Abstract
Congenital Anomalies of the Lower Urinary Tract (CALUT) are a family of birth defects of the ureter, the bladder and the urethra. CALUT includes ureteral anomalies such as congenital abnormalities of the ureteropelvic junction (UPJ) and ureterovesical junction (UVJ), and birth defects of the bladder and the urethra such as bladder-exstrophy-epispadias complex (BEEC), prune belly syndrome (PBS), and posterior urethral valves (PUV). CALUT is one of the most common birth defects and is often associated with antenatal hydronephrosis, vesicoureteral reflux (VUR), urinary tract obstruction, urinary tract infections (UTI), chronic kidney disease and renal failure in children. Here, we discuss the current genetic and molecular knowledge about lower urinary tract development and genetic basis of CALUT in both human and mouse models. We provide an overview of the developmental processes leading to the formation of the ureter, bladder, and urethra, and different genes and signaling pathways controlling these developmental processes. Human genetic disorders that affect the ureter, bladder and urethra and associated gene mutations are also presented. As we are entering the post-genomic era of personalized medicine, information in this article may provide useful interpretation for the genetic and genomic test results collected from patients with lower urinary tract birth defects. With evidence-based interpretations, clinicians may provide more effective personalized therapies to patients and genetic counseling for their families.
INTRODUCTION
Each year an estimated six percent of total births worldwide (~ 8 million children) including three percent of all live births in the United States (more than 120,000 babies) are born with a serious birth defect of genetic origin.1–3 Among these, as many as one percent of human fetuses have congenital anomalies of the kidney and urinary tract (CAKUT), which is a family of birth defects including kidney anomalies such as renal hypodysplasia and hydronephrosis, and lower urinary tract (LUT) anomalies such as vesicoureteral reflux (VUR), urinary tract obstruction, bladder and urethral abnormalities.4–6 Although CAKUT is a complex genetically heterogeneous developmental disorder with variable phenotype, it can be caused by mutations in a single gene that controls early kidney and lower urinary tract development.7–11 CAKUT is a leading cause of urinary tract infection (UTI), chronic kidney disease and renal failure in children and may also manifests as primary renal disease in adults as more children with urinary tract birth defects survive to adulthood.12–14 However, little is known about the contribution of congenital lower urinary tract malformations to chronic kidney disease and renal failure in CAKUT patients as we lack a comprehensive understanding of the genetic and molecular basis of the lower urinary tract development. Therefore, it is challenging to provide genetic counseling, molecular diagnosis, and personalized medical/surgical management to patients with these broad clinical conditions without a clear understanding of their developmental etiology.15, 16
The urinary system is a multi-component organ system, whose primary function is to produce, transport, store, and eliminate urine in order to maintain body homoeostasis by controlling the water and ionic balance of the blood. Anatomically, these functions are served by an upper unit, the kidney, which filters and modifies the blood to produce urine, and a lower unit consisting of the ureter, the bladder, and the urethra, which transports, stores and eliminates the urine to the outside. Normal development of the upper unit kidney and associated congenital renal anomalies have been well reviewed recently17–23. In this article, we focus on current genetic and molecular knowledge of lower urinary tract development and related birth defects of the ureter, the bladder and the urethra in both human and mouse models, which are collectively named CALUT (Congenital Anomalies of the Lower Urinary Tract) in this review. We will describe different molecular pathways controlling lower urinary tract development as well as human genetic disorders affecting the lower urinary tract. We believe that understanding the genetic basis of CALUT in patients can help scientists and clinicians to decipher the molecular mechanism of normal developmental processes of the lower urinary tract and discover more CALUT causative genes.
OVERVIEW OF LOWER URINARY TRACT DEVELOPMENT
Development of the ureter and ureteral peristaltic machinery
The kidney and ureter share a common ontogenic origin, the intermediate mesoderm, in early embryos when an epithelial outpouching called the ureteric bud (UB) sprouts from the caudal region of the Wolffian duct (also called mesonephric duct) and invades adjacent metanephric mesenchyme (MM) (Figure 1A). This process begins at around 4 weeks of gestation in human and at embryonic 10.5 days (E10.5) in mouse. After the ureteric bud invasion into the metanephric mesenchyme, the reciprocal interaction between the tip of the ureteric bud and the metanephric mesenchyme results in multiple rounds of UB branching morphogenesis to form the collecting system, while mesenchymal-to-epithelial transition (MET) of the MM leads to the formation of the nephron.24 These developmental processes ultimately give rise to a functional kidney that starts to produce urine at ~10 weeks of gestation in human and at ~E16.5 in mice. At the same time, the trunk of the ureteric bud (i.e. the UB portion remaining outside of the metanephric mesenchyme) gains a different fate and elongates without branching to form the ureter, a muscular tube structure transporting urine from the kidney to the bladder (Figure 1B, 1C).25 Together with the growth of the caudal part of the body during fetal development, the elongation of the ureter leads the ascent of the kidney to its final position at the level of upper lumber vertebrae.
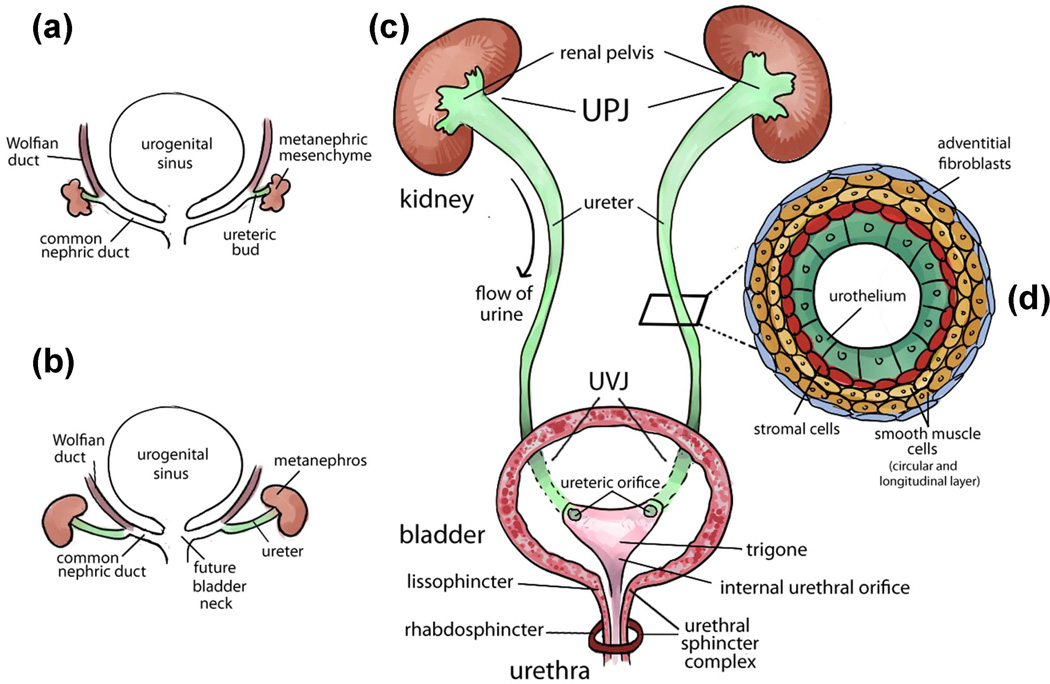
FIGURE 1.
Urinary tract development and structure. (a) Early development of the urinary tract (4th week of gestation in human and E10.5 in mice). An epithelial diverticulum called ureteric bud (UB) emanates from the Wolffian duct and grows into an adjacent group of mesenchymal cells (metanephric mesenchyme). (b) Elongation of the ureter and formation of the kidney (metanephros) during development. The common nephric ducts shorten, expand and integrate into the urogenital sinus (the future bladder) close to the region where the future bladder neck is located. (c) Structure of mature urinary tract in human and mice. Urine flows from the renal pelvis in the kidney through the ureter to the bladder for storage and eliminates to the outside through the urethra. The ureter is connected to the kidney at the ureteropelvic junction (UPJ) and is connected to the bladder at the ureterovesical junction (UVJ). Inside the bladder, two ureteric orifices and the internal urethral orifice form the trigone. The urethral sphincter complex includes the lissosphincter which is a continuation of the bladder smooth muscle and the rhabdosphincter which consists of striated muscles. (d) Transverse section of the mature ureter depicts four layers of cells: urothelium, stromal cells, smooth muscle cells and adventitial fibroblasts.
Similar to the kidney development that is guided by the reciprocal interaction between the epithelial cells in the UB tips and the mesenchymal cells in the MM, the morphogenesis of the ureter also requires a close interaction between the inner ureteral epithelial cells and the surrounding ureteral mesenchymal cells. In response to the molecular signals from the ureteral epithelial and mesenchymal cells, the early simple cuboidal ureteral epithelial cells differentiate into the multilayered urothelium (also called transitional epithelium, a part of mucosa after maturation). The urothelium is covered by urothelial plaques expressing uroplakin proteins and is impermeable to urine and its caustic effect.26–28 This process occurs around 10 weeks in human and E16.5 in mouse, which coincides with the beginning of urine production by the embryonic kidney. Meanwhile, the mesenchymal cells differentiate into the stromal cells, smooth muscle cells, and adventitial fibroblasts (also called serosa after maturation) (Figure 1D). Differentiated smooth muscle cells are further organized into layers with inner circular and outer longitudinal orientation, and are characterized with strong expression of α-smooth muscle actin.29–32 Interestingly, the ureter smooth muscle differentiation occurs in an ascending fashion from the distal ureter close to the bladder (i.e. ureterovesical junction - UVJ) to the proximal ureter that is next to the intrarenal collecting system (i.e. ureteropelvic junction - UPJ).30 This developmental process is in the opposite direction to the propagation of ureteral peristaltic waves that transport urine from the kidney to the bladder (Figure 1C).
Ureteral peristaltic waves are initiated in the renal pelvis and are propagated rhythmically through the ureter wall to the bladder. This contractile activity is triggered by special pacemaker cells located in the most proximal calyceal region of the pelvic-kidney junction, which produces regular pulsatile electrical signals transmitted along the electrically active “typical” smooth muscle cells (Typical SMC) in the ureter.33 Two types of special pacemaker cells with electrical rhythmicity have been identified in the renal pelvis and ureter. The primary pacemaker cells are also called “atypical” smooth muscle cells (Atypical SMC) because they contain fewer contractile filaments with weak α-smooth muscle actin expression compared to the regular “typical” smooth muscle cells that are most abundant in the ureter. Atypical SMCs are mainly located within the most proximal region of the renal pelvis and have many morphological features similar to those of the cardiac sinoatrial pacemaker cells.34 The other type of pacemaker cells in the renal pelvis and ureter is the interstitial cells of Cajal (ICC) – like cells (ICC-LCs), which are characterized by the expression of the proto-oncogene Kit and have thin and long cytoplasmic processes that are similar to those of the intestinal ICCs.35, 36 These ICC-LCs are located sparsely throughout the ureter (including renal pelvis, UPJ, UVJ), and among atypical SMCs, typical SMC (including inner circular and outer longitudinal muscle layers), the lamina propria underneath the urothelium, and neurons.36, 37 However, the distribution of ICC-LCs is not even, with most ICC-LCs located in the proximal renal pelvis and reduced cell density in the distal segments of the ureter.36, 37 The ICC-LCs can produce electrical slow-wave potentials that control the propagation of the unidirectional ureteral peristaltic activity.37
The molecular mechanism and physiological functions of the ureteral peristaltic machinery are still ill-defined. It has been suggested that atypical SMCs in the tip of renal pelvis act as the primary pacemaker to initiate spontaneous transient potentials that are propagated and modulated by ICC-LCs to the adjacent typical SMCs in order to trigger intermediate action potentials and ureteral smooth muscle contraction. The ICC-LCs may also produce additional autorhythmicity and can take over pacemaking in the renal pelvis and ureter in the absence of the primary pacemaker activity from the atypical SMCs.37, 38 The development of the ureteral peristaltic machinery is also not well understood. A recent study shows that mouse Kit-expressing ICC-LCs are first detected at E15.5 in a subset of renal epithelial cells and cells of the ureteropelvic adventitia.35 This developmental time point is followed by the beginning of urine production by the mouse embryonic kidney around E16.5 and is well before the initial ureteral peristaltic waves that are first observed around E18.5 in mice.35 It is still unclear when the ICC-LCs appear in human fetal ureter and at what embryonic stage human fetuses start to have rhythmic ureteral peristalsis.
Development of the bladder and urethra
Unlike the mesodermal ontogenetic origin of the kidney and ureter, the bladder and urethra arise from the endodermal urogenital sinus after the urorectal septum (i.e. Tourneux’s fold) partitions the embryonic cloaca into the ventral urogenital sinus and the dorsal rectum.39 At around 5 weeks of human gestation and at E11–12 in mouse, the urogenital sinus is further separated into the anterior vesicourethral canal and the posterior urogenital sinus. The anterior portion of the urogenital sinus (i.e. anterior vesicourethral canal) becomes the bladder, which has an open outflow tract at its apex that is connected to the allantois during early fetal life. This outflow tract is only functional at the early embryonic stage to drain the developing bladder to the allantois through the umbilical cord. By ~15 weeks of human gestation, the bladder separates from the umbilicus as the allantois regresses and becomes a remnant called the urachus, which is further stretched to become the median umbilical ligament. In the meantime, the posterior vesicourethral canal becomes the pelvic portion of urethra in the male (which can be further divided into three segments: pre-prostatic, prostatic, and membranous urethra) and the entire urethra in the female. The posterior portion of the urogenital sinus later develops into the phallic urethra (also called spongy or penile urethra) in the male and the lower portion of the vagina and vaginal vestibule with perineal urethra orifice in the female.39, 40
By about four and half weeks of gestation in human and ~E12.5 in mouse, the common nephric duct (i.e. the posterior portion of the Wolffian duct distal to the ureteric budding site) shortens, expands and integrates into the urogenital sinus close to the region where the future bladder neck is located (Figure 1A, 1B).41, 42 As the common nephric duct integrates into the bladder, it brings the ureteric budding site and anterior portion of the Wolffian duct with it.41, 42 Recent studies have demonstrated that this is a vitamin-A dependent developmental process involving apoptosis of the common nephric duct, which eventually results in the transposition of the ureteric budding site from the Wolffian duct to the urogenital sinus epithelium to form the ureteral orifice.43–45 The anterior portion of the Wolffian duct later becomes the vas deferens in males but regress in females. The openings of the vas deferens (Wolffian ducts) in males migrate gradually downward and medially and become the ejaculatory duct draining into the prostate portion of the urethra just below the bladder neck.39
After the integration of the common nephric ducts (CND) into the future bladder neck region in the urogenital sinus, the CND expands and moves the ureteric orifice anteriorly and separates it from the Wolffian duct. Subsequent developmental processes of CND apoptosis and expansion of the bladder body re-position the ureteric orifices in their final positions in the bladder wall.45 Together with the internal urethral orifice, they form a triangular region at the base of the bladder that is also called trigone (Figure 1C). Although the trigone is traditionally considered as the only region in the bladder that is structurally different from the rest of the bladder and urethra46, recent studies show that it is actually also derived from the endodermal bladder with only minor contribution from the mesodermal ureter.47–49
At the time that the early bladder is still part of the urogenital sinus, its lumen is lined by bilayered cuboidal and glycogen-rich epithelial cells surrounded by loose undifferentiated mesenchymal cells.50 However, the developmental origin of these glycogen-rich epithelial cells is largely unknown. Similar to the kidney and ureter development, the epithelial-mesenchymal interaction is critical for proper bladder development.51 At about 12 weeks of gestation in human and at E13.5 in mouse, bladder mesenchymal cells start to differentiate into smooth muscle cells.50, 52 By 21 weeks of gestation, the human fetal bladder acquires three to five layers of urothelial cells similar to the fully differentiated urothelium, and a well-developed smooth muscle coat consisting of three layers of longitudinal and circular smooth muscles (also called detrusor).50 In mouse, the urinary bladder becomes a fully developed organ after E15.5 with multi-layered urothelium expressing uroplakins and randomly oriented smooth muscle fibers that express α-smooth muscle actin.52, 53
The function of the human bladder is to store and empty urine. This requires normal bladder compliance for urine storage and urinary continence mechanism for emptying bladder in a coordinated and controlled manner. Bladder compliance increases during development as the thickness of the bladder muscle wall increases while the amount of collagen content decreases.54, 55 A well developed human adult bladder normally can hold about 500 ml of urine when it is full.56, 57 However, we usually experience desire to micturate when the bladder contains only about 200–300 ml of urine. The molecular basis of bladder compliance formation and development is largely unknown.
The urinary continence mechanism is formed in a similar way in both male and female by a combination of muscles from the bladder detrusor, trigone, and urethral sphincter complex.58 The urethral sphincter complex is derived from the sphincter urethrae primordium, an embryonic structure of mesenchymal condensation that can be identified in the urogenital sinus at nine weeks of gestation in human after the division of the cloaca.59, 60 At about 13–15 weeks of gestation, the sphincter urethrae primordium starts to differentiate into two components of urethral sphincter complex that include the inner smooth muscle fibers (also called lissosphincter) and the outer striated muscle fibers (also called rhabdosphincter) (Figure 1C).60 After 20 weeks of gestation, both lissosphincter and rhabdosphincter develop into an omega-shaped muscle coat surrounding the urethra with a narrow posterior connective tissue raphe that attaches to the lateral wall of the prostate in the male and the vagina in the female.58–61 The smooth muscle lissosphincter also intermixes with the bladder detrusor and is abundant only in the proximal two thirds of the urethra below the bladder neck, whereas the striated muscle rhabdosphincter is predominantly located in the distal two thirds of the pelvic urethra.60, 62 The urethral sphincter muscles are innervated by both autonomic nerves (pelvic plexus: regulates the proximal part of the urethra) and somatic nerves (pudendal nerves: control the contraction of the distal part of the urethra).63–65 Normal development and innervation of both urethral sphincter muscles are likely to play a critical role in maintaining urinary continence after birth. The neural control of urinary continence has been thoroughly reviewed recently.66
As the bladder muscle mature, it also forms a muscle coat around the ureteric orifices in the trigone and functions as an “ureterovesical sphincter” that contracts in response to bladder contraction during voiding and subsequently relaxes following the closure of the external urethral sphincter complex during bladder filling.67, 68 The contraction of the muscle coat around the ureteric orifices in the trigone acts as an “active” ostial-valve anti-reflux mechanism to prevent the retrograde urine flow from the bladder back to the ureter and kidney.69–71 When the ureteric orifice establishes its final position in the bladder trigone, it guides the ureter to perforate the bladder muscle laterally in an oblique direction and proceed between the bladder mucosa and detrusor muscle to form an intravesical tunnel structure at the UVJ (Figure 1C). The intravesical portion of the ureter collapses during bladder voiding and creates a second anti-reflux mechanism also called the “passive” flap-valve mechanism.72, 73 The active and passive anti-reflux mechanisms act together as a one-way valve allowing ejection of the bolus of urine from the ureter into bladder lumen when its pressure is low during bladder filling, and preventing retrograde flow of urine back to the ureter and kidney when the bladder pressure is high during micturition.67, 68
Genes and signaling pathways in lower urinary tract development and disease
Although only a few genes have been discovered so far associated with lower urinary tract birth defects in human, results from basic science research in the past 20 years provide us rich biological insights at the molecular level on early urinary tract development. Especially, many genes and signaling pathways have been identified to play important roles in early ureteric budding (Figure 2) and ureter development (Figure 3). Many common molecular signaling pathways important in other organ systems have also been shown to play significant roles in the development of the lower urinary tract. These include the receptor tyrosine kinase (RTK) signaling pathway (key known genes: Gdnf, Ret),74 the Wnt signaling pathway (key known genes: Ctnnb1, Wnt7b, Wnt9b, Fzd1),75 the Hedgehog signaling pathway (key known genes: Shh. Gli3, Smo, Tshz3),31, 32, 76 the TGF-β signaling pathway (key known genes: Bmp4, Smad4),77–79 the retinoic acid mediated nuclear receptor signaling pathway (key known genes: Rara, Rarb),44, 45, 80 and the renin-angiotensin system (key known genes: Agt, Ren, Agtr1, Agtr2).81–85 (Figure 2 and 3; Table 1–4). Interestingly, not only are these signaling pathways required for the development of the lower urinary tract, but also the expression and activity of these genes and pathways are modified by lower urinary tract diseases. For example, recent global gene profiling studies have shown that UTI influences the activity of these signaling pathways.86, 87 Throughout this review, we will refer to these molecular signaling pathways and link them to the phenotype of patients and/or mice with lower urinary tract diseases when these pathways are disrupted. Understanding the signaling pathways involved in lower urinary tract development and diseases can help us illuminate new directions for future genetic studies and identify novel candidate genes associated with these disorders in patients.
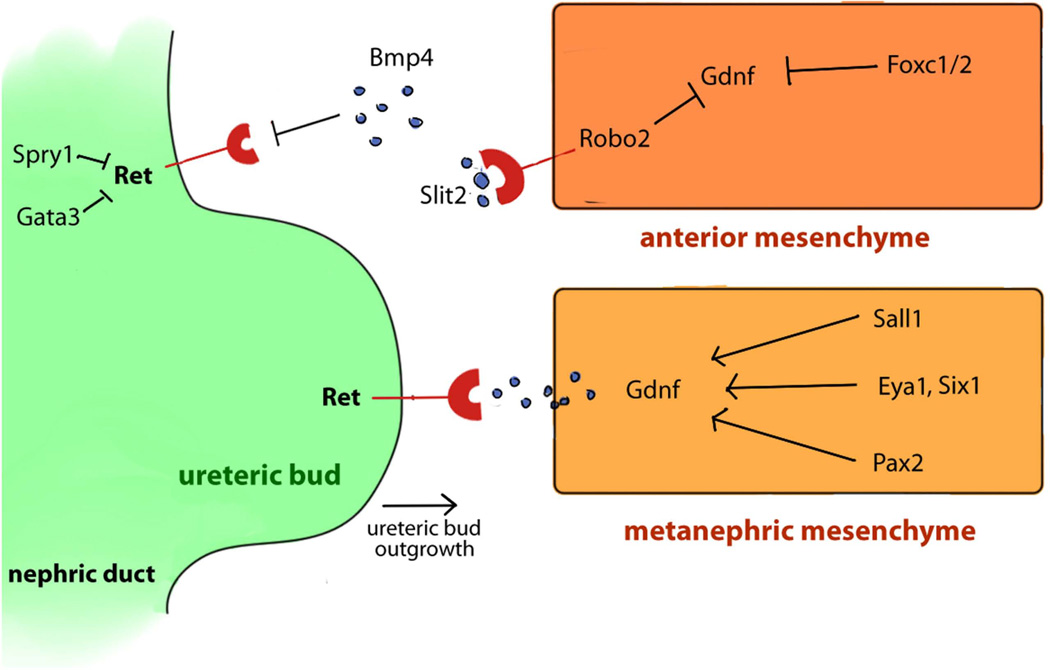
FIGURE 2.
Genes and signaling pathways involved in ureteric budding. Scheme of signaling pathways between the ureteric buds (green) and the metanephric mesenchyme (yellow-orange). The most important inducer of UB outgrowth is the Receptor Tyrosine Kinase signaling pathway mediated by Gdnf and its receptor Ret. Ret is expressed by the nephric duct (green) and ureteric buds (green). Gdnf is secreted by the metanephric mesenchyme (yellow). The coordination of different signaling pathways in the anterior mesenchyme and the metanephric mesenchyme play a crucial role in the development of a single ureteric bud.
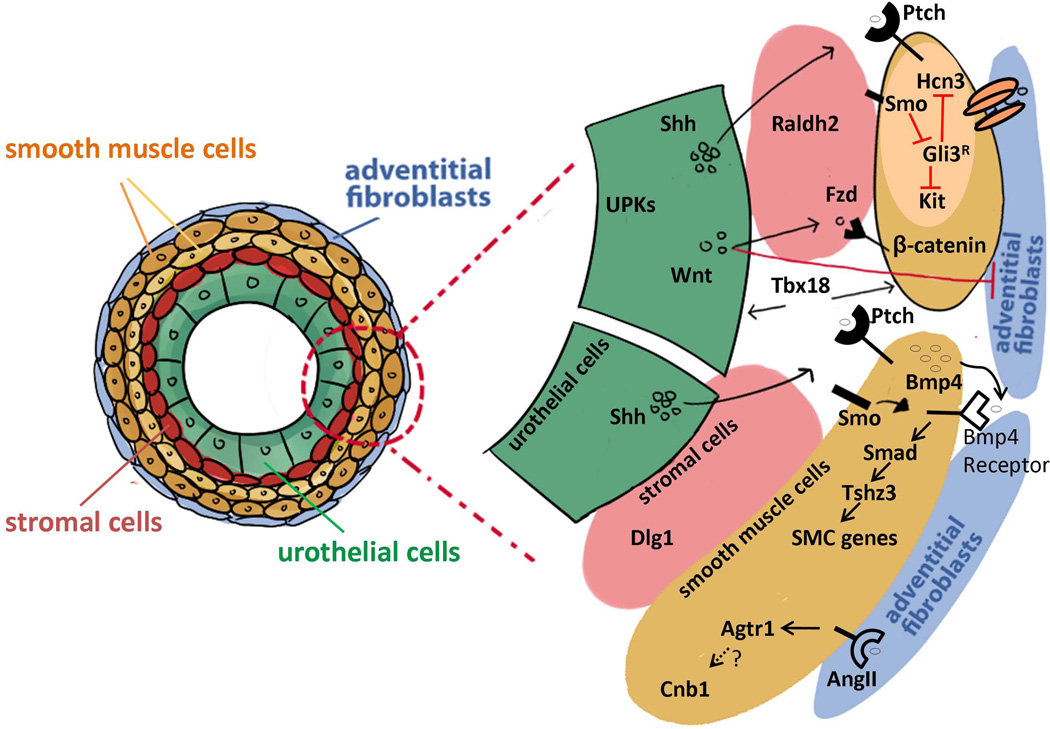
FIGURE 3.
Genes and signaling pathways involved in ureter development. Scheme of genes and signaling pathways involved in the development of the urothelial cells (green), the stromal cells (pink), the smooth muscle cells (yellow) and the adventitial fibroblasts (blue). The transcription factor Tbx18 is one of the major early genes in ureter differentiation. Tbx18 is expressed in undifferentiated ureter mesenchymal cells and promotes the differentiation of both urothelium and smooth muscle cells. Uroplakins (UPKs) are expressed on the apical surface of urothelial cells which also express Shh (sonic hedgehog) and Wnt molecules. Dlg1 plays an essential role in the differentiation of the stromal cells that express the marker gene Raldh2. The Hedgehog signaling (Shh and its receptor Ptch) plays a major role in the ureteric smooth muscle maturation through molecules in the TGF-β signaling pathway such as Bmp4 and Tshz3. Shh is also necessary for the differentiation of ureteric pacemaker cells by suppressing the Gli3 repressor (Gli3R) through Smo (Smoothened) which in turn activates the expression of Kit and Hcn3. The canonical Wnt signaling is necessary for the differentiation of smooth muscle cells and the repression of the adventitial fibroblast cell differentiation. The smooth muscle cells express the Wnt receptors Frizzled (Fzd) which activates β-catenin (Ctnnb1). Calcineurin b1 (Cnb1) is required in the mesenchyme and smooth muscle cells for the development of pyeloureteral peristaltic machinery. The Angiotensin pathway may activate Cnb1.
Table 1.
Genes Associated with Vesicoureteral Reflux
| Gene | Chr | Exp | Type of protein | Signaling | Human disease (OMIM) | Urinary tract defects in animal models |
Ref |
|---|---|---|---|---|---|---|---|
| Kal1 | Xp22.31 | Adhesion molecule | Kallmann syndrome (OMIM 308700) | 283 | |||
| Nfia | 1p31.3-p31.2 | ue, um | Transcription factor | Chromosome 1p32-p31 deletion syndrome (OMIM 613735): VUR | Duplex ureters, VUR, UPJ defects, hydroureter, hydronephrosis, megaureter | 284 | |
| Rpl11 | 1p36.1-p35 | Ribosomal protein | Diamond-Blackfan anemia 7 (OMIM 612562): VUR, horseshoe kidney | 285 | |||
| Robo2 | 3p12.3 | mm | Slit receptor, Ig superfamily | Robo/Slit | Vesicoureteral reflux 2 (OMIM 610878) | Ectopic UB, multiple ureters, hydroureter, hydronephrosis | 10, 119 |
| Nipbl | 5p13.2 | chromosomal adherin | Cornelia de Lange syndrome 1 (OMIM 122470): VUR | 286 | |||
| Nsd1 | 5q35 | nuclear receptor | Sotos syndrome (OMIM 117550): VUR | 287 | |||
| Gli3 | 7p13 | zinc finger | hedgehog | Pallister-Hall syndrome (OMIM 146510): VUR | Peristalsis defect, hydroureter, and hydronephrosis | 76, 288 | |
| Micro-deletion | 7q11.2-q21.3 | EEC1 syndrome (OMIM 129900):; VUR ureterocele and atretic ureter in the ectrodactyly, ectodermal dysplasia, and cleft lip/palate | 289 | ||||
| Micro-deletion Fgfr1 | 8p11.23-p11.22 | Kallmann syndrome 2 (OMIM 147950): VUR and ureter duplication | 283 | ||||
| Sox17 | 8q11.23 | ub, mm | HMGbox transcription factor | VUR3 (OMIM 613674) | 138 | ||
| Trps1, Ext1 | 8q24.11-q24.13 | Langer-Giedion syndrome (OMIM 150230): VUR | 290 | ||||
| Ret | 10q11.21 | Receptor | RTK | VUR, hydronephrosis, renal dysplasia | 140, 141 | ||
| Pax2 | 10q24 | wd, ue, mm | Transcription factor | Papillorenal syndrome (OMIM 120330) | Caudal ureteric bud, reflux, hydroureter | 291 | |
| Fgfr2 | 10q26.13 | FGF receptor | VUR | 122 | |||
| Upk2 | 11q23 | uro | Trans-membrane glycocalyx component | VUR, hydroureter, hydronephrosis | 28 | ||
| Six1 | 14q23.1 | Transcription activator | Deafness, autosomal dominant 23 (OMIM 605192): VUR | 155, 180, 292 | |||
| Micro-deletion | 16p11.2 | VUR, dilation and tortuosity of the ureter, (OMIM 613444) | 293 | ||||
| Micro-deletion | 17q21.31 | Mental retardation, autosomal dominant 17 (OMIM 610443): cryptorchidism, hypospadias, VUR, duplex kidney | 294 | ||||
| Sall4 | 20q13.2 | Transcription factor | Duane-radial ray syndrome (OMIM 607323): VUR and bladder diverticula | 295 | |||
| Micro-deletion | 22q11.2 | Opitz GBBB syndrome (OMIM 145410): VUR and hypospadias | 296 | ||||
| Upk3a | 22q13.31 | uro | Transmembrane glycocalyx component | VUR, hydroureter, hydronephrosis | 27 |
Abbreviations: Chr: chromosomal location; Exp: expression in the urinary tract; Signaling: signaling pathway; Ref: References; ue: ureteric epithelium; um: ureteric mesenchyme; smc: smooth muscle cells; UVJ: ureterovesical junction; uro: urothelium; TGF-β: transforming growth factor-β; UPJ: ureteropelvic junction; VUR: vesicoureteric reflux; wd: Wolffian duct; ub: ureteric bud; RAS: Renin-angiotensin system.
Table 4.
Genes and Genomic Loci Associated with Urethra Anomalies
| Gene | Chr | Exp | Type of protein |
Signaling | Human disease (OMIM) | urinary tract defects in animal models |
Ref |
|---|---|---|---|---|---|---|---|
| Ar | Xq12 | Androgen receptor | Hypospadias (OMIM 300633) | 315 | |||
| Mid1 | Xp22 | RING finger protein | Opitz syndrome (OMIM 300000): hypospadias | 316 | |||
| Mamld1 | Xq28 | transcriptional co-activator | Hypospadias 2, X-linked (OMIM 300758) | 317 | |||
| EphB2 | 1p36.1-p35 | Ephrin receptor | Hypospadias | 318 | |||
| Srd5a2 | 2p23 | Steroid 5-alpha-reductase | Pseudovaginal perineoscrotal hypospadias (OMIM 264600) | 319 | |||
| Tp63 | 3q28 | up | Transcription factor | Ectrodactyly, ectodermal dysplasia, and cleft lip/palate syndrome 3 (OMIM 604292) and split-hand/foot malformation 4 (OMIM 605289) | Hypospadias | 320, 321 | |
| Fras1 | 4q21.21 | ub | putative extracellular matrix protein | Fraser syndrome (OMIM 219000): anterior urethral atresia | Dilated ureteric bud, cystic kidney | 322, 323 | |
| Dlx5, Dlx6 | 7q22 | up | Transcription factor | Hypospadias | 321 | ||
| Gli3 | 7p13 | zinc finger | hedgehog | Pallister-Hall syndrome (OMIM 146510) | 76, 288 | ||
| Hoxa13 | 7p15.2 | Transcription factor | Wnt | Hand-foot-uterus syndrome (OMIM 140000): duplication of the genital tract in female and hypospadias in male | 324 | ||
| Microdeletion | 7q11.23 | Williams syndrome (OMIM 194050): urethral stenosis, bladder diverticula, VUR | 239, 240 | ||||
| Shh | 7q36 | uro | Secreted Sonic Hedgehog | Hedgehog | hypoplasia of external genitalia, internal urethra (pelvic urethra) and bladder | 94 | |
| Pitx2 | 4q25 | Transcription factor | Axenfeld-Rieger syndrome, type 1 (OMIM 180500): hypospadias | 325 | |||
| Eya1 | 8q13.3 | Transcription activator | Hypospadias, hypoplastic genitalia and persistent cloaca | 257 | |||
| Plec | 8q24 | Intermediate filament-binding protein | Epidermolysis Bullosa (OMIM 226670): urethral strictures | 326 | |||
| Gata3 | 10p15 | Transcription factor | Wnt | Hypoparathyroidism-deafness-renal syndrome (OMIM 146255) | Ectopic UB, duplex kidneys, enlargement of the vas deferens, loss of uterus | 154 | |
| Fgfr2 | 10q26.13 | FGF receptor | Hypospadias | 327 | |||
| Fkbp4 | 12p13.33 | Macro immunophilin | Hypospadias | 328 | |||
| Frem2 | 13q13.3 | Membrane protein- Fras1 family | Fraser syndrome (OMIM 219000): anterior urethral atresia | Renal cysts | 322, 329 | ||
| Six1 | 14q23.1 | Transcription activator | Hypospadias, hypoplastic genitalia and persistent cloaca | 257 | |||
| Wnt3 | 17q21 | Secreted protein | Wnt | Tetraamelia (OMIM 273395): urethra atresia, persistence of cloaca | 330 | ||
| Mks1 | 17q22 | Basal body protein | Hedgehog | Meckel syndrome type 1 (OMIM 249000): urethral atresia | Renal cysts | 331, 332 | |
| Bmp7 | 20q13 | up | Secreted protein TGF-β family | TGF-β | Hypospadias | 321 | |
| Bbs | Bardet-Biedl syndrome (OMIM 209900): persistent urogenital sinus, ectopic urethra in female BBS patients | 333 | |||||
| Unknown gene | Cervical ribs, sprengel anomaly, anal atresia, and urethral obstruction (OMIM 601389) | 334 | |||||
| Unknown gene | Vitiligo, progressive, with mental retardation and urethral duplication (OMIM 277465) | 335 | |||||
| Unknown gene | Fryns syndrome (OMIM 229850): Cryptorchidism, megaureter, hydroureter, cystic ureter, ectopic or blind urethral opening | 310 |
Abbreviations: Chr: chromosomal location; Exp: expression in the urinary tract; Signaling: signaling pathway; Ref: References; up: urethral plate; TGF-β: transforming growth factor-β; VUR: vesicoureteral reflux.
For the last 20 years, animal model studies have provided us an enormous amount of new knowledge regarding genes and signaling pathways involved in lower urinary tract development and congenital anomalies. The best animal model in the field is the mouse since lower model organisms have different developmental structures of the lower urinary tract compared to human. The high similarities between the human and mouse genomes together with mature genetic engineering technologies and large accessible mutant mouse resources provide excellent research tools to characterize the effects of human mutations in vivo.88 The research tools in mouse genetics have evolved from positional cloning of spontaneous single gene mutations, ENU-mutagenesis screening, conventional gene knockouts, to spatially and temporally controlled induction of gene expressions and gene deletions in mice.88, 89 The cell-specific deletions of genes in the lower urinary tract of mice using the Cre-lox system have revolutionized scientist’s capacity to understand the role of major genes in urinary tract development, which often cause embryonic lethal phenotype in mice with germline mutations. For example, the mouse strain carrying the Hoxb7-Cre transgenic construct has enabled scientists to specifically knockout genes of interest in the epithelial cells of the ureteric bud lineage,32, 90 while the Tbx18-Cre transgene enables researchers to specifically delete genes in the ureteral mesenchymal cells.91 The Cre-lox system also enables scientists to perform fate mapping and cell lineage analysis in mice in order to understand the developmental origins of different cell types of the lower urinary tract system. These include cell lineage analysis of the epithelial cells of the Wolffian duct and ureteric bud,92 the ureteral mensenchymal cells,49, 78 the muscle cells of the bladder detrusor,93, 94 and the distal urethra.95
GENETIC BASIS OF CONGENITAL ANOMALIES OF THE LOWER URINARY TRACT (CALUT)
Common congenital anomalies of the ureter
The major function of the ureter is to transport urine from the kidney to the bladder in a unidirectional manner. Therefore, congenital anomalies of the ureter and their associated junction structures (i.e. UPJ and UVJ) often cause abnormal urine transport including urinary obstruction (i.e. the blockage of urine transport from the kidney to the bladder that can be either physical or functional obstruction) and vesicoureteral reflux (VUR: retrograde flow of urine from the bladder back to the ureter and kidney). Both urinary obstruction and VUR elevate the pressure in the ureter and renal pelvis and can co-exist in the same patient.96 Chronic and persistent urinary obstruction or VUR eventually cause dilatation of the renal pelvis (i.e. hydronephrosis) and dilatation of the ureter (i.e. hydroureter) (Figure 4A), which may facilitate colonization and grow of bacteria such as E. Coli in urine and predispose CALUT patients to recurrent UTI.97
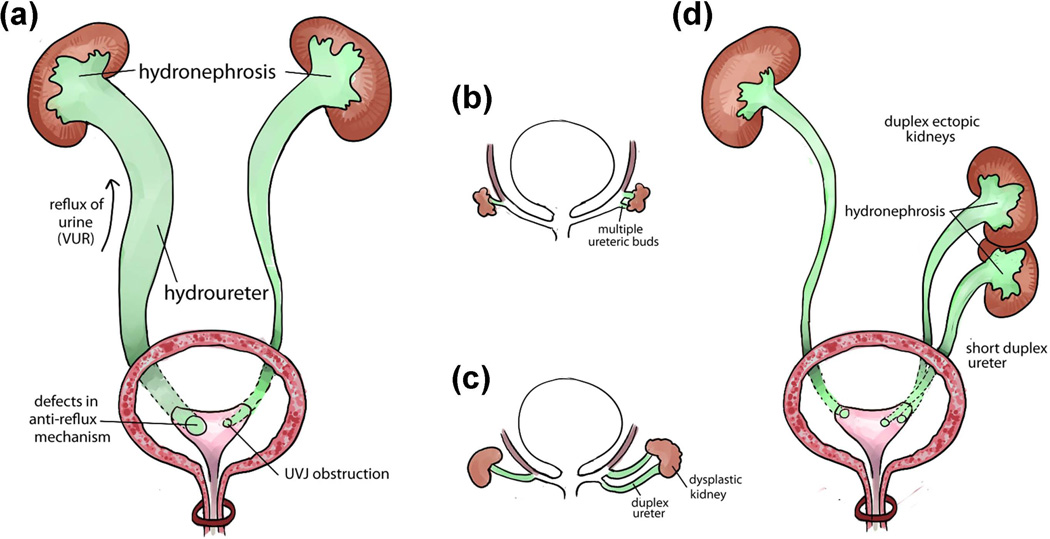
FIGURE 4.
Common congenital anomalies of the ureter. (a) Two main causes of hydronephrosis and hydroureter: vesicoureteral reflux (VUR) caused by defects of anti-reflux mechanism is on the left side and urinary obstruction caused by abnormal structure of the ureterovesical junction (UVJ) is on the right side. (b–d) Early abnormal ureteric budding can lead to congenital anomalies of the ureter: Abnormal multiple ureteric buds formation from the right Wolffian duct (b) can lead to abnormal phenotypes including duplex ureter, dysplastic kidney (c), ectopic kidney, duplex kidney, duplex ureter, short ureter, which are often associated with hydronephrosis phenotype on the right side (d), compared to the normal ureteric buds development on the left side. Each of these malformations can be found separately or coexist with other types of anomalies.
The overall incidence of urinary obstruction and VUR in children is estimated at greater than 1% and is one of the most common problems encountered by pediatric nephrologists and urologists.98–100 Patients with urinary obstruction and VUR at a young age may develop obstructive or reflux nephropathies featuring recurrent UTI, renal scarring, nephron loss, and compensatory hypertrophy of remnant nephrons.101 Obstructive and reflux nephropathies ultimately cause proteinuria, degeneration of remnant nephrons, glomerulosclerosis, and tubular atrophy, which leads to chronic kidney insufficiency and end stage renal disease (ESRD).102 It has been reported that about 10% of patients with reflux nephropathy will progress to chronic kidney insufficiency and ESRD, and eventually require dialysis or kidney transplantation.103
The pathogenesis of reflux nephropathy is not well understood, and it remains unclear why only a subset of patients progress to develop chronic kidney insufficiency and ESRD. The progression from reflux nephropathy to ESRD is almost always associated with proteinuria,104 and most patients with reflux nephropathy and ESRD have focal segmental glomerulosclerosis (FSGS).105 Over the past 30 years, the prevailing view about the pathogenesis of reflux nephropathy has been that high pressure from the refluxing urinary stream and recurrent UTI can result in renal injury and kidney parenchyma fibrosis or scarring also called “acquired” reflux nephropathy, which impairs kidney development and growth.106–110 The pathogenesis of proteinuria and glomerulosclerosis in patients with “acquired” reflux nephropathy and progressive renal insufficiency remains controversial. At least four mechanisms have been proposed, which include immunologic injury, macromolecular trapping and mesangial dysfunction, vascular alterations, and glomerular hypertension.111 On the other hand, the clinical course resulting in reflux nephropathy and ESRD does not appear to be altered by either surgical correction of VUR or control of UTI and hypertension.104, 112–114 Many scientists and clinicians believe that many VUR children with urinary tract congenital abnormalities actually develop congenital renal scar before birth.115 These patients will progress to “congenital” reflux nephropathy and renal insufficiency due to abnormal ureteral and kidney development even in the absence of UTI and associated inflammatory reaction in the kidney.116
Genetic basis of vesicoureteral reflux (VUR)
VUR can be caused by a variety of birth defects affecting the lower urinary tract development. These include defects in ureteric budding, ureter differentiation and elongation, peristalsis, UVJ formation, and bladder and urethra development. In patients with primary VUR, the location of the ureteric orifice (i.e. UVJ) of a ureter tends to be located laterally and more cephalad in the bladder.41, 117 This results in a shortening of the submucosal ureteric segment and a weakening of the flap-valve anti-reflux mechanism and VUR ensues. The degree of reflux may correlate with the degree of ureteric orifice laterality and inversely with the length of the intravesical submucosal ureter.118 The lateral ectopia of the ureteric orifice may be related to abnormal development of the embryonic ureteric bud (Figure 4B–4D), which is also called Machie and Stephens hypothesis or “bud theory”.41
The “bud theory” developed by Machie and Stephens proposes that the ureteral orifice derives from the original ureteric budding site on the Wolffian duct during embryonic development (Figure 1A). When the ureteric buds arise at abnormal sites of the Wolffian duct, such as multiple ureteric buds (Figure 4B), the final sites of the ureteral orifices may also be abnormal (Figure 4C, 4D), resulting in defective ureterovesical junctions, VUR or UVJ obstruction. Machie and Stephens suggest that the final sites of the ureteral orifices in the bladder are determined by the insertion of the common nephric duct into the bladder and its expansion to form the trigone. However, recent cell lineage studies show that the common nephric duct does not differentiate into the trigone but instead undergoes apoptosis.44 Subsequent expansion of the bladder repositions the ureteral orifices to their final positions in the bladder trigone.44, 45 In addition, multiple ureteric buds may also lead to short duplex ureters, duplex kidneys, ectopic and dysplastic kidneys (Figure 4A, 4C, 4D).10, 41, 44, 45, 77, 119–122 Therefore, mutations of genes controlling early ureteric buds formation and positioning often cause ureteral phenotypes such like urinary obstruction, VUR and hydronephrosis during fetal life and after birth in both human and mouse (Figure 2, Table 1–2).25, 42, 122–124
Table 2.
Genes Associated with Hydronephrosis and Hydroureter due to Lower Urinary Tract Defects
| Gene | Chr | Exp | Type of protein | Signaling | Human disease (OMIM) |
Urinary tract defects in animal models |
Ref |
|---|---|---|---|---|---|---|---|
| L1cam | Xq28 | ue | Adhesion molecule | Ectopic UB, duplex ureters, megaureter, hydronephrosis | 297 | ||
| Agtr2 | Xq22-q23 | um | G-protein coupled angiotensin II receptor | RAS | X-linked mental retardation-88 (OMIM 300852) | Ectopic UB, duplex ureters, hydroureter, hydronephrosis | 82 |
| Ptprf | 1p34 | Receptor Tyrosine Phosphatase | Hydroureter, hydronephrosis, and ureterocele | 178 | |||
| Nfia | 1p31.3-p31.2 | ue, um | Nuclear Factor 1 transcription factor | Chromosome 1p32-p31 deletion syndrome (OMIM 613735): VUR | duplex ureters, VUR, UPJ defects, hydroureter, hydronephrosis, megaureter | 284 | |
| Agt | 1q42.2 | k | Secreted angiotensinogen | RAS | Peristalsis defect, hydronephrosis | 298 | |
| Id2 | 2p25 | ue, um | bHLH DNA binding factor | ID | UPJ defect, hydronephrosis | 299 | |
| Ppp3r1 | 2p15 | um | Protein phosphatase | Peristalsis defect, hydronephrosis | 193 | ||
| Hoxd13 | 2q31.1 | Transcription factor | Wnt | Hydroureter and hydronephrosis | 300 | ||
| Robo2 | 3p12.3 | mm | Slit receptor, Ig superfamily | Robo/Slit | Vesicoureteral reflux 2 (OMIM 610878) | Ectopic UB, multiple ureters, hydroureter, hydronephrosis | 10, 119 |
| Ctnnb1 | 3p21 | β-catenin | Wnt | Hydroureter and hydronephrosis | 301 | ||
| Rarb2 | 3p24.2 | ue | Retinoic acid receptor | Retinoic acid signaling | Ectopia of distal ureter ends, hydroureter, megaureter, hydronephrosis | 43 | |
| Gata2 | 3q21.3 | ue, um | Zinc Finger transcription factor | Wnt | Megaureter, hydroureter, hydronephrosis, hypoplastic kidneys | 302 | |
| Agtr1a/b | 3q24 | um | Angiotensin 2 receptor | RAS | Peristalsis defect, hydroureter, hydronephrosis, pelvis agenesis | 199 | |
| Dlg1 | 3q29 | ue, um | Membrane-associated guanylate kinase scaffolding protein | p38 | Congenital hydronephrosis, smooth muscle orientation defect, peristalsis defect | 190 | |
| Slit2 | 4p15.2 | ue | Secreted protein -Robo ligand | Robo/Slit | Ectopic UB, multiple ureters, hydroureter, hydronephrosis | 119 | |
| Wfs1 | 4p16.1 | transmembrane protein | Wolfram syndrome (OMIM 222300): hydronephrosis, dilated ureters, distended bladder without VUR | 303 | |||
| Scarb2 | 4q21.1 | glycoprotein | Kidney and ureter duplication, UPJ obstruction, hydroureter, and hydronephrosis | 304 | |||
| Spry1 | 4q28.1 | wd, mm | Receptor Tyrosine Kinase antagonist | GDNF/RET | Ectopic UB, multiple ureters, hydroureter, hydronephrosis | 120 | |
| Bmp5 | 6p12.1 | um | Secreted molecule | TGF-beta | Hydroureter, hydronephrosis | 305 | |
| Foxc1 | 6p25 | um | Forkhead transcription factor | Foxc | Axenfeld-Rieger syndrome type 3 (OMIM 602482) | Ectopic UB, duplex ureters, hydroureter, hydronephrosis | 121, 174 |
| Unknown gene | 6p | Multicystic renal dysplasia, bilateral; MCRD (OMIM 143400): UPJ obstruction, hydronephrosis | 306 | ||||
| Tbx18 | 6q14-q15 | um | T-box transcription factor | Bmp, wnt, hedgehog | Lack of smooth muscles in the ureter, short ureter, hydronephrosis | 29 | |
| Gli3 | 7p13 | zinc finger | hedgehog | Pallister-Hall syndrome (OMIM 146510) | peristalsis defect, hydroureter, and hydronephrosis | 76, 288 | |
| Smo | 7q32.3 | G protein-coupled receptor | Hedgehog | Ureter dyskinesia, functional obstruction, hydroureter and hydronephrosis | 76 | ||
| Shh | 7q36 | ue | Secreted Sonic Hedgehog | Hedgehog | Smooth muscle defects, short hydroureter, hydronephrosis | 32 | |
| Ret | 10q11.21 | Receptor | RTK | Hydronephrosis, megaureters, renal dysplasia | Abnormal distal ureter maturation | 43, 141 | |
| Upk2 | 11q23 | uro | Transmembrane glycocalyx component | VUR, hydroureter, hydronephrosis | 28 | ||
| Aqp2 | 12q12-q13 | Water channel protein | Hydronephrosis | 187 | |||
| Bmp4 | 14q22-q23 | um | Secreted molecule-TGF-beta family | TGF-beta | Renal hypoplasia | Ectopic UB, duplex ureters, ectopic UVJ, hydroureter | 77 |
| Aldh1a2 | 15q21.3 | Retinaldehyde dehydrogenase | Urogenital sinus abnormalities hydronephrosis and megaureter | 44 | |||
| Stra6 | 15q24.1 | A receptor for retinol/retinol binding protein complexes | Retinoic acid signaling | Hydronephrosis | 307 | ||
| Foxc2 | 16q24.1 | um | Forkhead transcription factor | Foxc | Ectopic UB, duplex ureters, hydroureter, ureter agenesis | 121 | |
| Rara | 17q21.2 | Retinoic acid receptor | Retinoic acid signaling | Ectopia of distal ureter ends, hydroureter, megaureter | 43, 44 | ||
| Smad4 | 18q21.1 | ue, um | TGF-β | Hydroureter, hydronephrosis | 79 | ||
| Tshz3 | 19q12 | Transcription factor | Hedgehog | Smooth muscle differentiation, congenital hydronephrosis | 31 | ||
| Adamts1 | 21q21.2 | um | Secreted protease | enlarged renal calices; ureteropelvic junction obstruction | 308 | ||
| Upk3a | 22q13.31 | uro | Trans-membrane glycocalyx component | VUR, hydroureter, hydronephrosis | 27 | ||
| Unknown gene | Hardihar syndrome (OMIM 612726): hydroureter, hydronephrosis | 309 | |||||
| Unknown gene | Fryns syndrome (OMIM 229850): Cryptorchidism, megaureter, hydroureter, cystic ureter, ectopic or blind urethral opening | 310 |
Abbreviations: Chr: chromosomal location; Exp: expression in the urinary tract; Signaling: signaling pathway; Ref: References; k: kidney; ue: ureteric epithelium; um: ureteric mesenchyme; uro: urothelium; TGF-β: transforming growth factor-β; UPJ: ureteropelvic junction; VUR: vesicoureteric reflux; RAS: Renin-angiotensin system.
Many syndromes have VUR as one of the phenotypes. These include Sotos, Cornelia de Lange, Diamon Blackfan, Duane Radial ray, Langer-Giedion, Kallmann, EEC1 syndrome, etc. (Table 1). Although for some of them the underlying genes have been identified, the genetic basis of primary nonsyndromic VUR remains ill-defined.125 Family studies show familial clustering of reflux and imply a genetic origin for primary VUR. About 45–50% percent of children with primary VUR are from families where at least one additional family member is affected.126, 127 The disease often occurs in two or more generations with up to a 65% transmission rate from parents to children,128, 129 and 34–45% of an affected patient’s siblings will have reflux.130–132 One study has showed that 80% of identical twins and 35% of fraternal twins develop primary VUR.126 These data strongly support a genetic basis for primary VUR and are consistent with an autosomal dominant mode of inheritance, albeit with incomplete penetrance.133
Segregation analysis has concluded that primary VUR is caused by a major dominantly inherited allele.134 Mutations in PAX2 on chromosome 10q cause the coloboma-ureteric-renal syndrome (also called Papillorenal syndrome, OMIM 120330), in which VUR is part of the phenotype.7, 135 Recently, mutations in several other genes have also been identified associated with primary VUR.136 These include ROBO2 (VUR2, OMIM 610878),10, 136, 137 SOX17 (VUR3, OMIM 613674),138, UPK3A139, and RET140, 141 (Table 1). However, all these VUR genes have been excluded as major players in primary nonsyndromic VUR.136, 142–147
Human ROBO2 mutations have been identified in patients with VUR from several unrelated families.10, 137 Robo2 is a member of the immunoglobulin superfamily and encodes a cell adhesion molecule involved in axonal guidance and neurogenesis.148, 149 It is a receptor for the Slit2 ligand,150 and Slit2-Robo2 signaling acts as a chemorepulsive guidance cue to control axon pathfinding and neuron migration during nervous system development.151 Slit2-Robo2 signaling also plays crucial roles in early ureteric buds outgrowth and positioning. Mouse knockouts that lack either Slit2 or Robo2 develop supernumerary ureteric buds, duplex kidney and hydroureter phenotype.10, 119 A recent study further demonstrates that Robo2 is critical for the formation of normal ureteral orifices and for the maintenance of both active and passive anti-reflux mechanisms.123 Interestingly, Robo2 signaling has also recently been shown to act as a negative regulator on nephrin to influence podocyte foot process architecture in kidney glomeruli.152
Mutations in genes controlling early ureteric buds formation
Gdnf is the most important inducer of UB outgrowth through the receptor tyrosine kinase (RTK) signaling pathway and is mediated by its receptor Ret (Figure 2).17, 74 The Gdnf gene encodes a highly conserved secreted protein in the metanephric mesenchyme and induces ureteric buds outgrowth during early kidney and ureter development.17, 74 Loss of Gdnf in mice causes absence of ureteric buds formation and renal agenesis.153 Therefore, mutations in genes associated with the Gdnf/Ret pathway, like Spry1, Gata3, Bmp4, Slit2/Robo2, Foxc1/2, Pax2, Eya1/Six1, and Sall1, all cause abnormal ureteric budding phenotypes in mice (Figure 2, Table 1–2).10, 77, 119–121, 154–158
The Ret gene encodes a receptor tyrosine kinase in the UB and interacts with its ligand Gdnf secreted from MM (Figure 2). Mutations in RET have been identified in patients with VUR, ureteral obstruction, megaureter, duplex kidney, renal abnormalities, as well as Hirschsprung's disease and cancer.141, 159–161 Yang and colleagues have observed a significant association between primary VUR and a G691S polymorphism (rs1799939) in the RET gene among French Canadian VUR patients.140 In mice, Ret mutations cause similar defects as in humans, which include renal agenesis, hypoplasia, ectopic ureter termination, and enteric nervous system defects.162–165 Interestingly, a study shows that overexpression of Ret in mice also causes VUR phenotype.166 Spry1 is a negative regulator of RTK signaling and acts as a negative feedback to balance the Gdnf/Ret signaling in the UB. In mice, mutations in Spry1 cause multiple ureteric buds and hydroureter.120 Accordingly, loss of Spry1 is able to rescue mouse Gdnf and Ret knockout phenotypes.167, 168 However, no SPRY1 mutations have been identified so far in human VUR or other lower urinary tract birth defects.169
Mutations in GATA3 in human have been identified as a cause of Hypoparathyroidism, sensorineural Deafness, and Renal disease (HDR: OMIM 146255), which includes renal dysplasia, unilateral kidney agenesis and VUR phenotypes.170, 171 In mice, loss of Gata3 leads to ectopic ureteric budding, duplex kidney, hydroureter, as well as vas deferens hyperplasia and uterine agenesis.154 Gata3 is a transcription factor of the GATA family that is expressed in the ureteric buds.172 It is regulated by the Pax2 and Pax8 genes and is a key regulator of the Wolffian duct morphogenesis.173 Molecular analysis has further placed Gata3 upstream of Ret but downstream of β-catenin, preventing ectopic ureter budding and premature cell differentiation in the Wolffian duct (Figure 2).154
The Axenfeld-Rieger syndrome (OMIM 602482) is caused by mutations in FOXC1, a transcription factor of the forkhead family that is highly expressed in the metanephric mesenchyme. Recently, Weisschuh and collaborators have described a ureteral stenosis phenotype in patients with Axenfeld-Rieger syndrome.174 Interestingly, in mouse, mutations in Foxc1 and its family member Foxc2 result in the expansion of the Gdnf expression domain in the metanephric mesenchyme, which leads to a urinary tract phenotype including ectopic ureteric buds, duplex ureters, and hydroureters.121 In addition, mutations in the transcription factor FOXF1, another family member of the forkhead box genes, cause the “Alveolar Capillary Dysplasia with Misalignment of Pulmonary Veins” syndrome (ACDMPV: OMIM 265380). ACDMPV is associated with ureteral valve-like constriction at the UPJ, tortuous ureters, and hydronephrosis.174, 175 FOXF1 is located at the same chromosomal locus as FOXC2, and is a part of the sonic hedgehog and TGF-β signaling pathways.176 Other genes in the sonic hedgehog and TGF-β signaling pathways also play critical roles in lower urinary tract development and congenital anomalies (Figure 3). FOXF1 is the only gene so far that has been associated with UPJ obstruction in human.
In mice, mutations in several genes have been shown to cause UPJ obstruction and ureter-bladder connection defects.43, 44, 177, 178 For example, loss of both retinoic acid receptor alpha (Rara) and beta (Rarb) in mice lead to megaureter and hydronephrosis owing to abnormal apoptosis activity mediated by vitamin A signaling at the ureter-bladder insertion site.43, 44 In mice with double knockout of the genes encoding the protein tyrosine phosphatase receptor type F and S (Ptprf and Prpts), the regression of the common nephric duct is delayed resulting in inappropriate tissue survival and delayed distal ureter maturation. These ureter-bladder connection defects cause urinary obstruction, hydroureter and hydronephrosis in Ptprf;Prpts double mutant embryos.178 A recent study also shows that the Gata3-Raldh2-Ret molecular network plays a crucial role in regulating the proper insertion of the nephric duct into the mouse developing bladder.177 Absence of Ret, Gata3, or Raldh2 can cause similar distal ureter insertion defects with urinary obstruction and hydronephrosis phenotypes in mice.177
Interestingly, despite the common ontogenetic origin of the kidney and the ureter, some transcription factors in the early metanephric mesenchyme enabling the ureteric buds outgrowth may induce the ureter formation but not the kidney. For example, mutations in SALL1, a transcriptional factor expressed in the MM that enhances the canonical Wnt signaling pathway, is responsible for the Townes-Brocks Syndrome (TBS: OMIM 107480).179 Homozygous deletion of Sall1 in mice results in apoptosis of the mesenchyme and renal agenesis but normal blind-ended ureter. This phenotype can be rescued by lowering beta-catenin levels in the Sall1 mutant.157 Heterozygous SIX1 mutations are known to cause the Branchiootic Syndrome 3 (BOS3: OMIM 608389). However, mice homozygotes for Six1 mutations develop ureters in the absence of kidneys180, but the ureteral mesenchymal precursors fail to condense and differentiate into normal smooth muscle in the ureters.155 Interestingly, loss of the Eya1 gene (the transcriptional activator interacting with Six1 in the MM) causes agenesis of both the ureters and kidneys due to a failure of the ureteric buds outgrowth and metanephric induction156. Mutations in EYA1 in humans cause Branchio-oto-renal Syndrome (BOR: OMIM 113650) which is characterized by renal tract abnormalities ranging from mild renal hypoplasia to a complete absence of the kidney, as well as duplex ureters, VUR and ureteropelvic junction stenosis.181
Mutations in genes controlling ureter development
Ureter developmental malformations often lead to hydronephrosis (excessive water inside the renal pelvis and calyces). Antenatal hydronephrosis (ANH) is one of the most common congenital abnormalities detected with prenatal ultrasonography in more than 1% of all pregnancies.182, 183 ANH can be caused by a wide spectrum of renal and urological conditions ranging from transient hydronephrosis that resolves spontaneously after birth to clinically significant VUR or urinary tract obstruction that leads to renal failure.184, 185 Because the advancement of latest medical technologies has not provided a gold standard for the diagnosis of clinically significant hydronephrosis, the evaluation and treatment of ANH is an area of considerable controversy among medical professionals.186 Much of the controversy that surrounds the diagnosis and management of ANH stems from a lack of understanding of the molecular etiologies and developmental origins of this common birth defect.
Recent genetic studies in both human and mice have identified many genes associated with hydronephrosis due to lower urinary tract abnormalities (Table 2). Antenatal hydronephrosis can originate from kidney defects (e.g. mutations in AQP2 gene that encodes aquaporin 2 water channel in the kidney collecting tubule187), or from defects in the ureter such as VUR or urinary obstruction.186, 188 Mutations in many genes controlling the developmental processes of different components of the ureter have been shown to cause hydronephrosis in mice (Figure 3). For example, deletions of uroplakins (Upk3a and Upk2) in the urothelium in mice cause loss of superficial umbrella cell layer, overgrowth of the urothelium, urothelial leakage, which lead to hydronephrosis and VUR.27, 28 In addition, mutations in UPK3A have been found in patients with dysplastic kidney, hydronephrosis and VUR.139 Stromal cells (marked by Raldh2 expression) are also important for normal ureteral development and function.189, 190 Loss of Discs-large homolog 1 (Dlg1) has been shown to cause absence of the stromal cell layer in the ureter, which leads to abnormal ureteral smooth muscle orientation, impaired ureteral peristalsis, and severe antenatal hydronephrosis.189, 190 Dlg1 is the only gene identified so far that is required for ureteral stromal cell formation.
Many genes associated with hydronephrosis in mice are genes controlling ureteral typical smooth muscle cell development (Figure 3). These include genes in the sonic hedgehog pathway (e.g. Shh, Gli3, Smo, Tshz3),31, 32, 76 the TGF-β pathway (e.g. Bmp4, Smad4),77–79 and the Wnt pathway (e.g. Ctnnb1).75 A recent study from Trowe and colleagues also shows that canonical Wnt signaling is required for the ureteral adventitial fibroblast differentiation.75 In mice, loss of the T-box transcription factor Tbx18 causes a failure of the ureteral mesenchymal cells to differentiate into ureteral smooth muscle cells as well as an abnormal differentiation of the urothelium, leading to UVJ obstruction, short hydroureter, and antenatal hydronephrosis.29 The abnormal smooth muscle phenotype in Tbx18 mutant mice might be caused by the downregulation of the sonic hedgehog signaling (e.g. Ptch1) in the ureteral mesenchyme and Bmp4 expression in the ureter.29, 191 Taken together, these results underscore the importance of normal development of the typical smooth muscle cells in the ureter, which produce contractile forces to propel urine from the kidney to the bladder.192
Several recent studies also demonstrate the importance of genes controlling ureteral pacemaker cell development and peristalsis machinery in the pathogenesis of hydronephrosis.35, 76, 193, 194 In the urinary tract, the proto-oncogene Kit marks interstitial cells of Cajal (ICC) – like cells (ICC-LCs).35 Recently, Hcn3 (hyperpolarization-activated cation channel 3) has also been identified as playing a fundamental role to trigger and coordinate proximal-to-distal ureter peristalsis.194 In mice, inactivation of Smo (Smoothened) and upregulation of the Gli3 repressor, two components of the sonic hedgehog signaling pathway, lead to abnormal ureteral peristalsis, nonobstructive hydronephrosis and hydroureter before birth.76 Although the urothelium and smooth muscle cells develop normally in these mutant mice, the number of ureteral pacemaker cells (marked by Kit and Hcn3 expression) in the renal pelvis and the ureter are significantly reduced.76 This study provides a strong evidence that sonic hedgehog signaling controls ureteral pacemaker cell development and defective pacemaker cell differentiation can lead to abnormal ureteral peristalsis and hydronephrosis.76, 195 Consistent with this finding, mutations in GLI3 gene have been identified in patients with the Pallister-Hall syndrome (PHS, OMIM: 146510), which includes urinary tract phenotypes like hydronephrosis and hydroureter.196–198
In another study, Chang and colleagues have shown that expression of one of the calcineurin subunit B isoform gene Cnb1 (also called Ppp3r1) is required in the mouse urinary tract mesenchyme for the development of the pyeloureteral peristaltic machinery.193 Tissue specific knockout of Cnb1 in mice cause an abnormal formation of the renal pelvis and ureter as well as defective pyeloureteral peristaltic waves, which lead to progressive renal tract obstruction and hydronephrosis after birth.193 The angiotensin type 1 receptor (Agtr1) has also been shown to play a role in ureteral peristalsis since Agtr1 knockout mice have abnormal renal pelvis and lack ureteral peristaltic waves, and subsequently develop hydronephrosis phenotype.199 However, no causal mutations in either CNB1 or AGTR1 have been reported in patients with lower urinary tract anomalies although mutations in AGTR1 are associated with autosomal recessive renal tubular dysgenesis (RTD).81, 200
Genetic basis of congenital anomalies of the bladder
Compared with known causative genes identified in kidney anomalies and hydronephrosis, the knowledge of the genetic basis for bladder congenital anomalies is very limited (Table 3). Congenital anomalies of the bladder range from severe life-threatening birth defects like bladder agenesis, megacystis, bladder-exstrophy-epispadias complex (BEEC), prune belly syndrome (PBS) to relative mild dysfunctions of the bladder muscle, bladder diverticula, and dysfunctional urinary voiding.201 These birth defects of the bladder can have a significant deleterious effect on the kidney function in children.202 Bladder agenesis is extremely rare and often associated with urethral agenesis as well as other urinary anomalies including hydronephrosis, duplex kidney, and ectopic ureter draining into the vagina, uterus or rectum.203 The genetic basis of bladder agenesis is unknown. The vast majority of patients reported are female infants although two viable male infants have also been reported.204
Table 3.
Genes Associated with Bladder Malformations and Dysfunctions
| Gene | Chr | Exp | Type of protein | Signaling | Human disease (OMIM) | Urinary tract defects in animal models |
Ref |
|---|---|---|---|---|---|---|---|
| Atp7a | Xq21.1 | Transmembrane copper-transporting P-type ATPase | Occipital horn syndrome (OMIM 304150): bladder diverticula | 236 | |||
| Chrm3 | 1q43 | Muscarinic acetylcholine receptor | Prune Belly syndrome (OMIM 100100) | Male distended bladders, impaired contractility of the detrusor smooth muscle | 230, 231 | ||
| Gli2 | 2q14 | Transcription factor | Hedgehog | Hypoplasia of the internal urethra and bladder | 52, 94, 311, 312 | ||
| Tp63 | 3q28 | bl, uro | Transcription factor | bladder exstrophy | 219 | ||
| Micro-deletion | 7q11.23 | Williams syndrome (OMIM 194050): urethral stenosis, bladder diverticula, vesicoureteral reflux | 239, 240 | ||||
| Shh | 7q36 | uro | Secreted Sonic Hedgehog | Hedgehog | hypoplasia of external genitalia, internal urethra (pelvic urethra) and bladder | 94 | |
| Hpse2 | 10q23-q24 | Heparanase | Urofacial syndrome (OMIM 236730) | 248 | |||
| Atca2 | 10q23.31 | actin | Prune-belly sequence | 313 | |||
| Aldh1a2 | 15q21.3 | Retinaldehyde dehydrogenase | Urogenital sinus abnormalities hydronephrosis and megaureter | 44 | |||
| Col5a2, Col5a1, Col1a1 | 2q14-q32 9q34.2-q34.3 17q21.33 |
Collagen | Ehlers-Danlos syndrome (OMIM 130000) | 241, 242 | |||
| Ltbp4 | 19q13 | latent TGF-β -binding protein | TGF-β | Cutis laxa syndrome with severe pulmonary, gastrointestinal, and urinary abnormalities” (OMIM 613177): bladder diverticula, hydronephrosis | 243 | ||
| Microduplication | 22q11.21 | Myosin | Bladder exstrophy | 217, 218 | |||
| Ubp1 | 22q11.23 | Beta-ureidopropionase | Beta-ureidopropionase deficiency (OMIM 606673): has bladder exstrophy phenotype | 314 |
Abbreviations: Chr: chromosomal location; Exp: expression in the urinary tract; Signaling: signaling pathway; Ref: References; bl uro: bladder urothelium; m: mesenchyme; uro: urothelium; TGF-β: transforming growth factor-β.
Megacystis (abnormally large or distended bladder) are often associated with chromosome abnormalities such as trisomy 13 and trisomy 18.205 It can also manifest as one of the phenotypes in male fetuses with lower urinary tract obstruction as a consequence of urethral obstructive congenital anomalies such as posterior urethral valves (PUVs) or urethral atresia.206 In female fetuses, megacystis can be caused by cloacal plate anomalies such as megacystis-microcolon-intestinal hypoperistalsis syndrome (MMIH, OMIM: 249210), which is a rare genetic disease with autosomal recessive inheritance.207, 208 The causal gene for MMIH has not been identified. A unique transgenic mouse model called mgb develops megabladder, hydronephrosis, obstructive uropathy, and renal failure. The homozygous mgb mice nearly completely lack the bladder detrusor muscle and develop prenatal megacystis with altered bladder smooth muscle development.209, 210 The megabladder phenotype in mgb mouse is believed to be caused by the disruption of an endogenous gene due to a random insertion of a transgene construct into a region of mouse chromosome 16 that then translocates to chromosome 11.209 So far, the cloning of the endogenous causal gene disrupted by the transgene insertion or the chromosome translocation in mgb mouse has not been reported.
The bladder-exstrophy-epispadias complex (BEEC) represents a spectrum of congenital anomalies characterized by defects with different severities in the closure of the lower abdominal wall and the bladder.211 Depending on the severity, BEEC can be subdivided into Epispadias (E: the mildest form), Classical Bladder Exstrophy (CBE: intermediate severity), and Cloacal Exstrophy (CE: the most severe form).211, 212 Epispadias and bladder exstrophy occur more often in males while the majority of patients with cloacal exstrophy are females. Epispadias is characterized by an open male urethral plate or a cleft in the female’s urethra.212 Patients with bladder exstrophy have the bladder and related structure (e.g. bladder mucosa, trigone, bladder neck and urethra) everted through the ventral wall of the abdomen and is visible from the outside between the umbilicus and symphysis pubis.213 In cloacal exstrophy, in addition to bladder exstrophy, most patients also have omphalocele (intestine or other abdominal organs protrude into the navel) and imperforate anus, while others have spine defects and renal malformations (also called OEIS complex – Omphalocele, bladder Exstrophy, Imperforate anus, Spine defects).214 The etiology of BEEC has not been resolved. Studies suggest that genetic factors may play a role and several familial BEEC cases have been reported.211 Although BEEC can occur as a part of syndromes, most of the reported cases are isolated.211, 215 Cytogenetic and array-CGH analyses have revealed several BEEC cases with chromosomal abnormalities including a de novo reciprocal chromosomal translocation between 8p11.2 and 9q13 disrupting CNTNAP3 gene that encodes a cell adhesion and recognition molecule of the NCP (Neurexin-IV/Caspr/Paranodin) family.216 Recently, a chromosomal microduplication at 22q11.2 has also been identified in three bladder exstrophy patients by two research groups using array-CGH and genome-wide SNP arrays.217, 218 However, no causal relationship of any single gene mutations and BEEC has been established in human so far. A mouse model with Tp63 (a transcription factor of p53 tumor protein family) deletion has recently been shown to have bladder exstrophy and absence of abdominal and ventral bladder walls with increased apoptosis in the ventral bladder urothelium.219 However, no TP63 mutations have been reported in human BEEC cases.
Prune belly syndrome (PBS) is a rare lower urinary tract birth defect affecting about 1 in 30,000 births.220 PBS is characterized by three major defects that include a partial or complete lack of abdominal muscle with a dry prune like wrinkly skin appearance of the abdomen (so-called prune belly), urinary tract dilatation such as distended thin-walled bladder with disorganized detrusor muscle, bilateral hydroureter and hydronephrosis, and cryptorchidism.221 The majority of PBS patients are males and often have coexisting morbidities such as pulmonary hypoplasia, VUR, urethra abnormalities, chronic pyelonephritis and dysplastic kidney, which commonly lead to renal failure and kidney transplantation.221, 222 Several cases of familial PBS have been reported with an autosomal recessive mode of inheritance.223 A deletion of HNF1B (hepatocyte nuclear factor 1B) has also been reported in a few PBS cases.224, 225 Although mutations in HNF1B are known causes for renal cysts and diabetes syndrome226, familial glomerulocystic kidney disease,227 and renal hypodysplasia.228, it is still unclear if HNF1B is one of the causative genes for PBS.229 Recently, a homozygous nonsense mutation of CHRM3 (muscarinic cholinergic receptor 3) has been identified in six male patients from one family with congenital bladder malformation associated with a prune-belly-like syndrome.230 CHRM3 is expressed in human and mouse bladder urothelial and detrusor muscle cells.230 In mice, Chrm3 has been shown to play a key role in bladder detrusor contractions and Chrm3 knockout mice develop distended bladder with thin bladder smooth muscle layer that resembles the bladder phenotype in PBS patients.231 It would be interesting to see whether CHRM3 mutations will be identified in other PBS cases.
Congenital bladder diverticulum is characterized by a bladder mucosa herniation through muscular fibers of the bladder wall. It often occurs in the region of the bladder where the detrusor muscle is thin due to abnormal bladder development.232, 233 The majority of congenital bladder diverticula are located close to the ureteral orifice (so-called paraureteral diverticula), which can disrupt the anti-reflux mechanism and lead to VUR, UTI, and hydronephrosis.234 About 10% of congenital bladder diverticula occur in the posterolateral region of the bladder, which can grow very large and cause bladder outlet obstruction.235 The genetic basis of congenital bladder diverticula is unclear. Since the diverticula are often associated with incompetent ureteral orifices, it could be caused by mutations in genes controlling early ureteric budding and UVJ formation.232 Bladder diverticula can also occur as part of a number of congenital syndromes, such as occipital horn syndrome (OMIM: 304150, can be phenotypically overlap with Menkes disease – OMIM: 309400),236, 237 Williams-Beuren syndrome (OMIM: 194050),238–240 Ehlers-Danlos syndrome (OMIM: 130000),241, 242 and cutis laxa syndrome with severe pulmonary, gastrointestinal, and urinary abnormalities (OMIM: 613177)243 (Table 3).
The urofacial syndrome (USF, OMIM: 236730, also called Ochoa syndrome) is a rare autosomal recessive disorder characterized by a severe and early-onset urinary voiding dysfunction, bowel dysfunction, and a unique inverted facial grimacing expression when patients attempt to smile.244 USF patients often have neurogenic bladder like symptoms such as urinary incontinence, bladder-sphincter dysfunction, UTI, constipation or encopresis, but without apparent neurological or obstructive pathology. If USF is not diagnosed and treated early, the disease often impairs urine flow causing severe VUR, recurrent UTI, kidney damage, hypertension, and renal failure.244–246 Recently, mutations in the HPSE2 gene have been identified as causing USF. Both microdeletion and nonsense point mutations in HPSE2 have been identified in multiple unrelated familial USF cases by different research groups.247–249 HPSE2 encodes Heparanase 2 which is an endoglycosidase that degrades heparin sulfate proteoglycans and is located on the extracellular matrix and cell surface.250 HPSE2 is highly expressed in human and mouse tissues of the bladder, ureter, kidney and brain.248 However, it is unclear how absence of HPSE2 expression in the urinary tract and brain tissues causes USF phenotype in human. There is no Hpse2 mutant animal model currently available for further mechanistic study.
Genetic basis of congenital anomalies of the urethra
The anatomical structures of the urethra are different between males and females. Because additional developmental processes are involved in the formation of male phallic urethra, congenital anomalies of the urethra occur more often in male infants, and include hypospadias, posterior urethral valve (PUV), and anterior urethra abnormalities. Female infants can develop congenital anomalies of the urethra as well, and they are often more severe than males and are associated with congenital defects of the bladder, the vagina, or the rectum. For example, epispadias, which is part of bladder-exstrophy-epispadias complex (BEEC) described previously, can occur in female children.251 Persistent cloaca, one of the most severe types of anorectal malformation with defects in the urethra, the vagina, and the rectum, is seen exclusively in girls.252–254 It is characterized by a single common channel in the perineum for the drainage of the urethra, the vagina, and the rectum, owing to the failure of proper cloaca separation in early embryonic development.252 Persistent cloaca is often associated with other urinary tract abnormalities including VUR, hydroureter, hydronephrosis, UPJ obstruction, renal dysplasia, which all may lead to chronic kidney insufficiency and renal failure.255 Persistent cloaca can be detected prenatally,256 however, the genetic basis of this congenital anomaly is presently unknown. In mice, deletion of either or both Eya1 and Six1 genes has recently been shown to cause persistent cloaca,257 although it has not been reported in patients with branchio-oto-renal syndrome (caused by mutations in Eya1 or Six1).
Hypospadias is one of the most frequent urogenital birth defects in male newborns with an incidence ranging from 1/1000 to 1/100 births.258, 259 It is defined as a midline fusion defect of the male urethra and penis that results in an ectopic opening of the urethral meatus along the ventral region of the male urethra.260 The causes of hypospadias are considered multifactorial, involving both genetic and environmental factors and have been extensively reviewed recently.261–263 Intensive investigations in the past 20 years have identified many genes that are associated with hypospadias (Table 4). Single gene mutations have been found in WT1, SF1, BMP4, BMP7, FGF8, FGFR2, AR, HSD3B2, SRD5A2, ATF3, MAMLD1, MID1 and BNC2.263 In addition, hypospadias has been associated with polymorphisms in many other genes including FGF8, FGFR2, AR, HSD17B3, SRD5A2, ESR1, ESR2, ATF3, MAMLD1, DGKK, MID1, CYP1A1, GSTM1 and GSTT1.263 Studies of gene expression in patients with hypospadias further identify CTGF, CYR61 and EGF as potential new candidate genes.263 Hypospadias can also be part of syndromes like the Hand-Foot-Genital syndrome (OMIM: 140000), which is caused by mutations in HOXA13.264 The environment risk factors for hypospadias may include low birth weight, maternal hypertension, pre-eclampsia, and maternal exposure of exogenous endocrine-disrupting chemicals.263 However, the majority of isolated hypospadias cases remain unexplained and the major genetic or environmental risk factors for hypospadias are still elusive.
Posterior urethral valve (PUV) is the most common cause of lower urinary tract obstruction in male infants. It is characterized by the formation of sail-like membrane folds from the verumontanum in the posterior urethra at early embryonic stage, which causes obstruction of urine flow and persistent high pressure in the bladder, the ureter and the kidney throughout development.265, 266 PUV is often diagnosed in fetuses with antenatal hydronephrosis during ultrasound examination and is associated with megacystis, thickened bladder wall, and posterior urethra dilatation (observed collectively as a keyhole sign in the bladder neck by ultrasound). If PUV is not corrected early with urethral catheter and cystoscopic valve ablation, it can cause severe VUR, reflux nephropathy, chronic kidney disease and renal failure.267, 268 The fetal origin of PUV formation is still ill-defined. It is thought to occur due to an abnormal insertion of the Wolffian duct into the cloaca during early development resulting in the formation of abnormal ridges or folds in the posterior urethra.266 So far, no causative genes have been identified for PUV although familial inheritance of this birth defect has been reported.269 Recently, a genetic association has been reported between renal damage in PUV and polymorphisms of two renin-angiotensin system genes, ACE (angiotensin converting enzyme and AGTR2 (angiotensin II receptor type 2).270 Urethral valve can also occur in the anterior region of the urethra (so-called anterior urethral valve).271, 272 Together with urethral diverticulum and megalourethra, they constitute a group of rare congenital anomalies of the anterior urethra.273–275 Overall the renal outcome and prognosis of congenital anomalies of the anterior urethra are generally good. The genetic basis of these urethral birth defects is currently unknown.276, 277
CONCLUSION AND FUTURE DIRECTIONS
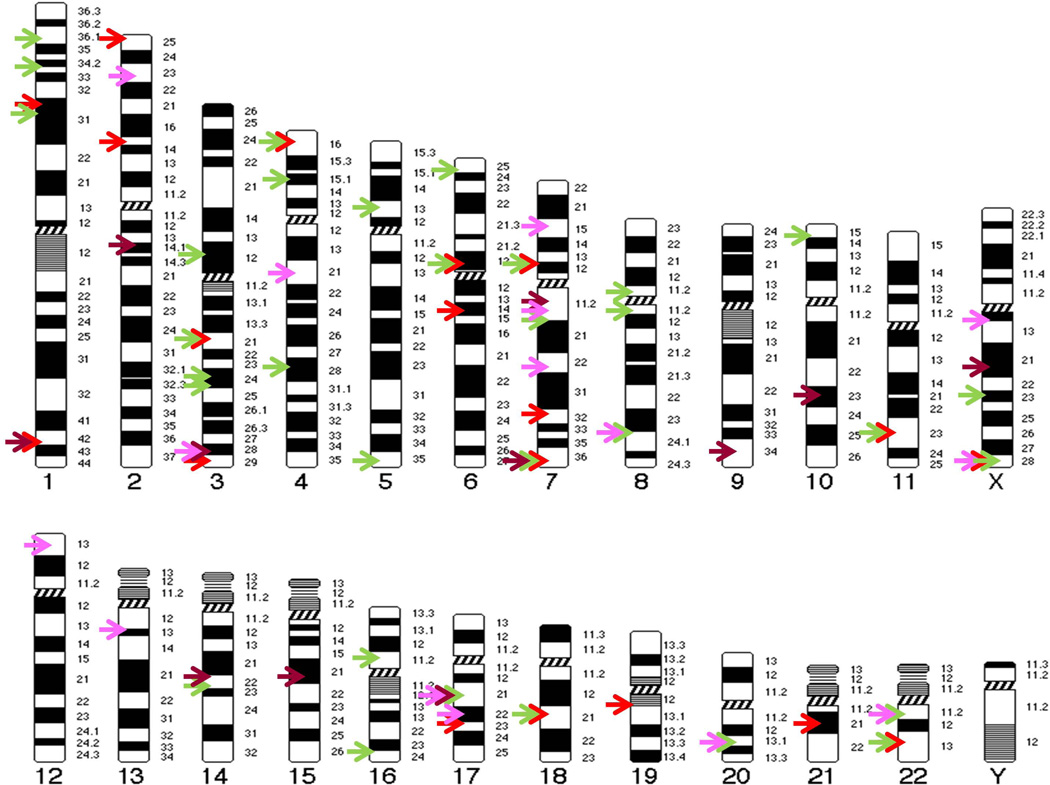
Congenital anomalies of the lower urinary tract account for 20–30% of all anomalies identified in the prenatal period. During pregnancy, routine prenatal ultrasound examination enables early detection of many lower urinary tract anomalies in fetuses (e.g. antenatal hydronephrosis) prior to the development of renal tract complications such as UTI, kidney stones and renal insufficiency.186 Although some lower urinary tract phenotypes (such as antenatal hydronephrosis) will resolve spontaneously in many fetuses after birth, the prenatal detection of hydronephrosis during pregnancy brings a heavy psychological burden on the parents. When an anomaly is detected during obstetric ultrasound scan, a decision regarding the pregnancy is strongly influenced by the definitive diagnosis and prognosis of the condition.278 Since a long list of genetic syndromes and chromosomal abnormalities is associated with hydronephrosis (Figure 5), the decision regarding a pregnancy and fetal intervention with antenatal hydronephrosis can be difficult. Especially, only few causative genes have been identified so far in patients with isolated or non-syndromic lower urinary tract birth defects that may lead to progressive renal injury and chronic kidney disease after birth. Therefore, it is important to identify new causative genes for lower urinary tract birth defects and related signaling pathways and biological processes that may affect renal outcome and prognosis.
FIGURE 5.
Chromosome map of genomic loci associated with congenital anomalies of the lower urinary tract. Each arrow indicates the physical mapping position of a single locus. Different malformations are represented by different colors as following: hydronephrosis (Red), vesicoureteral reflux (Green), bladder anomalies (Brown), urethra anomalies (Purple). See Table 1–4 for details about gene names, chromosome locations, and associated phenotypes, etc.
In the field of human genetics, tests using DNA microarrays were developed in the late 1990s and introduced into the clinic at the beginning of this century. This genomic approach enables clinicians and scientists to uncover chromosome microdeletions and microduplications and to identify genomic loci associated with a range of human diseases. In the past few years, exome and whole genome sequencing have been developed and introduced in human genetic research and clinical diagnosis. These new genetic and genomic diagnostic technologies have revolutionized the field of personalized medicine as well as genetic counseling in both prenatal and postnatal care settings. However, the large amounts of genetic and genomic data that are gathered from these diagnostic tests are very difficult to interpret even by medical professionals. Many copy number variations (CNVs) and single nucleotide polymorphisms (SNPs) have been discovered in patients as well as healthy controls. The causality of these variants is hard to prove with the tools of human genetics alone.
The knowledge derived from patients and model organisms such as mice is complementary since human genetics can identify candidate genes associated with lower urinary tract birth defects, while mouse models can provide additional validation for that causal association and further uncover the disease mechanism at the molecular level. Knowledge gained by comprehending the molecular mechanism can also lead to new candidate genes. As data on gene expression and mutant strains of the laboratory mice are increasingly available to the scientific community, now is the most exciting time to perform high-throughput mouse model phenotype analysis to verify candidate genes for human lower urinary tract birth defects279–282. Knowledge deduced from these studies may have long term implications on preventive interventions aimed at reducing the incidence of lower urinary tract birth defects and minimize further renal injury caused by CALUT (e.g. progressive hydronephrosis or high-grade VUR) and preserve remaining renal function. Understanding the signaling pathways involved in the lower urinary tract development and function may also fill an important scientific knowledge gap at the junction of basic science research and clinical implications, which would lead to new opportunities for prenatal screening, diagnosis and therapy.
ACKNOWLEDGMENTS
We thank Lily Lu for help with art work of the figures and Dr. Herbert T. Cohen for critical reading of the manuscript. This work is supported by NIH grants R01DK078226 and R01HD060050, and is also supported in part by research grant #1-FY12-426 from the March of Dimes Foundation.
Footnotes
The authors have declared that no competing interests exist.
REFERENCES
- 1.Christianson A, Howson CP, Modell B. March of Dimes global report on birth defects. 2006 Pages Retrieved from http://www.marchofdimes.com/downloads/Birth_Defects_Report-PF.pdf.
- 2.CDC. Update on overall prevalence of major birth defects--Atlanta, Georgia, 1978–2005. MMWR Morb Mortal Wkly Rep. 2008;57:1–5. [PubMed] [Google Scholar]
- 3.Yoon PW, Olney RS, Khoury MJ, Sappenfield WM, Chavez GF, Taylor D. Contribution of birth defects and genetic diseases to pediatric hospitalizations. A population-based study. Arch Pediatr Adolesc Med. 1997;151:1096–1103. doi: 10.1001/archpedi.1997.02170480026004. [DOI] [PubMed] [Google Scholar]
- 4.Pope JCt, Brock JW, 3rd, Adams MC, Stephens FD, Ichikawa I. How they begin and how they end: classic and new theories for the development and deterioration of congenital anomalies of the kidney and urinary tract, CAKUT. J Am Soc Nephrol. 1999;10:2018–2028. doi: 10.1681/ASN.V1092018. [DOI] [PubMed] [Google Scholar]
- 5.Miyazaki Y, Ichikawa I. Ontogeny of congenital anomalies of the kidney and urinary tract, CAKUT. Pediatr Int. 2003;45:598–604. doi: 10.1046/j.1442-200x.2003.01777.x. [DOI] [PubMed] [Google Scholar]
- 6.CDC. Hospital stays, hospital charges, and in-hospital deaths among infants with selected birth defects--United States, 2003. MMWR Morb Mortal Wkly Rep. 2007;56:25–29. [PubMed] [Google Scholar]
- 7.Sanyanusin P, McNoe LA, Sullivan MJ, Weaver RG, Eccles MR. Mutation of PAX2 in two siblings with renal-coloboma syndrome. Hum Mol Genet. 1995;4:2183–2184. doi: 10.1093/hmg/4.11.2183. [DOI] [PubMed] [Google Scholar]
- 8.Edghill EL, Bingham C, Ellard S, Hattersley AT. Mutations in hepatocyte nuclear factor-1beta and their related phenotypes. J Med Genet. 2006;43:84–90. doi: 10.1136/jmg.2005.032854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ulinski T, Lescure S, Beaufils S, Guigonis V, Decramer S, Morin D, Clauin S, Deschenes G, Bouissou F, Bensman A, et al. Renal phenotypes related to hepatocyte nuclear factor-1beta (TCF2) mutations in a pediatric cohort. J Am Soc Nephrol. 2006;17:497–503. doi: 10.1681/ASN.2005101040. [DOI] [PubMed] [Google Scholar]
- 10.Lu W, van Eerde AM, Fan X, Quintero-Rivera F, Kulkarni S, Ferguson HL, Kim H, Fan Y, Xi Q, Li QG, et al. Disruption of ROBO2 is associated with urinary tract anomalies and confers risk of vesicoureteral reflux. Am J Hum Genet. 2007;80:616–632. doi: 10.1086/512735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song R, Yosypiv IV. Genetics of congenital anomalies of the kidney and urinary tract. Pediatr Nephrol. 2011;26:353–364. doi: 10.1007/s00467-010-1629-4. [DOI] [PubMed] [Google Scholar]
- 12.Neild GH. Primary renal disease in young adults with renal failure. Nephrol Dial Transplant. 2010;25:1025–1032. doi: 10.1093/ndt/gfp653. [DOI] [PubMed] [Google Scholar]
- 13.Sanna-Cherchi S, Ravani P, Corbani V, Parodi S, Haupt R, Piaggio G, Innocenti ML, Somenzi D, Trivelli A, Caridi G, et al. Renal outcome in patients with congenital anomalies of the kidney and urinary tract. Kidney Int. 2009;76:528–533. doi: 10.1038/ki.2009.220. [DOI] [PubMed] [Google Scholar]
- 14.Toka HR, Toka O, Hariri A, Nguyen HT. Congenital anomalies of kidney and urinary tract. Semin Nephrol. 2010;30:374–386. doi: 10.1016/j.semnephrol.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 15.Chevalier RL. When is one kidney not enough? Kidney Int. 2009;76:475–477. doi: 10.1038/ki.2009.244. [DOI] [PubMed] [Google Scholar]
- 16.Kerecuk L, Schreuder MF, Woolf AS. Renal tract malformations: perspectives for nephrologists. Nat Clin Pract Nephrol. 2008;4:312–325. doi: 10.1038/ncpneph0807. [DOI] [PubMed] [Google Scholar]
- 17.Costantini F, Kopan R. Patterning a complex organ: branching morphogenesis and nephron segmentation in kidney development. Dev Cell. 2010;18:698–712. doi: 10.1016/j.devcel.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dressler GR. Advances in early kidney specification, development and patterning. Development. 2009;136:3863–3874. doi: 10.1242/dev.034876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Faa G, Gerosa C, Fanni D, Monga G, Zaffanello M, Van Eyken P, Fanos V. Morphogenesis and molecular mechanisms involved in human kidney development. J Cell Physiol. 2012;227:1257–1268. doi: 10.1002/jcp.22985. [DOI] [PubMed] [Google Scholar]
- 20.Little M, Georgas K, Pennisi D, Wilkinson L. Kidney development: two tales of tubulogenesis. Curr Top Dev Biol. 2010;90:193–229. doi: 10.1016/S0070-2153(10)90005-7. [DOI] [PubMed] [Google Scholar]
- 21.Little MH, McMahon AP. Mammalian kidney development: principles, progress, and projections. Cold Spring Harb Perspect Biol. 2012:4. doi: 10.1101/cshperspect.a008300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quaggin SE, Kreidberg JA. Development of the renal glomerulus: good neighbors and good fences. Development. 2008;135:609–620. doi: 10.1242/dev.001081. [DOI] [PubMed] [Google Scholar]
- 23.Hildebrandt F. Genetic kidney diseases. Lancet. 2010;375:1287–1295. doi: 10.1016/S0140-6736(10)60236-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saxen L. Organogenesis of the kidney. Cambridge: Cambridge University Press; 1987. [Google Scholar]
- 25.Airik R, Kispert A. Down the tube of obstructive nephropathies: the importance of tissue interactions during ureter development. Kidney Int. 2007;72:1459–1467. doi: 10.1038/sj.ki.5002589. [DOI] [PubMed] [Google Scholar]
- 26.Deng FM, Liang FX, Tu L, Resing KA, Hu P, Supino M, Hu CC, Zhou G, Ding M, Kreibich G, et al. Uroplakin IIIb, a urothelial differentiation marker, dimerizes with uroplakin Ib as an early step of urothelial plaque assembly. J Cell Biol. 2002;159:685–694. doi: 10.1083/jcb.200204102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hu P, Deng FM, Liang FX, Hu CM, Auerbach AB, Shapiro E, Wu XR, Kachar B, Sun TT. Ablation of uroplakin III gene results in small urothelial plaques, urothelial leakage, and vesicoureteral reflux. J Cell Biol. 2000;151:961–972. doi: 10.1083/jcb.151.5.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kong XT, Deng FM, Hu P, Liang FX, Zhou G, Auerbach AB, Genieser N, Nelson PK, Robbins ES, Shapiro E, et al. Roles of uroplakins in plaque formation, umbrella cell enlargement, and urinary tract diseases. J Cell Biol. 2004;167:1195–1204. doi: 10.1083/jcb.200406025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Airik R, Bussen M, Singh MK, Petry M, Kispert A. Tbx18 regulates the development of the ureteral mesenchyme. J Clin Invest. 2006;116:663–674. doi: 10.1172/JCI26027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baker LA, Gomez RA. Embryonic development of the ureter and bladder: acquisition of smooth muscle. J Urol. 1998;160:545–550. [PubMed] [Google Scholar]
- 31.Caubit X, Lye CM, Martin E, Core N, Long DA, Vola C, Jenkins D, Garratt AN, Skaer H, Woolf AS, et al. Teashirt 3 is necessary for ureteral smooth muscle differentiation downstream of SHH and BMP4. Development. 2008;135:3301–3310. doi: 10.1242/dev.022442. [DOI] [PubMed] [Google Scholar]
- 32.Yu J, Carroll TJ, McMahon AP. Sonic hedgehog regulates proliferation and differentiation of mesenchymal cells in the mouse metanephric kidney. Development. 2002;129:5301–5312. doi: 10.1242/dev.129.22.5301. [DOI] [PubMed] [Google Scholar]
- 33.Lang RJ, Tonta MA, Zoltkowski BZ, Meeker WF, Wendt I, Parkington HC. Pyeloureteric peristalsis: role of atypical smooth muscle cells and interstitial cells of Cajal-like cells as pacemakers. J Physiol. 2006;576:695–705. doi: 10.1113/jphysiol.2006.116855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lang RJ, Hashitani H, Tonta MA, Bourke JL, Parkington HC, Suzuki H. Spontaneous electrical and Ca2+ signals in the mouse renal pelvis that drive pyeloureteric peristalsis. Clin Exp Pharmacol Physiol. 2010;37:509–515. doi: 10.1111/j.1440-1681.2009.05226.x. [DOI] [PubMed] [Google Scholar]
- 35.David SG, Cebrian C, Vaughan ED, Jr., Herzlinger D. c-kit and ureteral peristalsis. J Urol. 2005;173:292–295. doi: 10.1097/01.ju.0000141594.99139.3d. [DOI] [PubMed] [Google Scholar]
- 36.Metzger R, Schuster T, Till H, Stehr M, Franke FE, Dietz HG. Cajal-like cells in the human upper urinary tract. J Urol. 2004;172:769–772. doi: 10.1097/01.ju.0000130571.15243.59. [DOI] [PubMed] [Google Scholar]
- 37.Di Benedetto A, Arena S, Nicotina PA, Mucciardi G, Gali A, Magno C. Pacemakers in the upper urinary tract. Neurourol Urodyn. 2012 doi: 10.1002/nau.22310. [DOI] [PubMed] [Google Scholar]
- 38.Lang RJ, Hashitani H, Tonta MA, Parkington HC, Suzuki H. Spontaneous electrical and Ca2+ signals in typical and atypical smooth muscle cells and interstitial cell of Cajal-like cells of mouse renal pelvis. J Physiol. 2007;583:1049–1068. doi: 10.1113/jphysiol.2007.137034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Park JM. Normal development of the genitourinary tract. In: Wein AJ, editor. Campbell-Walsh Urology. 10th ed. Vol. 4. Philadelphia, PA: Elsevier Saunders; 2012. pp. 2975–3001. [Google Scholar]
- 40.Woolf AS, Winyard PJ, Hermanns MM, Welham SJ. Maldevelopment of the human kidney and lower urinary tract: an overview. In: Vize PD, Woolf AS, Bard JBL, editors. The Kidney: From Normal Development to Congenital Disease. London, UK: Academic Press; 2003. pp. 377–393. [Google Scholar]
- 41.Mackie GG, Awang H, Stephens FD. The ureteric orifice: the embryologic key to radiologic status of duplex kidneys. J Pediatr Surg. 1975;10:473–481. doi: 10.1016/0022-3468(75)90187-6. [DOI] [PubMed] [Google Scholar]
- 42.Murawski IJ, Gupta IR. Vesicoureteric reflux and renal malformations: a developmental problem. Clin Genet. 2006;69:105–117. doi: 10.1111/j.1399-0004.2005.00562.x. [DOI] [PubMed] [Google Scholar]
- 43.Batourina E, Choi C, Paragas N, Bello N, Hensle T, Costantini FD, Schuchardt A, Bacallao RL, Mendelsohn CL. Distal ureter morphogenesis depends on epithelial cell remodeling mediated by vitamin A and Ret. Nat Genet. 2002;32:109–115. doi: 10.1038/ng952. [DOI] [PubMed] [Google Scholar]
- 44.Batourina E, Tsai S, Lambert S, Sprenkle P, Viana R, Dutta S, Hensle T, Wang F, Niederreither K, McMahon AP, et al. Apoptosis induced by vitamin A signaling is crucial for connecting the ureters to the bladder. Nat Genet. 2005;37:1082–1089. doi: 10.1038/ng1645. [DOI] [PubMed] [Google Scholar]
- 45.Mendelsohn C. Using mouse models to understand normal and abnormal urogenital tract development. Organogenesis. 2009;5:306–314. doi: 10.4161/org.8173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weiss JP. Embryogenesis of ureteral anomalies: a unifying theory. Aust N Z J Surg. 1988;58:631–638. doi: 10.1111/j.1445-2197.1988.tb07573.x. [DOI] [PubMed] [Google Scholar]
- 47.Tanaka ST, Ishii K, Demarco RT, Pope JCt, Brock JW, 3rd, Hayward SW. Endodermal origin of bladder trigone inferred from mesenchymal-epithelial interaction. J Urol. 2010;183:386–391. doi: 10.1016/j.juro.2009.08.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thomas JC, DeMarco RT, Pope JCt. Molecular biology of ureteral bud and trigonal development. Curr Urol Rep. 2005;6:146–151. doi: 10.1007/s11934-005-0084-4. [DOI] [PubMed] [Google Scholar]
- 49.Viana R, Batourina E, Huang H, Dressler GR, Kobayashi A, Behringer RR, Shapiro E, Hensle T, Lambert S, Mendelsohn C. The development of the bladder trigone, the center of the anti-reflux mechanism. Development. 2007;134:3763–3769. doi: 10.1242/dev.011270. [DOI] [PubMed] [Google Scholar]
- 50.Newman J, Antonakopoulos GN. The fine structure of the human fetal urinary bladder. Development and maturation. A light, transmission and scanning electron microscopic study. J Anat. 1989;166:135–150. [PMC free article] [PubMed] [Google Scholar]
- 51.Baskin LS, Hayward SW, Young P, Cunha GR. Role of mesenchymal-epithelial interactions in normal bladder development. J Urol. 1996;156:1820–1827. [PubMed] [Google Scholar]
- 52.Shiroyanagi Y, Liu B, Cao M, Agras K, Li J, Hsieh MH, Willingham EJ, Baskin LS. Urothelial sonic hedgehog signaling plays an important role in bladder smooth muscle formation. Differentiation. 2007;75:968–977. doi: 10.1111/j.1432-0436.2007.00187.x. [DOI] [PubMed] [Google Scholar]
- 53.Erman A, Veranic P, Psenicnik M, Jezernik K. Superficial cell differentiation during embryonic and postnatal development of mouse urothelium. Tissue Cell. 2006;38:293–301. doi: 10.1016/j.tice.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 54.Kim KM, Kogan BA, Massad CA, Huang YC. Collagen and elastin in the normal fetal bladder. J Urol. 1991;146:524–527. doi: 10.1016/s0022-5347(17)37843-6. [DOI] [PubMed] [Google Scholar]
- 55.Baskin L, Meaney D, Landsman A, Zderic SA, Macarak E. Bovine bladder compliance increases with normal fetal development. J Urol. 1994;152:692–695. doi: 10.1016/s0022-5347(17)32682-4. discussion 696-697. [DOI] [PubMed] [Google Scholar]
- 56.Kaefer M, Zurakowski D, Bauer SB, Retik AB, Peters CA, Atala A, Treves ST. Estimating normal bladder capacity in children. J Urol. 1997;158:2261–2264. doi: 10.1016/s0022-5347(01)68230-2. [DOI] [PubMed] [Google Scholar]
- 57.Wahl EF, Lahdes-Vasama TT, Churchill BM. Estimation of glomerular filtration rate and bladder capacity: the effect of maturation, ageing, gender and size. BJU Int. 2003;91:255–262. doi: 10.1046/j.1464-410x.2003.04053.x. [DOI] [PubMed] [Google Scholar]
- 58.Yucel S, Baskin LS. An anatomical description of the male and female urethral sphincter complex. J Urol. 2004;171:1890–1897. doi: 10.1097/01.ju.0000124106.16505.df. [DOI] [PubMed] [Google Scholar]
- 59.Bourdelat D, Barbet JP, Butler-Browne GS. Fetal development of the urethral sphincter. Eur J Pediatr Surg. 1992;2:35–38. doi: 10.1055/s-2008-1063397. [DOI] [PubMed] [Google Scholar]
- 60.Sebe P, Fritsch H, Oswald J, Schwentner C, Lunacek A, Bartsch G, Radmayr C. Fetal development of the female external urinary sphincter complex: an anatomical and histological study. J Urol. 2005;173:1738, 1742. doi: 10.1097/01.ju.0000154616.51979.da. discussion 1742. [DOI] [PubMed] [Google Scholar]
- 61.Ludwikowski B, Oesch Hayward I, Brenner E, Fritsch H. The development of the external urethral sphincter in humans. BJU Int. 2001;87:565–568. doi: 10.1046/j.1464-410x.2001.00086.x. [DOI] [PubMed] [Google Scholar]
- 62.Colleselli K, Stenzl A, Eder R, Strasser H, Poisel S, Bartsch G. The female urethral sphincter: a morphological and topographical study. J Urol. 1998;160:49–54. doi: 10.1016/s0022-5347(01)63025-8. [DOI] [PubMed] [Google Scholar]
- 63.Karam I, Droupy S, Abd-Alsamad I, Korbage A, Uhl JF, Benoit G, Delmas V. The precise location and nature of the nerves to the male human urethra: histological and immunohistochemical studies with three-dimensional reconstruction. Eur Urol. 2005;48:858–864. doi: 10.1016/j.eururo.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 64.Karam I, Droupy S, Abd-Alsamad I, Uhl JF, Benoit G, Delmas V. Innervation of the female human urethral sphincter: 3D reconstruction of immunohistochemical studies in the fetus. Eur Urol. 2005;47:627–633. doi: 10.1016/j.eururo.2005.01.001. discussion 634. [DOI] [PubMed] [Google Scholar]
- 65.Strasser H, Ninkovic M, Hess M, Bartsch G, Stenzl A. Anatomic and functional studies of the male and female urethral sphincter. World J Urol. 2000;18:324–329. doi: 10.1007/s003450000145. [DOI] [PubMed] [Google Scholar]
- 66.Fowler CJ, Griffiths D, de Groat WC. The neural control of micturition. Nat Rev Neurosci. 2008;9:453–466. doi: 10.1038/nrn2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shafik A. Ureterovesical junction inhibitory reflex and vesicoureteral junction excitatory reflex: description of two reflexes and their role in the ureteric antireflux mechanism. Urol Res. 1996;24:339–343. doi: 10.1007/BF00389790. [DOI] [PubMed] [Google Scholar]
- 68.Shafik A. Study of the effect of external urethral sphincter contraction on the mechanical activity of the ureterovesical junction and urinary bladder: recognition of the sphinctero-ureterovesical reflex. Urology. 1997;50:949–952. doi: 10.1016/S0090-4295(97)00405-6. [DOI] [PubMed] [Google Scholar]
- 69.Oswald J, Brenner E, Deibl M, Fritsch H, Bartsch G, Radmayr C. Longitudinal and thickness measurement of the normal distal and intravesical ureter in human fetuses. J Urol. 2003;169:1501–1504. doi: 10.1097/01.ju.0000057047.82984.7f. [DOI] [PubMed] [Google Scholar]
- 70.Arena S, Fazzari C, Arena F, Scuderi MG, Romeo C, Nicotina PA, Di Benedetto V. Altered 'active' antireflux mechanism in primary vesico-ureteric reflux: a morphological and manometric study. BJU Int. 2007;100:407–412. doi: 10.1111/j.1464-410X.2007.06921.x. [DOI] [PubMed] [Google Scholar]
- 71.Oswald J, Brenner E, Schwentner C, Deibl M, Bartsch G, Fritsch H, Radmayr C. The intravesical ureter in children with vesicoureteral reflux: a morphological and immunohistochemical characterization. J Urol. 2003;170:2423–2427. doi: 10.1097/01.ju.0000097146.26432.9a. [DOI] [PubMed] [Google Scholar]
- 72.Paquin AJ., Jr. Ureterovesical anastomosis: the description and evaluation of a technique. J Urol. 1959;82:573–583. doi: 10.1016/S0022-5347(17)65934-2. [DOI] [PubMed] [Google Scholar]
- 73.Tanagho EA, Hutch JA, Meyers FH, Rambo ON., Jr. Primary Vesicoureteral Reflux: Experimental Studies of Its Etiology. J Urol. 1965;93:165–176. doi: 10.1016/S0022-5347(17)63742-X. [DOI] [PubMed] [Google Scholar]
- 74.Costantini F. GDNF/Ret signaling and renal branching morphogenesis: From mesenchymal signals to epithelial cell behaviors. Organogenesis. 2010;6:252–262. doi: 10.4161/org.6.4.12680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Trowe MO, Airik R, Weiss AC, Farin HF, Foik AB, Bettenhausen E, Schuster-Gossler K, Taketo MM, Kispert A. Canonical Wnt signaling regulates smooth muscle precursor development in the mouse ureter. Development. 2012;139:3099–3108. doi: 10.1242/dev.077388. [DOI] [PubMed] [Google Scholar]
- 76.Cain JE, Islam E, Haxho F, Blake J, Rosenblum ND. GLI3 repressor controls functional development of the mouse ureter. J Clin Invest. 2011;121:1199–1206. doi: 10.1172/JCI45523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Miyazaki Y, Oshima K, Fogo A, Hogan BL, Ichikawa I. Bone morphogenetic protein 4 regulates the budding site and elongation of the mouse ureter. J Clin Invest. 2000;105:863–873. doi: 10.1172/JCI8256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Brenner-Anantharam A, Cebrian C, Guillaume R, Hurtado R, Sun TT, Herzlinger D. Tailbud-derived mesenchyme promotes urinary tract segmentation via BMP4 signaling. Development. 2007;134:1967–1975. doi: 10.1242/dev.004234. [DOI] [PubMed] [Google Scholar]
- 79.Tripathi P, Wang Y, Casey AM, Chen F. Absence of canonical Smad signaling in ureteral and bladder mesenchyme causes ureteropelvic junction obstruction. J Am Soc Nephrol. 2012;23:618–628. doi: 10.1681/ASN.2011060566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kam RK, Deng Y, Chen Y, Zhao H. Retinoic acid synthesis and functions in early embryonic development. Cell Biosci. 2012;2:11. doi: 10.1186/2045-3701-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gribouval O, Gonzales M, Neuhaus T, Aziza J, Bieth E, Laurent N, Bouton JM, Feuillet F, Makni S, Ben Amar H, et al. Mutations in genes in the renin-angiotensin system are associated with autosomal recessive renal tubular dysgenesis. Nat Genet. 2005;37:964–968. doi: 10.1038/ng1623. [DOI] [PubMed] [Google Scholar]
- 82.Nishimura H, Yerkes E, Hohenfellner K, Miyazaki Y, Ma J, Hunley TE, Yoshida H, Ichiki T, Threadgill D, Phillips JA, 3rd, et al. Role of the angiotensin type 2 receptor gene in congenital anomalies of the kidney and urinary tract, CAKUT, of mice and men. Mol Cell. 1999;3:1–10. doi: 10.1016/s1097-2765(00)80169-0. [DOI] [PubMed] [Google Scholar]
- 83.Oliverio MI, Kim HS, Ito M, Le T, Audoly L, Best CF, Hiller S, Kluckman K, Maeda N, Smithies O, et al. Reduced growth, abnormal kidney structure, and type 2 (AT2) angiotensin receptor-mediated blood pressure regulation in mice lacking both AT1A and AT1B receptors for angiotensin II. Proc Natl Acad Sci U S A. 1998;95:15496–15501. doi: 10.1073/pnas.95.26.15496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tsuchida S, Matsusaka T, Chen X, Okubo S, Niimura F, Nishimura H, Fogo A, Utsunomiya H, Inagami T, Ichikawa I. Murine double nullizygotes of the angiotensin type 1A and 1B receptor genes duplicate severe abnormal phenotypes of angiotensinogen nullizygotes. J Clin Invest. 1998;101:755–760. doi: 10.1172/JCI1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yosypiv IV. Renin-angiotensin system in ureteric bud branching morphogenesis: insights into the mechanisms. Pediatr Nephrol. 2011;26:1499–1512. doi: 10.1007/s00467-011-1820-2. [DOI] [PubMed] [Google Scholar]
- 86.Duell BL, Carey AJ, Tan CK, Cui X, Webb RI, Totsika M, Schembri MA, Derrington P, Irving-Rodgers H, Brooks AJ, et al. Innate transcriptional networks activated in bladder in response to uropathogenic Escherichia coli drive diverse biological pathways and rapid synthesis of IL-10 for defense against bacterial urinary tract infection. J Immunol. 2012;188:781–792. doi: 10.4049/jimmunol.1101231. [DOI] [PubMed] [Google Scholar]
- 87.Tan CK, Carey AJ, Cui X, Webb RI, Ipe D, Crowley M, Cripps AW, Benjamin WH, Jr., Ulett KB, Schembri MA, et al. Genome-wide mapping of cystitis due to Streptococcus agalactiae and Escherichia coli in mice identifies a unique bladder transcriptome that signifies pathogen-specific antimicrobial defense against urinary tract infection. Infect Immun. 2012;80:3145–3160. doi: 10.1128/IAI.00023-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Skarnes WC, Rosen B, West AP, Koutsourakis M, Bushell W, Iyer V, Mujica AO, Thomas M, Harrow J, Cox T, et al. A conditional knockout resource for the genome-wide study of mouse gene function. Nature. 2011;474:337–342. doi: 10.1038/nature10163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lewandoski M. Conditional control of gene expression in the mouse. Nat Rev Genet. 2001;2:743–755. doi: 10.1038/35093537. [DOI] [PubMed] [Google Scholar]
- 90.Zhao H, Kegg H, Grady S, Truong HT, Robinson ML, Baum M, Bates CM. Role of fibroblast growth factor receptors 1 and 2 in the ureteric bud. Dev Biol. 2004;276:403–415. doi: 10.1016/j.ydbio.2004.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang Y, Tripathi P, Guo Q, Coussens M, Ma L, Chen F. Cre/lox recombination in the lower urinary tract. Genesis. 2009;47:409–413. doi: 10.1002/dvg.20515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chi X, Michos O, Shakya R, Riccio P, Enomoto H, Licht JD, Asai N, Takahashi M, Ohgami N, Kato M, et al. Ret-dependent cell rearrangements in the Wolffian duct epithelium initiate ureteric bud morphogenesis. Dev Cell. 2009;17:199–209. doi: 10.1016/j.devcel.2009.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Haraguchi R, Matsumaru D, Nakagata N, Miyagawa S, Suzuki K, Kitazawa S, Yamada G. The hedgehog signal induced modulation of bone morphogenetic protein signaling: an essential signaling relay for urinary tract morphogenesis. PLoS One. 2012;7:e42245. doi: 10.1371/journal.pone.0042245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Haraguchi R, Motoyama J, Sasaki H, Satoh Y, Miyagawa S, Nakagata N, Moon A, Yamada G. Molecular analysis of coordinated bladder and urogenital organ formation by Hedgehog signaling. Development. 2007;134:525–533. doi: 10.1242/dev.02736. [DOI] [PubMed] [Google Scholar]
- 95.Seifert AW, Harfe BD, Cohn MJ. Cell lineage analysis demonstrates an endodermal origin of the distal urethra and perineum. Dev Biol. 2008;318:143–152. doi: 10.1016/j.ydbio.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hollowell JG, Altman HG, Snyder HM, 3rd, Duckett JW. Coexisting ureteropelvic junction obstruction and vesicoureteral reflux: diagnostic and therapeutic implications. J Urol. 1989;142:490–493. doi: 10.1016/s0022-5347(17)38793-1. discussion 501. [DOI] [PubMed] [Google Scholar]
- 97.Quirino IG, Diniz JS, Bouzada MC, Pereira AK, Lopes TJ, Paixao GM, Barros NN, Figueiredo LC, Cabral AC, Simoes e Silva AC, et al. Clinical course of 822 children with prenatally detected nephrouropathies. Clin J Am Soc Nephrol. 2012;7:444–451. doi: 10.2215/CJN.03400411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sargent MA. What is the normal prevalence of vesicoureteral reflux? Pediatr Radiol. 2000;30:587–593. doi: 10.1007/s002470000263. [DOI] [PubMed] [Google Scholar]
- 99.Gargollo PC, Diamond DA. Therapy insight: What nephrologists need to know about primary vesicoureteral reflux. Nat Clin Pract Nephrol. 2007;3:551–563. doi: 10.1038/ncpneph0610. [DOI] [PubMed] [Google Scholar]
- 100.Peters CA, Skoog SJ, Arant BS, Jr., Copp HL, Elder JS, Hudson RG, Khoury AE, Lorenzo AJ, Pohl HG, Shapiro E, et al. Summary of the AUA Guideline on Management of Primary Vesicoureteral Reflux in Children. J Urol. 2010;184:1134–1144. doi: 10.1016/j.juro.2010.05.065. [DOI] [PubMed] [Google Scholar]
- 101.Bailey RR. The relationship of vesico-ureteric reflux to urinary tract infection and chronic pyelonephritis-reflux nephropathy. Clin Nephrol. 1973;1:132–141. [PubMed] [Google Scholar]
- 102.Bailey RR, Lynn KL, Robson RA. End-stage reflux nephropathy. Ren Fail. 1994;16:27–35. doi: 10.3109/08860229409044845. [DOI] [PubMed] [Google Scholar]
- 103.Martinell J, Lidin-Janson G, Jagenburg R, Sivertsson R, Claesson I, Jodal U. Girls prone to urinary infections followed into adulthood. Indices of renal disease. Pediatr Nephrol. 1996;10:139–142. doi: 10.1007/BF00862054. [DOI] [PubMed] [Google Scholar]
- 104.Torres VE, Velosa JA, Holley KE, Kelalis PP, Stickler GB, Kurtz SB. The progression of vesicoureteral reflux nephropathy. Ann Intern Med. 1980;92:776–784. doi: 10.7326/0003-4819-92-6-776. [DOI] [PubMed] [Google Scholar]
- 105.Bhathena DB, Weiss JH, Holland NH, McMorrow RG, Curtis JJ, Lucas BA, Luke RG. Focal and segmental glomerular sclerosis in reflux nephropathy. Am J Med. 1980;68:886–892. doi: 10.1016/0002-9343(80)90218-1. [DOI] [PubMed] [Google Scholar]
- 106.Hodson CJ, Maling TM, McManamon PJ, Lewis MG. The pathogenesis of reflux nephropathy (chronic atrophic pyelonephritis) Br J Radiol. 1975;(Suppl 13):1–26. [PubMed] [Google Scholar]
- 107.Ransley PG, Risdon RA. The pathogenesis of reflux nephropathy. Contrib Nephrol. 1979;16:90–97. doi: 10.1159/000402880. [DOI] [PubMed] [Google Scholar]
- 108.Ransley PG, Risdon RA, Godley ML. High pressure sterile vesicoureteral reflux and renal scarring: an experimental study in the pig and minipig. Contrib Nephrol. 1984;39:320–343. doi: 10.1159/000409261. [DOI] [PubMed] [Google Scholar]
- 109.Swerkersson S, Jodal U, Sixt R, Stokland E, Hansson S. Relationship among vesicoureteral reflux, urinary tract infection and renal damage in children. J Urol. 2007;178:647–651. doi: 10.1016/j.juro.2007.04.004. discussion 650-641. [DOI] [PubMed] [Google Scholar]
- 110.Coulthard MG, Keir MJ. Reflux nephropathy in kidney transplants, demonstrated by dimercaptosuccinic acid scanning. Transplantation. 2006;82:205–210. doi: 10.1097/01.tp.0000226165.06196.84. [DOI] [PubMed] [Google Scholar]
- 111.Cotran RS. Nephrology Forum. Glomerulosclerosis in reflux nephropathy. Kidney Int. 1982;21:528–534. doi: 10.1038/ki.1982.57. [DOI] [PubMed] [Google Scholar]
- 112.Salvatierra O, Jr., Tanagho EA. Reflux as a cause of end stage kidney disease: report of 32 cases. J Urol. 1977;117:441–443. doi: 10.1016/s0022-5347(17)58492-x. [DOI] [PubMed] [Google Scholar]
- 113.Senekjian HO, Stinebaugh BJ, Mattioli CA, Suki WN. Irreversible renal failure following vesicoureteral reflux. Jama. 1979;241:160–162. [PubMed] [Google Scholar]
- 114.Toffolo A, Ammenti A, Montini G. Long-term clinical consequences of urinary tract infections during childhood: a review. Acta Paediatr. 2012;101:1018–1031. doi: 10.1111/j.1651-2227.2012.02785.x. [DOI] [PubMed] [Google Scholar]
- 115.Cendron M. Reflux nephropathy. J Pediatr Urol. 2008;4:414–421. doi: 10.1016/j.jpurol.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 116.Peters C, Rushton HG. Vesicoureteral reflux associated renal damage: congenital reflux nephropathy and acquired renal scarring. J Urol. 2010;184:265–273. doi: 10.1016/j.juro.2010.03.076. [DOI] [PubMed] [Google Scholar]
- 117.Tanagho EA, Guthrie TH, Lyon RP. The intravesical ureter in primary reflux. J Urol. 1969;101:824–832. doi: 10.1016/s0022-5347(17)62433-9. [DOI] [PubMed] [Google Scholar]
- 118.King LR, Kazmi SO, Belman AB. Natural history of vesicoureteral reflux. Outcome of a trial of nonoperative therapy. Urol Clin North Am. 1974;1:441–455. [PubMed] [Google Scholar]
- 119.Grieshammer U, Le M, Plump AS, Wang F, Tessier-Lavigne M, Martin GR. SLIT2-mediated ROBO2 signaling restricts kidney induction to a single site. Dev Cell. 2004;6:709–717. doi: 10.1016/s1534-5807(04)00108-x. [DOI] [PubMed] [Google Scholar]
- 120.Basson MA, Akbulut S, Watson-Johnson J, Simon R, Carroll TJ, Shakya R, Gross I, Martin GR, Lufkin T, McMahon AP, et al. Sprouty1 is a critical regulator of GDNF/RET-mediated kidney induction. Dev Cell. 2005;8:229–239. doi: 10.1016/j.devcel.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 121.Kume T, Deng K, Hogan BL. Murine forkhead/winged helix genes Foxc1 (Mf1) and Foxc2 (Mfh1) are required for the early organogenesis of the kidney and urinary tract. Development. 2000;127:1387–1395. doi: 10.1242/dev.127.7.1387. [DOI] [PubMed] [Google Scholar]
- 122.Hains DS, Sims-Lucas S, Carpenter A, Saha M, Murawski I, Kish K, Gupta I, McHugh K, Bates CM. High incidence of vesicoureteral reflux in mice with Fgfr2 deletion in kidney mesenchyma. J Urol. 2010;183:2077–2084. doi: 10.1016/j.juro.2009.12.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wang H, Li Q, Liu J, Mendelsohn C, Salant DJ, Lu W. Noninvasive assessment of antenatal hydronephrosis in mice reveals a critical role for Robo2 in maintaining anti-reflux mechanism. PLoS One. 2011;6:e24763. doi: 10.1371/journal.pone.0024763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Uetani N, Bouchard M. Plumbing in the embryo: developmental defects of the urinary tracts. Clin Genet. 2009;75:307–317. doi: 10.1111/j.1399-0004.2009.01175.x. [DOI] [PubMed] [Google Scholar]
- 125.Murawski IJ, Gupta IR. Gene discovery and vesicoureteric reflux. Pediatr Nephrol. 2008;23:1021–1027. doi: 10.1007/s00467-007-0704-y. [DOI] [PubMed] [Google Scholar]
- 126.Kaefer M, Curran M, Treves ST, Bauer S, Hendren WH, Peters CA, Atala A, Diamond D, Retik A. Sibling vesicoureteral reflux in multiple gestation births. Pediatrics. 2000;105:800–804. doi: 10.1542/peds.105.4.800. [DOI] [PubMed] [Google Scholar]
- 127.Van den Abbeele AD, Treves ST, Lebowitz RL, Bauer S, Davis RT, Retik A, Colodny A. Vesicoureteral reflux in asymptomatic siblings of patients with known reflux: radionuclide cystography. Pediatrics. 1987;79:147–153. [PubMed] [Google Scholar]
- 128.Noe HN, Wyatt RJ, Peeden JN, Jr., Rivas ML. The transmission of vesicoureteral reflux from parent to child. J Urol. 1992;148:1869–1871. doi: 10.1016/s0022-5347(17)37053-2. [DOI] [PubMed] [Google Scholar]
- 129.Scott JE, Swallow V, Coulthard MG, Lambert HJ, Lee RE. Screening of newborn babies for familial ureteric reflux. Lancet. 1997;350:396–400. doi: 10.1016/s0140-6736(97)01515-8. [DOI] [PubMed] [Google Scholar]
- 130.Peeden JN, Jr., Noe HN. Is it practical to screen for familial vesicoureteral reflux within a private pediatric practice? Pediatrics. 1992;89:758–760. [PubMed] [Google Scholar]
- 131.Noe HN. The long-term results of prospective sibling reflux screening. J Urol. 1992;148:1739–1742. doi: 10.1016/s0022-5347(17)37017-9. [DOI] [PubMed] [Google Scholar]
- 132.Connolly LP, Treves ST, Connolly SA, Zurakowski D, Share JC, Bar-Sever Z, Mitchell KD, Bauer SB. Vesicoureteral reflux in children: incidence and severity in siblings. J Urol. 1997;157:2287–2290. doi: 10.1016/s0022-5347(01)64764-5. [DOI] [PubMed] [Google Scholar]
- 133.Feather SA, Malcolm S, Woolf AS, Wright V, Blaydon D, Reid CJ, Flinter FA, Proesmans W, Devriendt K, Carter J, et al. Primary, nonsyndromic vesicoureteric reflux and its nephropathy is genetically heterogeneous, with a locus on chromosome 1. Am J Hum Genet. 2000;66:1420–1425. doi: 10.1086/302864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chapman CJ, Bailey RR, Janus ED, Abbott GD, Lynn KL. Vesicoureteric reflux: segregation analysis. Am J Med Genet. 1985;20:577–584. doi: 10.1002/ajmg.1320200403. [DOI] [PubMed] [Google Scholar]
- 135.Sanyanusin P, Schimmenti LA, McNoe LA, Ward TA, Pierpont ME, Sullivan MJ, Dobyns WB, Eccles MR. Mutation of the PAX2 gene in a family with optic nerve colobomas, renal anomalies and vesicoureteral reflux. Nat Genet. 1995;9:358–364. doi: 10.1038/ng0495-358. [DOI] [PubMed] [Google Scholar]
- 136.van Eerde AM, Duran K, van Riel E, de Kovel CG, Koeleman BP, Knoers NV, Renkema KY, van der Horst HJ, Bokenkamp A, van Hagen JM, et al. Genes in the ureteric budding pathway: association study on vesico-ureteral reflux patients. PLoS One. 2012;7:e31327. doi: 10.1371/journal.pone.0031327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bertoli-Avella AM, Conte ML, Punzo F, de Graaf BM, Lama G, La Manna A, Polito C, Grassia C, Nobili B, Rambaldi PF, et al. ROBO2 gene variants are associated with familial vesicoureteral reflux. J Am Soc Nephrol. 2008;19:825–831. doi: 10.1681/ASN.2007060692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gimelli S, Caridi G, Beri S, McCracken K, Bocciardi R, Zordan P, Dagnino M, Fiorio P, Murer L, Benetti E, et al. Mutations in SOX17 are associated with congenital anomalies of the kidney and the urinary tract. Hum Mutat. 2010;31:1352–1359. doi: 10.1002/humu.21378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Jenkins D, Bitner-Glindzicz M, Malcolm S, Hu CC, Allison J, Winyard PJ, Gullett AM, Thomas DF, Belk RA, Feather SA, et al. De novo Uroplakin IIIa heterozygous mutations cause human renal adysplasia leading to severe kidney failure. J Am Soc Nephrol. 2005;16:2141–2149. doi: 10.1681/ASN.2004090776. [DOI] [PubMed] [Google Scholar]
- 140.Yang Y, Houle AM, Letendre J, Richter A. RET Gly691Ser mutation is associated with primary vesicoureteral reflux in the French-Canadian population from Quebec. Hum Mutat. 2008;29:695–702. doi: 10.1002/humu.20705. [DOI] [PubMed] [Google Scholar]
- 141.Chatterjee R, Ramos E, Hoffman M, Vanwinkle J, Martin DR, Davis TK, Hoshi M, Hmiel SP, Beck A, Hruska K, et al. Traditional and targeted exome sequencing reveals common, rare and novel functional deleterious variants in RET-signaling complex in a cohort of living US patients with urinary tract malformations. Hum Genet. 2012 doi: 10.1007/s00439-012-1181-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Choi KL, McNoe LA, French MC, Guilford PJ, Eccles MR. Absence of PAX2 gene mutations in patients with primary familial vesicoureteric reflux. J Med Genet. 1998;35:338–339. doi: 10.1136/jmg.35.4.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Yoneda A, Cascio S, Green A, Barton D, Puri P. Angiotensin II type 2 receptor gene is not responsible for familial vesicoureteral reflux. J Urol. 2002;168:1138–1141. doi: 10.1016/S0022-5347(05)64611-3. [DOI] [PubMed] [Google Scholar]
- 144.Kelly H, Ennis S, Yoneda A, Bermingham C, Shields DC, Molony C, Green AJ, Puri P, Barton DE. Uroplakin III is not a major candidate gene for primary vesicoureteral reflux. Eur J Hum Genet. 2005;13:500–502. doi: 10.1038/sj.ejhg.5201322. [DOI] [PubMed] [Google Scholar]
- 145.Zu S, Bartik Z, Zhao S, Sillen U, Nordenskjold A. Mutations in the ROBO2 and SLIT2 genes are rare causes of familial vesico-ureteral reflux. Pediatr Nephrol. 2009;24:1501–1508. doi: 10.1007/s00467-009-1179-9. [DOI] [PubMed] [Google Scholar]
- 146.Cordell HJ, Darlay R, Charoen P, Stewart A, Gullett AM, Lambert HJ, Malcolm S, Feather SA, Goodship TH, Woolf AS, et al. Whole-genome linkage and association scan in primary, nonsyndromic vesicoureteric reflux. J Am Soc Nephrol. 2010;21:113–123. doi: 10.1681/ASN.2009060624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Jiang S, Gitlin J, Deng FM, Liang FX, Lee A, Atala A, Bauer SB, Ehrlich GD, Feather SA, Goldberg JD, et al. Lack of major involvement of human uroplakin genes in vesicoureteral reflux: implications for disease heterogeneity. Kidney Int. 2004;66:10–19. doi: 10.1111/j.1523-1755.2004.00703.x. [DOI] [PubMed] [Google Scholar]
- 148.Dickson BJ, Gilestro GF. Regulation of commissural axon pathfinding by slit and its Robo receptors. Annu Rev Cell Dev Biol. 2006;22:651–675. doi: 10.1146/annurev.cellbio.21.090704.151234. [DOI] [PubMed] [Google Scholar]
- 149.Fricke C, Lee JS, Geiger-Rudolph S, Bonhoeffer F, Chien CB. astray, a zebrafish roundabout homolog required for retinal axon guidance. Science. 2001;292:507–510. doi: 10.1126/science.1059496. [DOI] [PubMed] [Google Scholar]
- 150.Brose K, Bland KS, Wang KH, Arnott D, Henzel W, Goodman CS, Tessier-Lavigne M, Kidd T. Slit proteins bind Robo receptors and have an evolutionarily conserved role in repulsive axon guidance. Cell. 1999;96:795–806. doi: 10.1016/s0092-8674(00)80590-5. [DOI] [PubMed] [Google Scholar]
- 151.Tessier-Lavigne M, Goodman CS. The molecular biology of axon guidance. Science. 1996;274:1123–1133. doi: 10.1126/science.274.5290.1123. [DOI] [PubMed] [Google Scholar]
- 152.Fan X, Li Q, Pisarek-Horowitz A, Rasouly HM, Wang X, Bonegio RG, Wang H, McLaughlin M, Mangos S, Kalluri R, et al. Inhibitory Effects of Robo2 on Nephrin: A Crosstalk between Positive and Negative Signals Regulating Podocyte Structure. Cell Rep. 2012;2:52–61. doi: 10.1016/j.celrep.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Sanchez MP, Silos-Santiago I, Frisen J, He B, Lira SA, Barbacid M. Renal agenesis and the absence of enteric neurons in mice lacking GDNF. Nature. 1996;382:70–73. doi: 10.1038/382070a0. [DOI] [PubMed] [Google Scholar]
- 154.Grote D, Boualia SK, Souabni A, Merkel C, Chi X, Costantini F, Carroll T, Bouchard M. Gata3 acts downstream of beta-catenin signaling to prevent ectopic metanephric kidney induction. PLoS Genet. 2008;4:e1000316. doi: 10.1371/journal.pgen.1000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Nie X, Xu J, El-Hashash A, Xu PX. Six1 regulates Grem1 expression in the metanephric mesenchyme to initiate branching morphogenesis. Dev Biol. 2011;352:141–151. doi: 10.1016/j.ydbio.2011.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Xu PX, Adams J, Peters H, Brown MC, Heaney S, Maas R. Eya1-deficient mice lack ears and kidneys and show abnormal apoptosis of organ primordia. Nat Genet. 1999;23:113–117. doi: 10.1038/12722. [DOI] [PubMed] [Google Scholar]
- 157.Kiefer SM, Robbins L, Stumpff KM, Lin C, Ma L, Rauchman M. Sall1-dependent signals affect Wnt signaling and ureter tip fate to initiate kidney development. Development. 2010;137:3099–3106. doi: 10.1242/dev.037812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Nishinakamura R, Matsumoto Y, Nakao K, Nakamura K, Sato A, Copeland NG, Gilbert DJ, Jenkins NA, Scully S, Lacey DL, et al. Murine homolog of SALL1 is essential for ureteric bud invasion in kidney development. Development. 2001;128:3105–3115. doi: 10.1242/dev.128.16.3105. [DOI] [PubMed] [Google Scholar]
- 159.Jain S. The many faces of RET dysfunction in kidney. Organogenesis. 2009;5:95–108. doi: 10.4161/org.5.4.10048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Pini Prato A, Musso M, Ceccherini I, Mattioli G, Giunta C, Ghiggeri GM, Jasonni V. Hirschsprung disease and congenital anomalies of the kidney and urinary tract (CAKUT): a novel syndromic association. Medicine (Baltimore) 2009;88:83–90. doi: 10.1097/MD.0b013e31819cf5da. [DOI] [PubMed] [Google Scholar]
- 161.Takahashi M. The GDNF/RET signaling pathway and human diseases. Cytokine Growth Factor Rev. 2001;12:361–373. doi: 10.1016/s1359-6101(01)00012-0. [DOI] [PubMed] [Google Scholar]
- 162.de Graaff E, Srinivas S, Kilkenny C, D'Agati V, Mankoo BS, Costantini F, Pachnis V. Differential activities of the RET tyrosine kinase receptor isoforms during mammalian embryogenesis. Genes Dev. 2001;15:2433–2444. doi: 10.1101/gad.205001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Jain S, Encinas M, Johnson EM, Jr., Milbrandt J. Critical and distinct roles for key RET tyrosine docking sites in renal development. Genes Dev. 2006;20:321–333. doi: 10.1101/gad.1387206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Schuchardt A, D'Agati V, Pachnis V, Costantini F. Renal agenesis and hypodysplasia in ret-k- mutant mice result from defects in ureteric bud development. Development. 1996;122:1919–1929. doi: 10.1242/dev.122.6.1919. [DOI] [PubMed] [Google Scholar]
- 165.Skinner MA, Safford SD, Reeves JG, Jackson ME, Freemerman AJ. Renal aplasia in humans is associated with RET mutations. Am J Hum Genet. 2008;82:344–351. doi: 10.1016/j.ajhg.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Yu OH, Murawski IJ, Myburgh DB, Gupta IR. Overexpression of RET leads to vesicoureteric reflux in mice. Am J Physiol Renal Physiol. 2004;287:F1123–F1130. doi: 10.1152/ajprenal.00444.2003. [DOI] [PubMed] [Google Scholar]
- 167.Rozen EJ, Schmidt H, Dolcet X, Basson MA, Jain S, Encinas M. Loss of Sprouty1 rescues renal agenesis caused by Ret mutation. J Am Soc Nephrol. 2009;20:255–259. doi: 10.1681/ASN.2008030267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Michos O, Cebrian C, Hyink D, Grieshammer U, Williams L, D'Agati V, Licht JD, Martin GR, Costantini F. Kidney development in the absence of Gdnf and Spry1 requires Fgf10. PLoS Genet. 2010;6:e1000809. doi: 10.1371/journal.pgen.1000809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.van Eerde AM, Koeleman BP, van de Kamp JM, de Jong TP, Wijmenga C, Giltay JC. Linkage study of 14 candidate genes and loci in four large Dutch families with vesico-ureteral reflux. Pediatr Nephrol. 2007;22:1129–1133. doi: 10.1007/s00467-007-0492-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Benetti E, Murer L, Bordugo A, Andreetta B, Artifoni L. 10p12.1 deletion: HDR phenotype without DGS2 features. Exp Mol Pathol. 2009;86:74–76. doi: 10.1016/j.yexmp.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 171.Van Esch H, Groenen P, Nesbit MA, Schuffenhauer S, Lichtner P, Vanderlinden G, Harding B, Beetz R, Bilous RW, Holdaway I, et al. GATA3 haplo-insufficiency causes human HDR syndrome. Nature. 2000;406:419–422. doi: 10.1038/35019088. [DOI] [PubMed] [Google Scholar]
- 172.Labastie MC, Catala M, Gregoire JM, Peault B. The GATA-3 gene is expressed during human kidney embryogenesis. Kidney Int. 1995;47:1597–1603. doi: 10.1038/ki.1995.223. [DOI] [PubMed] [Google Scholar]
- 173.Grote D, Souabni A, Busslinger M, Bouchard M. Pax 2/8-regulated Gata 3 expression is necessary for morphogenesis and guidance of the nephric duct in the developing kidney. Development. 2006;133:53–61. doi: 10.1242/dev.02184. [DOI] [PubMed] [Google Scholar]
- 174.Weisschuh N, Wolf C, Wissinger B, Gramer E. A novel mutation in the FOXC1 gene in a family with Axenfeld-Rieger syndrome and Peters' anomaly. Clin Genet. 2008;74:476–480. doi: 10.1111/j.1399-0004.2008.01025.x. [DOI] [PubMed] [Google Scholar]
- 175.Yu S, Shao L, Kilbride H, Zwick DL. Haploinsufficiencies of FOXF1 and FOXC2 genes associated with lethal alveolar capillary dysplasia and congenital heart disease. Am J Med Genet A. 2010;152A:1257–1262. doi: 10.1002/ajmg.a.33378. [DOI] [PubMed] [Google Scholar]
- 176.Astorga J, Carlsson P. Hedgehog induction of murine vasculogenesis is mediated by Foxf1 and Bmp4. Development. 2007;134:3753–3761. doi: 10.1242/dev.004432. [DOI] [PubMed] [Google Scholar]
- 177.Chia I, Grote D, Marcotte M, Batourina E, Mendelsohn C, Bouchard M. Nephric duct insertion is a crucial step in urinary tract maturation that is regulated by a Gata3-Raldh2-Ret molecular network in mice. Development. 2011;138:2089–2097. doi: 10.1242/dev.056838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Uetani N, Bertozzi K, Chagnon MJ, Hendriks W, Tremblay ML, Bouchard M. Maturation of ureter-bladder connection in mice is controlled by LAR family receptor protein tyrosine phosphatases. J Clin Invest. 2009;119:924–935. doi: 10.1172/JCI37196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Kohlhase J, Wischermann A, Reichenbach H, Froster U, Engel W. Mutations in the SALL1 putative transcription factor gene cause Townes-Brocks syndrome. Nat Genet. 1998;18:81–83. doi: 10.1038/ng0198-81. [DOI] [PubMed] [Google Scholar]
- 180.Bush KT, Vaughn DA, Li X, Rosenfeld MG, Rose DW, Mendoza SA, Nigam SK. Development and differentiation of the ureteric bud into the ureter in the absence of a kidney collecting system. Dev Biol. 2006;298:571–584. doi: 10.1016/j.ydbio.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 181.Pierides AM, Athanasiou Y, Demetriou K, Koptides M, Deltas CC. A family with the branchio-oto-renal syndrome: clinical and genetic correlations. Nephrol Dial Transplant. 2002;17:1014–1018. doi: 10.1093/ndt/17.6.1014. [DOI] [PubMed] [Google Scholar]
- 182.Lee RS, Cendron M, Kinnamon DD, Nguyen HT. Antenatal hydronephrosis as a predictor of postnatal outcome: a meta-analysis. Pediatrics. 2006;118:586–593. doi: 10.1542/peds.2006-0120. [DOI] [PubMed] [Google Scholar]
- 183.Ek S, Lidefeldt KJ, Varricio L. Fetal hydronephrosis; prevalence, natural history and postnatal consequences in an unselected population. Acta Obstet Gynecol Scand. 2007;86:1463–1466. doi: 10.1080/00016340701714802. [DOI] [PubMed] [Google Scholar]
- 184.Anderson NG, Allan RB, Abbott GD. Fluctuating fetal or neonatal renal pelvis: marker of high-grade vesicoureteral reflux. Pediatr Nephrol. 2004;19:749–753. doi: 10.1007/s00467-004-1425-0. [DOI] [PubMed] [Google Scholar]
- 185.Grazioli S, Parvex P, Merlini L, Combescure C, Girardin E. Antenatal and postnatal ultrasound in the evaluation of the risk of vesicoureteral reflux. Pediatr Nephrol. 2010;25:1687–1692. doi: 10.1007/s00467-010-1543-9. [DOI] [PubMed] [Google Scholar]
- 186.Nguyen HT, Herndon CD, Cooper C, Gatti J, Kirsch A, Kokorowski P, Lee R, Perez-Brayfield M, Metcalfe P, Yerkes E, et al. The Society for Fetal Urology consensus statement on the evaluation and management of antenatal hydronephrosis. J Pediatr Urol. 2010;6:212–231. doi: 10.1016/j.jpurol.2010.02.205. [DOI] [PubMed] [Google Scholar]
- 187.McDill BW, Li SZ, Kovach PA, Ding L, Chen F. Congenital progressive hydronephrosis (cph) is caused by an S256L mutation in aquaporin-2 that affects its phosphorylation and apical membrane accumulation. Proc Natl Acad Sci U S A. 2006;103:6952–6957. doi: 10.1073/pnas.0602087103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Mendelsohn C. Functional obstruction: the renal pelvis rules. J Clin Invest. 2004;113:957–959. doi: 10.1172/JCI21402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Iizuka-Kogo A, Ishidao T, Akiyama T, Senda T. Abnormal development of urogenital organs in Dlgh1-deficient mice. Development. 2007;134:1799–1807. doi: 10.1242/dev.02830. [DOI] [PubMed] [Google Scholar]
- 190.Mahoney ZX, Sammut B, Xavier RJ, Cunningham J, Go G, Brim KL, Stappenbeck TS, Miner JH, Swat W. Discs-large homolog 1 regulates smooth muscle orientation in the mouse ureter. Proc Natl Acad Sci U S A. 2006;103:19872–19877. doi: 10.1073/pnas.0609326103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Mendelsohn C. Going in circles: conserved mechanisms control radial patterning in the urinary and digestive tracts. J Clin Invest. 2006;116:635–637. doi: 10.1172/JCI27985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Lye CM, Fasano L, Woolf AS. Ureter myogenesis: putting Teashirt into context. J Am Soc Nephrol. 2010;21:24–30. doi: 10.1681/ASN.2008111206. [DOI] [PubMed] [Google Scholar]
- 193.Chang CP, McDill BW, Neilson JR, Joist HE, Epstein JA, Crabtree GR, Chen F. Calcineurin is required in urinary tract mesenchyme for the development of the pyeloureteral peristaltic machinery. J Clin Invest. 2004;113:1051–1058. doi: 10.1172/JCI20049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Hurtado R, Bub G, Herzlinger D. The pelvis-kidney junction contains HCN3, a hyperpolarization-activated cation channel that triggers ureter peristalsis. Kidney Int. 2010;77:500–508. doi: 10.1038/ki.2009.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Herzlinger D. Upper urinary tract pacemaker cells join the GLI club. J Clin Invest. 2011;121:836–838. doi: 10.1172/JCI46400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Johnston JJ, Olivos-Glander I, Killoran C, Elson E, Turner JT, Peters KF, Abbott MH, Aughton DJ, Aylsworth AS, Bamshad MJ, et al. Molecular and clinical analyses of Greig cephalopolysyndactyly and Pallister-Hall syndromes: robust phenotype prediction from the type and position of GLI3 mutations. Am J Hum Genet. 2005;76:609–622. doi: 10.1086/429346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Kang S, Graham JM, Jr., Olney AH, Biesecker LG. GLI3 frameshift mutations cause autosomal dominant Pallister-Hall syndrome. Nat Genet. 1997;15:266–268. doi: 10.1038/ng0397-266. [DOI] [PubMed] [Google Scholar]
- 198.Pallister PD, Hecht F, Herrman J. Three additional cases of the congenital hypothalamic "hamartoblastoma" (Pallister-Hall) syndrome. Am J Med Genet. 1989;33:500–501. doi: 10.1002/ajmg.1320330417. [DOI] [PubMed] [Google Scholar]
- 199.Miyazaki Y, Tsuchida S, Nishimura H, Pope JCt, Harris RC, McKanna JM, Inagami T, Hogan BL, Fogo A, Ichikawa I. Angiotensin induces the urinary peristaltic machinery during the perinatal period. J Clin Invest. 1998;102:1489–1497. doi: 10.1172/JCI4401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Gubler MC, Antignac C. Renin-angiotensin system in kidney development: renal tubular dysgenesis. Kidney Int. 2010;77:400–406. doi: 10.1038/ki.2009.423. [DOI] [PubMed] [Google Scholar]
- 201.Yiee J, Wilcox D. Abnormalities of the fetal bladder. Semin Fetal Neonatal Med. 2008;13:164–170. doi: 10.1016/j.siny.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 202.Penna FJ, Elder JS. CKD and bladder problems in children. Adv Chronic Kidney Dis. 2011;18:362–369. doi: 10.1053/j.ackd.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 203.Chen CY, Tsao TF, Chang HM, Chen SL, Chen SM, Hung TW, Lue KH, Sheu JN. Bladder agenesis and bilateral ectopic ureters draining into the vagina in a female infant: demonstrated by MR imaging. Surg Radiol Anat. 2012;34:89–92. doi: 10.1007/s00276-011-0838-2. [DOI] [PubMed] [Google Scholar]
- 204.Weight CJ, Chand D, Ross JH. Single system ectopic ureter to rectum subtending solitary kidney and bladder agenesis in newborn male. Urology. 2006;68:1344 e1341–1343. doi: 10.1016/j.urology.2006.09.048. [DOI] [PubMed] [Google Scholar]
- 205.Liao AW, Sebire NJ, Geerts L, Cicero S, Nicolaides KH. Megacystis at 10–14 weeks of gestation: chromosomal defects and outcome according to bladder length. Ultrasound Obstet Gynecol. 2003;21:338–341. doi: 10.1002/uog.81. [DOI] [PubMed] [Google Scholar]
- 206.Lissauer D, Morris RK, Kilby MD. Fetal lower urinary tract obstruction. Semin Fetal Neonatal Med. 2007;12:464–470. doi: 10.1016/j.siny.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 207.Anneren G, Meurling S, Olsen L. Megacystis-microcolon-intestinal hypoperistalsis syndrome (MMIHS), an autosomal recessive disorder: clinical reports and review of the literature. Am J Med Genet. 1991;41:251–254. doi: 10.1002/ajmg.1320410224. [DOI] [PubMed] [Google Scholar]
- 208.Gosemann JH, Puri P. Megacystis microcolon intestinal hypoperistalsis syndrome: systematic review of outcome. Pediatr Surg Int. 2011;27:1041–1046. doi: 10.1007/s00383-011-2954-9. [DOI] [PubMed] [Google Scholar]
- 209.Singh S, Robinson M, Nahi F, Coley B, Robinson ML, Bates CM, Kornacker K, McHugh KM. Identification of a unique transgenic mouse line that develops megabladder, obstructive uropathy, and renal dysfunction. J Am Soc Nephrol. 2007;18:461–471. doi: 10.1681/ASN.2006040405. [DOI] [PubMed] [Google Scholar]
- 210.Ingraham SE, Saha M, Carpenter AR, Robinson M, Ismail I, Singh S, Hains D, Robinson ML, Hirselj DA, Koff SA, et al. Pathogenesis of renal injury in the megabladder mouse: a genetic model of congenital obstructive nephropathy. Pediatr Res. 2010;68:500–507. doi: 10.1203/PDR.0b013e3181f82f15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Ludwig M, Ching B, Reutter H, Boyadjiev SA. Bladder exstrophy-epispadias complex. Birth Defects Res A Clin Mol Teratol. 2009;85:509–522. doi: 10.1002/bdra.20557. [DOI] [PubMed] [Google Scholar]
- 212.Ebert AK, Reutter H, Ludwig M, Rosch WH. The exstrophy-epispadias complex. Orphanet J Rare Dis. 2009;4:23. doi: 10.1186/1750-1172-4-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Siffel C, Correa A, Amar E, Bakker MK, Bermejo-Sanchez E, Bianca S, Castilla EE, Clementi M, Cocchi G, Csaky-Szunyogh M, et al. Bladder exstrophy: an epidemiologic study from the International Clearinghouse for Birth Defects Surveillance and Research, and an overview of the literature. Am J Med Genet C Semin Med Genet. 2011;157C:321–332. doi: 10.1002/ajmg.c.30316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Feldkamp ML, Botto LD, Amar E, Bakker MK, Bermejo-Sanchez E, Bianca S, Canfield MA, Castilla EE, Clementi M, Csaky-Szunyogh M, et al. Cloacal exstrophy: an epidemiologic study from the International Clearinghouse for Birth Defects Surveillance and Research. Am J Med Genet C Semin Med Genet. 2011;157C:333–343. doi: 10.1002/ajmg.c.30317. [DOI] [PubMed] [Google Scholar]
- 215.Ludwig M, Ruschendorf F, Saar K, Hubner N, Siekmann L, Boyadjiev SA, Reutter H. Genome-wide linkage scan for bladder exstrophy-epispadias complex. Birth Defects Res A Clin Mol Teratol. 2009;85:174–178. doi: 10.1002/bdra.20512. [DOI] [PubMed] [Google Scholar]
- 216.Boyadjiev SA, South ST, Radford CL, Patel A, Zhang G, Hur DJ, Thomas GH, Gearhart JP, Stetten G. A reciprocal translocation 46,XY,t(8;9)(p11.2;q13) in a bladder exstrophy patient disrupts CNTNAP3 and presents evidence of a pericentromeric duplication on chromosome 9. Genomics. 2005;85:622–629. doi: 10.1016/j.ygeno.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 217.Draaken M, Reutter H, Schramm C, Bartels E, Boemers TM, Ebert AK, Rosch W, Schroder A, Stein R, Moebus S, et al. Microduplications at 22q11.21 are associated with non-syndromic classic bladder exstrophy. Eur J Med Genet. 2010;53:55–60. doi: 10.1016/j.ejmg.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 218.Lundin J, Soderhall C, Lunden L, Hammarsjo A, White I, Schoumans J, Lackgren G, Kockum CC, Nordenskjold A. 22q11.2 microduplication in two patients with bladder exstrophy and hearing impairment. Eur J Med Genet. 2010;53:61–65. doi: 10.1016/j.ejmg.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 219.Cheng W, Jacobs WB, Zhang JJ, Moro A, Park JH, Kushida M, Qiu W, Mills AA, Kim PC. DeltaNp63 plays an anti-apoptotic role in ventral bladder development. Development. 2006;133:4783–4792. doi: 10.1242/dev.02621. [DOI] [PubMed] [Google Scholar]
- 220.Routh JC, Huang L, Retik AB, Nelson CP. Contemporary epidemiology and characterization of newborn males with prune belly syndrome. Urology. 2010;76:44–48. doi: 10.1016/j.urology.2009.12.072. [DOI] [PubMed] [Google Scholar]
- 221.Hassett S, Smith GH, Holland AJ. Prune belly syndrome. Pediatr Surg Int. 2012;28:219–228. doi: 10.1007/s00383-011-3046-6. [DOI] [PubMed] [Google Scholar]
- 222.Noh PH, Cooper CS, Winkler AC, Zderic SA, Snyder HM, 3rd, Canning DA. Prognostic factors for long-term renal function in boys with the prune-belly syndrome. J Urol. 1999;162:1399–1401. [PubMed] [Google Scholar]
- 223.Ramasamy R, Haviland M, Woodard JR, Barone JG. Patterns of inheritance in familial prune belly syndrome. Urology. 2005;65:1227. doi: 10.1016/j.urology.2004.12.050. [DOI] [PubMed] [Google Scholar]
- 224.Haeri S, Devers PL, Kaiser-Rogers KA, Moylan VJ, Jr., Torchia BS, Horton AL, Wolfe HM, Aylsworth AS. Deletion of hepatocyte nuclear factor-1-beta in an infant with prune belly syndrome. Am J Perinatol. 2010;27:559–563. doi: 10.1055/s-0030-1248943. [DOI] [PubMed] [Google Scholar]
- 225.Murray PJ, Thomas K, Mulgrew CJ, Ellard S, Edghill EL, Bingham C. Whole gene deletion of the hepatocyte nuclear factor-1beta gene in a patient with the prune-belly syndrome. Nephrol Dial Transplant. 2008;23:2412–2415. doi: 10.1093/ndt/gfn169. [DOI] [PubMed] [Google Scholar]
- 226.Mefford HC, Clauin S, Sharp AJ, Moller RS, Ullmann R, Kapur R, Pinkel D, Cooper GM, Ventura M, Ropers HH, et al. Recurrent reciprocal genomic rearrangements of 17q12 are associated with renal disease, diabetes, and epilepsy. Am J Hum Genet. 2007;81:1057–1069. doi: 10.1086/522591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Bingham C, Bulman MP, Ellard S, Allen LI, Lipkin GW, Hoff WG, Woolf AS, Rizzoni G, Novelli G, Nicholls AJ, et al. Mutations in the hepatocyte nuclear factor-1beta gene are associated with familial hypoplastic glomerulocystic kidney disease. Am J Hum Genet. 2001;68:219–224. doi: 10.1086/316945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Thomas R, Sanna-Cherchi S, Warady BA, Furth SL, Kaskel FJ, Gharavi AG. HNF1B and PAX2 mutations are a common cause of renal hypodysplasia in the CKiD cohort. Pediatr Nephrol. 2011;26:897–903. doi: 10.1007/s00467-011-1826-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Granberg CF, Harrison SM, Dajusta D, Zhang S, Hajarnis S, Igarashi P, Baker LA. Genetic basis of prune belly syndrome: screening for HNF1beta gene. J Urol. 2012;187:272–278. doi: 10.1016/j.juro.2011.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Weber S, Thiele H, Mir S, Toliat MR, Sozeri B, Reutter H, Draaken M, Ludwig M, Altmuller J, Frommolt P, et al. Muscarinic Acetylcholine Receptor M3 Mutation Causes Urinary Bladder Disease and a Prune-Belly-like Syndrome. Am J Hum Genet. 2011;89:668–674. doi: 10.1016/j.ajhg.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Matsui M, Motomura D, Karasawa H, Fujikawa T, Jiang J, Komiya Y, Takahashi S, Taketo MM. Multiple functional defects in peripheral autonomic organs in mice lacking muscarinic acetylcholine receptor gene for the M3 subtype. Proc Natl Acad Sci U S A. 2000;97:9579–9584. doi: 10.1073/pnas.97.17.9579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Psutka SP, Cendron M. Bladder diverticula in children. J Pediatr Urol. 2012 doi: 10.1016/j.jpurol.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 233.Alexander R, Kum JB, Idrees M. Bladder diverticulum: Clinicopathologic spectrum in pediatric patients. Pediatr Dev Pathol. 2012 doi: 10.2350/12-02-1154-OA.1. [DOI] [PubMed] [Google Scholar]
- 234.Tokunaka S, Koyanagi T, Matsuno T, Gotoh T, Tsuji I. Paraureteral diverticula: clinical experience with 17 cases with associated renal dysmorphism. J Urol. 1980;124:791–796. doi: 10.1016/s0022-5347(17)55667-0. [DOI] [PubMed] [Google Scholar]
- 235.Shukla AR, Bellah RA, Canning DA, Carr MC, Snyder HM, Zderic SA. Giant bladder diverticula causing bladder outlet obstruction in children. J Urol. 2004;172:1977–1979. doi: 10.1097/01.ju.0000140450.50242.50. [DOI] [PubMed] [Google Scholar]
- 236.Das S, Levinson B, Vulpe C, Whitney S, Gitschier J, Packman S. Similar splicing mutations of the Menkes/mottled copper-transporting ATPase gene in occipital horn syndrome and the blotchy mouse. Am J Hum Genet. 1995;56:570–576. [PMC free article] [PubMed] [Google Scholar]
- 237.Oshio T, Hino M, Kirino A, Matsumura C, Fukuda K. Urologic abnormalities in Menkes' kinky hair disease: report of three cases. J Pediatr Surg. 1997;32:782–784. doi: 10.1016/s0022-3468(97)90035-x. [DOI] [PubMed] [Google Scholar]
- 238.Game X, Panicker J, Fowler CJ. Williams-Beuren syndrome. N Engl J Med. 2010;362:1449. doi: 10.1056/NEJMc1001965. author reply 1450. [DOI] [PubMed] [Google Scholar]
- 239.Pober BR. Williams-Beuren syndrome. N Engl J Med. 2010;362:239–252. doi: 10.1056/NEJMra0903074. [DOI] [PubMed] [Google Scholar]
- 240.Schulman SL, Zderic S, Kaplan P. Increased prevalence of urinary symptoms and voiding dysfunction in Williams syndrome. J Pediatr. 1996;129:466–469. doi: 10.1016/s0022-3476(96)70086-0. [DOI] [PubMed] [Google Scholar]
- 241.Jorion JL, Michel M. Spontaneous rupture of bladder diverticula in a girl with Ehlers-Danlos syndrome. J Pediatr Surg. 1999;34:483–484. doi: 10.1016/s0022-3468(99)90506-7. [DOI] [PubMed] [Google Scholar]
- 242.Levard G, Aigrain Y, Ferkadji L, Elghoneimi A, Pichon J, Boureau M. Urinary bladder diverticula and the Ehlers-Danlos syndrome in children. J Pediatr Surg. 1989;24:1184–1186. doi: 10.1016/s0022-3468(89)80115-0. [DOI] [PubMed] [Google Scholar]
- 243.Urban Z, Hucthagowder V, Schurmann N, Todorovic V, Zilberberg L, Choi J, Sens C, Brown CW, Clark RD, Holland KE, et al. Mutations in LTBP4 cause a syndrome of impaired pulmonary, gastrointestinal, genitourinary, musculoskeletal, and dermal development. Am J Hum Genet. 2009;85:593–605. doi: 10.1016/j.ajhg.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Ochoa B. Can a congenital dysfunctional bladder be diagnosed from a smile? The Ochoa syndrome updated. Pediatr Nephrol. 2004;19:6–12. doi: 10.1007/s00467-003-1291-1. [DOI] [PubMed] [Google Scholar]
- 245.Derbent M, Melek E, Arman A, Uckan S, Baskin E. Urofacial (ochoa) syndrome: can a facial gestalt represent severe voiding dysfunction? Ren Fail. 2009;31:589–592. doi: 10.1080/08860220903003370. [DOI] [PubMed] [Google Scholar]
- 246.Stamatiou K, Tyritzis S, Karakos C, Skolarikos A. Urofacial syndrome: a subset of neurogenic bladder dysfunction syndromes? Urology. 2011;78:911–913. doi: 10.1016/j.urology.2010.12.061. [DOI] [PubMed] [Google Scholar]
- 247.Al Badr W, Al Bader S, Otto E, Hildebrandt F, Ackley T, Peng W, Xu J, Li J, Owens KM, Bloom D, et al. Exome capture and massively parallel sequencing identifies a novel HPSE2 mutation in a Saudi Arabian child with Ochoa (urofacial) syndrome. J Pediatr Urol. 2011;7:569–573. doi: 10.1016/j.jpurol.2011.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Daly SB, Urquhart JE, Hilton E, McKenzie EA, Kammerer RA, Lewis M, Kerr B, Stuart H, Donnai D, Long DA, et al. Mutations in HPSE2 cause urofacial syndrome. Am J Hum Genet. 2010;86:963–969. doi: 10.1016/j.ajhg.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Pang J, Zhang S, Yang P, Hawkins-Lee B, Zhong J, Zhang Y, Ochoa B, Agundez JA, Voelckel MA, Fisher RB, et al. Loss-of-function mutations in HPSE2 cause the autosomal recessive urofacial syndrome. Am J Hum Genet. 2010;86:957–962. doi: 10.1016/j.ajhg.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.McKenzie E, Tyson K, Stamps A, Smith P, Turner P, Barry R, Hircock M, Patel S, Barry E, Stubberfield C, et al. Cloning and expression profiling of Hpa2, a novel mammalian heparanase family member. Biochem Biophys Res Commun. 2000;276:1170–1177. doi: 10.1006/bbrc.2000.3586. [DOI] [PubMed] [Google Scholar]
- 251.Tantibhedhyangkul J, Copland SD, Haqq AM, Price TM. A case of female epispadias. Fertil Steril. 2008;90:2017 e2011–2013. doi: 10.1016/j.fertnstert.2007.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Warne SA, Hiorns MP, Curry J, Mushtaq I. Understanding cloacal anomalies. Arch Dis Child. 2011;96:1072–1076. doi: 10.1136/adc.2009.175034. [DOI] [PubMed] [Google Scholar]
- 253.Warne SA, Wilcox DT, Creighton S, Ransley PG. Long-term gynecological outcome of patients with persistent cloaca. J Urol. 2003;170:1493–1496. doi: 10.1097/01.ju.0000086702.87930.c2. [DOI] [PubMed] [Google Scholar]
- 254.Warne SA, Wilcox DT, Ransley PG. Long-term urological outcome of patients presenting with persistent cloaca. J Urol. 2002;168:1859–1862. doi: 10.1097/01.ju.0000030712.17096.0d. discussion 1862. [DOI] [PubMed] [Google Scholar]
- 255.Warne SA, Wilcox DT, Ledermann SE, Ransley PG. Renal outcome in patients with cloaca. J Urol. 2002;167:2548–2551. discussion 2551. [PubMed] [Google Scholar]
- 256.Cacciaguerra S, Lo Presti L, Di Leo L, Grasso S, Gangarossa S, Di Benedetto V, Di Benedetto A. Prenatal diagnosis of cloacal anomaly. Scand J Urol Nephrol. 1998;32:77–80. doi: 10.1080/003655998750014783. [DOI] [PubMed] [Google Scholar]
- 257.Wang C, Gargollo P, Guo C, Tang T, Mingin G, Sun Y, Li X. Six1 and Eya1 are critical regulators of peri-cloacal mesenchymal progenitors during genitourinary tract development. Dev Biol. 2011;360:186–194. doi: 10.1016/j.ydbio.2011.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Manson JM, Carr MC. Molecular epidemiology of hypospadias: review of genetic and environmental risk factors. Birth Defects Res A Clin Mol Teratol. 2003;67:825–836. doi: 10.1002/bdra.10084. [DOI] [PubMed] [Google Scholar]
- 259.Nassar N, Bower C, Barker A. Increasing prevalence of hypospadias in Western Australia, 1980–2000. Arch Dis Child. 2007;92:580–584. doi: 10.1136/adc.2006.112862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Baskin LS. Hypospadias and urethral development. J Urol. 2000;163:951–956. [PubMed] [Google Scholar]
- 261.Kalfa N, Philibert P, Baskin LS, Sultan C. Hypospadias: interactions between environment and genetics. Mol Cell Endocrinol. 2011;335:89–95. doi: 10.1016/j.mce.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 262.Kojima Y, Kohri K, Hayashi Y. Genetic pathway of external genitalia formation and molecular etiology of hypospadias. J Pediatr Urol. 2010;6:346–354. doi: 10.1016/j.jpurol.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 263.van der Zanden LF, van Rooij IA, Feitz WF, Franke B, Knoers NV, Roeleveld N. Aetiology of hypospadias: a systematic review of genes and environment. Hum Reprod Update. 2012;18:260–283. doi: 10.1093/humupd/dms002. [DOI] [PubMed] [Google Scholar]
- 264.Goodman FR, Bacchelli C, Brady AF, Brueton LA, Fryns JP, Mortlock DP, Innis JW, Holmes LB, Donnenfeld AE, Feingold M, et al. Novel HOXA13 mutations and the phenotypic spectrum of hand-foot-genital syndrome. Am J Hum Genet. 2000;67:197–202. doi: 10.1086/302961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Hodges SJ, Patel B, McLorie G, Atala A. Posterior urethral valves. ScientificWorldJournal. 2009;9:1119–1126. doi: 10.1100/tsw.2009.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Nasir AA, Ameh EA, Abdur-Rahman LO, Adeniran JO, Abraham MK. Posterior urethral valve. World J Pediatr. 2011;7:205–216. doi: 10.1007/s12519-011-0289-1. [DOI] [PubMed] [Google Scholar]
- 267.Cozzi DA, Morgante D, Frediani S, Iaconelli R, Ceccanti S, Mele E, Cozzi F. Posterior urethral valves: relationship between vesicoureteral reflux and renal function. Urology. 2011;77:1209–1212. doi: 10.1016/j.urology.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 268.Heikkila J, Rintala R, Taskinen S. Vesicoureteral reflux in conjunction with posterior urethral valves. J Urol. 2009;182:1555–1560. doi: 10.1016/j.juro.2009.06.057. [DOI] [PubMed] [Google Scholar]
- 269.Borzi PA, Beasley SW, Fowler R. Posterior urethral valves in non-twin siblings. Br J Urol. 1992;70:201. doi: 10.1111/j.1464-410x.1992.tb15704.x. [DOI] [PubMed] [Google Scholar]
- 270.Laksmi NK, Khullar M, Kaur B, Ahuja M, Mahajan JK, Mittal BR, Bhattacharya A, Medhi B. Association of angiotensin converting enzyme and angiotensin type 2 receptor gene polymorphisms with renal damage in posterior urethral valves. J Pediatr Urol. 2010;6:560–566. doi: 10.1016/j.jpurol.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 271.Kajbafzadeh AM, Jangouk P, Ahmadi Yazdi C. Anterior urethral valve associated with posterior urethral valves. J Pediatr Urol. 2005;1:433–435. doi: 10.1016/j.jpurol.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 272.Kibar Y, Coban H, Irkilata HC, Erdemir F, Seckin B, Dayanc M. Anterior urethral valves: an uncommon cause of obstructive uropathy in children. J Pediatr Urol. 2007;3:350–353. doi: 10.1016/j.jpurol.2007.01.197. [DOI] [PubMed] [Google Scholar]
- 273.Amsalem H, Fitzgerald B, Keating S, Ryan G, Keunen J, Pippi Salle JL, Berger H, Aiello H, Otano L, Bernier F, et al. Congenital megalourethra: prenatal diagnosis and postnatal/autopsy findings in 10 cases. Ultrasound Obstet Gynecol. 2011;37:678–683. doi: 10.1002/uog.8862. [DOI] [PubMed] [Google Scholar]
- 274.Gupta DK, Srinivas M. Congenital anterior urethral diverticulum in children. Pediatr Surg Int. 2000;16:565–568. doi: 10.1007/s003830000430. [DOI] [PubMed] [Google Scholar]
- 275.Karnak I, Senocak ME, Buyukpamukcu N, Hicsonmez A. Rare congenital abnormalities of the anterior urethra. Pediatr Surg Int. 1997;12:407–409. doi: 10.1007/BF01076951. [DOI] [PubMed] [Google Scholar]
- 276.Coplen DE, Austin PF. Prenatal diagnosis and neonatal management of congenital urethral diverticulum. J Urol. 2007;177:2330–2332. doi: 10.1016/j.juro.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 277.Routh JC, McGee SM, Ashley RA, Reinberg Y, Vandersteen DR. Predicting renal outcomes in children with anterior urethral valves: a systematic review. J Urol. 2010;184:1615–1619. doi: 10.1016/j.juro.2010.03.119. [DOI] [PubMed] [Google Scholar]
- 278.Deshpande C, Hennekam RC. Genetic syndromes and prenatally detected renal anomalies. Semin Fetal Neonatal Med. 2008;13:171–180. doi: 10.1016/j.siny.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 279.Blake JA, Bult CJ, Kadin JA, Richardson JE, Eppig JT. The Mouse Genome Database (MGD): premier model organism resource for mammalian genomics and genetics. Nucleic Acids Res. 2011;39:D842–D848. doi: 10.1093/nar/gkq1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.McMahon AP, Aronow BJ, Davidson DR, Davies JA, Gaido KW, Grimmond S, Lessard JL, Little MH, Potter SS, Wilder EL, et al. GUDMAP: the genitourinary developmental molecular anatomy project. J Am Soc Nephrol. 2008;19:667–671. doi: 10.1681/ASN.2007101078. [DOI] [PubMed] [Google Scholar]
- 281.Ringwald M, Iyer V, Mason JC, Stone KR, Tadepally HD, Kadin JA, Bult CJ, Eppig JT, Oakley DJ, Briois S, et al. The IKMC web portal: a central point of entry to data and resources from the International Knockout Mouse Consortium. Nucleic Acids Res. 2011;39:D849–D855. doi: 10.1093/nar/gkq879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Diez-Roux G, Banfi S, Sultan M, Geffers L, Anand S, Rozado D, Magen A, Canidio E, Pagani M, Peluso I, et al. A high-resolution anatomical atlas of the transcriptome in the mouse embryo. PLoS Biol. 2011;9:e1000582. doi: 10.1371/journal.pbio.1000582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Levy CM, Knudtzon J. Kallmann syndrome in two sisters with other developmental anomalies also affecting their father. Clin Genet. 1993;43:51–53. doi: 10.1111/j.1399-0004.1993.tb04451.x. [DOI] [PubMed] [Google Scholar]
- 284.Lu W, Quintero-Rivera F, Fan Y, Alkuraya F, Donovan DJ, Xi Q, Turbe-Doan A, Li QG, Campbell CG, Shanske AL, et al. NFIA haploinsufficiency is associated with a CNS malformation syndrome and urinary tract defects. PLoS Genet. 2007;3:e80. doi: 10.1371/journal.pgen.0030080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Gazda HT, Sheen MR, Vlachos A, Choesmel V, O'Donohue MF, Schneider H, Darras N, Hasman C, Sieff CA, Newburger PE, et al. Ribosomal protein L5 and L11 mutations are associated with cleft palate and abnormal thumbs in Diamond-Blackfan anemia patients. Am J Hum Genet. 2008;83:769–780. doi: 10.1016/j.ajhg.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Selicorni A, Sforzini C, Milani D, Cagnoli G, Fossali E, Bianchetti MG. Anomalies of the kidney and urinary tract are common in de Lange syndrome. Am J Med Genet A. 2005;132:395–397. doi: 10.1002/ajmg.a.30445. [DOI] [PubMed] [Google Scholar]
- 287.Robertson SP, Bankier A. Sotos syndrome and cutis laxa. J Med Genet. 1999;36:51–56. [PMC free article] [PubMed] [Google Scholar]
- 288.Narumi Y, Kosho T, Tsuruta G, Shiohara M, Shimazaki E, Mori T, Shimizu A, Igawa Y, Nishizawa S, Takagi K, et al. Genital abnormalities in Pallister-Hall syndrome: Report of two patients and review of the literature. Am J Med Genet A. 2010;152A:3143–3147. doi: 10.1002/ajmg.a.33720. [DOI] [PubMed] [Google Scholar]
- 289.Frick H, Munger DM, Fauchere JC, Stallmach T. Hypoplastic thymus and T-cell reduction in EECUT syndrome. Am J Med Genet. 1997;69:65–68. [PubMed] [Google Scholar]
- 290.Partington MW, Rae J, Payne MJ. Haematometra in the Langer-Giedion syndrome. J Med Genet. 1991;28:644–645. doi: 10.1136/jmg.28.9.644-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Murawski IJ, Myburgh DB, Favor J, Gupta IR. Vesico-ureteric reflux and urinary tract development in the Pax2 1Neu+/− mouse. Am J Physiol Renal Physiol. 2007;293:F1736–F1745. doi: 10.1152/ajprenal.00221.2007. [DOI] [PubMed] [Google Scholar]
- 292.Ruf RG, Xu PX, Silvius D, Otto EA, Beekmann F, Muerb UT, Kumar S, Neuhaus TJ, Kemper MJ, Raymond RM, Jr., et al. SIX1 mutations cause branchio-oto-renal syndrome by disruption of EYA1-SIX1-DNA complexes. Proc Natl Acad Sci U S A. 2004;101:8090–8095. doi: 10.1073/pnas.0308475101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Bachmann-Gagescu R, Mefford HC, Cowan C, Glew GM, Hing AV, Wallace S, Bader PI, Hamati A, Reitnauer PJ, Smith R, et al. Recurrent 200-kb deletions of 16p11.2 that include the SH2B1 gene are associated with developmental delay and obesity. Genet Med. 2010;12:641–647. doi: 10.1097/GIM.0b013e3181ef4286. [DOI] [PubMed] [Google Scholar]
- 294.Tan TY, Aftimos S, Worgan L, Susman R, Wilson M, Ghedia S, Kirk EP, Love D, Ronan A, Darmanian A, et al. Phenotypic expansion and further characterisation of the 17q21.31 microdeletion syndrome. J Med Genet. 2009;46:480–489. doi: 10.1136/jmg.2008.065391. [DOI] [PubMed] [Google Scholar]
- 295.Halal F, Homsy M, Perreault G. Acro-renal-ocular syndrome: autosomal dominant thumb hypoplasia, renal ectopia, and eye defect. Am J Med Genet. 1984;17:753–762. doi: 10.1002/ajmg.1320170406. [DOI] [PubMed] [Google Scholar]
- 296.Wilson GN, Oliver WJ. Further delineation of the G syndrome: a manageable genetic cause of infantile dysphagia. J Med Genet. 1988;25:157–163. doi: 10.1136/jmg.25.3.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Debiec H, Kutsche M, Schachner M, Ronco P. Abnormal renal phenotype in L1 knockout mice: a novel cause of CAKUT. Nephrol Dial Transplant. 2002;17 Suppl 9:42–44. doi: 10.1093/ndt/17.suppl_9.42. [DOI] [PubMed] [Google Scholar]
- 298.Niimura F, Labosky PA, Kakuchi J, Okubo S, Yoshida H, Oikawa T, Ichiki T, Naftilan AJ, Fogo A, Inagami T, et al. Gene targeting in mice reveals a requirement for angiotensin in the development and maintenance of kidney morphology and growth factor regulation. J Clin Invest. 1995;96:2947–2954. doi: 10.1172/JCI118366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Aoki Y, Mori S, Kitajima K, Yokoyama O, Kanamaru H, Okada K, Yokota Y. Id2 haploinsufficiency in mice leads to congenital hydronephrosis resembling that in humans. Genes Cells. 2004;9:1287–1296. doi: 10.1111/j.1365-2443.2004.00805.x. [DOI] [PubMed] [Google Scholar]
- 300.Warot X, Fromental-Ramain C, Fraulob V, Chambon P, Dolle P. Gene dosage-dependent effects of the Hoxa-13 and Hoxd-13 mutations on morphogenesis of the terminal parts of the digestive and urogenital tracts. Development. 1997;124:4781–4791. doi: 10.1242/dev.124.23.4781. [DOI] [PubMed] [Google Scholar]
- 301.Marose TD, Merkel CE, McMahon AP, Carroll TJ. Beta-catenin is necessary to keep cells of ureteric bud/Wolffian duct epithelium in a precursor state. Dev Biol. 2008;314:112–126. doi: 10.1016/j.ydbio.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Hoshino T, Shimizu R, Ohmori S, Nagano M, Pan X, Ohneda O, Khandekar M, Yamamoto M, Lim KC, Engel JD. Reduced BMP4 abundance in Gata2 hypomorphic mutant mice result in uropathies resembling human CAKUT. Genes Cells. 2008;13:159–170. doi: 10.1111/j.1365-2443.2007.01158.x. [DOI] [PubMed] [Google Scholar]
- 303.Salih MA, Tuvemo T. Diabetes insipidus, diabetes mellitus, optic atrophy and deafness (DIDMOAD syndrome). A clinical study in two Sudanese families. Acta Paediatr Scand. 1991;80:567–572. doi: 10.1111/j.1651-2227.1991.tb11908.x. [DOI] [PubMed] [Google Scholar]
- 304.Gamp AC, Tanaka Y, Lullmann-Rauch R, Wittke D, D'Hooge R, De Deyn PP, Moser T, Maier H, Hartmann D, Reiss K, et al. LIMP-2/LGP85 deficiency causes ureteric pelvic junction obstruction, deafness and peripheral neuropathy in mice. Hum Mol Genet. 2003;12:631–646. [PubMed] [Google Scholar]
- 305.King JA, Marker PC, Seung KJ, Kingsley DM. BMP5 and the molecular, skeletal, and soft-tissue alterations in short ear mice. Dev Biol. 1994;166:112–122. doi: 10.1006/dbio.1994.1300. [DOI] [PubMed] [Google Scholar]
- 306.Fryns JP, Kleczkowska A, Moerman P, Vandenberghe K. Hereditary hydronephrosis and the short arm of chromosome 6. Hum Genet. 1993;91:514–515. doi: 10.1007/BF00217787. [DOI] [PubMed] [Google Scholar]
- 307.Pasutto F, Sticht H, Hammersen G, Gillessen-Kaesbach G, Fitzpatrick DR, Nurnberg G, Brasch F, Schirmer-Zimmermann H, Tolmie JL, Chitayat D, et al. Mutations in STRA6 cause a broad spectrum of malformations including anophthalmia, congenital heart defects, diaphragmatic hernia, alveolar capillary dysplasia, lung hypoplasia, and mental retardation. Am J Hum Genet. 2007;80:550–560. doi: 10.1086/512203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Shindo T, Kurihara H, Kuno K, Yokoyama H, Wada T, Kurihara Y, Imai T, Wang Y, Ogata M, Nishimatsu H, et al. ADAMTS-1: a metalloproteinase-disintegrin essential for normal growth, fertility, and organ morphology and function. J Clin Invest. 2000;105:1345–1352. doi: 10.1172/JCI8635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Poley JR, Proud VK. Hardikar syndrome: new features. Am J Med Genet A. 2008;146A:2473–2479. doi: 10.1002/ajmg.a.32266. [DOI] [PubMed] [Google Scholar]
- 310.Slavotinek AM. Fryns syndrome: a review of the phenotype and diagnostic guidelines. Am J Med Genet A. 2004;124A:427–433. doi: 10.1002/ajmg.a.20381. [DOI] [PubMed] [Google Scholar]
- 311.Kimmel SG, Mo R, Hui CC, Kim PC. New mouse models of congenital anorectal malformations. J Pediatr Surg. 2000;35:227–230. doi: 10.1016/s0022-3468(00)90014-9. discussion 230-221. [DOI] [PubMed] [Google Scholar]
- 312.Cheng W, Yeung CK, Ng YK, Zhang JR, Hui CC, Kim PC. Sonic Hedgehog mediator Gli2 regulates bladder mesenchymal patterning. J Urol. 2008;180:1543–1550. doi: 10.1016/j.juro.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 313.Richer J, Milewicz DM, Gow R, de Nanassy J, Maharajh G, Miller E, Oppenheimer L, Weiler G, O'Connor M. R179H mutation in ACTA2 expanding the phenotype to include prune-belly sequence and skin manifestations. Am J Med Genet A. 2012;158A:664–668. doi: 10.1002/ajmg.a.35206. [DOI] [PubMed] [Google Scholar]
- 314.Yaplito-Lee J, Pitt J, Meijer J, Zoetekouw L, Meinsma R, van Kuilenburg AB. Beta-ureidopropionase deficiency presenting with congenital anomalies of the urogenital and colorectal systems. Mol Genet Metab. 2008;93:190–194. doi: 10.1016/j.ymgme.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 315.Sutherland RW, Wiener JS, Hicks JP, Marcelli M, Gonzales ET, Jr., Roth DR, Lamb DJ. Androgen receptor gene mutations are rarely associated with isolated penile hypospadias. J Urol. 1996;156:828–831. doi: 10.1097/00005392-199608001-00077. [DOI] [PubMed] [Google Scholar]
- 316.De Falco F, Cainarca S, Andolfi G, Ferrentino R, Berti C, Rodriguez Criado G, Rittinger O, Dennis N, Odent S, Rastogi A, et al. X-linked Opitz syndrome: novel mutations in the MID1 gene and redefinition of the clinical spectrum. Am J Med Genet A. 2003;120A:222–228. doi: 10.1002/ajmg.a.10265. [DOI] [PubMed] [Google Scholar]
- 317.Fukami M, Wada Y, Miyabayashi K, Nishino I, Hasegawa T, Nordenskjold A, Camerino G, Kretz C, Buj-Bello A, Laporte J, et al. CXorf6 is a causative gene for hypospadias. Nat Genet. 2006;38:1369–1371. doi: 10.1038/ng1900. [DOI] [PubMed] [Google Scholar]
- 318.Dravis C, Yokoyama N, Chumley MJ, Cowan CA, Silvany RE, Shay J, Baker LA, Henkemeyer M. Bidirectional signaling mediated by ephrin-B2 and EphB2 controls urorectal development. Dev Biol. 2004;271:272–290. doi: 10.1016/j.ydbio.2004.03.027. [DOI] [PubMed] [Google Scholar]
- 319.Jenkins EP, Andersson S, Imperato-McGinley J, Wilson JD, Russell DW. Genetic and pharmacological evidence for more than one human steroid 5 alpha-reductase. J Clin Invest. 1992;89:293–300. doi: 10.1172/JCI115574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Maas SM, de Jong TP, Buss P, Hennekam RC. EEC syndrome and genitourinary anomalies: an update. Am J Med Genet. 1996;63:472–478. doi: 10.1002/(SICI)1096-8628(19960614)63:3<472::AID-AJMG11>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 321.Suzuki K, Haraguchi R, Ogata T, Barbieri O, Alegria O, Vieux-Rochas M, Nakagata N, Ito M, Mills AA, Kurita T, et al. Abnormal urethra formation in mouse models of split-hand/split-foot malformation type 1 and type 4. Eur J Hum Genet. 2008;16:36–44. doi: 10.1038/sj.ejhg.5201925. [DOI] [PubMed] [Google Scholar]
- 322.Andiran F, Tanyel FC, Hicsonmez A. Fraser syndrome associated with anterior urethral atresia. Am J Med Genet. 1999;82:359–361. doi: 10.1002/(sici)1096-8628(19990212)82:4<359::aid-ajmg17>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 323.McGregor L, Makela V, Darling SM, Vrontou S, Chalepakis G, Roberts C, Smart N, Rutland P, Prescott N, Hopkins J, et al. Fraser syndrome and mouse blebbed phenotype caused by mutations in FRAS1/Fras1 encoding a putative extracellular matrix protein. Nat Genet. 2003;34:203–208. doi: 10.1038/ng1142. [DOI] [PubMed] [Google Scholar]
- 324.Halal F. The hand-foot-genital (hand-foot-uterus) syndrome: family report and update. Am J Med Genet. 1988;30:793–803. doi: 10.1002/ajmg.1320300312. [DOI] [PubMed] [Google Scholar]
- 325.Chisholm IA, Chudley AE. Autosomal dominant iridogoniodysgenesis with associated somatic anomalies: four-generation family with Rieger's syndrome. Br J Ophthalmol. 1983;67:529–534. doi: 10.1136/bjo.67.8.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Shimizu H, Takizawa Y, Pulkkinen L, Murata S, Kawai M, Hachisuka H, Udono M, Uitto J, Nishikawa T. Epidermolysis bullosa simplex associated with muscular dystrophy: phenotype-genotype correlations and review of the literature. J Am Acad Dermatol. 1999;41:950–956. doi: 10.1016/s0190-9622(99)70252-5. [DOI] [PubMed] [Google Scholar]
- 327.Petiot A, Perriton CL, Dickson C, Cohn MJ. Development of the mammalian urethra is controlled by Fgfr2-IIIb. Development. 2005;132:2441–2450. doi: 10.1242/dev.01778. [DOI] [PubMed] [Google Scholar]
- 328.Chen H, Yong W, Hinds TD, Jr., Yang Z, Zhou Y, Sanchez ER, Shou W. Fkbp52 regulates androgen receptor transactivation activity and male urethra morphogenesis. J Biol Chem. 2010;285:27776–27784. doi: 10.1074/jbc.M110.156091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Jadeja S, Smyth I, Pitera JE, Taylor MS, van Haelst M, Bentley E, McGregor L, Hopkins J, Chalepakis G, Philip N, et al. Identification of a new gene mutated in Fraser syndrome and mouse myelencephalic blebs. Nat Genet. 2005;37:520–525. doi: 10.1038/ng1549. [DOI] [PubMed] [Google Scholar]
- 330.Niemann S, Zhao C, Pascu F, Stahl U, Aulepp U, Niswander L, Weber JL, Muller U. Homozygous WNT3 mutation causes tetra-amelia in a large consanguineous family. Am J Hum Genet. 2004;74:558–563. doi: 10.1086/382196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Salonen R. The Meckel syndrome: clinicopathological findings in 67 patients. Am J Med Genet. 1984;18:671–689. doi: 10.1002/ajmg.1320180414. [DOI] [PubMed] [Google Scholar]
- 332.Weatherbee SD, Niswander LA, Anderson KV. A mouse model for Meckel syndrome reveals Mks1 is required for ciliogenesis and Hedgehog signaling. Hum Mol Genet. 2009;18:4565–4575. doi: 10.1093/hmg/ddp422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Stoler JM, Herrin JT, Holmes LB. Genital abnormalities in females with Bardet-Biedl syndrome. Am J Med Genet. 1995;55:276–278. doi: 10.1002/ajmg.1320550306. [DOI] [PubMed] [Google Scholar]
- 334.Frydman M, Cohen HA, Ashkenazi A, Varsano I. Familial segregation of cervical ribs, Sprengel anomaly, preaxial polydactyly, anal atresia, and urethral obstruction: a new syndrome? Am J Med Genet. 1993;45:717–720. doi: 10.1002/ajmg.1320450611. [DOI] [PubMed] [Google Scholar]
- 335.Labrune P, Assathiany R, Penso D, Odievre M. Progressive vitiligo, mental retardation, facial dysmorphism, and urethral duplication without chromosomal breakage or immunodeficiency. J Med Genet. 1992;29:592–594. doi: 10.1136/jmg.29.8.592. [DOI] [PMC free article] [PubMed] [Google Scholar]