Abstract
Opioid and excitatory amino acid receptors contribute to morphine dependence, but there are no studies of their role in heroin dependence. Thus, mice injected with acute or chronic heroin doses in the present study were pretreated with one of the following selective antagonists: 7-benzylidenenaltrexone (BNTX), naltriben (NTB), nor-binaltorphimine (nor-BNI; δ1, δ2, and κ opioid receptors, respectively), MK-801, or LY293558 (NMDA and AMPA excitatory amino acid receptors, respectively). Naloxone-precipitated withdrawal jumping frequency, shown here to be a reliable index of heroin dependence magnitude, was reduced by BNTX or NTB in mice injected with both acute and chronic heroin doses. In contrast, nor-BNI did not alter jumping frequencies in mice injected with an acute heroin dose but significantly increased them in mice receiving chronic heroin injections. Continuous MK-801 or LY293558 infusion, but not injection, reduced jumping frequencies during withdrawal from acute heroin treatment. Their delivery by injection was nonetheless effective against chronic heroin dependence, suggesting mechanisms not simply attributable to NMDA or AMPA blockade. These data indicate that whereas δ1, δ2, NMDA, and AMPA receptors enable acute and chronic heroin dependence, κ receptor activity limits the dependence liability of chronic heroin. With the exception of δ1 receptors, the apparent role of these receptors to heroin dependence is consistent with their contribution to morphine dependence, indicating that there is substantial physiological commonality underlying dependence to both heroin and morphine. The ability of κ receptor blockade to differentially alter acute and chronic dependence supports previous assertions from studies with other opioids that acute an chronic opioid dependence are, at least in part, mechanistically distinct. Elucidating the substrates contributing to heroin dependence, and identifying their similarities and differences with those of other opioids such as morphine, may yield effective treatment strategies to the problem of heroin dependency.
Keywords: Heroin, naloxone-precipitated withdrawal, opioid receptors, excitatory amino acid receptors
1. Introduction
Exposure to opiate drugs such as morphine can result in dependence. Manifested by a characteristic withdrawal syndrome of multiple aversive physical signs, dependence remains a primary concern of physicians, leading them to under-medicate pain patients (Breivik, 2001). For narcotic drug abusing populations, the aversive withdrawal symptomology is regarded as a causative factor in their continued opioid use, engendering dangerous drug seeking behavior (Jasinski, 1977). Although physical dependence is commonly associated with chronic opioid intake, acute opioid treatment comprised of even a single exposure can cause dependence in a wide variety of species (Smits, 1975; Jasinski, 1977; McLemore et al. 1997; Kest et al. 2001). Despite their qualitatively similar withdrawal symptoms, there is behavioral and biochemical evidence that acute and chronic dependence are mediated by distinct mechanisms (Nehmad et al. 1982; McLemore et al. 1997; Kest et al. 2001).
Research into the mechanism of opioid dependence in rodents has been facilitated by precipitating withdrawal by injecting the wide-spectrum naloxone. Among naloxone-precipitated withdrawal signs in mice, frequency of uncontrollable stereotypical jumping (i.e., a hyperactivity response) is widely considered the most sensitive and reliable index of withdrawal intensity and is by far the most commonly used (Saelens et al. 1971; Smits, 1975; El-Kadi and Sharif, 1994; Kest et al., 2002). For a variety of opioids, jumping frequencies in mice during naloxone-precipitated withdrawal correlate well with their known physical dependence liability in man (Saelens et al. 1971).
Although all opioids have some dependence liability, our current understanding of opioid dependence is based predominantly on studies using morphine (Nestler, 1994). For example, the recent cloning of three separate genes encoding opioid receptors supports pharmacological studies indicating that opioids exert their effects at μ, δ, and κ receptor types (Knapp et al., 1995). Although morphine dependence has been shown to be a consequence of activity at the μ type (Matthes et al., 1996; Berrendero et al., 2002), δ and κ receptors have a modulatory role. The δ receptor type has been further resolved into subtypes, each with a specific contribution to morphine dependence. For example, blocking the δ2-subtype with naltriben (NTB) or naltrindole 5′-isothiocyanate attenuated the jumping, body shakes and weight loss accompanying naloxone-precipitated withdrawal after chronic morphine treatment. In contrast, δ1-receptor antagonists, including 7-benzylidenenaltrexone (BNTX), were either without effect on any withdrawal measure or blocked the withdrawal-induced changes in body weight only (Miyamoto et al., 1994; Suzuki et al. 1997b). Antisense oligodeoxynucleotides targeting δ2-receptors similarly attenuated naloxone-precipitated withdrawal jumping after acute and chronic morphine treatment (Kest et al., 1996; Suzuki et al. 1997a). The exact role of kappa opioid receptors is more equivocal. Whereas mice with a disruption in the κ-opioid receptor gene appear to have attenuated withdrawal signs including naloxone-precipitated withdrawal jumping following chronic morphine administration (Simonin et al. 1998), administration of the κ-receptor antagonist nor-binaltorphimine (nor-BNI) increased naloxone-precipitated withdrawal weight loss in mice (Suzuki et al., 1992). Additionally, administration of dynorphin A (1-13), an opioid peptide with greater relative affinity for κ than μ and δ receptors, immediately prior to naloxone-precipitated withdrawal significantly suppressed the withdrawal jumping response in mice made chronically dependent on morphine (Takemori et al., 1992). The contribution of κ-opioid receptors to acute morphine dependence has not been reported.
By activating N-methyl-D-aspartate (NMDA) and α-amino-3-hydroxy-5-methylisoxazole-4- propionic acid (AMPA) receptors, excitatory amino acids such as glutamate and aspartate also make a critical contribution to morphine dependence. For example, continuous infusion or repeated injection of MK-801 attenuated naloxone-precipitated withdrawal jumping frequencies in mice rendered dependent on morphine after acute or chronic administration, respectively (Trujillo and Akil, 1991; Verma and Kulkarni, 1995; McLemore et al. 1997). These data collectively indicate a role for NMDA receptors in mechanisms contributing to both acute and chronic morphine dependence. The contribution of AMPA receptors to morphine dependence has been much less studied, but a contribution to morphine dependence is nonetheless indicated. Chronic infusion of the selective antagonist LY293558 attenuated jumping frequencies in mice subject to acute morphine dependence (McLemore et al. 1997). Although the same LY293558 infusion dose was ineffective in mice chronically treated with morphine, “knock-out” mice lacking the AMPA GluR-A subunit show reduced jumping frequencies under these conditions (Vekovischeva et al., 2001).
It has commonly thought that heroin, which is rapidly converted to 6-monoacetylmorphine (6-MAM) and then morphine in vivo (Inturrisi et al. 1983), would logically have a pharmacological profile similar to that of morphine. Indeed, both opioids preferentially bind and activate μ opioid receptors (Watson et al., 1996; Selley et al., 2001). However, recent data indicate mechanistic differences between morphine and heroin with respect to analgesia processes. For example, although morphine analgesia is reduced in μ opioid receptor deficient CXBK mice (Ikeda et al., 1999), heroin analgesia is uncompromised (Rossi et al. 1996). Studies using an antisense strategy to target different exons of the μ opioid receptor gene have also demonstrated that different splice variants mediate morphine and heroin analgesia (Rossi et al. 1995). Since there are very few studies of heroin dependence, it is unknown whether - like analgesia – the mechanisms underlying heroin and morphine dependence are somewhat distinct. Specifically, it is possible that opioid and excitatory amino acid receptors make different contributions to heroin and morphine dependence. There is currently no data that can address this possibility.
Thus, the aim of the present study is twofold. First, we sough to assess whether jumping during naloxone-precipitated withdrawal from heroin is a reliable measure of dependence and to determine heroin-naloxone doses and injection intervals yielding optimal responding. Thus, we first obtained time- and dose- response data across a range of heroin and naloxone doses and injection intervals after both acute and chronic heroin injection. Second, we assessed the contribution of δ and κ opioid as well as NMDA and AMPA types of excitatory amino acid receptors to heroin dependence by using selective antagonists previously used in studies with morphine.
2. Materials and methods
2.1 Subjects
Adult male CD-1 mice (Charles Rivers, Kingston, NY) were maintained on a 12:12-h light/dark cycle in a climate-controlled room with free access to food and tap water. Each mouse was used once and for all groups, n ≥ 6. Mice were subject to experimental protocols approved by the Queen College Institutional Animal Care and Use Committee.
2.2 Drugs
Heroin hydrochloride and morphine sulfate (both gift of NIDA), naloxone hydrochloride (Sigma-Aldrich, St. Louis, MO), the respective δ1, δ2, and κ opioid receptor antagonists BNTX, naltriben, and nor-BNI (all gifts of NIDA), and the respective NMDA and AMPA receptor antagonists (5R,10S)-(-)-5-Methyl-10,11-dihydro-5H-dibenzo[a,d]cylcohepten-5,10-imine maleate (MK-801; Sigma-Aldrich, St. Louis, MO) and (3S,4aR,6R,8aR)-6-[2-(1(2)H-tetrazole-5yl)ethyl] decahydroisoquinoline-3carboxylic acid (LY293558; gift of Eli Lily Co., Indianapolis, IN) were delivered in a 0.9% physiological saline vehicle. Administration was by the subcutaneous (s.c.) route, either by injection (10 ml/kg injection volume) or, for excitatory amino acid receptor antagonists only, continuous infusion via osmotic pumps (Alzet Model 2001, Alza, Mountain View, CA) surgically implanted through a small dorsal midline incision in mice under oxygen/isoflourane inhalant anesthesia. Heroin and morphine were always injected at 09:00 h with additional daily injections during chronic treatment at 13:00 and 17:00 h. Withdrawal was always precipitated between 4 and 8 h into the light cycle (lights on at 06:00 h).
2.3 Naloxone-precipitated withdrawal
Immediately after naloxone injection, subjects were placed into individual Plexiglas observation cylinders (25 × 11 cm), and the frequency of jumps for each subject was tallied over the next 15 min. Among withdrawal measures, only the jumping response—defined as the simultaneous removal of all four paws from the horizontal surface—was consistently observed and found to be dose-dependent in previous studies of both acute and chronic morphine dependence (Smits, 1975; El-Kadi and Sharif, 1994; Kest et al. 2001). Thus, although symptoms such as diarrhea, ptosis, wet-dog shakes, lacrimation were occasionally observed in the present study, they were excluded from analysis.
2.3 Time-response studies
In the acute heroin dependence protocol, mice were injected once with heroin (50 mg/kg). To study chronic dependence, heroin does of 10, 20, and 40 mg/kg were injected t.i.d. on Days 1, 2, and 3, respectively. A final heroin injection (40 mg/kg) was made before naloxone-precipitated withdrawal on day 4. At the completion of heroin treatment, mice were injected with a single bolus dose of naloxone (50 mg/kg) and jumping frequencies were tallied at various hourly intervals. The heroin and naloxone doses chosen are those previously shown to elicit maximal jumping responses in the study of acute and chronic morphine dependence in mice (El-Kadi and Sharif, 1994; Kest et al., 2002). Heroin control mice were injected with saline instead of naloxone and naloxone control mice were injected with saline instead of heroin at the heroin-naloxone interval eliciting the greatest jumping frequency.
2.4 Dose-response studies
2.4.1 Heroin
The relationship between heroin dose and naloxone-precipitated withdrawal jumping was determined for acute and chronic dependence paradigms. For acute dependence, mice were injected with a single heroin dose (2-50 mg/kg) followed by naloxone (50 mg/kg). We used a heroin-naloxone interval (2 h) determined from the above time-response studies to yield maximal jumping responses following acute heroin injection.
For chronic dependence studies, heroin doses of 5, 10, and 20 or 10, 20, and 40 mg/kg were injected into separate groups of mice t.i.d. on Days 1, 2, and 3 respectively. On Day 4, mice received a final single 40 mg/kg heroin injection followed by a naloxone (50 mg/kg) injection using an interval determined from the above time-response studies to yield maximal jumping responses following chronic heroin treatment (1 h).
Heroin control mice in both acute and chronic treatment paradigms were injected with saline instead of heroin using the naloxone dose eliciting the greatest jumping frequency.
2.4.2 Naloxone
The relationship between naloxone dose and naloxone-precipitated withdrawal jumping was determined for acute and chronic heroin dependence paradigms. For acute dependence, mice were injected once with heroin (50.0 mg/kg). For the chronic treatment condition, mice were injected with 5, 10, and 20 mg/kg of heroin on days 1, 2, and 3 respectively, followed by a final heroin injection of 20 mg/kg on day 4. A range of naloxone doses (0.3-50.0 mg/kg) were injected after the end of heroin treatment using a heroin-naloxone interval shown in the time-response studies to elicit maximal jumping frequencies (acute: 2 h; chronic: 1 h).
Naloxone control mice in both acute and chronic treatment paradigms were injected with saline instead of naloxone using the heroin dose eliciting the greatest jumping frequency.
2.5 Opioid and excitatory amino acid receptor antagonist studies
The acute and chronic heroin dependence paradigms used in these studies were those shown in the above time- and dose- response studies to elicit maximal naloxone-precipitated withdrawal jumping frequencies.
2.5.1 Acute heroin dependence
Groups of mice were injected with a single bolus dose of heroin (50 mg/kg) followed 2 h later by naloxone (50 mg/kg). Some groups were injected 30 minutes prior to heroin with either BNTX (0.5 mg/kg), naltriben (1 mg/kg), MK-801 (0.05 mg/kg), or LY293558 (5 mg/kg). The BNTX and naltriben doses used are those previously reported as effective in the morphine dependence literature (Suzuki et al., 1997b). MK-801 and LY293558 doses were based on our pilot studies and were the maximal allowable dose not causing severe motor impairment and/or lethality. Since McLemore et al. (1997) have shown that MK-801 and LY293558 can attenuate acute morphine dependence after their continuous subcutaneous infusion, separate groups received these drugs using delivery protocols described in that study. Specifically, pumps infusing cumulative daily doses of 1 and 60 mg/kg containing MK-801 and LY293558, respectively, were implanted 16 hours prior to acute heroin injection and removed 30 min prior. nor-BNI (10 mg/kg) was injected 8 hours prior to heroin, corresponding to its maximal blockade of κ receptors (Endoh et al., 1992). An antagonist control group was injected with saline instead of an antagonist.
2.5.2 Chronic heroin dependence
Heroin doses of 10, 20, and 40 mg/kg were injected into separate groups of mice t.i.d. on days 1, 2, and 3, respectively. A final 40 mg/kg heroin dose injection on Day 4 was followed 1 h later by naloxone (50 mg/kg). BNTX, naltriben, MK-801, and LY 293558 doses identical to those used for the acute heroin study above were injected 30 minutes prior to each heroin injection. We also injected the identical nor-BNI dose, but since this drug is an irreversible antagonist and a single injection provides κ opioid receptor blockade for up to 96 hours (Endoh et al., 1992), it was injected 8 h prior to the first heroin injection on days 1 and 3 only. Antagonist controls were injected with saline instead of antagonist.
2.5.3 Acute and chronic morphine dependence
Previous studies report that the δ1 receptor plays no role in morphine dependence (Miyamoto et al., 1994; Suzuki et al. 1997b). However, in the above studies (2.5.1 and 2.5.2), the δ1 receptor antagonist BNTX nonetheless attenuated both acute and chronic heroin NPW. To confirm this apparent dissociation between heroin and morphine dependence, we additionally tested acute and chronic morphine dependence in BNTX treated mice using protocols virtually identical with those used to study heroin dependence. Acute morphine dependence was induced by injecting mice with a single morphine dose (50 mg/kg). Chronic morphine dependence was induced by injecting mice t.i.d. with morphine doses of 10, 20, and 40 mg/kg on days 1, 2, and 3, respectively. A final 40 mg/kg morphine dose was injected on Day 4. A single BNTX dose (0.5 mg/kg) preceded each morphine injection by 30 minutes. For both paradigms, the final morphine dose was followed 3 h later by naloxone (50 mg/kg), corresponding to the morphine-naloxone interval yielding maximal jumping frequencies (El-Kadi and Sharif, 1994; Kest et al., 2001; Smits, 1975). Separate antagonist control groups injected with saline instead of BNTX were also tested.
2.6 Data analysis
Jumping frequencies between groups within the various studies were subject to an independent samples t-test or one-way ANOVA. Fisher's LSD test was used for post-hoc comparisons. An α level of 0.05 was used throughout.
3. Results
3.1 Time-response studies
3.1.1 Acute heroin injection
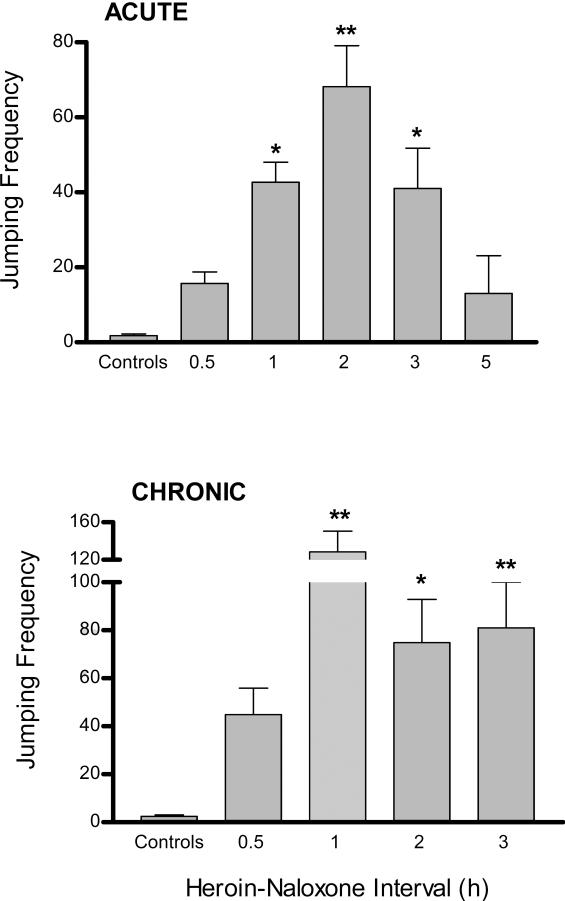
Jumping frequencies after injecting the acute heroin bolus dose was maximal when naloxone was injected 2 h later (Fig. 1A format). Thus, this heroin-naloxone interval was used to test heroin and naloxone control mice. Since the mean jumping frequencies of the two control groups were not significantly different (heroin controls: 1.3 jumps; naloxone controls: 2.3 jumps), they were pooled into a single control group. Relative to control values, significant naloxone-precipitated withdrawal jumping was observed when naloxone was injected 1, 2, or 3 h after heroin. Nonetheless, values obtained at 2 h were significantly greater than those obtained at 1 or 3 h. Jumping frequencies obtained at heroin-naloxone intervals of 0.5 and 5 h were not significantly different than those obtained in controls.
Figure 1.
Time-response study of naloxone precipitated withdrawal from acute and chronic heroin treatment. ACUTE (top figure): mice were injected with a single heroin dose (50 mg/kg). CHRONIC (bottom figure): mice were injected t.i.d. for three days with escalating heroin doses (10, 20, and 40 mg/kg on treatment Days 1, 2, and 3, respectively) and a final 40 mg/kg heroin dose on Day 4. Withdrawal was precipitated by injecting naloxone (50 mg/kg) at various intervals after heroin treatment was completed. Control mice were subject to the identical acute or chronic heroin treatment protocols but received saline injections in place of either heroin or naloxone and tested at the heroin-naloxone interval yielding maximal frequencies. Significant differences from control values (* P <0.05; ** P <0.01) are indicated.
3.1.2 Chronic heroin injection
For chronic heroin treatment, maximal jumping responses were obtained at a heroin-naloxone interval of 1 h (Fig. 1B format), and therefore both heroin and naloxone control groups were tested at that time. Again, since the mean jumping frequencies of the two control groups were virtually identical (heroin controls: 2.6 jumps; naloxone controls: 2.2 jumps), they were pooled. Significant jumping relative to control mice was also observed at heroin-naloxone intervals of 2 and 3 h, but not 0.5 h. There was no significant difference between jumping values obtained at 1, 2, and 3 h.
3.2 Dose-Response Studies
3.2.1 Acute heroin doses
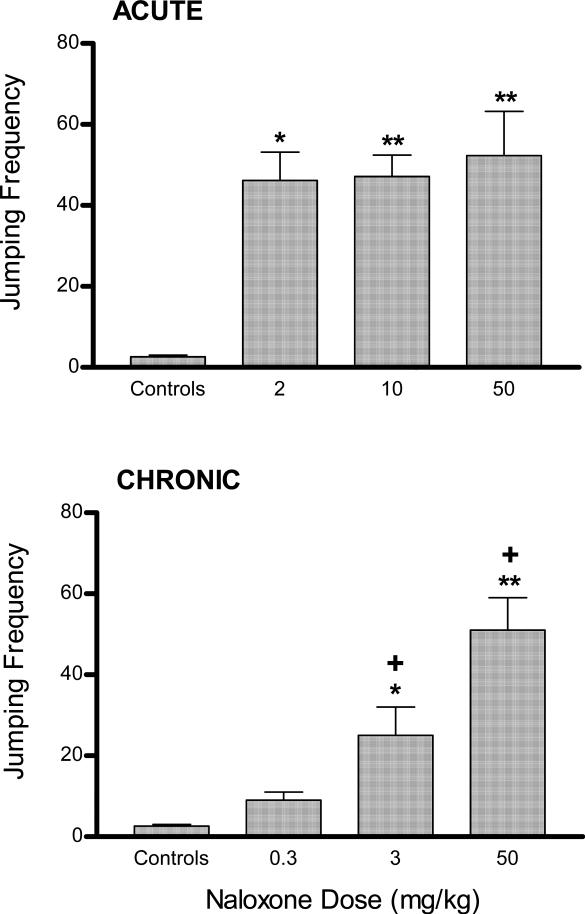
There was a positive relationship between heroin dose and naloxone-precipitated withdrawal jumping, with the acute 50 mg/kg heroin dose causing the greatest mean jumping frequency (Fig. 2A format). Accordingly, mice in the heroin control group were injected with the identical heroin dose (but without subsequent naloxone). Although all heroin doses increased jumping frequency relative to heroin controls, these differences were significant only after acute heroin injections of 10 and 50, but not 2, mg/kg. Although there was no significant difference between mice treated with the 10 and 50 mg/kg heroin doses, their frequency values were significantly greater (between 45%-50%) than that obtained after 2 mg/kg heroin.
Figure 2.
Dose-response relationship between acute and chronic heroin doses and jumping frequency. ACUTE (top figure): Mice received a single injection of various heroin doses followed 2 h later by naloxone. CHRONIC (bottom figure): Mice were injected t.i.d. for three days with escalating heroin doses (5, 10, and 20 or 10, 20, and 40 mg/kg on treatment Days 1, 2, and 3, respectively) and a final single heroin dose (40 mg/kg) on Day 4. Withdrawal was precipitated by injecting naloxone (50 mg/kg). Heroin control mice in both treatment paradigms were injected with saline instead of naloxone after injecting the heroin dose eliciting the greatest jumping frequency. Significant differences from control group (**P <0.01) and all other non-control groups (+) are indicated.
3.2.2 Chronic heroin doses
For chronic heroin treatment, maximal jumping responses were obtained after treatment with the higher dosing regimen of 10, 20, and 40 mg/kg t.i.d. on days 1, 2, and 3, respectively. (Fig. 2B format). Relative to the heroin control group, who thus received the identical heroin dosing regimen but without subsequent naloxone-precipitated withdrawal, both chronic heroin dose groups displayed significantly greater jumping frequencies. Although mice treated with the greater heroin doses jumped more (~30%) than those treated with the lower chronic doses, this difference was not significant.
3.2.3 Naloxone doses: acute heroin treatment
After an acute 50 mg/kg heroin injection, the 50 mg/kg naloxone dose elicited the greatest mean jumping frequency (Fig. 3A format) and so naloxone control mice were injected with this naloxone dose (without prior heroin treatment). Relative to these controls, all three naloxone doses (2, 10, and 50 mg/kg) elicited significant jumping, and there was no significant difference between the groups.
Figure 3.
Dose-response relationship between naloxone doses after heroin treatment and jumping frequency. ACUTE (top figure): mice were injected with a single heroin dose (50 mg/kg). CHRONIC (bottom figure): mice were injected t.i.d. for three days with escalating heroin doses (5, 10, and 20 mg/kg on treatment Days 1, 2, and 3, respectively) and a final 40 mg/kg heroin dose on Day 4. Naloxone doses were injected 2 and 1 h after acute and chronic heroin treatment, respectively. Naloxone control mice in both treatment paradigms were injected with saline instead of heroin followed by an injection of the naloxone dose eliciting the greatest jumping frequency. Significant differences from control group (*P <0.05; ** P <0.01) and the lower preceding dose (+) are indicated.
3.2.4 Naloxone doses: Chronic heroin treatment
There was a clear positive relationship between naloxone doses and jumping frequency, with the largest naloxone dose (50 mg/kg) eliciting the greatest mean jumping frequency (Fig. 3B format) after chronic heroin treatment. Thus, naloxone control mice were injected with 50 mg/kg naloxone only. Relative to these controls, significant jumping was elicited after naloxone doses of 3 and 50, but not 0.3, mg/kg. Furthermore, each naloxone dose significantly increased jumping frequencies between 2- and 2.5- fold relative to the preceding lower dose.
3.3 Opioid and excitatory amino acid receptor antagonists
3.3.1 Acute heroin injection
The respective δ1 and δ2 opioid receptor antagonists BNTX and naltriben, but not the κ antagonist nor-BNI, significantly reduced mean heroin naloxone-precipitated withdrawal jumping frequencies by ~35%-50% relative to antagonist controls (Fig. 4A). Although neither the respective NMDA nor AMPA receptor antagonists MK-801 or LY293558 altered jumping frequencies after their acute s.c. injection, both drugs caused marked and significant frequency reductions of between 60%-80% when they were delivered by continuous infusion.
Figure 4.
Effect of opioid and excitatory amino acid receptor blockade on withdrawal jumping after acute and chronic heroin treatment. ACUTE (left figure): mice were injected with a single heroin dose (50 mg/kg). CHRONIC (right figure): mice were injected t.i.d. for three days with escalating heroin doses (10, 20, and 40 mg/kg on treatment Days 1, 2, and 3, respectively) and a final 40 mg/kg heroin dose on Day 4. All mice were also injected with one of the following antagonists: BNTX, NTB, nor-BNI (δ1, δ2, and κ opioid receptor antagonists, respectively), MK-801 and LY293558 (NMDA and AMPA receptor antagonists, respectively: see text for antagonist doses and injection schedule). MK-801 and LY293558 were also delivered by continuous infusion during acute heroin treatment. Withdrawal was precipitated by naloxone (50 mg/kg) injection 2 and 1 h after completing acute and chronic heroin treatment, respectively. Control mice in both treatment paradigms were injected with saline instead of antagonist. Significant differences from control group are indicated (*P <0.05).
3.3.2 Chronic heroin injection
With the exception of nor-BNI, all antagonists significantly reduced mean heroin naloxone-precipitated withdrawal jumping frequencies by about 50 - 60% relative to antagonist controls (Fig. 4A). In contrast, nor-BNI increased jumping relative to controls by ~70%.
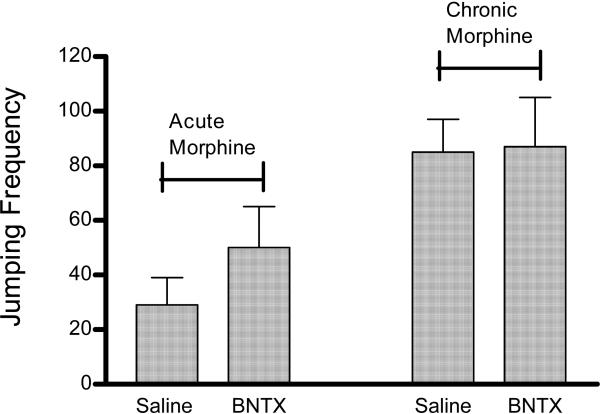
3.3.3 Effect of BNTX on acute and chronic morphine dependence
As illustrated in Fig. 5, mice subject to our acute and chronic morphine treatment displayed jumping responses during NPW that were similar in frequency to values obtained in mice subject to acute and chronic heroin treatment. However, in contrast to heroin treated mice (3.3.1 and 3.3.2), the δ1 opioid receptor antagonist BNTX did not attenuate jumping frequencies relative to saline-injected controls when mice were subjected to acute or chronic morphine injection.
Figure 5.
Effect of δ1 opioid receptor blockade on withdrawal jumping after acute and chronic morphine treatment. Mice subject to acute morphine treatment (left) were injected with a single morphine dose (50 mg/kg) whereas those subject to chronic morphine treatment (right) were injected t.i.d. for three days with escalating morphine doses (10, 20, and 40 mg/kg on treatment Days 1, 2, and 3, respectively) and a final 40 mg/kg morphine dose on Day 4. All mice were also injected with the δ1 opioid receptor antagonist BNTX (0.5 mg/kg) 30 min prior to every morphine injection. Withdrawal was precipitated by naloxone (50 mg/kg) injection 3 h after completing acute and chronic morphine treatment. Antagonist control mice in both treatment paradigms were injected with saline instead of BNTX. There were no significant differences between saline and BNTX treated groups (P >0.05).
4. Discussion
Previous studies have demonstrated a positive dose-response relationship between morphine dose and subsequent jumping frequencies during naloxone-precipitated withdrawal (Marshall and Weinstock, 1969; Wiley and Downs, 1979; Kest et al., 2001). On the basis of these and other studies, naloxone-precipitated withdrawal jumping frequency is widely considered a reliable and sensitive index of morphine withdrawal severity and, accordingly, morphine dependence. The present study sought to determine whether there is a similar relationship between heroin treatment and naloxone-precipitated withdrawal jumping responses. Since there has yet to be a demonstration of a dose-response relationship between morphine and other observable measures typical of opioid withdrawal such as ptosis, diarrhea, wet-dog shakes, or rearing, these were not studied here. The data show a positive dose-response relationship between either acute or chronic heroin doses and naloxone-precipitated withdrawal jumping frequencies. Although the increased frequency between a particular dose and the one preceding or following was not always significant, this may simply reflect the doses chosen for study. That is, the trend in the data indicate that eliminating some doses and/or testing additional higher or lower doses than those presently employed would have likely resulted in additional significant differences between doses. In order to minimize the total number of mice studied and lethality associated with large opioid bolus doses, these additional doses were not tested. In contrast to the experimental mice treated with heroin followed by naloxone, jumping frequencies tallied in heroin control mice were minimal (mean: 2-4 jumps/15 min). In fact, these already low mean values are overestimates as the overwhelming majority of subjects were non-responders, regardless of the heroin injection protocol or dose used. Collectively, these data indicate that the responses of experimental mice were a consequence of their heroin dependency, and that, like morphine dependence, jumping frequency is a reliable and sensitive index of heroin withdrawal magnitude in mice.
Previous studies have also reported a positive dose-response relationship between naloxone doses used to precipitate withdrawal after acute and chronic morphine treatment and jumping frequency (Smits 1975; El-Kadi and Sharif, 1994; Kest et al., 2001). Here, increasing the naloxone dose also caused significant concomitant increases in jumping frequencies following chronic, but not acute, heroin administration. At present we have no explanation as to why increasing naloxone dose after an acute heroin injection did not concomitantly increase jumping frequencies, particularly since such increases are evident after acute morphine injection (Kest et al., 2001), and the present acute heroin protocols utilized highly similar experimental methodologies including the range of naloxone doses tested. Although the interval between morphine and naloxone injection of 3 h in that study was slightly longer than the 2 h heroin-naloxone interval used here, this difference is unlikely to be critical since in both studies jumping frequencies were tallied immediately after naloxone injection and there is no reason to suspect that differences in naloxone pharmacokinetics between morphine and heroin treated mice that would cause differences in naloxone bioavailability or efficacy.
The time-response data indicate that maximal naloxone-precipitated withdrawal responding is obtained after acute and chronic heroin treatment using a heroin-naloxone interval of 2 and 1 h, respectively. It is possible that prolonged heroin exposure during chronic treatment caused pharmacodynamic changes at opioid receptors themselves or recruited additional and/or distinct adaptive changes in any of several neural adaptations that underlie dependence subsequent to their activation. Additional studies are needed to distinguish between these possibilities. Despite their differences, the heroin-naloxone interval yielding maximal responses after acute (2 h) and chronic (1h) heroin are still smaller than those demonstrated for acute (3h) and chronic (3h) morphine treatment (Wiley and Downs, 1979; El-Kadi and Sharif, 1994). It seems logical to us that ability of heroin to more rapidly cross the blood-brain barrier and penetrate the brain relative to morphine (Oldendorf, 1972) may underlie this difference.
In the present study, the respective δ1 and δ2 opioid receptor antagonists BNTX and NTB significantly reduced naloxone-precipitated withdrawal jumping frequencies relative to controls after both acute and chronic heroin injection, suggesting that activity at either δ receptor subtype enables heroin dependence. Although our finding with NTB parallels the results from studies of acute and chronic morphine dependence, the ability of BNTX to reduce heroin withdrawal jumping frequencies does not. That is, BNTX doses identical to or even greater than that used here are ineffective in reducing withdrawal jumping in mice after chronic morphine treatment (Suzuki et al., 1997b). Our confidence in this previous finding is greatly enhanced by our own demonstration here that the BNTX dose attenuating heroin jumping frequencies did not similarly reduce frequencies in morphine treated mice. Furthermore, it does not seem likely that the ability of BNTX to differentially alter heroin and morphine dependence is due to some intrinsic property of BNTX as another δ1 receptor selective antagonist, DALCE, is also ineffective in reducing jumping frequencies after chronic morphine treatment (Miyamoto et al., 1994). Instead, the prevailing evidence indicates that δ1 receptors do not contribute to morphine dependence and, with the present data, suggest that the δ opioid receptor modulation of morphine and heroin dependence is not identical. Converging lines of evidence indicate a close association of μ and δ binding sites in an opioid receptor complex, and this association is thought to underlie the ability of δ2 opioid receptors to attenuate morphine dependence (George et al, 2000; Daniels et al., 2005). We suspect that the ability of NTB to attenuate withdrawal jumping after heroin, which also acts primarily at the μ receptor (Liu et al., 2003), results from the same mechanism. However, since there are no similar studies reporting an anatomical association or functional interaction between μ and δ1 receptors, the basis by which δ1 receptors modulate heroin dependence is not obvious to us. Furthermore, any such mechanism would have to be distinct from that mediating morphine dependence.
In contrast to δ opioid receptor antagonists, the selective and irreversible κ-opioid receptor antagonist nor-BNI was without effect on naloxone-precipitated withdrawal jumping frequencies after acute heroin injection. We do not believe this finding resulted from using an insufficiently large nor-BNI dose since this dose has been shown to provide effective κ-opioid receptor blockade on several behavioral measures (Narita et al., 1990), including chronic morphine dependence (Suzuki et al., 1992). Indeed, here, injecting the identical nor-BNI dose only twice over 4 days significantly increasing jumping frequencies in mice injected repeatedly and chronically with heroin over 4 days. Instead, we believe that κ-opioid receptors act exclusively to restrict withdrawal jumping after chronic but not acute heroin treatment. That acute and chronic heroin dependence may be mediated by distinct substrates is an assertion previously advocated regarding acute and chronic morphine dependence (Nehmad et al., 1982; McLemore et al. 1997; Kest et al., 2001). Interestingly, the identical nor-BNI dose tested here significantly increased weight loss in mice and rats consequent to withdrawal after chronic morphine treatment (Suzuki et al., 1992). Similarly, weight loss, “wet-dog” shakes, and teeth chattering were also increased after intrathecal nor-BNI injection in rats chronically treated with morphine (Cui et al., 2000). These findings parallel the increased jumping caused by nor-BNI in our mice chronically injected with heroin. The mechanism by which κ-opioid receptor blockade increases naloxone-precipitated withdrawal symptoms is not well understood (however, see Suzuki et al., 1992). nor-BNI can act as a μ opioid receptor antagonist (Endoh et al., 1992), which must be considered when evaluating data generated by this drug. Nonetheless, it is very unlikely that any μ receptor blockade of nor-BNI contributed to its effects in the present study for the following reasons. First, nor-BNI injection provides only a transient 2 to 4 hr blockade of μ receptors in mice, but effectively blocks κ opioid receptors for up to 96 hours (Endoh et al., 1992). Here, nor-BNI was injected 8 h prior to heroin injection and 10 h prior to naloxone-precipitated withdrawal in the acute heroin dependence protocol, corresponding to a time when it acts solely as a κ opioid receptor antagonist. Similarly, nor-BNI was injected 8 h prior to the first heroin injection on days 1 and 3, and ~25 h prior to precipitated withdrawal in mice subject to chronic heroin treatment. Second, the effect of nor-BNI on heroin withdrawal jumping frequencies in the present study is inconsistent with μ receptor blockade. Specifically, blocking μ receptors during the injection of a μ receptor opioid such as heroin should decrease or even abolish the development of dependence and subsequent withdrawal responding. Here, in contrast, nor-BNI had no effect on withdrawal jumping after acute heroin treatment and significantly increased jumping frequencies in mice chronically injected with heroin.
NMDA receptor activity is critically important to neuronal plasticity, resulting in adaptive changes after morphine exposure that results in dependence (Trujillo and Akil, 1991; Inoue et al., 2003). Indeed, MK-801 has been reported to attenuate naloxone-precipitated withdrawal symptoms when delivered prior to acute and chronic morphine injections (Trujillo and Akil, 1991; Verma and Kulkarni, 1995; McLemore et al., 1997). Here, MK-801 was always injected 30 min prior to heroin in both the acute and chronic heroin treatment protocols, presumably before neuronal adaptive processes have been initiated. After an acute heroin injection, we indeed found that NMDA receptor blockade reduced jumping frequencies, but only when MK-801 was delivered via continuous infusion starting 16 h before heroin injection and not when it was delivered as an acute bolus dose 30 min before. It is unlikely that the acute MK-801 bolus dose was not sufficiently large since the same dose was effective against chronic dependence when injected prior to each heroin injection. Furthermore, MK-801 delivery by infusion was terminated 30 min prior to the acute heroin injection, corresponding exactly with the interval between acute MK-801 and heroin injection. Thus, MK-801 delivery by infusion was no more proximal to the acute heroin injection than was MK-801 delivery by acute bolus injection. Nonetheless, only MK-801 infusion was effective in reducing the subsequent withdrawal jumping. These data suggest that the ability of MK-801 to attenuate heroin dependence might not result from the blockade of NMDA receptors per se, but from physiological and/or neuronal adaptations resulting from prolonged MK-801 treatment. For example, chronic MK-801 administration or NMDA receptor blockade can reduce the density of the glutamate binding site within the NMDA receptor (Manallack et al., 1989; Beart & Lodge, 1990), which would presumably down-regulate NMDA receptor activity and the consequent biochemical cascade that underlies dependence., and alter GABAA and dopamine D2 receptors that contribute to opioid dependence (Micheletti et al., 1992; Lannes et al., 1995). This supposition is also consistent with the finding that mice injected daily with MK-801 followed by saline for 8 consecutive days show attenuated withdrawal jumping and weight loss in response to an acute morphine injection on Day 9 (Koyuncuoglu et al., 1999). Such an adaptive process may similarly underlie the efficacy with which repeated and chronic acute MK-801 bolus doses injected prior to each heroin injection were effective against chronic heroin dependence.
Like NMDA receptors, AMPA receptors mediate long-term central nervous system changes (Bettler and Mulle, 1995). Not surprisingly then, these receptors contribute to morphine dependence (McLemore et al., 1997; Vandergriff and Rasmussen, 1999) and, as demonstrated here, they apparently do so with respect to heroin dependence as well. We observed that LY293558 delivery by continuous infusion - but not acute bolus dose injection - prior to acute heroin injection reduced naloxone-precipitated withdrawal jumping, and injecting a bolus dose of LY293558 t.i.d. over 4 days also attenuated jumping frequencies in the chronic heroin treatment protocol. The finding that continuous LY293558 infusion reduces acute heroin dependence parallels the finding that it attenuates withdrawal jumping under identical acute morphine dosing and delivery protocols (McLemore et al., 1997). Although pharmacological, electrophysiological, and molecular cloning studies have demonstrated that excitatory amino acid receptors are functionally and constitutively distinct (Watkins and Collingridge, 1994; Bettler and Mulle, 1995), the effect of LY293558 on heroin dependence was identical to our present finding with MK-801. Accordingly, we suggest that, like MK-801, it is possible that only prolonged LY293558 treatment will reduce withdrawal jumping. Unlike for chronic MK-801 treatment and NMDA receptor blockade, however, we are not aware of any studies reporting that chronic LY293558 administration or AMPA receptor blockade causes adaptive changes that might obviously impact heroin or opioid dependence. It is important to note that AMPA receptors have a highly overlapping anatomical distribution with NMDA receptors and are thought to assist in their activation by providing the postsynaptic depolarization necessary to remove the voltage-sensitive Mg2+ blockade of NMDA receptors (Bliss and Collingridge, 1993; Patel and McCulloch, 1995). Since MK-801 and LY293558 were effective only under the identical specific delivery protocols, we can not rule out the possibility that blockade of AMPA receptors here by LY293558 reduced heroin dependence only indirectly, via an AMPA receptor-mediated reduction in NMDA receptor activity. Mechanism notwithstanding, the present data indicate that activation of the excitatory amino acid receptors NMDA or AMPA during acute and chronic heroin treatment enables heroin dependence.
Finally, it should be noted that the antagonist injection protocols used here make it almost certain that these drugs were pharmacologically active during heroin injection and subsequent naloxone-precipitated withdrawal. Thus, it is possible that the opioid and excitatory amino acid receptor antagonists altered jumping frequencies by altering the mechanisms underlying the induction of heroin dependence, the expression of withdrawal per se, or both. Clearly needed are studies of opioid dependence that discern between these alternative possibilities, which will likely have practical implications in addressing the utility of these drugs in treating populations already opioid-addicted.
Acknowledgements
Supported by the CSI/IBR Center for Developmental Neuroscience (AJ, BK), PSC/CUNY (BK), NIDA grant DA001457 (CEI), and NIDA center grant DA005130 (CEI).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Beart PM, Lodge D. Chronic administration of MK-801 and the NMDA receptor: further evidence for reduced sensitivity of the primary acceptor site from studies with the cortical wedge preparation. J. Pharm. Pharmacol. 1990;42:354–355. doi: 10.1111/j.2042-7158.1990.tb05426.x. [DOI] [PubMed] [Google Scholar]
- Berrendero F, Kieffer BL, Maldonado R. Attenuation of nicotine-induced antinociception, rewarding effects, and dependence in mu-opioid receptor knock-out mice. J. Neurosci. 2002;22:10935–10940. doi: 10.1523/JNEUROSCI.22-24-10935.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettler B, Mulle C. Review: neurotransmitter receptors. II. AMPA and kainate receptors. Neuropharmacology. 1995;34:123–139. doi: 10.1016/0028-3908(94)00141-e. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Breivik H. Opioids in cancer and chronic non-cancer pain therapy - indications and controversies. Acta Anesthes. Scandinavia. 2001;45:1059–1066. doi: 10.1034/j.1399-6576.2001.450902.x. [DOI] [PubMed] [Google Scholar]
- Cui CL, Wu LZ, Han JS. Spinal kappa-opioid system plays an important role in suppressing morphine withdrawal syndrome in the rat. Neurosci. Lett. 2000;295:45–48. doi: 10.1016/s0304-3940(00)01593-7. [DOI] [PubMed] [Google Scholar]
- Daniels DJ, Lenard NR, Etienne CL, Law PY, Roerig SC, Portoghese PS. Opioid-induced tolerance and dependence in mice is modulated by the distance between pharmacophores in a bivalent ligand series. Proc. Natl. Acad. Sci. 2005;102:19208–19213. doi: 10.1073/pnas.0506627102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endoh T, Matsuura H, Tanaka C, Nagase H. Nor-binaltorphimine: a potent and selective kappa-opioid receptor antagonist with long-lasting activity in vivo. Arch. Int. Pharmacodyn. Ther. 1993;316:30–42. [PubMed] [Google Scholar]
- El-Kadi AO, Sharif SI. The influence of various experimental conditions on the expression of naloxone-induced withdrawal symptoms in mice. Gen. Pharmacol. 1994;25:1505–1510. doi: 10.1016/0306-3623(94)90181-3. [DOI] [PubMed] [Google Scholar]
- George SR, Fan T, Xie Z, Tse R, Tam V, Varghese G, O'Dowd BF. Oligomerization of mu- and delta-opioid receptors. Generation of novel functional properties. J. Biol. Chem. 2000;275:26128–26135. doi: 10.1074/jbc.M000345200. [DOI] [PubMed] [Google Scholar]
- Ikeda K, Ichikawa T, Kobayashi T, Kumanishi T, Oike S, Yano R. Unique behavioural phenotypes of recombinant-inbred CXBK mice: partial deficiency of sensitivity to mu- and kappa-agonists. Neurosci. Res. 1999;34:149–155. doi: 10.1016/s0168-0102(99)00047-4. [DOI] [PubMed] [Google Scholar]
- Inoue M, Mishina M, Ueda H. Locus-specific rescue of GluRepsilon1 NMDA receptors in mutant mice identifies the brain regions important for morphine tolerance and dependence. J. Neurosci. 2003;23:6529–6536. doi: 10.1523/JNEUROSCI.23-16-06529.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inturrisi CE, Schultz M, Shin S, Umans JG, Angel L, Simon EJ. Evidence from opiate binding studies that heroin acts through its metabolites. Life Sci. 1983;33:773–776. doi: 10.1016/0024-3205(83)90616-1. [DOI] [PubMed] [Google Scholar]
- Jasinski DR. Assessment of the abuse potentiality of morphine like drugs (methods used in man). In: Martin WR, editor. Handbook of Experimental Pharmacology. Springer-Verlag; Berlin: 1977. pp. 197–258. [Google Scholar]
- Kest B, Palmese CA, Hopkins E, Adler M, Juni A. Assessment of acute and chronic morphine dependence in male and female mice. Pharmacol. Biochem. Behav. 2001;70:149–156. doi: 10.1016/s0091-3057(01)00600-1. [DOI] [PubMed] [Google Scholar]
- Kest B, Palmese CA, Hopkins E, Adler M, Juni A, Mogil JS. Naloxone-precipitated withdrawal jumping in 11 inbred mouse strains: evidence for common genetic mechanisms in acute and chronic morphine physical dependence. Neuroscience. 2002;115:463–469. doi: 10.1016/s0306-4522(02)00458-x. [DOI] [PubMed] [Google Scholar]
- Kest B, Lee CE, McLemore GL, Inturrisi CE. An antisense oligodeoxynucleotide to the delta opioid receptor (DOR-1) inhibits morphine tolerance and acute dependence in mice. Brain Res. Bull. 1996;39:185–188. doi: 10.1016/0361-9230(95)02092-6. [DOI] [PubMed] [Google Scholar]
- Knapp RJ, Malatynska E, Collins N, Fang L, Wang JY, Hruby VJ, Roeske WR, Yamamura HI. Molecular biology and pharmacology of cloned opioid receptors. FASEB J. 1995;9:516–525. doi: 10.1096/fasebj.9.7.7737460. [DOI] [PubMed] [Google Scholar]
- Koyuncuoglu H, Nurten A, Yamanturk P, Nurten R. The importance of the number of NMDA receptors in the development of supersensitivity or tolerance to and dependence on morphine. Pharmacol. Res. 1999;39:311–319. doi: 10.1006/phrs.1998.0443. [DOI] [PubMed] [Google Scholar]
- Lannes B, Bernard V, Bloch B, Micheletti G. Chronic treatment with dizocilpine maleate increases the number of striatal neurons expressing the d-2 receptor gene. Neuroscience. 1995;65:431–438. doi: 10.1016/0306-4522(94)00501-u. [DOI] [PubMed] [Google Scholar]
- Liu ZH, He Y, Jin WQ, Chen XJ, Zhang HP, Shen QX, Chi ZQ. Binding affinity to and dependence on some opioids in Sf9 insect cells expressing human mu-opioid receptor. Acta Pharmacol. Sin. 2003;24:859–863. [PubMed] [Google Scholar]
- Manallack DT, Lodge D, Beart PM. Subchronic administration for MK-801 in the rat decreases cortical binding of [3H]D-AP5, suggesting down-regulation of the cortical N-methyl-D-aspartate receptors. Neuroscience. 1989;30:87–94. doi: 10.1016/0306-4522(89)90355-2. [DOI] [PubMed] [Google Scholar]
- Marshall I, Weinstock M. A quantitative method for the assessment of physical dependence on narcotic analgesics in mice. Br. J. Pharmacol. 1969;37:505P–506P. [PMC free article] [PubMed] [Google Scholar]
- Matthes HW, Maldonado R, Simonin F, Valverde O, Slowe S, Kitchen I, Befort K, Dierich A, Le Meur M, Dolle P, Tzavara E, Hanoune J, Roques BP, Kieffer BL. Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the mu-opioid-receptor gene. Nature. 1996;383:819–823. doi: 10.1038/383819a0. [DOI] [PubMed] [Google Scholar]
- McLemore GL, Kest B, Inturrisi CE. The effects of LY293558, an AMPA receptor antagonist, on acute and chronic morphine dependence. Brain Res. 1997;778:120–126. doi: 10.1016/s0006-8993(97)00985-2. [DOI] [PubMed] [Google Scholar]
- Micheletti G, Lannes B, Haby C, Borrelli E, Kempf E, Warter JM, Zwiller J. Chronic administration of NMDA antagonists induces D2 receptor synthesis in rat striatum. Mol. Brain Res. 1992;14:363–368. doi: 10.1016/0169-328x(92)90105-k. [DOI] [PubMed] [Google Scholar]
- Miyamoto Y, Bowen WD, Portoghese PS, Takemori AE. Lack of involvement of delta-1 opioid receptors in the development of physical dependence on morphine in mice. J. Pharmacol. Exp. Ther. 1994;270:37–39. [PubMed] [Google Scholar]
- Narita M, Suzuki T, Misawa M, Nagase H. Effects of κ opioids on the morphine-induced pharmacological actions. Eur. J. Pharmacol. 1990;183:2328. [Google Scholar]
- Nehmad R, Nadler H, Simantov R. Effects of acute and chronic morphine treatment of calmodulin activity of rat brain. Mol. Pharmacol. 1982;22:389–394. [PubMed] [Google Scholar]
- Nestler EJ, Alreja M, Aghajanian GK. Molecular and cellular mechanisms of opiate action: studies in the rat locus coeruleus. Brain Res. Bull. 1994;35:521–528. doi: 10.1016/0361-9230(94)90166-x. [DOI] [PubMed] [Google Scholar]
- Oldendorf WH, Hyman S, Braun L, Oldendorf SZ. Blood-brain barrier: penetration of morphine, codeine, heroin, and methadone after carotid injection. Science. 1972;178:984–986. doi: 10.1126/science.178.4064.984. [DOI] [PubMed] [Google Scholar]
- Patel TR, McCulloch J. AMPA receptor antagonism attenuates MK-801-induced hypermetabolism in the posterior cingulate cortex. Brain Res. 1995;686:254–258. doi: 10.1016/0006-8993(95)00483-7. [DOI] [PubMed] [Google Scholar]
- Rossi GC, Brown GP, Leventhal L, Yang K, Pasternak GW. Novel receptor mechanisms for heroin and morphine-6 beta-glucuronide analgesia. Neurosci. Lett. 1996;216:1–4. doi: 10.1016/0304-3940(96)12976-1. [DOI] [PubMed] [Google Scholar]
- Rossi GC, Pan YX, Brown GP, Pasternak GW. Antisense mapping the MOR-1 opioid receptor: evidence for alternative splicing and a novel morphine-6 beta-glucuronide receptor. FEBS Lett. 1995;369:192–196. doi: 10.1016/0014-5793(95)00757-z. [DOI] [PubMed] [Google Scholar]
- Saelens JK, Granat FR, Sawyer WK. The mouse jumping test--a simple screening method to estimate the physical dependence capacity of analgesics. Arch. Int. Pharmacodyn. Ther. 1971;190:213–218. [PubMed] [Google Scholar]
- Selley DE, Caob C-C, Sexton T, Schwegel JA, Martin TJ, Childers SR. Mu opioid receptor-mediated G-protein activation by heroin metabolites: evidence for greater efficacy of 6-monoacetylmorphine compared with morphine. Biochem. Pharm. 2001;4:447–455. doi: 10.1016/s0006-2952(01)00689-x. [DOI] [PubMed] [Google Scholar]
- Simonin F, Valverde O, Smadja C, Slowe S, Kitchen I, Dierich A, Le MM, Roques BP, Maldonado R, Kieffer BL. Disruption of the kappa-opioid receptor gene in mice enhances sensitivity to chemical visceral pain, impairs pharmacological actions of the selective kappa-agonist U-50,488H and attenuates morphine withdrawal. EMBO J. 1998;17:886–897. doi: 10.1093/emboj/17.4.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits SE. Quantitation of physical dependence in mice by naloxone-precipitated jumping after a single dose of morphine. Res. Commun. Chem. Pathol. Pharmacol. 1975;10:651–661. [PubMed] [Google Scholar]
- Suzuki T, Ikeda H, Tsuji M, Misawa M, Narita M, Tseng LF. Antisense oligodeoxynucleotide to delta opioid receptors attenuates morphine dependence in mice. Life Sci. 1997a;61:165–170. doi: 10.1016/s0024-3205(97)00620-6. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Narita M, Takahashi Y, Misawa M, Nagase H. Effects of nor-binaltorphimine on the development of analgesic tolerance to and physical dependence on morphine. Eur. J. Pharmacol. 1992;213:91–97. doi: 10.1016/0014-2999(92)90237-x. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Tsuji M, Mori T, Misawa M, Nagase H. Involvement of delta 1 and delta 2 opioid receptor subtypes in the development of physical dependence on morphine in mice. Pharmacol. Biochem. Behav. 1997b;57:293–299. doi: 10.1016/s0091-3057(96)00319-x. [DOI] [PubMed] [Google Scholar]
- Takemori AE, Loh HH, Lee NM. Suppression by dynorphin A-( l-13) of the expression of opiate withdrawal and tolerance in mice. Eur. J. Pharmacol. 1992;221:223–226. doi: 10.1016/0014-2999(92)90705-9. [DOI] [PubMed] [Google Scholar]
- Trujillo KA, Akil H. Inhibition of morphine tolerance and dependence by the NMDA receptor antagonist MK-801. Science. 1991;251:85–87. doi: 10.1126/science.1824728. [DOI] [PubMed] [Google Scholar]
- Vandergriff J, Rasmussen K. The selective mGlu2/3 receptor agonist LY354740 attenuates morphine-withdrawal-induced activation of locus coeruleus neurons and behavioral signs of morphine withdrawal. Neuropharmacology. 1999;38:217–222. doi: 10.1016/s0028-3908(98)00196-8. [DOI] [PubMed] [Google Scholar]
- Vekovischeva OY, Zamanillo D, Echenko O, Seppala T, Uusi-Oukari M, Honkanen A, Seeburg PH, Sprengel R, Korpi ER. Morphine-induced dependence and sensitization are altered in mice deficient in AMPA-type glutamate receptor-A subunits. J. Neurosci. 2001;21:4451–4459. doi: 10.1523/JNEUROSCI.21-12-04451.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma A, Kulkarni SK. Role of D1/D2 dopamine and N-methyl-D-aspartate (NMDA) receptors in morphine tolerance and dependence in mice. Eur. Neuropsychopharmacol. 1995;5:81–87. doi: 10.1016/0924-977X(94)00140-7. [DOI] [PubMed] [Google Scholar]
- Watkins J, Collingridge G. Phenylglycine derivatives as antagonists of metabotropic glutamate receptors. Trends Pharmacol. Sci. 1994;15:333–342. doi: 10.1016/0165-6147(94)90028-0. [DOI] [PubMed] [Google Scholar]
- Watson B, Meng F, Akil H. A chimeric analysis of the opioid receptor domains critical for the binding selectivity of mu opioid ligands. Neurobiol. Dis. 1996;3:87–96. doi: 10.1006/nbdi.1996.0009. [DOI] [PubMed] [Google Scholar]
- Wiley JN, Downs DA. Naloxone-precipitated jumping in mice pretreated with acute injections of opioids. Life Sci. 1979;25:797–801. doi: 10.1016/0024-3205(79)90525-3. [DOI] [PubMed] [Google Scholar]
- Zarrindast MR, Habibi M, Borzabadi S, Fazli-Tabaei S, Hossein Yahyavi S, Rostamin P. The effects of dopamine receptor agents on naloxone-induced jumping behaviour in morphine-dependent mice. Eur. J. Pharmacol. 2002;45:287–293. doi: 10.1016/s0014-2999(02)02149-0. [DOI] [PubMed] [Google Scholar]