Abstract
Antibody-based immunotherapy has become an integral part of cancer therapeutics. However, monoclonal antibodies have their limitations as identifying an antigen selectively expressed on malignant cells and developing a high-affinity antibody may not by itself alter tumor growth. This is illustrated in the case of CD30; CD30 epitomizes many properties of an ideal pharmacologic target such as high expression on malignant cells and limited expression on normal tissues. However, until the advent of brentuximab vedotin, CD30 remained an elusive target as antibody-based anti-CD30 immunotherapy had been largely clinically unsuccessful. Brentuximab vedotin (cAC10-vcMMAE, SGN-35) is an antibody-drug conjugate consisting of a chimeric anti-CD30 monoclonal antibody whereupon the potent microtubule inhibitor monomethyl auristatin E (MMAE) is attached via a valine–citrulline linker. Once bound to CD30, brentuximab vedotin is internalized and MMAE is released with the action of lysosomal enzymes on the linker. In phase I studies in relapsed or refractory Hodgkin lymphoma and anaplastic large cell lymphoma, brentuximab vedotin induced unprecedented responses with manageable toxicity. In phase II studies, brentuximab vedotin induced overall response rates of 75% and 86% in relapsed or refractory Hodgkin lymphoma and anaplastic large cell lymphoma, respectively. The results of these trials led to the accelerated approval of the drug by the US Food and Drug Administration in a patient population with few other alternative options. Brentuximab vedotin has overall manageable toxicity profile; however, cumulative peripheral neuropathy constitutes an important clinical consideration as it may limit prolonged administration of the drug. The mechanism by which brentuximab vedotin exerts its antitumor activity is not entirely clear. Diffusion of MMAE in the tumor microenvironment and cytotoxicity on bystander cells may in part explain its activity, especially in Hodgkin lymphoma. Herein, we review the biology of CD30 and brentuximab vedotin, and the clinical data that has accumulated thus far with SGN-35.
Keywords: anaplastic large cell lymphoma, antibody-drug conjugate, brentuximab vedotin, cAC10-vcMMAE, CD30, CD30 targeting, Hodgkin’s lymphoma, SGN-35
Introduction
The introduction of monoclonal antibodies in cancer therapeutics has changed significantly the clinical outlook of many malignancies. Monoclonal antibodies exert their lytic effects on cancer cells by inducing complement-mediated and antibody-dependent cell-mediated cytotoxicity and by blocking signaling cascades initiated by their respective targets. The obvious advantage of antibody-based cancer immunotherapy is the selective targeting of tumor cells whereas normal tissues are relatively spared. Nonetheless monoclonal antibodies have their limitations as not infrequently, identifying a target protein selectively expressed on malignant cells and developing a high-affinity antibody may not be enough to significantly alter tumor growth. In an attempt to enhance the efficacy of monoclonal antibodies, several strategies have been undertaken including administration of higher doses of the monoclonal antibodies [Khouri et al. 2005] as well as combination with cytokines to potentiate antibody-dependent cell-mediated cytotoxicity [Ansell et al. 2006; Khan et al. 2006]. The most promising strategies, however, have been the fusion of monoclonal antibodies with toxins, and conjugation with radioisotopes or cytotoxic drugs [Teicher and Chari, 2011], thereby utilizing the high selectivity of antibody-based immunotherapy for targeted delivery of toxins, radiation therapy, or toxic agents, respectively, to the tumor sites.
Unlike radioimmunotherapy which has already been incorporated into the treatment algorithms of non-Hodgkin lymphoma [Fisher et al. 2005; Morschhauser et al. 2008; Witzig et al. 2002], antibody–drug conjugate (ADC) therapeutics had to overcome many hurdles before reaching broad clinical applications. The immunogenicity of the early compounds was overcome by replacing murine antibodies with humanized or fully human ones, potency was enhanced by the conjugation of agents considerably more cytotoxic compared with the agents used earlier, and selectivity was improved by better antibody and target selection [Teicher and Chari, 2011]. Advances in linker technology have improved the pharmacodynamics and, consequently, the therapeutic window of ADCs by minimizing the release of the toxic conjugate in the circulation and optimizing its selective delivery in tumor sites. Also, progress in monoclonal antibody engineering has addressed the compositional heterogeneity and inconsistency emanating from variable antibody:drug stoichiometry of the early compounds [Teicher and Chari, 2011]. These advances have paved new avenues in cancer therapeutics with unprecedented potential. This is clearly illustrated in the case of brentuximab vedotin, an ADC targeting CD30, which demonstrated significant efficacy in relapsed or refractory Hodgkin lymphoma (HL) and anaplastic large cell lymphoma (ALCL). Before the advent of brentuximab vedotin, both conditions had few therapeutic options, all of limited efficacy, and their prognosis was overall dismal.
Biology of CD30 antigen
CD30 (tumor necrosis factor receptor superfamily, member 8; TNFRSF8) is a type I transmembrane receptor that shares sequence homology in the extracellular domain with other members of the tumor necrosis factor (TNF) receptor superfamily [Locksley et al. 2001; Younes and Kadin, 2003]. It adopts an elongated structure by a scaffold of disulfide bonds that form between highly conserved cysteine residues within its six cysteine-rich domains. Its cytoplasmic tail is of moderate length and functions as a docking site for signaling molecules [Locksley et al. 2001]. There is no sequence homology in the intracellular domain among the TNF receptor superfamily members, underlying their diverse context-dependent biologic functions [Younes and Aggarwall, 2003]. The ligand for CD30 (CD30L or CD153) is a type II transmembrane protein which can exist in a membrane-embedded and a cleaved, soluble form [Locksley et al. 2001]. In their active conformation, both CD30 and CD30L form self-assembling noncovalent trimers. CD30 signals through TNF receptor-associated factors (TRAFs): upon stimulation by its cognate ligand, CD30 recruits TRAF2 and TRAF5 via interaction with its TRAF binding motif in the cytoplasmic domain. CD30 can also display noncanonical functions that do not require the binding of CD30L trimer [Locksley et al. 2001]. There is a crosstalk between CD30 and other members of the TNF receptor superfamily that contain Death Domain motifs in their cytoplasmic tail, such as TNF-receptor 1 [Duckett and Thompson, 1997]. The basis for this crosstalk is TRAF2, a common signaling intermediate that undergoes degradation upon CD30 stimulation while, upon stimulation of TNFR1, TRAF2 interacts with TNFR1 and TNF receptor-associated death domain (TRADD) leading ultimately to the activation of the nuclear factor (NF)-κB pathway (Figure 1). NF-κB is a transcription factor family that regulates the expression of various genes with critical roles in apoptosis, tumorigenesis, and inflammation. Activation of NF-κB (as can occur with cytotoxic chemotherapy and radiation) has been shown to block apoptosis and promote proliferation [Wang et al. 1998].
Figure 1.
CD30 signals through TRAFs: upon stimulation by CD30L, CD30 recruits TRAF2 via interaction with its TRAF binding motif, resulting in TRAF2 degradation. TNFR1, on the other hand, signals through TRADD leading to activation of caspases and ultimately apoptosis or activation of the classical NF-κB pathway and ultimately survival and proliferation. Signaling through CD30 depletes intracellular TRAF2 diverting signaling through TNFR1 to apoptosis [Mir et al. 2000]. Not all TRAF2 undergoes degradation upon CD30 stimulation. In certain ALCL cells, TRAF2 translocates to a detergent-insoluble cellular compartment, and this translocation has been associated with NF-κB pathway activation [Wright et al. 2007]. Activation of the classical NF-κB pathway involves activation of the IKK complex and proteasomal degradation of the IκB proteins. The proteasomal degradation of TRAF2, TRAF3, and c-IAP-1/2 which hold NIK upon recruitment by a subset of TNF receptor superfamily proteins, notably CD40, leads to the activation of the alternative NF-κB pathway via liberation and stabilization of NIK [Staudt, 2010]. Activation of the alternative NF-κB pathway involves activation of IKK1 and proteolytic processing of the p100 precursor protein to the p52 subunit of NF-κB. (Illustration courtesy of Alessandro Baliani. Copyright © 2012.) CD30, cluster of differentiation 30; CD40, cluster of differentiation 40; CD30L, CD30 ligand; c-IAP1/2, cellular inhibitor of apoptosis protein; IκB, inhibitor of kappa B; IKK1/2, IκB kinase; NF-κB, nuclear factor kappa B; NIK, NF-κB inducible kinase; P, phosphate; p50, p52, p65 (RelA), RelB, p50, p52, p65 (RelA), RelB subunits of NF-κB; p100, p52 precursor subunit of NF-κB; TNF, tumor necrosis factor; TNFR1, TNF receptor 1; TRADD, TNF receptor-associated death domain; TRAF2 and TRAF3, TNF receptor-associated factor 2 and 3, respectively; Ub, ubiquitin.
The normal functions of CD30 are not entirely clear as no human disease has been associated with defects in CD30/CD30L and studies in transgenic mice generated conflicting data. Its role in thymic negative selection and expansion of autoreactive T lymphocytes has been a matter of controversy [Amakawa et al. 1996; Chiarle et al. 1999; DeYoung et al. 2000; Kurts et al. 1999]. In ALCL cell lines, CD30 activation led to rapid induction of apoptosis via degradation and intracellular depletion of TRAF2, thereby diverting TNFR1 signaling from TNFR1–TRADD–TRAF2-mediated NF-κB activation to induction of apoptosis [Mir et al. 2000]. HL cells, on the other hand, were insensitive to CD30 activation secondary to constitutive NF-κB pathway activation [Mir et al. 2000]. It is now clear, however, that upon CD30 stimulation by its cognate ligand, not all ALCL cells undergo apoptosis. Instead of undergoing degradation, in some cells TRAF2 translocates to a detergent-insoluble cellular compartment, and this translocation has been associated with NF-κB pathway activation [Wright et al. 2007]. Furthermore, the proteasomal degradation of TRAF2 and TRAF3 upon recruitment by a subset of TNF receptor superfamily proteins has also been associated with activation of the alternative NF-κB pathway via liberation and stabilization of the NF-κB inducible kinase (NIK, Figure 1) [Staudt, 2010].
Like other members of the TNF receptor superfamily, CD30 can undergo ectodomain shedding generating an 85 kDa soluble form of CD30 (sCD30) which can be identified in culture supernatants of CD30+ cell lines as well as sera of patients harboring CD30+ tumors [Younes and Aggarwall, 2003]. In fact, high serum levels of sCD30 correlated with the presence of B symptoms, higher stage and tumor burden in HL [Nadali et al. 1998], as well as poor clinical outcome in both HL [Nadali et al. 1998] and ALCL [Zinzani et al. 1998].
CD30 is an attractive pharmacologic target due to its restricted presence on normal cells unlike its uniform, high-level expression pattern on the malignant cells of HL and ALCL [Katz et al. 2011] (Figure 2). Normally, CD30 expression is limited to activated B and T lymphocytes and virally infected lymphocytes [Horie and Watanabe, 1998]. CD30+ lymphocytes can be found in the parafollicular areas, rim of the follicular centers, proliferating germinal centers, and thymic medulla [Horie and Watanabe, 1998; Younes, 2011]. Other hematologic malignancies with CD30 expression besides HL and ALCL include the primary cutaneous ALCL, lymphomatoid papulosis [Kempf et al. 2011] and pagetoid reticulosis, primary mediastinal B-cell lymphoma, primary effusion lymphoma associated with human herpes virus-8, multiple myeloma, adult T-cell leukemia/lymphoma [Younes, 2011; Younes and Carbone, 1999], mast cell malignancies [Sotlar et al. 2011], and mycosis fungoides [Edinger et al. 2009]. Unlike its receptor, CD30L has a broader spectrum of expression in hematopoietic malignancies [Gattei et al. 1997] (Table 1).
Figure 2.
CD30 immunohistochemical staining in HL, ALCL, and reactive lymphadenopathy. (A) classical HL with Hodgkin cells (arrowhead) and Reed–Sternberg cells (arrow) in a background of nonneoplastic cells (hematoxylin and eosin staining). (B) CD30 staining in HL. (C) ALK-negative ALCL with multiple pleomorphic large ‘hallmark’ cells (hematoxylin and eosin staining). (D) CD30 staining in ALK-negative ALCL. (E) Reactive lymphadenopathy (hematoxylin and eosin). (F) CD30 staining in reactive lymphadenopathy. Note the faint, nonspecific staining as compared with HL and ALCL. (Courtesy of Dr Xiaojun Wu, Department of Pathology, University of Alabama at Birmingham.)
Table 1.
Properties and expression patterns of CD30 and CD30L [Edinger et al. 2009; Gattei et al. 1997; Horie and Watanabe, 1998; Pileri et al. 2003; Romagnani et al. 1998; Sotlar et al. 2011; Stein et al. 1985; Younes, 2011; Younes and Carbone, 1999].
| CD30 | CD30L (CD153) | |
|---|---|---|
| Gene locus | 1p36 | 9q33 |
| Molecular weight | 120 kDa | 26 kDa |
| Expression by hematopoietic cells | ||
| Benign | Activated B and T lymphocytes Virally infected lymphocytes [Horie and Watanabe, 1998] |
Medullary epithelial cells and Hassal’s corpuscles of the thymus [Romagnani et al. 1998] Activated T cells and resting B cells, granulocytes [Horie and Watanabe, 1998] |
| Malignant | HRS cells of HL [Stein et al. 1985] ALCL and primary cutaneous ALCL Lymphomatoid papulosis and pagetoid reticulosis Primary effusion lymphoma associated with HHV8 Multiple myeloma Adult T-cell leukemia/lymphoma [Younes, 2011; Younes and Carbone, 1999], Primary mediastinal B-cell lymphoma [Pileri et al. 2003], Neoplastic mast cells [Sotlar et al. 2011] Mycosis Fungoides [Edinger et al. 2009] |
Acute myelogenous leukemia B-lineage acute lymphoblastic leukemia Hairy cell leukemia High grade B-cell NHL Chronic lymphocytic leukemia Multiple myeloma T-cell prolymphocytic leukemia Peripheral T-cell lymphoma Adult T-cell leukemia/lymphoma [Gattei et al. 1997] |
ALCL, anaplastic large cell lymphoma; HHV8, human herpes virus-8; HL, Hodgkin’s lymphoma; HRS, Hodgkin and Reed–Sternberg; NHL, non-Hodgkin lymphoma.
Classical Hodgkin’s lymphoma
With an estimated 8490 new cases in 2010 [Jemal et al. 2010], HL is a relatively uncommon human malignancy. One of the histologic hallmarks of HL is the scarcity of neoplastic cells which are dispersed in a background of abundant heterogeneous inflammatory and accessory cells (Figure 2A and B). The pathognomonic large mononucleated (Hodgkin cells) and multinucleated tumor cells (Reed–Sternberg cells) derive from mature B cells at a germinal center or post-germinal center stage of differentiation that have acquired disadvantageous immunoglobulin variable region gene mutations and normally would have undergone apoptosis [Kuppers, 2009].
With the advances made in frontline multiagent chemotherapy, HL is deemed to be a highly curable disease. Nonetheless, advanced HL (defined as stage III or IV, presence of bulky disease or B symptoms) remains a challenge as 10% of patients will not achieve a complete remission and 20–30% of patients who initially responded, will experience a relapse [Kuruvilla et al. 2011]. Salvage chemotherapy followed by autologous stem-cell transplantation (SCT) has become the treatment of choice in relapsed [Schmitz et al. 2002] or refractory [Lazarus et al. 1999] disease. Several salvage regimens have been reported, mostly in single-arm phase II studies, and in the absence of comparative trials, there is no consensus on the optimal regimen [Kuruvilla et al. 2011]. With salvage chemotherapy, hematologic toxicities and compromised stem-cell mobilization are significant issues of concern. Despite the reported overall response rates of 69–89%, lack of response is not uncommon and proceeding to autologous SCT in this setting is controversial [Kuruvilla et al. 2011]: evidence from randomized trials supports autologous SCT only in chemosensitive disease [Schmitz et al. 2002] and outcomes in the absence of complete response have been inferior [Lazarus et al. 1999]. Myeloablative allogeneic SCT has been fraught with high treatment-related mortality and early studies failed to show an advantage over autologous SCT [Milpied et al. 1996]. Nonmyeloablative allogeneic SCT on the other hand has lower treatment-related mortality but the incidence of relapsed/refractory disease is high (40–63% in 2 years) [Burroughs et al. 2008]. With an estimated median survival of less than 3 years in the setting of failure of first- and second-line therapy [Younes, 2009], and a proportion of patients ineligible for SCT, relapsed or refractory HL represents a significant therapeutic challenge.
Anaplastic large cell lymphoma
ALCL is a rare CD30+ T-cell lymphoma that constitutes 10–15% and 2–8% of non-Hodgkin lymphomas in children and adults, respectively [Merkel et al. 2011]. The neoplastic cells are pleomorphic, typically large, with eccentric, horseshoe or kidney-shaped nuclei. They are consistently present in all histologic variants of ALCL, hence termed ‘hallmark’ cells (Figure 2C and D). Two clinically and genetically distinct but indistinguishable on morphologic grounds alone ALCL entities are recognized based on the expression of anaplastic lymphoma kinase (ALK), ALK-positive and ALK-negative ALCL. ALK expression results from translocations involving ALK on chromosome 2 with various partner genes, most commonly nucleophosmin on chromosome 5, whereby the tyrosine kinase domain of ALK fuses with the N-terminal part of the partner gene. The fusion partner introduces dimerization motifs in the chimeric protein leading to the constitutive activation of ALK which results in oncogenic transformation and lymphomagenesis [Amin and Lai, 2007].
Unlike its primary cutaneous variant that has an excellent prognosis, systemic ALCL has an aggressive clinical course with frequent involvement of extranodal sites. Overall, ALK-negative ALCL has a consistently worse prognosis compared with its ALK-positive counterpart [Corradini et al. 2006; Savage et al. 2008]. A multiagent, anthracycline-containing regimen is the mainstay first-line therapy for remission induction, although the addition of an anthracycline in the treatment of ALK-negative ALCL is debatable [Vose et al. 2008]. The treatment of relapsed or refractory disease has been challenging as few agents have consistently shown activity such as pralatrexate (overall response rate 35%) [O’Connor et al. 2011]. Five-year overall survival as high as 55% has been reported for young patients who undergo allogeneic SCT, but refractory or progressive disease at the time of transplantation are significant adverse prognostic factors [Le Gouill et al. 2008].
Brentuximab vedotin: composition and mechanism of action
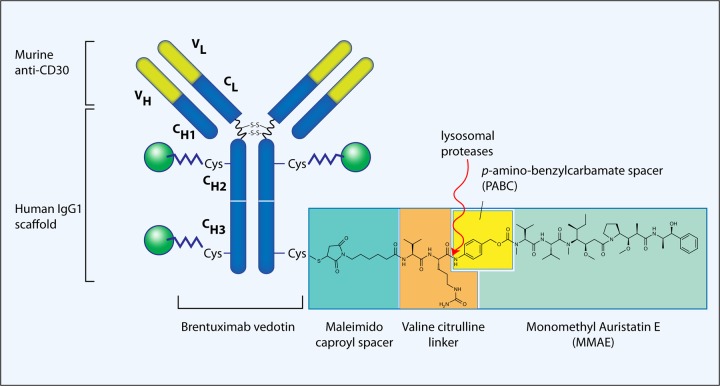
Brentuximab vedotin is an ADC consisting of the monoclonal antibody cAC10 and the cytotoxic agent monomethyl auristatin E (MMAE, Figure 3) [Francisco et al. 2003].
Figure 3.
Composition of brentuximab vedotin (cAC10-vcMMAE, SGN-35). SGN-30 (cAC10, the parent naked antibody) is a chimeric anti-CD30 monoclonal antibody derived from the fusion of the variable heavy and light region of the murine anti-CD30 antibody AC10, with the constant gamma1-heavy and kappa-light region of the human immunoglobulin [Wahl et al. 2002]. An average of 4 (2–8) MMAE molecules are attached to the SGN-30 scaffold. The points of MMAE attachment are –SH groups of cysteine residues produced by mild reduction of the interchain disulfide bonds. The linker consists of a thiolreactive maleimidocaproyl spacer, the dipeptide valine–citrulline linker, and a PABC spacer [Francisco et al. 2003]. The site of action of the lysosomal proteases is also shown [Sutherland et al. 2006]. (Illustration courtesy of Alessandro Baliani. Copyright © 2012.) MMAE, monomethyl auristatin E; PABC, p-aminobenzylcarbamate.
cAC10 (SGN-30) is a chimeric anti-CD30 monoclonal antibody derived from the fusion of the variable heavy and light region of the murine anti-CD30 antibody AC10, with the constant gamma1-heavy and kappa-light region of the human immunoglobulin [Wahl et al. 2002]. Unlike previous anti-CD30 antibodies that have been active against ALCL [Tian et al. 1995] but not HL cell lines (probably because of the constitutively active NF-κB pathway), both SGN-30 and its murine predecessor AC10 have shown activity against HL [Wahl et al. 2002]. It is not entirely clear why these particular antibodies have shown activity in vitro as well as in immunodeficient mouse models of HL where antibody-dependent cell-mediated and complement-mediated cytotoxicity are expectedly compromised. A possible explanation may involve the crosslinking properties of SGN-30 and resultant clustering of SGN-30-CD30 complexes on the surface of the cells [Wahl et al. 2002]. This, in turn, activates the NF-κB pathway but the resultant changes in the transcriptome lead to growth arrest and apoptosis [Cerveny et al. 2005; Wahl et al. 2002]. While active by itself, SGN-30 was shown to synergize well with conventional chemotherapeutics used to treat HL [Cerveny et al. 2005].
In an attempt to enhance the antitumor properties of SGN-30, MMAE was conjugated to the monoclonal antibody generating the cAC10-vcMMAE ADC (SGN-35, brentuximab vedotin) [Wahl et al. 2002]. MMAE is a synthetic derivative of dolastatin 10, a natural cytostatic pseudopeptide originally isolated from the marine shell-less mollusk Dorabella auricularia. MMAE exerts its potent cytostatic effect by inhibiting microtubule assembly, tubulin-dependent GTP hydrolysis and polymerization [Bai et al. 1990]. By themselves, both dolastatin 10 and MMAE have shown significant activity against various cell lines of hematopoietic tumors in vitro [Bai et al. 1990; Francisco et al. 2003; Gerber et al. 2009]. The points of MMAE attachment on SGN-30 scaffold are –SH groups of cysteine residues produced by mild reduction of the interchain disulfide bonds. The linker consists of a thiolreactive maleimidocaproyl spacer, the dipeptide valine–citrulline linker, and a self-immolative p-aminobenzylcarbamate spacer [Francisco et al. 2003] (Figure 3). The peptide-based linker provides a highly stable bond between the antibody and the cytotoxic compound under physiologic conditions while it facilitates the rapid and efficient drug cleavage upon internalization of the ADC by the target tumor cell [Doronina et al. 2003]. Indeed, after a 10-day incubation in human plasma at 37ºC, less than 2% of the MMAE was released [Francisco et al. 2003]. Two to eight MMAE molecules are attached on each antibody with an average MMAE:cAC10 stoichiometry of 4:1.
In vitro, cAC10-vcMMAE was shown to bind with the same affinity to CD30 as the parent naked antibody [Francisco et al. 2003]. Once bound, the ADC is internalized by clathrin-mediated endocytosis [Sutherland et al. 2006]. The vesicle fuses with lysosomes where MMAE is released with the action of cathepsin B and other lysosomal cysteine proteases [Sutherland et al. 2006] (Figure 4), thereby causing cell cycle arrest followed by apoptotic cell death [Francisco et al. 2003]. Owing to plasma membrane permeability, part of the MMAE is released in the tumor microenvironment exerting a cytotoxic effect on bystander cells [Okeley et al. 2010]. The efficacy of cAC10-vcMMAE was evaluated in mouse models of ALCL and HL where the ADC was shown to be highly selective to the tumor cells inducing durable and complete regressions in doses significantly lower than the maximum tolerated dose (MTD). Both in vitro and in vivo, cAC10-vcMMAE was superior to the unconjugated parent antibody [Francisco et al. 2003]. In mouse studies combining cAC10-vcMMAE with conventional regimens for HL such as ABVD (doxorubicin, bleomycin, vinblastine, dacarbazine), a significant synergism was observed [Oflazoglu et al. 2008]. Moreover, durable remissions were achieved even when the inoculated tumors were allowed to reach large proportions, recapitulating disease with high tumor burden [Oflazoglu et al. 2008].
Figure 4.
SGN-35 binds to CD30 and is internalized by clathrin-mediated endocytosis [Sutherland et al. 2006]. The vesicle fuses with lysosomes where MMAE is released with the action of cathepsin B and other lysosomal cysteine proteases [Sutherland et al. 2006]. MMAE inhibits microtubule assembly and polymerization thereby causing G2/M cell cycle arrest followed by apoptotic cell death [Francisco et al. 2003]. Small amounts of free MMAE may be diffused in the microenvironment where they exert potent cytotoxic effects on cells surrounding the malignant ALCL cells, Hodgkin and Reed–Sternberg cells. (Illustration courtesy of Alessandro Baliani. Copyright © 2012.) Lys, lysosome; MMAE, monomethyl auristatin E.
Phase I studies
As CD30 was recognized early to be an appealing therapeutic target, several variably successful efforts have been undertaken over the years to target this receptor with the first proof-of-concept clinical investigations dating as far back as 1992 [Falini et al. 1992]. Table 2 provides a list of the clinical studies published thus far with naked antibodies [Ansell et al. 2007; Bartlett et al. 2008; Forero-Torres et al. 2009], radioimmunoconjugates [Schnell et al. 2005], immunotoxins [Falini et al. 1992; Schnell et al. 2002], bispecific antibodies [Borchmann et al. 2002], Fc-engineered antibodies [Blum et al. 2009], while other strategies, such as CD30L fusion toxins [Huhn et al. 2001], have also been investigated in vitro or in vivo. Studies with the unconjugated parent antibody, SGN-30, have also been undertaken [Bartlett et al. 2008; Forero-Torres et al. 2009], but further clinical development was curtailed because of the low response rates [Katz et al. 2011]. Objective responses were seen only in ALCL and, while no objective responses were seen in HL, 19–29% of patients achieved stable disease [Bartlett et al. 2008; Forero-Torres et al. 2009].
Table 2.
Clinical trials with anti-CD30 monoclonal antibodies.
| Drug (Phase) | Disease | Evaluable patients | Response | Notes |
|---|---|---|---|---|
| Ber-H2/SO6 (proof-of-concept) [Falini et al. 1992] | HL | 4 | PR, 75% | With this antiCD30 immunotoxin, 3/4 patients experienced rapid (5–7 days) reduction in tumor masses but responses were transient (6–10 weeks). |
| Ki-4.dgA (I) [Schnell et al. 2002] | HL, ALCL | 15 | PR, 7% MR + SD, 20% |
This immunotoxin had low MTD and only moderate efficacy. DLTs included myalgias, fatigue, and vascular leak syndrome. |
| H22xKi-4 (I) [Borchmann et al. 2002] | HL | 10 | CR, 10% PR, 30% SD, 40% |
This bispecific antibody targets CD30 and CD64 in an attempt to recruit immuno-competent CD64+ effector cells. MTD was not reached |
| Iodine-131-Ki-4 (I) [Schnell et al. 2005] | HL | 22 | CR, 4.5% PR, 23% MR + SD, 18% |
One CR lasted 5 months; median duration of PR was 4 months. This radioimmuno-conjugate was associated with significant hematologic toxicity. |
| MDX-060 (I/II) [Ansell et al. 2007] | HL, ALCL, CD30+ TCL | 72 (HL:63, ALCL:7) |
CR, 5.5% (HL 3%, ALCL 29%) PR, 3% SD, 35% |
53% experienced PD by month 2. SD lasted 2 – 18 months. MTD was not reached. MDX-060 had minimal toxicity but also limited efficacy as single agent. |
| SGN-30 (I) [Bartlett et al. 2008] | HL, CD30+ NHL |
24 (HL:21) |
CR, 4% (1 pt with cut-ALCL) SD, 25% SD in HL, 19% |
71% experienced PD. Adverse events were common but only mild to moderate. MTD was not reached. SGN-30 was safe but clinical activity was modest. |
| SGN-30 (II) [Forero-Torres et al. 2009] | HL, ALCL | 79 (HL: 38, ALCL: 41) |
CR, 2.5% PR, 6.3% SD (ALCL), 15% SD (HL), 29% |
All responses were seen in patients with ALCL. No objective responses seen in HL although SD of at least 2 months was achieved in 29%. |
| SGN-30 (II) [Duvic et al. 2009] | cut-ALCL, LyP, transformed MF | 23 | CR, 43% PR, 26% SD, 17% |
SGN-30 showed promising activity and safety profile in primary cutaneous lymphoproliferative CD30+ disorders. Most common adverse events included fatigue, diarrhea, back pain, insomnia, and pruritus. |
| XmAb2513 (I) [Blum et al. 2009] | HL | 13 | PR, 7% | MTD not reached. This humanized antibody with the Fc fragment engineered to enhance Fcγ-receptor mediated effector cell functions was well tolerated; no immunogenicity observed. |
| Clinical trials with SGN-35 | ||||
| SGN-35 q3 weeks (I) [Younes et al. 2010] | CD30+ hematologic malignancies | 44 (HL: 41, ALCL: 2 AITL: 1) |
CR, 25% PR, 13.6% SD, 43% |
The MTD was determined at 1.8 mg/kg q3 weeks. SGN-35 was well tolerated with primarily mild to moderate adverse events. Peripheral neuropathy is common. SGN-35 showed unprecedented responses in CD30+ hematologic malignancies. |
| SGN-35 weekly (I) [Fanale et al. 2011] | CD30+ hematologic malignancies | 41 (HL: 38, ALCL: 5 PTCL: 1) |
CR, 34% PR, 24% SD, 32% |
The MTD was determined at 1.2 mg/kg weekly q3 weeks of a 4-week cycle. CR rate may be higher as compared with a q3 week regimen. Earlier-onset, cumulative peripheral neuropathy may limit prolonged therapy. |
| SGN-35 weekly or q3 weeks (case series) [Bartlett et al. 2010] | HL, ALCL | 8 | CR, 25% PR, 50% SD, 25% |
In patients who previously achieved tumor reductions with brentuximab vedotin but relapsed, retreatment induced an objective response rate of 75%. |
| SGN-35 q3 weeks (II) [Chen et al. 2011] | HL | 98 | CR, 34% PR, 40% |
Brentuximab vedotin showed remarkable antitumor activity and induced durable remissions in heavily pretreated patients with manageable toxicity. |
| SGN-35 q3 weeks (II) [Pro et al. 2011] | ALCL | 58 | CR, 53% PR, 33% |
Brentuximab vedotin showed significant antitumor activity in ALCL, both ALK(+) and ALK(-), with an objective response rate of 86% and manageable toxicity. |
AITL, angioimmunoblastic T-cell lymphoma; ALCL, anaplastic large cell lymphoma; CD, cluster of differentiation; CR, complete remission; cut-ALCL, primary cutaneous anaplastic large cell lymphoma; DLT, dose limiting toxicity; HL, Hodgkin’s lymphoma; LyP, lymphomatoid papulosis; MF, mycosis fungoides; MTD, maximum tolerated dose; PD, progressive disease; PR, partial response; PTCL, peripheral T-cell lymphoma; SD, stable disease.
Two phase I studies investigating two different regimens of brentuximab vedotin were undertaken [Fanale et al. 2011; Younes et al. 2010].
In the first study, the ADC was administered intravenously every 3 weeks at doses of 0.1 to 3.6 mg/kg according to a traditional dose-escalation design followed by a cohort expansion phase [Younes et al. 2010]. The primary objective was to determine the MTD, while the secondary objectives included assessment of antitumor activity. The study enrolled a total of 45 patients: 42 with HL, 2 with ALK(-positive) ALCL and one with angioimmunoblastic T-cell lymphoma. All participants had relapsed or refractory disease to multiple prior regimens; the median number of prior chemotherapies was 3 (range, 1–7) while 73% had failed autologous SCT. The MTD and the dose for further clinical investigations was determined at 1.8 mg/kg as at the 2.7 mg/kg dose level 3 of 12 patients experienced dose limiting toxicities and the single patient treated at 3.6 mg/kg developed febrile neutropenia that contributed to his subsequent death. The most common adverse events included fatigue, pyrexia, diarrhea, nausea, neutropenia and were mostly mild to moderate in severity. Nonetheless, dose delays and treatment withdrawal due to adverse events occurred in 36% and 27% of patients, respectively. Cumulative dose-related peripheral neuropathy, probably associated with unconjugated MMAE, was clinically important as it occurred in 36% of patients after a median of three cycles. Although it resolved in most patients, 37% of patients had persistent symptoms at the last safety assessment and 25% of drug discontinuations due to adverse events were associated with peripheral neuropathy [Younes et al. 2010].
The highlight of the study though was the unprecedented responses observed in a patient population with very few other treatment options [Younes et al. 2010]. The overall objective response rate was 38.6% (25% complete and 13.6% partial response rate), whereas 81% of patients with disease-related symptoms at baseline experienced resolution regardless of response status. At the MTD, the overall response rate was 50% (67% according to independent review). The median duration of response was at least 9.7 months and the progression-free survival 5.9 months [Younes et al. 2010].
In the second phase I study, brentuximab vedotin was administered weekly for three weeks of a 4-week cycle at doses of 0.4 to 1.4 mg/kg [Fanale et al. 2011]. The hypothesis was that weekly administration of the ADC would augment its antitumor activity without compromising its safety. This study also followed a traditional dose-escalation design with cohort expansion phase. The primary objectives were the determination of MTD and safety profile of a weekly regimen, while secondary objectives included assessment of antitumor efficacy. The study enrolled 44 patients with relapsed (55%) or refractory (45%) CD30+ hematologic malignancies: 38 patients with HL, 5 with ALCL (1 ALK-positive, 4 ALK-negative), and 1 patient with peripheral T-cell lymphoma not otherwise specified. The patient characteristics were similar with the prior phase I study: the median number of prior systemic therapies was 3 (range, 1–8) and 68% of patients had failed prior autologous SCT. With the dose of the investigational drug escalated at smaller increments around the MTD compared with the prior study, the MTD was determined at 1.2 mg/kg. Hyperglycemia and diarrhea constituted the dose-limiting toxicities in the immediately higher cohort (1.4 mg/kg); no dose-limiting toxicities were seen at the MTD. The nature of the most common adverse events was similar between weekly and q3-week administration of brentuximab vedotin; however, the incidence of fatigue, nausea, arthralgia, diarrhea, upper respiratory tract infection, infusion reactions, and primarily peripheral neuropathy (sensory and motor) was higher with the weekly regimen. Overall, 73% of patients developed peripheral neuropathy, mostly mild to moderate; grade III peripheral neuropathy developed in 14% (as compared with 36% incidence of any grade and 2.2% incidence of grade III peripheral neuropathy with the q3-week administration) [Fanale et al. 2011].
This study showed that a weekly regimen is at least as efficacious as q3-week administration in inducing durable remissions [Fanale et al. 2011]. The overall response rate was 59% with complete remissions achieved in 34%. At the MTD, the overall and complete response rates were 58% and 25%, respectively. With a median follow up of 11 months, the median progression-free survival was approximately 7 months, whereas the median overall survival has not been reached [Fanale et al. 2011]. Although more patients achieved complete remissions when compared with the prior phase I study, the results are not directly comparable as the number of patients who received therapeutic doses of the investigational drug are different. Both studies have indicated a dose-response therapeutic effect and efficacy was not their primary objective. Weekly infusion of brentuximab vedotin may be advantageous over a q3-week regimen in inducing rapid responses in patients with bulky or symptomatic disease, but cumulative peripheral neuropathy may limit prolonged administrations.
Both phase I studies allowed retreatment with brentuximab vedotin in patients who achieved reductions in tumor volume to the investigational therapy but relapsed [Bartlett et al. 2010]. The regimen consisted of 1.8 mg/kg q3 weeks or 1 mg/kg weekly, depending on the prior study. Preliminary data on seven patients (six with HL and one with ALCL) who received eight retreatments have been reported. Since the last administration of brentuximab vedotin, half of the retreatment cases did not receive any therapy, whereas the other half received one to three chemotherapy regimens; one patient underwent autologous SCT in the interim. Responses were remarkable even in this setting, as 2/8 and 4/8 retreatment cases achieved complete and partial remissions, respectively. The other two cases achieved stable disease. Adverse events were similar to those reported in the phase I studies including peripheral neuropathy and upper respiratory tract infections [Bartlett et al. 2010].
Phase II studies/pivotal clinical trials
The encouraging results of the phase I studies set the stage for the conduction of two single-arm, multicenter phase II clinical trials with brentuximab vedotin at 1.8 mg/kg q3 weeks up to 16 cycles in relapsed or refractory HL [Chen et al. 2011] and ALCL [Pro et al. 2011]. In both studies, the primary objective was objective response rate as assessed by independent review.
The phase II study in HL enrolled a total of 102 patients with relapsed or refractory disease having failed prior autologous SCT [Chen et al. 2011]. The median age of the participants was 31 years (range, 15–77) and the median number of prior treatments was 3.5 (range, 1–13) in addition to high-dose chemotherapy followed by autologous SCT. A total of 71% of patients had primary refractory disease (defined as failure to achieve complete remission or progression within 3 months of completing frontline therapy) and 42% had not responded to their most recent prior treatment. The objective response rate was 75% with complete remission rate of 34%. A total of 79 of 102 patients (95%) achieved reductions in tumor size and 83% of patients with B symptoms at baseline, experienced resolution of these symptoms. With a median follow up of 9 months, the median duration of response in patients who achieved complete remission has not been reached and the reported estimated 12-month overall survival was 88%. The most common adverse events of any grade were peripheral neuropathy, fatigue, nausea, diarrhea, and neutropenia, whereas common grade III adverse events included peripheral neuropathy and cytopenias; 18% of patients discontinued treatment due to adverse events. Overall, brentuximab vedotin showed remarkable antitumor activity in a heavily pretreated population with manageable toxicities [Chen et al. 2011].
The phase II study in ALCL enrolled a total of 58 patients with relapsed or refractory disease [Pro et al. 2011]. The median age of the participants was 52 years (range, 14–76) and the median number of prior treatments was 2 (range, 1–6). Consistent with the aggressive nature of ALCL, especially ALK-negative ALCL which was overrepresented in the study (72%), the incidence of primary refractory disease and disease refractory to the most recent treatment was high (62% and 50%, respectively). Moreover, 22% of patients never responded to any prior therapy. In this difficult-to-treat population, the objective response rate was 86% with complete remission rate of 53%. More than 90% of patients with secondary cutaneous involvement experienced resolution of the lesions after a median of 4.9 weeks. At the time of presentation of the results, the median duration of response was not reached but ranged between 0.3 and 45.3 weeks. Interestingly, the response pattern was similar in ALK-positive and ALK-negative disease. The treatment-related adverse events were similar in nature and incidence to those reported in the phase II study in HL with cytopenias and peripheral neuropathy constituting the most common grade III toxicities. Overall, brentuximab vedotin showed significant antitumor activity in ALCL, both ALK-positive and ALK-negative, and induced a high proportion of durable complete remissions with manageable toxicity [Pro et al. 2011].
The final results of those two pivotal trials have not been published yet. Nonetheless, given the unprecedented antitumor efficacy in a patient population with few other treatment options, the Food and Drug Administration (FDA) granted accelerated approval of brentuximab vedotin in relapsed or refractory HL and ALCL [FDA, 2011]. In HL, the indication includes failure of autologous SCT or at least two prior multiagent chemotherapy regimens in patients ineligible for SCT. Given the paucity of effective second-line regimens in ALCL (especially ALK-negative ALCL), the indication in ALCL includes failure of at least one prior multiagent chemotherapy. Owing to cumulative toxicities, the treatment should not exceed 16 cycles and, although effective [Bartlett et al. 2010], retreatment with brentuximab vedotin should be considered investigational.
Conclusions and future directions
It is important to highlight the significant difference in clinical activity between the naked parent antibody SGN-30 and the ADC counterpart, SGN-35 [Forero-Torres et al. 2009; Younes et al. 2010]. It is evident that conjugation of SGN-30 with a potent cytotoxin, enhanced significantly its antitumor activity. Possible explanations for the modest activity of the naked antibody may include impaired antibody-dependent cell-mediated cytotoxicity in the immunosuppressing tumor microenvironment and sequestration by the soluble CD30. The naked antibody may be active in its own right as shown in vitro and in mouse models of HL [Wahl et al. 2002], but in practice, the addition of a cytotoxic agent may be required to significantly potentiate its antitumor efficacy. On the other hand, the cytotoxic effect of free MMAE diffused from CD30+ malignant cells on bystander cells may in part account for the significant antitumor activity of SGN-35 [Katz et al. 2011], especially in HL where the tumor microenvironment is essential for the proliferation and survival of the malignant Hodgkin and Reed–Sternberg cells [Kuppers, 2009].
Several studies of brentuximab vedotin are currently ongoing. The AETHERA trial (ADC Empowered Trial for Hodgkin to Evaluate PRogression after Autologous SCT) is a phase III randomized, double-blind study comparing brentuximab vedotin plus best supportive care versus placebo plus best supportive care in patients with HL at high risk of relapse after autologous SCT [ClinicalTrials.gov identifier: NCT01100502]. The results of this study will provide the basis for full FDA approval. Building on the encouraging results of a case series [Bartlett et al. 2010], a phase II trial is evaluating the potential for retreatment with brentuximab vedotin in patients who have relapsed after discontinuing previous therapy with the same agent [ClinicalTrials.gov identifier: NCT00947856].
Given the significant antitumor activity and the favorable toxicity profile of brentuximab vedotin, many clinical trials are investigating its efficacy in earlier stages of HL. A phase I, two-arm, open-label, dose-escalation study is investigating the combination of brentuximab vedotin with multiagent chemotherapy in front-line treatment of HL. The treatment arms consist of brentuximab vedotin in combination with ABVD or AVD (doxorubicin, vinblastine, dacarbazine) [ClinicalTrials.gov identifier: NCT01060904]. Another phase II study is evaluating the efficacy of four courses of brentuximab vedotin in patients with recurrent HL prior to autologous SCT [ClinicalTrials.gov identifier: NCT01393717]. Lastly, a phase II study is evaluating the efficacy of brentuximab vedotin as frontline therapy in elderly patients (older than 60 years of age) with HL. Treatment consists of a lead-in phase with two cycles of brentuximab vedotin q3 weeks, followed by six cycles of AVD. Patients who achieve complete remission will receive another 4 cycles of brentuximab vedotin as consolidation [ClinicalTrials.gov identifier: NCT01476410].
As other malignancies besides HL and ALCL may express CD30, ongoing clinical trials are currently investigating the potential of SGN-35 in other CD30+ neoplasms. A phase I trial is evaluating brentuximab vedotin given sequentially or concurrently with multiagent chemotherapy as front-line treatment in patients with CD30+ mature T-cell or NK-cell neoplasms including ALCL. The study has three arms: brentuximab vedotin given sequentially with cyclophosphamide, prednisone, doxorubicin, and vincristine and brentuximab vedotin given concurrently or sequentially with cyclophosphamide, doxorubicin, and prednisone [ClinicalTrials.gov identifier: NCT01309789]. A phase II study is evaluating the antitumor efficacy of brentuximab vedotin in CD30+ primary cutaneous lymphoproliferative disorders [ClinicalTrials.gov identifer: NCT01352520], whereas another phase II study is evaluating brentuximab vedotin in CD30+ nonlymphomatous malignancies, including solid tumors [ClinicalTrials.gov identifier: NCT01461538]. Lastly, the efficacy and safety of brentuximab vedotin as a single agent in CD30+ T-cell and B-cell non-Hodgkin lymphomas is under clinical investigation in a multicenter, open-label phase II study [ClinicalTrials.gov identifier: NCT01421667].
In conclusion, CD30 epitomizes many properties of an ideal pharmacologic target. However, until the advent of brentuximab vedotin, antibody-based anti-CD30 immunotherapy had been largely unsuccessful. Brentuximab vedotin showed significant antitumor activity in relapsed or refractory HL and ALCL, leading to its accelerated approval by the FDA. Before the advent of brentuximab vedotin, both conditions had limited alternative options and an overall unfavorable prognosis. A major clinical consideration is cumulative peripheral neuropathy which may limit prolonged administrations. As other hematologic malignancies may express CD30 and brentuximab vedotin has a favorable toxicity profile, its indications are anticipated to expand.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare no conflicts of interest in preparing this article.
Contributor Information
Christos Vaklavas, Division of Hematology/Oncology, Comprehensive Cancer Center, University of Alabama at Birmingham, AL, USA.
Andres Forero-Torres, Comprehensive Cancer Center, University of Alabama at Birmingham, NP 2540, 1530 3rd Avenue South, Birmingham, AL 35294-3300, USA.
References
- Amakawa R., Hakem A., Kundig T.M., Matsuyama T., Simard J.J., Timms E., et al. (1996) Impaired negative selection of T cells in Hodgkin’s disease antigen CD30-deficient mice. Cell 84: 55–562 [DOI] [PubMed] [Google Scholar]
- Amin H.M., Lai R. (2007) Pathobiology of ALK+ anaplastic large-cell lymphoma. Blood 110: 2259–2267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansell S.M., Geyer S.M., Maurer M.J., Kurtin P.J., Micallef I.N., Stella P., et al. (2006) Randomized phase II study of interleukin-12 in combination with rituximab in previously treated non-Hodgkin’s lymphoma patients. Clin Cancer Res 12: 6056–6063 [DOI] [PubMed] [Google Scholar]
- Ansell S.M., Horwitz S.M., Engert A., Khan K.D., Lin T., Strair R., et al. (2007) Phase I/II study of an anti-CD30 monoclonal antibody (MDX-060) in Hodgkin’s lymphoma and anaplastic large-cell lymphoma. J Clin Oncol 25: 2764–2769 [DOI] [PubMed] [Google Scholar]
- Bai R., Pettit G.R., Hamel E. (1990) Dolastatin 10, a powerful cytostatic peptide derived from a marine animal. Inhibition of tubulin polymerization mediated through the vinca alkaloid binding domain. Biochem Pharmacol 39: 1941–1949 [DOI] [PubMed] [Google Scholar]
- Bartlett N., Grove L.E., Kennedy D.A., Sievers E.L., Forero-Torres A. (2010) Objective responses with brentuximab vedotin (SGN-35) retreatment in CD30-positive hematologic malignancies: A case series. J Clin Oncol 28(15 Suppl.): 8062 [Google Scholar]
- Bartlett N.L., Younes A., Carabasi M.H., Forero A., Rosenblatt J.D., Leonard J.P., et al. (2008) A phase 1 multidose study of SGN-30 immunotherapy in patients with refractory or recurrent CD30+ hematologic malignancies. Blood 111: 1848–1854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum K.A., Smith M., Fung H., Zalevsky J., Combs D., Ramies D.A., et al. (2009) Phase I study of an anti-CD30 Fc engineered humanized monoclonal antibody in Hodgkin lymphoma (HL) or anaplastic large cell lymphoma (ALCL) patients: Safety, pharmacokinetics (PK), immunogenicity, and efficacy. J Clin Oncol 27(15 Suppl.): 8531 [Google Scholar]
- Borchmann P., Schnell R., Fuss I., Manzke O., Davis T., Lewis L.D., et al. (2002) Phase 1 trial of the novel bispecific molecule H22xKi-4 in patients with refractory Hodgkin lymphoma. Blood 100: 3101–3107 [DOI] [PubMed] [Google Scholar]
- Burroughs L.M., O’Donnell P.V., Sandmaier B.M., Storer B.E., Luznik L., Symons H.J., et al. (2008) Comparison of outcomes of HLA-matched related, unrelated, or HLA-haploidentical related hematopoietic cell transplantation following nonmyeloablative conditioning for relapsed or refractory Hodgkin lymphoma. Biol Blood Marrow Transplant 14: 1279–1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerveny C.G., Law C.L., McCormick R.S., Lenox J.S., Hamblett K.J., Westendorf L.E., et al. (2005) Signaling via the anti-CD30 mAb SGN-30 sensitizes Hodgkin’s disease cells to conventional chemotherapeutics. Leukemia 19: 1648–1655 [DOI] [PubMed] [Google Scholar]
- Chen R.W., Gopal A.K., Smith S.E., Ansell S.M., Rosenblatt J.D., Savage K.J., et al. (2011) Results from a pivotal phase II study of brentuximab vedotin (SGN-35) in patients with relapsed or refractory Hodgkin lymphoma (HL). In: J Clin Oncol 29: 8031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiarle R., Podda A., Prolla G., Podack E.R., Thorbecke G.J., Inghirami G. (1999) CD30 overexpression enhances negative selection in the thymus and mediates programmed cell death via a Bcl-2-sensitive pathway. J Immunol 163: 194–205 [PubMed] [Google Scholar]
- Corradini P., Tarella C., Zallio F., Dodero A., Zanni M., Valagussa P., et al. (2006) Long-term follow-up of patients with peripheral T-cell lymphomas treated up-front with high-dose chemotherapy followed by autologous stem cell transplantation. Leukemia 20: 1533–1538 [DOI] [PubMed] [Google Scholar]
- DeYoung A.L., Duramad O., Winoto A. (2000) The TNF receptor family member CD30 is not essential for negative selection. J Immunol 165: 6170–6173 [DOI] [PubMed] [Google Scholar]
- Doronina S.O., Toki B.E., Torgov M.Y., Mendelsohn B.A., Cerveny C.G., Chace D.F., et al. (2003) Development of potent monoclonal antibody auristatin conjugates for cancer therapy. Nat Biotechnol 21: 778–784 [DOI] [PubMed] [Google Scholar]
- Duckett C.S., Thompson C.B. (1997) CD30-dependent degradation of TRAF2: implications for negative regulation of TRAF signaling and the control of cell survival. Genes Dev 11: 2810–2821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvic M., Reddy S.A., Pinter-Brown L., Korman N.J., Zic J., Kennedy D.A., et al. (2009) A phase II study of SGN-30 in cutaneous anaplastic large cell lymphoma and related lymphoproliferative disorders. Clin Cancer Res 15: 6217–6224 [DOI] [PubMed] [Google Scholar]
- Edinger J.T., Clark B.Z., Pucevich B.E., Geskin L.J., Swerdlow S.H. (2009) CD30 expression and proliferative fraction in nontransformed mycosis fungoides. Am J Surg Pathol 33: 1860–1868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falini B., Bolognesi A., Flenghi L., Tazzari P.L., Broe M.K., Stein H., et al. (1992) Response of refractory Hodgkin’s disease to monoclonal anti-CD30 immunotoxin. Lancet 339: 1195–1196 [DOI] [PubMed] [Google Scholar]
- Fanale M.A., Forero-Torres A., Rosenblatt J.D., Advani R.H., Franklin A.R., Kennedy D.A., et al. (2011) A phase I weekly dosing study of brentuximab vedotin in patients with relapsed/refractory CD30-positive hematologic malignancies. Clin Cancer Res, in press [DOI] [PubMed] [Google Scholar]
- FDA (2011) ADCETRIS™ (brentuximab vedotin) for injection. Highlights of prescribing information. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/125388s000,125399s000lbl.pdf
- Fisher R.I., Kaminski M.S., Wahl R.L., Knox S.J., Zelenetz A.D., Vose J.M., et al. (2005) Tositumomab and iodine-131 tositumomab produces durable complete remissions in a subset of heavily pretreated patients with low-grade and transformed non-Hodgkin’s lymphomas. J Clin Oncol 23: 7565–7573 [DOI] [PubMed] [Google Scholar]
- Forero-Torres A., Leonard J.P., Younes A., Rosenblatt J.D., Brice P., Bartlett N.L., et al. (2009) A Phase II study of SGN-30 (anti-CD30 mAb) in Hodgkin lymphoma or systemic anaplastic large cell lymphoma. Br J Haematol 146: 171–179 [DOI] [PubMed] [Google Scholar]
- Francisco J.A., Cerveny C.G., Meyer D.L., Mixan B.J., Klussman K., Chace D.F., et al. (2003) cAC10-vcMMAE, an anti-CD30-monomethyl auristatin E conjugate with potent and selective antitumor activity. Blood 102: 1458–1465 [DOI] [PubMed] [Google Scholar]
- Gattei V., Degan M., Gloghini A., De Iuliis A., Improta S., Rossi F.M., et al. (1997) CD30 ligand is frequently expressed in human hematopoietic malignancies of myeloid and lymphoid origin. Blood 89: 2048–2059 [PubMed] [Google Scholar]
- Gerber H.P., Kung-Sutherland M., Stone I., Morris-Tilden C., Miyamoto J., McCormick R., et al. (2009) Potent antitumor activity of the anti-CD19 auristatin antibody drug conjugate hBU12-vcMMAE against rituximab-sensitive and -resistant lymphomas. Blood 113: 4352–4361 [DOI] [PubMed] [Google Scholar]
- Horie R., Watanabe T. (1998) CD30: expression and function in health and disease. Semin Immunol 10: 457–470 [DOI] [PubMed] [Google Scholar]
- Huhn M., Sasse S., Tur M.K., Matthey B., Schinkothe T., Rybak S.M., et al. (2001) Human angiogenin fused to human CD30 ligand (Ang-CD30L) exhibits specific cytotoxicity against CD30-positive lymphoma. Cancer Res 61: 8737–8742 [PubMed] [Google Scholar]
- Jemal A., Siegel R., Xu J., Ward E. (2010) Cancer statistics, 2010. CA Cancer J Clin 60: 277–300 [DOI] [PubMed] [Google Scholar]
- Katz J., Janik J.E., Younes A. (2011) Brentuximab Vedotin (SGN-35). Clin Cancer Res 17(20): 6428–6436 [DOI] [PubMed] [Google Scholar]
- Kempf W., Pfaltz K., Vermeer M.H., Cozzio A., Ortiz-Romero P.L., Bagot M., et al. (2011) EORTC, ISCL, and USCLC consensus recommendations for the treatment of primary cutaneous CD30-positive lymphoproliferative disorders: lymphomatoid papulosis and primary cutaneous anaplastic large-cell lymphoma. Blood 118: 4024–4035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan K.D., Emmanouilides C., Benson D.M., Jr., Hurst D., Garcia P., Michelson G., et al. (2006) A phase 2 study of rituximab in combination with recombinant interleukin-2 for rituximab-refractory indolent non-Hodgkin’s lymphoma. Clin Cancer Res 12: 7046–7053 [DOI] [PubMed] [Google Scholar]
- Khouri I.F., Saliba R.M., Hosing C., Okoroji G.J., Acholonu S., Anderlini P., et al. (2005) Concurrent administration of high-dose rituximab before and after autologous stem-cell transplantation for relapsed aggressive B-cell non-Hodgkin’s lymphomas. J Clin Oncol 23: 2240–2247 [DOI] [PubMed] [Google Scholar]
- Kuppers R. (2009) The biology of Hodgkin’s lymphoma. Nat Rev Cancer 9: 15–27 [DOI] [PubMed] [Google Scholar]
- Kurts C., Carbone F.R., Krummel M.F., Koch K.M., Miller J.F., Heath W.R. (1999) Signalling through CD30 protects against autoimmune diabetes mediated by CD8 T cells. Nature 398: 341–344 [DOI] [PubMed] [Google Scholar]
- Kuruvilla J., Keating A., Crump M. (2011) How I treat relapsed and refractory Hodgkin lymphoma. Blood 117: 4208–4217 [DOI] [PubMed] [Google Scholar]
- Lazarus H.M., Rowlings P.A., Zhang M.J., Vose J.M., Armitage J.O., Bierman P.J., et al. (1999) Autotransplants for Hodgkin’s disease in patients never achieving remission: a report from the Autologous Blood and Marrow Transplant Registry. J Clin Oncol 17: 534–545 [DOI] [PubMed] [Google Scholar]
- Le Gouill S., Milpied N., Buzyn A., De Latour R.P., Vernant J.P., Mohty M., et al. (2008) Graft-versus-lymphoma effect for aggressive T-cell lymphomas in adults: a study by the Societe Francaise de Greffe de Moelle et de Therapie Cellulaire. J Clin Oncol 26: 2264–2271 [DOI] [PubMed] [Google Scholar]
- Locksley R.M., Killeen N., Lenardo M.J. (2001) The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell 104: 487–501 [DOI] [PubMed] [Google Scholar]
- Merkel O., Hamacher F., Sifft E., Kenner L., Greil R. (2011) Novel therapeutic options in anaplastic large cell lymphoma: molecular targets and immunological tools. Mol Cancer Ther 10: 1127–1136 [DOI] [PubMed] [Google Scholar]
- Milpied N., Fielding A.K., Pearce R.M., Ernst P., Goldstone A.H. (1996) Allogeneic bone marrow transplant is not better than autologous transplant for patients with relapsed Hodgkin’s disease. European Group for Blood and Bone Marrow Transplantation. J Clin Oncol 14: 1291–1296 [DOI] [PubMed] [Google Scholar]
- Mir S.S., Richter B.W., Duckett C.S. (2000) Differential effects of CD30 activation in anaplastic large cell lymphoma and Hodgkin disease cells. Blood 96: 4307–4312 [PubMed] [Google Scholar]
- Morschhauser F., Radford J., Van Hoof A., Vitolo U., Soubeyran P., Tilly H., et al. (2008) Phase III trial of consolidation therapy with yttrium-90-ibritumomab tiuxetan compared with no additional therapy after first remission in advanced follicular lymphoma. J Clin Oncol 26: 5156–5164 [DOI] [PubMed] [Google Scholar]
- Nadali G., Tavecchia L., Zanolin E., Bonfante V., Viviani S., Camerini E., et al. (1998) Serum level of the soluble form of the CD30 molecule identifies patients with Hodgkin’s disease at high risk of unfavorable outcome. Blood 91: 3011–3016 [PubMed] [Google Scholar]
- O’Connor O.A., Pro B., Pinter-Brown L., Bartlett N., Popplewell L., Coiffier B., et al. (2011) Pralatrexate in patients with relapsed or refractory peripheral T-cell lymphoma: results from the pivotal PROPEL study. J Clin Oncol 29: 1182–1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oflazoglu E., Kissler K.M., Sievers E.L., Grewal I.S., Gerber H.P. (2008) Combination of the anti-CD30-auristatin-E antibody-drug conjugate (SGN-35) with chemotherapy improves antitumour activity in Hodgkin lymphoma. Br J Haematol 142: 69–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okeley N.M., Miyamoto J.B., Zhang X., Sanderson R.J., Benjamin D.R., Sievers E.L., et al. (2010) Intracellular activation of SGN-35, a potent anti-CD30 antibody-drug conjugate. Clin Cancer Res 16: 888–897 [DOI] [PubMed] [Google Scholar]
- Pileri S.A., Zinzani P.L., Gaidano G., Falini B., Gaulard P., Zucca E., et al. (2003) Pathobiology of primary mediastinal B-cell lymphoma. Leuk Lymphoma 44(Suppl. 3): S21–S26 [DOI] [PubMed] [Google Scholar]
- Pro B., Advani R., Brice P., Bartlett N., Rosenblatt J.D., Illidge T., et al. (2011) Durable remissions with brentuximab vedotin (SGN-35): Updated results of a phase II study in patients with relapsed or refractory systemic anaplastic large cell lymphoma (sALCL). J Clin Oncol 29: 8032. [DOI] [PubMed] [Google Scholar]
- Romagnani P., Annunziato F., Manetti R., Mavilia C., Lasagni L., Manuelli C., et al. (1998) High CD30 ligand expression by epithelial cells and Hassal’s corpuscles in the medulla of human thymus. Blood 91: 3323–3332 [PubMed] [Google Scholar]
- Savage K.J., Harris N.L., Vose J.M., Ullrich F., Jaffe E.S., Connors J.M., et al. (2008) ALK- anaplastic large-cell lymphoma is clinically and immunophenotypically different from both ALK+ ALCL and peripheral T-cell lymphoma, not otherwise specified: report from the International Peripheral T-Cell Lymphoma Project. Blood 111: 5496–5504 [DOI] [PubMed] [Google Scholar]
- Schmitz N., Pfistner B., Sextro M., Sieber M., Carella A.M., Haenel M., et al. (2002) Aggressive conventional chemotherapy compared with high-dose chemotherapy with autologous haemopoietic stem-cell transplantation for relapsed chemosensitive Hodgkin’s disease: a randomised trial. Lancet 359: 2065–2071 [DOI] [PubMed] [Google Scholar]
- Schnell R., Dietlein M., Staak J.O., Borchmann P., Schomaecker K., Fischer T., et al. (2005) Treatment of refractory Hodgkin’s lymphoma patients with an iodine-131-labeled murine anti-CD30 monoclonal antibody. J Clin Oncol 23: 4669–4678 [DOI] [PubMed] [Google Scholar]
- Schnell R., Staak O., Borchmann P., Schwartz C., Matthey B., Hansen H., et al. (2002) A Phase I study with an anti-CD30 ricin A-chain immunotoxin (Ki-4.dgA) in patients with refractory CD30+ Hodgkin’s and non-Hodgkin’s lymphoma. Clin Cancer Res 8: 1779–1786 [PubMed] [Google Scholar]
- Sotlar K., Cerny-Reiterer S., Petat-Dutter K., Hessel H., Berezowska S., Mullauer L., et al. (2011) Aberrant expression of CD30 in neoplastic mast cells in high-grade mastocytosis. Mod Pathol 24: 585–595 [DOI] [PubMed] [Google Scholar]
- Staudt L.M. (2010) Oncogenic activation of NF-kappaB. Cold Spring Harb Perspect Biol 2(6): a000109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein H., Mason D.Y., Gerdes J., O’Connor N., Wainscoat J., Pallesen G., et al. (1985) The expression of the Hodgkin’s disease associated antigen Ki-1 in reactive and neoplastic lymphoid tissue: evidence that Reed–Sternberg cells and histiocytic malignancies are derived from activated lymphoid cells. Blood 66: 848–858 [PubMed] [Google Scholar]
- Sutherland M.S., Sanderson R.J., Gordon K.A., Andreyka J., Cerveny C.G., Yu C., et al. (2006) Lysosomal trafficking and cysteine protease metabolism confer target-specific cytotoxicity by peptide-linked anti-CD30-auristatin conjugates. J Biol Chem 281: 10540–10547 [DOI] [PubMed] [Google Scholar]
- Teicher B.A., Chari R.V. (2011) Antibody conjugate therapeutics: challenges and potential. Clin Cancer Res 17: 6389–6397 [DOI] [PubMed] [Google Scholar]
- Tian Z.G., Longo D.L., Funakoshi S., Asai O., Ferris D.K., Widmer M., et al. (1995) In vivo antitumor effects of unconjugated CD30 monoclonal antibodies on human anaplastic large-cell lymphoma xenografts. Cancer Res 55: 5335–5341 [PubMed] [Google Scholar]
- Vose J., Armitage J., Weisenburger D. (2008) International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol 26: 4124–4130 [DOI] [PubMed] [Google Scholar]
- Wahl A.F., Klussman K., Thompson J.D., Chen J.H., Francisco L.V., Risdon G., et al. (2002) The anti-CD30 monoclonal antibody SGN-30 promotes growth arrest and DNA fragmentation in vitro and affects antitumor activity in models of Hodgkin’s disease. Cancer Res 62: 3736–3742 [PubMed] [Google Scholar]
- Wang C.Y., Mayo M.W., Korneluk R.G., Goeddel D.V., Baldwin A.S., Jr (1998) NF-kappaB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science 281: 1680–1683 [DOI] [PubMed] [Google Scholar]
- Witzig T.E., Gordon L.I., Cabanillas F., Czuczman M.S., Emmanouilides C., Joyce R., et al. (2002) Randomized controlled trial of yttrium-90-labeled ibritumomab tiuxetan radioimmunotherapy versus rituximab immunotherapy for patients with relapsed or refractory low-grade, follicular, or transformed B-cell non-Hodgkin’s lymphoma. J Clin Oncol 20: 2453–2463 [DOI] [PubMed] [Google Scholar]
- Wright C.W., Rumble J.M., Duckett C.S. (2007) CD30 activates both the canonical and alternative NF-kappaB pathways in anaplastic large cell lymphoma cells. J Biol Chem 282: 10252–10262 [DOI] [PubMed] [Google Scholar]
- Younes A. (2009) Novel treatment strategies for patients with relapsed classical Hodgkin lymphoma. Hematology Am Soc Hematol Educ Program: 507–519 [DOI] [PubMed] [Google Scholar]
- Younes A. (2011) CD30-targeted antibody therapy. Curr Opin Oncol 23: 587–593 [DOI] [PubMed] [Google Scholar]
- Younes A., Aggarwall B.B. (2003) Clinical implications of the tumor necrosis factor family in benign and malignant hematologic disorders. Cancer 98: 458–467 [DOI] [PubMed] [Google Scholar]
- Younes A., Bartlett N.L., Leonard J.P., Kennedy D.A., Lynch C.M., Sievers E.L., et al. (2010) Brentuximab vedotin (SGN-35) for relapsed CD30-positive lymphomas. N Engl J Med 363: 1812–1821 [DOI] [PubMed] [Google Scholar]
- Younes A., Carbone A. (1999) CD30/CD30 ligand and CD40/CD40 ligand in malignant lymphoid disorders. Int J Biol Markers 14: 135–143 [DOI] [PubMed] [Google Scholar]
- Younes A., Kadin M.E. (2003) Emerging applications of the tumor necrosis factor family of ligands and receptors in cancer therapy. J Clin Oncol 21: 3526–3534 [DOI] [PubMed] [Google Scholar]
- Zinzani P.L., Pileri S., Bendandi M., Buzzi M., Sabattini E., Ascani S., et al. (1998) Clinical implications of serum levels of soluble CD30 in 70 adult anaplastic large-cell lymphoma patients. J Clin Oncol 16: 1532–1537 [DOI] [PubMed] [Google Scholar]