Abstract
The radial artery approach to coronary catheterization is considered to be superior to femoral artery access in terms of vascular complications. The authors describe two patients who developed pseudoaneurysm following radial artery access for cardiac catheterization. The patients underwent surgical exploration with good results. Although rare, radial artery pseudo-aneurysms may complicate cardiac catheterization and have serious clinical consequences.
Keywords: Cardiac catheterization, Coronary artery disease, Pseudoaneurysm, Radial artery
A transradial approach to vascular access is rapidly becoming preferable to traditional femoral artery access for both diagnostic coronary angiography and percutaneous coronary intervention (PCI). This trend is explained by the significant reduction in the occurrence of access-site complications observed when selecting a transradial over transfemoral technique – reported to be 0.6% and 1.5%, respectively, in a large single-centre study (1). This is of particular relevance to patients who are at high risk for femoral access-site complications, including patients with a high body mass index and individuals receiving a glycoprotein IIb/IIIa inhibitor (1–3).
METHODS
Two patients who developed radial artery pseudoaneurysms complicating diagnostic cardiac catheterization via the radial artery approach are described. Both patients were managed surgically with exploration and repair.
Case 1
An 83-year-old woman with a history of paroxysmal atrial fibrillation, moderate aortic valve stenosis, with preserved left ventricular function and chronic stable angina, underwent cardiac catheterization after presenting with acute coronary syndrome. On diagnosis, the patient was administered 300 mg of dual antiplatelet therapy (acetylsalicylic acid and clopidogrel), both of which were continued at a dose of 75 mg/day. In addition, the patient received a single dose of 2.5 mg fondaparinux. The patient underwent cardiac catheterization the following day. The patient was administered 3000 IU of heparin and the procedure was performed via right radial artery access using a 6 Fr Terumo sheath (Terumo Interventional Systems, USA). A diagnostic catheter (Tiger Radial TIG 4.0, Terumo Interventional Systems, USA) was used for angiographic imaging, which demonstrated severe left distal main stem and moderate right coronary artery disease. The left anterior descending artery exhibited mild mid-vessel disease. At the end of the procedure, the radial-vascular sheath was partially removed and a TR band (Terumo Interventional Systems, USA) was applied over the access site. The band was inflated with 15 mL of air and the sheath was fully removed. An additional 1 mL of air was used to further inflate the band to maintain hemostatic control. The TR band was deflated gradually and removed after 4 h to 5 h; no bleeding was observed during this time. Within 24 h of the procedure, a painless, pulsatile mass was identified at the location of the procedure access site, preceding a painful swelling of the right forearm due to extension of the hematoma. Duplex ultrasonography revealed a 29.7 mm × 16.4 mm × 23.6 mm pseudoaneurysm of the right radial artery at the level of the wrist (Figures 1 and 2), with some intrapseudoaneurysm thrombus formation.
Figure 1).
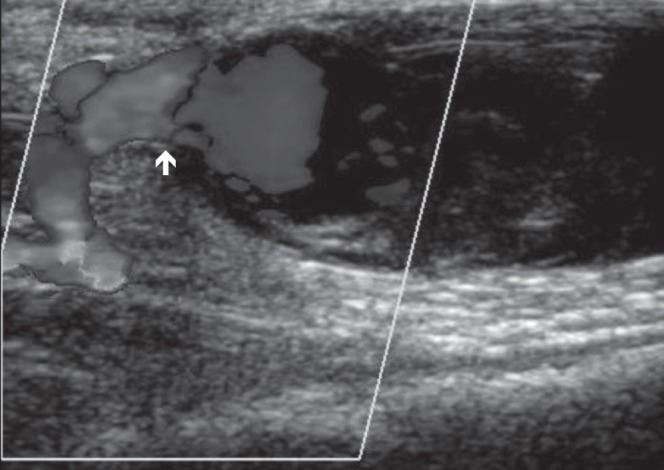
Doppler ultrasound of pseudoaneurysm (longitudinal view). Biphasic flow demonstrated at neck (arrow) of pseudoaneurysm
Figure 2).
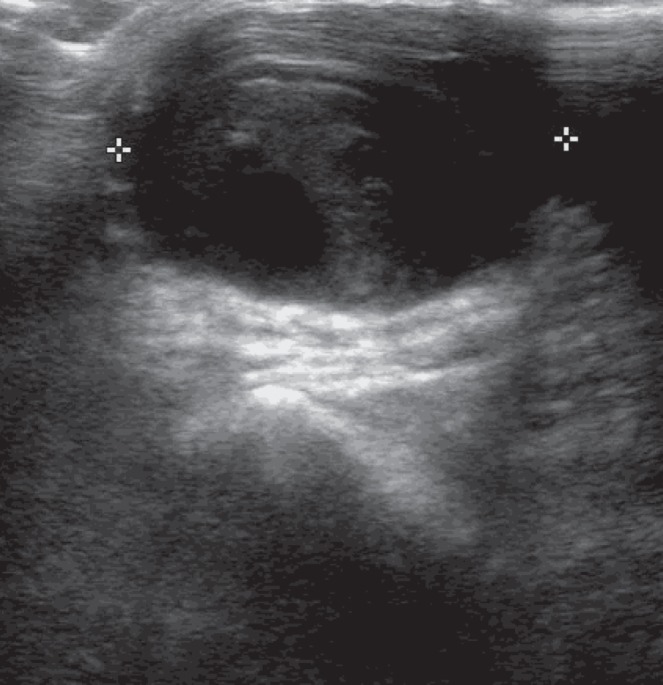
Doppler ultrasound of pseudoaneurysm (transverse view). Aneurysmal sac dimension 29.7 mm × 16.4 mm × 23.6 mm
The patient underwent surgical exploration of the hematoma and the radial artery was repaired using three 6/0 prolene sutures. The hematoma was evacuated and fasciotomies were performed on the flexor and extensor surfaces of the forearm. Postprocedure ultrasonography confirmed good radial artery duplex signals with preserved neurological function.
The patient recovered completely within 10 days and was subsequently discharged home and later underwent outpatient PCI of the right coronary artery and left main stem via right femoral artery approach. No access-site complication occurred during PCI. She remains asymptomatic at five months follow-up.
Case 2
An 80-year-old woman was admitted with troponin-positive acute coronary syndrome. Following diagnosis, she was administered 300 mg of dual antiplatelet therapy (acetylsalicylic acid and clopidogrel), both of which were continued at a dose of 75 mg/day. In addition, the patient received a single dose of fondaparinux 2.5 mg and subsequently underwent cardiac catheterization the following day. Her medical history included type 2 diabetes and hypertension.
The patient was administered 3000 IU heparin and the procedure was performed via right radial artery access using a 6 Fr Terumo sheath. A 6 Fr JL 3.5 and JR 4 (Judkins, USA) diagnostic catheter was used for coronary angiography. This identified severe calcified three-vessel disease with preserved left ventricular function. A TR band was applied as the vascular sheath was partially removed and inflated with 15 mL of air; the sheath was then completely removed. Adequate hemostatic control was observed at this point. The TR band was deflated gradually and removed over 4 h to 5 h. No significant bleeding was observed during deflation or TR band removal. Forty-eight hours following the procedure, a pulsatile swelling developed at the puncture site. The patient was reviewed by the surgical team and underwent surgical exploration of the swelling. The right radial artery revealed a small puncture in the anterior wall, with extensive hematoma formation. The hematoma was evacuated and the radial artery was debrided and patched using a small section of cephalic vein. Ultrasonography showed good radial artery flow postprocedure.
The patient subsequently underwent outpatient coronary artery bypass surgery.
DISCUSSION
The increasing practice of transradial cardiac catheterization accounts for the significant reduction in access-site complication rates. Complications of transradial catheterization include radial artery occlusion, nonocclusive injury, spasm, hand ischemia, nerve damage, bleeding and pseudoaneurysm formation (2).
A pseudoaneurysm is described as a tear through all of the layers of the artery with persistent flow outside of the artery contained by the surrounding tissue (4). Clinically significant pseudoaneurysm occurs in 0.05% to 1.0% of diagnostic and up to 6% of interventional transfemoral procedures (5,6). Post-transradial catheterization pseudoaneurysm is rare, with an incidence <0.1% reported in a large case series (7).
Factors predisposing to the development of radial artery pseudoaneurysm include multiple puncture attempts, ongoing systemic anticoagulation, inadequate hemostasis/postprocedure compression, vascular site infection and the use of larger catheter sheath sizes (2,3,8–11). Neither of the two cases reported above received pre- or postprocedure anticoagulation therapy, nor was recovery affected by access-site infection.
The use of a small-diameter catheter sheath size, as studied by Lefevre et al (11), was associated with a reduction in access-site complication rates. Additionally, in cases where a 4 Fr catheter sheath was selected over a 6 Fr, both a reduction in procedure time and time to ambulation was identified, with acceptable angiographic results (11). In both of the cases described above, a 6 Fr catheter sheath was used; the choice of this size sheath may have contributed to the development of the pseudoaneurysms outlined.
Postprocedure hemostatic compression devices are frequently used for the prevention of pseudoaneurysm formation (12). In the cases described above, a Terumo TR band was used, with no immediate bleeding observed following removal. Potential problems originating from TR band use include both incorrect band positioning and inadequate compression pressure required to prevent vascular complications. To address these issues, both care in application of the TR band and rigorous assessment of early bleeding following sheath removal must be undertaken with additional band inflation where appropriate. The number of reported cases in the literature is, to date, insufficient to enable an assessment of the efficacy among various hemostatic compression devices in preventing radial artery pseudoaneurysm.
Following cardiac catheterization, it is recommended that frequent assessment by attending nurses and physicians undertake observation of the vascular access site. Large pseudoaneurysms, similar to that described in case 1, may be identified early on clinical examination. Following clinical suspicion, confirmation of pseudoaneurysm with Doppler ultrasonography or explorative surgery and subsequent intervention is paramount to preventing evolution of a hematoma and associated comorbidity, similar to that described in case 1.
An individualized approach to management based on the severity of the pseudoaneurysm is recommended (2,3). When the defect is small, compression with a view to thrombosis of the false aneurysm may suffice; when larger, surgical intervention becomes necessary. Surgical technique is determined by the width of the neck of the pseudoaneurysm. Less impressive cases will allow for direct ligation of the defect. In cases where a larger neck diameter is observed, excision of the vessel wall with vein patch angioplasty may be required, as in case 2. Where there is evidence of adequate collateral arterial flow to the hand, ligation and excision of the pseudoaneurysm and radial artery may be permitted (13).
Management using thrombin injection has also been reported (13). Although deemed safe and effective, given the anatomical location of a radial artery pseudoaneurysm and the ease of surgical access, this approach has been deemed unlikely to offer significant advantage over surgical intervention (3).
CONCLUSION
Although pseudoaneurysms of the radial artery are rare, they are significant and, in some cases, may result in serious consequences. Prevention must be focused on obtaining both optimal individualized postprocedure compression pressure and duration. A balance must be struck between preventing pseudoaneurysm development and avoiding overenthusiastic compression resulting in occlusion of the artery.
REFERENCES
- 1.Eichhofer J, Horlick E, Ivanov J, et al. Decreased complication rates using the transradial compared to the transfemoral approach in percutaneous coronary intervention in the era of routine stenting and glycoprotein platelet IIb/IIIa inhibitor use: A large single-center experience. Am Heart J. 2008;156:864–70. doi: 10.1016/j.ahj.2008.06.044. [DOI] [PubMed] [Google Scholar]
- 2.Kanei Y, Kwan T, Nakra NC, et al. Transradial cardiac catheterization: A review of access site complications. Catheter Cardiovasc Interven. 2011;78:840–6. doi: 10.1002/ccd.22978. [DOI] [PubMed] [Google Scholar]
- 3.Collins N, Wainstein R, Ward M, Bhagwandeen R, Dzavik V. Pseudoaneurysm after transradial cardiac catheterization: Case series and review of the literature. Catheter Cardiovasc Interv. 2012;80:283–7. doi: 10.1002/ccd.23216. [DOI] [PubMed] [Google Scholar]
- 4.Kalapatapu VR, Shelton KR, Ali AT, Moursu MM, Eidt JF. Pseudoaneuysm: A review. Curr Treat Cardiovasc Med. 2008;2:173–83. doi: 10.1007/s11936-008-0019-8. [DOI] [PubMed] [Google Scholar]
- 5.Katzenschlager R, Ugurluoglu A, Ahmadi A, et al. Incidence of pseudoaneurysm after diagnostic and therapeutic angiography. Radiology. 1995;195:463–6. doi: 10.1148/radiology.195.2.7724767. [DOI] [PubMed] [Google Scholar]
- 6.Kresowik TF, Khoury MD, Miller BV, et al. A prospective study of the incidence and natural history of femoral vascular complications after percutaneous coronary angioplasty. J VascSurg. 1991;13:328–36. [PubMed] [Google Scholar]
- 7.Sanmartín M, Cuevas D, Goicolea J, Ruiz-Salmerón R, Gómez M, Argibay V. Vascular complications associated with radial artery access for cardiac catheterization. Rev Esp Cardiol. 2004;57:581–4. [PubMed] [Google Scholar]
- 8.Gilchrist IC. Laissez-faire hemostasis and transradial injuries. Catheter Cardiovasc Interv. 2009;73:473–4. doi: 10.1002/ccd.22006. [DOI] [PubMed] [Google Scholar]
- 9.Ganchi PA, Wilhelmi BJ, Fujita K, Lee WP. Ruptured pseudoaneurysm complicating an infected radial artery catheter: Case report and review of the literature. Ann Plast Surg. 2001;46:647–50. doi: 10.1097/00000637-200106000-00015. [DOI] [PubMed] [Google Scholar]
- 10.Tsao JW, Neymark E, Gooding GA. Radial artery mycoticpseudoaneurysm: An unusual complication of catheterization. J Clin Ultrasound. 2000;28:414–6. doi: 10.1002/1097-0096(200010)28:8<414::aid-jcu6>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 11.Lefevre T, Morice MC, Bonan R, et al. Coronary angiography using 4 or 6 French diagnostic catheters: A prospective, randomized study. J Invasive Cardiol. 2001;13:674–7. [PubMed] [Google Scholar]
- 12.Liou M, Tung F, Kanei Y, Kwan T. Treatment of radial artery pseudoaneurysm using a novel compression device. J Invasive Cardiol. 2010;22:293–5. [PubMed] [Google Scholar]
- 13.D’Achille A, Sebben RA, Davies RP. Percutaneous ultrasound-guided thrombin injection for coagulation of post-traumatic pseudoaneurysms. Australas Radiol. 2001;45:218–21. doi: 10.1046/j.1440-1673.2001.00906.x. [DOI] [PubMed] [Google Scholar]


