Abstract
Heparin-induced thrombocytopenia (HIT) is a drug-mediated, prothrombotic disorder caused by immunization against platelet factor 4 (PF4) after complex formation with heparin or other polyanions. After their binding to PF4/heparin complexes on the platelet surface, HIT antibodies are capable of intravascular platelet activation by cross-linking Fcγ receptor IIA leading to a platelet count decrease and/or thrombosis. Diagnosis of HIT is often difficult. This, and the low specificity of the commercially available immunoassays, leads currently to substantial overdiagnosis of HIT. Timing of onset, the moderate nature of thrombocytopenia, and the common concurrence of thrombosis are very important factors, which help to differentiate HIT from other potential causes of thrombocytopenia. A combination of a clinical pretest scoring system and laboratory investigation is usually necessary to diagnose HIT. Although HIT is considered to be a rare complication of heparin treatment, the very high number of hospital inpatients, and increasingly also hospital outpatients receiving heparin, still result in a considerable number of patients developing HIT. If HIT occurs, potentially devastating complications such as life-threatening thrombosis make it one of the most serious adverse drug reactions. If HIT is strongly suspected, all heparin must be stopped and an alternative nonheparin anticoagulant started at a therapeutic dose to prevent thromboembolic complications. However, the nonheparin alternative anticoagulants bear a considerable bleeding risk, especially if given to patients with thrombocytopenia due to other reasons than HIT. While established drugs for HIT are disappearing from the market (lepirudin, danaparoid), bivalirudin, fondaparinux and potentially the new anticoagulants such as dabigatran, rivaroxaban and apixaban provide new treatment options.
Keywords: heparin, thrombocytopenia, thrombosis
Introduction
Heparin-induced thrombocytopenia (HIT) is a prothrombotic, immune-mediated adverse reaction that occurs after exposure to unfractionated heparin (UFH), low molecular weight heparin (LMWH), or other polyanions such as hypersulfated chondroitin sulfate [Greinacher, 2006; Warkentin et al. 2008b]. The in vivo formation of highly immunizing multimolecular complexes consisting of the negatively charged polyanions and the cationic protein platelet factor 4 (PF4) results in antibody formation in many heparin-exposed patients [Amiral et al. 1992; Greinacher et al. 1994]. Only a minority of these patients, however, develop HIT characterized by a fall in platelet count beginning after 5–10 days of heparin therapy with or without thromboembolic events [Greinacher, 2006; Warkentin, 2003]. The risk of immunization depends on the type of heparin (UFH >> LMWH) [Warkentin, 2003], length of heparin administration (>5 days >> 1–4 days), underlying disease (major surgery > minor surgery > medical patients) [Lubenow et al. 2010; Prandoni et al. 2005a, 2005b], and gender (female > male) [Warkentin et al. 2006].
Diagnosis of HIT is often difficult. Two approaches can help the primary treating physician to confirm or to rule out HIT: (a) systematic assessment of the clinical presentation using scoring models that determine the pretest probability of HIT; and (b) in vitro demonstration of PF4/heparin antibodies. Here a negative antigen assay usually rules out HIT, while a positive functional assay increases the likelihood for the presence of HIT [Warkentin et al. 2006].
Antibodies are responsible for the prothrombotic nature of HIT. They induce platelet activation by cross-linking platelet Fcγ receptor IIA (CD32a) leading to the release of procoagulant platelet-derived microparticles, which provide the catalytic surface for excess thrombin generation. Therefore, when HIT is strongly suspected, heparin should be stopped and an alternative nonheparin anticoagulant started to prevent new thromboembolic complications.
Unfortunately, as the alternative anticoagulants argatroban, lepirudin, danaparoid are only approved for the niche indication HIT, many physicians have limited experience handling these drugs. This increases the risk for both, bleeding due to overdosing or thrombotic events due to underdosing. In addition, the excessive use of these alternative anticoagulants can result in a considerable cost burden. Therefore, reliable diagnosis of ‘true’ HIT is very important for safe and cost effective patient management.
This review provides an overview of current diagnostic and treatment approaches for the management of HIT, and also summarizes the evidence for evolving new therapeutic options.
HIT, an acute, drug-induced procoagulatory disorder with autoimmune features
New concepts on the immune pathogenesis of HIT
Recent insights into the pathogenesis of HIT indicate that HIT has some characteristics of an autoimmune disease. While little is still known about the mechanisms, which modulate the immune response to platelet factor 4 (PF4)/heparin complexes, the pathway of platelet activation, induction of thrombin generation, and finally predispositions for clinical HIT (thrombocytopenia and thromboembolic events) are increasingly understood.
The immune response in HIT is triggered by the interaction of heparin with a specific platelet protein, PF4 [Amiral et al. 1995]. When PF4 interacts with polyanions, both molecules form multimolecular complexes. The size of the resulting complexes depends on grade of sulfation and chain length of the polyanion. Large molecules such as UFH form bigger complexes whereas shorter polyanions such as LMWH most likely form smaller complexes [Greinacher et al. 2006]. In addition, formation of the immunogenic multimolecular complexes occurs at optimal ratios of PF4 and polyanions [Greinacher et al. 2008]. The result is formation of neoepitoes on PF4 [Ziporen et al. 1998], which can lead to the production of antibodies.
Accumulating evidence suggests that PF4 interacts with bacteria forming PF4/polyanion complexes on the bacterial surface. Heparin-induced antibodies cross-react with PF4 coated bacteria and lead to increased phagocytosis of PF4/ heparin-antibody-coated bacteria. Furthermore, bacterial sepsis is associated with formation of these antibodies in a mouse model [Krauel et al. 2011b], and in humans there is an association between the presence of PF4/heparin antibodies and periodontitis, a chronic infection in which bacteremia frequently occurs [Krauel et al. 2011a]. These observations may explain why anti-PF4/heparin IgG antibodies can occur as early as day 5 of heparin treatment, even in patients who have never received heparin before. These patients presumably had been preimmunized by PF4-coated bacteria, allowing a rapid secondary immune response to PF4/heparin complexes.
Important for current treatment concepts is the observation that, in acute HIT, the PF4/heparin antibodies can cross-react with PF4 bound to platelets without the need of additional heparin. When sera of patients with acute HIT are incubated with platelets, they frequently induce platelet activation even in the absence of any heparin [Socher et al. 2008], and in some patients, HIT occurs only several days after cessation of heparin (so-called delayed onset HIT [Warkentin and Kelton, 2001a]. These antibodies activate platelets in vivo and obviously also in vitro. This autoimmune feature of acute HIT explains why cessation of heparin alone is not sufficient to prevent further progression of HIT [Kelton and Warkentin, 1996] and is the rational for not only stopping heparin in acute HIT, but starting an alternative anticoagulant in therapeutic dose to counteract the procoagulatory effects of these autoantibodies. Very rarely, HIT occurs spontaneously [Krauel et al. 2011b; Lo et al. 2006], these patients have either another autoimmune disorder (such as systemic lupus erythematosus) or they had recent bacterial infections, supporting the concept that HIT is likely a misdirected bacterial defense mechanism.
Interestingly, antibodies against PF4/heparin being present before surgery are also associated with an increased risk of nonthrombotic perioperative complications and cardiovascular mortality in patients who did not develop HIT [Selleng et al. 2010a; Williams et al. 2003]. These nonthrombotic complications are correlated to anti-PF4/heparin IgM but not to anti-PF4/heparin IgG antibodies [Selleng et al. 2010b]. This is very different to HIT, which depends in most cases on PF4/heparin IgG antibodies. Although some authors described rare cases of pathogenic anti-PF4/heparin IgM and IgA antibodies [Selleng et al. 2010b], the clinical relevance of these IgM and IgA antibodies is currently debated. One explanation for the association between anti-PF4/heparin IgM antibodies and adverse outcomes is that the IgM antibodies are a surrogate marker for comorbidities such as infections, which may also cause a predisposition for other complications postsurgery.
PF4/heparin IgM antibodies have also been found in the normal population [Hursting et al. 2010]. For these findings two explanations are currently debated. Either these antibodies are an artifact caused by background signals in the antigen assay, or heparin is not the sole inducer of PF4/heparin antibodies. While the clinical relevance of these non-heparin-induced antibodies is still unclear, HIT is likely an extreme form of this immune response. It is an intriguing hypothesis that (still unknown) cosignals associated with tissue trauma during surgery are stimulating the B-cell population to produce high-titer PF4/heparin IgG antibodies. This would nicely explain the higher prevalence of HIT in surgical patients compared with medical patients.
Clinical presentation of HIT
Patients with HIT can present with a wide spectrum of symptoms ranging from an isolated decrease in platelet counts, typically between days 5 and 14 after starting heparin, to life-threatening thromboembolic complications [Greinacher, 2006; Warkentin, 2003]. About half of the patients with acute HIT develop a new thrombotic complication [Warkentin et al. 1994b]. Lower extremity deep vein thrombosis (DVT) and pulmonary embolism are the predominant thrombotic manifestations, outnumbering arterial events by approximately 2:1 [Greinacher et al. 2005] with the exception of patients with arterial disease in whom there is a slight predisposition to arterial occlusions. HIT-associated thrombosis shows a propensity to occur at sites of vessel injury, e.g. in patients with central venous catheters, arterial lines, and other vascular interventions [Hong et al. 2003]. In about 25–30% of patients the thrombotic event precedes onset of thrombocytopenia by 1–3 days [Greinacher and Warkentin, 2006]. In these patients enhanced platelet consumption is likely compensated by increased platelet production. In retrospect, often a slight decrease in platelet counts can usually be seen in these patients at the time thrombosis occurred. Typically platelet counts then decrease rapidly when the heparin dose is increased to treat the thrombosis.
Other (rare) complications include skin necrosis at the heparin injection sites and adrenal hemorrhagic necrosis (most often seen in intensive care HIT patients, and caused by adrenal vein thrombosis) as well as occlusion of vascular grafts, fistulae, and extracorporeal circuits [Rosenberger et al. 2011; Schindewolf et al. 2010]. In contrast erythematous, nonnecrotizing lesions at the heparin injection site are usually delayed type IV reactions and not HIT related [Ludwig et al. 2006; Schindewolf et al. 2009].
Identifying the pretest risk of HIT
Substituting heparin with an alternative anticoagulant is expensive, and is associated with major bleeding in a relevant percentage of patients [Lubenow et al. 2006]. Therefore, the diagnosis of HIT must be established promptly but also carefully.
HIT usually features a platelet count fall >50% (from the highest value after start of heparin treatment). While a mean nadir between 50–80 × 109/l was most often found in larger patient series, in individual patients the platelet count values can range between 20 and 150 × 109/l, depending on the highest platelet count value after start of heparin. The platelet count fall is associated with a new thrombosis in about 50% of HIT patients. Both, the platelet count fall and the onset of thrombosis occurs typically 5–14 days after the start of heparin. The onset of HIT is independent from dosage, schedule, and route of administration of heparin [Castelli et al. 2007]. Patients with early-onset thrombocytopenia or a platelet count <20 × 109/l usually do not have HIT unless they present with superimposed disseminated intravasal coagulopathy (DIC). A platelet count fall within the first hours after the start of heparin (= rapid onset HIT) is sometimes observed in preimmunized patients who had received heparin within the past 30 days. According to these characteristic features, the probability for HIT as a cause for thrombocytopenia or new thrombosis can be evaluated using different pretest scoring systems.
The most thoroughly evaluated clinical scoring system is the 4T’s score (for Thrombocytopenia, Timing, Thrombosis and oThers). The 4T’s score allows us to estimate the pretest probability of HIT. It has a high negative predictive value, i.e. it is helpful in identifying patients who are unlikely to have HIT. In case of a 4T’s score below 4, the underlying cause for thrombocytopenia is most probably not HIT. At a higher 4T’s score, HIT should be considered as a potential cause of thrombocytopenia, but the positive predictive value of the score varies largely depending on the experience of the physician applying the score.
More recently, the HIT expert probability (HEP) score has been introduced. It relies on eight features, clinical and laboratory, to identify the risk of having HIT. In the first evaluation study, the HEP score had greater sensitivity and specificity compared to the 4T’s score, and more importantly, higher interobserver agreement [Cuker et al. 2010]. Another simple score to exclude HIT in patient receiving heparin was suggested by Messmore and colleagues [Messmore et al. 2011]. The system is designed to arrive at low (0) or possible (1) probability scores depending on the presence or absence of typical HIT manifestation without knowledge of laboratory test results (except platelet counts) to avoid delays. This scoring system seems to be efficiently capable of identifying patients who do not have HIT and more useful for non-HIT expert clinicians. Both of these novel scores require more validation in larger prospective studies before firm conclusion can be drawn.
In general, all systematic approaches to assess the likelihood of HIT are easier to apply for the nonexpert compared with clinical judgment, and including one of these scoring systems into the hospital’s standard operating procedures may reduce overdiagnosis of HIT.
In vitro demonstration of HIT antibodies
As HIT can often not be excluded on clinical grounds, laboratory testing for heparin-dependent antibodies is a mainstay of the diagnosis. Two different classes of assays are available: immunoassays, which detect binding of PF4/heparin antibodies and functional laboratory assays, which investigate the capability of these antibodies to activate platelet in the presence of heparin.
Detection of PF4/heparin antibodies
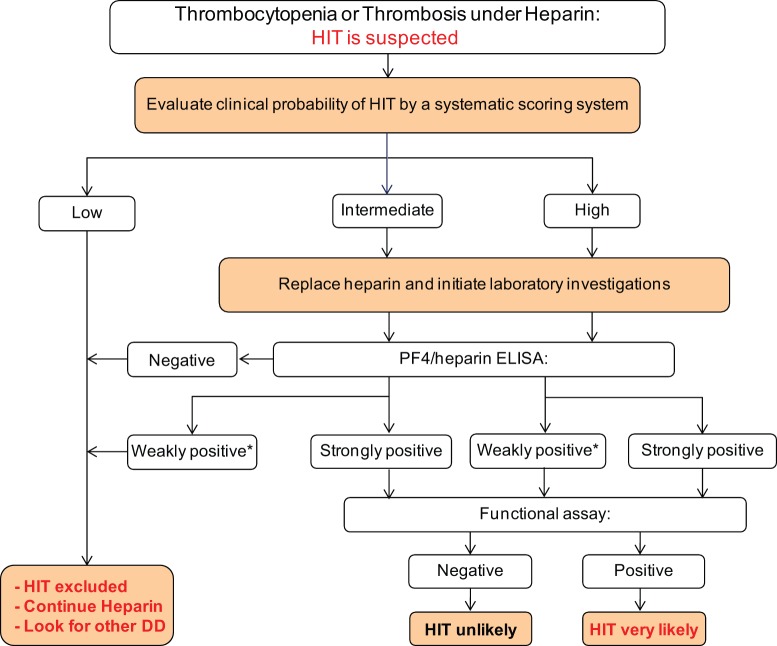
Immunoassays employ PF4/heparin or PF4/polyvinylsulfonate complexes immobilized to a microtiter plate or to another carrier as target antigens [Eichler et al. 2002; Meyer et al. 1999; Visentin et al. 2001]. Immunoassays are highly sensitive. Because of their excellent negative predictive values (NPVs), they are helpful in excluding HIT. However, these assays do not differentiate between pathogenic antibodies and clinically irrelevant antibodies. Immunoassays detecting IgG antibodies only have improved operating characteristics by increased diagnostic specificity for pathogenic antibodies [Bakchoul et al. 2009; Moore et al. 2008; Pouplard et al. 2010], however, still only ~50% of anti-PF4/heparin IgG antibodies are platelet activating. Another approach to increase test specificity is a confirmation step using heparin in high concentration [Warkentin et al. 2008c; Whitlatch et al. 2008, 2010]. While the high heparin step increases specificity and positive predictive value (PPV), it has to be applied with caution. It is reasonable to apply the inhibition step for low-titer antibodies (optical density [OD] <1.0), while some of the clinically most relevant high-titer antibodies with strong platelet activating capacity are not inhibited [Althaus et al. 2011; Bakchoul et al. 2011]. Furthermore, the magnitude of the OD values correlates with the likelihood of HIT determined by a scoring system and the capability of the antibodies to activate platelets [Warkentin et al. 2008b]. Both parameters, inhibition by high heparin and the magnitude of the OD value, should be included into interpretation of antigen assays (Figure 1). These measures may also contribute to reduce overdiagnosis of HIT.
Figure 1.
A suggested approach to diagnosis and initial management of patients with suspected HIT.This approach to the diagnosis and initial management of patients with suspected HIT is based on clinical assessment supported by complementary laboratory investigations. Results of Immunoassays can be devided into neagtive, weakly positive (OD < 1.0) and strong positive (OD>1.0). The decision whether weakly positive results need to be further verfied using functional assays or not depends on the clinical probabilty. As indeterminate results may be occasionally obtained using laboratory tests, re-evaluating the clinical probability of HIT in an individual patient may be helpful to overcome some diagnostic uncertainty.
Despite their wide availability, immunoassays care mostly tailored for testing batches rather than single patient samples. Thus, samples are often sent to referral laboratories or, in some settings, collected in the local laboratory for batch testing. This results in a delay in the availability of test results. Since alternative anticoagulants are costly and associated with a risk of major bleeding of about 1% per treatment day, the period between discontinuation of heparin and obtaining a reliable laboratory test result should be as short as possible.
Lateral flow point of care tests can improve the turnaround time for negative test results. In a recent study, the use of such an assay to detect HIT antibodies was evaluated. The test system is designed to investigate one patient sample and can be performed within less than 15 minutes without the need for special equipment. Interestingly, not only a high NPV but also a satisfactory specificity and PPV were observed by testing a large retrospective cohort of HIT patients [Sachs et al. 2011].
Detection of antibody capability to activate platelets
The functional assays, although not available commercially, are the gold standard in the laboratory diagnosis of HIT. They employ washed platelets and detect their activation by heparin-dependent antibodies by measuring platelet aggregation or release of serotonin [Greinacher et al. 1991; Warkentin et al. 1992]. Both assays have similar operating characteristics [Savi et al. 2005; Warkentin and Sheppard, 2006]. The specificity of the functional assays is increased by inhibition of platelet activation by adding heparin in excess (proving the heparin dependency), and showing Fcγ receptor IIA-dependent activation using a blocking monoclonal antibody (clone IV.3). As these assays are performed on microtiter plates, they allow inclusion of appropriate weak positive and appropriate negative controls.
In addition to PF4/heparin antibodies, also platelet activating, heparin-dependent antibodies to other chemokines such as interleukin-8 or neutrophil activating peptide 2 (NAP-2) are detected by the functional assays but not by the antigen assays [Bounameaux et al. 2007; Regnault et al. 2003].
Figure 1 shows a diagnostic algorithm showing the sequence of laboratory tests and their interpretation in patients suspected to have HIT (Figure 1).
Management of patients with suspected HIT
Diagnostic pathways
Given that HIT is an immune reaction enhanced by heparin, it is mandatory to discontinue all heparin when HIT is suspected. However, as outlined above, due to the partly autoimmune nature of HIT, discontinuation of heparin alone is not adequate. In fact, up to 50% of patients, who had no HIT-associated thrombosis at the time of diagnosis, developed a new thrombosis within a month after stopping heparin therapy if they did not receive an alternative anticoagulant [Lubenow et al. 2004; Warkentin et al. 1994a]. Patients with high clinical suspicion of HIT should be promptly treated with a nonheparin anticoagulant, while awaiting laboratory confirmation or exclusion of the diagnosis. However, also in patients with moderate clinical suspicion of HIT, diagnosis of HIT should be definitively confirmed or ruled out using sensitive and specific laboratory investigations. These patients are often readmitted to hospital due to their underlying disease. As the antibodies in HIT are transient and disappear within 100 days in the majority of patients, mentioning of suspected HIT in the patient file may prompt unnecessary use of nonheparin anticoagulants or even delay or withholding of treatment.
The following section addresses the different anticoagulants currently used to treat patients with HIT.
Activated factor X inhibitors
Danaparoid
Danaparoid, a heparinoid with predominantly antifactor Xa activity and some antifactor IIa activity, is a mixture of three glycosaminoglycans (heparan sulfate, dermatan sulfate, and chondroitin sulfate). Danaparoid is efficient in preventing new, progressive, or recurrent thromboembolic complications (including thrombotic death) or limb amputation in HIT [Lubenow et al. 2006]. It has a low cross-reactivity rate with HIT antibodies in vitro, and in vivo cross-reactivity is very rare. Danaparoid harbors the unique property of specific suppression of HIT antibody-induced platelet activation by replacing PF4/heparin complexes from the platelet surface [Chong et al. 1989] and by disrupting PF4/heparin complexes [Krauel et al. 2008]. For therapeutic dose anticoagulation, danaparoid is given i.v. as a bolus of 2250 units, followed by an infusion of 400 units/h for 4 h, 300 units/h for 4 h, and then 200 units/h. Dosage should be adjusted to maintain a plasma anti-Xa level between 0.5 and 0.8 anti-Xa units/ml (performed with a danaparoid standard curve). The drug is not available in the US, while it had been the most widely used drug for HIT in the EU and Australia for many years. Unfortunately, an ongoing worldwide shortage of danaparoid limits its use during the past 2 years.
Fondaparinux
Fondaparinux is a synthetic pentasaccharide with potent indirect anti-Xa inhibitor properties. The pharmacokinetics of fondaparinux depends strongly on renal function, and may accumulate in patients with renal impairment, making fondaparinux a problematic drug in this subset of patients.
Fondaparinux is very well suited in patients with a history of HIT, requiring anticoagulation for an underlying disorder for which fondaparinux is approved. Although fondaparinux has not been systematically investigated in acute HIT, several case series indicate that it is effective and reasonably safe in these patients [Goldfarb and Blostein, 2011; Warkentin et al. 2011b]. In a recent study, fondaparinux was compared with enoxaparin in nearly 4000 clinically and laboratory well-characterized patients with acute thrombosis among whom 14 were found to have heparin-dependent platelet activating antibodies at baseline. These antibodies presumably were caused by recent heparin exposure, without overt clinical manifestations of HIT. Ten of these patients were treated with fondaparinux and did not develop HIT. The remaining four, conversely, received LMWH or UFH and all developed HIT [Warkentin et al. 2011a]. This difference in the risk of developing adverse effects after the start of anticoagulation was highly significant (p < 0.001) despite the low numbers of patients.
These findings raised some uncertainty in the view of previous reports visualizing formation of multimolecular complexes of PF4 and fondaparinux [Greinacher et al. 2006]; studies showing that fondaparinux induces PF4/heparin antibodies as frequently as LMWH [Warkentin et al. 2005] and, most importantly, several cases of fondaparinux-associated HIT [Warkentin, 2010]. A potential explanation is the dissociation between immunogenicity and cross-reactivity. Considerations for this dissociation are: the number of multimolecular PF4/fondaparinux complexes is presumably rather low. This number may be sufficient to induce an immune response, but might be not sufficient to activate enough platelets to trigger the prothrombotic disorder. Alternatively, fondaparinux is an additional bystander, which together with other negatively charged polyanions, e.g. heparansulfate, allows close approximation of PF4 tetramers with the result of triggering an immune reaction.
In the few patients with fondaparinux induced HIT, potentially heparin independent antibodies, like in delayed-onset HIT had been present, which cross-react with PF4 bound to platelets and cause platelet activation even in the absence of any heparin [Greinacher, 2011].
Direct thrombin inhibitors
Argatroban
Argatroban is a synthetic direct thrombin inhibitor (DTI) derived from L-arginine that reversibly binds to the thrombin active site. Argatroban’s anticoagulant effects include prevention of fibrin formation; activation of coagulation factors V, VIII, and XIII; activation of protein C; and platelet aggregation. Argatroban is capable of inhibiting both free and clot-associated thrombin. Steady-state levels of anticoagulant effects are typically attained within 1–3 hours after start of i.v. infusion. In adult patients with acute HIT and no hepatic impairment, the recommended initial dose of argatroban is 2 μg/kg/min administered as a continuous infusion [Baron et al. 2008]. The activated partial thromboplastin time (aPTT) should be checked 2 hours after the initiation of therapy (or dosage adjustment), until the steady-state aPTT is 1.5–3.0 times the initial baseline value. While no initial dose adjustment for argatroban is required in patients with renal impairment, attention should be paid to patients with hepatic impairment or impairment of hepatic perfusion, in whom the initial dose should be reduced to 0.5 μg/kg/min with close aPTT monitoring. Since no specific antidote to argatroban is available, caution is advised during the use of argatroban in conditions or circumstances that increase the risk of hemorrhage, such as severe hypertension; immediately following lumbar puncture; spinal anesthesia; major surgery, especially involving the brain, spinal cord, or eye; and hematologic conditions associated with increased bleeding tendencies.
Argatroban dose-dependently increases the aPTT, the activated clotting time (ACT), the prothrombin time, and the international normalized ratio (INR). Therefore, when switching to vitamin K antagonists (VKAs), the potential for combined effects of argatroban and VKAs on the INR should be taken into consideration. The INR values should be measured daily while coadministering (bridging) these agents and argatroban can be discontinued when the INR is >4. The INR should be measured again 4–6 hours after discontinuation of argatroban, and if the repeat INR is below the therapeutic range, argatroban infusion may be resumed. This procedure should be repeated daily until the desired therapeutic INR is achieved with vitamin K antagonists alone.
A recent consensus statement summarizes detailed dosing recommendations for argatroban in various patient groups [Alatri et al. 2011].
Lepirudin and desirudin
Lepirudin and desirudin are recombinant hirudins, which are direct, specific, and irreversible inhibitors of thrombin. They can be given i.v. and s.c. Their biggest drawbacks are the strong dependency of pharmacokinetics from renal function and immunogenicity. Hirudins require strict laboratory monitoring, when given in therapeutic dose, especially in patients with impaired renal function, even when moderate. Anaphylactic reactions have been reported in patients re-exposed to this drug by an i.v. bolus [Carlsson et al. 2003]. Initial doses of lepirudin should be low (0.05 to 0.10 mg/kg/h), particularly when there is renal dysfunction. The initial i.v. bolus should be omitted unless there is a perceived life-threatening or limb-threatening thrombosis. Lepirudin, which is approved for treatment of HIT in many countries, will be no longer available beginning in spring 2012. However, desirudin (Canyon Pharmaceuticals) will still be available. Although not approved for HIT, its pharmacokinetics and mode of action are very similar to lepirudin and it might therefore be used as an alternative. In a small study, s.c. low-dose desirudin has been compared with argatroban in patients suspected to have HIT. While the number of patients is too small to draw any conclusions on efficacy, no increase in major bleeding complications was observed [Kennedy et al. 2011].
Outside Europe and North America the recombinant hirudin RB-hirudin variant (Rhein-Minapharm) is often the only alternative anticoagulant available and lessons learned from the use of lepirudin might be used to guide treatment with these drugs [Greinacher and Warkentin, 2008].
Bivalirudin
Bivalirudin is a synthetic peptide composed of two short hirudin peptide fragments. For the treatment of HIT, a reasonable dosage regimen is 0.15 mg/kg/h, with subsequent adjustments made to keep the aPTT between 1.5- and 2.5-fold higher than the baseline [Warkentin et al. 2008a]. In patients with renal impairment the starting dose should be reduced by 30–50% to avoid overdosage. Bivalirudin is the best investigated alternative anticoagulant in non-HIT patients with coronary disease, including acute coronary syndrome, requiring coronary intervention.
Partial thromboplastin time for monitoring of DTI therapy
The partial thromboplastin time (PTT), a global coagulation assay, is most often used for monitoring of DTI therapy. However, results obtained with the aPTT or ECT may be inaccurate in patients whose plasma has a reduced concentration of prothrombin (e.g. severe liver disease, disseminated intravascular coagulation [DIC], treatment with VKAs) or in patients with fibrinogen depletion (e.g. postthrombolysis, hemodilution during CPB) [Greinacher and Warkentin, 2008; Lindhoff-Last et al. 2000; de Denus and Spinier, 2002; Alatri et al. 2011]. In particular, in DIC this can result in supratherapeutic PTTs despite low plasma levels of the DTI. This often results in inappropriate DTI dose interruptions or reductions due to the in vitro artifact of PTT-prolonging effects of the combination of low prothrombin levels and DTI.
This PTT confounding was first recognized in patients with HIT who were already receiving warfarin. In these patients supratherapeutic PTT levels were reached in a short time after starting DTIs. When DTI therapy was then interrupted, this resulted in a rapid progression of microvascular thrombosis and multiple limb necrosis [Greinacher and Warkentin, 2008; Warkentin et al. 2006; Alatri et al. 2011]. Subsequently, similar courses were also seen in severe HIT-associated DIC [Socher et al. 2008].
Enzym immunoassay (EIAs) which measure the plasma concentration of r-hirudin independent of prothrombin concentration and the presence of a lupus anticoagulant heparin can overcome this problem for r-hirudins, while the chromogenic ecarin assay can also be used for the other DTIs [Socher et al. 2008]. Both assays are commercially available in Europe.
The new oral direct factor Xa and thrombin inhibitors
Rivaroxaban, apixaban, and dabigatran
Rivaroxaban and apixaban directly inhibit activated factor X, while dabigatran is a direct thrombin inhibitor [Bauer, 2011; DeLoughery, 2011; Hankey and Eikelboom, 2011; Turun et al. 2011]. All three drugs are given orally and have been shown to be effective anticoagulants in various indications such as thrombosis prophylaxis after major surgery, deep vein thrombosis, and atrial fibrillation. Owing to their structure they do not interact with PF4, which has been systematically assessed for rivaroxaban and dabigatran [Krauel et al. 2011a]. Once they are approved for certain indications, these drugs are ideal replacements for heparin in patients with a history of HIT. They may also be appropriate for treatment of patients with acute HIT based on theoretical considerations. However, the lack of a validated tool for monitoring of these new drugs may cause problems in the situation of acute HIT, which is so prothrombotic that aggressive treatment is required, while at the same time close monitoring is needed to avoid overdosing in these often severely ill patients.
Platelet transfusion in HIT
Although platelet transfusion has recently been shown to be ‘safe’ in four patients with clinically suspected HIT and a positive serotonin release assay (SRA) [Hopkins and Goldfinger, 2008], we recommend caution with platelet transfusions in patients with acute HIT. Thrombocytopenia in HIT patients is caused by intravascular platelet activation via anti-PF4/heparin antibodies. These antibodies together with PF4 and heparin circulate in the patient’s blood for hours after heparin administration has been stopped. In this situation transfusion of platelets might be dangerous. In fact, in the nonbleeding patient, platelet transfusions are usually not needed. This typically accounts for vascular surgery to remove an arterial clot in acute HIT. Even with platelet counts <50,000/µl, bleeding is usually not an issue in this situation and prophylactic platelet transfusion should be avoided.
Patients with symptomatic bleeding most likely do not have HIT, or bleeding is caused by an overdose of alternative anticoagulants. In these bleeding patients platelet transfusions might be considered [Refaai et al. 2010].
Glycoprotein IIb/IIIa inhibitors
Neither a direct anticoagulant effect nor inhibition of Fcγ receptor IIA-mediated platelet activation can be expected by the administration of GPIIb/IIIa inhibitors in HIT patients. Moreover, the combination of a GPIIb/IIIa inhibitor and new anticoagulants likely increased the bleeding risk [Cruz-Gonzalez et al. 2008]. GPIIb/IIIa inhibitors should not be used as a sole therapy for HIT but may be cautiously used in patients with acute HIT and acute coronary syndrome in combination with a nonheparin anticoagulant.
Subsequent management of HIT patients
Bridging to VKAs
In patients with acute HIT, VKAs can induce venous limb gangrene because of VKA-induced protein C depletion [Warkentin et al. 2008b]. Therefore, it is essential to postpone this therapy until the platelet count has recovered ideally to a stable plateau but at least to 150 × 109/l, since platelet recovery resembles a marker that HIT is under control. VKA start requires an overlap with an alternative anticoagulant for at least 5 days. Duration of alternative anticoagulation in patients in whom HIT was not complicated by thrombosis remains unclear. In the prospective lepirudin studies, there were no new thromboses after a mean treatment duration with lepirudin of 10 days or until the platelet count recovered [Lubenow et al. 2004]. Other experts recommend to anticoagulate these patients for 4 weeks, using VKAs after the platelet count has normalized [Arepally and Ortel, 2006].
Re-exposure to heparin
The immunology of HIT differs from classic immune reactions. Approximately 100 days after the onset of HIT, antibodies against PF4/heparin usually disappear from the patient’s circulation, which is much faster than observed for other drug-induced antibodies. Furthermore, in the case of re-exposure with heparin, an anamnestic response of antibody formation is very rare. This is consistent with the observation that these patients do not form typical memory B cells specific for PF4/heparin [Selleng et al. 2010a]. However, as HIT may reoccur by chance again during repeated use of heparin [Warkentin and Greinacher, 2004], patients with previous HIT should be treated with an alternative anticoagulant whenever possible.
The low likelihood for recurrence of HIT during re-exposure can be used to manage patients in clinical situations in which heparin has major advantages over other anticoagulants due to easy monitoring and the possibility of antagonization with protamine. Before re-exposure with heparin, absence of platelet activating antibodies should be documented by a sensitive functional test and heparin should be used only for a short period, e.g. during cardiac surgery and then replaced by an alternative anticoagulant. In the unlikely case of an anamnestic response, B cells require several days to produce sufficient quantities of PF4/heparin antibodies for platelet activation and if heparin is only given for several hours, it is no longer present in the circulation, when the boosted antibodies reach clinically relevant levels.
Cardiac surgery
In patients with acute HIT, cardiac surgery should be postponed, when possible until HIT-antibody tests become negative. Then the patient can be re-exposed to heparin during surgery. In the case of acute HIT with circulating activating antibodies (antibody still detectable by washed platelet activation assay and urgent cardiac surgery), an alternative anticoagulant is needed during cardiac surgery. Alternatively heparin plus the antiplatelet agent epoprostenol, or heparin plus the antiplatelet agent tirofiban may be used [Warkentin et al. 2008b]. There are anecdotal reports that patients, in whom a sensitive washed platelet assay became negative can also be re-exposed safely to heparin during cardiac surgery even if PF4/heparin antigen test is still positive [Selleng et al. 2008]. This situation typically occurs in patients with a cardiac assist device awaiting cardiac transplant and re-exposure to heparin can then facilitate heart transplantation.
Hemodialysis
Antibodies against PF4/heparin are not a rare finding in patients on chronic renal replacement therapy [Hutchison and Dasgupta, 2007]. However, these antibodies are usually clinically irrelevant [Asmis et al. 2008; Carrier et al. 2007]. Nevertheless, HIT is an issue during initiation of chronic dialysis. HIT should be suspected when thrombocytopenia and/or clot formation occur during the first 2–3 weeks after start of hemodialysis and during the first 2 weeks when patients on chronic dialysis undergo major surgery [Tholl et al. 1997]. Major surgery seems to ‘reset the clock’ for the susceptibility for HIT in dialysis patients.
In patients with confirmed HIT, heparin re-exposure should then be avoided when possible as long as HIT antibodies are detectable in the immunoassay. After PF4/heparin antibodies have disappeared, some small observational studies indicate that hemodialysis can again be performed safely with heparin [Matsuo et al. 2003, 2007; Matsuo and Wanaka, 2008; Wanaka et al. 2010; Warkentin and Kelton, 2001b]. If these preliminary findings will be confirmed by larger studies, this would greatly facilitate management of patients on chronic renal replacement therapy and a history of HIT.
Future perspective
While the incidence of HIT in uncomplicated patients will likely further decline due to the increasing use of alternative, nonheparin anticoagulants, HIT will remain an issue in patients after cardiac surgery and in severely sick patients requiring therapeutic dose anticoagulation. In both situations unfractionated heparin has major advantages over all other anticoagulants as it can be easily monitored and antagonized. As thrombocytopenia is rather frequent in these patient populations, the combination of clinical assessment of the likelihood of HIT together with rapidly available assays with a high negative predictive value will help to reduce overtreatment of suspected HIT. However, until assays with a higher positive predictive value for relevant PF4/heparin antibodies become available, overdiagnosis of HIT will not only remain a major issue for patient safety but also a major cost burden.
In addition to its direct clinical relevance, HIT is a fascinating model for studying mechanisms of the immune system in humans. The wide use of heparin and the frequent occurrence of anti-PF4/heparin antibodies provide a relatively unique chance to study the underlying immune mechanisms in humans rather than in mice. This is especially interesting for the immune response in HIT, as this immune reaction is somewhat atypical. Even high-titer IgG antibodies decline within 100 days to levels below detectability and there is only rarely an anamnestic response after re-exposure. The occurrence of anti-PF4/polyanion antibodies after bacterial infection hints towards a potential network between the platelet-derived chemokine PF4 and antibodies in bacterial host defense. Very recently another plasma protein involved in a procoagulatory autoimmune disorder, beta2 glycoprotein I, has also been shown to bind to bacteria and to interact with lipopolysaccharide (LPS) [Ağar et al. 2011; Van Os et al. 2011]. Potentially PF4 and beta 2 glycoprotein I are two examples of an evolutionary old pathogen defense system using endogenous proteins to label pathogens. The intriguing aspect is that one antibody class, e.g. anti-PF4/polyanion antibodies can recognize a wide variety of pathogens [Greinacher, 2010]. Solving the mechanisms which lead to a loss of self-tolerance in those patients, in whom the antibodies then become autoantibodies, activating platelets even in the absence of heparin, may provide important insights into mechanisms of autoimmunity. It is already known that there is no clear HLA-dependent disposition for HIT [Greinacher and Mueller-Eckhardt, 1993].
Accumulating evidence suggests an important role of monocytes in the pathogenesis of HIT. Potentially monocytes also play an important role in other autoimmune disorders and HIT might be a model to further investigate this. In the same context, the role of IgA and IgM antibodies in the pathogenesis of HIT is unresolved. Antibodies against PF4/heparin of the IgM class were found to be associated with nonthromboembolic complications and length of stay of patients undergoing cardiac surgery. Potentially these effects are mediated via monocytes rather than platelets.
Conclusion
The use of heparin places patients at increased risk for developing HIT. HIT is a prothrombotic syndrome initiated by platelet-activating antibodies that recognize PF4/heparin complexes [Greinacher, 2006]. Once the diagnosis of HIT is suspected, discontinuation of heparin and treatment with an alternative anticoagulant are mandatory. The diagnosis of HIT is based on clinical criteria and confirmed by in vitro demonstration of PF4/heparin antibodies using functional and immunological methods [Warkentin and Greinacher, 2004]. Currently antigen assays for PF4/heparin antibodies are commonly used in routine laboratories, if combined with a scoring system they can be used to guide management of patients until the results of the functional assay are available (Figure 1). Although functional assays represent the gold standard in the serological diagnosis of HIT, they are technically challenging and in many countries not readily available [Warkentin and Sheppard, 2006]. In this regard Germany and France are examples showing that it is feasible to establish a network of laboratories in a country providing access to these functional assays on a 24 h turnaround basis (5/7 days per week) for all hospitals.
Since HIT is a prothrombotic disorder, an effective alternative anticoagulant is essentially required in the management of HIT beside cessation heparin treatment. However, a considerable bleeding risk is associated with the alternative non-heparin anticoagulants. While two of the approved alternative anticoagulants, lepirudin and danaparoid are either retrieved from the market or face supply problems, fondaparinux and bivalirudin, and potentially also new drugs such as dabigatran, rivaroxaban, or apixaban provide new options to treat patients with HIT.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: T. Bakchoul has no conflict of interest to declare. A. Greinacher has received consultant fees, honoraria for lectures, and research support from companies whose products are mentioned in this review. Drugs for treatment of HIT: Danaparoid (MSD); Argatroban (Mutsubishi Pharma); Rivaroxaban (Bayer); Dabigatran (Boehringer Ingelheim); Fondaparinux (GSK). Assays for detection of PF4/heparin antibodies: GTI; Biotest, Instrumentation Laboratory
References
- Ağar Ç., de Groot P., Marquart J.A., Meijers J.C. (2011) Evolutionary conservation of the lipopolysaccharide binding site of β2-glycoprotein I. Thromb Haemost 106: 1069-1075 [DOI] [PubMed] [Google Scholar]
- Alatri A., Armstrong A.E., Greinacher A., Koster A., Kozek-Langenecker S.A., Lance M.D., et al. (2011) Results of a consensus meeting on the use of argatroban in patients with heparin-induced thrombocytopenia requiring antithrombotic therapy - An European Perspective. Thromb Res, in press, http://www.ncbi.nlm.nih.gov/pubmed/22178575 [DOI] [PubMed]
- Althaus K., Strobel U., Warkentin T.E., Greinacher A. (2011) Combined use of the high heparin step and optical density to optimize diagnostic sensitivity and specificity of an anti-PF4/heparin enzyme-immunoassay. Thromb Res 128: 256–260 [DOI] [PubMed] [Google Scholar]
- Amiral J., Bridey F., Dreyfus M., Vissoc A.M., Fressinaud E., Wolf M., et al. (1992) Platelet factor 4 complexed to heparin is the target for antibodies generated in heparin-induced thrombocytopenia. Thromb Haemost 68: 95–96 [PubMed] [Google Scholar]
- Amiral J., Bridey F., Wolf M., Boyer-Neumann C., Fressinaud E., Vissac A.M., et al. (1995) Antibodies to macromolecular platelet factor 4-heparin complexes in heparin-induced thrombocytopenia: a study of 44 cases. Thromb Haemost 73: 21–28 [PubMed] [Google Scholar]
- Arepally G.M., Ortel T.L. (2006) Clinical practice. Heparin-induced thrombocytopenia. N Engl J Med 355: 809–817 [DOI] [PubMed] [Google Scholar]
- Asmis L.M., Segal J.B., Plantinga L.C., Fink N.E., Kerman J.S., Kickler T.S., et al. (2008) Heparin-induced antibodies and cardiovascular risk in patients on dialysis. Thromb Haemost 100: 498–504 [PubMed] [Google Scholar]
- Bakchoul T., Giptner A., Bein G., Santoso S., Sachs U.J. (2011) Performance characteristics of two commercially available IgG-specific immunoassays in the assessment of heparin-induced thrombocytopenia (HIT). Thromb Res 127: 345–348 [DOI] [PubMed] [Google Scholar]
- Bakchoul T., Giptner A., Najaoui A., Bein G., Santoso S., Sachs U.J. (2009) Prospective evaluation of PF4/heparin immunoassays for the diagnosis of heparin-induced thrombocytopenia. J Thromb Haemost 7: 1260–1265 [DOI] [PubMed] [Google Scholar]
- Baron S.J., Yeh R.W., Cruz-Gonzalez I., Healy J.L., Pomerantsev E., Garasic J., et al. (2008) Efficacy and safety of argatroban in patients with heparin induced thrombocytopenia undergoing endovascular intervention for peripheral arterial disease. Catheter Cardiovasc Interv 72: 116–120 [DOI] [PubMed] [Google Scholar]
- Bauer K.A. (2011) Recent progress in anticoagulant therapy: oral direct inhibitors of thrombin and factor Xa. J Thromb Haemost 9(Suppl. 1): 12–19 [DOI] [PubMed] [Google Scholar]
- Bounameaux C., Boehlen F., Membre A., Genne D., Pouplard C., Regnault V., et al. (2007) Heparin-induced thrombocytopenia associated with interleukin-8-dependent platelet activation in a patient with antiphospholipid syndrome. Eur J Haematol 79: 550–553 [DOI] [PubMed] [Google Scholar]
- Carlsson L.E., Lubenow N., Blumentritt C., Kempf R., Papenberg S., Schroder W., et al. (2003) Platelet receptor and clotting factor polymorphisms as genetic risk factors for thromboembolic complications in heparin-induced thrombocytopenia. Pharmacogenetics 13: 253–258 [DOI] [PubMed] [Google Scholar]
- Carrier M., Knoll G.A., Kovacs M.J., Moore J.C., Fergusson D., Rodger M.A. (2007) The prevalence of antibodies to the platelet factor 4 -heparin complex and association with access thrombosis in patients on chronic hemodialysis. Thromb Res 120: 215–220 [DOI] [PubMed] [Google Scholar]
- Castelli R., Cassinerio E., Cappellini M.D., Porro F., Graziadei G., Fabris F. (2007) Heparin induced thrombocytopenia: pathogenetic, clinical, diagnostic and therapeutic aspects. Cardiovasc Hematol Disord Drug Targets 7: 153–162 [DOI] [PubMed] [Google Scholar]
- Chong B.H., Ismail F., Cade J., Gallus A.S., Gordon S., Chesterman C.N. (1989) Heparin-induced thrombocytopenia: studies with a new low molecular weight heparinoid, Org 10172. Blood 73: 1592–1596 [PubMed] [Google Scholar]
- Cruz-Gonzalez I., Sanchez-Ledesma M., Baron S.J., Healy J.L., Watanabe H., Osakabe M., et al. (2008) Efficacy and safety of argatroban with or without glycoprotein IIb/IIIa inhibitor in patients with heparin induced thrombocytopenia undergoing percutaneous coronary intervention for acute coronary syndrome. J Thromb Thrombolysis 25: 214–218 [DOI] [PubMed] [Google Scholar]
- Cuker A., Arepally G., Crowther M.A., Rice L., Datko F., Hook K., et al. (2010) The HIT Expert Probability (HEP) Score: a novel pre-test probability model for heparin-induced thrombocytopenia based on broad expert opinion. J Thromb Haemost 8: 2642–2650 [DOI] [PubMed] [Google Scholar]
- de Denus S., Spinler S. A. (2002) Clinical monitoring of direct thrombin inhibitors using the ecarin clotting time. Pharmacotherapy 22: 433–435 [DOI] [PubMed] [Google Scholar]
- DeLoughery T.G. (2011) Practical aspects of the oral new anticoagulants. Am J Hematol 86: 586–590 [DOI] [PubMed] [Google Scholar]
- Eichler P., Raschke R., Lubenow N., Meyer O., Schwind P., Greinacher A. (2002) The new ID-heparin/PF4 antibody test for rapid detection of heparin-induced antibodies in comparison with functional and antigenic assays. Br J Haematol 116: 887–891 [DOI] [PubMed] [Google Scholar]
- Goldfarb M.J., Blostein M.D. (2011) Fondaparinux in acute heparin-induced thrombocytopenia: a case series. J Thromb Haemost 9: 2501–2503 [DOI] [PubMed] [Google Scholar]
- Greinacher A. (2006) Heparin-induced thrombocytopenia: frequency and pathogenesis. Pathophysiol Haemost Thromb 35: 37–45 [DOI] [PubMed] [Google Scholar]
- Greinacher A. (2010) Opposites attract. Blood 115: 440–441 [DOI] [PubMed] [Google Scholar]
- Greinacher A. (2011) Immunogenic but effective: the HIT-fondaparinux brain puzzler. J Thromb Haemost 9: 2386–2388 [DOI] [PubMed] [Google Scholar]
- Greinacher A., Alban S., Omer-Adam M.A., Weitschies W., Warkentin T.E. (2008) Heparin-induced thrombocytopenia: a stoichiometry-based model to explain the differing immunogenicities of unfractionated heparin, low-molecular-weight heparin, and fondaparinux in different clinical settings. Thromb Res 122: 211–220 [DOI] [PubMed] [Google Scholar]
- Greinacher A., Farner B., Kroll H., Kohlmann T., Warkentin T.E., Eichler P. (2005) Clinical features of heparin-induced thrombocytopenia including risk factors for thrombosis. A retrospective analysis of 408 patients. Thromb Haemost 94: 132–135 [DOI] [PubMed] [Google Scholar]
- Greinacher A., Gopinadhan M., Gunther J.U., Omer-Adam M.A., Strobel U., Warkentin T.E., et al. (2006) Close approximation of two platelet factor 4 tetramers by charge neutralization forms the antigens recognized by HIT antibodies. Arterioscler Thromb Vasc Biol 26: 2386–2393 [DOI] [PubMed] [Google Scholar]
- Greinacher A., Michels I., Kiefel V., Mueller-Eckhardt C. (1991) A rapid and sensitive test for diagnosing heparin-associated thrombocytopenia. Thromb Haemost 66: 734–736 [PubMed] [Google Scholar]
- Greinacher A., Mueller-Eckhardt G. (1993) Heparin-associated thrombocytopenia: no association of immune response with HLA. Vox Sang 65: 151–153 [DOI] [PubMed] [Google Scholar]
- Greinacher A., Potzsch B., Amiral J., Dummel V., Eichner A., Mueller-Eckhardt C. (1994) Heparin-associated thrombocytopenia: isolation of the antibody and characterization of a multimolecular PF4-heparin complex as the major antigen. Thromb Haemost 71: 247–251 [PubMed] [Google Scholar]
- Greinacher A., Warkentin T.E. (2006) Recognition, treatment, and prevention of heparin-induced thrombocytopenia: review and update. Thromb Res 118: 165–176 [DOI] [PubMed] [Google Scholar]
- Greinacher A., Warkentin T.E. (2008) The direct thrombin inhibitor hirudin. Thromb Haemost 99: 819–829 [DOI] [PubMed] [Google Scholar]
- Hankey G.J., Eikelboom J.W. (2011) Dabigatran etexilate: a new oral thrombin inhibitor. Circulation 123: 1436–1450 [DOI] [PubMed] [Google Scholar]
- Hong A.P., Cook D.J., Sigouin C.S., Warkentin T.E. (2003) Central venous catheters and upper-extremity deep-vein thrombosis complicating immune heparin-induced thrombocytopenia. Blood 101: 3049–3051 [DOI] [PubMed] [Google Scholar]
- Hopkins C.K., Goldfinger D. (2008) Platelet transfusions in heparin-induced thrombocytopenia: a report of four cases and review of the literature. Transfusion 48: 2128–2132 [DOI] [PubMed] [Google Scholar]
- Hursting M.J., Pai P.J., McCracken J.E., Hwang F., Suvarna S., Arepally G.M., et al. (2010) Platelet factor 4/heparin antibodies in blood bank donors. Am J Clin Pathol 134: 774–780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison C.A., Dasgupta I. (2007) National survey of heparin-induced thrombocytopenia in the haemodialysis population of the UK population. Nephrol Dial Transplant 22: 1680–1684 [DOI] [PubMed] [Google Scholar]
- Kelton J.G., Warkentin T.E. (1996) Heparin-induced thrombocytopenia: what the serologists have taught us. J Lab Clin Med 128: 346–348 [DOI] [PubMed] [Google Scholar]
- Kennedy K., Steinke D., King S., Poe K., Reeves J. (2011) Evaluation of a standardized protocol using lepirudin or argatroban for heparin-induced thrombocytopenia. Cardiovasc Hematol Agents Med Chem, in press, http://www.ncbi.nlm.nih.gov/pubmed/21902659 [DOI] [PubMed]
- Krauel K., Hackbarth C., Furll B., Greinacher A. (2011a) Heparin-induced thrombocytopenia: in vitro studies on the interaction of dabigatran, rivaroxaban, and low-sulfated heparin, with platelet factor 4 and anti-PF4/heparin antibodies. Blood, in press, http://www.ncbi.nlm.nih.gov/pubmed/22049520 [DOI] [PubMed]
- Krauel K., Potschke C., Weber C., Kessler W., Furll B., Ittermann T., et al. (2011b) Platelet factor 4 binds to bacteria, [corrected] inducing antibodies cross-reacting with the major antigen in heparin-induced thrombocytopenia. Blood 117: 1370–1378 [DOI] [PubMed] [Google Scholar]
- Lindhoff-Last E., Piechottka G. P., Rabe F., Bauersachs R. (2000) Hirudin determination in plasma can be strongly influenced by the prothrombin level. Thromb Res 100: 55–60 [DOI] [PubMed] [Google Scholar]
- Lo G.K., Juhl D., Warkentin T.E., Sigouin C.S., Eichler P., Greinacher A. (2006) Evaluation of pretest clinical score (4 T’s) for the diagnosis of heparin-induced thrombocytopenia in two clinical settings. J Thromb Haemost 4: 759–765 [DOI] [PubMed] [Google Scholar]
- Lubenow N., Eichler P., Lietz T., Farner B., Greinacher A. (2004) Lepirudin for prophylaxis of thrombosis in patients with acute isolated heparin-induced thrombocytopenia: an analysis of 3 prospective studies. Blood 104: 3072–3077 [DOI] [PubMed] [Google Scholar]
- Lubenow N., Hinz P., Thomaschewski S., Lietz T., Vogler M., Ladwig A., et al. (2010) The severity of trauma determines the immune response to PF4/heparin and the frequency of heparin-induced thrombocytopenia. Blood 115: 1797–1803 [DOI] [PubMed] [Google Scholar]
- Lubenow N., Warkentin T.E., Greinacher A., Wessel A., Sloane D.A., Krahn E.L., et al. (2006) Results of a systematic evaluation of treatment outcomes for heparin-induced thrombocytopenia in patients receiving danaparoid, ancrod, and/or coumarin explain the rapid shift in clinical practice during the 1990s. Thromb Res 117: 507–515 [DOI] [PubMed] [Google Scholar]
- Ludwig R.J., Schindewolf M., Utikal J., Lindhoff-Last E., Boehncke W.H. (2006) Management of cutaneous type IV hypersensitivity reactions induced by heparin. Thromb Haemost 96: 611–617 [PubMed] [Google Scholar]
- Matsuo T., Kusano H., Wanaka K., Ishihara M., Oyama A. (2007) Heparin-induced thrombocytopenia in a uremic patient requiring hemodialysis: an alternative treatment and reexposure to heparin. Clin Appl Thromb Hemost 13: 182–187 [DOI] [PubMed] [Google Scholar]
- Matsuo T., Matuo M., Wanaka K., Sakai R. (2003) Heparin re-exposure after heparin-induced thrombocytopenia in a chronic hemodialysis patient. Clin Lab Haematol 25: 333–334 [DOI] [PubMed] [Google Scholar]
- Matsuo T., Wanaka K. (2008) Management of uremic patients with heparin-induced thrombocytopenia requiring hemodialysis. Clin Appl Thromb Hemost 14: 459–464 [DOI] [PubMed] [Google Scholar]
- Messmore H.L., Fabbrini N., Bird M.L., Choudhury A.M., Cerejo M., Prechel M., et al. (2011) Simple scoring system for early management of heparin-induced thrombocytopenia. Clin Appl Thromb Hemost 17: 197–201 [DOI] [PubMed] [Google Scholar]
- Meyer O., Salama A., Pittet N., Schwind P. (1999) Rapid detection of heparin-induced platelet antibodies with particle gel immunoassay (ID-HPF4). Lancet 354: 1525–1526 [DOI] [PubMed] [Google Scholar]
- Moore J.C., Arnold D.M., Warkentin T.E., Warkentin A.E., Kelton J.G. (2008) An algorithm for resolving ‘indeterminate’ test results in the platelet serotonin release assay for investigation of heparin-induced thrombocytopenia. J Thromb Haemost 6: 1595–1597 [DOI] [PubMed] [Google Scholar]
- Pouplard C., Leroux D., Regina S., Rollin J., Gruel Y. (2010) Effectiveness of a new immunoassay for the diagnosis of heparin-induced thrombocytopenia and improved specificity when detecting IgG antibodies. Thromb Haemost 103: 145–150 [DOI] [PubMed] [Google Scholar]
- Prandoni P., Siragusa S., Girolami B., Fabris F., Group B.I. (2005a) The incidence of heparin-induced thrombocytopenia in medical patients treated with low-molecular-weight heparin: a prospective cohort study. Blood 106: 3049–3054 [DOI] [PubMed] [Google Scholar]
- Prandoni P., Tormene D., Pesavento R. and Vesalio Investigators Group (2005b) High vs. low doses of low-molecular-weight heparin for the treatment of superficial vein thrombosis of the legs: a double-blind, randomized trial. J Thromb Haemost 3: 1152–1157 [DOI] [PubMed] [Google Scholar]
- Refaai M.A., Chuang C., Menegus M., Blumberg N., Francis C.W. (2010) Outcomes after platelet transfusion in patients with heparin-induced thrombocytopenia. J Thromb Haemost 8: 1419–1421 [DOI] [PubMed] [Google Scholar]
- Regnault V., de Maistre E., Carteaux J.P., Gruel Y., Nguyen P., Tardy B., et al. (2003) Platelet activation induced by human antibodies to interleukin-8. Blood 101: 1419–1421 [DOI] [PubMed] [Google Scholar]
- Rosenberger L.H., Smith P.W., Sawyer R.G., Hanks J.B., Adams R.B., Hedrick T.L. (2011) Bilateral adrenal hemorrhage: the unrecognized cause of hemodynamic collapse associated with heparin-induced thrombocytopenia. Crit Care Med 39: 833–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs U.J., Sachs U.J., von Hesberg J., Santoso S., Bein G., Bakchoul T. (2011) Evaluation of a new nanoparticle-based lateral-flow immunoassay for the exclusion of heparin-induced thrombocytopenia (HIT). Thromb Haemost 106: 1197–1202 [DOI] [PubMed] [Google Scholar]
- Savi P., Chong B.H., Greinacher A., Gruel Y., Kelton J.G., Warkentin T.E., et al. (2005) Effect of fondaparinux on platelet activation in the presence of heparin-dependent antibodies: a blinded comparative multicenter study with unfractionated heparin. Blood 105: 139–144 [DOI] [PubMed] [Google Scholar]
- Schindewolf M., Kroll H., Ackermann H., Garbaraviciene J., Kaufmann R., Boehncke W.H., et al. (2010) Heparin-induced non-necrotizing skin lesions: rarely associated with heparin-induced thrombocytopenia. J Thromb Haemost 8: 1486–1491 [DOI] [PubMed] [Google Scholar]
- Schindewolf M., Schwaner S., Wolter M., Kroll H., Recke A., Kaufmann R., et al. (2009) Incidence and causes of heparin-induced skin lesions. CMAJ 181: 477–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selleng K., Schutt A., Selleng S., Warkentin T.E., Greinacher A. (2010a) Studies of the anti-platelet factor 4/heparin immune response: adapting the enzyme-linked immunosorbent spot assay for detection of memory B cells against complex antigens. Transfusion 50: 32–39 [DOI] [PubMed] [Google Scholar]
- Selleng S., Haneya A., Hirt S., Selleng K., Schmid C., Greinacher A. (2008) Management of anticoagulation in patients with subacute heparin-induced thrombocytopenia scheduled for heart transplantation. Blood 112: 4024–4027 [DOI] [PubMed] [Google Scholar]
- Selleng S., Malowsky B., Itterman T., Bagemuhl J., Wessel A., Wollert H.G., et al. (2010b) Incidence and clinical relevance of anti-platelet factor 4/heparin antibodies before cardiac surgery. Am Heart J 160: 362–369 [DOI] [PubMed] [Google Scholar]
- Socher I., Kroll H., Jorks S., Santoso S., Sachs U.J. (2008) Heparin-independent activation of platelets by heparin-induced thrombocytopenia antibodies: a common occurrence. J Thromb Haemost 6: 197–200 [DOI] [PubMed] [Google Scholar]
- Tholl U., Greinacher A., Overdick K., Anlauf M. (1997) Life-threatening anaphylactic reaction following parathyroidectomy in a dialysis patient with heparin-induced thrombocytopenia. Nephrol Dial Transplant 12: 2750–2755 [DOI] [PubMed] [Google Scholar]
- Turun S., Banghua L., Yuan Y., Zhenhui L., Ying N., Jin C. (2011) A systematic review of rivaroxaban versus enoxaparin in the prevention of venous thromboembolism after hip or knee replacement. Thromb Res 127: 525–534 [DOI] [PubMed] [Google Scholar]
- Van Os G.M., Meijers J.C., Agar Ç., Seron M.V., Marquart J.A., Akesson P., et al. (2011) Induction of anti-β(2)-glycoprotein I autoantibodies in mice by protein H of Streptococcus pyogenes . J Thromb Haemost 9: 2447–2456 [DOI] [PubMed] [Google Scholar]
- Visentin G.P., Moghaddam M., Beery S.E., McFarland J.G., Aster R.H. (2001) Heparin is not required for detection of antibodies associated with heparin-induced thrombocytopenia/thrombosis. J Lab Clin Med 138: 22–31 [DOI] [PubMed] [Google Scholar]
- Wanaka K., Matsuo T., Matsuo M., Kaneko C., Miyashita K., Asada R., et al. (2010) Re-exposure to heparin in uremic patients requiring hemodialysis with heparin-induced thrombocytopenia. J Thromb Haemost 8: 616–618 [DOI] [PubMed] [Google Scholar]
- Warkentin T.E. (2003) Heparin-induced thrombocytopenia: pathogenesis and management. Br J Haematol 121: 535–555 [DOI] [PubMed] [Google Scholar]
- Warkentin T.E. (2010) Fondaparinux: does it cause HIT? Can it treat HIT? Expert Rev Hematol 3: 567–581 [DOI] [PubMed] [Google Scholar]
- Warkentin T.E., Cook R.J., Marder V.J., Sheppard J.A., Moore J.C., Eriksson B.I., et al. (2005) Anti-platelet factor 4/heparin antibodies in orthopedic surgery patients receiving antithrombotic prophylaxis with fondaparinux or enoxaparin. Blood 106: 3791–3796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warkentin T.E., Davidson B.L., Buller H.R., Gallus A., Gent M., Lensing A.W., et al. (2011a) Prevalence and risk of preexisting heparin-induced thrombocytopenia antibodies in patients with acute VTE. Chest 140: 366–373 [DOI] [PubMed] [Google Scholar]
- Warkentin T.E., Greinacher A. (2004) Heparin-induced thrombocytopenia: recognition, treatment, and prevention: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 126(3 Suppl.): 311S-337S. [DOI] [PubMed] [Google Scholar]
- Warkentin T.E., Greinacher A., Koster A. (2008a) Bivalirudin. Thromb Haemost 99: 830–839 [DOI] [PubMed] [Google Scholar]
- Warkentin T.E., Greinacher A., Koster A., Lincoff A.M. (2008b) Treatment and prevention of heparin-induced thrombocytopenia: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 133(6 Suppl.): 340S-380S [DOI] [PubMed] [Google Scholar]
- Warkentin T.E., Hayward C.P., Boshkov L.K., Santos A.V., Sheppard J.A., Bode A.P., et al. (1994a) Sera from patients with heparin-induced thrombocytopenia generate platelet-derived microparticles with procoagulant activity: an explanation for the thrombotic complications of heparin-induced thrombocytopenia. Blood 84: 3691–3699 [PubMed] [Google Scholar]
- Warkentin T.E., Hayward C.P., Smith C.A., Kelly P.M., Kelton J.G. (1992) Determinants of donor platelet variability when testing for heparin-induced thrombocytopenia. J Lab Clin Med 120: 371–379 [PubMed] [Google Scholar]
- Warkentin T.E., Hirte H.W., Anderson D.R., Wilson W.E., O’Connell G.J., Lo R.C. (1994b) Transient global amnesia associated with acute heparin-induced thrombocytopenia. Am J Med 97: 489–491 [DOI] [PubMed] [Google Scholar]
- Warkentin T.E., Kelton J.G. (2001a) Delayed-onset heparin-induced thrombocytopenia and thrombosis. Ann Intern Med 135: 502–506 [DOI] [PubMed] [Google Scholar]
- Warkentin T.E., Kelton J.G. (2001b) Temporal aspects of heparin-induced thrombocytopenia. N Engl J Med 344: 1286–1292 [DOI] [PubMed] [Google Scholar]
- Warkentin T.E., Pai M., Sheppard J.I., Schulman S., Spyropoulos A.C., Eikelboom J.W. (2011b) Fondaparinux treatment of acute heparin-induced thrombocytopenia confirmed by the serotonin-release assay: a 30-month, 16-patient case series. J Thromb Haemost 9: 2389–2396 [DOI] [PubMed] [Google Scholar]
- Warkentin T.E., Sheppard J.A. (2006) Testing for heparin-induced thrombocytopenia antibodies. Transfus Med Rev 20: 259–272 [DOI] [PubMed] [Google Scholar]
- Warkentin T.E., Sheppard J.A., Sigouin C.S., Kohlmann T., Eichler P., Greinacher A. (2006) Gender imbalance and risk factor interactions in heparin-induced thrombocytopenia. Blood 108: 2937–2941 [DOI] [PubMed] [Google Scholar]
- Warkentin T.E., Sheppard J.I., Moore J.C., Sigouin C.S., Kelton J.G. (2008c) Quantitative interpretation of optical density measurements using PF4-dependent enzyme-immunoassays. J Thromb Haemost 6: 1304–1312 [DOI] [PubMed] [Google Scholar]
- Whitlatch N.L., Kong D.F., Metjian A.D., Arepally G.M., Ortel T.L. (2010) Validation of the high-dose heparin confirmatory step for the diagnosis of heparin-induced thrombocytopenia. Blood 116: 1761–1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlatch N.L., Perry S.L., Ortel T.L. (2008) Anti-heparin/platelet factor 4 antibody optical density values and the confirmatory procedure in the diagnosis of heparin-induced thrombocytopenia. Thromb Haemost 100: 678–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R.T., Damaraju L.V., Mascelli M.A., Barnathan E.S., Califf R.M., Simoons M.L., et al. (2003) Anti-platelet factor 4/heparin antibodies: an independent predictor of 30-day myocardial infarction after acute coronary ischemic syndromes. Circulation 107: 2307–2312 [DOI] [PubMed] [Google Scholar]
- Ziporen L., Li Z.Q., Park K.S., Sabnekar P., Liu W.Y., Arepally G., et al. (1998) Defining an antigenic epitope on platelet factor 4 associated with heparin-induced thrombocytopenia. Blood 92: 3250–3259 [PubMed] [Google Scholar]