Abstract
Inadequate β-cell mass can lead to insulin insufficiency and diabetes. During times of prolonged metabolic demand for insulin, the endocrine pancreas can respond by increasing β-cell mass, both by increasing cell size and by changing the balance between β-cell proliferation and apoptosis. In this paper, we review recent advances in our understanding of the mechanisms that control the adaptive expansion of β-cell mass, focusing on the islet’s response to pregnancy, a physiological state of insulin resistance. Functional characterization of factors controlling both β-cell proliferation and survival might not only lead to the development of successful therapeutic strategies to enhance the response of the β-cell to increased metabolic loads, but also improve islet transplantation regimens.
β-cell mass and diabetes mellitus
The endocrine pancreas is a microorgan that is essential for glucose homeostasis. Of the five endocrine cell types in the pancreas, the β-cell is arguably the most important, as it produces and secretes the amount of insulin necessary for optimal control of glucose homeostasis. β-cell mass is determined by the product of the number and size of pancreatic β-cells. Once thought to be static and slow in turnover, it is now known that adult β-cells can dynamically respond to systemic increases in insulin demand (here defined as an increase in metabolic load) by dramatically expanding their functional mass, at least in rodents and possibly in humans, as seen during aging [1], pregnancy [2], obesity [3] and genetic insulin resistance [4].
Compensatory changes in β-cell mass are controlled by increases in cell size and adjustments to the rate of β-cell proliferation and death. Current evidence suggests that dysregulation of these mechanisms is an essential feature in the pathogenesis of diabetes mellitus, an increasingly prevalent metabolic disorder that is estimated to affect over 300 million people by 2025 [5]. Indeed, individuals with either type 1 or type 2 diabetes show decreases in β-cell mass and increases in β-cell apoptosis, outweighing that of β-cell growth [6,7]. Furthermore, studies of obese patients (including non-diabetic, prediabetic and diabetic groups) reported an inverse relationship between blood glucose levels and β-cell volume below a certain threshold [8], illustrating the importance of β-cell mass as a central factor determining insulin secretory capacity.
Knowledge of the mechanisms that control the balance between the production and loss of β-cell mass promises to be useful for the treatment of both type 1 and type 2 diabetes. For type 1 diabetes, being able to expand β-cell mass ex vivo, or in vivo after islet transplantation, could increase the number of patients that can be treated with a limited supply of donor islets and also improve the outcome after transplantation. For type 2 diabetes, the identification of targets and pathways that mediate proliferation and/or apoptosis might lead to the development of novel drugs that stimulate β-cell growth in the patient and thus allow for improved glycemic control. This review summarizes recent advances towards understanding the processes that allow for the adaptive expansion of β-cell mass during instances of increased metabolic load, and focuses specifically on the pregnancy paradigm as an example.
Homeostatic control of β-cell mass
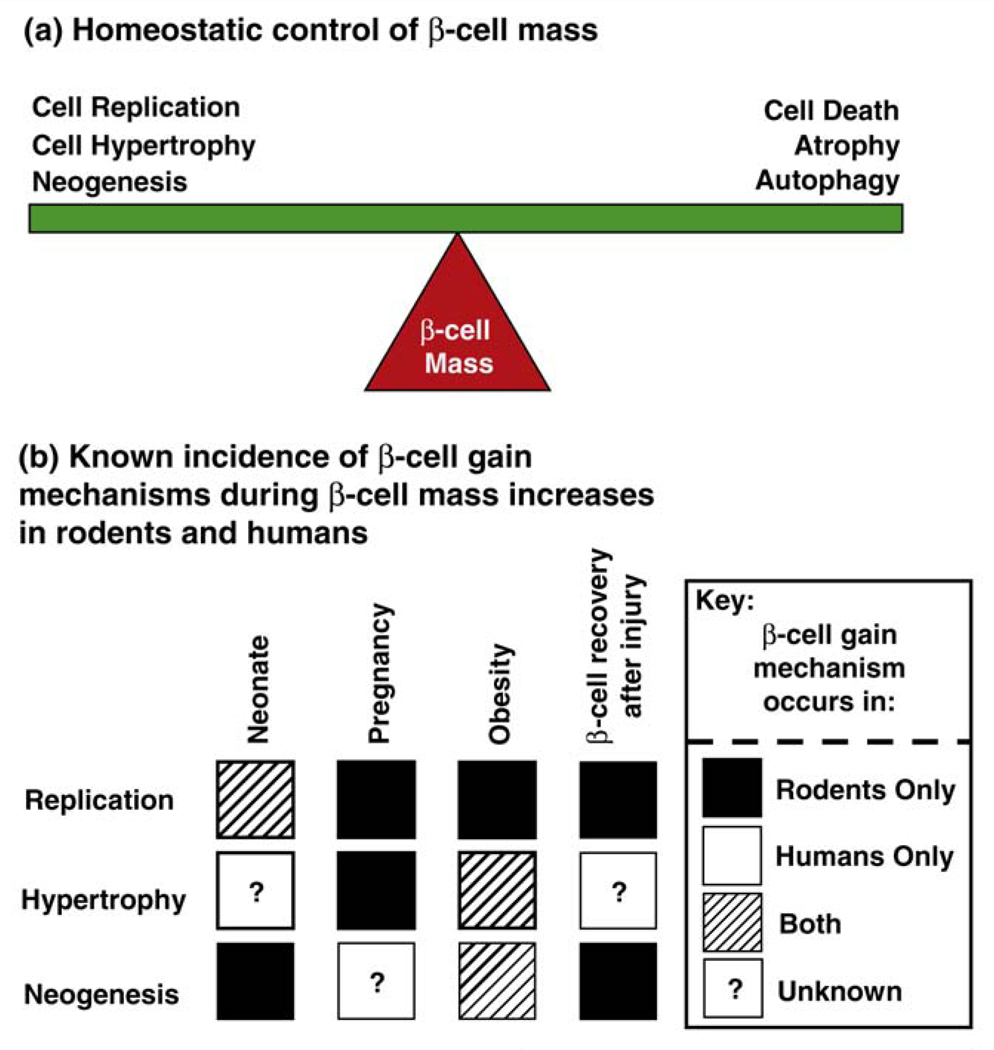
In adult mammals, β-cell mass is maintained by the balance between cell renewal and growth (cell replication, hypertrophy, neogenesis) and cell loss (cell death, atrophy, autophagy) (Figure 1a). There is now growing evidence suggesting that β-cell replication and hypertrophy, in both young mice and humans, occurs during periods of intense metabolic demand [6,9–11], e.g. in hyperglycemia after β-cell ablation [12,13] and in the neonatal period [14–18] (Figure 1b). Evidence for neogenesis (i.e. the production of new β-cells arising from the differentiation of progenitors) was for a long time limited to the detection of insulin-positive cells within the ductal epithelium [6,11,17,19] (Figure 1b). However, a recent genetic lineage tracing experiment showing that cells expressing Cre recombinase under the control of the carbonic anhydrase II promoter, a gene expressed at high levels in duct cells, strengthens the argument that a portion of β-cells can arise from the ductal compartment [20]. Furthermore, the fetal differentiation program, as marked by Cre recombinase under the control of the bHLH factor Ngn3, can be reactivated in the adult mouse by the extreme injury stimulus of pancreatic duct ligation [21]. As shown nearly ten years ago by Ferber and colleagues, and confirmed multiple times since then, forced expression of various pancreatic transcription factors and other agents can induce partial trans-differentiation of other endoderm-derived cells such as hepatocytes into insulin-producing cells in mice [22]. Regardless of whether and to what extent neogenesis or trans-differentiation might be exploitable to increase β-cell mass, it is clear that under most physiological circumstances the expansion of existing β-cell mass by hypertrophy and cell division is a major driver of postnatal islet expansion.
Figure 1.
Homeostatic control of β-cell mass in rodents and humans. (a) Control of β-cell mass (the fulcrum of the balance) is based on the relative contribution of processes that result in β-cell gain (replication, hypertrophy, neogenesis) and β-cell loss (death, atrophy, autophagy). A net increase in β-cell mass occurs when mechanisms involved in β-cell gain exceed those of β-cell loss. Improper regulation of this balance is a major contributor to the onset of diabetes. (b) Experimental evidence in rodents and humans of β-cell gain mechanisms (β-cell replication, hypertrophy and neogenesis) during adaptive increases in β-cell mass in response to various increases in metabolic loads (neonatal period, pregnancy, obesity and β-cell recovery after injury). Dark square, evidence for β-cell gain mechanism only in rodent models; white square, only in human autopsy pancreatic samples; striped square, evidence both in rodents and humans; question mark square, no current evidence. This highlights the plasticity of the β-cell’s ability to increase its mass during different physiological and pathological (hyperglycemic) states and the relatively large amount of knowledge that remains to be uncovered, especially with respect to human β-cell biology.
Postnatal β-cell mass is regulated by proliferation and apoptosis
Genetic lineage tracing studies performed in the young adult mouse indicate that the great majority of new β-cells are derived through replication of pre-existing β-cells and few, if any, newly formed β-cells stem from progenitor populations under normal circumstances, at least in rodents [23]. Additional genetic experiments in mice support this conclusion. For instance, when cell cycle arrest is induced specifically in β-cells by overexpression of p27 [24] or by deletion of Cdk4 postnatally, β-cell mass decreases or fails to expand, which would not be the case if neogenesis or transdifferentiation were major sources of new β-cells [25,26]. Of course, analogous studies cannot be performed in humans; however, it is worth noting that there are important differences in the activity profile of cell cycle genes between human and mouse islets. In a comprehensive study, Fiaschi-Taesch and colleagues catalogued the G1/S proteome of the human islet and found that Cdk4 and Cdk6 are expressed at comparable levels, whereas only Cdk4 was found in the mouse [27].
The capacity of the β-cell to expand in response to injury or a high-fat diet decreases with age in both humans and mice [28,29]. This decline correlates with epigenetic changes at the Cdkn2a locus, which encodes the cell cycle inhibitors, p16Ink4a and p19Arf [30,31]. In fact, manipulation of p16Ink4a expression in transgenic mice dramatically alters the proliferative capacity of β-cells, precisely as would be expected if p16Ink4a limits proliferation in aging β-cells [32]. Furthermore, gene expression studies uncovered a cell cycle regulatory module in islets that distinguishes between diabetes-resistant and diabetes-susceptible strains of leptin-deficient Ob/Ob mice, and successfully predicts their predisposition to diabetes [33]. Keller and colleagues performed one of the most comprehensive gene expression studies to date, investigating the effects of age, obesity and genetic predisposition to diabetes in multiple metabolic tissues. They identified a module of 217 genes involved in cell cycle regulation in islets. This module contains multiple Cdc (cell division cycle) and Mcm (minichromosome maintenance) homologues, as well as the cyclins A2, B1 and B2, the transcription factor FoxM1 and two E2F genes. Also among these differentially regulated genes is the anti-apoptotic gene Birc5, which we will discuss in more detail below as one of the differentially expressed genes in pregnancy. Strikingly, this gene expression module alone is sufficient to predict that islet cells can proliferate in response to obesity in C57Bl6 mice, but not in BTBR Ob/Ob mice, again stressing the importance of cell cycle regulation in the islet in adaptation to metabolic stress.
Cell cycle regulators are of course not only important in rodents, but also in humans. Strikingly, recent genome-wide association studies link the cyclin-dependent kinase inhibitors CDKN2A and CDKN2B (encoding p15Ink4b) and the cell cycle regulators CDC123 and CDKAL1 to the risk of type 2 diabetes [34–37]. These genetic data together indicate a role for β-cell proliferation at some time during ontogeny; they do not, however, address the question of when in life β-cell proliferation is important.
A subset of obese individuals is unable to compensate for insulin resistance, and thus develop type 2 diabetes mellitus, due in part to increased β-cell apoptosis [3,6]. Alternatively, a subset of individuals could have inadequate β-cell mass even before the onset of obesity and then fail to compensate. Intriguingly, pancreata from individuals with long-standing type 1 diabetes displayed not only measurable β-cell mass, but also evidence of β-cell apoptosis [7]. These findings were interpreted to suggest that even in autoimmune destruction of β-cells, regeneration of β-cell mass is attempted but balanced by cell death; however, it cannot be excluded that in these patients β-cell mass is simply lost more slowly (Figure 1a). Genetic models of obesity such as the Zucker diabetic fatty (ZDF) rat ( fa/fa) and transgenic mice overexpressing human islet amyloid polypeptide (IAPP) recapitulate the decrease in β-cell mass caused by increased β-cell apoptosis that accompanies the onset of type 2 diabetes in humans [11,38]. By contrast, the non-diabetic Zucker fatty (ZF) rat, which also becomes obese and resistant to insulin, adequately increases its β-cell mass through increased β-cell proliferation, hypertrophy and neogenesis without evidence of increased apoptosis [11]. Given the importance of maintaining β-cell mass, there is increased interest in understanding the mechanisms that control the regulation of β-cell hypertrophy, proliferation and apoptosis during times of increased metabolic demand.
Reversible β-cell mass expansion during pregnancy
It has been recognized for decades that increased β-cell mass is an adaptation to the progressive insulin resistance that develops during pregnancy in women. Placental lactogen and growth hormone increase hepatic gluconeogenesis and lipolysis, and maternal IGF-1 levels increase in response to increased growth hormone levels [2,39]. The precise mechanism of β-cell mass expansion, i.e. proliferation, neogenesis or increase in size, has been elucidated only in part [2,40,41] (Figure 2a). However, as with obese individuals, when compensatory β-cell mass expansion fails during gestation, diabetes results. Interestingly, long-term follow-up studies show that 70% of women who are diabetic during pregnancy develop type 2 diabetes later in life, emphasizing that the ability of β-cells to successfully adapt to increases in metabolic load is a common theme for preventing both gestational diabetes and type 2 diabetes [42].
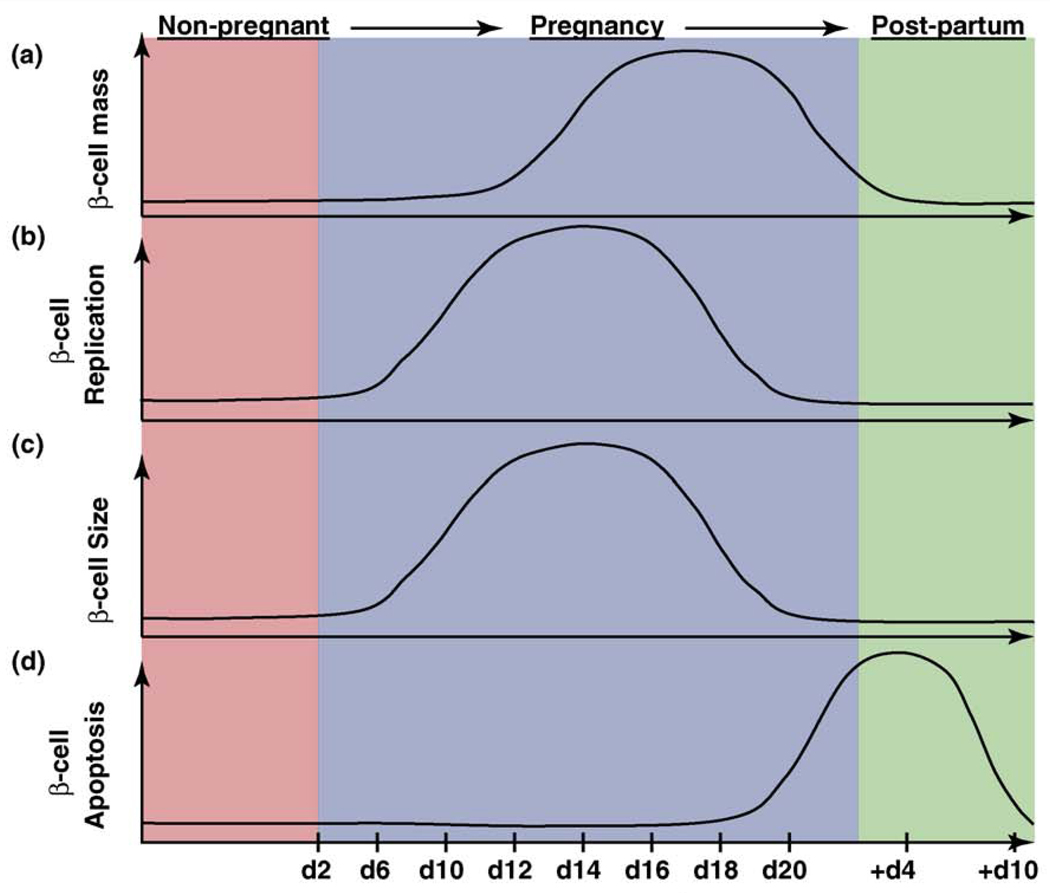
Figure 2.
β-cell mass dynamics during pregnancy in the mouse. (a) β-cell mass is increased by both (b) β-cell replication and (c) β-cell hypertrophy during the first two-thirds of gestation. After parturition, maternal β-cell mass returns to non-pregnant levels by (d) β-cell apoptosis, which increases through the end of pregnancy and is still detected 4–6 days after birth. The graphs represent approximate changes in these processes before pregnancy (red, non-pregnant), over the course of pregnancy (light purple) and post-partum (green), and show what is believed to occur during human pregnancy based on rodent studies. Serum lactogenic hormone levels during pregnancy are increased from gestational day 10 to 20, pointing to their key role in the adaptation of the islet to pregnancy [9].
Studies in rodents found a 3–4-fold increase in β-cell mass during gestation and demonstrated that in addition to substantial maternal β-cell hypertrophy [2,10,43], β-cell proliferation also increases dramatically during pregnancy [9,10] (Figure 2b,c). The peak of bromodeoxyuridine (BrdU) incorporation, an indicator of DNA synthesis during S-phase, occurs about two-thirds of the way through the gestational period, with labeling returning to pre-pregnancy levels shortly before parturition. Notably, this peak in DNA synthesis coincides with increased lactogen levels, suggesting that lactogenic activity is vital for the ability of β-cells to enhance proliferation and function in response to pregnancy [9]. Intriguingly, β-cell mass returns to normal levels within ten days after birth through increased β-cell apoptosis, decreased proliferation and reduced β-cell size [41] (Figure 2d). Although the mechanisms underlying these processes are not yet known, the pregnancy paradigm is a unique example of rapid and reversible β-cell mass expansion, with distinct bursts of both β-cell proliferation and β-cell apoptosis occurring in a physiological setting.
As a proof of principle for direct regulation of β-cell proliferation by prolactin (PRL) and placental lactogen (PL), overexpression of PL in the β-cell caused a dramatic increase in β-cell proliferation and β-cell mass, even resulting in hypoglycemia [44]. Similarly, global deletion of the prolactin receptor (PRLR), through which both PRL and PL signal, reduces β-cell mass and mildly impairs insulin secretion [45]. The requirement of the prolactin receptor for β-cell adaptation during pregnancy was demonstrated using pregnant mice heterozygous for the prolactin receptor null mutation. These mice exhibited reduced β-cell proliferation, decreased β-cell size and mass, and impaired glucose tolerance [46]. Interestingly, the maternal genotype had a significant effect on the phenotype of female offspring that became pregnant, as assessed by serum glucose levels [46], suggesting that in utero exposure to impaired glucose homeostasis alters the epigenetic memory of β-cells [47–49].
These findings provide a link to the well-known phenomenon that the intrauterine milieu affects glucose homeostasis in the adult (reviewed in [50]). Epidemiological studies in humans show very clearly how caloric intake by the mother affects the future glycemic health of the child. Intrauterine growth retardation in rodents is an experimental approach that has been used to investigate this phenomenon on the molecular level. In this model, epigenetic marks at the promoter of the β-cell transcription factor Pdx1 were found to be altered in the offspring of dams in which the uterine arteries had been ligated, causing intrauterine growth retardation [51]. Again, it is clear that the metabolic state of the fetus determines the epigenetic fate of the β-cell.
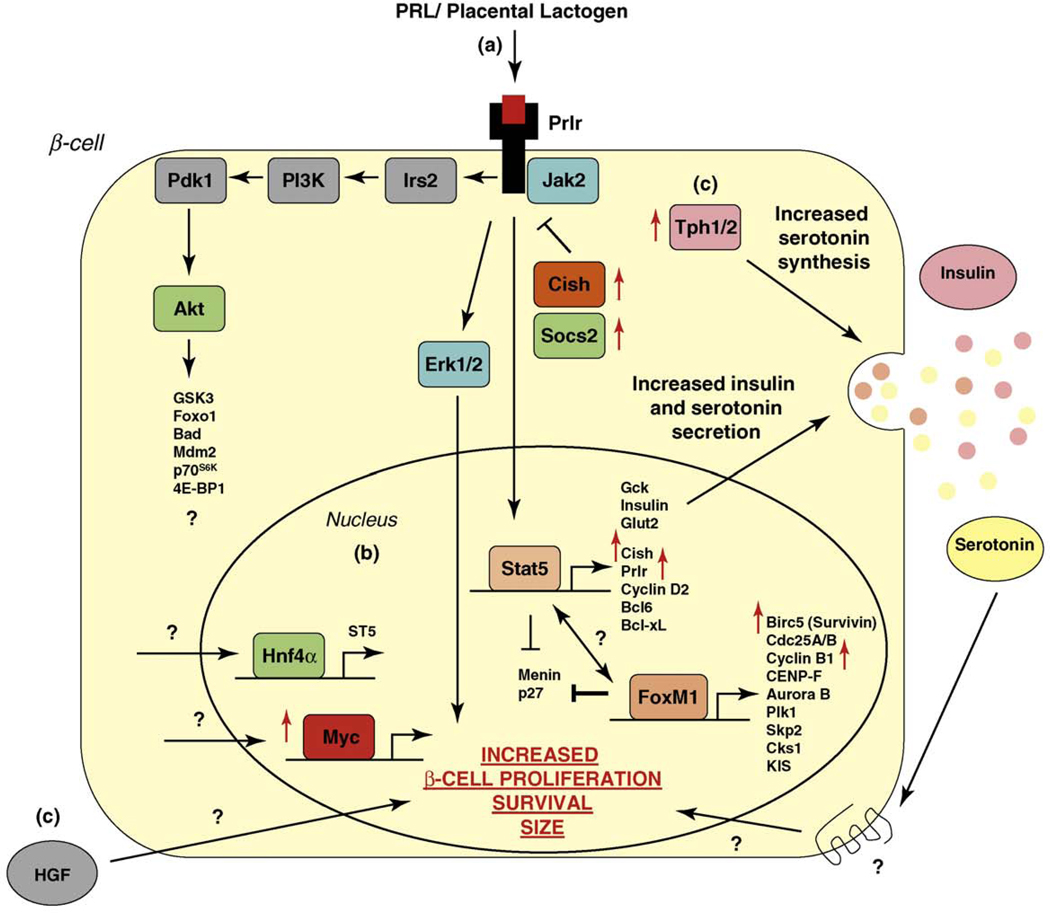
Although activation of multiple signaling pathways such as STAT5, MAPK and phosphatidylinositol 3-kinase and IRS1/2 enhances β-cell compensation downstream of the prolactin receptor in vitro [52–54], it is not clear whether PRL and/or PL stimulate these pathways in vivo. Additionally, a recent study showed that the orphan nuclear receptor hepatocyte nuclear factor-4 (HNF-4)α is required in β-cells for the proliferative response of pregnancy through activation of the Ras/ERK signaling cascade, at least in part through the regulation of the guanine nucleotide exchange factor ST5 (Figure 3) [55]. In addition, STAT5-dependent downregulation of the tumor suppressor gene menin (Men1) and subsequent inhibition of p18 and p27 are crucial events in β-cell expansion during pregnancy [56]. To complement the substantial progress that has been made in elucidating the contribution of selected genes to β-cell compensation, we recently reported the first systematic study investigating the global expression profile of islets in response to pregnancy. Gene expression analysis during the peak of β-cell DNA synthesis not only showed induction of Prlr and Cdk4 expression in the islets of pregnant mice, but also identified many genes that had not previously been reported to play a role in β-cell expansion, which we discuss further below [10].
Figure 3.
Known mechanisms responsible for β-cell gain during pregnancy. (a) Activation of PRL receptors upon binding of lactogens (PRL or placental lactogen) plays a pivotal role in the adaptation of the β-cell to pregnancy. Downstream signaling pathways of the PRL receptor include STAT5, phosphatidylinositol 3-kinase (PI3K) and MAPK pathways, targets of which have been implicated to lead to increased β-cell proliferation, survival and size. (b) Known transcription factors (listed with their target genes) that regulate the increase in β-cell mass during pregnancy. Red arrow indicates an increase in expression of genes in the islet during pregnancy day 14.5 [10]. (c) Possible PRL receptor independent mechanisms leading to β-cell gain mechanisms. For example, increased HGF levels in the islet endothelium correlates with increased β-cell proliferation in pregnant rats [83]. However, much is still to be discovered, as evidenced by the recent finding that upregulation of the developmental transcription factor MafB in β-cells occurs during pregnancy [84].
Both proliferative and survival signals are required for β-cell expansion
The onset of type 2 diabetes in both humans and rodents is accompanied by a progressive decrease in β-cell mass resulting from an increase in β-cell apoptosis. In this context, it is interesting that during the peak of β-cell proliferation in pregnancy, the antiapoptotic gene Birc5 was upregulated [10]. In pancreatic tissue sections from type 2 diabetics, apoptotic β-cells are often organized in pairs, a finding that has been interpreted as β-cell apoptosis following mitosis as a mechanism of β-cell death [57]. Thus, in this case, proliferation is attempted but leads to apoptosis of the proliferating cells. Studies in rodents [38,58] and primary human islets [57] also suggest that diabetes onset is a result of a failure of β-cell expansion, rather than a decrease in existing β-cell mass only (Figure 4).
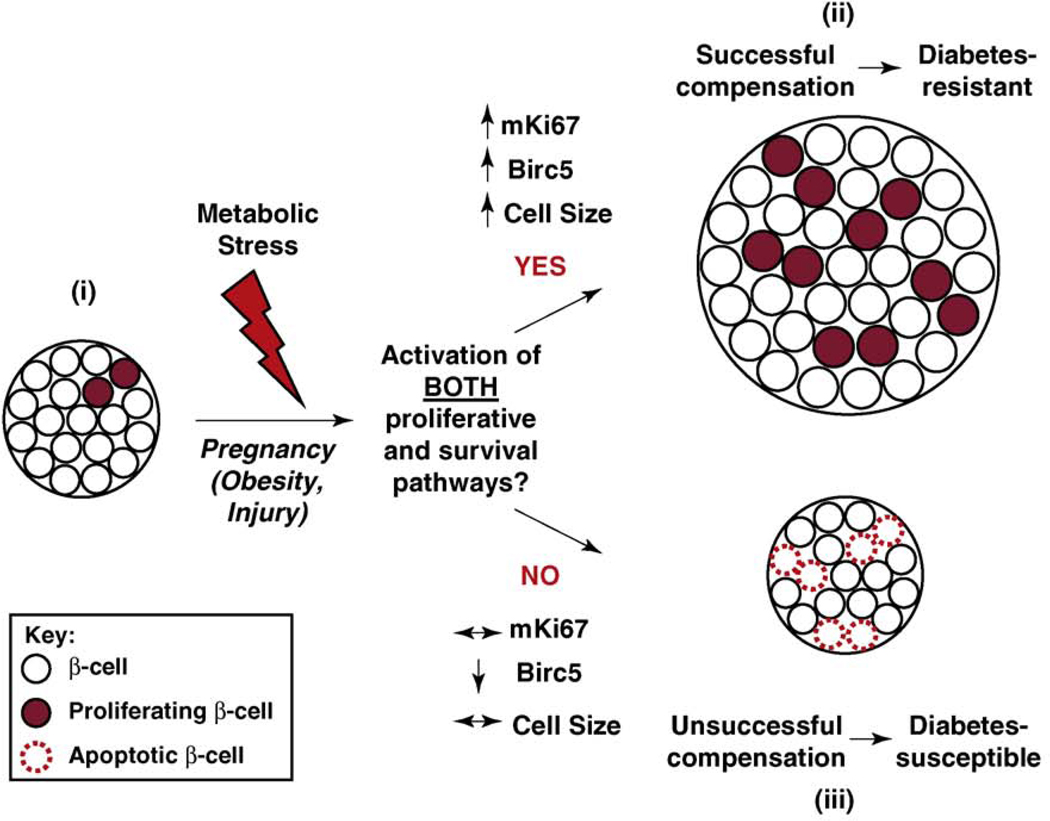
Figure 4.
β-cell expansion in response to increased metabolic loads. (i) Normally, β-cells in the mouse undergo very little turnover, exhibiting a low basal rate of replication. Pregnancy, obesity and β-cell recovery after injury are examples in which the systemic demand for insulin increases. (ii) To successfully compensate for the relative insulin deficiency that occurs during pregnancy, both proliferative and survival pathways are activated in β-cells, protecting against the onset of diabetes. Recent experimental evidence also suggests the same requirement during obesity and β-cell recovery after injury [10]. An increase in β-cell size also accompanies β-cell expansion occurring during pregnancy and obesity. (iii) If the expanding β-cell mass remains predisposed to β-cell apoptosis resulting from either an increased vulnerability during replication or from byproducts of increased metabolic load, β-cell compensation fails. If β-cell apoptosis overcomes β-cell renewal mechanisms and persists for a prolonged amount of time, diabetes might ensue. Apoptotic β-cells are often organized in pairs in pancreatic tissue sections from type 2 diabetics [57].
To successfully expand β-cell mass in response to increased metabolic load, the proliferating β-cell needs to induce mechanisms ensuring its survival through mitosis. A recent study showed a bifunctional role for Birc5 (survivin), controlling both proliferation and inhibition of apoptosis specifically in the β-cell in vivo [59]. Birc5-deficient animals progressively lose β-cell mass after 2 weeks of age, and Birc5-deficient cells exhibit dysmorphic nuclei, consistent with defective cell division [59]. Indeed, Birc5 gene expression in islets closely mirrors the proliferative profile as reflected in mRNA levels of Ki67 throughout pregnancy, obesity and β-cell recovery after injury [10]. These studies implicate Birc5 as a crucial and universal component that ensures successful expansion of β-cell mass in various conditions [60] (Figure 4). On the other hand, β-cell specific overexpression of c-Myc in transgenic mice not only increases β-cell proliferation, but also increases apoptosis, leading to the development of diabetes [61]. Simultaneous ablation of Caspase 3 in these transgenic mice protects the β-cell from c-Myc-induced apoptosis and diabetes, supporting the notion that the ability of β-cells to compensate during metabolic stress requires the simultaneous induction of both the proliferative and survival pathways [62]. Together, these studies suggest that if both proliferative and survival pathways are not induced simultaneously within the regenerating β-cell, the balance becomes skewed towards β-cell loss, accelerating the onset of diabetes.
Potential factors regulating β-cell proliferation, survival and function during pregnancy
Identifying the mediators affecting both β-cell proliferation and survival is a prerequisite for novel treatment options for diabetes. The current view of the major players in the pro-proliferative response of the β-cell to pregnancy is summarized schematically in Figure 3. Of the transcriptional mediators, Foxm1 has been shown to directly activate the transcription of Birc5, the antiapoptotic gene induced during pregnancy discussed previously in this review [63]. Indeed, Foxm1 is required for the maintenance of β-cell mass postnatally and during β-cell recovery after partial pancreatectomy [64]. Other known Foxm1 targets such as Skp2 have already been implicated in β-cell compensation in response to diet-induced obesity [65], and the proliferative response of the β-cell to pregnancy in mice is dependent on Foxm1 itself [66].
Both Cish and Socs2, members of the suppressor of cytokine signaling family of proteins, are robustly induced at the transcriptional level in pancreatic islets during pregnancy [10]. Each acts as a negative regulator of a variety of tyrosine kinases and receptors affecting β-cell proliferation, including the prolactin receptor [46]. Cish and Socs2 decrease activation of STAT5 [67,68], which has both pro-proliferative (Cyclin D2) [69] and anti-apoptotic (Bcl-xl, Bcl6) targets, as well as targets involved in β-cell function (Figure 3) [56,70]. Considering the underlying complexity and number of inhibitory pathways that normally restrain β-cell replication [71], inhibition of CISH and SOCS2 in patients with diabetes could induce both β-cell proliferation and survival. An analogous strategy, i.e. the inhibition of an inhibitor, has been used recently as a solution to enhance β-cell proliferation by attenuating signals that restrain GLP-1 action [72].
In addition to intracellular mediators, expression of signaling molecules such as the nerve growth factor receptor, Ngfr, is also dramatically upregulated in islets during pregnancy and during recovery after β-cell ablation [10]. Indeed, Ngf has been suggested to contribute to β-cell survival [73], and the outcome of islet transplantation is improved by pre-treating donor islets with Ngf [74]. Additionally, Ngf expression in skeletal muscle is strongly correlated with the islet cell cycle regulatory module activated during obesity, again suggesting a link to β-cell proliferation [33]. Similarly, hepatocyte growth factor, Hgf, increases β-cell proliferation and mass, and improves islet transplant outcomes in vivo [75,76]. Two of the genes with the most dramatic fold change in steady state mRNA levels in islets during pregnancy were tryptophan hydroxylase 1 and 2 (Tph1 and Tph2) [10]. Although it has been known for a long time that serotonin (5-hydroxytryptamine; 5-HT) is synthesized within β-cells and stored together with insulin in secretory granules [77,78], the physiological role of this synthesis and storage was unknown until very recently. Just this year, Paulmann and colleagues found impaired insulin secretion in mice deficient for Tph1 [79]. They further showed that 5-HT is coupled via the action of transglutaminases to two small GTPases, rendering the latter constitutively active and promoting insulin secretion. What remains to be established is why Tph1/2 expression is increased so dramatically in islets during pregnancy; however, it is tempting to speculate that it increases the insulin secretory response of the β-cell.
Conclusions
Comparison of several paradigms of β-cell expansion suggests that diverse mechanisms can be used by the islet to expand its mass depending on the metabolic setting [10]. This points to the potential development of several therapies to enhance β-mass expansion in patients with diabetes. However, many advances must be made to fully appreciate all the mechanisms available to enhance β-cell proliferation and/or hypertrophy, not only within the β-cell itself, but also encompassing signals originating from other organs, such as bone [80] and the central nervous system [81], or elicited by insulin-resistant states [82]. Furthermore, there is a growing need to effectively translate and confirm lessons learned in model organisms to humans. Some of the outstanding questions in the field are listed in Box 1.
Box 1. Outstanding questions.
How does our knowledge gained from animal models translate to human β-cell biology and into effective treatments for both gestational and type 2 diabetes mellitus?
Is there a genetic contribution to the risk of gestational diabetes mellitus? If so, are these genes related to the pathways activated in the proliferating β-cell during pregnancy?
Can new β-cells arising from the differentiation of progenitors contribute to the β-cell mass increase seen during pregnancy?
What are the specific mechanisms that the β-cell uses to decrease its mass after parturition?
What are the temporal changes in β-cell size throughout pregnancy? Does an increase in β-cell size affect the amount of insulin secretion?
What is the relative importance of the multiple putative signaling pathways downstream of the prolactin receptor?
The balance between β-cell growth and loss is tightly regulated and functional β-cell mass normally compensates for increased demands for insulin, for example, during pregnancy. However, if this equilibrium is skewed improperly away from normal induction of β-cell growth and survival during β-cell expansion, β-cell compensation can fail, resulting in gestational diabetes (Figure 4). Elucidating the mediators that control the successful adaptive expansion of β-cell mass during pregnancy will contribute to the development of new therapeutic targets that enhance β-cell proliferation, growth and survival of both transplanted islets for the treatment of type 1 diabetes and endogenous β-cells in type 2 diabetics.
Acknowledgements
We regret the omission of important contributions that we could not discuss because of space constraints. We thank Dr Jake Kushner, Dr John Le Lay and three anonymous reviewers for valuable suggestions on the manuscript. Work on the topic in the Kaestner lab is supported by the JDRF, NIDDK grant R01-DK055243 and the Beta Cell Biology Consortium.
References
- 1.Matveyenko AV, et al. Adaptations in pulsatile insulin secretion, hepatic insulin clearance, and beta-cell mass to age-related insulin resistance in rats. Am. J. Physiol. Endocrinol. Metab. 2008;295:E832–E841. doi: 10.1152/ajpendo.90451.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sorenson RL, Brelje TC. Adaptation of islets of Langerhans to pregnancy: beta-cell growth, enhanced insulin secretion and the role of lactogenic hormones. Horm. Metab. Res. 1997;29:301–307. doi: 10.1055/s-2007-979040. [DOI] [PubMed] [Google Scholar]
- 3.Kloppel G, et al. Islet pathology and the pathogenesis of type 1 and type 2 diabetes mellitus revisited. Surv. Synth. Pathol. Res. 1985;4:110–125. doi: 10.1159/000156969. [DOI] [PubMed] [Google Scholar]
- 4.Bruning JC, et al. Development of a novel polygenic model of NIDDM in mice heterozygous for IR and IRS-1 null alleles. Cell. 1997;88:561–572. doi: 10.1016/s0092-8674(00)81896-6. [DOI] [PubMed] [Google Scholar]
- 5.Zimmet P, et al. Global and societal implications of the diabetes epidemic. Nature. 2001;414:782–787. doi: 10.1038/414782a. [DOI] [PubMed] [Google Scholar]
- 6.Butler AE, et al. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52:102–110. doi: 10.2337/diabetes.52.1.102. [DOI] [PubMed] [Google Scholar]
- 7.Meier JJ, et al. Sustained beta cell apoptosis in patients with long-standing type 1 diabetes: indirect evidence for islet regeneration? Diabetologia. 2005;48:2221–2228. doi: 10.1007/s00125-005-1949-2. [DOI] [PubMed] [Google Scholar]
- 8.Ritzel RA, et al. Relationship between beta-cell mass and fasting blood glucose concentration in humans. Diabetes Care. 2006;29:717–718. doi: 10.2337/diacare.29.03.06.dc05-1538. [DOI] [PubMed] [Google Scholar]
- 9.Parsons JA, et al. Adaptation of islets of Langerhans to pregnancy: increased islet cell proliferation and insulin secretion correlates with the onset of placental lactogen secretion. Endocrinology. 1992;130:1459–1466. doi: 10.1210/endo.130.3.1537300. [DOI] [PubMed] [Google Scholar]
- 10.Rieck S, et al. The transcriptional response of the islet to pregnancy in mice. Mol. Endocrinol. 2009;23:1702–1712. doi: 10.1210/me.2009-0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pick A, et al. Role of apoptosis in failure of beta-cell mass compensation for insulin resistance and beta-cell defects in the male Zucker diabetic fatty rat. Diabetes. 1998;47:358–364. doi: 10.2337/diabetes.47.3.358. [DOI] [PubMed] [Google Scholar]
- 12.Nir T, et al. Recovery from diabetes in mice by beta cell regeneration. J. Clin. Invest. 2007;117:2553–2561. doi: 10.1172/JCI32959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cano DA, et al. Regulated beta-cell regeneration in the adult mouse pancreas. Diabetes. 2008;57:958–966. doi: 10.2337/db07-0913. [DOI] [PubMed] [Google Scholar]
- 14.Bonner-Weir S. Perspective: Postnatal pancreatic beta cell growth. Endocrinology. 2000;141:1926–1929. doi: 10.1210/endo.141.6.7567. [DOI] [PubMed] [Google Scholar]
- 15.Kaung HL. Growth dynamics of pancreatic islet cell populations during fetal and neonatal development of the rat. Dev. Dyn. 1994;200:163–175. doi: 10.1002/aja.1002000208. [DOI] [PubMed] [Google Scholar]
- 16.Meier JJ, et al. Beta-cell replication is the primary mechanism subserving the postnatal expansion of beta-cell mass in humans. Diabetes. 2008;57:1584–1594. doi: 10.2337/db07-1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scaglia L, et al. Apoptosis participates in the remodeling of the endocrine pancreas in the neonatal rat. Endocrinology. 1997;138:1736–1741. doi: 10.1210/endo.138.4.5069. [DOI] [PubMed] [Google Scholar]
- 18.Georgia S, Bhushan A. Beta cell replication is the primary mechanism for maintaining postnatal beta cell mass. J. Clin. Invest. 2004;114:963–968. doi: 10.1172/JCI22098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang ZV, et al. PANIC-ATTAC: a mouse model for inducible and reversible beta-cell ablation. Diabetes. 2008;57:2137–2148. doi: 10.2337/db07-1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inada A, et al. Carbonic anhydrase II-positive pancreatic cells are progenitors for both endocrine and exocrine pancreas after birth. Proc. Natl. Acad. Sci. U. S. A. 2008;105:19915–19919. doi: 10.1073/pnas.0805803105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu X, et al. Beta cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell. 2008;132:197–207. doi: 10.1016/j.cell.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 22.Ferber S, et al. Pancreatic and duodenal homeobox gene 1 induces expression of insulin genes in liver and ameliorates streptozotocin-induced hyperglycemia. Nat. Med. 2000;6:568–572. doi: 10.1038/75050. [DOI] [PubMed] [Google Scholar]
- 23.Dor Y, et al. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature. 2004;429:41–46. doi: 10.1038/nature02520. [DOI] [PubMed] [Google Scholar]
- 24.Uchida T, et al. Deletion of Cdkn1b ameliorates hyperglycemia by maintaining compensatory hyperinsulinemia in diabetic mice. Nat. Med. 2005;11:175–182. doi: 10.1038/nm1187. [DOI] [PubMed] [Google Scholar]
- 25.Rane SG, et al. Loss of Cdk4 expression causes insulin-deficient diabetes and Cdk4 activation results in beta-islet cell hyperplasia. Nat. Genet. 1999;22:44–52. doi: 10.1038/8751. [DOI] [PubMed] [Google Scholar]
- 26.Martin J, et al. Genetic rescue of Cdk4 null mice restores pancreatic beta-cell proliferation but not homeostatic cell number. Oncogene. 2003;22:5261–5269. doi: 10.1038/sj.onc.1206506. [DOI] [PubMed] [Google Scholar]
- 27.Fiaschi-Taesch N, et al. Survey of the human pancreatic beta-cell G1/S proteome reveals a potential therapeutic role for cdk-6 and cyclin D1 in enhancing human beta-cell replication and function in vivo. Diabetes. 2009;58:882–893. doi: 10.2337/db08-0631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tschen SI, et al. Age-dependent decline in beta-cell proliferation restricts the capacity of beta-cell regeneration in mice. Diabetes. 2009;58:1312–1320. doi: 10.2337/db08-1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rankin MM, Kushner JA. Adaptive beta-cell proliferation is severely restricted with advanced age. Diabetes. 2009;58:1365–1372. doi: 10.2337/db08-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dhawan S, et al. Bmi-1 regulates the Ink4a/Arf locus to control pancreatic beta-cell proliferation. Genes Dev. 2009;23:906–911. doi: 10.1101/gad.1742609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen H, et al. Polycomb protein Ezh2 regulates pancreatic beta-cell Ink4a/Arf expression and regeneration in diabetes mellitus. Genes Dev. 2009;23:975–985. doi: 10.1101/gad.1742509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krishnamurthy J, et al. p16INK4a induces an age-dependent decline in islet regenerative potential. Nature. 2006;443:453–457. doi: 10.1038/nature05092. [DOI] [PubMed] [Google Scholar]
- 33.Keller MP, et al. A gene expression network model of type 2 diabetes links cell cycle regulation in islets with diabetes susceptibility. Genome Res. 2008;18:706–716. doi: 10.1101/gr.074914.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zeggini E, et al. Replication of genome-wide association signals in UK samples reveals risk loci for type 2 diabetes. Science. 2007;316:1336–1341. doi: 10.1126/science.1142364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scott LJ, et al. A genome-wide association study of type 2 diabetes in Finns detects multiple susceptibility variants. Science. 2007;316:1341–1345. doi: 10.1126/science.1142382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saxena R, et al. Genome-wide association analysis identifies loci for type 2 diabetes and triglyceride levels. Science. 2007;316:1331–1336. doi: 10.1126/science.1142358. [DOI] [PubMed] [Google Scholar]
- 37.Zeggini E, et al. Meta-analysis of genome-wide association data and large-scale replication identifies additional susceptibility loci for type 2 diabetes. Nat. Genet. 2008;40:638–645. doi: 10.1038/ng.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Butler AE, et al. Increased beta-cell apoptosis prevents adaptive increase in beta-cell mass in mouse model of type 2 diabetes: evidence for role of islet amyloid formation rather than direct action of amyloid. Diabetes. 2003;52:2304–2314. doi: 10.2337/diabetes.52.9.2304. [DOI] [PubMed] [Google Scholar]
- 39.Alsat E, et al. Human placental growth hormone. Am J. Obstet. Gynecol. 1997;177:1526–1534. doi: 10.1016/s0002-9378(97)70103-0. [DOI] [PubMed] [Google Scholar]
- 40.Van Assche FA, et al. A morphological study of the endocrine pancreas in human pregnancy. Br. J. Obstet. Gynaecol. 1978;85:818–820. doi: 10.1111/j.1471-0528.1978.tb15835.x. [DOI] [PubMed] [Google Scholar]
- 41.Scaglia L, et al. Apoptosis contributes to the involution of beta cell mass in the post partum rat pancreas. Endocrinology. 1995;136:5461–5468. doi: 10.1210/endo.136.12.7588296. [DOI] [PubMed] [Google Scholar]
- 42.Kim C, et al. Gestational diabetes and the incidence of type 2 diabetes: a systematic review. Diabetes Care. 2002;25:1862–1868. doi: 10.2337/diacare.25.10.1862. [DOI] [PubMed] [Google Scholar]
- 43.Parsons JA, et al. Number and size of islets of Langerhans in pregnant, human growth hormone-expressing transgenic, and pituitary dwarf mice: effect of lactogenic hormones. Endocrinology. 1995;136:2013–2021. doi: 10.1210/endo.136.5.7720649. [DOI] [PubMed] [Google Scholar]
- 44.Vasavada RC, et al. Targeted expression of placental lactogen in the beta cells of transgenic mice results in beta cell proliferation, islet mass augmentation, and hypoglycemia. J. Biol. Chem. 2000;275:15399–15406. doi: 10.1074/jbc.275.20.15399. [DOI] [PubMed] [Google Scholar]
- 45.Freemark M, et al. Targeted deletion of the PRL receptor: effects on islet development, insulin production, and glucose tolerance. Endocrinology. 2002;143:1378–1385. doi: 10.1210/endo.143.4.8722. [DOI] [PubMed] [Google Scholar]
- 46.Huang C, et al. Prolactin receptor is required for normal glucose homeostasis and modulation of beta-cell mass during pregnancy. Endocrinology. 2009;150:1618–1626. doi: 10.1210/en.2008-1003. [DOI] [PubMed] [Google Scholar]
- 47.Aerts L, et al. The endocrine pancreas in virgin and pregnant offspring of diabetic pregnant rats. Diabetes Res. Clin. Pract. 1997;38:9–19. doi: 10.1016/s0168-8227(97)00080-6. [DOI] [PubMed] [Google Scholar]
- 48.Boloker J, et al. Gestational diabetes leads to the development of diabetes in adulthood in the rat. Diabetes. 2002;51:1499–1506. doi: 10.2337/diabetes.51.5.1499. [DOI] [PubMed] [Google Scholar]
- 49.Aerts L, Van Assche FA. Ultrastructural evaluation of B–cell recruitment in virgin and pregnant offspring of diabetic mothers. Diabetes Res. Clin. Pract. 1998;41:9–14. doi: 10.1016/s0168-8227(98)00063-1. [DOI] [PubMed] [Google Scholar]
- 50.Pinney SE, Simmons RA. Epigenetic mechanisms in the development of type 2 diabetes. Trends Endocrinol. Metab. 2009 doi: 10.1016/j.tem.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Park JH, et al. Development of type 2 diabetes following intrauterine growth retardation in rats is associated with progressive epigenetic silencing of Pdx1. J. Clin. Invest. 2008;118:2316–2324. doi: 10.1172/JCI33655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brelje TC, et al. Distinctive roles for prolactin and growth hormone in the activation of signal transducer and activator of transcription 5 in pancreatic islets of langerhans. Endocrinology. 2004;145:4162–4175. doi: 10.1210/en.2004-0201. [DOI] [PubMed] [Google Scholar]
- 53.Amaral ME, et al. Participation of prolactin receptors and phosphatidylinositol 3-kinase and MAP kinase pathways in the increase in pancreatic islet mass and sensitivity to glucose during pregnancy. J. Endocrinol. 2004;183:469–476. doi: 10.1677/joe.1.05547. [DOI] [PubMed] [Google Scholar]
- 54.Amaral ME, et al. Prolactin-signal transduction in neonatal rat pancreatic islets and interaction with the insulin-signaling pathway. Horm. Metab. Res. 2003;35:282–289. doi: 10.1055/s-2003-41303. [DOI] [PubMed] [Google Scholar]
- 55.Gupta RK, et al. Expansion of adult beta-cell mass in response to increased metabolic demand is dependent on HNF-4alpha. Genes Dev. 2007;21:756–769. doi: 10.1101/gad.1535507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Karnik SK, et al. Menin controls growth of pancreatic beta-cells in pregnant mice and promotes gestational diabetes mellitus. Science. 2007;318:806–809. doi: 10.1126/science.1146812. [DOI] [PubMed] [Google Scholar]
- 57.Ritzel RA, Butler PC. Replication increases beta-cell vulnerability to human islet amyloid polypeptide-induced apoptosis. Diabetes. 2003;52:1701–1708. doi: 10.2337/diabetes.52.7.1701. [DOI] [PubMed] [Google Scholar]
- 58.Donath MY, et al. Hyperglycemia-induced beta-cell apoptosis in pancreatic islets of Psammomys obesus during development of diabetes. Diabetes. 1999;48:738–744. doi: 10.2337/diabetes.48.4.738. [DOI] [PubMed] [Google Scholar]
- 59.Jiang Y, et al. Postnatal expansion of the pancreatic beta-cell mass is dependent on survivin. Diabetes. 2008;57:2718–2727. doi: 10.2337/db08-0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dohi T, et al. Inhibition of apoptosis by survivin improves transplantation of pancreatic islets for treatment of diabetes in mice. EMBO Rep. 2006;7:438–443. doi: 10.1038/sj.embor.7400640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Laybutt DR, et al. Overexpression of c-Myc in beta-cells of transgenic mice causes proliferation and apoptosis, downregulation of insulin gene expression, and diabetes. Diabetes. 2002;51:1793–1804. doi: 10.2337/diabetes.51.6.1793. [DOI] [PubMed] [Google Scholar]
- 62.Radziszewska A, et al. Absence of caspase-3 protects pancreatic {beta}-cells from c-Myc-induced apoptosis without leading to tumor formation. J. Biol. Chem. 2009;284:10947–10956. doi: 10.1074/jbc.M806960200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang IC, et al. Forkhead box M1 regulates the transcriptional network of genes essential for mitotic progression and genes encoding the SCF (Skp2-Cks1) ubiquitin ligase. Mol. Cell Biol. 2005;25:10875–10894. doi: 10.1128/MCB.25.24.10875-10894.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ackermann Misfeldt A, et al. Beta-cell proliferation, but not neogenesis, following 60% partial pancreatectomy is impaired in the absence of FoxM1. Diabetes. 2008;57:3069–3077. doi: 10.2337/db08-0878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhong L, et al. Essential role of Skp2-mediated p27 degradation in growth and adaptive expansion of pancreatic beta cells. J. Clin. Invest. 2007;117:2869–2876. doi: 10.1172/JCI32198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang H, et al. Gestational diabetes resulting from impaired {beta}-cell compensation in the absence of FoxM1, a novel downstream effector of placental lactogen. Diabetes. 2009 doi: 10.2337/db09-0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Endo T, et al. CIS1 interacts with the Y532 of the prolactin receptor and suppresses prolactin-dependent STAT5 activation. J. Biochem. 2003;133:109–113. doi: 10.1093/jb/mvg004. [DOI] [PubMed] [Google Scholar]
- 68.Greenhalgh CJ, et al. Growth enhancement in suppressor of cytokine signaling 2 (SOCS-2)-deficient mice is dependent on signal transducer and activator of transcription 5b (STAT5b) Mol. Endocrinol. 2002;16:1394–1406. doi: 10.1210/mend.16.6.0845. [DOI] [PubMed] [Google Scholar]
- 69.Friedrichsen BN, et al. Signal transducer and activator of transcription 5 activation is sufficient to drive transcriptional induction of cyclin D2 gene and proliferation of rat pancreatic beta-cells. Mol. Endocrinol. 2003;17:945–958. doi: 10.1210/me.2002-0356. [DOI] [PubMed] [Google Scholar]
- 70.Fujinaka Y, et al. Lactogens promote beta cell survival through JAK2/STAT5 activation and Bcl-XL upregulation. J. Biol. Chem. 2007;282:30707–30717. doi: 10.1074/jbc.M702607200. [DOI] [PubMed] [Google Scholar]
- 71.Cozar-Castellano I, et al. The cell cycle inhibitory protein p21cip is not essential for maintaining beta-cell cycle arrest or beta-cell function in vivo. Diabetes. 2006;55:3271–3278. doi: 10.2337/db06-0627. [DOI] [PubMed] [Google Scholar]
- 72.Klinger S, et al. Increasing GLP-1-induced beta-cell proliferation by silencing the negative regulators of signaling cAMP response element modulator-alpha and DUSP14. Diabetes. 2008;57:584–593. doi: 10.2337/db07-1414. [DOI] [PubMed] [Google Scholar]
- 73.Navarro-Tableros V, et al. Autocrine regulation of single pancreatic beta-cell survival. Diabetes. 2004;53:2018–2023. doi: 10.2337/diabetes.53.8.2018. [DOI] [PubMed] [Google Scholar]
- 74.Miao G, et al. In vitro and in vivo improvement of islet survival following treatment with nerve growth factor. Transplantation. 2006;81:519–524. doi: 10.1097/01.tp.0000200320.16723.b3. [DOI] [PubMed] [Google Scholar]
- 75.Garcia-Ocana A, et al. Hepatocyte growth factor overexpression in the islet of transgenic mice increases beta cell proliferation, enhances islet mass, and induces mild hypoglycemia. J. Biol. Chem. 2000;275:1226–1232. doi: 10.1074/jbc.275.2.1226. [DOI] [PubMed] [Google Scholar]
- 76.Fiaschi-Taesch NM, et al. Hepatocyte growth factor enhances engraftment and function of nonhuman primate islets. Diabetes. 2008;57:2745–2754. doi: 10.2337/db08-1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ekholm R, et al. Monoamines in the pancreatic islets of the mouse. Subcellular localization of 5-hydroxytryptamine by electron microscopic autoradiography. Diabetologia. 1971;7:339–348. doi: 10.1007/BF01219468. [DOI] [PubMed] [Google Scholar]
- 78.Richmond JE, et al. Calcium- and barium-dependent exocytosis from the rat insulinoma cell line RINm5F assayed using membrane capacitance measurements and serotonin release. Pflugers Arch. 1996;432:258–269. doi: 10.1007/s004240050132. [DOI] [PubMed] [Google Scholar]
- 79.Paulmann N, et al. Intracellular serotonin modulates insulin secretion from pancreatic beta-cells by protein serotonylation. PLoS Biol. 2009;7 doi: 10.1371/journal.pbio.1000229. e1000229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lee NK, et al. Endocrine regulation of energy metabolism by the skeleton. Cell. 2007;130:456–469. doi: 10.1016/j.cell.2007.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Imai J, et al. Regulation of pancreatic beta cell mass by neuronal signals from the liver. Science. 2008;322:1250–1254. doi: 10.1126/science.1163971. [DOI] [PubMed] [Google Scholar]
- 82.Flier SN, et al. Evidence for a circulating islet cell growth factor in insulin-resistant states. Proc. Natl. Acad. Sci. U. S. A. 2001;98:7475–7480. doi: 10.1073/pnas.131192998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Johansson M, et al. Islet endothelial cells and pancreatic beta-cell proliferation: studies in vitro and during pregnancy in adult rats. Endocrinology. 2006;147:2315–2324. doi: 10.1210/en.2005-0997. [DOI] [PubMed] [Google Scholar]
- 84.Pechhold S, et al. Transcriptional analysis of intracytoplasmically stained. FACS-purified cells by high-throughput, quantitative nuclease protection. Nat. Biotechnol. 2009;27:1038–1042. doi: 10.1038/nbt.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]