Summary
The purpose of this study is to evaluate the perfusional state of cerebral aneurysms treated by platinum detachable coils using three different techniques of MR angiography (MRA), and to compare the results of each MRA technique. Thirty examinations were investigated in twelve patients. They were three men and nine women, and their average age was 67y.o. They were all treated by platinum detachable coils for cerebral aneurysms. We obtained three different types of MRA on the same day; 2D-FSPGR Gd-DTPA enhanced dynamic MRA, 3D-TOF MRA with and without Gd-DTPA enhancement. On 2D FSPGR enhanced dynamic MRA, we used the first pass arterial phase for judgement that did not overlap the venous phase. In each study, we evaluated parent artery flow, branch artery flow, residual flow in coils, and residual neck. Digital subtraction angiography (DSA) was used as gold standard. On 3D-TOF MRA examinations without enhancement, parent artery flow was correctly identified with an accuracy of 96.7% with DSA confirmation. Branch artery flow was identified with an accuracy of 91.3%. Flow in the coils was correctly identified with an accuracy of 86.7%. Residual neck was correctly evaluated with an accuracy of 83.3%. On 3D-TOF MRA with enhancement, parent artery flow was correctly identified with an accuracy of 96.7%. Branch artery flow was identified with an accuracy of 91.3%. Flow in the coils was correctly identified with an accuracy of 93.3%. Residual neck was correctly identified with an accuracy of 86.7%. On 2D FSPGR enhanced dynamic MRA, parent artery flow was correctly identified with an accuracy of 100%. Branch artery flow was identified with an accuracy of 94.2%. Flow in the coils was correctly identified with an accuracy of 96.7%. Residual neck was correctly evaluated with an accuracy of 100%. Parent artery flow, branch artery flow, residual flow in coils, and residual necks were seen more accurately with 2D-FSPGR Gd-DTPA enhanced dynamic MRA than 3D-TOF MRA with and without enhancement. With T1 shortening effect of Gd-DTPA and first pass arterial phase of 2D-FSPGR enhanced dynamic MRA techniques, we could evaluate more accurately the perfusional status of platinum-coil-treated cerebral aneurysms and arteries adjacent to the aneurysms than with non enhanced or enhanced 3D TOF MRA.
Key words: intracranial aneurysms, Guglielmi detachable coil, interlocking detachable coil, 2D fast spoiled gradiant echo enhanced dynamic MR angiography
Introduction
Endovascular embolization with platinum detachable coils (Guglielmi and interlocking detachable coils (GDC and IDC); Boston Scientific / Target Therapeutics, Boston, Mass) is considered to be useful for the treatment of intracranial aneurysms and other vascular abnormalities 1-3. The concept of this technique is to exclude the aneurysm from the circulation by filling it with platinum microcoils. Preliminary data suggested a durable result when the aneurysm is completely packed with coils 4. However, the long-term occlusion rates have not yet been established. In some patients the aneurysm may recur, either because of coil compaction or because of regrowth of residual aneurysmal neck. Moreover, some aneurysms cannot be completely packed with coils. In such cases residual filling within the interstices of the coil mass or a residual flow in the neck is present after treatment.
Patients treated with GDC and IDC are studied after therapy with conventional digital subtraction angiography (DSA) in order to assess the durability of the initial treatment and to determine the need for further therapy. Arteriography is often required in acute management after treatment. DSA is considered to be a gold standard for evaluating platinum-coil-treated aneurysms. But, the DSA is more invasive for most of the patients than CT angiography or MRA. Recently, MR compatibility of platinum detachable coils was examined in vitro and in vivo5-7. Safety and usefulness of 3D-TOF MRA and 3D-TOF MRA with ultra-short echo time, with the aim of reducing the platinum induced susceptibility artifact, were also evaluated 8-9. However, in some cases it is very difficult to evaluate the perfusion state (regrowth of residual neck, coil compaction, loose packing) and adjacent vessel patency, due to vortex flow, turbulent flow, and promoted thrombus formation. This drawback of traditional MRA may be overcome by using sequences with relatively short TE. The purpose of this study is to evaluate three MRA techniques to determine the best way to study postoperative patients.
Material and Methods
Out of 30 patients that the platinum coil embolizations were performed for cerebral aneurysms in our hospital and related hospitals from 1996 July to 1999 November, 12 patients were investigated.
Twelve patients showed minimal changes of coils` forms by follow up skull roentgen films or showed questionable signals within the interstices of the coil mass by follow up MR T1 and T2 weighted axial images. One patient had five times follow up MR and DSA examinations, two patients had four times, three patients had three times, and two patients had twice follow up. A total of 30 pairs of MR imaging, MR angiography (2D-FSPGR and 3D-TOF MRA) and DSA studies were obtained. Signa Horizon 1.0 T MR machine (Signa; GE Medical Systems, Milwaukee, Wis) was used for evaluations. In all patients, Tlweighted images (T1WI), T2 weighted images (T2WI), 3D-TOF MRA with, and without Gd-DTPA enhancement, and 2D-FSPGR enhanced dynamic MR angiography and these subtraction images were obtained. Three MRA techniques used in the same day. DSA studies (BV 3000; machine Philips Integris, Best, the Netherland) were performed within 1 week before or after doing the MRA studies. Results of the DSA studies were summarized in table 1. Details of the MR sequences were as follows; T1WI=Spin Echo method, Axial images, TR620, TE10, FOV 24x18 cm, Matrix 256x192, NEX1 Thickness 5.0 mm; T2WI=Spin Echo method, Axial images, TR4200, TE9l, FOV 24x18 cm, Matrix 256x192, NEX2 Thickness 5.0 mm; 3D TOF MRA=TR4O, TE3.8, FOV 16.0x14.4 cm, Matrix 256x192, NEX1, Flip angle 30, Source images and MIP reconstruction images; 2D-FSPGR enhanced dynamic MRA and MRDSA=(2D FSPGR method sagittal images and coronal images) TR 8-9 msec, TE 1.2 msec-1.8 msec, FOV 24x18.5 cm, Matrix 256x192, NEX1, Flip angle 30°, thickness 20 mm, Scanning begun exactly 5 sec after beginning intravenous bolus administration of 10 cc Gd-DTPA and 20 cc saline in succession (2cc/sec) from elbow site. Thirty frames images were obtained in 30-40 sec. Evaluations were mainly done by first pass arterial phase that did not overlap the venous phase images.
Table 1.
Patient age at the time of TAE, sex, time interval from TAE to MRA (follow up DSA) investigation, perfusional state at the time of TAE and at the time MRA were performed.
| Patient Age,y/Sex Exam number |
AN Position/size |
Extent of AN occlusion TAE evaluated by DSA |
Follow up DSA Time interval/ State |
|---|---|---|---|
| 1) 1-1 54/F | Lt ICPC/ L | complete | 5month/complete |
| 2) 1-follow | 7month/complete | ||
| 3) 1-2nd follow | 1year/complete | ||
| 4) 1-3rd follow | 1y5month/coil compaction | ||
| 5) 1-4th follow | 1y6month/coil compaction | ||
| 6) 2-1 64/F | Basilar top/ S | complete | 1 day/complete |
| 7) 2-follow | 2month/complete | ||
| 8) 2-2nd follow | 7month/complete | ||
| 9) 2-3rd follow | 1y2month/complete | ||
| 10) 3-1 74/M | Basilar top/ L | complete | 8month/complete |
| 11) 3-follow | 11 month/complete | ||
| 12) 3-2nd follow | 1year/coil compaction | ||
| 13) 4-1 67/M | Basilar top/L | neck remnant | 9month/neck remnant |
| 14) 4-follow | 11month/neck remnant | ||
| 15) 4-2nd follow | 1year/ neck remnant | ||
| 16) 4-3rd follow | ly5month/ neck remnant | ||
| 17) 5-1 70/F | Basilar top / S | bleb TAE | 2y2month/ blebTAE |
| 18) 5-follow | 2y9month/ blebTAE | ||
| 19) 6 68/M | Lt IC-opthalmic/ L | complete | 2y8month/complete |
| 20) 7-1 68/F | Lt IC-PC/ S | complete | 3month/complete |
| 21) 7-follow | 6month/complete | ||
| 22) 7-2nd follow | 10month/complete | ||
| 23) 8 74/F L | t IC-PC / L | loose packing | 8days/loose packing |
| 24) 8-follow | 2month/loose packing | ||
| 25) 8-2nd follow | 5month/loose packing | ||
| 26) 9-1 64/F | Lt IC-PC/ S | complete | 3month/complete |
| 27) 9 -follow | 5month/complete | ||
| 28) 10 78/F | Rt IC-PC/ S | complete | 4month/complete |
| 29) 11 68/F-7 | Lt IC-PC / S | complete | 6month/complete |
| 30) 12 51/F | Lt IC-PC / S | complete | 2month/complete |
|
Note---AN indicates aneurysms, S indicates small aneurysm that had a diameter of less than12mm, L indicates large aneurysm the di- ameter of it was 12-25mm. Additional TAE’s were done just before doing MRA examinations no. 3, 10, and 11, because coil com- pactions occurred. | |||
The MRA images including source images were evaluated by 2 reviewers (TH.JA). They evaluated parent vessel patency, branch vessel patency, residual flow within the interstices of the coil mass, and residual or reccurent aneurysmal neck. DSA studies were used as a gold standard for comparing three different methods of MRA. The presence or absence of these findings was determined by the consensus of the reviewers. Patency of branch vessels was only assessed in those aneurysms whose origin involved or was in proximity to the origin of major branch vessels. For example, the patency of both superior cerebellar and posterior cerebral arteries was assessed for treated basilar top aneurysms. Similarly, branch vessel patency was evaluated for aneurysms of the internal carotid artery bifurcation (two branches), the middle cerebral artery trifurcation (three branches), the anterior communicating artery (two branches), and the posterior communicating artery (one branch). The parent vessels were considered patent if some continuity of high signal was observed. Parent vessels were considered absent if any continuity of high signal was not observed. Residual flow within the aneurysm and the presence of an aneurysmal neck were assessed in a similar manner.
Focal high signal within the coil mass or at the neck the aneurysm beyond the expected confinement of the parent artery on the source and MIP images or subtraction images was considered to represent intra-aneurysmal flow. The case histories of all patients were analized retrospectively.
Results
The data were summarized in table 2 and table 3. The sensitivity, specificity, and accuracy of the 3 different techniques of MRA on every 4 points we evaluated were shown in table 3. On 3D-TOF MRA examinations without enhancement, parent artery flow was correctly identified at the accuracy of 96.7% with 1 false-negative and no false-positive with DSA confirmation. 63 of 69 patent branch artery flow was identified at the accuracy of 91.3% with 6 false-negatives and no false-positive. Flow in the coils was correctly identified in 26 of 30 aneurysms at the accuracy of 86.7% with 4 false-negatives and no false-positive. Residual neck was correctly evaluated in 25 of 30 at the accuracy of 83.3% with 5 false-negatives and no false-positive. On 3D TOF MRA with enhancement, parent artery flow was correctly identified on 29 of 30 at the accuracy of 96.7% with 1 false-negative and no false-positive. Sixty-three of sixty-nine patent branch artery flow was identified at the accuracy of 91.3% with 6 false-negative and no false-positive. Flow in the coils were correctly identified in 28 of 30 at the accuracy of 93.3% with 1 false-negative and 1 false-positive. Residual neck was correctly identified in 26 of 30 at the accuracy of 86.7% with 2 false-negatives and 2 false-positives. On 2D FSPGR enhanced dynamic MRA, parent artery flow was correctly identified on 30 of 30 at the accuracy of 100% with no false-negative or false-positive. 65 of 69 branch artery flow was identified at the accuracy of 94.2% with 4 false-negatives and no false-positive. Flow in the coils were correctly identified in 29 of 30 at the accuracy of 96.7% with no false-negative and 1 false-positive. Residual neck was correctly evaluated in 30 of 30 aneurysms at the accuracy of 100% with no false-negative and no false-positive.2D-FSPGR enhanced dynamic MRA were better than comparatively short echo time 3D-TOF MRA with and without enhancement.
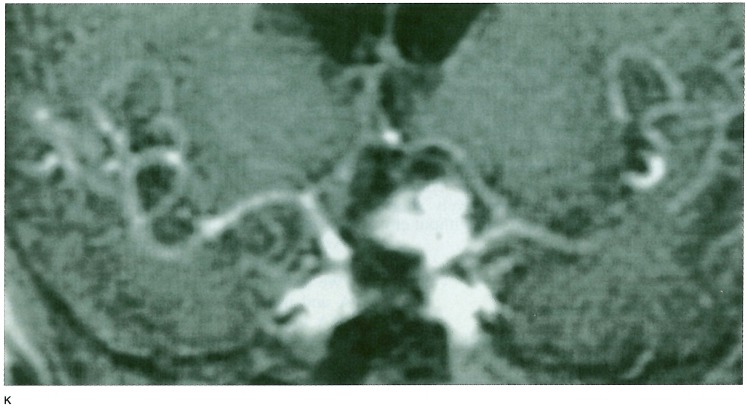
We showed a case in figure 1.
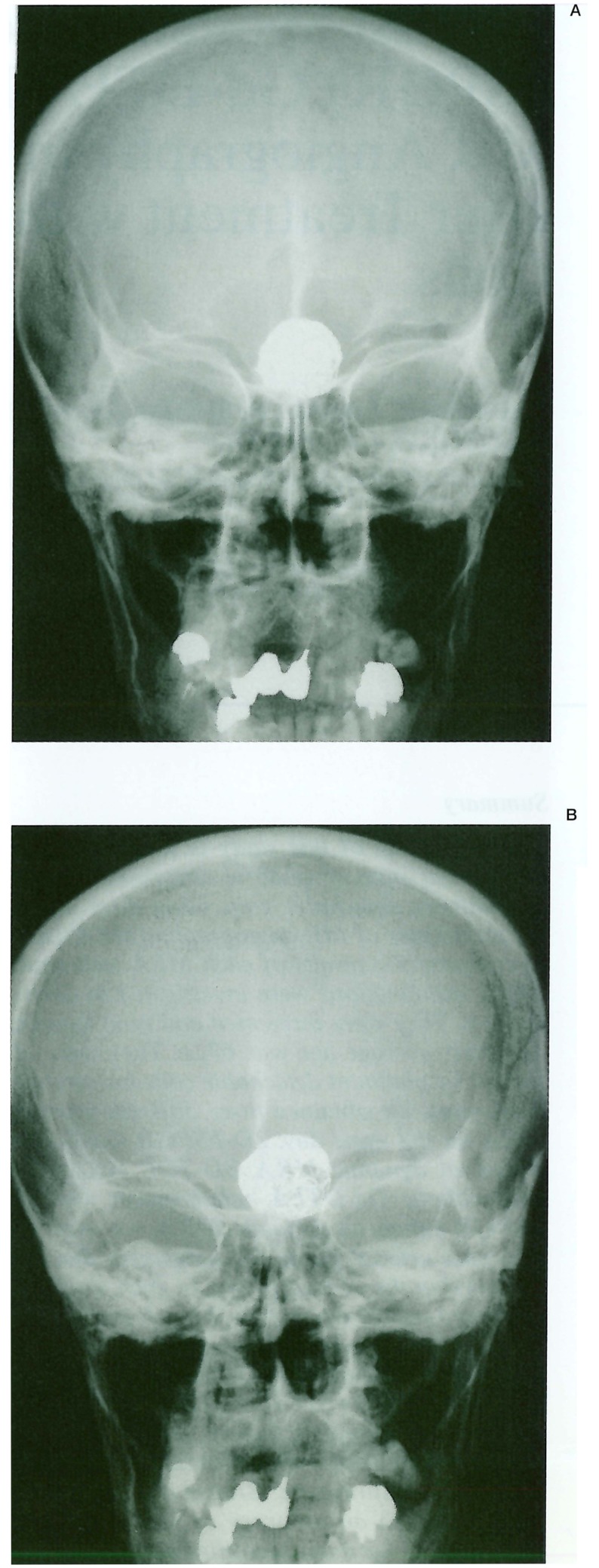
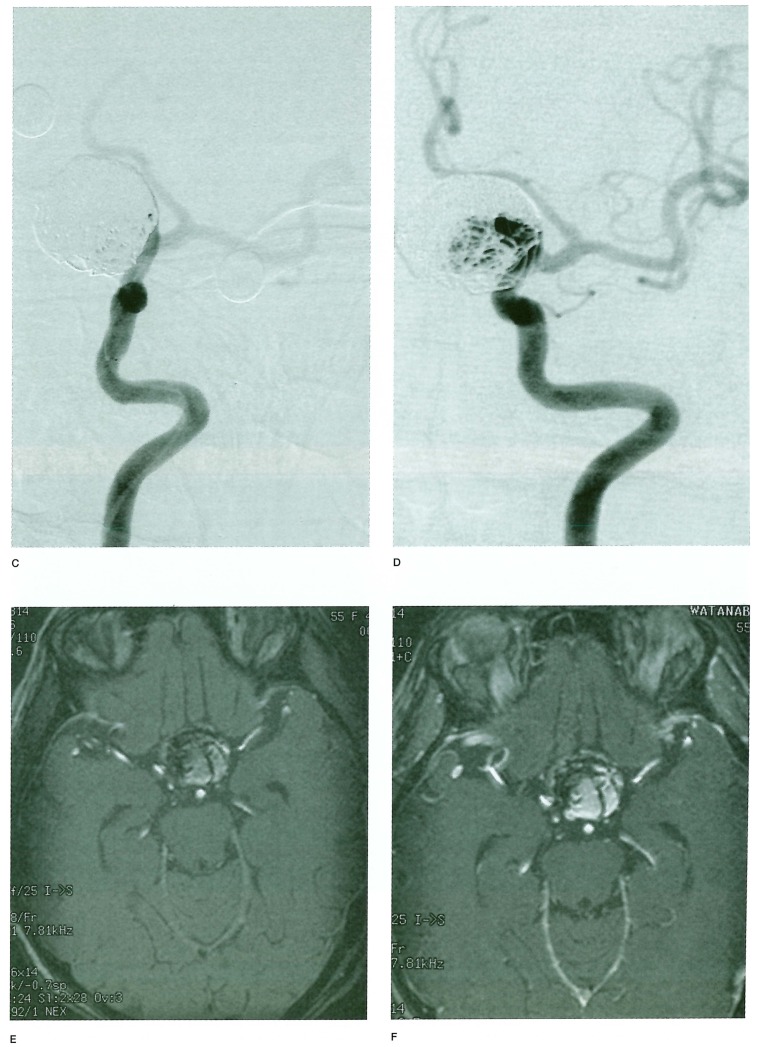
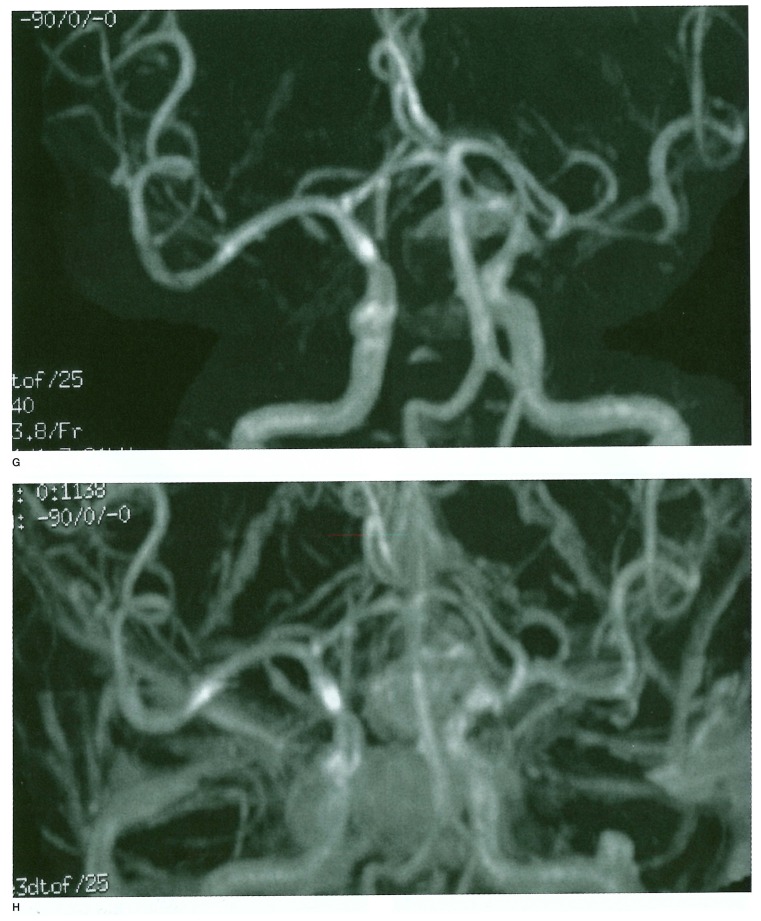
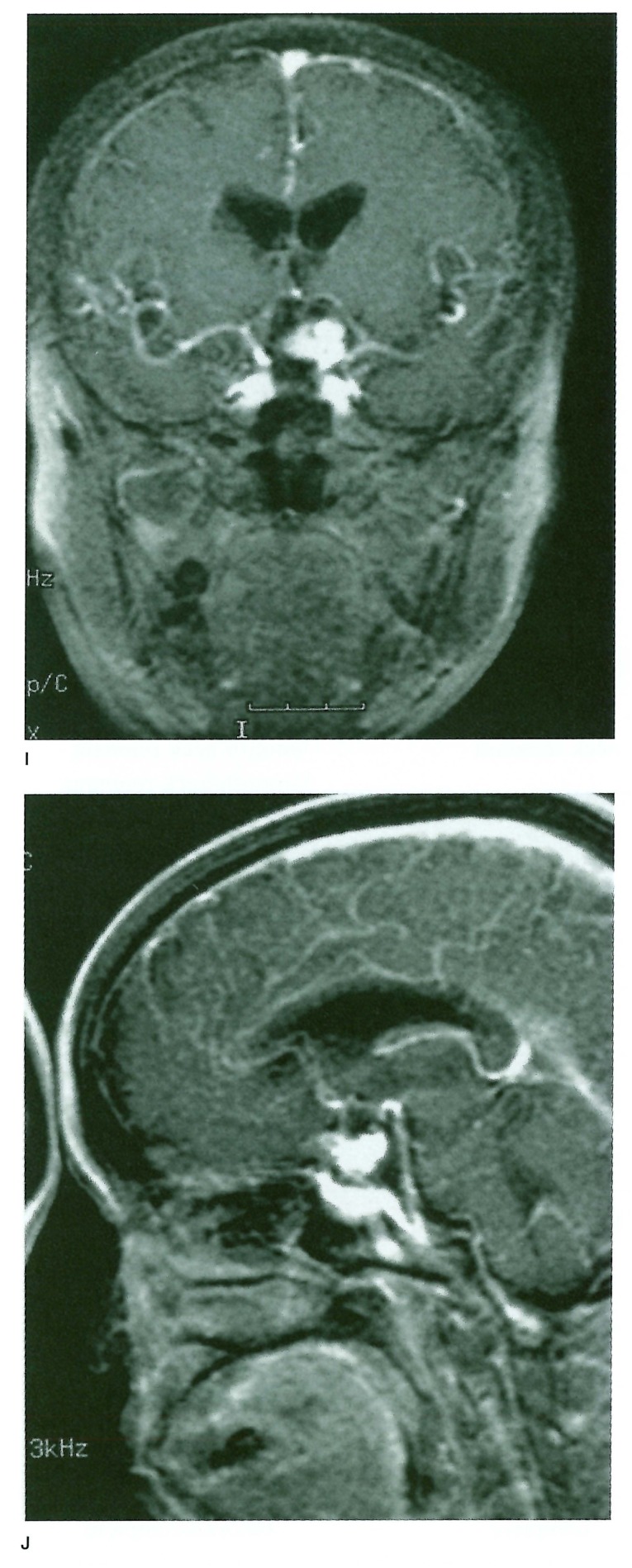
Figure 1.
A-D) Raptured 12 mm IC-PC aneurysm in a 54-year old woman treated with GDC placement, (table 1 Exam 4 patient) A) A-P view of the skull immediately after TAE. B) 1.5 years after TAE. C) Frontal DSA views of the left internal carotid artery immediately after TAE. D) 1.5 years after TAE. These views show regrowth of the aneurysm. Figure 1 E-H) 3D TOF MRA with and without Gd-DTPA enhancement. E) Non-enhanced 3D TOF MRA source image. F) Enhanced 3D TOF MRA source image. G) Non-enhanced 3D TOF MRA MIP reconstruction image. H) Enhanced 3D TOF MRA MIP reconstruction image. 3D TOF MRA without enhancement showed no distinct high signal in the aneurysm either in source or MIP reconstruction images. 3D TOF with enhancement shows a higher signal in the aneurysm in source image that suggested residual flow in the aneurysm, but in MIP reconstruction images residual flow and neck were obscured by overlapping cerebral veins. Figure 1 I-K) 2D FSPGR enhanced dynamic MRA. I) Coronal view. J) Sagittal view. K) A-P spot view focusing on aneurysm. Note prominent high signal in the aneurysm and peripheral low signal areas that suggested compacted coils.
Discussion and Conclusion
The treatment of intracranial saccular aneurysms with GDCs and IDCs is still in its infancy. Much more knowledge regarding the durability of this therapy as well as the clinical importance of residual aneurysmal neck and flow within the interstices of the coil mass has yet to be gained10-11. But the treatments would be considerd to be one of the excellent method in the future. Some data of mid-term follow up studies were also published.
Derdeyn and coworkers first evaluated platinum coil treated cerebral aneurysms by 3D-TOF MRA and, Gonner and coworkers evaluated platinum coil treated cerebral aneurysms using ultrashort echo time 3D TOF MR angiography9,12, But these studies were based on time of flight method, and in some cases it was difficult to evaluate the coil conditions by susceptibility artifact of platinum coils, turbulent or vortex flow around the aneurysms, and promoted thrombus formations.
We utilized T1 shortening effect of Gd-DTPA and using 2D FSPGR dynamic enhanced MRA method by bolus injection we got the good resuluts. We believe that the new technique of MR angiography will be clinically useful because by using the method, we can obtain the images in shorter time than using 3D-TOF MRA method, less invasive than DSA, and can obtain the accurate images very close to the DSA.
References
- 1.Klein GE, Szolar DH, et al. Extracranial aneurysm and arteriovenous fistula: embolization with the Guglielmi detachable coil. Radiology. 1996;201:489–494. doi: 10.1148/radiology.201.2.8888247. [DOI] [PubMed] [Google Scholar]
- 2.Guglielmi G, Vineula F, et al. Electrothrombosis of saccular aneurysms via endovascular approach. I. Electrochemical basis, technique, and experimental results. J Neurosurg. 1991;75:1–7. doi: 10.3171/jns.1991.75.1.0001. [DOI] [PubMed] [Google Scholar]
- 3.Gobin YP, Vineuela F, et al. Treatment of large and fusiform intracranial aneurysms with Guglielmi detachable coils. J Neurosurg. 1996;84:55–62. doi: 10.3171/jns.1996.84.1.0055. [DOI] [PubMed] [Google Scholar]
- 4.Mawad M, Mawad J, et al. Long-term histopathologic changes in canaine aneurysms embolized with Guglielmi detachable coils. Am J Neuroradiol. 1995;16:7–13. [PMC free article] [PubMed] [Google Scholar]
- 5.Mylon W Marshall, George P Teitlbaum, et al. Ferromagnetism and magnetic resonance artifacts of platinum embolization microcoils. Cardiovasc Intervent Radiol. 1991;14:163–166. doi: 10.1007/BF02577720. [DOI] [PubMed] [Google Scholar]
- 6.Shellock FG, Detrick MD, et al. MR compatibility of Guglielmi detachable coils. Radiology. 1997;203:568–570. doi: 10.1148/radiology.203.2.9114123. [DOI] [PubMed] [Google Scholar]
- 7.Hartman J, Nguyen T, et al. MR artifact, heat production, and ferromagnetism of Guglielmi detachable coils. Am J Neuroradiol. 1997;18:497–501. [PMC free article] [PubMed] [Google Scholar]
- 8.Derdeyn CP, Graves VB, et al. MR angiography of saccular aneurysms after treatment with Guglielmi detachable coils: Preliminary experience. Am J Neuroradiol. 1997;18:279–286. [PMC free article] [PubMed] [Google Scholar]
- 9.Gonner F, Heid O, et al. MR angiography with ultrashort echo time in cerebral aneurysms treated with Guglielmi detachable coils. Am J Neuroradiol. 1998;19:1324–1328. [PMC free article] [PubMed] [Google Scholar]
- 10.Richling B, Bavinski G, et al. Early clinical outcome of patients with ruptured cerebral aneurysms treated endovascular (GDC) or microsurgical techniques: a single center experience. Interventional Neuroradiology. 1995;1:19–27. doi: 10.1177/159101999500100105. [DOI] [PubMed] [Google Scholar]
- 11.Gravis VB, Strother CM, et al. Early treatment of ruptured aneurysms with Guglielmi detachable coils: effect on subsequent rebleeding. Neurosurgery. 1995;37:640–648. doi: 10.1227/00006123-199510000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Derdeyn CP, Graves VB, et al. MR angiography of saccular aneurysms after treatment with Guglielmi detachable coils: preliminary experience. Am J Neuroradiol. 1997;18:279–286. [PMC free article] [PubMed] [Google Scholar]