Summary
There are some problems such as a narrow therapeutic time window and severe side effects of fibrinolytics in the therapy of cerebral embolisms. Therefore, it is necessary to develop a new method to remove a cerebral thrombus more rapidly with fewer fibrinolytics.
A Q-switch pulsed holmium (Ho): YAG laser with 86 mJ/pulse, pulse duration of 200ns and wavelength of 2.1 mm was used. The laser beam was transmitted through a 0.6 mm diameter quartz optical fiber. Experiments were conducted in a stainless steel container equipped with observation windows .The test chamber was filled with distilled water at 283K. At first, the formation of laser-induced bubbles in a 4 mm diameter glass tube was observed. The bubble gradually expanded and reached a maximum size at about lms after irradiation. A shock wave induced by ignition of silver azide pellet was interacted with it at 500µs before Ho:YAG laser irradiation, which resulted in forming a liquid jet. This liquid jet penetrated into an artificial thrombus made of gelatin, and its maximum penetration depth was 4.2 mm, which was nearly twice deeper than the laser irradiation only (2.2 mm).
Combination of this liquid jet and fibrinolytics will realize more rapid recanalization with fewer drugs.
Key words: cerebral embolism, fibrinolysis, holmium: YAG laser, liquid jet, shock wave
Introduction
As advancing neurological images such as diffusion magnetic resonance imaging 1,2 and single photon emission computed tomography3, the patients having cerebral infarction within several hours after onset have been detectable. Since the therapeutic time window of cerebral infarction is generally thought to be within several hours4,5, it is necessary to accomplish recanalization of occluded cerebral arteries as soon as possible. The advent of fibrinolytics such as urokinase (UK) and tissue-plasminogen activator (t-PA) had brought great changes to the therapy of cerebral infarction. Intravenous application of fibrinolytics raises the recanalisation rate to 34-47% within 24 hours while spontaneous recanalization occurs in nearly 20% of patients6. Moreover, local intraarterial application of fibrinolytics with endovascular therapy increases recanalization rate up to 75% within several hours7. However, fibrinolytics promotes general bleeding tendency such as gastrointestinal bleedings and intracranial hemorrhages8. Nicole et A1 also reported that t-PA could increase neuronal damage after focal cerebral ischemia9. For these reasons, the amount of used fibrinolytics should be restricted as small as possible.
Recently, fibrinolysis with a micro liquid jet produced by interaction between a bubble and a shock wave was attempted in vitro 10. Though this method raised the fibrinolysis rate to twofold in comparison with the case of only fibrinolytics, some problems had prevented it from applying to clinical fields. For example, injection of air bubble into cerebral arteries may cause another cerebral embolism, and uneven distribution of a bubble in liquid by buoyancy influenced on the direction of a liquid jet.
Ho: YAG laser is a kind of solid-state laser, which has a mid-infrared wavelength (2.1 µm) near the high light-absorption spectrum of water. Hence, it has strong effects on substances containing much water and came into wide use in many medical fields. Especially, this laser irradiation in water results in forming a large cavitation bubble, which oscillates and induces shock waves 11,12. In the present study, a liquid jet produced by interaction between a laser-induced bubble and a shock wave was investigated to develop a new effective method of fibrinolysis.
Methods
Liquid jet formation
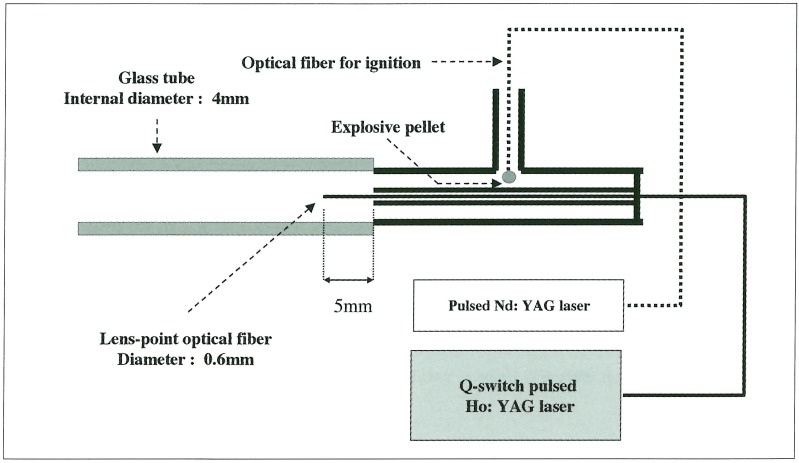
The Laser source in this experiment was a Q-switch pulsed Ho: YAG laser (Ho: YAG laser QNIH102: Nippon Infrared Industries Co. Ltd., Tokyo, Japan) whose wavelength, pulse-duration time, and laser energy were 2.1 µm, 200 ns, and 36~150 mJ / pulse, respectively. The laser light was transmitted through a quartz optical fiber whose diameter was 0.6 mm. The experiment was carried out in a stainless steel chamber (110 mm x 110 mm x 130 mm) equipped with optical windows at room temperature of 29OK under atmosphere pressure. The chamberwas filled with pure water of 29l~292K. A glass tube that had internal diameter of 4 mm was filled with pure water and immersed in the chamber. An end of the tube was fixed on a wall of the chamber with the device for liquid jet generation (figure 1), in which air bubbles were removed. An optical fiber for bubble formation was inserted into the center of the device. A micro explosive pellet; AgN3 of 150 µg was glued to the tip of another optical fiber, which was inserted into the lateral canal of the device (figure 1).
Figure 1.
Schematic diagram of the experimental device to produce a liquid jet by interaction between a laser-induced bubble and an explosion-induced shock wave.
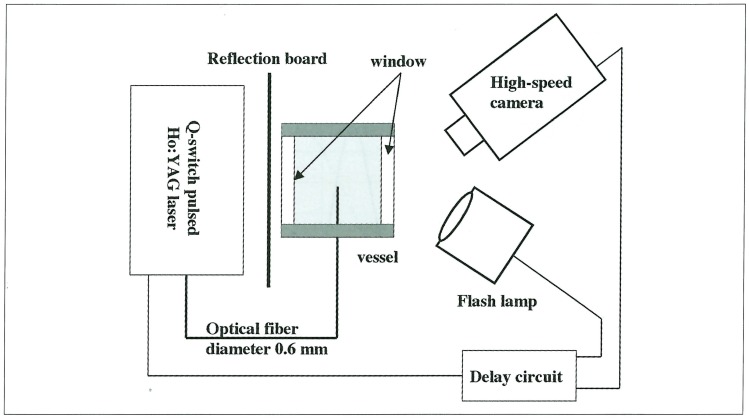
The explosive was ignited by Nd: YAG laser (pulse duration: 7 ns, laser energy: 25 mJ/Pulse) irradiation through another optical fiber. The distance between the end of the optical fiber for bubble formation and the exit of the device was adjusted to 5 mm, where the overpressure of explosive shock wave was about 10 MPa. At first, a bubble formation in the glass tube was observed. The process of bubble or liquid-jet formation was recorded with a high-speed camera (ISIS Prototype CCD Camera: Shimadzu Co. Ltd., Kyoto, Japan) in framing mode; the frame rate could be varied from 1.9 frames / s to 1.0 x 106 frames / s. A commercial strobe flash with 3.3 ms pulse duration was used as a light source (figure 2). The time sequence of observation was controlled with a delay generator.
Figure 2.
Schematic diagram of the experimental set-up to observe the behavior of a laser-induced bubble or a liquid jet. A high-speed camera whose frame rate was varied from 1.9 frames / s to 1.0 x 106 frames / s. and a commercial strobe flash with 3.3 ms pulse duration were used.
Penetration of a liquid jet into an artificial thrombus
A gelatin (077-03155: Wako Pure Chemical Industries Co. Ltd., Osaka, Japan) was dissolved in pure water of temperature less than 323K on the concentration of 10% (w/v). We regarded the 10% (w/v) gelatin as a reasonable artificial thrombus, because its Young's modulus is 0.3 x 104 Nm-2 at 294K13 which corresponds to that of a previously reported artificial thrombus 14. In order to observe penetration of a liquid jet, the gelatin was added to a glass tube whose length and internal diameter were 60 mm and 4 mm, respectively. The length of gelatin layer in the tube was 10mm, which was preserved in a refrigerator at 277K and exposed to ambient condition just before the experiment. Water was injected into the glass tube with a syringe in order to remove air bubbles, and it was immersed in the test chamber as did in the above mentioned experiment. An optical fiber for Ho: YAG laser was inserted into the glass tube. At first, the end of the optical fiber was attached to the surface of an artificial thrombus, and the laser was irradiated without explosion.
After switching the artificial thrombus for another, the distance between the fiber end and the new artificial thrombus bottom was adjusted to 2 mm in order to keep movable space of the bubble. The following operation was performed by the same way of the liquid jet formation.
Results
Liquid jet formation
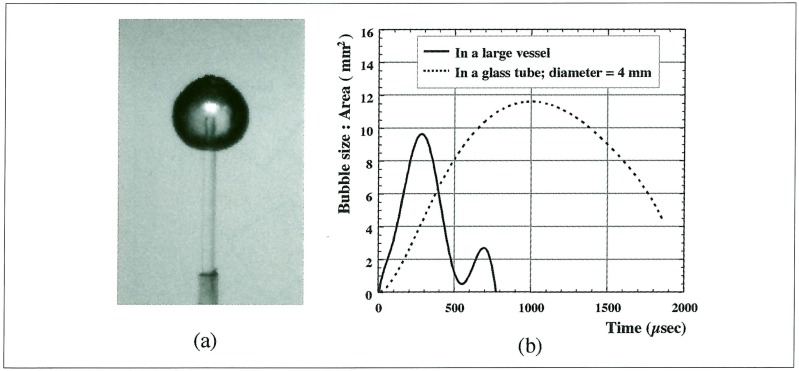
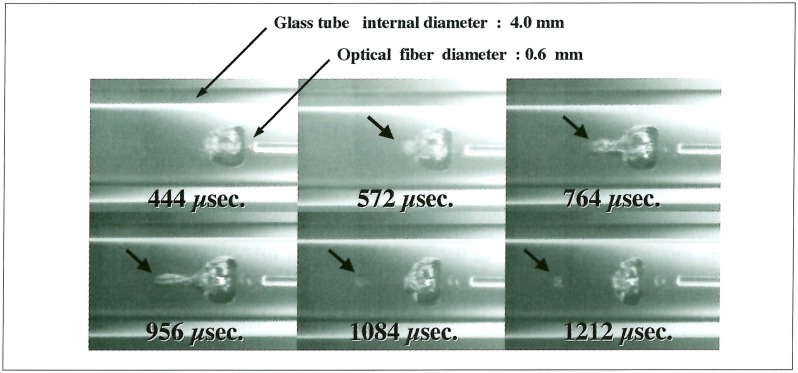
A laser-induced bubble in a large vessel attained a maximum size at about 300 µs and completely vanished at about 800µs after the laser irradiation. Meanwhile, in a glass tube whose internal diameter was 4 mm, it reached a maximum size at about 1 ms, and its lifetime was extended to more than 2 ms (figure 3). Since an air bubble put in water was transformed to a long liquid jet nearly 500 µs after interaction of a shock wave, the explosive pellet was ignited 400 µs after Ho: YAG laser irradiation in order to employ a larger bubble. After interaction of a shock wave, a laser-induced bubble had left the optical-fiber end at the speed of 1.7 m/s. At 572 µs after laser irradiation, the top of the bubble began to protrude and reached a most distant point at 956 µs, whose growing velocity was 5.2 m/s (figure 4).
Figure 3.
Profile of laser-induced bubble. Laser energy is 86 mJ/pulse. (a): A photograph of maximum bubble taken by highspeed camera. The shape of a bubble looks almost spherical, (b): In a glass tube whose internal diameter was 4 mm, a laser-induced bubble reached a maximum size at about 1 ms, and its lifetime was extended to more than 2 ms.
Figure 4.
The explosive pellet was ignited 400 µs after Ho: YAG laser irradiation. The top of the bubble began to protrude at 572 µs after laser irradiation, and a liquid jet had been formed.
Penetration of a liquid jet into an artificial thrombus
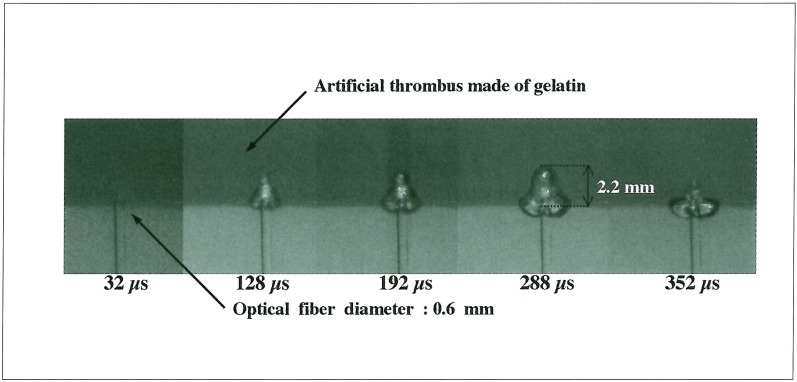
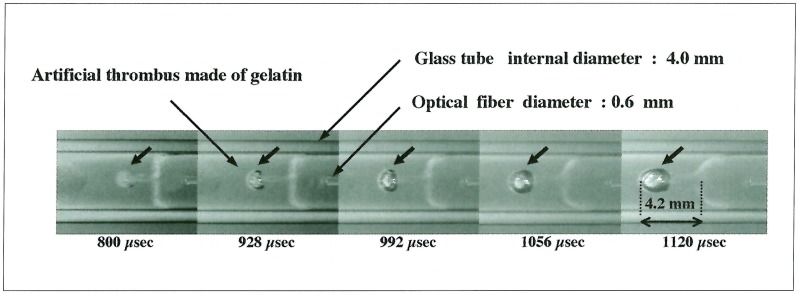
A laser-induced bubble without interaction of a shock wave, penetrated into an artificial thrombus, and its maximum depth was 2.2 mm (figure 5). Meanwhile, the bubble influenced by a shock wave formed a liquid jet and penetrated into an artificial thrombus while gradually expanding. The penetration depth finally attained a maximum value; 4.2 mm at 1120 µs (figure 6).
Figure 5.
The end of the optical fiber was attached to the surface of an artificial thrombus, and the laser was irradiated without explosion, which penetrated into an artificial thrombus; its maximum depth was 2.2 mm.
Figure 6.
Penetration of a liquid jet into an artificial thrombus. The bubble influenced by a shock wave formed a liquid jet and penetrated into an artificial thrombus. It finally attained a maximum penetration depth of 4.2 mm.
Discussion
The Ho: YAG laser is a solid-state laser with a mid-infrared wavelength (2.1 µm) near the light-absorption spectrum of water. It therefore has strong effects on substances containing large volume of water and has come into wide use in many medical fields. One of its most important characteristics is that, when activated in water, this laser irradiation forms a large cavitation bubble that oscillates and induces shock waves 11,12. Some reports have indicated that thrombi might be destroyed by mid-infrared-laser-induced mechanical actions such as bubble expansion15 or the shearing force of shock waves 16. In this study, a simple laser-induced bubble penetrated into an artificial thrombus. After ignition of explosives, liquid flow was induced behind a shock wave, and this bubble could be transformed to a liquid jet. In the experiment on artificial thrombi, the penetration depth of this liquid jet became nearly twice as much as that of simple laser irradiation. Hence, with fibrinolytic drugs such as urokinase and tissue-plasminogen activator, this jet could drive them deeply into a thrombus and increase the area contacted with these drugs. The other present experimental findings suggested that ultrasound accelerated enzymatic fibrinolysis by increasing the transport of fibrinolytics due to micro-cavitation-related mechanisms 17,18. Similarly, this liquid jet is also regarded as a kind of drug delivery system.
In order to apply this system to clinical fields, some problems remain to be resolved before clinical application.
In particular, usage of explosives may cause tissue damages. Moreover, though its successive exposure can be expected to result in even more effective fibrinolysis with lower doses of fibrinolytics, it is difficult to repeat ignition of explosives. Fortunately, since the Q-switch pulsed Ho: YAG laser emits a strong shock wave in water, this laser can be also used as a source of shock waves. If the endovascular device equipped with the improved system is investigated, more rapid fibrinolysis with lower dose of fibrinolytics will be realized in the treatment of cerebral embolisms.
Conclusions
Interaction between a laser-induced bubble and a shock wave caused a liquid jet formation, which deeply penetrated into an artificial thrombus. This jet is a kind of drug delivery systems and may promoted fibrinolysis with use of only a small amount of fibrinolytics.
References
- 1.Sorensen AG, Buonanno FS, et al. Hyperacute stroke: evaluation with combined multisection diffusion-weighted and hemodynamically weighted echo-planar MR imaging. Radiology; 1996;199:391–401. doi: 10.1148/radiology.199.2.8668784. [DOI] [PubMed] [Google Scholar]
- 2.van Everdingen KJ, van der Grond J, et al. Diffusion-weighted magnetic resonance imaging in acute stroke. Stroke; 1998;29:1783–1790. doi: 10.1161/01.str.29.9.1783. [DOI] [PubMed] [Google Scholar]
- 3.Umemura A, Suzuka T, Yamada K. Quantitative measurement of cerebral blood flow by 99m Tc-HMPAO SPECT in acute ischaemic stroke: usefulness in determining therapeutic options. J Neurol Neurosurg Psychiatry; 2000;69:472–478. doi: 10.1136/jnnp.69.4.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bozzao L, Fantozzi LM, et al. Early collateral blood supply and late parenchymal brain damage in patients with middle cerebral artery occlusion. Stroke. 1989;20:735–740. doi: 10.1161/01.str.20.6.735. [DOI] [PubMed] [Google Scholar]
- 5.Hatazawa J, Shimosegawa, et al. cerebral blood volume in acute brain infarction: A combined study with dynamic susceptibility contrast MRI and 99mTc-HMPAO-SPECT. Stroke. 1999;30:800–806. doi: 10.1161/01.str.30.4.800. [DOI] [PubMed] [Google Scholar]
- 6.Mori E, Yoneda Y, et al. Intravenous recombinant tissue plasminogen activator in acute carotid artery territory stroke Neurology. 1992;42:976–982. doi: 10.1212/wnl.42.5.976. [DOI] [PubMed] [Google Scholar]
- 7.del Zoppo GJ, Ferbert A, et al. Local intra-arterial fibrinolytic therapy in acute carotid territory stroke. A pilot study. Stroke. 1988;19:307–313. doi: 10.1161/01.str.19.3.307. [DOI] [PubMed] [Google Scholar]
- 8.NINDS; The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 9.Nicole O, Docagne F, et al. The proteolytic activity of tissue-plasminogen activator enhances NMD A receptormediated signaling. Nature Medicine. 2001;7(1):59–64. doi: 10.1038/83358. [DOI] [PubMed] [Google Scholar]
- 10.Kodama T, Tatsuno M, et al. Liquid jets, accelerated thrombolysis: a study for revascularization of cerebral embolism. Ultrasound Med Biol. 1999;25:977–983. doi: 10.1016/s0301-5629(99)00050-2. [DOI] [PubMed] [Google Scholar]
- 11.Asshauer T, Rink K, Delacretaz G. Acoustic transient generation by holmium-laser-induced cavitation bubbles. J Appl Phys. 1994;76(9):5007–5013. [Google Scholar]
- 12.Asshauer T, Delacretaz G, et al. Pulsed holmium laser ablation of tissue phantoms: correlation between bubble formation and acoustic transients. Appl Phys B. 1997;65:647–657. [Google Scholar]
- 13.Kodama T, Takayama K, Uenohara H. A new technology for revascularisation of cerebral enbolism using liquid impact. Phys Med Biol. 1997;42:2355–2367. doi: 10.1088/0031-9155/42/12/004. [DOI] [PubMed] [Google Scholar]
- 14.Rosenschein U, Frimerman A, et al. Study of the mechanism of ultrasound angioplasty from human thrombi and bovine aorta. Am J Cardiol. 1994;74:1263–1266. doi: 10.1016/0002-9149(94)90560-6. [DOI] [PubMed] [Google Scholar]
- 15.Leeuwen TG, Veen MJ, et al. Noncontact tissue ablation by Holmium: YSSG laser pulses in blood. Laser Surg Med. 1991;11:26–34. doi: 10.1002/lsm.1900110108. [DOI] [PubMed] [Google Scholar]
- 16.Topaz ON, Minisi AJ, et al. Photoacoustic fibrinolysis: pulsed-wave mid-infrared laser-clot interaction. J Thromb Thrombolysis. 1996;3(3):209–214. doi: 10.1007/BF00181663. [DOI] [PubMed] [Google Scholar]
- 17.Francis CW, Blinc A, et al. Ultrasound accelerates transport of recombinant tissue plasminogen activator into clots. Ultrasound Med Biol. 1995;21:419–424. doi: 10.1016/0301-5629(94)00119-x. [DOI] [PubMed] [Google Scholar]
- 18.Blinc A, Francis CW, et al. Characterization of ultrasound-potentiated fibriinolysis in vitro. Blood. 1993;81:2636–2643. [PubMed] [Google Scholar]