Abstract
BACKGROUND
The objective of this study was to test a low dose of (25 mg weekly) of the mammalian target of rapamycin kinase inhibitor temsirolimus for patients with relapsed mantle cell lymphoma (MCL).
METHODS
Patients with relapsed or refractory MCL were eligible to receive temsirolimus 25 mg intravenously every week as a single agent. Patients who had a tumor response after 6 cycles were eligible to continue drug for a total of 12 cycles or 2 cycles after complete remission and then were observed without maintenance.
RESULTS
Of 29 enrolled patients, 28 were evaluable for toxicity, and 27 were evaluable for efficacy. The median age was 69 years (range, 51–85 years), 86% of patients had stage IV disease, and 71% had ≥2 extranodal sites. Patients had received a median of 4 prior therapies (range, 1–9 prior therapies), and 50% were refractory to the last treatment. The overall confirmed response rate was 41% (11 of 27 patients; 90% confidence interval [CI], 22%–61%) with 1 complete response (3.7%) and 10 partial responses (37%). The median time to progression in all eligible patients was 6 months (95% CI, 3–11 months), and the median duration of response for the 11 responders was 6 months (range, 1–26 months). Hematologic toxicities were the most common, with 50% (14 of 28 patients) grade 3 and 4% (1 of 28 patients) grade 4 toxicities observed. Thrombocytopenia was the most frequent cause of dose reduction.
CONCLUSIONS
Single-agent temsirolimus at a dose of 25 mg weekly is an effective new agent for the treatment of MCL. The 25-mg dose level retained the antitumor activity of the 250-mg dose with less myelosuppression. Further studies of temsirolimus in combination with other active drugs for MCL and other lymphoid malignancies are warranted.
Keywords: mantle cell lymphoma, temsirolimus, CCI-779, rapamycin, mammalian target of rapamycin kinase
Mantle cell lymphoma (MCL) is an incurable, aggressive B-cell non-Hodgkin lymphoma (NHL) that represents about 6% of cases of NHL.1 Patients who relapse after conventional therapy or stem cell transplantation have a poor prognosis and are candidates for novel agents. A pathologic hallmark of MCL is the characteristic overexpression of cyclin D1 (CCND1) in the MCL tumor cells.2 CCND1 is 1 of the proteins in which translation is under the control of the phosphatidylinositol-3 kinase signal-transduction pathway and is downstream of the mammalian target of rapamycin kinase (mTOR).3 We hypothesized that temsirolimus, a dihydroester of the selective mTOR inhibitor rapamycin, would be an active antitumor agent in MCL. Indeed, in a previous phase 2 trial that tested a dose of 250 mg intravenously (iv) weekly for patients with relapsed MCL in the North Central Cancer Treatment Group (NCCTG), we observed a 38% overall response rate (ORR), with a 3% complete remission (CR) rate and a 35% partial remission (PR) rate.4 However, in this patient population, reversible myelosuppression was substantial: Twenty-five of 35 of patients (71%) experienced grade 3 hematologic toxicity, and 3 of 35 patients (9%) experienced grade 4 hematologic toxicity.
The United States Federal Drug Administration recently approved temsirolimus for renal cell carcinoma because of its demonstrated antitumor activity at a dose of 25 mg iv weekly.5,6 We performed a phase 2 trial of temsirolimus 25 mg iv weekly to determine whether the efficacy of temsirolimus in relapsed MCL could be maintained while reducing toxicity.
MATERIALS AND METHODS
A single-stage, phase 2 study with an interim analysis was conducted to assess the proportion of previously treated patients with MCL who achieved a PR or better after treatment with single-agent temsirolimus. This study was conducted through the NCCTG and was approved by the institutional review board of each treatment site. Patients were eligible for this trial if they had previously received therapy and had relapsed or were refractory to their last treatment. There was no limit on the number of prior therapies. Central pathology review confirmed the diagnosis of MCL based on morphology and phenotype. In addition, all tumors were positive for CCND1 by immunohistochemistry or demonstrated to have CCND1/IgH by fluorescence in situ hybridization. Patients were required to have measurable disease with a lymph node or tumor mass ≥2 cm or malignant lymphocytosis with an absolute lymphocyte count ≥5000/μL; a life expectancy ≥3 months; an Eastern Cooperative Oncology Group performance status of 0, 1, or 2; an absolute neutrophil count (ANC) ≥1000/μL; platelets ≥75,000 × 109/L; hemoglobin ≥8 g/dL; serum creatinine ≤2 times the upper limit of normal (UNL); serum bilirubin ≤1.5 the UNL; serum cholesterol ≤350 mg/dL; and triglycerides ≤400 mg/dL. Patients could not have known central nervous system involvement or human immunodeficiency virus infection.
Patients were treated with a flat dose of 25 mg of temsirolimus diluted in 250 mL of normal saline and delivered iv over 30 minutes. Patients were pretreated with diphenhydramine (25-50 mg iv). Treatment was weekly, and 4 weeks was considered 1 cycle. A complete blood count was performed each week. If the platelet count was ≥50,000/μL, and the ANC was ≥1000/μL, and there were no grade 3 or 4 nonhema-tologic toxicities (National Cancer Institute Common Toxicity Criteria, version 2), then the full dose of temsirolimus administered. Patients who did not meet the retreatment criteria had the dose held until recovery followed by a stepwise dose modification to 15 mg weekly, 10 mg weekly, and 10 mg every other week. Patients were not to receive prophylactic white blood cell growth factors to maintain dosing but could receive them at their physician’s discretion if neutropenia developed. Erythropoietin treatment for anemia also was permitted.
Patients were restaged after 1 cycle and every 3 cycles thereafter or at the physician’s discretion. Responses were categorized using the International Workshop Criteria.7 Patients who progressed at any time or patients who had stable disease after 6 cycles went off study. Patients who had a CR at 6 months were to receive 2 cycles past CR or a total of 12 months if they had a PR and then were observed without further therapy.
Statistical Design
The current trial was designed to test the null hypothesis that the true, confirmed ORR was at most 5%. The smallest ORR that would indicate that this regimen was worth further study in this population of patients with relapsed MCL was 20%. The design was generated based on the parameters and assumptions of a 2-stage Simon Min-Max design but without suspending accrual for the interim analysis.8 This study design required a maximum of 27 evaluable patients, and the interim analysis was performed after 13 patients had been accrued and followed for at least 24 weeks for response. An additional 2 patients were accrued to this cohort (for a maximum of 29 patients overall) to account for the possibilities of ineligibility, withdrawal from study before drug administration, or major violations. However, only the first 27 evaluable patients were to be used to evaluate the decision criteria for this design. At least 1 response in the first 13 evaluable patients needed to be observed in the interim analysis to continue accrual. At the time of the final analyses, ≥4 responses were required to indicate that the regimen warranted further evaluation in this patient population. The proportion of responses was calculated and the 90% exact binomial confidence interval (CI) for the true ORR was calculated (with all eligible patients accrued) assuming that the number of responses was distributed binomially.
The duration of response (DR) was defined as the time from the date of documented response to the date of progression. Patients who went off treatment because of other reasons (eg, adverse reactions, refusal of further treatment) were censored at that time. The time to progression (TTP) was defined as the time from registration to the date of progression. Patients who died without disease progression were censored at the date of their last evaluation. If a patient died without documentation of disease progression, then the patient was considered to have had disease progression at the time of death unless there was sufficient documented evidence to conclude that progression did not occur before death. The time to discontinuation of active treatment was defined as the time from registration to the date the decision was made to take the patient off active treatment. Patients who still were receiving treatment at the time of these analyses were censored at the date of their last evaluation. Overall survival (OS) was defined as the time from registration to death resulting from any cause. The distributions of these time-to-event endpoints were each estimated by using the Kaplan-Meier method.9
RESULTS
Patient Characteristics
In total, 29 patients were enrolled on this trial by the NCCTG sites from March 2004 to August 2005 (Table 1). One patient received bortezomib 18 days before enrollment and was ineligible for the analysis of efficacy but was included in the analysis of safety. One additional patient had a major violation from the protocol (received concomitant corticosteroids) and was excluded from all results. The patients tended to be older adults (median age, 69 years; range, 51–85 years). Most patients (86%) had stage IV disease and were heavily pretreated (median, 4 prior therapies; mean, 4 prior therapies; range, 1–9 prior therapies). The majority of patients had failed previous rituximab (96%); an alkylator agent, such as cyclophosphamide (96%); and an anthracycline, such as doxorubicin (79%).
TABLE 1.
Patient Characteristics (N = 28)
| Characteristic | No. of patients (%) |
|---|---|
| Age, y | |
| Median | 69 |
| Range | 51–85 |
| Sex, men | 19 (68) |
| Performance status | |
| 0 | 11 (39) |
| 1 | 11 (39) |
| 2 | 6 (22) |
| Lymphoma stage at study entry | |
| I/II | 1 (3) |
| III/IV | 27 (97) |
| Bone marrow involvement | 21 (75) |
| Blastoid morphology | 4 (14) |
| B symptoms | 2 (7) |
| No. of extranodal sites | |
| 0–1 | 8 (29) |
| ≥2 | 20 (71) |
| Disease status | |
| Relapsed | 14 (50) |
| Refractory* | 14 (50) |
| No. of prior therapy treatments | |
| Mean | 4 |
| Median [range] | 4 [1–9] |
| Type of prior therapy | |
| Rituximab | 27 (96) |
| Alkylator | 27 (96) |
| Anthracycline | 22 (79) |
| Purine nucleoside analog | 12 (43) |
| Platinum analog | 8 (29) |
| Radiotherapy | 8 (29) |
| Stem cell transplantation | 7 (25) |
Lack of a complete or partial remission of >1 month duration to the last regimen before protocol entry.
Clinical Outcomes
The ORR was 41% (11 of 27 patients; 90% CI, 22%–61%) with 1 CR and 10 PRs (Fig. 1). The tumor responses occurred rapidly, with a median time to response of 1 month (range, 1–6 months). Eight responses occurred after 1 cycle, 2 responses were documented after 3 cycles, and 1 response was documented after 6 cycles. In addition, 1 patient achieved a PR after 12 cycles but was not considered in the ORR because the statistical design required the response to occur within 6 months (see above). Three of the 7 patients (43%) who had a history of prior stem cell transplantation responded. There were no responses in the 4 patients who had blastoid morphology. The median time to discontinuation of treatment was 4 months (95% CI, 2–6 months).
FIGURE 1.
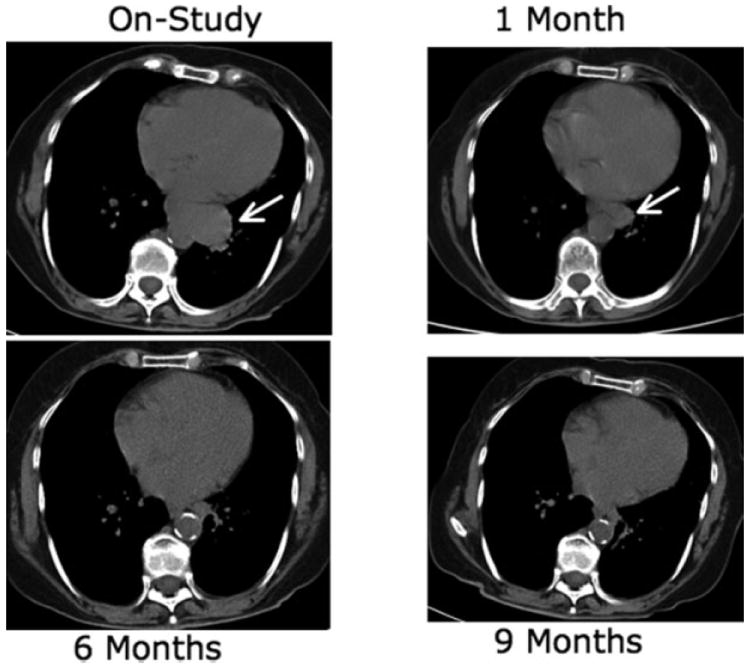
Computed tomography scans of a woman aged 73 years who responded to treatment with weekly temsirolimus at an intravenous dose of 25 mg.
Nineteen patients had dose reductions or treatment delays. Dose reductions were because of thrombocytopenia in 5 patients, neutropenia in 2 patients, thrombocytopenia/neutropenia in 2 patients, and surgery in 1 patient. Treatment delays were because of thrombocytopenia in 1 patient, neutropenia in 1 patient, infection in 3 patients, fever in 1 patient, pneumonia in 1 patient, surgery in 1 patient, fatigue in 1 patient, and pleural effusion in 1 patient. Overall, 15 patients were able to receive 25 mg weekly for at least the first cycle of treatment, with a median of 4 cycles at this full dose (range, 1-12 cycles); 8 patients required dose reductions in the first cycle, and 5 patients did not complete the first cycle of treatment (4 patients went off for either disease progression or clinical deterioration, and 1 patient died 8 days after starting treatment because of infection with neutropenia, which was deemed unrelated to temsirolimus). Of the 12 patients who received more than 1 cycle at the full dose level, 2 patients eventually required a dose reduction in subsequent cycles, and 6 patients had treatment delayed because of either adverse events or hospitalization. Of the 10 patients who had dose reductions, 5 patients were reduced to 15 mg weekly, 3 patients were reduced to 10 mg weekly, and 2 patients were reduced to 10 mg every other week. Across all patients, the median dose received per month on study was 60 mg, with 100 mg in responding patients and 60 mg in nonresponders. In addition, the patient who was deemed ineligible for assessment of efficacy received the full dose (25 mg weekly) for 8 weeks.
The median TTP (Fig. 2) was 6 months (95% CI, 3–11 months). The median OS from study entry was 14 months (95% CI, 10–27 months). The median duration of response for the 11 responders was 6 months (range, 1–26 months). The median follow-up of living patients was 30 months (range, from 22 months to >39 months). Overall, 24 patients had disease progression, and 19 patients died. Three patients had documented death without disease progression.
FIGURE 2.
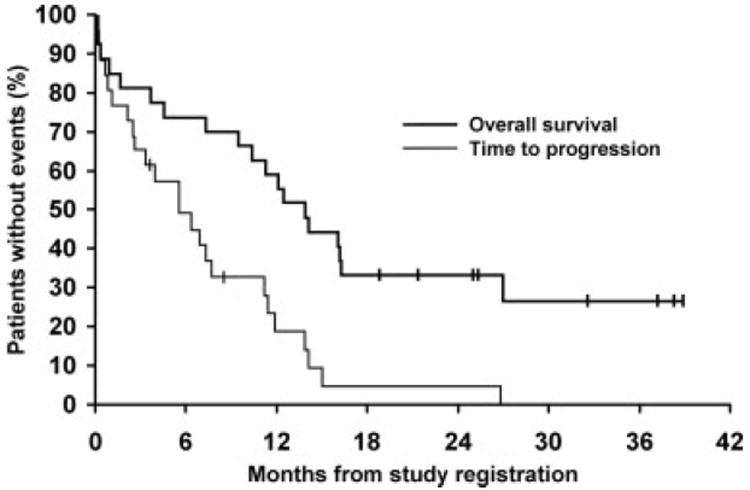
The time to progression and overall survival in 27 eligible patients who received with single-agent temsirolimus.
Safety and Tolerability
All patients (except the 1 patient with a major protocol violation) were included in the analysis of safety and tolerability. In general, temsirolimus was tolerated well, and the primary toxicity was reversible myelosuppression. There were no significant toxicities during the 30-minute infusion of temsirolimus. The most common adverse events (irrespective of attribution) of all grades were thrombocytopenia (82%), fatigue (75%), hyperglycemia (71%), hypertriglyceridemia (71%), and anemia and neutropenia (57%). Other adverse events included nausea (39%), stomatitis (39%), anorexia (36%), rash (36%), infection without concomitant neutropenia (32%), and hypercholesterolemia (28%). Although 9 of 28 patients (32%) reported an adverse event of sensory neuropathy, 7 events were grade 1, with 1 event was grade 2, and 1 event was grade 3. Eight of the 9 events (including the grade 3 event) were considered unrelated to temsirolimus, and 1 grade 1 event was considered possibly related. Two patients died on treatment (grade 5) of causes deemed unrelated to temsirolimus—1 from infection without neutropenia and the other of progressive MCL.
Table 2 shows the occurrences of severe toxicities (grade ≥3) that were considered at least possibly related to temsirolimus were low. Thrombocytopenia was the cause of most dose reductions and was rapidly reversible with drug delays of typically only 1 week. One patient required platelet transfusions. Three patients experienced infection (grade 3) without concomitant neutropenia, and 1 patient had infection (grade 3) with neutropenia. Three patients had grade 3 dyspnea, and 1 of those patients also had grade 3 hypoxia (Table 2). One of those patients was a woman aged 85 years who also had fatigue at the same time that the dyspnea was noted. Her computed tomography (CT) scan did not reveal evidence of infection or interstitial lung disease. The other 2 patients had evidence on CT scans for progression of MCL at the time of the dyspnea. In 1 of those patients, a large pleural effusion was noted and was positive by cytology for MCL. In the other patient, pneumonia was suspected: The patient was treated with antibiotics and steroids, and the dyspnea resolved. In summary, it is difficult in these 3 patients to definitively attribute the dyspnea to temsirolimus.
TABLE 2.
Grade 3 and 4 Toxicity/Adverse Events Considered at Least Possibly Related to Temsirolimus*
| Toxicity type | % of patients (No.)
|
Grade 4 |
|---|---|---|
| Grade 3 | ||
| General | ||
| Fatigue | 18 (5) | 7 (2) |
| Muscular hypoplasia | 4 (1) | 0 |
| Hematologic | ||
| Thrombocytopenia | 39 (11) | 0 |
| Platelet transfusions | 4 (1) | 0 |
| Leukopenia | 7 (2) | |
| Neutropenia | 18 (5) | 0 |
| Lymphopenia | 4 (1) | 0 |
| Anemia | 11 (3) | 4 (1) |
| Erythrocyte transfusions | 4 (1) | 0 |
| Infection | ||
| Without ANC | 11 (3) | 0 |
| With ANC | 4 (1) | 0 |
| Gastrointestinal | ||
| Dehydration | 7 (2) | 0 |
| Diarrhea | 4 (1) | 0 |
| Anorexia | 4 (1) | 0 |
| Nausea | 7 (2) | 0 |
| Vomiting | 4 (1) | 0 |
| Metabolic | ||
| Hypertriglyceridemia | 7 (2) | 0 |
| Hyperglycemia | 11 (3) | 0 |
| Neurologic | ||
| Dizziness | 4 (1) | 0 |
| Hallucinations | 4 (1) | 0 |
| Pulmonary | ||
| Dyspnea | 11 (3) | 0 |
| Pleural effusion | 4 (1) | 0 |
| Hypoxia | 4 (1) | 0 |
| Cardiovascular | ||
| Hypertension | 4 (1) | 0 |
| Hypotension | 4 (1) | 0 |
| Left ventricular fail | 4 (1) | 0 |
| Sinus tachycardia | 4 (1) | 0 |
| Hemorrhage | ||
| Melena | 4 (1) | 0 |
| Maximum overall toxicity gradey† | 71 (20) | 11 (3) |
ANC indicates absolute neutrophil count.
Grade 3 and 4 toxicity (adverse events considered at least possibly related to temsirolimus) was observed in 23 of 28 patients (82%).
The maximum overall toxicity grade refers to the percentage (number) of patients with the respective grade of toxicity across all toxicity types.
DISCUSSION
There is a need for new agents for the treatment of all phases of MCL. Before integrating a new drug with conventional agents or using it in previously untreated patients, it is important to determine its single-agent activity and toxicities. In the current trial, we tested a lower dose, 25 mg iv weekly, of the mTOR inhibitor temsirolimus in patients with relapsed MCL and demonstrated ORR, TTP, and duration of response similar to those reported in a previous trial that studied the 250-mg dose level.4 The current trial again demonstrated the activity of mTOR inhibitors in MCL and provided further rationale to study combinations of temsirolimus with other agents. Everolimus, another mTOR kinase inhibitor, has also demonstrated activity against MCL lines in vitro10 and activity in patients with relapsed MCL.11 There was less hematologic toxicity with the lower dose of temsirolimus, as expected. The rates of grade 3 thrombocytopenia were 63% and 39% in the 250-mg and 25-mg dose level cohorts, respectively. Likewise, 23% of patients had grade 3 and 6% of patients had grade 4 neutropenia in the 250-mg dose cohort compared with 18% grade 3 and 0% grade 4 neutropenia in the 25-mg cohort. The thrombocytopenia observed at the 25-mg dose level was tolerable but still resulted in dose reductions and delays in this population of patients with relapsed MCL. This may be less of a problem in previously untreated patient with healthier bone marrow, but it probably will be an issue when temsirolimus is combined with other myelosuppressive agents. Currently, we are combining temsirolimus at the 25-mg weekly dose with rituximab in an ongoing phase 2 trial (N038H) in the NCCTG.
The other toxicities of temsirolimus were manageable. The hyperlipidemia that occurs in patients who receive the drug for extended lengths of time usually requires treatment with antilipid agents, such as statins. We observed 11% grade 3 dyspnea, which is similar to the 9% dyspnea observed in the temsirolimus study In renal cell carcinoma.12 Pulmonary symptoms often are difficult to distinguish from tumor or infection, and both must be ruled out before attributing symptoms to the drug. The initial presentation is usually asymptomatic interstitial pulmonary infiltrates on CT scans performed for the purpose of assessing tumor response. We did not reduce or discontinue temsirolimus unless the patient developed symptoms like cough or dyspnea on exertion. These symptoms usually resolved with cessation of temsirolimus or dose reduction. Future trials of mTOR inhibitors in patients with NHL will need to incorporate monitoring and treatment plans for pulmonary toxicity. The mTOR inhibitors join the list of other new agents, including bortezomib,13-15 bendamustine,16 and the immunomodulatory agents thalidomide17 and lenalidomide,18 that have single-agent activity in relapsed MCL. Bendamustine19-21 and thalidomide17 already have entered trials in combination with other agents. Studies combining bortezomib with other agents also have been initiated. It is anticipated that these new agents and the subsequent combinations with each other and with established agents will increase the survival of patients with MCL.
Acknowledgments
This study was conducted as a collaborative trial of the North Central Cancer Treatment Group and Mayo Clinic and was supported in part by Public Health Service grants CA-25224, CA-37404, CA-15083, CA-63826, CA-35195, CA-35267, CA-35101, CS-35431, CA-35090, CA-35113, CA-35415, CA-60276, CA-35448, CA-63848, CA97274, and CA112904 from the National Cancer Institute, Department of Heath and Human Services.
Additional institutions that participated in this study include: Medcenter One Health Systems, Bismarck, ND (Edward Wos, MD); Hematology and Oncology of Dayton, Inc. Dayton, Ohio (Howard M. Gross, MD); Illinois Oncology Research Association Community Clinical Oncology Program (CCOP), Peoria, Ill (John W. Kugler, MD); Toledo Community Hospital Oncology Program CCOP, Toledo, Ohio (Paul L. Schaefer, MD); Scottsdale CCOP, Scottsdale, Ariz (Tom R. Fitch, MD); Geisinger Clinic and Medical Center CCOP, Danville, Penn (Albert Bernath, MD); Ann Arbor Regional CCOP, Ann Arbor, Mich (Philip J. Stella, MD); Sioux Community Cancer Consortium, Sioux Falls, SD (Loren K. Tschetter, MD); and Upstate Carolina CCOP, Spartanburg, SC (Eric C. Nelson, MD).
References
- 1.A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin’s lymphoma. The Non-Hodgkin’s Lymphoma Classification Project. Blood. 1997;89:3909–3918. No authors listed. [PubMed] [Google Scholar]
- 2.Jaffe ES, Harris NL, Stein H, Vardiman JW World Health Organization Classification of Tumors. Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: IARC Press; 2001. [Google Scholar]
- 3.Witzig TE, Kaufmann SH. Inhibition of the phosphatidylinositol 3-kinase/mammalian target of rapamycin pathway in hematologic malignancies. Curr Treat Options Oncol. 2006;7:285–294. doi: 10.1007/s11864-006-0038-1. [DOI] [PubMed] [Google Scholar]
- 4.Witzig TE, Geyer SM, Ghobrial I, et al. Phase II trial of single-agent temsirolimus (CCI-779) for relapsed mantle cell lymphoma. J Clin Oncol. 2005;23:5347–5356. doi: 10.1200/JCO.2005.13.466. [DOI] [PubMed] [Google Scholar]
- 5.Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356:2271–2281. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 6.Atkins MB, Hidalgo M, Stadler WM, et al. Randomized phase II study of multiple dose levels of CCI-779, a novel mammalian target of rapamycin kinase inhibitor, in patients with advanced refractory renal cell carcinoma. J Clin Oncol. 2004;22:909–918. doi: 10.1200/JCO.2004.08.185. [DOI] [PubMed] [Google Scholar]
- 7.Cheson B, Horning S, Coiffier B, et al. Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphoma. J Clin Oncol. 1999;17:1244–1253. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- 8.Simon R. Optimal 2-stage designs for phase II clinical trials. Control Clin Trials. 1989;10:1–10. doi: 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- 9.Kaplan E, Meier P. Nonparametric estimation for incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 10.Haritunians T, Mori A, O’Kelly J, Luong QT, Giles FJ, Koeffler HP. Antiproliferative activity of RAD001 (everolimus) as a single agent and combined with other agents in mantle cell lymphoma. Leukemia. 2007;21:333–339. doi: 10.1038/sj.leu.2404471. [DOI] [PubMed] [Google Scholar]
- 11.Reeder CB, Gornet MK, Habermann TM, et al. A phase II trial of the oral mTOR inhibitor everolimus (RAD001) in relapsed aggressive non-Hodgkin lymphoma. Blood. 2007;110 [abstract] Abstract 121. [Google Scholar]
- 12.Hudes G, Carducci M, Tomczak P, et al. A phase III, randomized, 3-arm study of temsirolimus (TEMSR) or interferon-alpha (IFN) or the combination of TEMSR + IFN in the treatment of first-line, poor-risk patients with advanced renal cell carcinoma (adv RCC). J Clin Oncol; 2006 ASCO Annual Meeting Proceedings; 2006. [abstract] Abstract LBA4. [Google Scholar]
- 13.Fisher RI, Bernstein SH, Kahl BS, et al. Multicenter phase II study of bortezomib in patients with relapsed or refractory mantle cell lymphoma. J Clin Oncol. 2006;24:4867–4874. doi: 10.1200/JCO.2006.07.9665. [DOI] [PubMed] [Google Scholar]
- 14.Belch A, Kouroukis CT, Crump M, et al. A phase II study of bortezomib in mantle cell lymphoma: the National Cancer Institute of Canada Clinical Trials Group Trial IND 150. Ann Oncol. 2007;18:116–121. doi: 10.1093/annonc/mdl316. [DOI] [PubMed] [Google Scholar]
- 15.Kane RC, Dagher R, Farrell A, et al. Bortezomib for the treatment of mantle cell lymphoma. Clin Cancer Res. 2007;13(18 pt 1):5291–5294. doi: 10.1158/1078-0432.CCR-07-0871. [DOI] [PubMed] [Google Scholar]
- 16.Rummel MJ, Al-Batran SE, Kim SZ, et al. Bendamustine plus rituximab is effective and has a favorable toxicity profile in the treatment of mantle cell and low-grade non-Hodgkin’s lymphoma. J Clin Oncol. 2005;23:3383–3389. doi: 10.1200/JCO.2005.08.100. [DOI] [PubMed] [Google Scholar]
- 17.Kaufmann H, Raderer M, Wohrer S, et al. Anti-tumor activity of rituximab plus thalidomide in patients with relapsed/refractory mantle cell lymphoma. Blood. 2004;104:2269–2271. doi: 10.1182/blood-2004-03-1091. [DOI] [PubMed] [Google Scholar]
- 18.Wiernik PH, Lossos I, Tuscano JM, et al. Preliminary results from a phase II study of lenalidomide monotherapy in relapsed/refractory aggressive non-Hodgkins lymphoma. Blood. 2006;108 [abstract] Abstract 531. [Google Scholar]
- 19.Weide R, Hess G, Koppler H, et al. High anti-lymphoma activity of bendamustine/mitoxantrone/rituximab in rituximab pretreated relapsed or refractory indolent lymphomas and mantle cell lymphomas. A multicenter phase II study of the German Low Grade Lymphoma Study Group (GLSG) Leuk Lymphoma. 2007;48:1299–1306. doi: 10.1080/10428190701361828. [DOI] [PubMed] [Google Scholar]
- 20.Koenigsmann M, Knauf W, Herold M, et al. Fludarabine and bendamustine in refractory and relapsed indolent lymphoma—a multicenter phase I/II trial of the east German Society of Hematology and Oncology (OSHO) Leuk Lymphoma. 2004;45:1821–1827. doi: 10.1080/1042819042000223822. [DOI] [PubMed] [Google Scholar]
- 21.Herold M, Schulze A, Niederwieser D, et al. Bendamustine, vincristine and prednisone (BOP) versus cyclophosphamide, vincristine and prednisone (COP) in advanced indolent non-Hodgkin’s lymphoma and mantle cell lymphoma: results of a randomised phase III trial (OSHO no. 19) J Cancer Res Clin Oncol. 2006;132:105–112. doi: 10.1007/s00432-005-0023-2. [DOI] [PubMed] [Google Scholar]


