Abstract
The hippocampus receives two streams of information, spatial and nonspatial, via major afferent inputs from the medial (MEC) and lateral entorhinal cortexes (LEC). The MEC and LEC projections in the temporoammonic pathway are topographically organized along the transverse-axis of area CA1. The potential for functional segregation of area CA1, however, remains relatively unexplored. Here, we demonstrated differential novelty-induced c-Fos expression along the transverse-axis of area CA1 corresponding to topographic projections of MEC and LEC inputs. We found that, while novel place exposure induced a uniform c-Fos expression along the transverse-axis of area CA1, novel object exposure primarily activated the distal half of CA1 neurons. In hippocampal slices, we observed distinct presynaptic properties between LEC and MEC terminals, and application of either DA or NE produced a largely selective influence on one set of inputs (LEC). Finally, we demonstrated that differential c-Fos expression along the transverse axis of area CA1 was largely abolished by an antagonist of neuromodulatory receptors, clozapine. Our results suggest that neuromodulators can control topographic TA projections allowing the hippocampus to differentially encode new information along the transverse axis of area CA1.
Keywords: temporoammonic pathway, medial/lateral entorhinal cortex, spatial/nonspatial information, dopamine, norepinephrine
INTRODUCTION
The brain has the capacity for parallel information processing, in which sensory information received from the environment is segregated and independently processed based on particular features. For example, it is generally accepted that visual information is processed in two distinct information streams (Ungerleider and Haxby, 1994): a ventral stream that subserves object recognition, or “what” perception and a dorsal stream that primarily represents spatial information, or “where” perception. These distinct streams of information must be integrated somewhere in the brain for coherent perception (Engel and Singer, 2001). A number of studies indicate that the hippocampus, a brain structure important for episodic/declarative memory formation (Scoville and Milner, 1957; Squire et al., 2004), is one such integrative area that combines the two streams of information (Witter and Amaral, 2004; Manns and Eichenbaum, 2006).
As initially described by Cajal (1911), the hippocampus receives major afferent inputs from its adjacent structure, the entorhinal cortex (EC). Anatomically, the EC can be further divided into two subdivisions, the medial and lateral areas (MEC and LEC), based on cytoarchitecture and projection patterns (Blackstad, 1956; Dolorfo and Amaral, 1998; Witter and Amaral, 2004; Canto et al., 2008). Recent studies further indicate that the MEC and LEC are functionally distinct. For example, strong spatial modulation of neuronal activities is observed in the MEC (Fyhn et al., 2004; Hafting et al., 2005), but not in the LEC (Hargreaves et al., 2005). LEC neurons are, in contrast, likely to be involved in nonspatial information processing related to specific objects or cues in the environment (Knierim et al., 2006). Targeted lesions of each subarea/projection led to distinct behavioral deficits (Gauthier et al., 1983; Ferbinteanu et al., 1999; Hunsaker et al., 2007).
The projections from the MEC and LEC terminate in distinct parts of the hippocampus (Steward, 1976; Wyss, 1981; Witter and Amaral, 2004) (see Fig. 2). In the perforant pathway (originating from layer II EC neurons), the fibers from the LEC terminate in the outer third of the molecular layer in the dentate gyrus (DG) and the superficial layer of stratum lacunosum moleculare (SLM) in area CA3, but the axons from the MEC make synapses in the middle third of the DG molecular layer and the deep layer of the CA3 SLM. On the other hand, in the temporoammonic (TA) projection (originating from layer III EC neurons) to area CA1, the fibers from the LEC make synapses in the distal part (close to the subiculum) of the CA1 SLM, and the axons from the MEC terminate in the proximal part (close to area CA3). The topographic organization of LEC and MEC inputs suggests that neurons in proximal or distal CA1 receive predominantly one set of entorhinal–cortical inputs via the TA pathway, implying the existence of functional division along the transverse axis of area CA1. This contrasts with the laminar organization of the perforant pathway, where each neuron in the DG or area CA3 receives both MEC and LEC inputs at different dendritic locations.
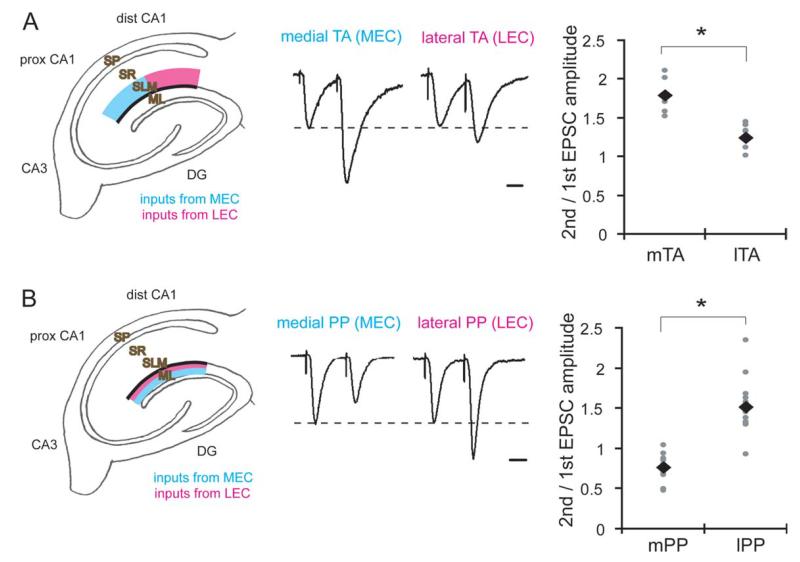
FIGURE 2.
Differences in paired-pulse facilitation between LEC and MEC synapses. (A) Whole-cell voltage-clamp recordings from pyramidal neurons in proximal or distal CA1 under blockade of fast inhibitory transmission (bicuculline; 10 μM). Internal pipette solution contained QX314 (5 mM), TEA (10 mM), and 4AP (1 mM) to block voltage-gated sodium and potassium channels. A paired-stimulus was applied, and paired pulse facilitation was quantified at medial and lateral TA-CA1 synapses (second/first EPSC amplitudes, interstimulus interval: 50 ms) (scale bar = 20 ms). Right figure represents data from individual experiments (gray circles) and the mean (black diamonds) (n = 7 for each) (*P < 0.05). (B) Whole-cell voltage-clamp recordings from granule cells in the DG. Recording conditions were same as in (A). A paired-stimulus was applied to either medial or lateral PP and paired pulse facilitation was quantified (second/first EPSC amplitudes, interstimulus interval: 50 ms) (scale bar = 20 ms). Right figure represents data from individual experiments (gray circles) and the mean (black diamonds) (n = 13 for each) (*P < 0.05).
Although the EC provides the hippocampus with both spatial and nonspatial information, hippocampal neurons are not just passive responders to environmental stimuli. One prominent feature of hippocampal neuron activity is a clear sensitivity to stimulus novelty (Knight, 1996; Stern et al., 1996; Dolan and Fletcher, 1997; Vinogradova, 2001; Rutishauser et al., 2006), suggesting that the hippocampus may act as a novelty detector (Parkin, 1997; Kumaran and Maguire, 2007). Supporting this idea, novelty exposure influences synaptic plasticity in the hippocampus (Manahan-Vaughan and Braunewell, 1999; Lemon and Manahan-Vaughan, 2006), and hippocampal-lesioned animals display deficits in novelty detection (Buhusi et al., 1998; Allen et al., 2002; Hunsaker et al., 2008). The novelty-dependent activation of hippocampal neurons is likely to be a critical feature for learning, allowing circuit modifications that optimize stimulus prediction. How the hippocampus acquires information about the novelty of stimulus or context is still unclear, but a number of studies have indicated a critical role of neuromodulators (Hasselmo, 1995; Ranganath and Rainer, 2003; Lisman and Grace, 2005).
Neuromodulators play a key role in controlling information flow among brain areas [see Ito and Schuman (2008) for a review]. Midbrain dopaminergic neurons in ventral tegmental area and substantial nigra compacta show increased activity after exposure to novel stimuli (Schultz, 1998; Horvitz, 2000). The release of another major neuromodulator in the brain, norepinephrine (NE), is also controlled by novel stimuli (Vankov et al., 1995; Harley, 2004; Aston-Jones and Cohen, 2005; Sara, 2009). The axons of these neuromodulator-releasing neurons project to the hippocampus (Swanson, 1987; Gasbarri et al., 1997) and release dopamine (DA) or NE after animals are exposed to a novel environment (Ihalainen et al., 1998). A number of studies have indicated that DA or NE plays an important role in hippocampal-dependent learning (Gasbarri et al., 1996; El-Ghundi et al., 1999; Li et al., 2003; Murchison et al., 2004).
The interactions between neuromodulators and the hippocampus may be crucial for constructing or updating representations of environmental context, which requires the integration of spatial and nonspatial information. Here, we investigated how neurons in the hippocampus are activated by different types of stimulus novelty and examined if there is a functional division in area CA1 afforded by the anatomical organization of LEC and MEC inputs. We then examined the effects of NE and DA at entorhinal–cortical inputs as a potential mechanism for the independent control of spatial and nonspatial information processing in the hippocampus.
MATERIALS AND METHODS
Hippocampal Slice Preparation
Slices were prepared from 21 to 30-day-old Sprague–Dawley rats (Charles River). A vibrating microtome (Leica VT1000S) was used to make horizontal sections of the hippocampus (500-μm thickness for extracellular recordings and 300 μm for whole-cell recordings) in ice-cold oxygenated artificial cerebrospinal fluid (ACSF) containing (in mM) 119 NaCl, 2.5 KCl, 1.3 MgSO4, 2.5 CaCl, 1.0 NaH2PO4, 26.2 NaHCO3, and 11.0 glucose. Slices made from 4 to 4.5 mm below the ventral surface of the brain were used for all electrophysiology and immunohistochemistry experiments (see Supporting Information Fig. 2). Slices were recovered at room temperature for at least 2 h in an interface chamber and then transferred to a submerged recording chamber perfused with ACSF at 24.5–25.5°C or 32–34°C (for frequency-dependent analysis). For TA pathway recordings, the DG and CA3 were removed to eliminate the possible activation of the trisynaptic pathway or perforant path projection to area CA3. Concentric bipolar tungsten electrodes (FHC: no. CBBRC75 or CABRC75; the inner pole diameter was 25 μm, and the outer pole diameter was 200 μm for the TA path or 125 μm for the perforant path stimulation.) and stimulus isolators (Axon Instruments) were used for the stimulation.
Electrophysiology
Extracellular field potential recordings were made with 1–3 MΩ resistance microelectrodes filled with 3 M NaCl using a bridge amplifier (Axoclamp 2B, Molecular Devices). We followed previous reports for the recordings from lateral TA-CA1 synapses (Otmakhova and Lisman, 1999; Ito and Schuman, 2007); the stimulating electrode was placed in the SLM of the CA1 border near the subiculum or the superficial molecular layer of the proximal subiculum (close to area CA1), and the recording electrode was inserted in the distal half of the CA1 SLM. To record from medial TA-CA1 synapses, the stimulating electrode was placed in the SLM of the CA1 border near area CA2, and the recording electrode was inserted in the proximal half of the CA1 SLM (see Fig. 3). The distance between each pair of stimulating and recording electrodes was 200–300 μm. We note here that although not explicitly stated, our previous work (Ito and Schuman, 2007) described recordings exclusively from the lateral TA-CA1 synapses. The evoked synaptic potentials in the above recording configuration represent synaptic activity derived from EC axons, but also from synapses with the nucleus reuniens (Herkenham, 1978; Wouterlood et al., 1990; Dolleman-Van Der Weel and Witter, 1996), postrhinal (Naber et al., 2001), or perirhinal cortex (Kosel et al., 1983; Naber et al., 1999). The reuniens inputs, however, uniformly project to proximal and distal parts of the CA1 SLM, thus will not contribute to the functional differences along the transverse-axis of CA1. The postrhinal and perirhinal projections to CA1 are topographically organized as similar to those from MEC and LEC; however, these projections are relatively weak and make synapses at the extreme borders of CA1 (Naber et al., 1999, 2001), which we avoided in the placement of the recording electrodes. Whole-cell voltage-clamp recordings from CA1 pyramidal neurons or DG granule cells were obtained without visualization with an Axopatch 200B (Molecular Devices). Internal solution of whole-cell patch pipettes was (in mM) 115 cesium gluconate, 20 KCl, 10 sodium phosphocreatine, 10 HEPES, 0.2 EGTA, 2 MgATP, and 0.3 NaGTP (pH 7.3). In addition, to minimize the possible postsynaptic current modulation by DA or NE, pipette solutions contained (in mM) 5 QX314, 10 TEA, and 1 4AP. Membrane voltage was clamped at −60 mV (without liquid junction potential correction). Membrane capacitance was cancelled, and series resistance was compensated (60–70%). Recordings were discarded when the series resistance was over 20 MΩ or either series or membrane resistance changed more than 30% during data acquisition. For the analysis of frequency-dependent signal modulation, 100 pulses were applied at the indicated frequencies after baseline responses were stable for at least 10 min. The long-term potentiation (LTP) induction protocol was 100 pulses at 100 Hz, repeated twice with a 30-s interval. All stimulus pulses were of the same length and amplitude as test pulses. Test pulses were applied once every 30 s for extracellular field recordings and every 10 s for whole cell recordings. Drugs were applied by dilution of concentrated stock solutions into the perfusion medium. The final concentration of bath-applied DA or NE was 20 or 10 μM, respectively. DA and NE were obtained from Sigma. All other drugs were obtained from Tocris.
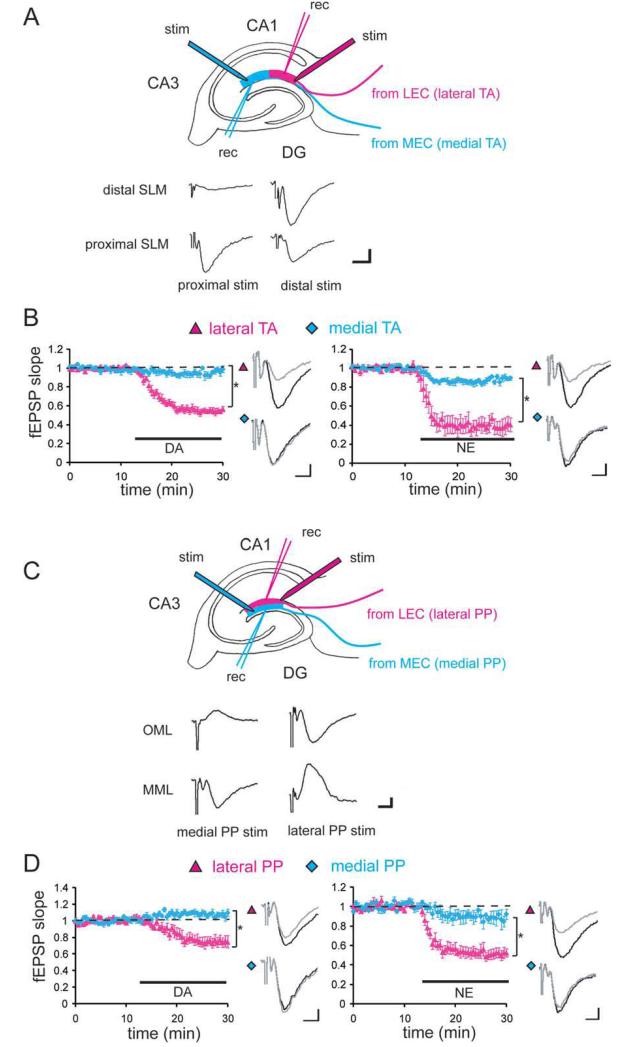
FIGURE 3.
Differential modulation of LEC inputs by DA and NE in two different hippocampal pathways. (A) Scheme of entorhinal cortical inputs to SLM of area CA1. MEC inputs primarily make synapses at the proximal (relative to CA3) CA1 SLM (medial TA pathway), but LEC inputs are enriched in the distal CA1 SLM (lateral TA pathway). Appropriate positioning of recording electrode in either proximal or distal parts of CA1 SLM allows the measurement of synaptic responses derived from each input (see Methods section). Two stimulating electrodes were positioned, so that the distance between stimulating and recording electrodes was equal for each input. Representative waveforms show field potentials in proximal and distal SLM after the stimulation of TA axons by each stimulating electrode (scale bar = 0.1 mV and 5 ms). (B) Either DA (20 μM) or NE (10 μM) application caused a large depression of the field EPSP resulting from lateral, but not medial TA-CA1 synapses (DA: n = 7; NE: n = 5). Field EPSP waveforms before (black) and after (gray) DA or NE application are shown (scale bar = 0.1 mV and 5 ms). (C) Scheme of entorhinal cortical inputs to the molecular layer of DG. LEC inputs make synapses in the outer molecular layer (OML; lateral PP), but MEC inputs project the middle molecular layer (MML; medial PP). Appropriate positioning of stimulating and recording electrodes allows the measurement of synaptic responses from each pathway. Pathway selectivity was further confirmed by sink-source waveform analysis of field potentials. Representative field potentials depict negative-going potentials in MML and positive-going potentials in the OML in response to medial PP stimulation. On the other hand, lateral PP stimulation induces a negative-going potential in the OML and a positive-going potential in the MML (scale bar = 0.1 mV and 5 ms). (D) Either DA or NE application induced large synaptic depression at the lateral PP–DG, but not in medial PP-DG synapse (DA: n = 6; NE: n = 5). Field EPSP waveforms before (black) and after (gray) DA or NE application are shown (scale bar = 0.1 mV and 5 ms).
Behavioral Analysis
Animals used for behavioral analysis were male Sprague–Dawley rats, 24–30 days old. All the behavioral manipulations were carried out at night (0–4:00 A.M.) to maximize active exploration of the environment. The objects used for novel object exposure were three small children’s toys, made of either plastic or wood. The new home cage for novel place exposure was in the same color and shape as the original cage, but had new woodchip flooring and did not have a food box on the ceiling. In DA -antagonist experiments, 250 μl of saline or clozapine (10 mg/kg, diluted in saline) was injected intraperitoneally in animals. Clozapine blocks every subtype of DA receptors, but also has a small antagonistic effect on serotonin receptors and α-2 adrenergic receptors (Baldessarini and Frankenburg, 1991).
Data Analysis
Electrophysiology
Data were collected using a custom-written program in LabView (National Instruments) for extracellular recordings, or DigiData 1200 and pClamp 9 (Molecular Devices) for whole-cell recordings. All numerical values listed represent mean ± s.e.m. For synaptic plasticity experiments, the baseline fEPSP was normalized to the depressed state. For analysis of the waveforms, during 100 pulse stimulation, stimulation artifacts and fiber volleys were excluded, and the gaps were linearly connected, and the last excitatory potential or current (100th stimulus response) was measured by a custom program in Matlab (MathWorks). A Wilcoxon rank sum test was performed to analyze the statistical significance of the data.
Immunohistochemistry
Slices (500 μm thickness) were prepared using the same procedure as for electrophysiology recordings. After cutting, slices were quickly fixed in 4% paraformaldehyde in phosphate-buffered saline for at least 2 days. Thin (50 μm) sections were cut with a vibrating microtome (Leica VT1000S). The sections were incubated overnight with either of 1:250 concentration of antic-Fos (sc-52) (Santa Cruz), 1:1,000 of anti-NeuN (Millipore), 1:1,000 of anti-Synapsin I (Millipore), and 1:1,000 of anti-Bassoon (Stressgen) antibodies. The incubation was carried out at room temperature in Tris-buffered saline containing 0.2% Triton X-100, BSA 2%, and NGS 4%, followed by 4 h of secondary-antibody incubation with 1:1,000 of Alexa 488-conjugated antirabbit and 1:1,000 of Alexa 543-conjugated anti-mouse antibodies (Invitrogen). For the analysis of immunohistochemistry experiments, images were obtained with Zeiss LSM 510 laser scanning confocal microscopes using a Plan-Neofluor 10×/0.3 air objective. Alexa 488 and 546 were visualized by excitation with the 488 line of an argon ion laser and the 543 nm line of a He–Ne laser, respectively. The optical section was 20 μm, and fluorescent signals were acquired throughout the slice thickness (50 μm). Each 50-μm slice was obtained from a different 500-μm section, and two slices were analyzed from each animal. Slices were obtained from the same septotemporal position in all experiments. To count the number of c-Fos positive neurons, fluorescent signals less than the mean + 2SD were excluded (see Supporting Information Fig. 1C). Then, automated particle analysis was carried out using ImageJ (NIH) based on the following criteria: particle size must be larger than 56 μm2 and the circularity larger than 0.5. For the analysis of dentate granule cells, particle sizes larger than 39 μm2, instead 56 μm2, were used due to the smaller size of granule cells. Statistical differences between animals groups were assessed by ANOVA. Regional differences were statistically analyzed by a Wilcoxon rank sum test.
RESULTS
Differential Activation of Distal and Proximal CA1 by Exposure to Novel Object or Place
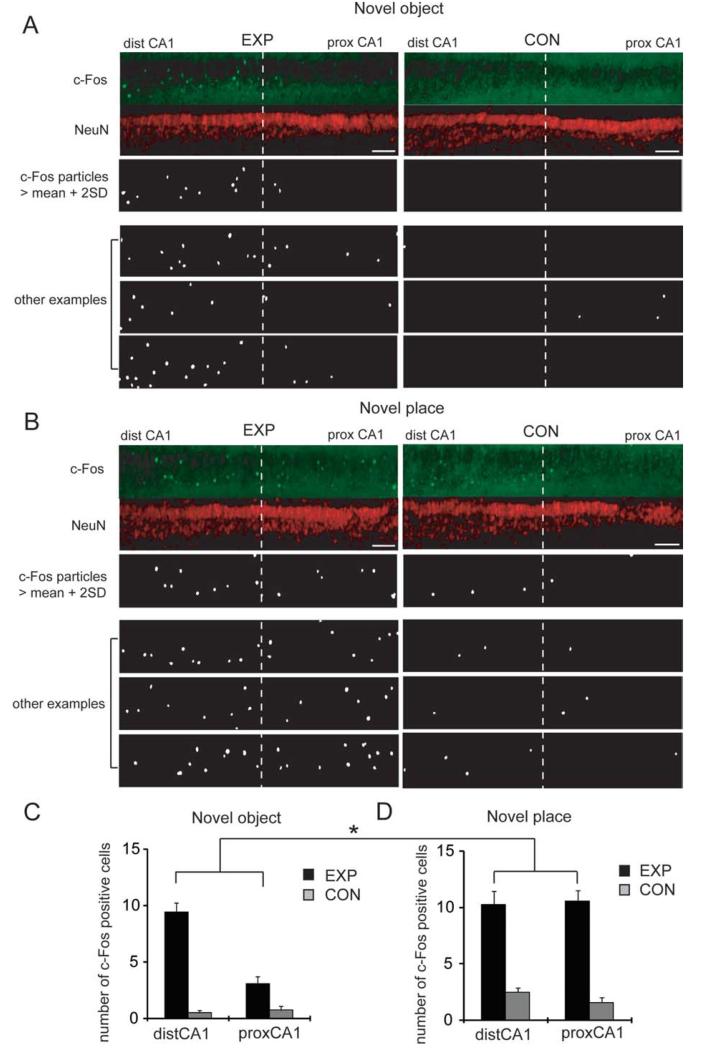
Neurons in the hippocampus exhibit differential firing based on the novelty or familiarity of stimuli encountered in the environment. We examined whether exposure to novel stimuli leads to activation of neurons in area CA1, and if so, whether there is differential activation along the transverse-axis of area CA1, due to the topographic organization of LEC and MEC projections in the TA pathway. Following home-cage exposure for several days, pairs of individually housed rats were subjected to one of the following conditions: exposure to novel objects in the home cage (experimental group: EXP) or sham exposure (cage opened but no objects introduced, control group: CON). After 2 h of novel object exposure, animals were sacrificed, and immunohistochemistry was performed on brain slices. To observe activation of CA1 neurons, we stained with antibodies for an immediate early gene product, c-Fos (Morgan and Curran, 1991; Guzowski et al., 2005), together with a neuronal nuclear marker protein, NeuN (Fig. 1A). Animals exposed to the novel objects exhibited a significantly higher number of c-Fos-positive neurons in area CA1, when compared with control animals. As the EC projection to area CA1 shows topographic organization, we counted the number c-Fos-positive neurons in distal (receiving LEC inputs) versus proximal (receiving MEC inputs) CA1. We observed a larger number of c-Fos positive neurons in distal CA1 compared to proximal CA1 (c-Fos positive cells: distal 9.4 ± 0.8, proximal 3.1 ± 0.6; P = 0.0001 in Wilcoxon rank sum test; Fig. 1C) in brain slices from EXP, but not control, animals.
FIGURE 1.
Differential c-Fos expression between proximal and distal CA1 after exposure to a novel object or place. (A) Pairs of animals were individually housed in a home cage for at least 2 days. For the experimental animal, the cage was opened, and three toys were placed inside (“EXP”). For the control animal, the cage was opened, but no object was inserted (“CON”). After 2 h, hippocampal slices were prepared from both animals, fixed, and immunostained with c-Fos and NeuN antibodies. c-Fos particles were detected and analyzed (see Methods section). Representative images show the pyramidal layer of area CA1 of “EXP” and “CON” animals (straightened by ImageJ; scale bar = 100 μm). (B) A pair of animals was individually housed in a home cage for at least 2 days. For one of animals, the cage was opened, and the animal was carried up and placed in a different cage (“EXP”). For another animal, the cage was opened, and the animals were carried up but placed back in the original cage (“CON”). After 2 h, slices were prepared and immunstained as in (A). Images shown are from the pyramidal layer of area CA1 of “EXP” and “CON” animals (straightened by ImageJ; scale bar = 100 μm). (C) The number of c-Fos positive cells was analyzed in the pyramidal layer of area CA1 in each 50 μm slice. Area CA1 was equally divided into two parts, representing distal and proximal CA1. The number of c-Fos positive cells was significantly higher in distal CA1 following exposure to novel objects, when compared with proximal CA1 (P = 0.0001 in Wilcoxon rank sum test; n = 6 pairs of animals). The total integrated NeuN signal in same areas used for c-Fos expression analysis did not differ between groups (Supporting Information Fig. 1B). D: The number of c-Fos positive cells was analyzed in the pyramidal layer of area CA1 in each slice. The number of c-Fos positive cells was high in both distal and proximal CA1 following exposure to a novel place (n = 6 pairs of animals). The total integrated NeuN signals in same areas used for c-Fos expression analysis did not differ between groups (Supporting Information Fig. 1B). For the analysis of novelty-induced c-Fos expression, a two-way ANOVA was performed with two variables, novelty type (object vs. place) and CA1 subregion (distal vs. proximal), and revealed a significant interaction (P = 0.0008).
To further examine the potential topographic representation of environmental signals to area CA1, we examined c-Fos expression after animals were exposed to a novel environment. Using the same design as earlier, one animal was removed from his home cage and placed in a new cage (“EXP” group), and the paired control was removed and then reintroduced to his home cage (“CON” group). Exposure to a novel place significantly enhanced c-Fos expression in neurons in both proximal and distal CA1 (c-Fos positive cells: distal 10.3 ± 1.2, proximal 10.6 ± 0.9; P = 0.82 in Wilcoxon rank sum test; Figs. 1B,D). These data indicate that proximal and distal CA1 can be differentially activated depending on the different type of stimulus novelty.
Differences in Presynaptic Properties Between LEC and MEC Inputs
The above difference in c-Fos immunoreactivity between proximal and distal CA1 indicates that topographic projections in the TA pathway are behaviorally relevant. We next investigated whether any differences can be detected between LEC and MEC inputs at the synaptic scale. Because neurons in different brain regions often express different level of presynaptic proteins (Oyler et al., 1989; Melloni et al., 1993; Richter et al., 1999), we performed immunohistochemistry on hippocampal slices to examine whether there are differences in presynaptic protein expression corresponding to LEC and MEC synapses. We found that synaptic terminal proteins, synapsin I, and bassoon showed differential distribution along the transverse axis of the CA1 SLM (Supporting Information Fig. 2). The transition of these differences occurs roughly at the midline of the CA1 transverse axis (Supporting Information Fig. 2D), corresponding to the slice section level, we chose (see Methods section), which mapped onto the previously reported anatomical distinction between areas receiving LEC and MEC inputs (Steward, 1976; Wyss, 1981; Witter and Amaral, 2004). These immunostaining results suggest that proximal and distal parts of the CA1 SLM possess different axon terminals, providing support for the topographic LEC and MEC projection.
Next, we examined paired pulse facilitation at synapses made in the proximal and distal half of the CA1 SLM, to assess the presynaptic function of LEC and MEC terminals. We found that paired-pulse facilitation was significantly larger at synapses made in the proximal SLM (receiving MEC projection; i.e., medial TA-CA1 synapses), compared to synapses made in the distal SLM (receiving LEC projection; i.e., lateral TA-CA1 synapses) (medial TA: 1.79 ± 0.08, lateral TA: 1.24 ± 0.07) (Fig. 2A). In the perforant pathway, lateral PP synapses showed larger facilitation than medial PP synapses (mPP: 0.76 ± 0.05, lPP: 1.51 ± 0.10) (Fig. 2B), as previously reported (McNaughton, 1980). Thus, our results indicate that LEC and MEC terminals have distinct functional properties, providing a potential substrate for the differential control of LEC and MEC synapses in vivo.
Differential Influence of DA and NE on Inputs from the LEC and MEC
Novel signals or stimuli in an animal’s environment are known to influence the firing of DA and NE-releasing neurons (Schultz, 1998; Harley, 2004). Thus, we asked whether DA and/or NE modulate LEC and MEC inputs to bring about the observed differences between distal and proximal CA1 activation. To examine this, we obtained extracellular field potentials from lateral and medial TA-CA1 synapses in hippocampal slices (Fig. 3A). When DA (20 μM) was bath applied, the fEPSP at lateral TA-CA1 synapses was largely depressed (55% ± 3%, mean percent of baseline 15–20 min after drug application) (Otmakhova and Lisman, 1999; Ito and Schuman, 2007), but the fEPSP at medial TA-CA1 synapses was only marginally influenced (95% ± 3%) (Fig. 3B). We also tested another neuromodulator, NE, and found that bath application of NE (10 μM) also induced significantly larger depression at lateral TA-CA1 synapses (40% ± 7%), compared to medial TA-CA1 synapses (89% ± 2%) (Fig. 3B). No significant influence of DA or NE was observed at Schaffer-collateral (SC) synapses made with either the proximal or distal part of CA1 (proximal SC: DA 101% ± 2%, NE 97% ± 2%; distal SC: DA 102% ± 2%, NE 98% ± 2%; mean percent of baseline 15–20 min after drug application) (Supporting Information Fig. 3A). Thus, bath application of DA or NE primarily modulated lateral TA-CA1 synapses, while their influence on other synapses in area CA1 was minimal. We hypothesized that this differential modulation by neuromodulators is due to the different origins of synaptic inputs (MEC vs. LEC). To test this idea, we also examined the neuromodulators’ effect on the perforant path (PP)–DG synaptic transmission.
We placed both stimulating and recording electrodes in either the outer or middle third of the molecular layer of the DG (Fig. 3C). When DA was bath applied, the fEPSP at lateral PP-DG synapses was depressed to 75% ± 4% of baseline; however, fEPSP at medial PP-DG synapses was not depressed but rather slightly enhanced (111% ± 6%) (Fig. 3D). Similarly, NE also largely depressed synaptic inputs from lateral PP (52% ± 5%), but had lesser effects on medial PP-DG synapses (88% ± 5%) (Fig. 3D). As previously demonstrated, DA- and NE-mediated synaptic depression at lateral TA-CA1 synapses is reversible after the washout of neuromodulators (Otmakhova and Lisman, 1999; Otmakhova et al., 2005; Ito and Schuman, 2007). Here, we confirmed that neuromodulator-mediated control of PP-DG synapses was also reversible (Supporting Information Fig. 3B). Taken together, our results indicate that the neuromodulators, DA, and NE primarily influence synapses made with LEC inputs, providing differential and temporally controlled regulation of two streams of information (e.g., spatial and nonspatial) from the EC to the hippocampus.
Presynaptic Inhibition of LEC Inputs by DA and NE
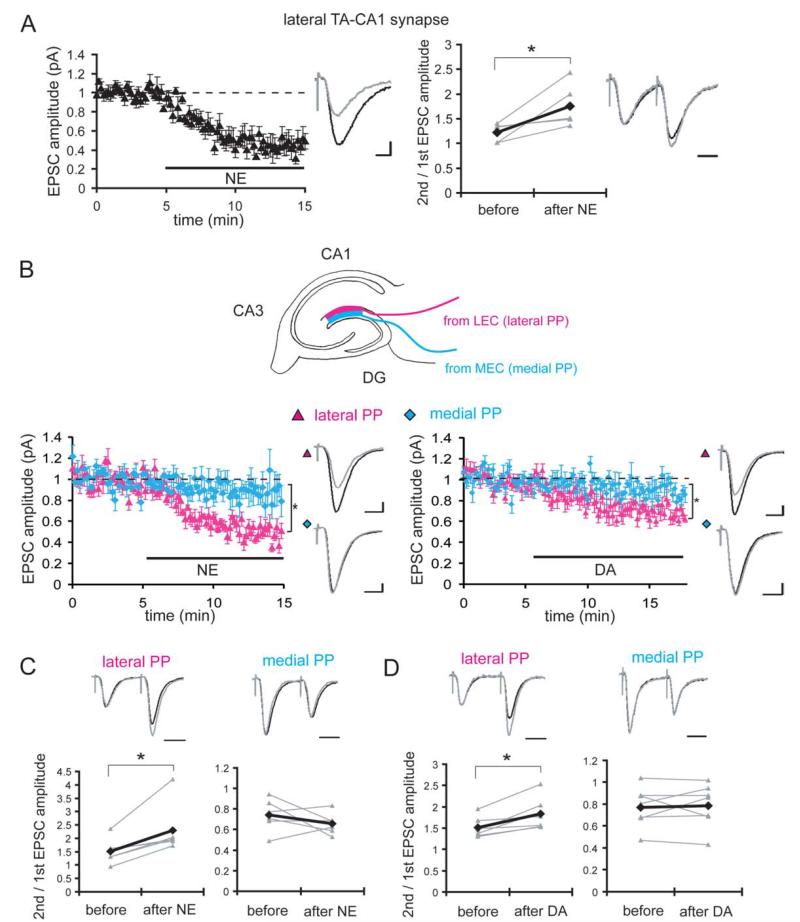
To examine the synaptic locus of this differential modulation of LEC versus MEC inputs, we conducted whole-cell voltage clamp recordings from pyramidal neurons in distal CA1 and measured the synaptic responses evoked by stimulation in SLM (Fig. 4A). When fast inhibitory synaptic transmission (bicuculline: 10 μM) as well as postsynaptic voltage-gated sodium/potassium channels (intracellular QX314: 5 mM, TEA: 10 mM, and 4AP: 1 mM) were blocked, NE still significantly depressed the EPSC derived from lateral TA-CA1 synapses (45% ± 8%, mean percent of baseline 7–10 min after drug application) and enhanced paired-pulse facilitation (1.22 ± 0.08 to 1.76 ± 0.20), suggesting a mechanism of presynaptic inhibition. We previously examined DAs influence on these synapses (Ito and Schuman, 2007) and found similar results (EPSC amplitude: 58% ± 2%, paired-pulse facilitation: 1.47 ± 0.05 to 1.90 ± 0.08 after DA application), suggesting that DA also reduces presynaptic release probability. Similar results were obtained with extracellular field recordings in the presence of GABAA and B receptor antagonists, bicuculline and CGP55845 (Supporting Information Fig. 4). We also conducted whole-cell voltage-clamp recording from granule cells in the DG and measured synaptic responses from lateral and medial PP inputs (Fig. 4B). Both NE and DA largely depressed the EPSC evoked by lateral PP stimulation (NE: 46% ± 5%, DA: 71% ± 6%), but only slightly depressed EPSC from medial PP (NE: 88% ± 10%, DA: 90% ± 4%). Paired-pulse facilitation was significantly increased by both DA and NE at lateral PP synapses (NE: 1.51 ± 0.20 to 2.29 ± 0.38 and DA: 1.51 ± 0.09 to 1.83 ± 0.14) but not at medial PP synapses (NE: 0.74 ± 0.6 to 0.66 ± 0.04 and DA: 0.77 ± 0.07 to 0.79 ± 0.07) (Fig. 4C). Taken together, our results suggest that neuromodulators act on LEC inputs via, at least in part, a presynaptic mechanism.
FIGURE 4.
DA and NE induce presynaptic inhibition of LEC inputs. (A) Whole-cell voltage-clamp recordings from pyramidal neurons in distal CA1 under blockade of fast inhibitory transmission (bicuculline; 10 μM; n = 5). Internal pipette solution contained QX314 (5 mM), TEA (10 mM), and 4AP (1 mM) to block voltage-gated sodium and potassium channels. NE application significantly depressed the EPSC evoked by lateral TA-CA1 synapses and enhanced paired pulse facilitation (second/first EPSC amplitudes (interstimulus interval: 50 ms). EPSC waveforms before (black) and after (gray) NE application are shown (scale bar = 20 pA and 10 ms) (*P < 0.05). (B) Whole-cell voltage-clamp recordings from granule cells in the DG. Recording conditions were same as in (A). NE application significantly depressed the lateral, but not medial PP inputs (n = 6). DA application also selectively depressed lateral PP inputs (n = 7). EPSC waveforms of before (black) and after DA or NE application (gray) are shown (scale bar = 20 pA and 10 ms). (C) NE application selectively enhanced paired pulse facilitation at lateral PP synapses (interstimulus interval: 50 ms) (gray: individual experiment, black: average). EPSC waveforms before (black) and after (gray) NE application are shown (scale bar = 20 ms) (*P < 0.05). (D) DA application selectively enhanced paired pulse facilitation at lateral PP synapses (interstimulus interval: 50 ms) (gray: individual experiment, black: average). EPSC waveforms before (black) and after (gray) DA application are shown (scale bar = 20 ms) (*P < 0.05).
We also examined the receptor subtype contribution to the neuromodulator-mediated control of entorhinal–hippocampal connections, using a variety of receptor antagonists. These studies suggest the contribution of both DA D1-like and D2-like receptors to DA-induced synaptic depression and the α-2 adrenergic receptor to NE-induced depression at both the TA and perforant pathway synapses (Supporting Information Fig. 4). Thus, the TA and perforant pathway appear to utilize a similar signal transduction pathway for the neuromodulator-mediated control of LEC inputs, irrespective of the origin differences between the TA pathway (layer III of the EC) and the perforant pathway (layer II of the EC). The differential control of lateral and medial perforant path by NE, we observed here, is mediated by a distinct mechanism from previous reports on β adrenergic receptors (Dahl and Sarvey, 1989; Pelletier et al., 1994). Because α-2 adrenergic receptors are primarily located at presynaptic terminals in the hippocampus (Milner et al., 1998; Hillman et al., 2005), our results are consistent with the idea that differential control of MEC and LEC synapses is mediated by presynaptic terminal differences.
Differential Modulation of High-Frequency Signal Transmission by DA or NE at Lateral TA-CA1 Synapses
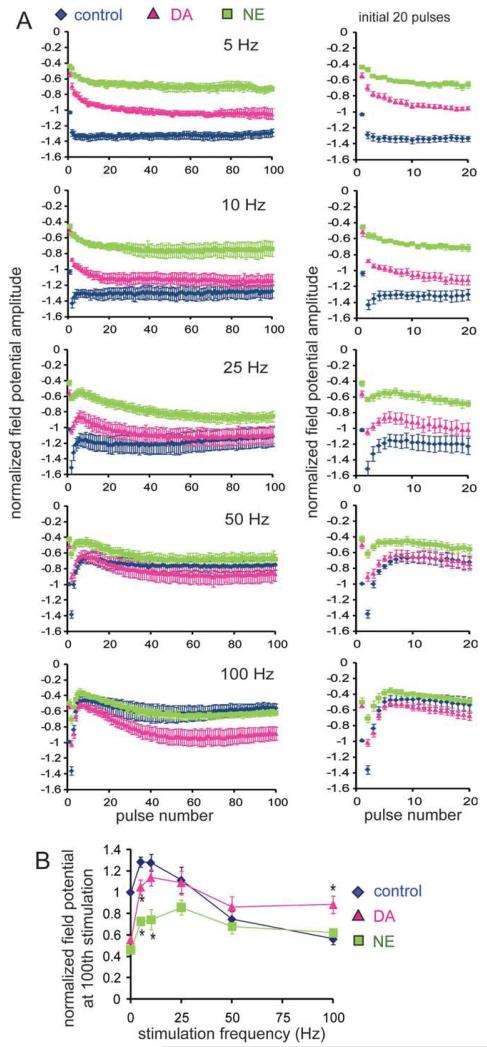
Signal transmission in neuronal networks is intrinsically nonlinear and strongly influenced by the frequency of the input signals (Markram et al., 1998). Dynamic changes in the amplitude, frequency, and phase-coordination of oscillations in vivo appear to be functionally linked to animal behavior (Buzsaki and Draguhn, 2004), suggesting that oscillatory activities may participate in signal gating (Laurent, 2002; Ito and Schuman, 2008). As DA modulates frequency-dependent signal transmission at distal TA-CA1 synapses by enhancing high-frequency input signals but depressing low-frequency inputs (Ito and Schuman, 2007; Fig. 5), we next examined how NE influences frequency-dependent signal transmission at these same synapses.
FIGURE 5.
Differences in DA or NE elicited frequency dependent modulation at lateral TA-CA1 synapses. (A) Normalized peak field potentials (at each pulse of a 100-pulse stimulation epoch) at frequencies ranging from 5 to 100 Hz. All experiments were conducted at near physiological temperature (32–34°C). Field potentials were normalized to the baseline fEPSP amplitude before neuromodulator application (from 5 to 100 Hz, n = 6, 5, 6, 7, and 8 for control; n = 7, 5, 5, 5, and 8 for DA; n = 6, 4, 4, 4, and 7 for NE). (B) Analysis of normalized peak field potentials at the 100th pulse. Both DA and NE depressed responses during low-frequency stimulation, but only DA enhanced responses during high-frequency stimulation (HFS) when compared with control.
We applied 100 pulses of stimulation to the TA pathway at different ranges of stimulation frequency from 5 to 100 Hz (Fig. 5A). As in our previous report (Ito and Schuman, 2007), we focused on the analysis of steady-state potentials, because transient potentials during the first few stimuli can be influenced by the prestimulus state of the neuronal network. When low-frequency (<50 Hz) stimulation was applied, NE strongly depressed the steady-state potentials when compared with control. However, as the stimulation frequency increased, the difference between control and NE became smaller (Fig. 5B), with no modulation observed at high frequencies (>50 Hz). Although the depression of low-frequency signals by NE is similar to DA-induced modulation, a major difference appeared during high-frequency stimulation: only DA enhanced high-frequency signals.
Thus, although no difference between DA- and NE-mediated synaptic modulation was evident in our analysis of basal synaptic transmission (0.033 Hz) (Fig. 3B), the frequency-response analysis revealed a clear difference in high-frequency signal modulation by DA and NE at lateral TA-CA1 synapses. We also examined frequency-dependent signal transmission at medial TA-CA1 synapses and found that neither DA nor NE influences the transmission of 5–100 Hz signals at these synapses (Supporting Information Fig. 5).
Selective Influence of DA or NE on LTP at Medial and Lateral TA-CA1 Synapses
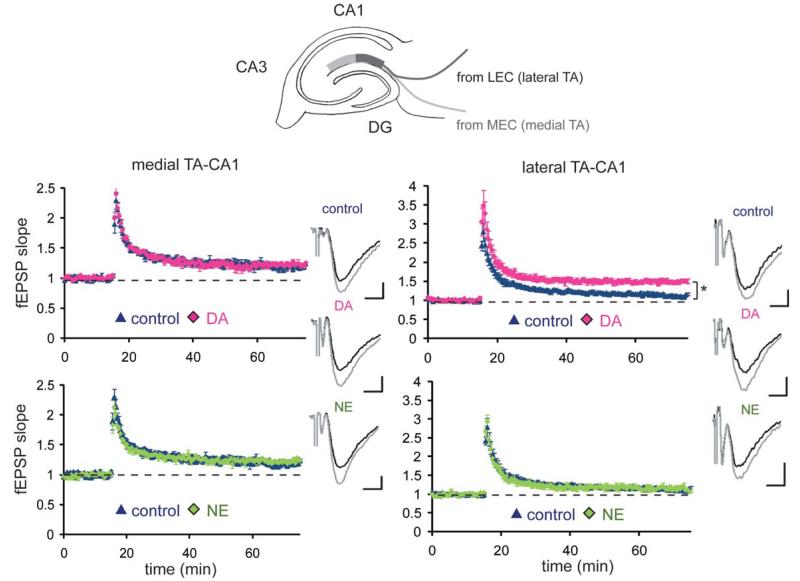
The above differences in frequency-response modulation between NE and DA may influence synaptic plasticity induction, due to the differential handling of high frequency signals like those commonly used to elicit long-term potentiation (LTP) (e.g., 100 Hz). We tested this idea by recording from lateral TA-CA1 synapses (Fig. 6). The application of high-frequency (100 Hz) stimulation induced LTP of a modest magnitude at these synapses (control: 112% ± 3%, mean percent of baseline 55–60 min after LTP induction). On the other hand, slices exposed to DA exhibited LTP of a greater magnitude, as we previously reported (Ito and Schuman, 2007) (DA: 149% ± 4%; Fig. 6, upper right). On the contrary, NE application did not enhance LTP magnitude, when compared with control (NE: 114% ± 3%; Fig. 6, lower right). This is predicted by the differences in high-frequency signal modulation between DA and NE that we observed (Fig. 5). We also tested whether neuromodulators influence LTP induction at medial TA-CA1 synapses, but neither DA nor NE altered LTP magnitude at these synapses (control: 119% ± 3%, DA: 122% ± 3%, NE: 122% ± 3%; Fig. 6, left). Thus, neither DA nor NE influences the transmission of 5–100 Hz signals (Supporting Information Fig. 5) or plasticity at medial TA-CA1 synapses. In contrast, DA, but not NE, modulated synaptic plasticity at lateral TA-CA1 synapses.
FIGURE 6.
Differential influence of DA or NE on LTP induction at TA-CA1 synapses. LTP was induced at medial or lateral TA-CA1 synapses. Neuromodulators were present throughout the experiments. Before the experiments, the stimulation amplitude was adjusted to obtain equivalent fEPSP slope as original baselines to examine the LTP magnitude independent of the depression of basal synaptic transmission by neuromodulators. The LTP induction protocol was 100 pulses at 100 Hz, repeated twice at a 30-s interval (n = 5 for each experimental group). Field EPSP waveforms before (black) and after (gray) NE or DA application are shown (scale bar = 0.1 mV and 5 ms) (*P < 0.05).
Although the above LTP experiments were done in the continuous presence of neuromodulators, we also determined whether an acute application of neuromodulators is sufficient to influence synaptic plasticity. An application of DA or NE at 10 s before the LTP induction still differentially controlled the LTP magnitude at lateral TA-CA1 synapses (Ito and Schuman, 2007; Supporting Information Fig. 6A), suggesting that a phasic release of neuromodulators in vivo could control synaptic plasticity at these synapses.
Clozapine Reduces Novelty-Induced Differential Activation of Distal and Proximal CA1
Our slice electrophysiology results suggest that neuromodulators primarily modulate synaptic transmission at lateral TA-CA1 synapses, raising the possibility that the novelty-induced enhancement of c-fos transcription is mediated by this influence. Thus, we asked whether an antagonist of neuromodulators influences the differential c-Fos expression along the transverse-axis of area CA1 observed in our behavioral experiments (Fig. 1). To test this, we examined c-Fos expression in animals treated with a broad spectrum receptor antagonist that blocks both DA and NE-mediated signaling, clozapine (Baldessarini and Frankenburg, 1991), before novelty exposure. We housed pairs of animals in the same cage for a baseline period of several days. Then, either saline or clozapine (10 mg/kg) was injected intraperitoneally in each animal. After 4 h, novel objects were placed in the home cage (novel object exposure), or both animals were transferred to a new cage (novel place exposure). In these experiments, the saline-treated animals still showed a differential c-Fos expression between proximal and distal CA1 following novel object or place exposure as observed in Figure 1 (Fig. 7). Clozapine-treatment at this dosage did not cause any apparent differences in animal mobility or novelty preference (Supporting Information Figs. 7D,E). We found, however, that clozapine-treatment caused a significant reduction in the global c-Fos expression in the hippocampus (c-Fos positive cells in the hippocampus: saline 52.4 ± 3.1, clozapine 38.6 ± 1.8; P = 0.001 in Wilcoxon rank sum test; Supporting Information Fig. 7A), consistent with previous studies, suggesting that DA plays an important role in novelty-induced hippocampal activation (Lisman and Grace, 2005). But more importantly, clozapine treatment appeared to have differential effects on c-Fos expression depending on whether the animals were exposed to novel objects or place. At distal CA1, c-Fos expression after the novel object exposure was significantly reduced by clozapine-treatment (c-Fos positive cells in distal CA1: saline 19.1 ± 1.7, clozapine 6.8 ± 1.1; P = 0.0001 in Wilcoxon rank sum test); however, c-Fos expression after novel place exposure did not show a significant decrease in clozapine-treated animals (c-Fos positive cells in distal CA1: saline 14.0 ± 1.5, clozapine 10.6 ± 0.8; P = 0.14 in Wilcoxon rank sum test) (Figs. 7B,C). These results imply that neuromodulators are more crucial for encoding information about objects than place in area CA1. Furthermore, because clozapine largely abolished the difference in c-Fos expression between proximal and distal CA1, neuromodulators are likely to play a key role in the differential activation of pyramidal neurons along the transverse-axis of area CA1, probably via largely selective modulation of LEC inputs.
FIGURE 7.
A neuromodulatory receptor antagonist prevents the enhanced c-fos immunostaining observed at distal CA1 following novel object exposure. (A) Pairs of animals were housed in the same home cage for at least 2 days. Then, either saline or clozapine (10 mg/kg) was intraperitoneally injected in each animal. After 4 h, both animals were exposed to either novel objects or novel place, as described in the Figure 1 legend. After 2 h, slices were prepared and stained as previously described. Images shown are from the pyramidal layer of area CA1 of saline-treated and clozapine-treated animals (straightened by ImageJ; scale bar = 100 μm). (B) The number of c-Fos positive cells after novel object exposure was analyzed in area CA1 as described in Figure 1. The total integrated NeuN signals in same areas used for c-Fos expression analysis did not differ between groups (Supporting Information Fig. 7B). The number of c-Fos positive cells at distal CA1 was significantly reduced by clozapine-treatment (P = 0.001 in Wilcoxon rank sum test; n = 6 pairs of animals). (C) The number of c-Fos positive cells after novel place exposure was analyzed in area CA1. The total integrated NeuN signals in same areas used for c-Fos expression analysis did not differ between groups (Supporting Information Fig. 7B). The saline-treated animals displayed differential c-Fos expression between proximal and distal CA1 following novel object or place exposure as observed in Figure 1 (a two-way ANOVA interaction: P = 0.0007; n = 7 pairs of animals for novel place exposure experiments). The number of c-Fos positive cells at distal CA1 did not show a significant decrease with clozapine-treatment (P = 0.14 in Wilcoxon rank sum test). For the comparison of c-Fos expression in distal CA1 after novel object and place exposure, a two-way ANOVA was performed with the two variables, novelty type (object vs. place) and drug treatment (saline vs. clozapine), showing a significant interaction (P = 0.0015).
DISCUSSION
Roles of the TA Pathway
Hippocampal area CA1 receives two major excitatory inputs, one from area CA3 (the SC pathway) and the other from the EC (the TA pathway). For many years, efforts to understand TA-CA1 synapses were dwarfed by studies of the SC input. Many recent studies, however, have elucidated the significance of this input. For example, in recordings from hippocampal slices, precisely timed TA inputs are critical for controlling dendritic signal propagation and synaptic plasticity at SC-CA1 synapses (Levy et al., 1998; Dvorak-Carbone and Schuman, 1999; Remondes and Schuman, 2002; Ang et al., 2005; Jarsky et al., 2005; Dudman et al., 2007). Furthermore, in behaving animals, the TA pathway appears to play a key role in particular hippocampal functions. For example, even after the lesions of SC inputs, CA1 neurons still show location-specific activities, and animals maintain spatial recognition memory (Brun et al., 2002). In addition, the TA pathway is essential for memory consolidation (Remondes and Schuman, 2004). On the other hand, the SC pathway is likely to be necessary for remote navigation memory or one-time contextual learning (Brun et al., 2002; Nakashiba et al., 2008). Thus, each pathway may play a distinct role in animal behavior and learning.
Because the TA pathway itself can maintain at least one form of spatial recognition memory, the interaction between the EC and area CA1 may be sufficient to carry out certain hippocampal functions. In support of this idea, reciprocal anatomical interactions between the EC and area CA1 have been described (Tamamaki and Nojyo, 1995). Neurons in proximal CA1, which receive MEC inputs via the TA pathway, send their projections back primarily to the MEC; on the other hand, neurons in distal CA1, which receive LEC inputs, project their efferents back to the LEC. Thus, two distinct TA-pathway-mediated information trajectories exist between the EC and area CA1; that is, MEC—proximal CA1—MEC and LEC—distal CA1—LEC. These functional loops could allow for the independent processing of spatial and nonspatial information. Our c-Fos expression analysis in the MEC and LEC indeed supports this idea, because both layer III (send afferents to area CA1) and layer V/VI (receive efferents from area CA1) neurons in the MEC and LEC show differential c-Fos expression (as observed in proximal and distal CA1), following novel object or place exposure (Supporting Information Figs. 1D,E). Thus, the EC-CA1 loop circuit allows for the differential handling of nonspatial and spatial information in the hippocampus (Witter et al., 2000; Eichenbaum and Lipton, 2008), which is in clear contrast to the trisynaptic circuit (Andersen et al., 1969) (i.e., EC—DG—CA3—CA1).
Differential c-Fos Expression in the Transverse-Axis of Area CA1
We demonstrated that exposure to a novel place induces high levels of c-Fos expression in both proximal and distal CA1, whereas novel object exposure primarily activates distal CA1 (Fig. 1). The c-Fos, an immediate early gene, requires a threshold level of activity for its transcription (Cole et al., 1989; Guzowski et al., 2005). In the hippocampus, the induction of c-Fos expression requires multiple trains of high-frequency stimulation, like that used to elicit LTP (Worley et al., 1993) and the deletion of the c-Fos gene impairs LTP (Fleischmann et al., 2003). Thus, the c-Fos positive cells in our study are not simply active cells, but rather the cells that received inputs at the appropriate intensity or frequency to trigger c-fos transcription and synaptic plasticity. The expression of c-Fos protein levels rise and reach a peak around 30 min to 1 h after the appropriate stimulus, and the half-life of c-Fos protein is about 2 h (Morgan and Curran, 1991). Our behavioral protocol was designed to detect changes in c-Fos expression associated with synaptic modifications that occurred during the 2-h exposure to stimulus novelty. Thus, the differential c-Fos expression between proximal and distal CA1 (Fig. 1) is likely to be a result of differential synaptic plasticity induction between these areas, which is controlled by proper coordination between SC- and TA-CA1 synapses (Remondes and Schuman, 2002; Ito and Schuman, 2007).
Our observation of the differential c-Fos expression between proximal and distal CA1 corresponds to anatomical projections from the MEC (spatial information) and the LEC (nonspatial information). These results, together with previous studies on c-Fos expression analysis in perirhinal and postrhinal cortexes (Wan et al., 1999; Vann et al., 2000), support the idea of two streams of information to the hippocampus (Manns and Eichenbaum, 2006). It is important to note that the exposure to novel objects or place does not simply correspond to a manipulation of either nonspatial or spatial information alone, because object recognition requires information about both the identity and location of the object, and the encoding of a novel place also requires both spatial and nonspatial information (e.g., novel wood-chip flooring or odor of the environment). We indeed observed strong c-Fos expression in both proximal and distal CA1 following novel place exposure, which probably results from the integration of nonspatial and spatial information to encode a new environment. The inputs from CA3 neurons (SC pathway) will also influence c-Fos expression in CA1 neurons. Recent in vivo recording studies indicate that the place-fields of CA3 neurons show differential place-field remapping, depending on the type of environmental manipulation (i.e., rate vs. global remapping) [see Colgin et al. (2008) for review]. According to this idea, exposure to a novel place will likely change the peak-firing location of CA3 neurons (i.e., global remapping), but exposure to a novel object will not. The changes in the peak-firing location of CA3 neurons will generate different temporal patterns of SC inputs, which may induce synaptic plasticity in both proximal and distal CA1 neurons via spike-timing-dependent mechanism [see Dan and Poo (2004) for review]. Here, in contrast to the TA pathway, the SC pathway is likely to provide a similar impact on both proximal and distal CA1 without differential modulation by DA or NE (Supporting Information Fig. 3A). Irrespective of the interpretation, our findings support the idea that new information is differentially encoded along the transverse-axis of area CA1 depending on the type of stimulus novelty.
Neuromodulators in the Hippocampus
A number of studies have indicated key roles for neuromodulators in hippocampal function (Hasselmo, 1995; Harley, 2004; Lisman and Grace, 2005). How neuromodulators exactly influence information processing is, however, unclear. Here, we demonstrated the dynamic influence of neuromodulators, DA and NE, on synaptic transmission at entorhinal–hippocampal connections. The neuromodulator-mediated effect on synaptic transmission and plasticity at these synapses specifically requires an exogenous application of neuromodulators (in μM range) (Supporting Information Fig. 6), although several studies have demonstrated the action of endogenous DA in acute hippocampal slices (Frey et al., 1990; Otmakhova and Lisman, 1996). It is important to note that DA-mediated signaling in the brain is not a simple on/off binary switch, but rather, DA concentration is dynamically regulated to various levels by the activity of dopaminergic neurons (e.g., tonic or phasic activation) (Grace, 1991). Indeed, microdialysis and voltametry studies show that there is residual DA in resting animals (in nM range) and that the concentration increases dramatically (to μM range) after animals are exposed to novel stimuli or following electrical stimulation of dopaminergic neurons (e.g., Garris et al., 1997; Ihalainen et al., 1999; Chen and Budygin, 2007). In addition, each subtype of DA receptor has a different affinity for DA (Gingrich and Caron, 1993). Thus, a phasic surge in DA concentration in vivo will influence a different subset of DA receptors, producing distinct modulatory actions.
The neuromodulators, DA and NE, primarily influence inputs from the LEC at both TA-CA1 and PP-DG synapses, in spite of the differences in neuronal morphology (Canto et al., 2008), presynaptic protein expression (Supporting Information Fig. 2), and PPF ratios (Fig. 2) between layer II (send axons to the DG) and III (send axons to area CA1) neurons in the EC. This implies that neuromodulator-mediated influence on the entorhinal–hippocampal connections might be based on the information-modality. We note here that we observed a different pattern of neuromodulator-mediated control in the mouse (C57BL/6J) hippocampus, where both DA and NE elicit significantly larger depression at medial than lateral TA-CA1 synapses (Ito et al., 2010). This modulatory difference in medial, and lateral TA-CA1 synapses implies a functional difference of the TA pathway between rat and mouse brain, which may explain the distinct behavioral deficits observed after the lesion/inactivation of SC-CA1 inputs in rat or mouse studies (Brun et al., 2002; Nakashiba et al., 2008).
In distal CA1, we previously demonstrated DA-induced disinhibition that reduces TA-pathway-mediated excitation of interneurons, resulting in frequency-dependent signal modulation as well as the enhancement of LTP at both TA- and SC-CA1 synapses (Ito and Schuman, 2007). The TA-pathway stimulation is known to evoke strong inhibitory responses in CA1 pyramidal neurons in slices (Empson and Heinemann, 1995; Dvorak-Carbone and Schuman, 1999); thus, disinhibition in this pathway will have a great impact on area CA1 output.
The EC, the origin of the TA pathway, is a major source of theta (4–12 Hz) and gamma (40–100 Hz) oscillatory activities (Chrobak et al., 2000); thus, the frequency-dependent effects, we observed, are predicted to modulate information flow in the circuit. We demonstrated the differential modulation of frequency-dependent signal transmission between DA and NE at lateral TA-CA1 synapses. Although both DA and NE similarly depressed low-frequency signals, only DA enhanced high-frequency signal transmission when compared with control. Thus, DA and NE may differentially gate a high-frequency range of oscillatory activities (e.g., gamma oscillations) in vivo.
We showed that the receptor antagonist clozapine abolished the differential c-Fos expression between proximal and distal CA1 observed following novel object or place exposure (Fig. 7), indicating the importance of neuromodulatory control in this circuit. We further observed a differential clozapine-sensitivity of c-Fos activation in distal CA1, depending on whether animals are exposed to novel objects or place (Fig. 7), which suggests that the hippocampus may use distinct encoding modes, with one mode being more neuromodulator-sensitive than the other. The main sites of clozapine’s action in our study are not clear, because it was administered systemically. We did not observe, however, a differential clozapine-sensitivity in proximal CA1 or CA3 between novel object and place exposure (Supporting Information Fig. 7A). Because neuromodulators primarily influence lateral TA-CA1 synapses in area CA1, these data are consistent with the idea that neuromodulators may allow differential encoding of nonspatial information in the hippocampus by controlling LEC inputs.
The hippocampus and its associated medial temporal lobe structures are thought to represent information about the environmental context (Myers and Gluck, 1994; Clark and Martin, 2005; Smith and Mizumori, 2006). To acquire such a representation, individual sensory inputs must be associated with the spatial geometry of the environment. Our data demonstrate that neuromodulators differentially control spatial and nonspatial information flow in entorhinal–hippocampal connections, which reveals a clear functional division along the transverse-axis of area CA1, emphasizing the importance of the EC-CA1 circuit. The differential gating of spatial and nonspatial information will extend the capacity of the hippocampus with optimal registering of new information in the circuit, allowing animals a more flexible adaptation to various changes in the environmental context.
Supplementary Material
Acknowledgments
We thank the members of the Schuman laboratory for discussions. E.M.S. was an Investigator of the Howard Hughes Medical Institute.
Footnotes
Additional Supporting Information may be found in the online version of this article.
REFERENCES
- Allen MT, Padilla Y, Myers CE, Gluck MA. Selective hippocampal lesions disrupt a novel cue effect but fail to eliminate blocking in rabbit eyeblink conditioning. Cogn Affect Behav Neurosci. 2002;2:318–328. doi: 10.3758/cabn.2.4.318. [DOI] [PubMed] [Google Scholar]
- Andersen P, Bliss TV, Lomo T, Olsen LI, Skrede KK. Lamellar organization of hippocampal excitatory pathways. Acta Physiol Scand. 1969;76:4A–5A. doi: 10.1111/j.1748-1716.1969.tb04499.x. [DOI] [PubMed] [Google Scholar]
- Ang CW, Carlson GC, Coulter DA. Hippocampal CA1 circuitry dynamically gates direct cortical inputs preferentially at theta frequencies. J Neurosci. 2005;25:9567–9580. doi: 10.1523/JNEUROSCI.2992-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: Adaptive gain and optimal performance. Annu Rev Neurosci. 2005;28:403–450. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- Baldessarini RJ, Frankenburg FR. Clozapine. A novel antipsychotic agent. N Engl J Med. 1991;324:746–754. doi: 10.1056/NEJM199103143241107. [DOI] [PubMed] [Google Scholar]
- Blackstad TW. Commissural connections of the hippocampal region in the rat, with special reference to their mode of termination. J Comp Neurol. 1956;105:417–537. doi: 10.1002/cne.901050305. [DOI] [PubMed] [Google Scholar]
- Brun VH, Otnass MK, Molden S, Steffenach HA, Witter MP, Moser MB, Moser EI. Place cells and place recognition maintained by direct entorhinal-hippocampal circuitry. Science. 2002;296:2243–2246. doi: 10.1126/science.1071089. [DOI] [PubMed] [Google Scholar]
- Buhusi CV, Gray JA, Schmajuk NA. Perplexing effects of hippocampal lesions on latent inhibition: A neural network solution. Behav Neurosci. 1998;112:316–351. doi: 10.1037//0735-7044.112.2.316. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Draguhn A. Neuronal oscillations in cortical networks. Science. 2004;304:1926–1929. doi: 10.1126/science.1099745. [DOI] [PubMed] [Google Scholar]
- Cajal SRY. Histologie du Systeme Nerveux de l’Hommes et des Vertebres. Maloine; Paris: 1911. [Google Scholar]
- Canto CB, Wouterlood FG, Witter MP. What does the anatomical organization of the entorhinal cortex tell us? Neural Plast. 2008;2008:381243. doi: 10.1155/2008/381243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen KC, Budygin EA. Extracting the basal extracellular dopamine concentrations from the evoked responses: Re-analysis of the dopamine kinetics. J Neurosci Methods. 2007;164:27–42. doi: 10.1016/j.jneumeth.2007.03.020. [DOI] [PubMed] [Google Scholar]
- Chrobak JJ, Lorincz A, Buzsaki G. Physiological patterns in the hippocampo-entorhinal cortex system. Hippocampus. 2000;10:457–465. doi: 10.1002/1098-1063(2000)10:4<457::AID-HIPO12>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Clark RE, Martin SJ. Interrogating rodents regarding their object and spatial memory. Curr Opin Neurobiol. 2005;15:593–598. doi: 10.1016/j.conb.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Cole AJ, Saffen DW, Baraban JM, Worley PF. Rapid increase of an immediate early gene messenger RNA in hippocampal neurons by synaptic NMDA receptor activation. Nature. 1989;340:474–476. doi: 10.1038/340474a0. [DOI] [PubMed] [Google Scholar]
- Colgin LL, Moser EI, Moser MB. Understanding memory through hippocampal remapping. Trends Neurosci. 2008;31:469–477. doi: 10.1016/j.tins.2008.06.008. [DOI] [PubMed] [Google Scholar]
- Dahl D, Sarvey JM. Norepinephrine induces pathway-specific long-lasting potentiation and depression in the hippocampal dentate gyrus. Proc Natl Acad Sci USA. 1989;86:4776–4780. doi: 10.1073/pnas.86.12.4776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dan Y, Poo MM. Spike timing-dependent plasticity of neural circuits. Neuron. 2004;44:23–30. doi: 10.1016/j.neuron.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Dolan RJ, Fletcher PC. Dissociating prefrontal and hippocampal function in episodic memory encoding. Nature. 1997;388:582–585. doi: 10.1038/41561. [DOI] [PubMed] [Google Scholar]
- Dolleman-Van Der Weel MJ, Witter MP. Projections from the nucleus reuniens thalami to the entorhinal cortex, hippocampal field CA1, and the subiculum in the rat arise from different populations of neurons. J Comp Neurol. 1996;364:637–650. doi: 10.1002/(SICI)1096-9861(19960122)364:4<637::AID-CNE3>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Dolorfo CL, Amaral DG. Entorhinal cortex of the rat: Organization of intrinsic connections. J Comp Neurol. 1998;398:49–82. doi: 10.1002/(sici)1096-9861(19980817)398:1<49::aid-cne4>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Dudman JT, Tsay D, Siegelbaum SA. A role for synaptic inputs at distal dendrites: Instructive signals for hippocampal long-term plasticity. Neuron. 2007;56:866–879. doi: 10.1016/j.neuron.2007.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvorak-Carbone H, Schuman EM. Patterned activity in stratum lacunosum moleculare inhibits CA1 pyramidal neuron firing. J Neurophysiol. 1999;82:3213–3222. doi: 10.1152/jn.1999.82.6.3213. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Lipton PA. Towards a functional organization of the medial temporal lobe memory system: Role of the parahippocampal and medial entorhinal cortical areas. Hippocampus. 2008;18:1314–1324. doi: 10.1002/hipo.20500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Ghundi M, Fletcher PJ, Drago J, Sibley DR, O’Dowd BF, George SR. Spatial learning deficit in dopamine D1 receptor knockout mice. Eur J Pharmacol. 1999;383:95–106. doi: 10.1016/s0014-2999(99)00573-7. [DOI] [PubMed] [Google Scholar]
- Empson RM, Heinemann U. The perforant path projection to hippocampal area CA1 in the rat hippocampal-entorhinal cortex combined slice. J Physiol. 1995;484(Pt 3):707–720. doi: 10.1113/jphysiol.1995.sp020697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel AK, Singer W. Temporal binding and the neural correlates of sensory awareness. Trends Cogn Sci. 2001;5:16–25. doi: 10.1016/s1364-6613(00)01568-0. [DOI] [PubMed] [Google Scholar]
- Ferbinteanu J, Holsinger RM, McDonald RJ. Lesions of the medial or lateral perforant path have different effects on hippocampal contributions to place learning and on fear conditioning to context. Behav Brain Res. 1999;101:65–84. doi: 10.1016/s0166-4328(98)00144-2. [DOI] [PubMed] [Google Scholar]
- Fleischmann A, Hvalby O, Jensen V, Strekalova T, Zacher C, Layer LE, Kvello A, Reschke M, Spanagel R, Sprengel R, Wagner EF, Gass P. Impaired long-term memory and NR2A-type NMDA receptor-dependent synaptic plasticity in mice lacking c-Fos in the CNS. J Neurosci. 2003;23:9116–9122. doi: 10.1523/JNEUROSCI.23-27-09116.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey U, Schroeder H, Matthies H. Dopaminergic antagonists prevent long-term maintenance of posttetanic LTP in the CA1 region of rat hippocampal slices. Brain Res. 1990;522:69–75. doi: 10.1016/0006-8993(90)91578-5. [DOI] [PubMed] [Google Scholar]
- Fyhn M, Molden S, Witter MP, Moser EI, Moser MB. Spatial representation in the entorhinal cortex. Science. 2004;305:1258–1264. doi: 10.1126/science.1099901. [DOI] [PubMed] [Google Scholar]
- Garris PA, Christensen JR, Rebec GV, Wightman RM. Realtime measurement of electrically evoked extracellular dopamine in the striatum of freely moving rats. J Neurochem. 1997;68:152–161. doi: 10.1046/j.1471-4159.1997.68010152.x. [DOI] [PubMed] [Google Scholar]
- Gasbarri A, Sulli A, Innocenzi R, Pacitti C, Brioni JD. Spatial memory impairment induced by lesion of the mesohippocampal dopaminergic system in the rat. Neuroscience. 1996;74:1037–1044. doi: 10.1016/0306-4522(96)00202-3. [DOI] [PubMed] [Google Scholar]
- Gasbarri A, Sulli A, Packard MG. The dopaminergic mesencephalic projections to the hippocampal formation in the rat. Prog Neuropsychopharmacol Biol Psychiatry. 1997;21:1–22. doi: 10.1016/s0278-5846(96)00157-1. [DOI] [PubMed] [Google Scholar]
- Gauthier M, Destrade C, Soumireu-Mourat B. Functional dissociation between lateral and medial entorhinal cortex in memory processes in mice. Behav Brain Res. 1983;9:111–117. doi: 10.1016/0166-4328(83)90017-7. [DOI] [PubMed] [Google Scholar]
- Gingrich JA, Caron MG. Recent advances in the molecular biology of dopamine receptors. Annu Rev Neurosci. 1993;16:299–321. doi: 10.1146/annurev.ne.16.030193.001503. [DOI] [PubMed] [Google Scholar]
- Grace AA. Phasic versus tonic dopamine release and the modulation of dopamine system responsivity: A hypothesis for the etiology of schizophrenia. Neuroscience. 1991;41:1–24. doi: 10.1016/0306-4522(91)90196-u. [DOI] [PubMed] [Google Scholar]
- Guzowski JF, Timlin JA, Roysam B, McNaughton BL, Worley PF, Barnes CA. Mapping behaviorally relevant neural circuits with immediate-early gene expression. Curr Opin Neurobiol. 2005;15:599–606. doi: 10.1016/j.conb.2005.08.018. [DOI] [PubMed] [Google Scholar]
- Hargreaves EL, Rao G, Lee I, Knierim JJ. Major dissociation between medial and lateral entorhinal input to dorsal hippocampus. Science. 2005;308:1792–1794. doi: 10.1126/science.1110449. [DOI] [PubMed] [Google Scholar]
- Harley CW. Norepinephrine and dopamine as learning signals. Neural Plast. 2004;11:191–204. doi: 10.1155/NP.2004.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME. Neuromodulation and cortical function: Modeling the physiological basis of behavior. Behav Brain Res. 1995;67:1–27. doi: 10.1016/0166-4328(94)00113-t. [DOI] [PubMed] [Google Scholar]
- Herkenham M. The connections of the nucleus reuniens thalami: Evidence for a direct thalamo-hippocampal pathway in the rat. J Comp Neurol. 1978;177:589–610. doi: 10.1002/cne.901770405. [DOI] [PubMed] [Google Scholar]
- Hillman KL, Knudson CA, Carr PA, Doze VA, Porter JE. Adrenergic receptor characterization of CA1 hippocampal neurons using real time single cell RT-PCR. Brain Res Mol Brain Res. 2005;139:267–276. doi: 10.1016/j.molbrainres.2005.05.033. [DOI] [PubMed] [Google Scholar]
- Horvitz JC. Mesolimbocortical and nigrostriatal dopamine responses to salient non-reward events. Neuroscience. 2000;96:651–656. doi: 10.1016/s0306-4522(00)00019-1. [DOI] [PubMed] [Google Scholar]
- Hunsaker MR, Mooy GG, Swift JS, Kesner RP. Dissociations of the medial and lateral perforant path projections into dorsal DG, CA3, and CA1 for spatial and nonspatial (visual object) information processing. Behav Neurosci. 2007;121:742–750. doi: 10.1037/0735-7044.121.4.742. [DOI] [PubMed] [Google Scholar]
- Hunsaker MR, Rosenberg JS, Kesner RP. The role of the dentate gyrus, CA3a, b, and CA3c for detecting spatial and environmental novelty. Hippocampus. 2008;18:1064–1073. doi: 10.1002/hipo.20464. [DOI] [PubMed] [Google Scholar]
- Ihalainen JA, Riekkinen P, Jr., Feenstra MG. Comparison of dopamine and noradrenaline release in mouse prefrontal cortex, striatum and hippocampus using microdialysis. Neurosci Lett. 1999;277:71–74. doi: 10.1016/s0304-3940(99)00840-x. [DOI] [PubMed] [Google Scholar]
- Ito HT, Schuman EM. Frequency-dependent gating of synaptic transmission and plasticity by dopamine. Front Neural Circuits. 2007;1:1. doi: 10.3389/neuro.04.001.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito HT, Schuman EM. Frequency-dependent signal transmission and modulation by neuromodulators. Front Neurosci. 2008;2:138–144. doi: 10.3389/neuro.01.027.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito HT, Smith SE, Hsiao E, Patterson PH. Maternal immune activation alters nonspatial information processing in the hippocampus of the adult offspring. Brain Behav Immun. 2010;24:930–941. doi: 10.1016/j.bbi.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarsky T, Roxin A, Kath WL, Spruston N. Conditional dendritic spike propagation following distal synaptic activation of hippocampal CA1 pyramidal neurons. Nat Neurosci. 2005;8:1667–1676. doi: 10.1038/nn1599. [DOI] [PubMed] [Google Scholar]
- Knierim JJ, Lee I, Hargreaves EL. Hippocampal place cells: Parallel input streams, subregional processing, and implications for episodic memory. Hippocampus. 2006;16:755–764. doi: 10.1002/hipo.20203. [DOI] [PubMed] [Google Scholar]
- Knight R. Contribution of human hippocampal region to novelty detection. Nature. 1996;383:256–259. doi: 10.1038/383256a0. [DOI] [PubMed] [Google Scholar]
- Kosel KC, Van Hoesen GW, Rosene DL. A direct projection from the perirhinal cortex (area 35) to the subiculum in the rat. Brain Res. 1983;269:347–351. doi: 10.1016/0006-8993(83)90144-0. [DOI] [PubMed] [Google Scholar]
- Kumaran D, Maguire EA. Which computational mechanisms operate in the hippocampus during novelty detection? Hippocampus. 2007;17:735–748. doi: 10.1002/hipo.20326. [DOI] [PubMed] [Google Scholar]
- Laurent G. Olfactory network dynamics and the coding of multidimensional signals. Nat Rev Neurosci. 2002;3:884–895. doi: 10.1038/nrn964. [DOI] [PubMed] [Google Scholar]
- Lemon N, Manahan-Vaughan D. Dopamine D1/D5 receptors gate the acquisition of novel information through hippocampal long-term potentiation and long-term depression. J Neurosci. 2006;26:7723–7729. doi: 10.1523/JNEUROSCI.1454-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy WB, Desmond NL, Zhang DX. Perforant path activation modulates the induction of long-term potentiation of the schaffer collateral–hippocampal CA1 response: Theoretical and experimental analyses. Learn Mem. 1998;4:510–518. doi: 10.1101/lm.4.6.510. [DOI] [PubMed] [Google Scholar]
- Li S, Cullen WK, Anwyl R, Rowan MJ. Dopamine-dependent facilitation of LTP induction in hippocampal CA1 by exposure to spatial novelty. Nat Neurosci. 2003;6:526–531. doi: 10.1038/nn1049. [DOI] [PubMed] [Google Scholar]
- Lisman JE, Grace AA. The hippocampal-VTA loop: Controlling the entry of information into long-term memory. Neuron. 2005;46:703–713. doi: 10.1016/j.neuron.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Manahan-Vaughan D, Braunewell KH. Novelty acquisition is associated with induction of hippocampal long-term depression. Proc Natl Acad Sci USA. 1999;96:8739–8744. doi: 10.1073/pnas.96.15.8739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manns JR, Eichenbaum H. Evolution of declarative memory. Hippocampus. 2006;16:795–808. doi: 10.1002/hipo.20205. [DOI] [PubMed] [Google Scholar]
- Markram H, Gupta A, Uziel A, Wang Y, Tsodyks M. Information processing with frequency-dependent synaptic connections. Neurobiol Learn Mem. 1998;70:101–112. doi: 10.1006/nlme.1998.3841. [DOI] [PubMed] [Google Scholar]
- McNaughton BL. Evidence for two physiologically distinct perforant pathways to the fascia dentata. Brain Res. 1980;199:1–19. doi: 10.1016/0006-8993(80)90226-7. [DOI] [PubMed] [Google Scholar]
- Melloni RH, Jr., Hemmendinger LM, Hamos JE, DeGennaro LJ. Synapsin I gene expression in the adult rat brain with comparative analysis of mRNA and protein in the hippocampus. J Comp Neurol. 1993;327:507–520. doi: 10.1002/cne.903270404. [DOI] [PubMed] [Google Scholar]
- Milner TA, Lee A, Aicher SA, Rosin DL. Hippocampal α2a-ad-renergic receptors are located predominantly presynaptically but are also found postsynaptically and in selective astrocytes. J Comp Neurol. 1998;395:310–327. [PubMed] [Google Scholar]
- Morgan JI, Curran T. Stimulus-transcription coupling in the nervous system: Involvement of the inducible proto-oncogenes fos and jun. Annu Rev Neurosci. 1991;14:421–451. doi: 10.1146/annurev.ne.14.030191.002225. [DOI] [PubMed] [Google Scholar]
- Murchison CF, Zhang XY, Zhang WP, Ouyang M, Lee A, Thomas SA. A distinct role for norepinephrine in memory retrieval. Cell. 2004;117:131–143. doi: 10.1016/s0092-8674(04)00259-4. [DOI] [PubMed] [Google Scholar]
- Myers CE, Gluck MA. Context, conditioning, and hippocampal rerepresentation in animal learning. Behav Neurosci. 1994;108:835–847. doi: 10.1037//0735-7044.108.5.835. [DOI] [PubMed] [Google Scholar]
- Naber PA, Witter MP, Lopes da Silva FH. Evidence for a direct projection from the postrhinal cortex to the subiculum in the rat. Hippocampus. 2001;11:105–117. doi: 10.1002/hipo.1029. [DOI] [PubMed] [Google Scholar]
- Naber PA, Witter MP, Lopez da Silva FH. Perirhinal cortex input to the hippocampus in the rat: evidence for parallel pathways, both direct and indirect. A combined physiological and anatomical study. Eur J Neurosci. 1999;11:4119–4133. doi: 10.1046/j.1460-9568.1999.00835.x. [DOI] [PubMed] [Google Scholar]
- Nakashiba T, Young JZ, McHugh TJ, Buhl DL, Tonegawa S. Transgenic inhibition of synaptic transmission reveals role of CA3 output in hippocampal learning. Science. 2008;319:1260–1264. doi: 10.1126/science.1151120. [DOI] [PubMed] [Google Scholar]
- Otmakhova NA, Lisman JE. D1/D5 dopamine receptor activation increases the magnitude of early long-term potentiation at CA1 hippocampal synapses. J Neurosci. 1996;16:7478–7486. doi: 10.1523/JNEUROSCI.16-23-07478.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otmakhova NA, Lisman JE. Dopamine selectively inhibits the direct cortical pathway to the CA1 hippocampal region. J Neurosci. 1999;19:1437–1445. doi: 10.1523/JNEUROSCI.19-04-01437.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otmakhova NA, Lewey J, Asrican B, Lisman JE. Inhibition of perforant path input to the CA1 region by serotonin and noradrenaline. J Neurophysiol. 2005;94:1413–1422. doi: 10.1152/jn.00217.2005. [DOI] [PubMed] [Google Scholar]
- Oyler GA, Higgins GA, Hart RA, Battenberg E, Billingsley M, Bloom FE, Wilson MC. The identification of a novel synaptosomal-associated protein, SNAP-25, differentially expressed by neuronal subpopulations. J Cell Biol. 1989;109(6, Pt 1):3039–3052. doi: 10.1083/jcb.109.6.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkin AJ. Human memory: Novelty, association and the brain. Curr Biol. 1997;7:R768–R769. doi: 10.1016/s0960-9822(06)00400-3. [DOI] [PubMed] [Google Scholar]
- Pelletier MR, Kirkby RD, Jones SJ, Corcoran ME. Pathway specificity of noradrenergic plasticity in the dentate gyrus. Hippocampus. 1994;4:181–188. doi: 10.1002/hipo.450040208. [DOI] [PubMed] [Google Scholar]
- Ranganath C, Rainer G. Neural mechanisms for detecting and remembering novel events. Nat Rev Neurosci. 2003;4:193–202. doi: 10.1038/nrn1052. [DOI] [PubMed] [Google Scholar]
- Remondes M, Schuman EM. Direct cortical input modulates plasticity and spiking in CA1 pyramidal neurons. Nature. 2002;416:736–740. doi: 10.1038/416736a. [DOI] [PubMed] [Google Scholar]
- Remondes M, Schuman EM. Role for a cortical input to hippocampal area CA1 in the consolidation of a long-term memory. Nature. 2004;431:699–703. doi: 10.1038/nature02965. [DOI] [PubMed] [Google Scholar]
- Richter K, Langnaese K, Kreutz MR, Olias G, Zhai R, Scheich H, Garner CC, Gundelfinger ED. Presynaptic cytomatrix protein bassoon is localized at both excitatory and inhibitory synapses of rat brain. J Comp Neurol. 1999;408:437–448. doi: 10.1002/(sici)1096-9861(19990607)408:3<437::aid-cne9>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Rutishauser U, Mamelak AN, Schuman EM. Single-trial learning of novel stimuli by individual neurons of the human hippocampus-amygdala complex. Neuron. 2006;49:805–813. doi: 10.1016/j.neuron.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Sara SJ. The locus coeruleus and noradrenergic modulation of cognition. Nat Rev Neurosci. 2009;10:211–223. doi: 10.1038/nrn2573. [DOI] [PubMed] [Google Scholar]
- Schultz W. Predictive reward signal of dopamine neurons. J Neurophysiol. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. J Neurochem. 1957;20:11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DM, Mizumori SJ. Hippocampal place cells, context, and episodic memory. Hippocampus. 2006;16:716–729. doi: 10.1002/hipo.20208. [DOI] [PubMed] [Google Scholar]
- Squire LR, Stark CE, Clark RE. The medial temporal lobe. Annu Rev Neurosci. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- Stern CE, Corkin S, Gonzalez RG, Guimaraes AR, Baker JR, Jennings PJ, Carr CA, Sugiura RM, Vedantham V, Rosen BR. The hippocampal formation participates in novel picture encoding: Evidence from functional magnetic resonance imaging. Proc Natl Acad Sci USA. 1996;93:8660–8665. doi: 10.1073/pnas.93.16.8660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward O. Topographic organization of the projections from the entorhinal area to the hippocampal formation of the rat. J Comp Neurol. 1976;167:285–314. doi: 10.1002/cne.901670303. [DOI] [PubMed] [Google Scholar]
- Swanson L. Handbook of Chemical neuroanatomy. Elsevier; Amsterdam: 1987. The Limbic Region I. The Septohippocampal System; pp. 125–227. [Google Scholar]
- Tamamaki N, Nojyo Y. Preservation of topography in the connections between the subiculum, field CA1, and the entorhinal cortex in rats. J Comp Neurol. 1995;353:379–390. doi: 10.1002/cne.903530306. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG, Haxby JV. ‘What’ and ‘where’ in the human brain. Curr Opin Neurobiol. 1994;4:157–165. doi: 10.1016/0959-4388(94)90066-3. [DOI] [PubMed] [Google Scholar]
- Vankov A, Herve-Minvielle A, Sara SJ. Response to novelty and its rapid habituation in locus coeruleus neurons of the freely exploring rat. Eur J Neurosci. 1995;7:1180–1187. doi: 10.1111/j.1460-9568.1995.tb01108.x. [DOI] [PubMed] [Google Scholar]
- Vann SD, Brown MW, Erichsen JT, Aggleton JP. Fos imaging reveals differential patterns of hippocampal and parahippocampal subfield activation in rats in response to different spatial memory tests. J Neurosci. 2000;20:2711–2718. doi: 10.1523/JNEUROSCI.20-07-02711.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinogradova OS. Hippocampus as comparator: Role of the two input and two output systems of the hippocampus in selection and registration of information. Hippocampus. 2001;11:578–598. doi: 10.1002/hipo.1073. [DOI] [PubMed] [Google Scholar]
- Wan H, Aggleton JP, Brown MW. Different contributions of the hippocampus and perirhinal cortex to recognition memory. J Neurosci. 1999;19:1142–1148. doi: 10.1523/JNEUROSCI.19-03-01142.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witter MP, Amaral DG. Hippocampal Formation. In: Paxinos G, editor. The Rat Nervous System. Elsevier; Amsterdam: 2004. [Google Scholar]
- Witter MP, Naber PA, van Haeften T, Machielsen WC, Rombouts SA, Barkhof F, Scheltens P, Lopes da Silva FH. Cortico-hippocampal communication by way of parallel parahippocampal-subicular pathways. Hippocampus. 2000;10:398–410. doi: 10.1002/1098-1063(2000)10:4<398::AID-HIPO6>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Worley PF, Bhat RV, Baraban JM, Erickson CA, McNaughton BL, Barnes CA. Thresholds for synaptic activation of transcription factors in hippocampus: Correlation with long-term enhancement. J Neurosci. 1993;13:4776–4786. doi: 10.1523/JNEUROSCI.13-11-04776.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wouterlood FG, Saldana E, Witter MP. Projection from the nucleus reuniens thalami to the hippocampal region: Light and electron microscopic tracing study in the rat with the anterograde tracer Phaseolus vulgaris-leucoagglutinin. J Comp Neurol. 1990;296:179–203. doi: 10.1002/cne.902960202. [DOI] [PubMed] [Google Scholar]
- Wyss JM. An autoradiographic study of the efferent connections of the entorhinal cortex in the rat. J Comp Neurol. 1981;199:495–512. doi: 10.1002/cne.901990405. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.