Abstract
Mouse models of myocardial ischemia and infarction are important in cardiovascular research. Reliable and reproducible assessment of the area at risk (AAR) and infarct size (IS) in mice is vital for deciphering mechanisms behind these common diseases, and for developing and evaluating treatment strategies. The present review will briefly describe and discuss the most common methods for determining the AAR and IS in mouse models of cardiovascular disease. Several methods exist for ex vivo assessment of IS. Conventional histological stains target the fibrous scar and require several days to pass from the time of infarct induction until the animal is euthanized, whereas triphenyltetrazolium-based techniques stain the viable tissue surrounding the infarct and can be performed on tissue harvested within a few hours after infarction. The AAR is usually stained by injecting a dye into the circulation. This dye subsequently distributes to perfused tissue but leaves the AAR unstained. In vivo assessment enables serial measurements of the IS and/or AAR and is sometimes preferable to ex vivo techniques. Echocardiography is usually the method of choice but magnetic resonance imaging-based techniques are also used. The aim of the present review was to provide basic researchers with an introduction to the various techniques used to assess and quantify the IS and AAR in experimental mouse models of myocardial ischemia-reperfusion and infarction.
Keywords: Area at risk, Echocardiography, Infarct size, Myocardial infarction
Small animal models of myocardial ischemia and infarction play an important role in cardiovascular research (1). Despite obvious dissimilarities in size and heart rate, with subsequent differences in cardiac hemodynamics and electrophysiology, the mouse heart is considered to resemble its human counterpart fairly well with regard to both anatomy and physiology. Due to the increased availability and relatively low cost of genetically modified mouse models, the mouse has emerged as the most widely used of the small animal models (2).
Infarct size (IS) and area at risk (AAR) are vital parameters in all research investigating IS limitation (3). The AAR represents the entire myocardial perfusion bed distal to an occluded coronary artery and is a major determinant of final IS and prognosis (4,5). During an acute coronary event, the myocardium in the AAR will progress into necrosis through the ‘wavefront’ phenomenon, beginning with the central subendocardial tissue and spreading epicardially and toward the periphery of the AAR (6). The IS:AAR ratio is a valuable parameter for evaluating the efficacy of various interventions aimed at reducing the final IS and studying the consequences of genetic manipulation on the ischemic tolerance of the myocardium.
With the vast amount of interest in cardiac conditioning phenomena and their cardioprotection during an ischemic insult (7), a reliable assessment of the IS and AAR is necessary for an animal model to be feasible (3).
The present article briefly reviews the different modalities used to assess these parameters in mouse models, and the benefits and drawbacks of each modality are discussed.
INDUCTION OF MYOCARDIAL ISCHEMIA AND INFARCTION IN SMALL ANIMAL MODELS
Surgical models for myocardial infarction and ischemia-reperfusion in mice are discussed elsewhere (8,9) and are only briefly mentioned here. In the classical model, the animal is intubated or tracheot-omized and connected to a mechanical ventilator. The heart is accessed through the fourth intercostal space, and the left anterior descending coronary artery (LAD) is either permanently or transiently occluded by placing a suture around the vessel as it emerges from below the left atrial appendage. Induction of infarction can be verified using echocardiography and by the visualization of a pale, hypokinetic ventricle distal to the occlusion (8,9). A new mouse model that does not require intubation has recently been described (10).
EVALUATION OF THE AAR AND IS IN SMALL ANIMALS
In mice, the anatomy of the coronary arteries is variable and highly debated (11,12). The left coronary artery courses to the apex as either one or two major vessels with several smaller branches. It is usually referred to as the LAD because the circumflex branch is considered to be unimportant in the mouse (13). The variability of both its branching pattern and of the myocardial vascular territories supplied by the LAD prevents reliable reproducibility in the size of the AAR and necessitates its subsequent assessment in experiments performed by expert surgeons with consistent positioning of the ligature (14–16).
EX VIVO METHODS FOR IS AND AAR ASSESSMENT
Ex vivo assessments of AAR and IS are inexpensive, reliable methods for quantification but necessitate euthanization of the animal and preclude long-term follow-up. Furthermore, these methods do not allow tissue samples to be used for other laboratory investigations. IS is usually measured using either conventional histological stains or 2,3,5-triphenyltetrazolium (TTC) staining, whereas the AAR may be determined by injecting a dye into the circulation after ligation of the artery. Injection of KCl immediately before the animals are euthanized arrests the heart in diastole, aiding subsequent quantification.
Conventional histology for IS assessment
After the animal has been euthanized, the heart is sectioned into slices 1 mm thick, fixed in formalin for several days and embedded in paraffin (17). The slices can then be further sectioned and stained using conventional agents such as the modified Masson’s trichrome stain (17,18). The IS can be calculated as a percentage of the entire left ventricle (see below). Care must be taken to allow time for the ventricle to remodel sufficiently for the conventional stains to delineate the infarct area (ie, fibrotic changes). Reliable IS assessment is possible only >72 h after coronary occlusion (19).
Although conventional histology has been considered the gold standard for IS assessment, the need for extensive sectioning and tissue processing, as well as the time necessary to allow fibrotic changes to occur, has led to the development of alternative methods (20).
TTC staining of IS
TTC staining is the method of choice in our laboratory for postmortem determination of IS. The heart is sliced transversally at a thickness of 1 mm and immersed in neutral TTC solution for 15 min to 20 min at 37°C.
The viable myocardium is stained as the water-soluble compound TTC is converted by active mitochondrial dehydrogenases into an insoluble red precipitate. The extent of staining correlates with the number of viable mitochondria and differentiates viable and nonviable tissue (21) (Figure 1). However, for optimal infarct delineation in acute ischemia-reperfusion experiments in which the LAD is transiently occluded, it is necessary to wait until the enzymes have been washed out from the infarcted territory (at least 2 h of reperfusion); the IS may otherwise be underestimated (22).
Figure 1).
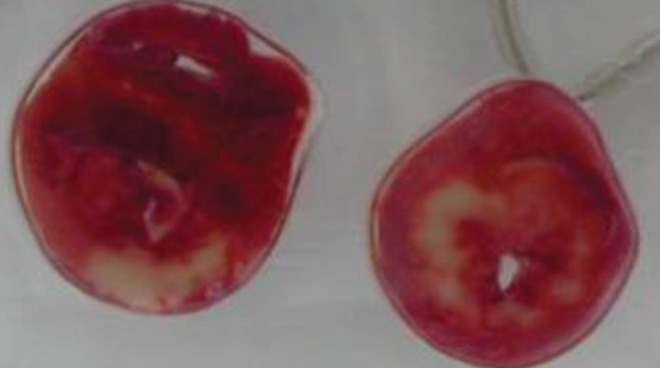
2,3,5-Triphenyltetrazolium staining after an experimentally induced myocardial infarction. Noninfarcted, viable tissue is stained red, whereas the infarcted region is left unstained. The image was provided by Dr J Downey, University of South Alabama (Mobile, Alabama, USA)
In the chronic setting, no residual NADH will persist in the infarct area, and reliable assessment can be performed without the necessity of reperfusion.
For better visualization, the heart slices may be stored for a variable period (eg, 24 h) in 10% formaldehyde or in phosphate-buffered saline. Once stained, the heart slices may then be scanned or photographed and the IS calculated as described below (Figure 1B).
Other ex vivo methods for delineating IS
Efforts have been made to introduce other techniques for IS assessment that would preclude the need for longer reperfusion times and tedious tissue processing, which has an inherent risk of introducing artefacts. For example, there has been renewed interest with regard to propidium iodide, a compound that emits red fluorescence as it intercalates with DNA, to which it gains access during and following cell death when the integrity of the cell membrane is disrupted. There is no need for longer reperfusion times using this method. Furthermore, the tissue does not require processing, but can be sliced and analyzed directly (23,24). However, despite recent demonstrations that this method yields results that strongly correlate with the results of TTC staining, this technique is not yet widely used.
Ex vivo AAR assessment using Evans Blue dye
Following occlusion of a coronary artery, the perfused myocardium may be stained by injecting a dye into the circulation of the animal, either in vivo or postmortem, through retrograde perfusion after cannulation of the aorta. The AAR will be left unstained (25). The heart may then be sectioned and the AAR quantified as the unstained area of each slice (Figure 1B). Each slice can then be further stained to delineate the IS within the AAR.
Evans Blue is an azo dye that, when injected into the circulation, binds tightly to plasma albumin and distributes to perfused tissue, staining that tissue dark blue (26). It is water soluble and has a tendency to smear out into unstained tissue, obscuring the border. This smearing can be prevented to a large extent by freezing the heart immediately after dye injection. Other, less water-soluble staining agents have been introduced to limit the extent of smearing (27).
Ex vivo AAR assessment using fluorescent microspheres
Fluorescent microspheres can be injected into the ventricular cavity following coronary occlusion. These microspheres will then distribute to perfused tissue and can be visualized using ultraviolet light. This approach allows for further evaluation of the tissue using other laboratory techniques (28).
Calculation of the IS and AAR
Regardless of which staining method is chosen, IS and AAR may be calculated using either area-based or length-based methods. In the area-based method, the area of the infarcted region and/or AAR is expressed as a percentage of the total area of that slice.
In the length-based method, the length of the infarcted segment and/or AAR, either at the epicardial or endocardial surface, is divided by the epicardial or endocardial circumference of each slice. Another approach is to use the midline length and circumference (29).
Regardless of whether an area- or length-based approach is chosen, the IS and AAR can be expressed as a percentage of the entire ventricle, and each slice should be corrected for weight.
In the chronic infarction model, in which the animal is euthanized several days or weeks after induction of myocardial infarction, considerable thinning of the infarcted segment may have occurred (30,31). In this model, the area-based method will underestimate the IS compared with the length-based method. However, both approaches have been found to be reliable provided they are not mixed (29).
IN VIVO METHODS FOR AAR AND IS QUANTIFICATION
In vivo quantification of the AAR and IS does not require euthanasia of the animal and is preferred over the postmortem methods described above in longitudinal studies of myocardial ischemia and/or reperfusion.
The IS can, of course, be quantified at the end of experiments, when animals are euthanized, using the in vitro techniques described above. However, the infarcted area undergoes significant remodelling and may differ substantially in size at different time points following infarction (30–32). Noninvasive methods enable serial assessment. Furthermore, the tissue, once harvested, may be used for other investigations.
The AAR can also be determined using in vitro techniques at the end of the experiments, following religation of the coronary artery immediately before dye injection. Again, IS expansion, extension or retraction, and other forms of myocardial remodelling may introduce significant bias (30).
Echocardiography
Echocardiography is a relatively inexpensive modality extensively used in phenotyping murine cardiac function (2,33). Provided the echocardiographic equipment is of sufficient quality, the small size of the mouse heart is compensated for by its proximity to the skin, and much higher ultrasonic frequencies can be used in the mouse compared with humans, yielding an image of good quality.
Segmental wall motion abnormalities (WMA) have been used to assess both the AAR and IS. Following transient ischemia, the myocardium shows reversible dysfunction, known as stunning (34). The stunned myocardium will be akinetic and regional WMA extending across the entire AAR can be observed on the two-dimensional echocardiogram. Evaluation of WMA in short-axis views, at the level of the mitral annulus, at the level of the papillary muscles and at the apical region of the ventricle, is used to calculate the AAR as the percentage of akinetic segments (25). Similarly, the infarcted myocardium is afunctional and akinetic.
In models of nonreperfused myocardial infarction, as well as in reperfused and reoccluded models (after several hours of reperfusion), the extent of hypokinesia correlates well with the AAR measured using microspheres (25,35). The extent of hypokinesia correlates less well with IS in reperfused hearts, especially at shorter ischemic times. This probably relates to the nontransmurality of such infarcts, in which there would persist viable and actively contracting tissue within the scar, rendering a given segment hypokinetic but not simply akinetic or normokinetic and precluding evaluation by the naked eye (25).
Transesophageal echocardiography may provide better image quality and has been shown to be superior to its transthoracic counterpart in assessing the AAR and IS using WMA (36).
Myocardial contrast echocardiography can be used to assess the AAR. First, contrast may be injected simply to better delineate the endocardial border, as described above. Second, microbubbles, when injected into the circulation, cause a signal intensity increase in the myocardial wall segments that remain perfused. The perfusion defect can then be quantified, accurately and less subjectively, to delineate the AAR (37).
A novel technique known as ‘speckle tracking’ has recently been developed, in which interference from backscattered ultrasound waves from neighbouring structures creates a speckled pattern in the myocardium that remains relatively constant during the cardiac cycle. Each small myocardial area, therefore, has its own speckle pattern and can be followed during the cardiac cycle (38). Thus, speckle tracking enables the measurement of strain and strain rate without angle dependence and without the need to correct for passive movement secondary to contraction of neighbouring myocardial segments or translation of the entire heart on the thoracic wall. Such considerations were necessary with earlier techniques (ie, tissue Doppler imaging) (39).
Peak systolic strain rate after myocardial infarction correlates with the transmurality of the infarction, and speckle tracking is a potential tool for assessing the IS in ischemia-reperfusion models, ie, when there is incomplete transmurality (39–42). Furtermore, automatized algorithms may make speckle tracking less subjective and less operator-dependent than visual WMA assessment (42).
Magnetic resonance imaging
Numerous magnetic resonance imaging (MRI)-based techniques have been developed for in vivo mouse studies (43). For an in-depth review of magnetic resonance concepts and applications, the reader is referred elsewhere (44).
With MRI being more expensive and less readily available than echocardiography, the latter is likely to be more important in assessing the IS and AAR in mice (45). High-field MRI scanners specialized for small animals are very expensive, but there have been attempts to validate lower-field scanners in assessments of mouse heart structure and function (46). MRI may be less operator-dependent and not as reliant on acoustic windows as echocardiography.
IS studies in mice have been performed using MRI protocols. Delayed-contrast enhanced MRI using gadolinium-based chelates, such as gadolinium-diethylenetriamine (Gd-DTPA), has emerged as the gold standard for detecting and quantifying infarction in humans and may also be used for the differentiation of viable from nonviable myocardium in mice (47,48). These agents shorten the T1 relaxation time of the neighbouring atoms (ie, the compartment in which the contrast agent is contained). Thus, on T1-weighted images, nonviable myocardium will be hyperintense when Gd-DPTA is used as a contrast agent because it is confined to the extracellular space and does not enter viable myocytes (44). There are other contrast agents in use and more are in the process of development.
In humans, the AAR can be reliably quantified using diffusion-weighted MRI. To our knowledge, it has not been used for AAR assessment in mice. This technique uses the principle of decreased diffusion in cytotoxic edema (due to cell swelling with concomitant decreased volume of the extracellular space). Several dephasing gradients are applied along one or both axes of a slice. When water molecules diffuse along these axes, they will acquire or lose phase (in a random manner) and their T2 relaxation time will decrease. Thus, areas of impaired diffusion (ie, ischemia) have higher signal intensity (44). This method is routinely used in imaging cerebral ischemia and infarcts in both humans and animals and has recently been used to detect ischemic changes in the human myocardium (49).
Other methods
Newly developed small animal multimodality molecular imaging techniques have been developed to study the cellular and molecular mechanism underlying myocardial infarction and remodelling (50).
Micro single-photon emission computed tomography (SPECT) and micro-SPECT/computed tomography (CT) systems have been developed for use in small animals (51).
99mTc-labelled radionucleotides are able to detect viable tissue in small animal models of ischemia-reperfusion. Furthermore, newly developed tracers specific for necrotic tissue may be used to delineate the IS. 99mTc-glucarate has a high affinity for histones, to which it gains access following loss of integrity of the plasma and nuclear membranes. Due to its rapid blood clearance, 99mTc-glucarate may be used to assess IS within minutes of coronary occlusion. In addition, apoptotic tracers, such as annexin A5, have been developed. A combination of SPECT with CT can improve these methods by defining the anatomy (52).
Positron emission tomography (PET) is still considered the gold standard for clinical assessment of myocardial viability and can also be used to assess both the IS and AAR in several animal models (53,54). 18Fluorodeoxyglucose is actively taken up by viable cells and allows for IS delineation. 13N-Ammonia is passively distributed by perfusion and can be used for AAR assessment. PET can be coupled to either MRI or CT for better visualization of the anatomy (50). PET scanners require an on-site cyclotron as well as well-trained operators and are expensive (55).
IS has recently been quantified in the mouse using micro-CT equipment (56), and efforts are continuing to introduce this technology in small animal laboratories (57).
Different optical imaging methods (58) are important in small animal models of cardiovascular disease but, as yet, these techniques have no place in the assessment of the AAR or IS.
DISCUSSION AND FUTURE PERSPECTIVES
Inexpensive, reliable and rapid assessment of the IS and AAR is desirable when studying acute myocardial infarction and interventions aimed at reducing its detrimental consequences on cardiac function and mortality.
Histology is inexpensive, rapid and reliable, but the need for euthanasia precludes serial measurements, a necessity in subacute and chronic models. In vivo techniques allow the IS:AAR ratio to be studied over time. In addition, the IS:AAR ratio is often not the only parameter of interest and it is desirable to use the tissue for other investigations. One drawback of some of the histological techniques is that they do not allow the tissue to be analyzed further.
Echocardiography, when performed by an experienced echocardiographer, is reliable, readily accessible, inexpensive and fast, making it ideal for serial measurements. Novel techniques, such as speckle tracking, appear likely to decrease the subjectivity in assessing the IS and AAR. We deem it likely that echocardiography will serve as the method of choice, in most circumstances, for in vivo assessment of the AAR and IS.
However, several additional parameters may be of interest depending on the hypothesis and study design. Modalities, such as MRI and molecular imaging methods as well as echocardiography, each have different strengths when it comes to evaluating cardiac structure and function, and progress is continuously being made in each field. The ability of a modality to provide specific additional information of interest may make that modality the method of choice for a given study.
Figure 2).
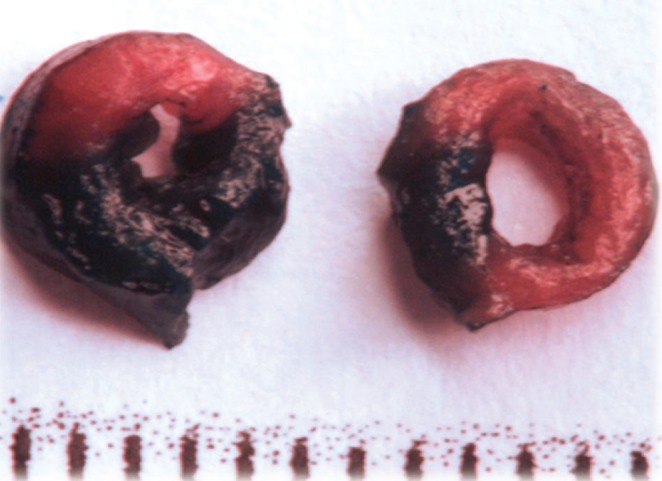
Area at risk (AAR) assessment using Evans Blue dye. For visualization of the AAR, Evans Blue dye is injected into the circulation and distributes to the perfused parts of the myocardium. Nonperfused areas, ie, the AAR, remain unstained
REFERENCES
- 1.Ytrehus K. The ischemic heart: Experimental models. Pharmacol Res. 2000;42:193–203. doi: 10.1006/phrs.2000.0669. [DOI] [PubMed] [Google Scholar]
- 2.Doevendans PA, Daemen MJ, de Muinck ED, Smits JF. Cardiovascular phenotyping in mice. Cardiovasc Res. 1998;39:34–49. doi: 10.1016/s0008-6363(98)00073-x. [DOI] [PubMed] [Google Scholar]
- 3.Liu Z, Kastis GA, Stevenson GD, et al. Quantitative analysis of acute myocardial infarct in rat hearts with ischemia-reperfusion using a high-resolution stationary SPECT system. J Nucl Med. 2002;43:933–9. [PMC free article] [PubMed] [Google Scholar]
- 4.Califf RM, Phillips HR, III, Hindman MC, et al. Prognostic value of a coronary artery jeopardy score. J Am Coll Cardiol. 1985;5:1055–63. doi: 10.1016/s0735-1097(85)80005-x. [DOI] [PubMed] [Google Scholar]
- 5.Graham MM, Faris PD, Ghali WA, et al. Validation of three myocardial jeopardy scores in a population-based cardiac catheterization cohort. Am Heart J. 2001;142:254–61. doi: 10.1067/mhj.2001.116481. [DOI] [PubMed] [Google Scholar]
- 6.Reimer KA, Lowe JE, Rasmussen MM, Jennings RB. The wavefront phenomenon of ischemic cell death. 1. Myocardial infarct size vs duration of coronary occlusion in dogs. Circulation. 1977;56:786–94. doi: 10.1161/01.cir.56.5.786. [DOI] [PubMed] [Google Scholar]
- 7.Lavi S, Lavi R. Conditioning of the heart: From pharmacological interventions to local and remote protection: Possible implications for clinical practice. Int J Cardiol. 2011;3:311–8. doi: 10.1016/j.ijcard.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 8.Tarnavski O, McMullen JR, Schinke M, Nie Q, Kong S, Izumo S. Mouse cardiac surgery: Comprehensive techniques for the generation of mouse models of human diseases and their application for genomic studies. Physiol Genomics. 2004;16:349–60. doi: 10.1152/physiolgenomics.00041.2003. [DOI] [PubMed] [Google Scholar]
- 9.Kolk MV, Meyberg D, Deuse T, et al. LAD-ligation: A murine model of myocardial infarction. J Vis Exp. 2009 doi: 10.3791/1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gao E, Lei YH, Shang X, et al. A novel and efficient model of coronary artery ligation and myocardial infarction in the mouse. Circ Res. 2010;107:1445–53. doi: 10.1161/CIRCRESAHA.110.223925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salto-Tellez M, Yung Lim S, El-Oakley RM, Tang TP, Almshergi ZA, Lim SK. Myocardial infarction in the C57BL/6J mouse: A quantifiable and highly reproducible experimental model. Cardiovasc Pathol. 2004;13:91–7. doi: 10.1016/S1054-8807(03)00129-7. [DOI] [PubMed] [Google Scholar]
- 12.Kumar D, Hacker TA, Buck J, et al. Distinct mouse coronary anatomy and myocardial infarction consequent to ligation. Coron Artery Dis. 2005;16:41–4. doi: 10.1097/00019501-200502000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Michael LH, Entman ML, Hartley CJ, et al. Myocardial ischemia and reperfusion: A murine model. Am J Physiol. 1995;269:H2147–54. doi: 10.1152/ajpheart.1995.269.6.H2147. [DOI] [PubMed] [Google Scholar]
- 14.Kanno S, Lerner DL, Schuessler RB, et al. Echocardiographic evaluation of ventricular remodeling in a mouse model of myocardial infarction. J Am Soc Echocardiogr. 2002;15:601–9. doi: 10.1067/mje.2002.117560. [DOI] [PubMed] [Google Scholar]
- 15.Patten RD, Aronovitz MJ, Deras-Mejia L, et al. Ventricular remodeling in a mouse model of myocardial infarction. Am J Physiol. 1998;274:H1812–20. doi: 10.1152/ajpheart.1998.274.5.H1812. [DOI] [PubMed] [Google Scholar]
- 16.Degabriele NM, Griesenbach U, Sato K, et al. Critical appraisal of the mouse model of myocardial infarction. Exp Physiol. 2004;89:497–505. doi: 10.1113/expphysiol.2004.027276. [DOI] [PubMed] [Google Scholar]
- 17.Pfeffer MA, Pfeffer JM, Fishbein MC, et al. Myocardial infarct size and ventricular function in rats. Circ Res. 1979;44:503–12. doi: 10.1161/01.res.44.4.503. [DOI] [PubMed] [Google Scholar]
- 18.Fletcher PJ, Pfeffer JM, Pfeffer MA, Braunwald E. Left ventricular diastolic pressure-volume relations in rats with healed myocardial infarction. Effects on systolic function. Circ Res. 1981;49:618–26. doi: 10.1161/01.res.49.3.618. [DOI] [PubMed] [Google Scholar]
- 19.Pfeffer JM, Pfeffer MA, Fletcher PJ, Braunwald E. Progressive ventricular remodeling in rat with myocardial infarction. Am J Physiol. 1991;260:H1406–14. doi: 10.1152/ajpheart.1991.260.5.H1406. [DOI] [PubMed] [Google Scholar]
- 20.Nachlas MM, Shnitka TK. Macroscopic identification of early myocardial infarcts by alterations in dehydrogenase activity. Am J Pathol. 1963;42:379–405. [PMC free article] [PubMed] [Google Scholar]
- 21.Ferrera R, Larese A, Berthod F, et al. Quantitative reduction of MTT by hearts biopsies in vitro is an index of viability. J Mol Cell Cardiol. 1993;25:1091–9. doi: 10.1006/jmcc.1993.1121. [DOI] [PubMed] [Google Scholar]
- 22.Eckle T, Grenz A, Kohler D, et al. Systematic evaluation of a novel model for cardiac ischemic preconditioning in mice. Am J Physiol Heart Circ Physiol. 2006;291:H2533–40. doi: 10.1152/ajpheart.00472.2006. [DOI] [PubMed] [Google Scholar]
- 23.Wolff RA, Chien GL, van Winkle DM. Propidium iodide compares favorably with histology and triphenyl tetrazolium chloride in the assessment of experimentally-induced infarct size. J Mol Cell Cardiol. 2000;32:225–32. doi: 10.1006/jmcc.1999.1074. [DOI] [PubMed] [Google Scholar]
- 24.Ito WD, Schaarschmidt S, Klask R, et al. Infarct size measurement by triphenyltetrazolium chloride staining versus in vivo injection of propidium iodide. J Mol Cell Cardiol. 1997;29:2169–75. doi: 10.1006/jmcc.1997.0456. [DOI] [PubMed] [Google Scholar]
- 25.Rodrigues ACT, Hataishi R, Ichinose F, et al. Relationship of systolic dysfunction to area at risk and infarction size after ischemia-reperfusion in mice. J Am Soc Echocardiogr. 2004;17:948–53. doi: 10.1016/j.echo.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 26.Stopa B, Rybarska J, Drozd A, et al. Albumin binds self-assembling dyes as specific polymolecular ligands. Int J Biol Macromol. 2006;40:1–8. doi: 10.1016/j.ijbiomac.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 27.Bohl S, Medway DJ, Schulz-Menger J, Schneider JE, Neubauer S, Lygate CA. Refined approach for quantification of in vivo ischemia-reperfusion injury in the mouse heart. Am J Physiol Heart Circ Physiol. 2009;297:H2054–8. doi: 10.1152/ajpheart.00836.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hale SL, Vivaldi MT, Kloner RA. Fluorescent microspheres: A new tool for visualization of ischemic myocardium in rats. Am J Physiol. 1986;251:H863–8. doi: 10.1152/ajpheart.1986.251.4.H863. [DOI] [PubMed] [Google Scholar]
- 29.Takagawa J, Zhang Y, Wong ML, et al. Myocardial infarct size measurement in the mouse chronic infarction model: Comparison of area- and length-based approaches. J Appl Physiol. 2007;102:2104–11. doi: 10.1152/japplphysiol.00033.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holmes JW, Yamashita H, Waldman LK, Covell JW. Scar remodeling and transmural deformation after infarction in the pig. Circulation. 1994;90:411–20. doi: 10.1161/01.cir.90.1.411. [DOI] [PubMed] [Google Scholar]
- 31.Ertl G, Frantz S. Healing after myocardial infarction. Cardiovasc Res. 2005;66:22–32. doi: 10.1016/j.cardiores.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 32.Sun Y. Intracardiac renin-angiotensin system and myocardial repair/remodeling following infarction. J Mol Cell Cardiol. 2010;48:483–9. doi: 10.1016/j.yjmcc.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Collins KA, Korcarz CE, Lang RM. Use of echocardiography for the phenotypic assessment of genetically altered mice. Physiol Genomics. 2003;13:227–39. doi: 10.1152/physiolgenomics.00005.2003. [DOI] [PubMed] [Google Scholar]
- 34.Heyndrickx GR, Millard RW, McRitchie RJ, Maroko PR, Vatner SF. Regional myocardial functional and electrophysiological alterations after brief coronary artery occlusion in conscious dogs. J Clin Invest. 1975;56:978–85. doi: 10.1172/JCI108178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suehiro K, Takuma S, Cardinale C, et al. Assessment of segmental wall motion abnormalities using contrast two-dimensional echocardiography in awake mice. Am J Physiol Heart Circ Physiol. 2001;280:H1729–35. doi: 10.1152/ajpheart.2001.280.4.H1729. [DOI] [PubMed] [Google Scholar]
- 36.Ramani R, Mathier M, Dawson J, McTiernan CF, Feldman AM. Assessment of infarct size and myocardial function in mice using transesophageal echocardiography. J Am Soc Echocardiogr. 2004;17:649–53. doi: 10.1016/j.echo.2004.02.019. [DOI] [PubMed] [Google Scholar]
- 37.Scherrer-Crosbie M, Steudel W, Ullrich R, et al. Echocardiographic determination of risk area size in a murine model of myocardial ischemia. Am J Physiol. 1999;277:H986–92. doi: 10.1152/ajpheart.1999.277.3.H986. [DOI] [PubMed] [Google Scholar]
- 38.Blessberger H, Binder T. Two dimensional speckle tracking echocardiography: Clinical applications. Heart. 2010;96:2032–40. doi: 10.1136/hrt.2010.199885. [DOI] [PubMed] [Google Scholar]
- 39.Artis NJ, Oxborough DL, Williams G, Pepper CB, Tan LB. Two-dimensional strain imaging: A new echocardiographic advance with research and clinical applications. Int J Cardiol. 2008;123:240–8. doi: 10.1016/j.ijcard.2007.02.046. [DOI] [PubMed] [Google Scholar]
- 40.Thibault H, Gomez L, Donal E, et al. Acute myocardial infarction in mice: assessment of transmurality by strain rate imaging. Am J Physiol Heart Circ Physiol. 2007;293:H496–502. doi: 10.1152/ajpheart.00087.2007. [DOI] [PubMed] [Google Scholar]
- 41.Weidemann F, Dommke C, Bijnens B, et al. Defining the transmurality of a chronic myocardial infarction by ultrasonic strain-rate imaging: Implications for identifying intramural viability: An experimental study. Circulation. 2003;107:883–8. doi: 10.1161/01.cir.0000050146.66577.4b. [DOI] [PubMed] [Google Scholar]
- 42.Sitia S, Tomasoni L, Turiel M. Speckle tracking echocardiography: A new approach to myocardial function. World J Cardiol. 2010;2:1–5. doi: 10.4330/wjc.v2.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Epstein FH. MR in mouse models of cardiac disease. NMR Biomed. 2007;20:238–55. doi: 10.1002/nbm.1152. [DOI] [PubMed] [Google Scholar]
- 44.Boesch C. Molecular aspects of magnetic resonance imaging and spectroscopy. Mol Aspects Med. 1999;20:185–318. doi: 10.1016/s0098-2997(99)00007-2. [DOI] [PubMed] [Google Scholar]
- 45.Rottman JN, Ni G, Brown M. Echocardiographic evaluation of ventricular function in mice. Echocardiography. 2007;24:83–9. doi: 10.1111/j.1540-8175.2006.00356.x. [DOI] [PubMed] [Google Scholar]
- 46.Voelkl JG, Haubner BJ, Kremser C, et al. Cardiac imaging using clinical 1.5 t MRI scanners in a murine ischemia/reperfusion model. J Biomed Biotechnol. 2011;2011:185683. doi: 10.1155/2011/185683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ojha N, Roy S, Radtke J, et al. Characterization of the structural and functional changes in the myocardium following focal ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2008;294:H2435–43. doi: 10.1152/ajpheart.01190.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bohl S, Lygate CA, Barnes H, et al. Advanced methods for quantification of infarct size in mice using three-dimensional high-field late gadolinium enhancement MRI. Am J Physiol Heart Circ Physiol. 2009;296:H1200–8. doi: 10.1152/ajpheart.01294.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Laissy JP, Serfaty JM, Messika-Zeitoun D, et al. [Cardiac diffusion MRI of recent and chronic myocardial infarction: Preliminary results] J Radiol. 2009;90:481–4. doi: 10.1016/s0221-0363(09)74007-7. [DOI] [PubMed] [Google Scholar]
- 50.Kramer CM, Sinusas AJ, Sosnovik DE, French BA, Bengel FM. Multimodality imaging of myocardial injury and remodeling. J Nucl Med. 2010;51(Suppl 1):107S–21S. doi: 10.2967/jnumed.109.068221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Golestani R, Wu C, Tio RA, et al. Small-animal SPECT and SPECT/CT: Application in cardiovascular research. Eur J Nucl Med Mol Imaging. 2010;37:1766–77. doi: 10.1007/s00259-009-1321-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Franc BL, Acton PD, Mari C, Hasegawa BH. Small-animal SPECT and SPECT/CT: Important tools for preclinical investigation. J Nucl Med. 2008;49:1651–63. doi: 10.2967/jnumed.108.055442. [DOI] [PubMed] [Google Scholar]
- 53.Shimada K, Yoshida K, Tadokoro H, et al. High-resolution cardiac PET in rabbits: Imaging and quantitation of myocardial blood flow. J Nucl Med. 1998;39:2022–7. [PubMed] [Google Scholar]
- 54.Lautamaki R, Schuleri KH, Sasano T, et al. Integration of infarct size, tissue perfusion, and metabolism by hybrid cardiac positron emission tomography/computed tomography: Evaluation in a porcine model of myocardial infarction. Circ Cardiovasc Imaging. 2009;2:299–305. doi: 10.1161/CIRCIMAGING.108.846253. [DOI] [PubMed] [Google Scholar]
- 55.Gropler RJ, Beanlands RS, Dilsizian V, Lewandowski ED, Villanueva FS, Ziadi MC. Imaging myocardial metabolic remodeling. J Nucl Med. 2010;51(Suppl 1):88S–101S. doi: 10.2967/jnumed.109.068197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nahrendorf M, Badea C, Hedlund LW, et al. High-resolution imaging of murine myocardial infarction with delayed-enhancement cine micro-CT. Am J Physiol Heart Circ Physiol. 2007;292:H3172–8. doi: 10.1152/ajpheart.01307.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sheikh AY, van der Bogt KE, Doyle TC, et al. Micro-CT for characterization of murine CV disease models. JACC Cardiovasc Imaging. 2010;3:783–5. doi: 10.1016/j.jcmg.2010.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Luker GD, Luker KE. Optical imaging: Current applications and future directions. J Nucl Med. 2008;49:1–4. doi: 10.2967/jnumed.107.045799. [DOI] [PubMed] [Google Scholar]


