=median).
(B). Correlation of breadth and magnitude: the number of positive pools (from Fig. 2A) compared with the magnitude of the ELISpot response (from Fig. 1A and 1B) are plotted.
We analyzed the 4 groups (3, 7 9 and 10) receiving the dose of 2.5 × 1010 vp of either vector up to week 4 and observed a median number of 5 pools recognized, with 5 donors recognizing all 6 pools.
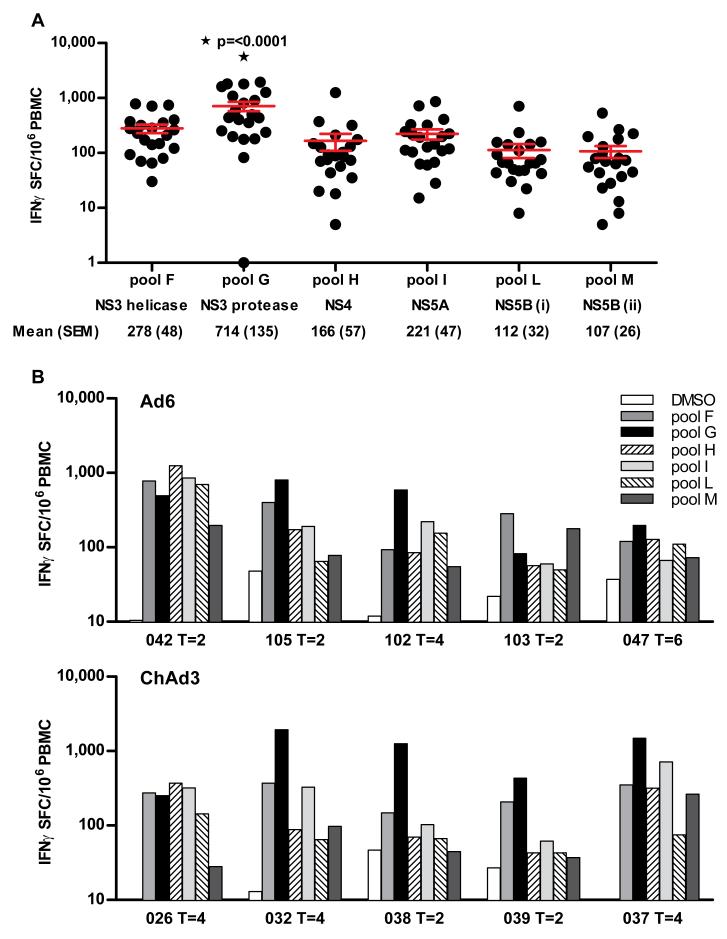
Amongst these 4 comparable groups, we assessed the specificity of the primed response. Although all pools were targeted, pool G (NS3) was immunodominant (Fig 3A)(p=<0.0001). Individual volunteer data (Fig 3B) showing the specificity at peak magnitude after priming with Ad6-NSmut and ChAd3-NSmut at 2.5 × 1010 vp is shown.
Figure 3. Specificity of T cell responses primed after vaccination with Ad6-NSmut or ChAd3-NSmut.
(A) Immunodominance of primed responses: Data are taken from the IFNγ ELISpot responses from high dose groups (3, 9; Ad6-NSmut 2.5 ×1010 vp and 7, 10; ChAd3 2.5 ×1010vp) at peak magnitude after prime. The magnitude of the HCV-specific T cell response to individual peptide pools (F-M), and the corresponding NS protein below is shown (Bars=mean +/− SEM, ▯=statistical significance p=0.0001).
(B) Individual volunteer data showing targeted pools after priming with Ad6-NSmut 2.5 ×1010 vp group 3 (upper panel) and ChAd3-NSmut 2.5 ×1010vp group 7 (lower panel) is shown.
Ad6-NSmut- and ChAd3-NSmut-primed functional CD4+ and CD8+ T cell responses in healthy volunteers
We next analyzed the functionality of the induced T cell responses and the contribution of CD4+ and CD8+ T cells. First, we analyzed CD4+ T cell proliferative responses against HCV recombinant proteins using 3H incorporation assays, comparable to those described in previous studies of natural protection13,14. We detected responses to multiple HCV proteins in the majority of volunteers in the high dose groups (Fig S2A). Reactivity was detectable against all antigens although maximal against HCV NS3-derived antigens (Fig S2B; NS3 and NS3-derived helicase; p<0.0002)
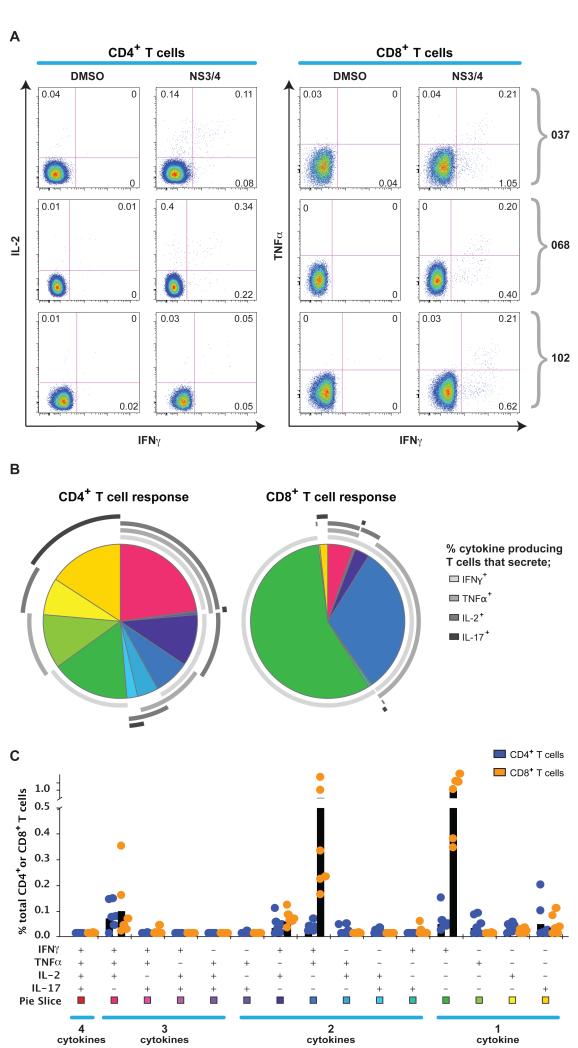
We also analyzed the functionality of the antiviral response induced by the vaccines using intracellular cytokine staining (ICS) for IFNγ, (tumour necrosis Factor-alpha) TNFα, interleukin (IL)-2 and IL-17. Responses were elicited using 2 pools of peptides (F+G+H =NS3/4, I+L+M=NS5A/B)(Fig 4). For both CD4+ and CD8+ T cell responses, we observed cytokine production, including IL-2, TNFα and IFNγ. For both vectors, the predominant population primed during the peak response were CD8+ T cells (Fig 4 and Fig S3). To assess whether cells were making a polyfunctional response, we analyzed the production of single and multiple cytokines in the primed responses. We observed populations with joint IFNγ/TNFα or IFNγ/IL2 production and co-production of all 3 cytokines; this was more balanced in the CD4+ T cell populations than the CD8+ T cell populations, where IFNγ single or IFNγ/TNFα double producing populations were more dominant. Fig 4 shows data from high dose groups (Ad6-NSmut and ChAd3-NSmut) and Fig S3 results from the dose titration. There were no significant differences between responses elicited by Ad6-NSmut compared to ChAd3-NSmut. We also analyzed IL17A secretion because of recent data on function of liver homing T cell populations15, but minimal response was seen.
Figure 4. Functionality of T cell responses primed after vaccination with Ad6-NSmut or ChAd3-NSmut.
A: Example FACS plots after Intracellular Cytokine Staining (ICS): Staining for IFNγ/IL-2, and IFNγ/TNF are shown for CD4+ and CD8+ T cell responses respectively, after stimulation with F+G+H (NS3/4) pooled peptides (see methods) in three donors (037 gp.7, 068 gp9, and 102 gp3; weeks 4-6). Plots are gated on live, CD3+, CD4+ or CD8+ T cell populations.
B and C: Group data for ICS after priming. SPICE analysis for combined ICS data (after stimulation with NS3/4 peptides) for CD8+ and CD4+ T cell responses after priming (2-4 weeks) from 6 volunteers each in high dose groups (3, 7, 9 and 10; responses >0.05%). The pie charts (B) represents the proportion of cytokine secreting cells that produce one, two, three or four cytokines (IFNγ, TNFα, IL17 and/or IL-2). CD4+ and CD8+ T cells are shown on left and right plots respectively. The graph in panel C shows individual cytokines produced alone and in combination, as % of total CD4+ and CD8+ T cells. Each dot (orange= CD8+, green=CD4+ T cells) represents responses in an individual. Black bars = mean response.
Cross-reactive T cell responses from Ad6-NSmut and ChAd3-NSmut priming
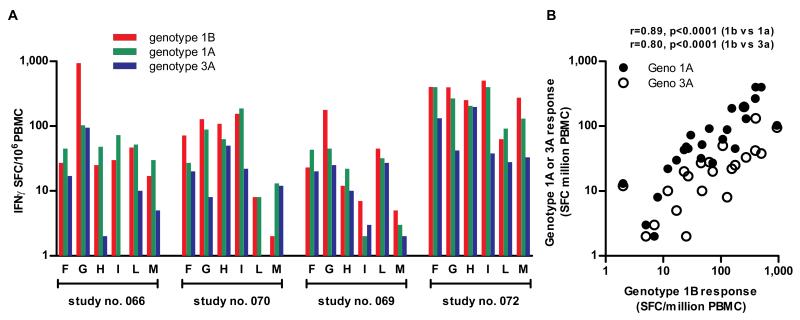
To assess whether the responses were cross-reactive against other HCV genotypes we performed ELISpots using peptide pools representing consensus sequences from genotypes 1B (vaccine immunogen), 1A and 3A. The latter is common in European intravenous drug using (IVDU) populations and is highly divergent from the vaccine sequence. This approach revealed a close relationship between the response to the priming genotype 1B, and the heterologous response (r=0.89, p<0.0001 1B compared with 1A, and r=0.80, p<0.0001 1B compared with 3A; Fig 5). Overall the response to genotype 1A was approximately half that of the response to genotype 1B, and that of genotype 3A approximately a fifth, still significantly above the response thresholds set using negative controls.
Figure 5. Cross-reactivity of T cell responses induced after vaccination with Ad6-NSmut or ChAd3-NSmut.
(A); Cross-reactivity measured by IFNγ ELISpot response ex vivo against Genotype 1B with Genotype 1A and 3A peptide pools (F-M) in volunteers from group 11 (7.5×1010 vp ChAd3-NSmut) tested at week 4.
(B); Correlation of IFNγ ELISpot response ex vivo against Genotype 1B with Genotype 1A and 3A peptide pools: ELISpot data from individuals in group 11 (7.5×1010 vp ChAd3-NSmut) tested at week 4. Each symbol represents a single pool response in a single volunteer.
Ad6-NSmut and ChAd3-NSmut as heterologous boosting vectors
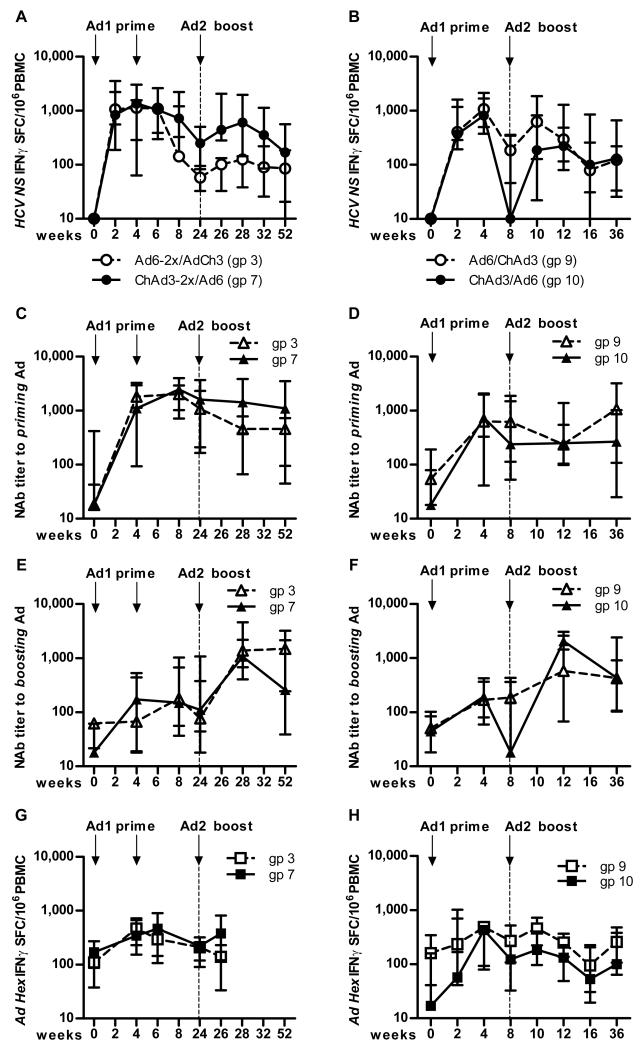
We next assessed the ability of the adenoviral vectors to boost in a heterologous manner–groups primed with ChAd3-NSmut vector were boosted with Ad6-NSmut vector (all at 2.5 × 1010 vp) and vice versa. Fig 6A shows the boosting from the two high dose double priming groups (groups 3 and 7). This figure demonstrates two features: First, there was some boosting of T cell frequencies seen compared to before boost (week 24), although the overall magnitude did not exceed that seen at priming. Second, the group primed with ChAd3-NSmut vector boosted better than the group primed with Ad6-NSmut: This was associated with a slightly higher number of pre-boost T cells in the former group. Similar observations were made in the single-primed groups (9 and 10; Fig 6B). Fig S4 shows boosting data from the low and medium prime dose groups. Across all dose-escalation groups a correlation was seen between the number of primed T cells and the number of boosted T cells (r=0.7 p<0.001; Fig S5).
Figure 6. Boosting of T cell responses primed after vaccination with Ad6-NSmut or ChAd3-NSmut.
(A and B). Boosting of primed responses: A. Group data from IFNγ ELISpot responses in high dose double prime groups (3; Ad6-NSmut 2.5 ×1010 vp and 7; ChAd3-NSmut 2.5 ×1010 vp) over time. The responses shown are the total positive IFNγ ELISpot response across all pools (see methods) presented as group medians and IQR. Open symbols indicate Ad6-NSmut priming (ChAd3-NSmut boost) and shaded symbols ChAd3-NSmut priming (Ad6-NSmut boost). B. Similar data for groups 9 and 10 (single prime groups)
(C-F) Neutralizing antibody titers (Nab): Homologous (against priming vector) (C, D) and heterologous (against boosting vector) (E, F) NAb titers are shown presented as group medians and IQR: Open symbols indicate Ad6-NSmut priming (ChAd3-NSmut boost) and shaded symbols ChAd3-NSmut priming (Ad6-NSmut boost). C and E show double-primed groups 3 and 7; D and F show single primed groups 9 and 10.
(G and H) Adenovirus specific responses after priming and boosting: Group data from IFNγ ELISpot responses in high dose groups (G double prime, and H single prime groups) over time. The responses shown are the total positive IFNγ ELISpot response across to Ad5 Hexon peptides (see methods) presented as group medians and IQR. Open symbols indicate Ad6-NSmut priming (ChAd3-NSmut boost) and shaded symbols ChAd3-NSmut priming (Ad6-NSmut boost).
These data raised the possibility that pre-existing anti-vector antibodies may limit boosting of both HCV-specific and adenovirus-specific T cells. We evaluated this by measuring neutralizing antibody (NAb) titres against Ad6-NSmut and ChAd3-NSmut. Interestingly, in both cases we found substantial levels of NAbs (titre >200) to both the homologous (Fig 6C and D), and more importantly to the heterologous vector (Fig 6E and F), although this did not differ substantially between the groups. These data support the idea that NAbs against the boosting vector may play some role in limiting the overall boosting effect: The differential impact on the order of the regimens is not fully elucidated but may potentially reflect the different susceptibility of the adenoviral vectors to neutralization in vivo.
We also assessed the adenovirus-specific IFNγ ELISpot responses in these groups using peptides representing the major adenoviral capsid Hexon protein. Adenovirus-specific T cell responses were present at baseline and increased substantially after vaccination. Adenovirus-specific T cell responses did not associate with reduction in primed or boosted HCV-specific responses; indeed, there was a positive correlation between the boosting of Adenovirus-specific responses and HCV-specific responses (Fig 6G and H).
Overall, we conclude the boosting of responses after heterologous adenovirus/adenovirus vaccination is possible and correlates with the level of T cell priming. However, it may be blunted by anti-vector immunity, likely mediated by cross-reactive NAbs, at least up to 24 weeks after priming.
Tracking peptide-specific T cell responses
The analysis of peptide specific responses was initiated by repeat testing of the PBMCs by ELISpot using smaller pools of peptides (“minipools”) (Table S2). In HLA-A2+ donors the dominant minipool was found to contain a previously described immunodominant peptide (NS3 A2 1406-15 KLSGLGINAV), and in HLA-A1+ donors a second pool contained a similarly immunodominant response (NS3 A1 ATDALMTGY).
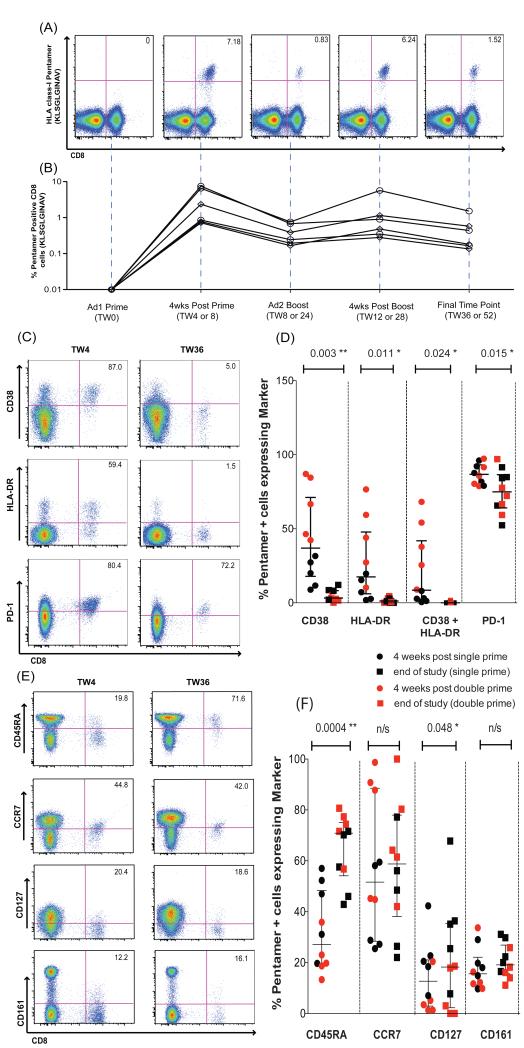
To track responses to these peptides, fluorescent HLA-peptide multimers were constructed and the frequency, phenotype, and function of peptide-specific populations were analyzed by flow cytometry. These data are summarized in Fig 7, which shows representative FACS plots of the NS3 A2 1406 response detected by multimer staining (Fig 7A). Responses of up to 7% of the total CD8+ T cell response were readily detectable and persisted over a year (6 months after boost; Fig 7B).
Figure 7. Characterization of epitope-specific T cell responses induced after vaccination with Ad6-NSmut or ChAd3-NSmut.
(A) Staining with tetramer A2-HCV-1406: KLSGLGINAV, in a representative volunteer (volunteer 60 gp 10) over the study time course. Gating is on live CD3+ (see methods). % pentamer+/CD8+ T cells is shown.
(B) Ex vivo tetramer + CD8+ T cell responses over time in 6 volunteers who had an ELISpot response to pool Gb that contains the A2 1406 peptide (Table S2 volunteers 32, 38, 105, 60, 64, and 68). Each received high dose (2.5 × 1010vp) ChAd3-NSmut or Ad6-NSmut.
The Activation (C and D) and memory differentiation status. (E and F) of pentamer + cells (gated on CD8+ T cells). FACS plots (C and E) show a representative volunteer (060 gp 10) at weeks 4 and 36 after ChAd3-NSmut prime (week 0) and Ad6-NSmut boost (week 8) (both 2.5 × 1010vp). % given is the proportion of tetramer + cells expressing the phenotypic y legend characteristic. The graphs (D and F) show % pentamer + cells (HLA-A2-HCV-1406 KLSGLGINAV, and HLA-A1-HCV-1435 ATDALMTY) in 9 individuals (HLA-A2; 032, 038, 060, 064, 068, 105 and HLA-A1; 19, 37, 66, 68) expressing the phenotypic markers given in the x axis, 4 weeks after single prime (red circles) or 4 weeks after second prime (black circles) and at the end of the study (week 52 for double prime groups and week 36 for single prime groups) (squares; red in the single prime and black in the double prime group) after ChAd3-NSmut prime (week 0) and Ad6-NSmut boost (week 8) (both 2.5 × 1010vp). Gates were set using the tetramer negative cell population as a reference.
Activation (CD38/HLA-DR) was only noted at the very earliest timepoints after the first priming vaccine, and remained low thereafter (Fig 7C/D and Fig S6B). The cells also expressed PD-1, a molecule that has been associated with both activation and exhaustion in persistent virus infection16, although the PD-1 levels declined over time in a manner analogous to that seen in acute resolving HCV infection17 (Fig 7C/D) and this was not associated with any clear dysfunction.
The responses had a mixed effector/central memory phenotype, which was sustained over time (Fig 7E/F); CD127, a molecule associated with long term stable memory was present on a proportion of the cells, with a significant increase over time (p<0.05). The cells included CD45RA−CCR7+ CD45RA−CCR7−, CD45RA+CCR7− and CD45RA+CCR7+ subsets, thus encompassing both effector and central memory populations (Fig S7). A stable proportion of the cells also expressed CD161 (15-20%); this molecule has also been observed as enriched on HCV-specific CD8+ T cells and its expression is linked to liver homing populations15 The cells appeared to contain cytolytic effector molecules, with high levels of granzymes. A and B, and variable levels of perforin (Fig S8).
Further analysis, using single peptide stimulations In ICS assays, showed that CD8+ T cell populations maintained strong effector function, with secretion of IFNγ, TNFα and MIP1β, together with degranulation (CD107a). Some IL-2 secretion was noted (Fig S8). Polyfunctionality was assessed as previously and was maintained at high levels (Figs S9). They also showed maintained proliferative capacity, being able to expand in response to boost vaccination in vivo (Fig S6) as well as to peptide in vitro (Fig S10).
These analyses confirm that epitope-specific memory populations maintained at high frequencies with a range of relevant antiviral effector functions
Discussion
We report the first trial of a T cell-based preventive vaccine for HCV. The approach taken was to test two vectors based on replication defective adenoviruses of rare serotypes. These were well tolerated, with a good safety profile. They were also highly immunogenic, with induction of CD4+ and CD8+ T cell responses targeting a wide range of antigens.
There were a number of important questions relating to a prophylactic vaccine. First, the responses need to be of sufficient magnitude to provide potential protection against persistent HCV infection. Our vectors were potent at priming responses, which often exceeded 1,000 SFC/million PBMC, and responses were readily detected at 1 year by ELISpot. Multimer staining revealed readily detectable and functional responses at 1 year, indicative of a long-term memory population.
Second, the issue of response breadth is very important for HCV. Responses found in chronic infection are typically both low in magnitude and narrowly focused on a limited number of antigens or epitopes facilitating viral escape. We observed an increase in the breadth of the response linked to increasing size of the response. Because of the diversity of viral strains even within a single genotype or subtype, such breadth improves the chances of peptide recognition of the incoming strain, as well as limiting further escape in vivo.
The third important feature is the generation of both CD4+ as well as CD8+ T cell responses. It is known that strong CD4+ T cell responses are required for induction and maintenance of functional CD8+ T cell memory, and in HCV it is clear that CD4+ T cell responses play a central role in host defence4. We observed CD4+ T cell responses by intracellular staining, although as expected these were at lower frequencies than the corresponding CD8+ T cell responses. We also analyzed proliferation, using recombinant protein antigens, an assay that is a correlate of protective immunity13. We observed strong and sustained CD4+ T cell responses targeting multiple antigens and comparable to those induced by natural infection and resolution.
Fourth, we assessed whether primed responses could target heterologous strains. The data here indicate that cross-strain recognition is possible, although of lower magnitude. To what extent a decrease in the T cell frequency would be associated with a decline in protection against challenge is not known. It is known that in natural infection the immune selection pressure against genotype 1 and genotype 3 are almost completely distinct, suggesting there may be many epitopes that are not shared and alternative vaccine approaches may be required18.
In terms of functionality of CD8+ T cells, we observed strong secretion of IFNγ and TNFα and sustained although less dominant secretion of IL-2, which is associated with “polyfunctional” populations linked to long term host defence19. The assays using peptide-specific analysis showed similar features of polyfunctionality, including degranulation (CD107a) and secretion of MIP1β, although direct cytotoxicity against infected hepatocytes is yet to be demonstrated. Similar cytokine secretion was demonstrated for CD4+ T cell populations.
The analysis of phenotype using Class I tetramers showed a distinct phenotype. Responses showed markers of activation (CD38, HLA-DR) at the earliest timepoints, and increasing levels of CD127 over time, consistent with emergence of at least a fraction of the cells as a long-term memory population20. Although we saw increasing levels of CCR7, associated with “central” memory, there was a mixture over time of “central” and “effector” memory pools observed including CD45RA+ (both CCR7+ and CCR7-TEMRA) cells. The overall functional significance of this balance is not known. In HIV we have observed an association between TEMRA frequencies and protection in acute HIV infection21 and recent data in models of protection against SIV suggests induction of long-lived effector memory pools by CMV vectors can show a very high degree of protection22. Importantly, the peptide specific cells demonstrated proliferative capacity both in vivo and in vitro, analogous to cells of a similar phenotype primed after Yellow Fever vaccination23.
In the face of priming of such strong and functional responses, even greater boosting might have been expected, but this effect was relatively modest. The limitations on the post-boosting peak were not fully defined but NAbs raised against the heterologous vector may limit host exposure to antigen. This was not the case seen in preclinical models11 but in experimental animals there is not extensive prior exposure to diverse adenoviral serotypes as in the volunteers. Heterologous NAbs were raised by both vectors, despite low pre-existing levels of antibody (a result of the screening procedure). The blunting of the ChAd3-NSmut boost (after Ad6-NSmut priming) was more striking in this trial than of the Ad6-NSmut boost, and because ChAd3-NSmut appears to be an excellent priming vector, this seems the appropriate priming choice for future studies. A single priming regimen may be sufficient, especially if a substantial boost can be obtained with different vectors.
Adenovirus/adenovirus prime boost regimes may give more striking boosts using alternative vector combinations. First, anti-vector T cells – which may be highly cross-reactive - did not seem to interfere with priming or boosting. Second, the adenoviral vectors used in this study belong to the same genetic subgroup11 where serologic cross-reactivity may remain more of a problem; use of adenovirus vectors from different genetic subgroups may provide a solution to this problem. As an alternative, a completely different boosting vector can be used and a trial to combine ChAd3-NSmut priming with the same insert in a modified vaccinia Ankara (MVA) construct is now underway (EUDRACT 2009- 018260-10).
The future development of a prophylactic vaccine will hinge not only on identifying the optimal priming/boosting regimen, but also identifying a suitable high risk cohort where such a strategy can be assessed in phase II clinical trials. Such cohorts have been identified in the USA, Canada, Australia, and the UK24. In the USA the predominant genotype in such groups is 1, and this will provide a crucial test of the success of this approach.
This vaccine may also be relevant to therapeutic strategies, potentially combined with other antiviral strategies. To what extent priming or boosting can be achieved in chronic HCV infection is now being assessed in an ongoing clinical study (EudraCT N. 2008-006127-32). Other therapeutic approaches using adjuvanted peptides (IC41)25 and and MVA-NS3/4A/5B vector (TG040)26 have induced T cell responses in the order of 100-500 SFC/million cells, associated with transient declines in viral load.
The ability to produce a vaccine is hampered by the huge diversity of HCV, its capacity to escape and to downregulate T cell immunity. However, a substantial fraction of those infected are able to control the virus spontaneously, a feature that makes this effort distinct from HIV vaccines. What such a vaccine should optimally achieve is to accelerate the generation of immunity (which may include NAbs) after exposure and to enhance the chances of clearance 8. Overall this work has shown it is possible to generate very strong, broad, long-lasting, and functional T cell responses against HCV in healthy donors using an adenovirus-based approach. The next critical step will be to test whether these populations can be protective in vivo, such as in a setting where HCV exposure is common, and therefore whether this or related strategies can provide an effective vaccine against HCV.
Materials and Methods
Vaccination protocols
The Ad6 and ChAd3 vectors encoding the NS3-5B region of genotype 1B (Ad6-NSmut, based on sequence accession number M58335) have been described previously9,27. The vaccine study was registered as clinical trial EudraCT N. 2007-004259-12 and with the ClinicalTrial.gov database (ID: NCT01070407). All volunteers gave written informed consent prior to participation and the studies were conducted according to the principles of the Declaration of Helsinki and in accordance with Good Clinical Practice (GCP). Volunteers were recruited at sites in Oxford and Birmingham UK. Those with pre-existing neutralizing titres against Ad6-NSmut or ChAd3-NSmut >200 were excluded. Vaccines were administered intramuscularly and the volunteer group protocols are described in Table S1.
Peptides and antigens
A set of 494 peptides 15 amino-acids (aa) in length, overlapping by 11 amino-acids and spanning the ORF from NS3-NS5B (1985) of HCV genotype 1b strain BK, were obtained from BEI resources. Peptides were initially dissolved in dimethyl sulfoxide (DMSO) and arranged into six pools (mean 82, range 73-112 peptides/pool) as indicated in Fig S11. Pools were used at a final concentration of 3μg/ml or 1μg/ml (each single peptide) in ELISpot and Intracellular staining, respectively. For cross-reactivity experiments, similar peptide pools derived from HCV genotype 1A (H77 strain) and genotype 3A (Genbank accession D28917) were also obtained and prepared identically. PepTivator-AdV5 Hexon pool (Miltenyi Biotec) was used at 1μg/ml final concentration.
ELISpot assays
Ex vivo IFNγ ELISpot assays were performed according to manufacturers’ instructions (Mabtech) on freshly isolated PBMC plated in triplicate at 2×105 PBMC per well. To determine robust cut-offs, we screened 74 healthy HCV seronegative volunteers and established mean and SD of responses to HCV genotype 1B pools (Fig S12). For a positive response; (i) the mean of antigen wells minus background was determined to be greater than 48 SFC(spot forming cells)/106 PBMC (mean + 3 SD) and (ii) to exceed 3× background. Background wells (medium only, cells + DMSO) were typically 0-4 spots. Internal positive controls included Concanavalin (Con) A, FEC (mixed HLA class –I restricted peptides from Flu, EBV and CMV) and CMV lysate. Total NS response was calculated by summing responses to all positive pools.
Proliferation assays
Ex vivo proliferation assays were performed on freshly isolated PBMC plated in triplicate at 2×105 PBMC per well using conventional Thymidine H3 incorporation methods and antigens (1μg/ml) as indicated in Fig S11 (Mikrogen). Data are displayed as SI (Stimulation Index; fold change above background). A positive response is defined as SI ≥3.
Ad6-NSmut and ChAd3-NSmut neutralizing antibody assays
Briefly, 3.5×104 HEK293 cells per well were seeded in a 96 well plate for 2 days. Each SEAP-expressing adenoviral vector28, incubated for 1h at 37°C alone or with serial dilutions of serum from trial volunteers, was then added to the 95-100% confluent HEK293 cells, incubated for 1h and washed. SEAP expression was measured 24h later using the chemiluminescent substrate (CSPD), from the Phospha-LightTM kit (Tropix Cat No T1016) without heat inactivation. Light emission (relative light units [RLU]) was monitored 45 min after the addition of the CSPD substrate using the Envision 2102 Multi-label reader (Perkin Elmer).
Intracellular cytokine stains (ICS)
Thawed PBMCs were stimulated using peptides in pool combinations (F+G+H=NS3/4, I+L+M=NS5A/B) or unstimulated (controlled for DMSO) or PMA/Ionomycin (50 and 500 ng/ml respectively). After overnight stimulation (Brefeldin A was added after 1 hr at 10μg/ml), cells were permeabilized (BD perm) and stained using the following antibodies: CD3-PO, CD4-Qdot 605, CD8-PerCP Cy5.5, IFNγ-AlexaFluor700, IL2-APC, TNFα-PE-Cy7, IL17-PE. Flow cytometry was performed using a BD LSRII and analysis by FlowJo (TreeStar). Analysis of polyfunctionality was performed using SPICE
Tetramer staining, short term cell lines and flow cytometry
For tetramer staining, PE-labeled pentamers loaded with HCV NS3 1406 (KLSALGINAV; HLA- A*0201) and HCV NS3 (ATDALMTGY, HLA-A*0101) were obtained from ProImmune. The cells were co-stained with combinations of the following antibodies: CD3-PO, CD8-PB, CCR7-PE Cy7, CD45RA-FITC, CD127-APC, CD38 – PerCp Cy5.5, HLA-DR Alexa700, Perforin-FITC, GzB- AlexaFluor700, GzA-PerCpCy5.5, CD161-APC, PD-1 Pe-Cy7. Short term cell lines were generated with peptide (10μg/ml), 100U/ml of IL-2 day 4 and 7, and cells were harvested day 10 or 11.
For analysis of peptide-specific function, PBMCs were stimulated with the respective peptide at 1μg/ml or control DMSO or PMA/Ionomycin in the presence of anti-CD107a PE-Cy5. ICS was then performed as described above, but staining with the following antibodies: CD3-PO, CD4-Qdot 605, CD8-PB, IFNγ-AlexaFluor700, IL2-APC, TNFα-PE-Cy7, MIP-1β-PE. Flow cytometry and analysis were performed as above.
Statistical analysis
Nonparametric tests were used, throughout, paired for within individual comparisons (Wilcoxon) and unpaired for group comparisons (Mann Whitney). For correlations a nonparametric test was used (Spearman). For multiple comparisons a one-way Anova with Bonferroni’s correction was used. Prism (v4.0 for Mac) was used throughout.
Supplementary Material
Acknowledgments
We thank staff of the CCVTM and CBF, Oxford, especially Eleanor Berrie, Alison Lawrie, Kathleen Gantlett and Ian Poulton. We would like to thank BEI for peptides. Funding: the European Union (Framework VI; HEPACIVAC) and also the MRC (UK), the Wellcome Trust, Oxford NIHR Biomedical Research Centre, the James Martin School for 21st Century, Oxford, the Wellcome Trust Clinical Research Facility, Birmingham and the NIHR Liver Biomedical Research Unit, Birmingham and the NIH grant 1U19AI082630-01.
Footnotes
Author contributions: EB, AF, SCa, SCo, GO, DA, AH, AN, and RC designed the study/protocols; EB, SCa, LSw, SA, AK, JM, RH, KS, RT, AB, RA, VA, MN, CW, GH, FG, MLE, LSi, and YO performed research and analysis; EB, AF, SCa, and PK wrote the manuscript; CT was the project manager; PK was the principal investigator.
Competing interests: S. Colloca, A. Folgori, R. Cortese and A. Nicosia are named inventors on patent applications covering HCV vectored vaccines and chimpanzee Adenovirus vectors (WO 2006133911 (A3) Hepatitis C virus nucleic acid vaccine, WO 2005071093 (A3) Chimpanzee adenovirus vaccine carriers, WO 03031588 (A2) Hepatitis C virus vaccine). PK has acted as a consultant to Tibotec and Pfizer on antiviral therapy. Authors from Okairos are employees of and/or shareholders in Okairos. The other authors declare that they have no competing interests.
References and Notes
- 1.Lauer GM, Walker BD. Hepatitis C virus infection. N Engl J Med. 2001;345:41–52. doi: 10.1056/NEJM200107053450107. [DOI] [PubMed] [Google Scholar]
- 2.Thimme R, Oldach D, Chang KM, Steiger C, Ray SC, Chisari FV. Determinants of viral clearance and persistence during acute hepatitis C virus infection. J Exp Med. 2001;194:1395–1406. doi: 10.1084/jem.194.10.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lechner F, Wong DK, Dunbar PR, Chapman R, Chung RT, Dohrenwend P, Robbins G, Phillips R, Klenerman P, Walker BD. Analysis of successful immune responses in persons infected with hepatitis C virus. J Exp Med. 2000;191:1499–1512. doi: 10.1084/jem.191.9.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grakoui A, Shoukry NH, Woollard DJ, Han JH, Hanson HL, Ghrayeb J, Murthy KK, Rice CM, Walker CM. HCV persistence and immune evasion in the absence of memory T cell help. Science. 2003;302:659–662. doi: 10.1126/science.1088774. [DOI] [PubMed] [Google Scholar]
- 5.Shoukry NH, Grakoui A, Houghton M, Chien DY, Ghrayeb J, Reimann KA, Walker CM. Memory CD8+ T cells are required for protection from persistent hepatitis C virus infection. J Exp Med. 2003;197:1645–1655. doi: 10.1084/jem.20030239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spada E, Mele A, Berton A, Ruggeri L, Ferrigno L, Garbuglia AR, Perrone MP, Girelli G, Del Porto P, Piccolella E, Mondelli MU, Amoroso P, Cortese R, Nicosia A, Vitelli A, Folgori A. Multispecific T cell response and negative HCV RNA tests during acute HCV infection are early prognostic factors of spontaneous clearance. Gut. 2004;53:1673–1681. doi: 10.1136/gut.2003.037788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thomas DL, Thio CL, Martin MP, Qi Y, Ge D, O’Huigin C, Kidd J, Kidd K, Khakoo SI, Alexander G, Goedert JJ, Kirk GD, Donfield SM, Rosen HR, Tobler LH, Busch MP, McHutchison JG, Goldstein DB, Carrington M. Genetic variation in IL28B and spontaneous clearance of hepatitis C virus. Nature. 2009;461:798–801. doi: 10.1038/nature08463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Osburn WO, Fisher BE, Dowd KA, Urban G, Liu L, Ray SC, Thomas DL, Cox AL. Spontaneous control of primary hepatitis C virus infection and immunity against persistent reinfection. Gastroenterology. 138:315–324. doi: 10.1053/j.gastro.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Folgori A, Capone S, Ruggeri L, Meola A, Sporeno E, Ercole BB, Pezzanera M, Tafi R, Arcuri M, Fattori E, Lahm A, Luzzago A, Vitelli A, Colloca S, Cortese R, Nicosia A. A T-cell HCV vaccine eliciting effective immunity against heterologous virus challenge in chimpanzees. Nat Med. 2006;12:190–197. doi: 10.1038/nm1353. [DOI] [PubMed] [Google Scholar]
- 10.Reyes-Sandoval A, Berthoud T, Alder N, Siani L, Gilbert SC, Nicosia A, Colloca S, Cortese R, Hill AV. Prime-boost immunization with adenoviral and modified vaccinia virus Ankara vectors enhances the durability and polyfunctionality of protective malaria CD8+ T-cell responses. Infect Immun. 78:145–153. doi: 10.1128/IAI.00740-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Colloca S, Barnes E, Folgori A, Ammendola V, Capone S, Siani L, Naddeo M, Grazioli F, Esposito M, Ambrosio M, Sparacino A, Bartiromo M, Meola A, Smith K, Kurioka A, O’Hara G, Ewer K, Hill AV, Traboni C, Klenerman P, Cortese R, Nicosia A. Generation and screening of a large collection of novel simian Adenovirus allows the identification of vaccine vectors inducing potent cellular immunity in humans. Science Translational medicine. 2011 doi: 10.1126/scitranslmed.3002925. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lauer GM, Barnes E, Lucas M, Timm J, Ouchi K, Kim AY, Day CL, Robbins GK, Casson DR, Reiser M, Dusheiko G, Allen TM, Chung RT, Walker BD, Klenerman P. High resolution analysis of cellular immune responses in resolved and persistent hepatitis C virus infection. Gastroenterology. 2004;127:924–936. doi: 10.1053/j.gastro.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 13.Diepolder HM, Gerlach JT, Zachoval R, Hoffmann RM, Jung MC, Wierenga EA, Scholz S, Santantonio T, Houghton M, Southwood S, Sette A, Pape GR. Immunodominant CD4+ T-cell epitope within nonstructural protein 3 in acute hepatitis C virus infection. J Virol. 1997;71:6011–6019. doi: 10.1128/jvi.71.8.6011-6019.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Missale G, Bertoni R, Lamonaca V, Valli A, Massari M, Mori C, Rumi M, Houghton M, Fiaccadori F, Ferrari C. Different clinical behaviours of acute HCV infection are associated with different vigor of the anti-viral T cell response. J Clin Invest. 1996;98:706–714. doi: 10.1172/JCI118842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Billerbeck E, Kang YH, Walker L, Lockstone H, Grafmueller S, Fleming V, Flint J, Willberg CB, Bengsch B, Seigel B, Ramamurthy N, Zitzmann N, Barnes EJ, Thevanayagam J, Bhagwanani A, Leslie A, Oo YH, Kollnberger S, Bowness P, Drognitz O, Adams DH, Blum HE, Thimme R, Klenerman P. Analysis of CD161 expression on human CD8+ T cells defines a distinct functional subset with tissue-homing properties. Proc Natl Acad Sci U S A. 107:3006–3011. doi: 10.1073/pnas.0914839107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 17.Kasprowicz V, Schulze Zur Wiesch J, Kuntzen T, Nolan BE, Longworth S, Berical A, Blum J, McMahon C, Reyor LL, Elias N, Kwok WW, McGovern BG, Freeman G, Chung RT, Klenerman P, Lewis-Ximenez L, Walker BD, Allen TM, Kim AY, Lauer GM. High level of PD-1 expression on hepatitis C virus (HCV)-specific CD8+ and CD4+ T cells during acute HCV infection, irrespective of clinical outcome. J Virol. 2008;82:3154–3160. doi: 10.1128/JVI.02474-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rauch A, James I, Pfafferott K, Nolan D, Klenerman P, Cheng W, Mollison L, McCaughan G, Shackel N, Jeffrey GP, Baker R, Freitas E, Humphreys I, Furrer H, Gunthard HF, Hirschel B, Mallal S, John M, Lucas M, Barnes E, Gaudieri S. Divergent adaptation of hepatitis C virus genotypes 1 and 3 to human leukocyte antigen-restricted immune pressure. Hepatology. 2009;50:1017–1029. doi: 10.1002/hep.23101. [DOI] [PubMed] [Google Scholar]
- 19.Seder RA, Darrah PA, Roederer M. T-cell quality in memory and protection: implications for vaccine design. Nat Rev Immunol. 2008;8:247–258. doi: 10.1038/nri2274. [DOI] [PubMed] [Google Scholar]
- 20.Wherry EJ, Day CL, Draenert R, Miller JD, Kiepiela P, Woodberry T, Brander C, Addo M, Klenerman P, Ahmed R, Walker BD. HIV-specific CD8 T cells express low levels of IL-7Ralpha: implications for HIV-specific T cell memory. Virology. 2006;353:366–373. doi: 10.1016/j.virol.2006.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Northfield JW, Loo CP, Barbour JD, Spotts G, Hecht FM, Klenerman P, Nixon DF, Michaelsson J. Human immunodeficiency virus type 1 (HIV-1)-specific CD8+ T(EMRA) cells in early infection are linked to control of HIV-1 viremia and predict the subsequent viral load set point. J Virol. 2007;81:5759–5765. doi: 10.1128/JVI.00045-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hansen SG, Ford JC, Lewis MS, Ventura AB, Hughes CM, Coyne-Johnson L, Whizin N, Oswald K, Shoemaker R, Swanson T, Legasse AW, Chiuchiolo MJ, Parks CL, Axthelm MK, Nelson JA, Jarvis MA, Piatak M, Jr., Lifson JD, Picker LJ. Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine. Nature. 2011;473:523–527. doi: 10.1038/nature10003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Akondy RS, Monson ND, Miller JD, Edupuganti S, Teuwen D, Wu H, Quyyumi F, Garg S, Altman JD, Del Rio C, Keyserling HL, Ploss A, Rice CM, Orenstein WA, Mulligan MJ, Ahmed R. The yellow fever virus vaccine induces a broad and polyfunctional human memory CD8+ T cell response. J Immunol. 2009;183:7919–7930. doi: 10.4049/jimmunol.0803903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cox AL, Page K, Bruneau J, Shoukry NH, Lauer GM, Kim AY, Rosen HR, Radziewicz H, Grakoui A, Fierer DS, Branch AD, Kaplan DE, Chang KM. Rare birds in North America: acute hepatitis C cohorts. Gastroenterology. 2009;136:26–31. doi: 10.1053/j.gastro.2008.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klade CS, Wedemeyer H, Berg T, Hinrichsen H, Cholewinska G, Zeuzem S, Blum H, Buschle M, Jelovcan S, Buerger V, Tauber E, Frisch J, Manns MP. Therapeutic vaccination of chronic hepatitis C nonresponder patients with the peptide vaccine IC41. Gastroenterology. 2008;134:1385–1395. doi: 10.1053/j.gastro.2008.02.058. [DOI] [PubMed] [Google Scholar]
- 26.Habersetzer F, Honnet G, Bain C, Maynard-Muet M, Leroy V, Zarski JP, Feray C, Baumert TF, Bronowicki JP, Doffoel M, Trepo C, Agathon D, Toh ML, Baudin M, Bonnefoy JY, Limacher JM, Inchauspe G. A poxvirus vaccine is safe, induces T-cell responses, and decreases viral load in patients with chronic hepatitis C. Gastroenterology. 141:890–899. e891–894. doi: 10.1053/j.gastro.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 27.Capone S, Meola A, Ercole BB, Vitelli A, Pezzanera M, Ruggeri L, Davies ME, Tafi R, Santini C, Luzzago A, Fu TM, Bett A, Colloca S, Cortese R, Nicosia A, Folgori A. A novel adenovirus type 6 (Ad6)-based hepatitis C virus vector that overcomes preexisting anti-ad5 immunity and induces potent and broad cellular immune responses in rhesus macaques. J Virol. 2006;80:1688–1699. doi: 10.1128/JVI.80.4.1688-1699.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aste-Amezaga M, Bett AJ, Wang F, Casimiro DR, Antonello JM, Patel DK, Dell EC, Franlin LL, Dougherty NM, Bennett PS, Perry HC, Davies ME, Shiver JW, Keller PM, Yeager MD. Quantitative adenovirus neutralization assays based on the secreted alkaline phosphatase reporter gene: application in epidemiologic studies and in the design of adenovector vaccines. Hum Gene Ther. 2004;15:293–304. doi: 10.1089/104303404322886147. [DOI] [PubMed] [Google Scholar]
- 29.Roederer M, Nozzi JL, Nason MC. SPICE: Exploration and analysis of post-cytofluorometric complex multivariate datasets. Cytometry Part A. 2011;79A:167–74. doi: 10.1002/cyto.a.21015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.