Abstract
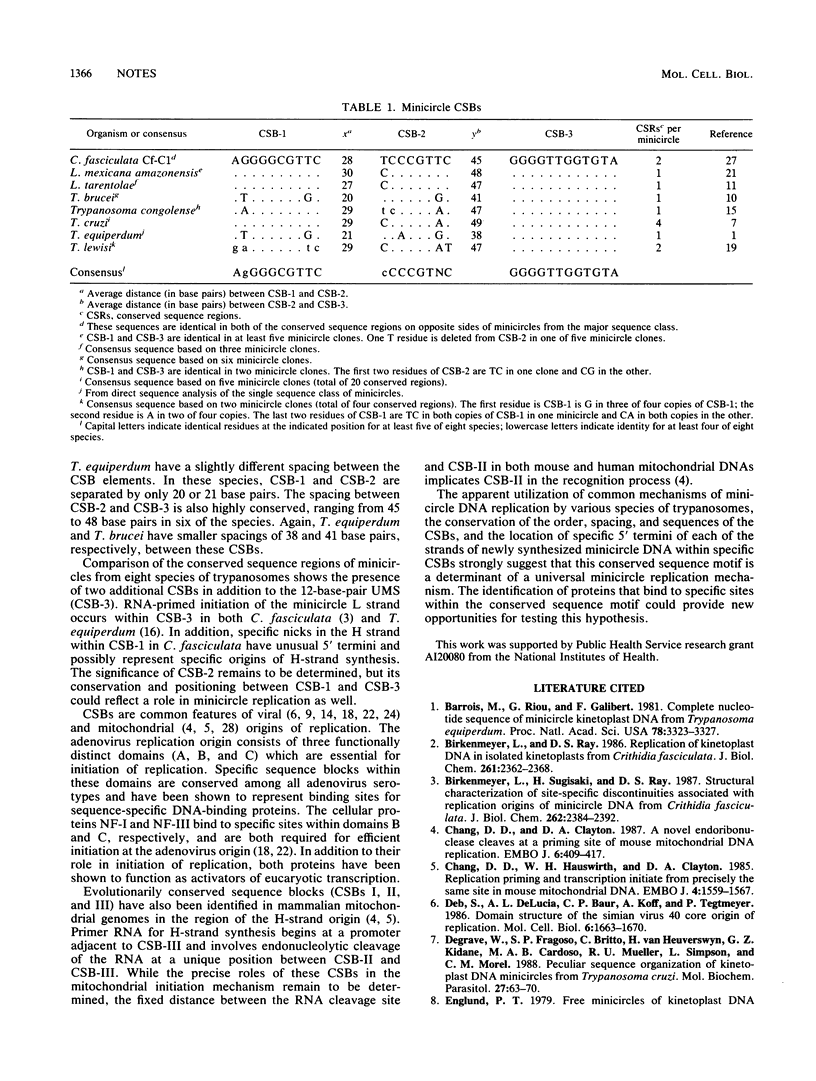
Kinetoplast DNA minicircles from various species of trypanosomes are heterogeneous in nucleotide sequence to various degrees but in all instances contain a conserved sequence region of 100 to 200 base pairs present in one, two, or four copies per minicircle. Comparison of the conserved sequence regions of minicircles from eight species of trypanosomes revealed a common sequence motif consisting of three conserved sequence blocks (CSBs) present in the same order and with similar spacing in all species. In addition to the invariant 12-base-pair universal minicircle sequence (CSB-3), a 10-base-pair sequence (CSB-1) and an 8-base-pair sequence (CSB-2) are highly conserved in all minicircles. The overlap of CSB-1 and CSB-3 with previously identified 5' termini of newly synthesized minicircle H and L strands, respectively, and the presence of this conserved sequence motif in minicircles from diverse species suggest that these CSBs may determine a common mechanism of minicircle replication.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrois M., Riou G., Galibert F. Complete nucleotide sequence of minicircle kinetoplast DNA from Trypanosoma equiperdum. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3323–3327. doi: 10.1073/pnas.78.6.3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkenmeyer L., Ray D. S. Replication of kinetoplast DNA in isolated kinetoplasts from Crithidia fasciculata. Identification of minicircle DNA replication intermediates. J Biol Chem. 1986 Feb 15;261(5):2362–2368. [PubMed] [Google Scholar]
- Birkenmeyer L., Sugisaki H., Ray D. S. Structural characterization of site-specific discontinuities associated with replication origins of minicircle DNA from Crithidia fasciculata. J Biol Chem. 1987 Feb 15;262(5):2384–2392. [PubMed] [Google Scholar]
- Chang D. D., Clayton D. A. A novel endoribonuclease cleaves at a priming site of mouse mitochondrial DNA replication. EMBO J. 1987 Feb;6(2):409–417. doi: 10.1002/j.1460-2075.1987.tb04770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang D. D., Hauswirth W. W., Clayton D. A. Replication priming and transcription initiate from precisely the same site in mouse mitochondrial DNA. EMBO J. 1985 Jun;4(6):1559–1567. doi: 10.1002/j.1460-2075.1985.tb03817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deb S., DeLucia A. L., Baur C. P., Koff A., Tegtmeyer P. Domain structure of the simian virus 40 core origin of replication. Mol Cell Biol. 1986 May;6(5):1663–1670. doi: 10.1128/mcb.6.5.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degrave W., Fragoso S. P., Britto C., van Heuverswyn H., Kidane G. Z., Cardoso M. A., Mueller R. U., Simpson L., Morel C. M. Peculiar sequence organization of kinetoplast DNA minicircles from Trypanosoma cruzi. Mol Biochem Parasitol. 1988 Jan 1;27(1):63–70. doi: 10.1016/0166-6851(88)90025-4. [DOI] [PubMed] [Google Scholar]
- Englund P. T. Free minicircles of kinetoplast DNA in Crithidia fasciculata. J Biol Chem. 1979 Jun 10;254(11):4895–4900. [PubMed] [Google Scholar]
- Fairman M. P., Stillman B. Cellular factors required for multiple stages of SV40 DNA replication in vitro. EMBO J. 1988 Apr;7(4):1211–1218. doi: 10.1002/j.1460-2075.1988.tb02933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasmer D. P., Stuart K. Conservation of kinetoplastid minicircle characteristics without nucleotide sequence conservation. Mol Biochem Parasitol. 1986 Mar;18(3):257–269. doi: 10.1016/0166-6851(86)90084-8. [DOI] [PubMed] [Google Scholar]
- Kidane G. Z., Hughes D., Simpson L. Sequence heterogeneity and anomalous electrophoretic mobility of kinetoplast minicircle DNA from Leishmania tarentolae. Gene. 1984 Mar;27(3):265–277. doi: 10.1016/0378-1119(84)90071-4. [DOI] [PubMed] [Google Scholar]
- Kitchin P. A., Klein V. A., Englund P. T. Intermediates in the replication of kinetoplast DNA minicircles. J Biol Chem. 1985 Mar 25;260(6):3844–3851. [PubMed] [Google Scholar]
- Kitchin P. A., Klein V. A., Fein B. I., Englund P. T. Gapped Minicircles. A novel replication intermediate of kinetoplast DNA. J Biol Chem. 1984 Dec 25;259(24):15532–15539. [PubMed] [Google Scholar]
- Li J. J., Peden K. W., Dixon R. A., Kelly T. Functional organization of the simian virus 40 origin of DNA replication. Mol Cell Biol. 1986 Apr;6(4):1117–1128. doi: 10.1128/mcb.6.4.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasir A., Cook G. A., Donelson J. E. Sequences of two kinetoplast minicircle DNAs of Trypanosoma (Nannomonas) congolense. Mol Biochem Parasitol. 1987 Jul;24(3):295–300. doi: 10.1016/0166-6851(87)90162-9. [DOI] [PubMed] [Google Scholar]
- Ntambi J. M., Englund P. T. A gap at a unique location in newly replicated kinetoplast DNA minicircles from Trypanosoma equiperdum. J Biol Chem. 1985 May 10;260(9):5574–5579. [PubMed] [Google Scholar]
- Ntambi J. M., Shapiro T. A., Ryan K. A., Englund P. T. Ribonucleotides associated with a gap in newly replicated kinetoplast DNA minicircles from Trypanosoma equiperdum. J Biol Chem. 1986 Sep 5;261(25):11890–11895. [PubMed] [Google Scholar]
- O'Neill E. A., Kelly T. J. Purification and characterization of nuclear factor III (origin recognition protein C), a sequence-specific DNA binding protein required for efficient initiation of adenovirus DNA replication. J Biol Chem. 1988 Jan 15;263(2):931–937. [PubMed] [Google Scholar]
- Ponzi M., Birago C., Battaglia P. A. Two identical symmetrical regions in the minicircle structure of Trypanosoma lewisi kinetoplast DNA. Mol Biochem Parasitol. 1984 Sep;13(1):111–119. doi: 10.1016/0166-6851(84)90105-1. [DOI] [PubMed] [Google Scholar]
- Ray D. S. Kinetoplast DNA minicircles: high-copy-number mitochondrial plasmids. Plasmid. 1987 May;17(3):177–190. doi: 10.1016/0147-619x(87)90026-6. [DOI] [PubMed] [Google Scholar]
- Rogers W. O., Wirth D. F. Generation of sequence diversity in the kinetoplast DNA minicircles of Leishmania mexicana amazonensis. Mol Biochem Parasitol. 1988 Jul;30(1):1–8. doi: 10.1016/0166-6851(88)90126-0. [DOI] [PubMed] [Google Scholar]
- Rosenfeld P. J., O'Neill E. A., Wides R. J., Kelly T. J. Sequence-specific interactions between cellular DNA-binding proteins and the adenovirus origin of DNA replication. Mol Cell Biol. 1987 Feb;7(2):875–886. doi: 10.1128/mcb.7.2.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan K. A., Shapiro T. A., Rauch C. A., Englund P. T. Replication of kinetoplast DNA in trypanosomes. Annu Rev Microbiol. 1988;42:339–358. doi: 10.1146/annurev.mi.42.100188.002011. [DOI] [PubMed] [Google Scholar]
- Sekimizu K., Bramhill D., Kornberg A. Sequential early stages in the in vitro initiation of replication at the origin of the Escherichia coli chromosome. J Biol Chem. 1988 May 25;263(15):7124–7130. [PubMed] [Google Scholar]
- Simpson L. Kinetoplast DNA in trypanosomid flagellates. Int Rev Cytol. 1986;99:119–179. doi: 10.1016/s0074-7696(08)61426-6. [DOI] [PubMed] [Google Scholar]
- Simpson L. The mitochondrial genome of kinetoplastid protozoa: genomic organization, transcription, replication, and evolution. Annu Rev Microbiol. 1987;41:363–382. doi: 10.1146/annurev.mi.41.100187.002051. [DOI] [PubMed] [Google Scholar]
- Sugisaki H., Ray D. S. DNA sequence of Crithidia fasciculata kinetoplast minicircles. Mol Biochem Parasitol. 1987 Apr;23(3):253–263. doi: 10.1016/0166-6851(87)90032-6. [DOI] [PubMed] [Google Scholar]
- Walberg M. W., Clayton D. A. Sequence and properties of the human KB cell and mouse L cell D-loop regions of mitochondrial DNA. Nucleic Acids Res. 1981 Oct 24;9(20):5411–5421. doi: 10.1093/nar/9.20.5411. [DOI] [PMC free article] [PubMed] [Google Scholar]


