Preface
Caspase-8 can initiate apoptosis, but it is also required for embryonic development and immune cell proliferation. While several non-apoptotic roles for caspase-8 have been proposed, recent work has indicated that the requirement for caspase-8 in development and proliferation is defined by suppression of RIPK3, a kinase that can trigger an alternative form of cell death called programmed necrosis. We will consider these recent findings, and how they can be reconciled with earlier work on the non-apoptotic roles of caspase-8.
Introduction
Caspase-8 is a cysteine protease that initiates apoptosis in response to cell surface receptors1. This process, referred to as “extrinsic apoptosis”, is mediated by a group of receptors of the Tumor Necrosis Factor (TNF) superfamily called the death receptors (DRs). These include TNF receptor-1 (TNFR1) and the related receptor CD95 (also called Fas and APO-1)2. Activation of the DRs can lead to cell death, but also to proliferation and enhanced NF-kB activation as discussed below. To trigger cell death, DRs recruit an adaptor protein, Fas associated protein with a death domain (FADD), to their cytoplasmic tails; FADD then recruits caspase-8 proenzymes and activates them by inducing them to dimerize, leading to caspase activation and apoptosis (See Box 1 and Figure 1). Apoptotic caspase-8 activation is prevented by another protein, FLICE-like inhibitory protein long (cFLIPL, hereafter referred to as FLIP), which is homologous to caspase-8 but lacks catalytic residues. FLIP is thereby able to form an inhibitory heterodimer with caspase-83 that limits apoptosis induction (Box 1). We will revisit the properties of the caspase-8-FLIP heterodimer below.
Box 1. The Mechanism of caspase-8 activation.
The caspases are present in inactive forms in most cells types. Caspases are synthesized as proenzymes composed of a central large subunit (~20 kilodaltons) and a C-terminal small subunit (~10 kilodaltons); in addition to these domains, the initiator caspases (such as caspases-8, 2 and 9, the enzymes that initiate apoptotic signaling) also possess an N-terminal prodomain that mediates protein-protein interactions76. Initiator caspase proenzymes remain monomeric until they are recruited to large activation platforms via interactions with their prodomains. These large molecular weight platforms include the DR- and RIPK1-associated complexes described herein for caspase-8, and the cytochrome-c/APAF-1 complex called the “apoptosome” for caspase-9. Once recruited to these platforms, caspase proenzymes are induced to dimerize, and this dimerization leads to enzyme activation77. In the case of caspase-8, this dimerization is followed by interdomain cleavage events, first between the large and small subunits and subsequently between the large subunit and the prodomain. These cleavage events stabilize the caspase-8 homodimer and remove the prodomain, leading to formation of a fully active enzyme composed of two large and two small subunits78. Importantly for the current topic, a point mutation that prevents the stabilizing cleavage of caspase-8 between the large and small subunits prevents activation of caspase-8 by prodomain-driven homodimerization79, 80. However, this non-cleavable mutant is still able to be activated by heterodimerization with the caspase-8-like protein FLIP45,48, 81. This caspase-8-FLIP heterodimer is implicated in carrying out the suppression of RIPK3 signaling that defines the non-apoptotic role of caspase-8. However, how this suppression takes place, and how the caspase-8-FLIP complex is prevented from inducing apoptosis remains unclear. Artificial formation of caspase-8-FLIP heterodimers independent of DR ligation using an inducible-dimerization system readily triggers apoptosis48, indicating that catalytic differences between the homo- and heterodimer are not sufficient to explain their distinct functions. It is likely that receptor-mediated signaling exerts additional controls on the caspase-8-FLIP complex, such as restricted localization or rapid degradation, that further limit its cleavage of apoptotic substrates.
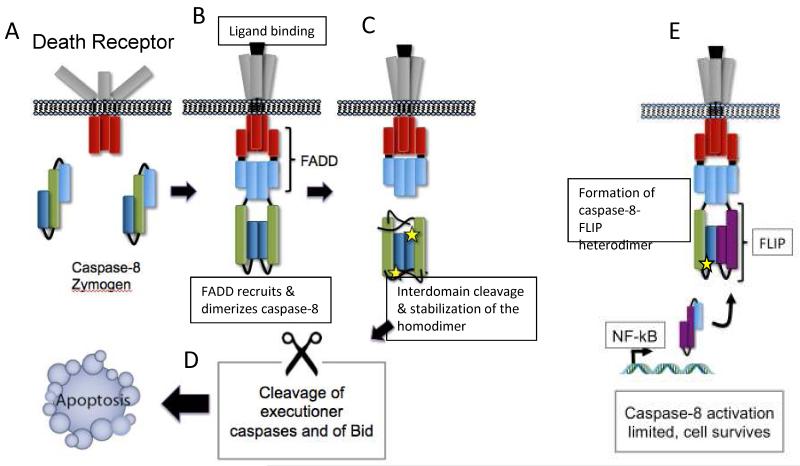
Fig. 1.
Activation of caspase-8 by homo- and heterodimerization.
This Figure depicts activation of caspase-8 at the receptor-associated DISC; however, similar homo- and heterodimerization events take place in the RIPK1-associated complex depicted in Fig. 2. A) Inactive caspase-8 zymogens are present in the cytosol of most healthy cells. These are composed of a prodomain (light blue), and one large and one small subunit (green and dark blue, respectively). B) Ligation of cell surface receptors such as CD95 leads to recruitment of the adapter FADD, which in turn recruits monomeric caspase-8 zymogens present in the cytosol via interactions with the caspase-8 prodomain. C) When FLIP levels are low, this recruitment leads to homodimerization, which is followed by cleavage of the interdomain linker regions. These cleavage events stabilize the homodimer and allow formation of the proteolytic active sites, symbolized by stars79. D) The fully active caspase-8 homodimer can then transduce the pro-apoptotic signal by activating downstream caspases, or by cleaving and activating the Bcl-2 family member Bid. E) When FLIP levels are high (e.g. following NF-kB activation by the TNF-R1-associated complex I; see Box 2), caspase-8 preferentially recruits and homodimerizes with FLIP45. The caspase-8-FLIP heterodimeric complex is catalytically active, and importantly FLIP can activate caspase-8 in the absence of interdomain cleavage events48. The activity of the caspase-8-FLIP complex does not trigger apoptosis, and is responsible for the suppression of RIPK1-RIPK3 signaling33. However, the key substrate(s) of this complex, as well as how FLIP limits caspase-8 activation in vivo, remain to be elucidated.
Apoptotic cell death is a means to delete superfluous or damaged cells, and as a consequence knockout of proteins involved in apoptosis often leads to defects associated with an overabundance of cells4-8. It is therefore surprising that knockout of caspase-8 in the mouse9, as well as that of FADD10, 11 or FLIP12, leads to embryonic lethality and a failure of yolk sac vascularization and hematopoiesis first observed around developmental day E10.5. This observation led to the hypothesis that these proteins play roles in cellular processes beyond apoptosis. Conditional deletion of FADD13, 14 or caspase-815 in lymphoid tissues reinforced this idea: T cells deficient for either protein—while resistant to apoptosis induced by CD95 ligation—failed to proliferate upon T-cell receptor (TCR) stimulation. The requirement of caspase-8 and FADD for cellular proliferation was extended to B cells stimulated with ligands for Toll-like receptors (TLRs) 3 and 414, 16, 17. Many studies and much effort have been devoted to explaining these observations, and several non-apoptotic roles have been assigned to caspase-8, FADD, and FLIP. All three have been reported to be involved in NF-kB activation17-20, and this requirement was proposed to explain the proliferative defects of caspase-8 deficient T and B cells17, 20. Caspase-8 has further been implicated in cell motility21, metastasis20, and suppression of inflammation22, 23, while FADD is reported to be required for normal cell cycle progression24-26.
Several years ago, it was observed that in cultured cells in which caspase-8 or FADD are absent or inhibited, TNF treatment causes a non-apoptotic form of cell death that involves cellular swelling and rupture, features commonly associated with necrotic (rather than apoptotic) cell death27. Like apoptosis, this alternate form of cell death was found to be “programmed,” in that it depends on activation of specific cellular enzymes: Receptor Interacting Protein Kinase-1 (RIPK1)28 and RIPK329-31. These observations led to the idea that the non-apoptotic roles of FADD and caspase-8 might involve the suppression of RIPK3-dependent programmed necrosis. This idea recently received strong genetic support, with the finding that concurrent ablation of RIPK1 or RIPK3 rescued the developmental and immune defects associated with FADD32 or caspase-833, 34 deficiency. In this Opinion piece, we will summarize recent work implicating suppression of RIPK signaling as the primary non-apoptotic role of caspase-8. We then revisit and cautiously reinterpret some previously proposed non-apoptotic roles of caspase-8, FADD, and FLIP in the context of RIPK signaling.
TNF: complexes and complexities
In considering how caspase-8 might exert its suppression of RIPK signaling, we must first discuss the pleiotropic nature of signaling by the cytokine TNF. Importantly, there is currently no genetic evidence that the defects observed in caspase-8 or FADD deficient animals are due to TNF signaling. Nonetheless, TNF signaling is the best-understood framework in which to consider the interconnected pathways of NF-kB activation, apoptosis, and programmed necrosis.
The initial events following ligation of the TNF receptor have been extensively reviewed elsewhere35, 36, and are summarized in Box 1. Importantly for the topic at hand, RIPK1 is recruited to the receptor complex (called “complex I”), and the NF-kB transcriptional program is activated. FLIP is a transcriptional target of NF-kB, and FLIP upregulation accounts for the non-apoptotic nature of the response to TNF in most cell types37; if FLIP upregulation is blocked, TNF signaling becomes potently pro-apoptotic37. As we will discuss below, FLIP is also implicated in the suppression of RIPK3-dependent signaling by caspase-8, and therefore represents a functional link between NF-kB signaling and the prevention of both apoptosis and RIPK3-dependent necrosis. (Fig. 2)
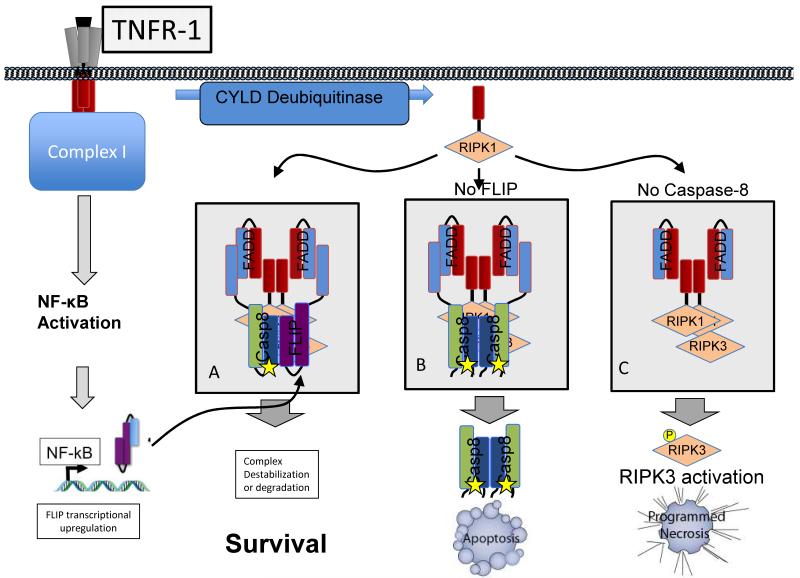
Fig. 2.
Recruitment of caspase-8 and FLIP to a RIPK1-containing complex determines cell fate. This Figure depicts the formation of the RIPK1 containing, RIPK3 activating complex II following TNFR1 ligation; however, recent evidence indicates that a similar complex can be induced by TLR-3 or -4 ligation or genotoxic stress40, 41. TNFR1 ligation initially triggers NF-kB activation and transcriptional upregulation of FLIP (see Box 2). RIPK1 is then deubiquitinated and translocates to the cytosol, where it can recruit FADD, caspase-8, and/or FLIP in a manner analogous to that depicted in Fig. 1. RIPK1 can also recruit and activate RIPK3 in this complex, a process that is controlled by FADD, caspase-8 and FLIP. A) When both caspase-8 and FLIP are present, these proteins are recruited to the RIPK1-containing complex. The caspase-8-FLIP heterodimer limits RIPK1-RIPK3 signaling, but does not trigger apoptosis. Importantly, the mechanism by which suppression of RIPK1-RIPK3 signaling by the caspase-8-FLIP complex is carried out remains controversial52, 53. B) When FLIP levels are low (for example, if NF-kB signaling is prevented), caspase-8 activation is unchecked, leading to apoptosis40, 41. FLIP is required not only to limit caspase-8 activation, but also to for suppression of RIPK signaling33, so reduced FLIP levels can also sensitize cells to RIPK3-dependnent necrosis if apoptosis is prevented. C) When caspase-8 (or FADD) is absent, apoptotic activation of caspase-8 is prevented, but RIPK signaling proceeds unchecked. The result is sensitization to RIPK3-dependent programmed necrosis.
Following the formation of complex I, RIPK1 translocates to a second cytosolic complex (complex II)38. This translocation depends on the deubiquitination of RIPK1 by the deubiquitinase CYLD, and complex II was initially described as a site of apoptotic caspase-8 activation38, 39. However, when it became clear that RIPK1 also activates RIPK3 to drive programmed necrosis, and that this signaling could be blocked by caspase-8, complex II was re-envisioned as a key site of interaction between the RIPK1-RIPK3 pathway and FADD, caspase-8, and FLIP29-31 (Fig. 2).
Two recent reports present compelling evidence that a RIPK1-containing complex capable of recruiting RIPK3, FADD, caspase-8 and FLIP may form in the cytosol independently of receptor activation40, 41 in response to multiple cellular signals and stresses. (Box 3) These studies indicate that this complex represents a signaling “module” that is able to activate either apoptosis (via caspase-8) or programmed necrosis (via RIPK3), and can be recruited to multiple signaling platforms including TNFR or the TLR adapter TRIF. These findings suggest that the balance between caspase-8, FLIP, and RIPK signaling can determine cell fate in response to many different types of cellular signaling and stress, a concept to which we will return.
Box 3. The role of ubiquitination.
In considering the dynamics of receptor activation, apoptosis, and programmed necrosis, it is useful to note the central role of ubiquitination in these processes. Both degradative and non-degradative ubquitination plays a central role in the control of NF-kB activation, as well as caspase-8 and RIPK1 signaling85, 86. Ubiquitin chains can be linked via several different lysine residues in the ubiquitin monomer, and recent reports show that RIPK1 is targeted by 4 separate types of ubiquitin linkages during receptor signaling87. The deubiquitinase CYLD is required remove ubiquitin chains from RIPK1, allowing formation of complex II39, and caspase-8 may block programmed necrosis by preventing this process53. More recent reports indicated that degradative ubiquitination by cIAP and XIAP prevent receptor-independent formation of a RIPK1-containing complex, termed the “ripoptosome,” that is capable of triggering caspase-8-mediated apoptosis as well as RIPK3-dependent necrosis40, 41. Furthermore, in the context of suppression of RIPK1-mediated inflammation at the RIG-I complex by caspase-8, it was reported that K63-linked RIPK1 ubiquitination is required to render RIPK1 susceptible to caspase-8-mediated cleavage, the mechanism by which RIPK1 signaling is reported to be suppressed in this context22. Caspase-8 has also been reported to be modified by non-degradative ubiquitination chains, and intriguingly this modification was reported to allow activation of non-cleavable caspase-8, a mutant able to suppress RIPK signaling but not trigger apoptosis85. It is therefore possible that dynamic ubiqutination is at least partially responsible for controlling the activation of caspase-8 by FLIP (see Box 1). Degradative ubiquitination of the caspase-8-FLIP complex has also been reported41, and the rapid proteosomal degradation of this complex could explain how it is prevented from triggering apoptosis. The study of the role of these and other posttranslational modifications in the dynamics of NF-kB activation, apoptosis and programmed necrosis will be crucial to understanding these interrelated processes.
RIPK3 & Caspase-8: Genetic evidence
RIPK3 is highly expressed in adult lymphoid tissue, and the first strong evidence that suppression of programmed necrosis might define the non-apoptotic role of caspase-8 was obtained in T cells. Initial reports held that caspase-8 deficient T-cells failed to accumulate due to proliferative defects; however, subsequent work showed that these cells proliferated normally, but did not accumulate due to increased rates of cell death42. Crucially, this cell death was found to be non-apoptotic, and proliferation could be restored in vitro by administration of the RIPK1 inhibitor Nec142.
Subsequent findings showed that concurrent ablation of caspase-8 and RIPK3 completely rescues developmental defects associated with caspase-8 deficiency33, 34 – mice lacking caspase-8 and RIPK3 are born at expected Medelian frequencies and display no overt phenotype. As expected, these animals are resistant to the lethal effects of CD95 ligation in vivo. Furthermore, their T cells proliferate and subsequently contract normally when activated in vivo by a bacterial superantigen33. As they age, caspase-8:RIPK3 double knockout animals display a lymphoaccumulative disorder similar to that observed in mice carrying mutations that inactivate CD95 or its ligand, characterized by accumulation of an aberrant population of CD3+ B220+ CD4- CD8- lymphoid cells33, 34. Further work showed that T-cell specific deletion of caspase-8 on a RIPK3-deficient genetic background recapitulates this disorder, indicating that this cell population arises from the T-cell lineage43. However, the selective events that yield this population remain to be elucidated.
Animals deficient for FADD display developmental and immune-cell defects similar to those observed in caspase-8 knockouts11. Initial work using an inducible Cre recombinase showed that deletion of FADD in the bone marrow leads to a dramatic decrease in hematopoietic precursor cells, as well as an impaired ability to generate both lymphoid and myeloid cells44. Subsequent work found that concurrent ablation of RIPK1 prevents the early embryonic lethality observed in FADD deficient animals32. Knockout of RIPK1 alone causes perinatal lethality, and the FADD-RIPK1 double knockout animal also die shortly after birth, precluding further analysis of FADD-deficient adults.
FLIPping the survival switch
Any discussion of the non-apoptotic role of caspase-8 must address the question of how the protease is activated in the non-apoptotic context. Given that caspase-8 is a protease whose activation leads to apoptotic cell death, how can its proteolitic activity suppress RIPK3 signaling without causing apoptosis?
Intriguingly, while FLIP inhibits apoptotic activation of caspase-8 by forming heterodimers with the caspase-8 proenzyme (Box 1, Figure 1), the resulting caspase-8-FLIP heterodimers possess catalytic activity45-47.In vitro and structural studies found that caspase-8-FLIP heterodimers form more readily than caspase-8 homodimers, because FLIP has greater affinity for the caspase-8 proenzyme than does the proenzyme itself 45. This property allows FLIP to activate caspase-8 even in the absence of the interdomain autocleavage events that are necessary for stabilization and activity of the caspase-8 homodimer45, 48. This is important because of the finding that a mouse expressing only a non-cleavable version of caspase-8 displays normal development and immune cell activation, but that cells and tissues from this mouse are resistant to apoptosis49. Thus, non-cleavable caspase-8—which can be activated by heterodimerization with FLIP, but not by homodimerization—can carry out the non-apoptotic functions of caspase-8. Reconstitution of caspase-8-deficient MEF cells with wild type or non-cleavable caspase-8 confirmed that non-cleavable caspase-8 is able to suppress RIPK3-dependent necrosis, but that it requires the presence of FLIP to do so33. (Fig. 2)
This work indicates that caspase-8 has distinct moieties with different functions: the caspase-8 homodimer causes apoptosis, and the catalytically active caspase-8-FLIP heterodimer, whose activity suppresses RIPK3 signaling and mediates the non-apoptotic role of caspase-833 (Figure 2). Importantly, when apoptosis is blocked downstream of caspase-8 activation, it was found that FLIP is nonetheless required to prevent RIPK3-dependent necrosis; that is, activation of the caspase-8 homodimer is not sufficient to prevent RIPK3-dependent necrosis33. Because FLIP is a transcriptional target of NF-kB, this work identifies FLIP upregulation as a key mechanism by which NF-kB signaling prevents both apoptosis and RIPK3 activation (Fig. 2). However, the mechanism by which the caspase-8-FLIP complex suppresses RIPK signaling is currently unknown. Both RIPK150 and RIPK351 have been described to be caspase-8 substrates, and a recent study showed that a cleavage mutant of RIPK1 could trigger necrosis even in the presence of caspase-8 activity52. Another recent study identified the deubiquitinase CYLD as a key substrate of caspase-8 in the prevention of RIPK-dependent necrosis53, though the complex in which the cleavage occurs in vivo remains to be elucidated. (Box 3)
The above treatment of FLIP signaling has considered only the long isoform of FLIP; however, a short version of FLIP (FLIPS), composed only of the prodomain, also exists54. FLIPS can inhibit caspase-8 activation in a dominant-negative fashion by competing for binding to FADD, but it does not possess the ability to activate caspase-8 by heterodimerization. Consistent with the model proposed above, FLIPS is not able to suppress RIPK activation55. This distinction is important because many viruses encode anti-apoptotic proteins homologous to FLIPS, and programmed necrosis has been implicated in anti-viral responses29, 56.
Caspase-8: roles beyond apoptosis
The data outlined above provide strong genetic support for the non-apoptotic functions of caspase-8 and FADD depending on the suppression of the RIPK1-RIPK3 signaling pathway32-34. In light of these findings, it is worthwhile to consider some non-apoptotic roles previously assigned to caspase-8 and FADD.
NF-kB signaling
Several reports have sought to explain the defects observed in caspase-8, FADD or FLIP deficient embryos and immune cells by implicating these proteins in the induction of NF-kB signaling (Box 2). The catalytic activity of caspase-8 was shown to be required for NF-kB activation in antigen-stimulated T cells, and caspase-8 was also found to associate with the CARMA-Bcl10-MALT-1 complex, which is required for NF-kB activation following antigen receptor ligation in T- and B-cells57. Caspase-8 was further shown to interact directly with TRAF family members, which promoted translocation of caspase-8 into lipid rafts58, and additional work implicated lipid rafts as the site of formation of the caspase-8-FLIP complex and its association with several proteins involved in NF-kB activation59. Indeed, FLIP has also been implicated in NF-kB activation, with a number of studies indicating that one or more of the FLIP cleavage fragments generated by association with caspase-8 are involved in NF-kB activation19, 60. Some caution must be used in interpreting these studies, as they present work from both murine and human systems. Unlike mice, humans have the close caspase-8 homologue caspase-1061,62, which displays regulatory and functional properties distinct from caspase-8. How the recent discoveries in murine systems will translate to human models remains an open question.
Box 2. Activation of NF-kB by TNF receptor.
When TNF binds to its trimeric receptor, it induces a conformational change that results in the recruitment of RIPK1 and the TNF receptor associated protein with a death domain (TRADD) to the cytoplasmic DD-containing tail of TNFR138 (1). These proteins subsequently recruit members of the TNFR-associated factor (TRAF) family, as well as cellular inhibitor of apoptosis (cIAP)-1 and -235. The cIAPs are E3 ubiquitin ligases which catalyze addition of ubiquitin moieties via non-degradative K63 linkages to RIPK1 and TRAF-2, as well as to the cIAPs themselves (2, black arrows); these modifications in turn allow recruitment of the linear ubiquitin chain assembly complex (LUBAC), which catalyzes addition of linear ubiquitin chains to multiple members of the complex, an event that is believed to stabilize these interactions and allow efficient, sustained signaling82, 83(3). NF-kB essential modulator (NEMO), the core component of the IkB kinase (IKK) complex, is among the ubiquitination targets of LUBAC; this modification stably recruits NEMO and the IKK complex. The IKK complex is then phosphorylated and thereby activated by kinases TAB2 and TAK1, which associate with ubiquitin linkages attached to RIPK184 (4). IKK complex activation leads to phosphorylation and proteasome-mediated degradation of the inhibitory molecule IkB, which in turn allows activation and nuclear translocation of the NF-kB transcption factor complexes35, and transcriptional upregulation of NF-kB targets, including FLIP. Following these events, ubiquitin chains are removed from RIPK1 by the deubiquitinase CYLD (5), which allows RIPK1 to translocate to the cytosol to form a second complex; this is believed to be the site of functional interaction between RIPK1, RIPK3, FADD, FLIP, and caspase-829, 39, 55 (Described in Fig. 2).
Nonetheless, the idea that caspase-8 plays a role in NF-kB activation has met with some recent setbacks. The timing and tissues involved in embryonic lethality of caspase-8-deficient mice are distinct from those observed in NF-kB-related knockouts—the latter die around E13-14 due to liver defects63, 64. Furthermore, caspase-8-RIPK3 double-knockout T-cells proliferate normally33, without any of the defects observed in T cells lacking NF-kB components63, Bcl10, CARMA-1 or MALT-165-67. Indeed a careful analysis of T cells indicated that caspase-8-deficient cells have normal NF-kB activation, and that the defects associated with caspase-8-deficeincy (initial proliferation followed by necrotic death) are distinct from those observed in cells lacking NF-kB signaling (lack of proliferation)42. These findings similarly raise questions about the proposed role of caspase-8-cleaved FLIP in NF-kB activation, as caspase-8 deficiency would presumably abolish this protein species. Indeed, careful analysis of FLIP-deficient T68 and B69 cells revealed that, while both cell types displayed an increased susceptibility to cell death, NF-kB signaling remained intact.
Beyond T-cell activation, both caspase-8 and FADD have also been reported to be required for NF-kB activation and proliferation in B cells, in response to the innate pattern recognition receptors TLR3 and TLR417, 70. In considering the role of FADD and caspase-8 in this context, it is interesting to note that both TLR-3 and TLR-4 signal through the adapter protein TRIF. This protein contains a Rip Homotypic Interaction Motif (RHIM) domain, and is able to recruit RIPK1 and RIPK371; it has also been reported to trigger FADD- and caspase-8 dependent apoptosis72. A recent report indicated that a RIPK1-containing complex capable of triggering both apoptosis and RIPK3-dependent necrosis is recruited to TRIF following TLR-3 ligation40. This report also showed the presence of FLIP in this complex, and a requirement for this molecule in limiting caspase-8 activation and suppressing RIPK3-dependent necrosis. Together, this work strongly implies that the requirement for FADD, caspase-8, and FLIP in TLR signaling is defined by suppression of RIPK3.
Caspase-8 and inflammation
Several reports have implicated caspase-8 in the suppression of inflammation. It was initially observed that, in mice in which caspase-8 was conditionally deleted in hepatocytes, partial hepatectomy led to a blunted proliferative response followed by chronic inflammation of the liver73. Subsequently, it was found that conditional deletion of caspase-8 in basal keratinocytes led to severe and fatal inflammatory skin disease, which could be partially ameliorated by knockout of TNF. Caspase-8 deficient epidermal cells displayed increased inflammatory signaling in response to transfected DNA, which raised the possibility that caspase-8 could function to suppress an inflammatory pathway downstream of an innate nucleotide sensor23. Subsequent work using sendai virus infection as a model system showed that caspase-8 suppresses activation of the pro-inflammatory transcription factor IRF-3 by the cytosolic RNA sensor RIG-I22. Significantly, this suppression was shown to be mediated by cleavage of RIPK1, which is recruited to a complex containing RIG-I and its adapter, MAVS (Box 3).
Here again, the phenotype of the caspase-8:RIPK3 knockout must be taken into account. Unlike the severe epidermal inflammation observed in skin-specific knockout of caspase-8, skin from the caspase-8-RIPK3 double knockout animal was entirely normal33. This implies that whatever defect provokes inflammation in the absence of caspase-8 is corrected by removal of RIPK3. Intriguingly, epidermal ablation of NF-kB signaling also causes severe inflammation74. FLIP connects NF-kB signaling to suppression of the RIPK1-RIPK3 pathway by caspase-8; the possibility therefore emerges that commonalities between epithelial deletion of caspase-8 and NF-kB signaling could be due to disregulation of RIPK1-RIPK3 signaling.
Recent findings from another epithelial model—the gut—also support these ideas75. This study found that FADD deletion in the gut led to inflammatory bowel disease, an effect that was rescued by concurrent ablation of RIPK3. Importantly, the inflammatory phenotype associated with FADD deletion was also ameliorated by deletion of TNF, the innate immune adapter Myd88, or elimination of gut microbiota. Similarly to the skin model discussed above, this finding presents the specter of unrestrained RIPK signaling leading to a multi-faceted, self-reinforcing inflammatory response, with TNF signaling and gut barrier breakdown both contributing. NF-kB deletion in this context would be expected to sensitize cells not only to RIPK3-dependent effects, but also to apoptosis—an outcome that caspase-8 or FADD deletion obviously prevents. Indeed, it was found that deletion of CYLD—which is required for RIPK activation downstream of TNF—prevented inflammation caused by intestinal deletion of FADD, but not of NF-kB signaling75. This implies that both apoptosis and RIPK-dependent necrosis contribute to the inflammatory effects of NF-kB deletion; analyzing these effects in a tissue other than the gut, where any cell death can cause barrier breakdown and thus severe inflammation, will be informative in delineating the relative inflammatory contributions of apoptotic and necrotic cell death.
Concluding Remarks
Recent work provides strong genetic evidence for the idea that suppression of RIPK1-RIPK3 signaling defines the non-apoptotic roles of FADD and caspase-8. Because caspase-8, FADD and FLIP are able to suppress RIPK1-RIPK3-mediated programmed necrosis in cultured cells, it is tempting to conclude that prevention of programmed necrosis defines the non-apoptotic role of these proteins. However, because the pathways of programmed necrosis are so poorly understood, all that can be said definitively is that the death of caspase-8-deficient embryos is RIPK3-dependent; what cellular processes are mediated by RIPK3 in this context, and what signaling events trigger them, remain unknown. The known roles or RIPK1 and RIPK3 in TNF and innate immune signaling make it tempting to reinterpret the proposed non-apoptotic roles for caspase-8, FADD and FLIP in these pathways, as we have done. However, we have not been exhaustive. FADD has been implicated in cell cycle progression24-26, while caspase-8 has been shown to promote cell motility and affect tumor metastasis21. It is possible that these functions are independent of RIPK1-RIPK3 signaling, but are simply not required for normal development and survival and therefore do not manifest in double-knockout mice. However, the intriguing possibility exists that these effects of FADD and caspase-8 are due to modulation of unknown effects of RIPK1-RIPK3 signaling. Rigorous reevaluation of possible roles for RIPK signaling in these contexts is needed, and until the downstream effects of the RIPK pathway are understood, we cannot rule out their involvement.
References
- 1.Muzio M, et al. FLICE, a novel FADD-homologous ICE/CED-3-like protease, is recruited to the CD95 (Fas/APO-1) death--inducing signaling complex. Cell. 1996;85:817–27. doi: 10.1016/s0092-8674(00)81266-0. [DOI] [PubMed] [Google Scholar]
- 2.Wilson NS, Dixit V&, Ashkenazi A. Death receptor signal transducers: nodes of coordination in immune signaling networks. Nat Immunol. 2009;10:348–55. doi: 10.1038/ni.1714. [DOI] [PubMed] [Google Scholar]
- 3.Irmler M, et al. Inhibition of death receptor signals by cellular FLIP. Nature. 1997;388:190–5. doi: 10.1038/40657. [DOI] [PubMed] [Google Scholar]
- 4.Watanabe-Fukunaga R, Brannan CI, Copeland NG, Jenkins NA, Nagata S. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature. 1992;356:314–7. doi: 10.1038/356314a0. [DOI] [PubMed] [Google Scholar]
- 5.Takahashi T, et al. Generalized lymphoproliferative disease in mice, caused by a point mutation in the Fas ligand. Cell. 1994;76:969–76. doi: 10.1016/0092-8674(94)90375-1. [DOI] [PubMed] [Google Scholar]
- 6.Hakem R, et al. Differential requirement for caspase 9 in apoptotic pathways in vivo. Cell. 1998;94:339–52. doi: 10.1016/s0092-8674(00)81477-4. [DOI] [PubMed] [Google Scholar]
- 7.Kuida K, et al. Reduced apoptosis and cytochrome c-mediated caspase activation in mice lacking caspase 9. Cell. 1998;94:325–37. doi: 10.1016/s0092-8674(00)81476-2. [DOI] [PubMed] [Google Scholar]
- 8.Pellegrini M, Belz G, Bouillet P, Strasser A. Shutdown of an acute T cell immune response to viral infection is mediated by the proapoptotic Bcl-2 homology 3-only protein Bim. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:14175–80. doi: 10.1073/pnas.2336198100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Varfolomeev EE, et al. Targeted disruption of the mouse Caspase 8 gene ablates cell death induction by the TNF receptors, Fas/Apo1, and DR3 and is lethal prenatally. Immunity. 1998;9:267–76. doi: 10.1016/s1074-7613(00)80609-3. [DOI] [PubMed] [Google Scholar]
- 10.Zhang J, Cado D, Chen A, Kabra NH, Winoto A. Fas-mediated apoptosis and activation-induced T-cell proliferation are defective in mice lacking FADD/Mort1. Nature. 1998;392:296–300. doi: 10.1038/32681. [DOI] [PubMed] [Google Scholar]
- 11.Yeh WC, et al. FADD: essential for embryo development and signaling from some, but not all, inducers of apoptosis. Science. 1998;279:1954–8. doi: 10.1126/science.279.5358.1954. [DOI] [PubMed] [Google Scholar]
- 12.Yeh WC, et al. Requirement for Casper (c-FLIP) in regulation of death receptorinduced apoptosis and embryonic development. Immunity. 2000;12:633–42. doi: 10.1016/s1074-7613(00)80214-9. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y, et al. Conditional Fas-associated death domain protein (FADD): GFP knockout mice reveal FADD is dispensable in thymic development but essential in peripheral T cell homeostasis. J Immunol. 2005;175:3033–44. doi: 10.4049/jimmunol.175.5.3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Imtiyaz HZ, et al. The Fas-associated death domain protein is required in apoptosis and TLR-induced proliferative responses in B cells. J Immunol. 2006;176:6852–61. doi: 10.4049/jimmunol.176.11.6852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salmena L, et al. Essential role for caspase 8 in T-cell homeostasis and T-cellmediated immunity. Genes Dev. 2003;17:883–95. doi: 10.1101/gad.1063703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beisner DR, Ch’en, IL, Kolla RV, Hoffmann A, Hedrick SM. Cutting edge: innate immunity conferred by B cells is regulated by caspase-8. J Immunol. 2005;175:3469–73. doi: 10.4049/jimmunol.175.6.3469. [DOI] [PubMed] [Google Scholar]
- 17.Su H, et al. Requirement for caspase-8 in NF-kappaB activation by antigen receptor. Science. 2005;307:1465–8. doi: 10.1126/science.1104765. [DOI] [PubMed] [Google Scholar]
- 18.Hu WH, Johnson H&, Shu HB. Activation of NF-kappaB by FADD, Casper, and caspase-8. The Journal of biological chemistry. 2000;275:10838–44. doi: 10.1074/jbc.275.15.10838. [DOI] [PubMed] [Google Scholar]
- 19.Golks A, Brenner D, Krammer PH, Lavrik IN. The c-FLIP-NH2 terminus (p22-FLIP) induces NF-kappaB activation. J Exp Med. 2006;203:1295–305. doi: 10.1084/jem.20051556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dohrman A, et al. Cellular FLIP (long form) regulates CD8+ T cell activation through caspase-8-dependent NF-kappa B activation. Journal of immunology. 2005;174:5270–8. doi: 10.4049/jimmunol.174.9.5270. [DOI] [PubMed] [Google Scholar]
- 21.Helfer B, et al. Caspase-8 promotes cell motility and calpain activity under nonapoptotic conditions. Cancer Res. 2006;66:4273–8. doi: 10.1158/0008-5472.CAN-05-4183. [DOI] [PubMed] [Google Scholar]
- 22.Rajput A, et al. RIG-I RNA helicase activation of IRF3 transcription factor is negatively regulated by caspase-8-mediated cleavage of the RIP1 protein. Immunity. 2011;34:340–51. doi: 10.1016/j.immuni.2010.12.018. [DOI] [PubMed] [Google Scholar]
- 23.Kovalenko A, et al. Caspase-8 deficiency in epidermal keratinocytes triggers an inflammatory skin disease. J Exp Med. 2009;206:2161–77. doi: 10.1084/jem.20090616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alappat EC, et al. Phosphorylation of FADD at serine 194 by CKIalpha regulates its nonapoptotic activities. Mol Cell. 2005;19:321–32. doi: 10.1016/j.molcel.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 25.Hua ZC, Sohn SJ, Kang C, Cado D, Winoto A. A function of Fas-associated death domain protein in cell cycle progression localized to a single amino acid at its Cterminal region. Immunity. 2003;18:513–21. doi: 10.1016/s1074-7613(03)00083-9. [DOI] [PubMed] [Google Scholar]
- 26.Zhang J, Kabra NH, Cado D, Kang C, Winoto A. FADD-deficient T cells exhibit a disaccord in regulation of the cell cycle machinery. J Biol Chem. 2001;276:29815–8. doi: 10.1074/jbc.M103838200. [DOI] [PubMed] [Google Scholar]
- 27.Vercammen D, et al. Inhibition of caspases increases the sensitivity of L929 cells to necrosis mediated by tumor necrosis factor. J Exp Med. 1998;187:1477–85. doi: 10.1084/jem.187.9.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Degterev A, et al. Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat Chem Biol. 2008;4:313–21. doi: 10.1038/nchembio.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cho YS, et al. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell. 2009;137:1112–23. doi: 10.1016/j.cell.2009.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.He S, et al. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell. 2009;137:1100–11. doi: 10.1016/j.cell.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 31.Zhang DW, et al. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science. 2009;325:332–6. doi: 10.1126/science.1172308. [DOI] [PubMed] [Google Scholar]
- 32.Zhang H, et al. Functional complementation between FADD and RIP1 in embryos and lymphocytes. Nature. 2011;471:373–6. doi: 10.1038/nature09878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oberst A, et al. Catalytic activity of the caspase-8-FLIP(L) complex inhibits RIPK3-dependent necrosis. Nature. 2011;471:363–7. doi: 10.1038/nature09852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaiser WJ, et al. RIP3 mediates the embryonic lethality of caspase-8-deficient mice. Nature. 2011;471:368–72. doi: 10.1038/nature09857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wajant H, Scheurich P. TNFR1-induced activation of the classical NF-kappaB pathway. The FEBS journal. 2011;278:862–76. doi: 10.1111/j.1742-4658.2011.08015.x. [DOI] [PubMed] [Google Scholar]
- 36.O’Donnell MA, Ting AT. RIP1 comes back to life as a cell death regulator in TNFR1 signaling. The FEBS journal. 2011;278:877–87. doi: 10.1111/j.1742-4658.2011.08016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Micheau O, Lens S, Gaide O, Alevizopoulos K, Tschopp J. NF-kappaB signals induce the expression of c-FLIP. Mol Cell Biol. 2001;21:5299–305. doi: 10.1128/MCB.21.16.5299-5305.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Micheau O, Tschopp J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell. 2003;114:181–90. doi: 10.1016/s0092-8674(03)00521-x. [DOI] [PubMed] [Google Scholar]
- 39.Wang L, Du F, Wang X. TNF-alpha induces two distinct caspase-8 activation pathways. Cell. 2008;133:693–703. doi: 10.1016/j.cell.2008.03.036. [DOI] [PubMed] [Google Scholar]
- 40.Feoktistova M, et al. cIAPs Block Ripoptosome Formation, a RIP1/Caspase-8 Containing Intracellular Cell Death Complex Differentially Regulated by cFLIP Isoforms. Molecular cell. 2011 doi: 10.1016/j.molcel.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tenev T, et al. The Ripoptosome, a Signaling Platform that Assembles in Response to Genotoxic Stress and Loss of IAPs. Molecular cell. 2011 doi: 10.1016/j.molcel.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 42.Ch’en IL, et al. Antigen-mediated T cell expansion regulated by parallel pathways of death. Proc Natl Acad Sci U S A. 2008;105:17463–8. doi: 10.1073/pnas.0808043105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ch’en IL, Tsau JS, Molkentin JD, Komatsu M, Hedrick SM. Mechanisms of necroptosis in T cells. The Journal of experimental medicine. 2011;208:633–41. doi: 10.1084/jem.20110251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rosenberg S, Zhang H, Zhang J. FADD deficiency impairs early hematopoiesis in the bone marrow. Journal of immunology. 2011;186:203–13. doi: 10.4049/jimmunol.1000648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boatright KM, Deis C, Denault JB, Sutherlin DP, Salvesen GS. Activation of caspases-8 and -10 by FLIP(L) Biochem J. 2004;382:651–7. doi: 10.1042/BJ20040809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chang DW, et al. c-FLIP(L) is a dual function regulator for caspase-8 activation and CD95-mediated apoptosis. EMBO J. 2002;21:3704–14. doi: 10.1093/emboj/cdf356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Micheau O, et al. The long form of FLIP is an activator of caspase-8 at the Fas deathinducing signaling complex. J Biol Chem. 2002;277:45162–71. doi: 10.1074/jbc.M206882200. [DOI] [PubMed] [Google Scholar]
- 48.Pop C, et al. FLIP(L) induces caspase 8 activity in the absence of interdomain caspase 8 cleavage and alters substrate specificity. The Biochemical journal. 2011;433:447–57. doi: 10.1042/BJ20101738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kang TB, et al. Mutation of a self-processing site in caspase-8 compromises its apoptotic but not its nonapoptotic functions in bacterial artificial chromosometransgenic mice. J Immunol. 2008;181:2522–32. doi: 10.4049/jimmunol.181.4.2522. [DOI] [PubMed] [Google Scholar]
- 50.Lin Y, Devin A, Rodriguez Y, Liu ZG. Cleavage of the death domain kinase RIP by caspase-8 prompts TNF-induced apoptosis. Genes & development. 1999;13:2514–26. doi: 10.1101/gad.13.19.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Feng S, et al. Cleavage of RIP3 inactivates its caspase-independent apoptosis pathway by removal of kinase domain. Cell Signal. 2007;19:2056–67. doi: 10.1016/j.cellsig.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 52.Lu JV, et al. Complementary roles of Fas-associated death domain (FADD) and receptor interacting protein kinase-3 (RIPK3) in T-cell homeostasis and antiviral immunity. Proceedings of the National Academy of Sciences of the United States of America. 2011 doi: 10.1073/pnas.1102779108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.O’Donnell MA, et al. Caspase 8 inhibits programmed necrosis by processing CYLD. Nature Cell Biology. 2011 doi: 10.1038/ncb2362. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thome M, et al. Viral FLICE-inhibitory proteins (FLIPs) prevent apoptosis induced by death receptors. Nature. 1997;386:517–21. doi: 10.1038/386517a0. [DOI] [PubMed] [Google Scholar]
- 55.Geserick P, et al. Cellular IAPs inhibit a cryptic CD95-induced cell death by limiting RIP1 kinase recruitment. J Cell Biol. 2009;187:1037–54. doi: 10.1083/jcb.200904158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Upton JW, Kaiser WJ, Mocarski ES. Virus inhibition of RIP3-dependent necrosis. Cell Host Microbe. 2010;7:302–13. doi: 10.1016/j.chom.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lemmers B, et al. Essential role for caspase-8 in Toll-like receptors and NFkappaB signaling. J Biol Chem. 2007;282:7416–23. doi: 10.1074/jbc.M606721200. [DOI] [PubMed] [Google Scholar]
- 58.Bidere N, Snow AL, Sakai K, Zheng L, Lenardo MJ. Caspase-8 regulation by direct interaction with TRAF6 in T cell receptor-induced NF-kappaB activation. Current biology: CB. 2006;16:1666–71. doi: 10.1016/j.cub.2006.06.062. [DOI] [PubMed] [Google Scholar]
- 59.Misra RS, et al. Caspase-8 and c-FLIPL associate in lipid rafts with NF-kappaB adaptors during T cell activation. The Journal of biological chemistry. 2007;282:19365–74. doi: 10.1074/jbc.M610610200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kataoka T, Tschopp J. N-terminal fragment of c-FLIP(L) processed by caspase 8 specifically interacts with TRAF2 and induces activation of the NF-kappaB signaling pathway. Molecular and cellular biology. 2004;24:2627–36. doi: 10.1128/MCB.24.7.2627-2636.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sprick MR, et al. Caspase-10 is recruited to and activated at the native TRAIL and CD95 death-inducing signalling complexes in a FADD-dependent manner but can not functionally substitute caspase-8. The EMBO journal. 2002;21:4520–30. doi: 10.1093/emboj/cdf441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fernandes-Alnemri T, et al. In vitro activation of CPP32 and Mch3 by Mch4, a novel human apoptotic cysteine protease containing two FADD-like domains. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:7464–9. doi: 10.1073/pnas.93.15.7464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Beg AA, Sha WC, Bronson RT, Ghosh S, Baltimore D. Embryonic lethality and liver degeneration in mice lacking the RelA component of NF-kappa B. Nature. 1995;376:167–70. doi: 10.1038/376167a0. [DOI] [PubMed] [Google Scholar]
- 64.Tanaka M, et al. Embryonic lethality, liver degeneration, and impaired NF-kappa B activation in IKK-beta-deficient mice. Immunity. 1999;10:421–9. doi: 10.1016/s1074-7613(00)80042-4. [DOI] [PubMed] [Google Scholar]
- 65.Jost PJ, et al. Bcl10/Malt1 signaling is essential for TCR-induced NF-kappaB activation in thymocytes but dispensable for positive or negative selection. Journal of immunology. 2007;178:953–60. doi: 10.4049/jimmunol.178.2.953. [DOI] [PubMed] [Google Scholar]
- 66.Ruland J, et al. Bcl10 is a positive regulator of antigen receptor-induced activation of NF-kappaB and neural tube closure. Cell. 2001;104:33–42. doi: 10.1016/s0092-8674(01)00189-1. [DOI] [PubMed] [Google Scholar]
- 67.Wang D, et al. A requirement for CARMA1 in TCR-induced NF-kappa B activation. Nature immunology. 2002;3:830–5. doi: 10.1038/ni824. [DOI] [PubMed] [Google Scholar]
- 68.Zhang N, He YW. An essential role for c-FLIP in the efficient development of mature T lymphocytes. The Journal of experimental medicine. 2005;202:395–404. doi: 10.1084/jem.20050117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang H, et al. A role for cFLIP in B cell proliferation and stress MAPK regulation. Journal of immunology. 2009;182:207–15. doi: 10.4049/jimmunol.182.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Imtiyaz HZ, et al. The Fas-associated death domain protein is required in apoptosis and TLR-induced proliferative responses in B cells. Journal of immunology. 2006;176:6852–61. doi: 10.4049/jimmunol.176.11.6852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kaiser WJ, Offermann MK. Apoptosis induced by the toll-like receptor adaptor TRIF is dependent on its receptor interacting protein homotypic interaction motif. Journal of immunology. 2005;174:4942–52. doi: 10.4049/jimmunol.174.8.4942. [DOI] [PubMed] [Google Scholar]
- 72.Weber A, et al. Proapoptotic signalling through Toll-like receptor-3 involves TRIFdependent activation of caspase-8 and is under the control of inhibitor of apoptosis proteins in melanoma cells. Cell death and differentiation. 2010;17:942–51. doi: 10.1038/cdd.2009.190. [DOI] [PubMed] [Google Scholar]
- 73.Ben Moshe T, et al. Role of caspase-8 in hepatocyte response to infection and injury in mice. Hepatology. 2007;45:1014–24. doi: 10.1002/hep.21495. [DOI] [PubMed] [Google Scholar]
- 74.Pasparakis M, et al. TNF-mediated inflammatory skin disease in mice with epidermis-specific deletion of IKK2. Nature. 2002;417:861–6. doi: 10.1038/nature00820. [DOI] [PubMed] [Google Scholar]
- 75.Welz PS, et al. FADD prevents RIP3-mediated epithelial cell necrosis and chronic intestinal inflammation. Nature. 2011 doi: 10.1038/nature10273. [DOI] [PubMed] [Google Scholar]
- 76.Pop C, Salvesen GS. Human caspases: activation, specificity, and regulation. J Biol Chem. 2009;284:21777–81. doi: 10.1074/jbc.R800084200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Boatright KM, et al. A unified model for apical caspase activation. Mol Cell. 2003;11:529–41. doi: 10.1016/s1097-2765(03)00051-0. [DOI] [PubMed] [Google Scholar]
- 78.Pop C, Fitzgerald P, Green DR, Salvesen GS. Role of proteolysis in caspase-8 activation and stabilization. Biochemistry. 2007;46:4398–407. doi: 10.1021/bi602623b. [DOI] [PubMed] [Google Scholar]
- 79.Oberst A, et al. Inducible dimerization and inducible cleavage reveal a requirement for both processes in caspase-8 activation. J Biol Chem. 2010;285:16632–42. doi: 10.1074/jbc.M109.095083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hughes MA, et al. Reconstitution of the death-inducing signaling complex reveals a substrate switch that determines CD95-mediated death or survival. Mol Cell. 2009;35:265–79. doi: 10.1016/j.molcel.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 81.Yu JW, Jeffrey PD, Shi Y. Mechanism of procaspase-8 activation by c-FLIPL. Proc Natl Acad Sci U S A. 2009;106:8169–74. doi: 10.1073/pnas.0812453106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tokunaga F, et al. Involvement of linear polyubiquitylation of NEMO in NF-kappaB activation. Nature cell biology. 2009;11:123–32. doi: 10.1038/ncb1821. [DOI] [PubMed] [Google Scholar]
- 83.Haas TL, et al. Recruitment of the linear ubiquitin chain assembly complex stabilizes the TNF-R1 signaling complex and is required for TNF-mediated gene induction. Molecular cell. 2009;36:831–44. doi: 10.1016/j.molcel.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 84.Blonska M, et al. TAK1 is recruited to the tumor necrosis factor-alpha (TNF-alpha) receptor 1 complex in a receptor-interacting protein (RIP)-dependent manner and cooperates with MEKK3 leading to NF-kappaB activation. The Journal of biological chemistry. 2005;280:43056–63. doi: 10.1074/jbc.M507807200. [DOI] [PubMed] [Google Scholar]
- 85.Jin Z, et al. Cullin3-based polyubiquitination and p62-dependent aggregation of caspase-8 mediate extrinsic apoptosis signaling. Cell. 2009;137:721–35. doi: 10.1016/j.cell.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 86.Newton K, et al. Ubiquitin chain editing revealed by polyubiquitin linkage-specific antibodies. Cell. 2008;134:668–78. doi: 10.1016/j.cell.2008.07.039. [DOI] [PubMed] [Google Scholar]
- 87.Gerlach B, et al. Linear ubiquitination prevents inflammation and regulates immune signalling. Nature. 2011;471:591–6. doi: 10.1038/nature09816. [DOI] [PubMed] [Google Scholar]