Abstract
Infective endocarditis is one of three common cardiac infections in the United Kingdom, in addition to myocarditis and pericarditis, with a reported incidence of 1.7 to 6.2 cases per 100,000 patient years. Infective endocarditis can often have serious consequences and a wide variety of organisms may be the causative pathogen. There are little published data regarding the exact spectrum of organisms that cause endocarditis in the United Kingdom and whether organisms such as streptococci still dominate. In the present study, all cases of endocarditis at the authors’ institution, representing a typical nontertiary centre, were retrospectively examined and audited to provide a snapshot of the organism spectrum in these patients.
The cases of more than 120 patients who were coded as having endocarditis by the institution’s clinical coding department during the period between December 2000 and January 2011 were examined. Microbiological tests and clinical case notes of all patients were reviewed. Of the 101 patients diagnosed with and treated for endocarditis, 64 were male, with a mean age of 60.57 years. The most common organisms identified were Streptococcus species (31%), Staphylococcus aureus (27%) and Enterococcus faecalis (21%). The organisms with the highest associated mortality rate were S aureus and the ‘other organism’ group, which included non-HACEK group (Haemophilus species, Actinobacillus actinomycetemcomitans, Cardiobacterium hominis, Eikenella corrodens and Kingella species) pathogens such as Candida albicans. Streptococcus species and S aureus remain the main cause of endocarditis in a typical hospital setting in the United Kingdom, although in a smaller proportion of cases than historical data suggests. Overall, mortality remains high, and the clinician should remain vigilant to endocarditis in any patient with a positive blood culture because the number of cases of endocarditis caused by less typical organisms are increasing.
Keywords: Endocarditis, Microbiology, Mortality, Outcomes
Over the past few decades, the population has evolved to become an older population with increased access to hospital resources. This has led to changes in the types of organisms involved in infections (eg, the emergence of Clostridium difficile). The clinical spectrum of infective endocarditis (IE) has also changed from the historical spectrum, with a reduction in rheumatic fever and a rise in intervention- and procedure-related secondary infections (1). In the 1970s, streptococci such as Streptococcus bovis or Streprococcus viridans were believed to account for up to 80% of all cases of IE, with Staphylococcus aureus comprising the majority of the remaining cases (1). In addition to the increasing age of the population, there have been changes in microbiological classifications such as when, importantly, Enterococcus faecalis was removed from the streptococcal group and classified as a separate organism in 1984 (2).
As mentioned above, as the population evolved, so did the micro-organisms involved in IE and, by the 1980s, the prevalence of streptococcal IE had diminished to less than 50% of cases, even in tertiary referral centres (3). In many areas, this trend has been related to an increase in the number of intravenous drug users (IVDUs) and an increase in the number of patients with degenerative valvular conditions who survive long enough to develop IE (4). What has not improved is the significant mortality rate associated with IE (11% to 26% [5]), which has even increased in some studies examining higher-risk populations (6). In addition, the overall incidence remains much the same as it has in previous years (3.6 per 100,000 per year [5]).
Because the most recent data published regarding the United Kingdom organism spectrum were recorded in the 1980s, we set out to determine whether changes continue to occur in the microbiological spectrum and, if so, whether this has affected the expected outcomes.
Methods
Data collection
The medical records of all patients who had been coded as having endocarditis between January 2000 and April 2011 were retrospectively collected. All case notes were reviewed and patients were included if they had been diagnosed with and treated for endocarditis, as identified by the attending physicians, and had either possible or probable IE according to the Duke criteria. Patients who did not meet the criteria and patients for whom the diagnosis was unclear were not included.
Possible endocarditis was defined as one major and one minor criteria or three minor criteria, and probable endocarditis as two major criteria, one major and three minor or five minor criteria.
The modified Duke criteria are shown below (7):
Major criteria
- Positive blood culture with typical IE microorganism, defined as one of the following:
- Typical microorganism consistent with IE from two separate blood cultures, such as Streptococci viridans, Streptococcus bovis, HACEK group organisms (Haemophilus species, Actinobacillus actinomy-cetemcomitans, Cardiobacterium hominis, Eikenella corrodens and Kingella species), Staphylococcus aureus or community-acquired enterococci, in the absence of a primary focus; or
- Microorganisms consistent with IE from persistently positive blood cultures, defined as:
- ○ Two positive cultures of blood samples drawn >12 h apart, or
- ○ All of three or a majority of four separate cultures of blood (with first and final samples drawn 1 h apart)
- ○ A single blood culture positive for Coxiella burnetii or antiphase I immuoglobulin G antibody titre >1:800
- Evidence of endocardial involvement
- Positive echocardiogram abscess, new dehiscence of a prosthetic valve or presence of an oscillating mass on the valve structures or other implanted material without alternative anatomical explanation
- New valvular regurgitation
Minor criteria
Predisposition to IE
Fever >38°C
Vascular phenomena such as an arterial embolus
Immunological phenomena such as a glomerulonephritis
Positive blood cultures that do not meet the major criteria or serological evidence of an organism consistent with IE
Data regarding patient age, sex, blood culture results, serological testing, echocardiogram results, existing valvular problems, length of stay and outcomes were also obtained. Some patients had more than one organism identified by blood culture; all culture growths fulfilling the criteria above were recorded for patients during their episode of IE. When a patient died, the organism identified was recorded to produce figures on mortality according to organism. In cases in which more than one organism was identified in a patient who died, the most repeatedly identified organism was used in mortality data analysis. Blood culture results were analyzed using commercially available automated systems and organism identification was performed in accordance with current National Health Service (NHS) systems.
Study population
The present study was conducted at Aintree University Hospitals NHS Foundation Trust, a large single-site hospital serving North Liverpool, South Sefton and Kirkby, United Kingdom. The hospital provides acute services to 330,000 individuals in a mostly urban area, including some areas of high social deprivation. The trust also offers some specialist services, such as sleep studies and hepatobiliary surgery, to a catchment area that includes approximately 1.5 million individuals in Merseyside, Cheshire, South Lancashire and North Wales.
Ethics approval
The present study was approved by the Research and Audit Department at Aintree University Hospitals NHS Foundation Trust. Formal ethics approval was not required.
RESULTS
Patient results
A total of 101 patients who had either possible or probable endocarditis according to the modified Duke criteria, and who had been diagnosed with and treated for IE by their attending physician, were identified over the 10-year period. Sixty-four patients were male and 47 were female, with a mean (± SD) age of 60.57±16.9 years and a mean length of stay of 45±15.95 days. The mean Duke score was 3.7±0.88. Only seven patients had evidence of right-sided or right ventricle pacing lead endocarditis, with all other cases affecting the aortic or mitral valve. Of the patients with IE, 21 were known IVDUs, 20 had prosthetic valves and 12 were known to have existing valve problems.
Microbiology results
The overall microbiological spectrum of blood culture isolates in the cohort of IE patients is presented in Figure 1. Overall, S aureus was the most commonly identified organism in the cohort over the entire time period, followed by the Streptococcus species, of which the most commonly identified organisms were S bovis and S viridans. Of the HACEK group organisms, Haemophilus aphrophilus and Actinobacillus actinomycetemcomitans were the only organisms identified in the present cohort. In the ‘other organism’ group, organisms identified in the context of acute endocarditis in multiple blood cultures included Klebsiella oxytoea, Erysipelothrix rhusiopathiae and C albicans.
Figure 1).
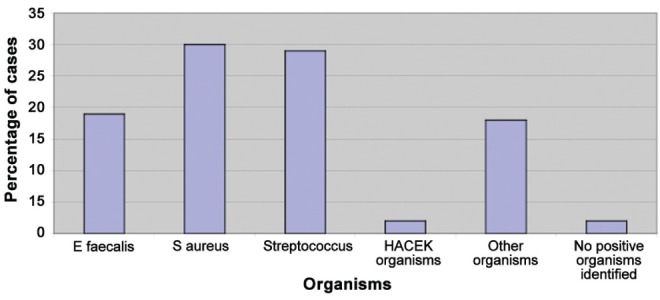
Overall organism spectrum, January 2000 to April 2011. E faecalis Enterococcus faecalis; HACEK Haemophilus species, Actinobacillus actinomycetemcomitans, Cardiobacterium hominis, Eikenella corrodens and Kingella species; S aureus Staphylococcus aureus
Figure 2 presents the variations in the incidence of various pathogens over three time periods of the present study. From these graphs, it is clear that there was a natural variation in the percentage of cases caused by a particular organism, with no specific trend for any organism over time in this institution. In the case of E faecalis, the period from 2004 to 2008 appeared to be associated with a high number of patients with prosthetic valves being admitted, with a corresponding reduction in native valve disease.
Figure 2).
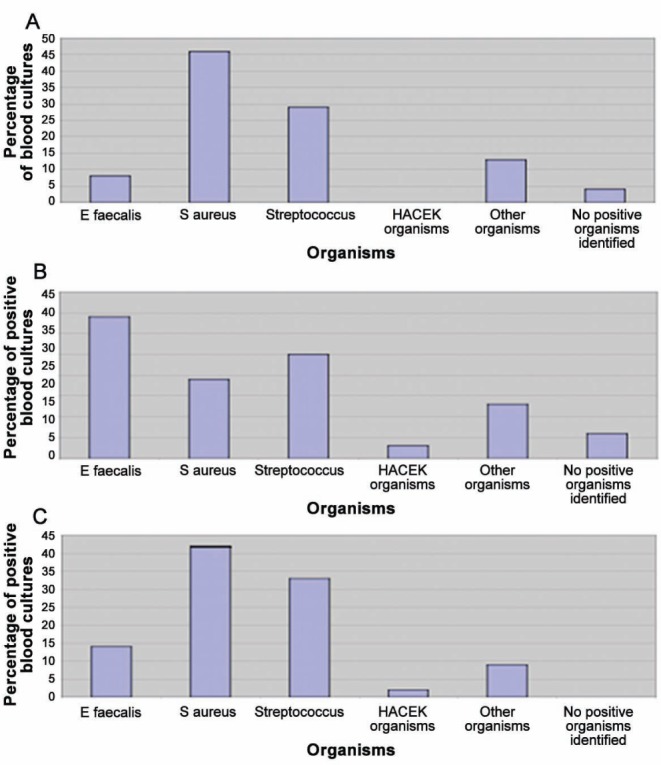
A Organism spectrum 2000 to 2004. B Organism spectrum 2004 to 2008. C Organism spectrum 2008 to 2011. E faecalis Enterococcus faecalis; HACEK Haemophilus species, Actinobacillus actinomycetemcomitans, Cardiobacterium hominis, Eikenella corrodens and Kingella species; S aureus Staphylococcus aureus
In higher-risk patients, such as IVDUs and individuals with known valve prostheses, virtually all cases were due to S aureus or Streptococcus species, except the cases of prosthetic valve endocarditis, which were mainly due to E faecalis (Figure 3).
Mortality data
In the present cohort, 30 deaths were related to episodes of IE, with 56% of these deaths occurring in individuals older than 70 years of age and 20% of these deaths in IVDUs. The mean Duke score in this group was 3.6, and of these 30 patients, 23 died during the initial presenting episode of overwhelming sepsis, three died due to a later recurrence of the same organism and four died immediately postoperatively, mainly from septic shock and circulatory collapse.
There tended to be a slightly more acute course in patients who died, with a mean time to death of 39±29.4 days, compared with the mean length of stay of 45 days for patients who survived.
It was difficult to estimate an initial point at which the disease process started and, therefore, a time to the start of treatment for patients who died because several patients had exhibited evidence of other potential infective processes such as positive urine cultures. In the present cohort, no patients were identified who had not received broad spectrum antibiotics within 48 h of admission, including patients who survived and patients who were relatively stable at the time of admission.
Figure 4 presents a breakdown of the organism spectrum in patients who died. In the ‘other organism’ group, nearly one-half of cases were due to C albicans, which is consistent with previously reported high mortality rates for fungal endocarditis (8).
DISCUSSION
The present analysis was the first audit of the microbiological spectrum in IE for many years. This is important because there has been a tendency for the most recent information in the United Kingdom regarding endocarditis to be in the form of either interesting case reports or assessment of treatment responses.
Our audit showed that IE remains a condition with a substantial mortality risk and that the spectrum of organisms involved has shifted over the years in our centre, which is fairly typical of the modern acute trust.
This shift does not appear to be continuing in our patient cohort, but appears to reflect general changes in the patient population and hospital experience in the past few decades that, in many other infective processes, have led to more infections with what were previously considered to be atypical organisms.
We also demonstrated that mortality differs among organisms and that, in particular, some of the less common organisms, such as C albicans, have a very high mortality rate compared with typical organisms such as streptococci. We also observed that in patients with prosthetic heart valves, E faecalis was the most common organism involved in IE, which may be expected due to the older age and multiple hospital visits of the typical valve replacement patient.
The present audit is important because continuous surveillance of the type of organism causing IE is necessary to enable clinicians to be aware of the significance of positive blood cultures and their likelihood of causing IE, even when the organism is one that is less typical.
The main change in recent years, both in the United Kingdom and elsewhere, appears to be a reduction in the incidence of IE caused by Streptococcus species and a rise in IE caused by S aureus and other bacteria (9–11). This is likely due to the increasing age of the population and an increase in the number of medical procedures patients undergo as part of their routine care (12). This aging population with multiple hospital attendances has led to an increase in the incidence of E faecalis IE in many countries (13), which was confirmed by the higher incidence of E faecalis in patients with prosthetic valves in our cohort.
In our cohort, the prevalence of culture-negative endocarditis was very low, with only three cases identified at our centre in 11 years. This is different from previously published studies showing that nearly 30% of cases were culture negative (14), although in studies from other countries the incidence has dropped to as low as 1% (15). In our centre, culture-negative cases are subjected to full immunological, polymerase chain reaction and serological testing. This, in addition to a global improvement in laboratory techniques in recent years, may help to explain our lower rates. Anecdotally, we also believe that increased performance of multiple blood cultures before antibiotics has helped increase our diagnostic yield. Because the microbiology spectrum varies from one country to another, it would be difficult to extrapolate our experience to other countries; this is demonstrated by the widely differing figures alluded to above.
Our overall mortality data are consistent with other published data from within the United Kingdom and similar countries regarding mortality from IE (16,17), with mortality rates of approximately 30%. This figure appears to have changed very little in the past few decades, highlighting the seriousness of the condition and the need to undertake regular audits of the microorganisms causing IE. It is likely that, for similar reasons to the proliferation of enterococcal IE (old age, increased morbidity, etc), mortality from IE will remain stubbornly high, especially considering the often insidious onset of the disease (18).
Limitations
Because the present analysis was a single-centre audit, we acknowledge that it is difficult to make generalizations to the rest of the United Kingdom regarding the microbiological changes in IE. However, because our data are consistent with previous data from the United Kingdom and more recent data from abroad (19,20), we can at least highlight to other trusts some of the changes that may be occurring in their local areas.
Another limitation of the present study is that we had only the recorded blood culture results to suggest that these organisms were the cause of our patients’ endocarditis. It is possible that this may not have been the case in some patients, and we do not have any tissue sample test results to compare with blood culture results.
We accept that IE due to separate organisms in blood cultures is highly unlikely, but in the case of patients with separate organisms being repeatedly cultured, we needed to record both in the absence of a tissue diagnosis to illustrate possible microbiological shifts.
CONCLUSIONS
IE in the United Kingdom remains a disease with a high mortality rate, and the microbiological spectrum causing this disease has shifted over time and will likely continue to do so. Doctors should be vigilant to this microbiological shift and the potential for some organisms to be more prevalent, such as S aureus in IVDUs or E faecalis in patients with prosthetic valves.
Figure 3).
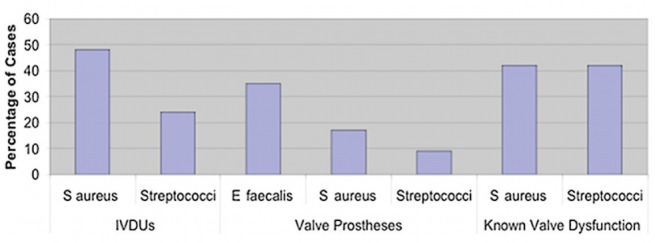
Organism spectrum in high-risk groups. E faecalis Enterococcus faecalis; IVDU Intravenous drug users; S aureus Staphylococcus aureus
Figure 4).
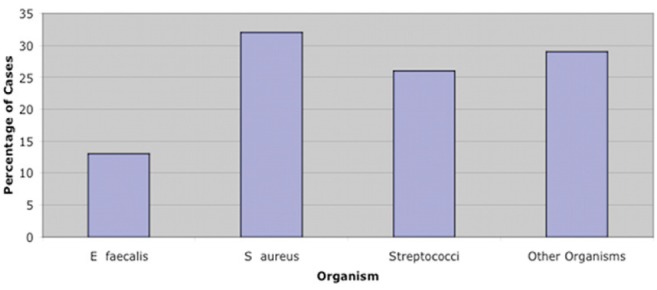
Major organism identified in blood cultures of patients who died from endocarditis, January 2000 to April 2011. E faecalis Enterococcus faecalis; S aureus Staphylococcus aureus
REFERENCES
- 1.Kaye D. Infective endocarditis. An overview. Am J Med. 1985;78:107–9. doi: 10.1016/0002-9343(85)90372-9. [DOI] [PubMed] [Google Scholar]
- 2.Schleifer KH, Kilpper-Balz R. Transfer of Streptococcus faecalis and Streptococcus faecium to the Genus Enterococcus nom. rev. as Enterococcus faecalis comb. nov. and Enterococcus faecium comb. nov. Int J Syst Bacteriol. 1984;34:31–4. [Google Scholar]
- 3.Dyson C, Barnes RA, Harrison GA. Infective endocarditis: An epidemiological review of 128 episodes. J Infect. 1999;38:87–93. doi: 10.1016/s0163-4453(99)90074-9. [DOI] [PubMed] [Google Scholar]
- 4.Cabell CH, Jollis JG, Peterson GE, et al. Changing patient characteristics and the effect on mortality in endocarditis. Arch Intern Med. 2002;162:90–4. doi: 10.1001/archinte.162.1.90. [DOI] [PubMed] [Google Scholar]
- 5.Moreillon P, Que YA. Infective endocarditis. Lancet. 2004;363:139–49. doi: 10.1016/S0140-6736(03)15266-X. [DOI] [PubMed] [Google Scholar]
- 6.Cicalini S, Puro V, Angeletti C, Chinello P, Macri G, Petrosillo N. Profile of infective endocarditis in a referral hospital over the last 24 years. J Infect. 2006;52:140–6. doi: 10.1016/j.jinf.2005.02.025. [DOI] [PubMed] [Google Scholar]
- 7.Li JS, Sexton DJ, Mick N, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis. 2000;30:633–8. doi: 10.1086/313753. [DOI] [PubMed] [Google Scholar]
- 8.Falcone M, Barzaghi N, Carosi G, et al. Candida infective endocarditis: Report of 15 cases from a prospective multicenter study. Medicine (Baltimore) 2009;88:160–8. doi: 10.1097/MD.0b013e3181a693f8. [DOI] [PubMed] [Google Scholar]
- 9.Tugcu A, Yildirimturk O, Baytaroglu C, et al. Clinical spectrum, presentation, and risk factors for mortality in infective endocarditis: A review of 68 cases at a tertiary care center in Turkey. Turk Kardiyol Dern Ars. 2009;37:9–18. [PubMed] [Google Scholar]
- 10.Giannitsioti E, Skiadas I, Antoniadou A, et al. Nosocomial vs. community-acquired infective endocarditis in Greece: Changing epidemiological profile and mortality risk. Clin Microbiol Infect. 2007;13:763–9. doi: 10.1111/j.1469-0691.2007.01746.x. [DOI] [PubMed] [Google Scholar]
- 11.Hill EE, Herijgers P, Claus P, Vanderschueren S, Herregods MC, Peetermans WE. Infective endocarditis: Changing epidemiology and predictors of 6-month mortality: A prospective cohort study. Eur Heart J. 2007;28:196–203. doi: 10.1093/eurheartj/ehl427. [DOI] [PubMed] [Google Scholar]
- 12.Fedeli U, Schievano E, Buonfrate D, Pellizzer G, Spolaore P. Increasing incidence and mortality of infective endocarditis: A population-based study through a record-linkage system. BMC Infect Dis. 2011;11:48. doi: 10.1186/1471-2334-11-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sy RW, Kritharides L. Health care exposure and age in infective endocarditis: Results of a contemporary population-based profile of 1536 patients in Australia. Eur Heart J. 2010;31:1890–7. doi: 10.1093/eurheartj/ehq110. [DOI] [PubMed] [Google Scholar]
- 14.Hricak V, Kovacik J, Marx P, et al. Etiology and risk factors of 180 cases of native valve endocarditis. Report from a 5-year national prospective survey in Slovak Republic. Diagn Microbiol Infect Dis. 1998;31:431–5. doi: 10.1016/s0732-8893(98)00030-3. [DOI] [PubMed] [Google Scholar]
- 15.Marks P, Gogova M, Kromery V., Jr Culture negative endocarditis: Data from the national survey in Slovakia. Postgrad Med J. 2002;78:61. doi: 10.1136/pmj.78.915.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McGivern D, Ispahani P, Banks D. Factors influencing mortality from infective endocarditis in two district general hospitals. Postgrad Med J. 1987;63:345–9. doi: 10.1136/pgmj.63.739.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chu J, Wilkins G, Williams M. Review of 65 cases of infective endocarditis in Dunedin Public Hospital. N Z Med J. 2004;117:U1021. [PubMed] [Google Scholar]
- 18.Lomas JM, Martinez-Marcos FJ, Plata A, et al. Healthcare-associated infective endocarditis: An undesirable effect of healthcare universalization. Clin Microbiol Infect. 2009;16:1683–90. doi: 10.1111/j.1469-0691.2009.03043.x. [DOI] [PubMed] [Google Scholar]
- 19.Khan NU, Farman MT, Sial JA, Achakzai AS, Saghir T, Ishaq M. Changing trends of infective endocarditis. J Pak Med Assoc. 2010;60:24–7. [PubMed] [Google Scholar]
- 20.Alonso-Valle H, Farinas-Alvarez C, Bernal-Marco JM, et al. The changing face of prosthetic valve endocarditis at a tertiary-care hospital: 1986–2005. Rev Esp Cardiol. 2010;63:28–35. doi: 10.1016/s1885-5857(10)70006-2. [DOI] [PubMed] [Google Scholar]


