Abstract
Reduced perception of respiratory sensations is associated with negative treatment outcome in asthma. We examined whether habituation in the neural processing of repeatedly experienced respiratory sensations may underlie subjective reports of reduced respiratory perception. Respiratory-related evoked potentials (RREP) elicited by inspiratory occlusions and reports of respiratory perception were compared between early and late experimental periods in healthy subjects. Reports of respiratory perception were reduced during late, compared to early, experimental periods. This was paralleled by reduced magnitudes in RREP components N1, P2, and P3 in late, compared to early, experimental periods. Habituation in the neural processing of respiratory sensations is a potential mechanism that underlies subjective reports of reduced respiratory perception and might represent a risk factor for reduced perception of respiratory sensations in asthma.
Descriptors: Asthma, Dyspnea, EEG, Habituation, Respiratory perception, Respiratory-related evoked potential
Respiratory sensations such as dyspnea are cardinal symptoms that patients with asthma and other respiratory diseases repeatedly experience in the course of their disease (American Thoracic Society, 1999; Global Initiative for Asthma [GINA], 2008). Although the perception of respiratory sensations is an impairing and frightening experience in many patients, accurate perception also provides the important motivational basis for seeking timely medical treatment (Banzett, Dempsey, O’Donnell, & Wamboldt, 2000; GINA, 2008). For example, reduced perception of initial bronchoconstriction and dyspnea in asthma patients has been shown to be associated with poor treatment outcome, including delayed or inadequate medication, frequent rehospitalisations or emergency department visits, and even near-fatal exacerbations (Barreiro, Gea, Sanjuas, Marcos, Broquetas, & Milic-Emili, 2004; Feldman et al., 2007; Kifle, Seng, & Davenport, 1997; Kikuchi et al., 1994; Magadle, Berar-Yanay, & Weiner, 2002).
Unfortunately, the mechanisms of reduced perception of respiratory sensations remain poorly understood; specifically the involved neural processes have rarely been examined. Only a few studies using electroencephalography (EEG) or functional magnetic resonance imaging (fMRI) are available, and these suggested reduced neural processing of respiratory signals to be associated with reduced respiratory perception (Davenport, Cruz, Stecenko, & Kifle, 2000; Fauroux et al., 2007; von Leupoldt et al., 2009; Webster & Colrain, 2002). Other findings suggested that habituation processes might play an additional important role by demonstrating reduced reports of perceived respiratory sensations after repeated or chronic respiratory stimulation (Bloch-Salisbury, Shea, Brown, Evans, & Banzett, 1996; Carrieri-Kohlman et al., 2001; Li et al., 2006; Wan, Van Diest, De Peuter, Bogaerts, & van den Bergh, 2009). However, the neural processes specifically underlying habituation effects of respiratory perception have not yet been studied in a controlled experimental setting, which was the aim of the present study.
We used the respiratory-related evoked potential (RREP) recorded from the EEG as a measure of cerebral cortical activity, which was elicited by short inspiratory occlusions (Chan & Davenport, 2010; Davenport et al., 2000; Davenport, Friedman, Thompson, & Franzen, 1986; Fauroux et al., 2007; Webster & Colrain, 2002). The early RREP components Nf, P1, and N1 (< 130 ms poststimulus) reflect the initial arrival and first-order sensory processing of afferent respiratory signals in sensorimotor regions. The later components P2 and P3 (> 150 ms poststimulus) characterize subsequent higher-order cognitive processing in other associative cortical areas (Chan & Davenport, 2010; von Leupoldt, Keil, et al., 2010). Specifically P2 and P3, and in some circumstances N1, are vulnerable to cognitive processes not related to respiration per se such as attentional distraction or emotion processing (Davenport, Chan, Zhang, & Chou, 2007; Harver, Squires, Bloch-Salisbury, & Katkin, 1995; von Leupoldt, Vovk, et al., 2010; Webster & Colrain, 2000).
We tested the hypotheses that repeated presentations of inspiratory occlusions in healthy individuals would not only result in reduced subjective reports of respiratory perception but also result in reduced neural processing of these respiratory sensations. Because the physical characteristics of the respiratory stimulus remained constant during the experiment, we expected habituation-related magnitude reductions in later, higher-order RREP components, but no changes in early, sensory components Nf and P1.
Methods
Participants
After providing informed written consent, 14 healthy, nonsmoking volunteers without history of significant psychological or medical conditions participated in this study (Table 1). Normal baseline lung function was confirmed by spirometry (Discovery, Futuremed America Inc., Granada Hills, CA) according to the joined ATS/ERS guidelines (Miller et al., 2005). The protocol was approved by the Institutional Review Board of the University of Florida. The present study is a follow-up analysis of an existing data set that examined the impact of viewing affective picture series on RREPs (reported elsewhere: von Leupoldt, Vovk, et al., 2010). The present analyses exclusively focus on habituation effects, that is, whether respiratory stimuli presented in the second half, compared to the first half, of the experiment would lead to reduced reports of subjective perception and reduced magnitudes of later RREP components.
Table 1.
Baseline Characteristics of Participants (Means, SD)
| Characteristics | Data |
|---|---|
| Age (years) | 19.7 (2.2) |
| Sex (female/male) | 6/8 |
| Weight (kg) | 69.7 (13.1) |
| Height (cm) | 177.3 (13.4) |
| Forced expiratory volume in 1 s (L) | 3.75 (0.67) |
| Forced expiratory volume in 1 s (% of predicted value) | 95.3 (10.6) |
| Forced vital capacity (L) | 4.43 (0.96) |
| Forced vital capacity (% of predicted value) | 97.1 (13.4) |
Measurement and Data Reduction of Respiratory-Related Evoked Potentials
Participants breathed via a mouthpiece through a non-rebreathing valve (Hans Rudolph Inc., Kansas City, MO). The inspiratory port of the valve was connected to a pressure-activated occluder. Inspiration was interrupted every two to six breaths for 500 ms by manual triggering of the occluder after the onset of inspiration, with a parallel marker signal being sent to the EEG recorder. Inspiratory onset was indicated by the continuously displayed mouth pressure signal recorded from the non-rebreathing valve center by a differential-pressure transducer (model MP-45, Validyne Engineering, Northridge, CA), which was connected to a PowerLab biosignal recording unit (ADInstruments, Bella Vista, Australia).
EEG data were recorded from the scalp using a 129-channel system (Electrical Geodesics Inc. [EGI], Eugene, OR) with a sampling rate of 250 Hz, vertex sensor as the reference electrode, and an online bandpass filter (0.1–100 Hz). Electrode impedances were kept below 70 kΩ. This threshold has been examined in empirical studies of signal-to-noise ratios in EGI dense-array systems under varying impedance levels (Ferree, Luu, Russell, & Tucker, 2001), which show that good signal quality can be achieved using those settings. All further processing was performed off-line, and data were converted to the average reference prior to all subsequent analyses, using functions built into BESA 5.1. After digital low-pass filtering (30 Hz) and artifact corrections, occlusion epochs were extracted (200 ms pre- and 1000 ms post-stimulus) and averaged across the first six experimental blocks and across the last six experimental blocks for each participant using a maximum of 200 μV as the cutoff amplitude (von Leupoldt, Vovk, et al., 2010). Based on previous reports and respective conventions in the RREP domain, which are based on established topographical localizations for each RREP component (Chan & Davenport, 2010; Davenport et al., 1986, 2007; Logie, Colrain, & Webster, 1998; von Leupoldt, Keil, et al., 2010), the RREP components were identified as follows: Nf = negative peak in the frontal region (sensors around F3/ F4; latency: 25–50 ms), P1 = positive peak in the centro-parietal region (sensors between Cz/Pz; latency: 45–65 ms), N1 = negative peak in the centro-lateral region (sensors around C3/Cz/ C4; latency: 85–125 ms), P2 = positive peak in the central region (sensors around Cz; latency: 160–230 ms), and P3 = positive peak in the centro-parietal region (sensors between Cz/Pz; latency: 250–350 ms). For each RREP component, means of the amplitudes across sensors in these clusters were calculated and entered into analyses.
Respiratory Motor Drive
To control for changes in the central respiratory motor output, the P0.1 (in cmH2O) was measured every 60 s during picture viewing. P0.1 is the negative inspiratory occlusion pressure at the mouth 100 ms after the onset of an inspiratory effort against a closed airway and reflects the summed motor output of the central respiratory controller (Whitelaw & Derenne, 1993). Values for P0.1 at rest in healthy participants are in the range of 1 cmH2O (Whitelaw & Derenne, 1993). P0.1 was derived off-line from the continuous Pm signal with the biosignal software package Labview (ADInstruments, Bella Vista, Australia). To avoid biases in the EEGsignal averaging, inspiratory occlusions for the measurement of P0.1 were not included in the RREP.
Respiratory Sensation
Participants rated the experienced intensity of the inspiratory occlusions after each experimental block on a horizontal visual analog scale (100 mm), ranging from 0 (not noticeable) to 100 (maximally imaginable intensity). To focus attention on the respiratory stimulus during all blocks, participants were instructed to press a button every time they perceived an inspiratory occlusion, and button presses were recorded.
Experimental Protocol
After standardized instructions and positioning of the EEG sensor net and nose clip, participants were seated in a recliner and started breathing through the breathing circuit. The experimental protocol was divided into 12 blocks. Each block started with a 1-min epoch of adaptation to the mouthpiece breathing during which no inspiratory occlusions were presented. A 6-min epoch with inspiratory occlusions followed while participants were instructed to view affective pictures presented on a monitor and to press a button every time they perceived an inspiratory occlusion. The number of occlusions that were presented every two to six breaths in each block varied between individuals because of the great interindividual variability in breathing patterns and durations of respiratory cycles. Immediately after each block, participants rated the perceived intensity of the respiratory stimulus on the visual analog scale, followed by a short rest period.
Data Analyses
Accuracy of occlusion detection was calculated as the proportion (%) of correct button presses from the total number of occlusions presented within one block. Outcome measures are reported as means ± standard deviations of the mean (SD) averaged across the first six experimental blocks (first half) and the second six experimental blocks (second half) and were compared with paired t tests. All analyses were calculated with SPSS 15.0 software (SPSS Inc., Chicago, IL) using a significance level of p<.05.
Results
Respiratory Sensation
Ratings of the perceived intensity of inspiratory occlusions showed significant reductions for the second half (43.9 ± 17.8 mm) compared to the first half of the experiment (51.2 ± 17.5 mm), t(13) = 3.08, p<.01. No difference was observed in the accuracy of detecting respiratory occlusions, suggesting a similar attentional focus to the inspiratory occlusions for the second (98.1 ± 2.6%) and first experimental half (97.9 ± 3.6%), t(13) = −0.54, n.s.
Respiratory-Related Evoked Potentials
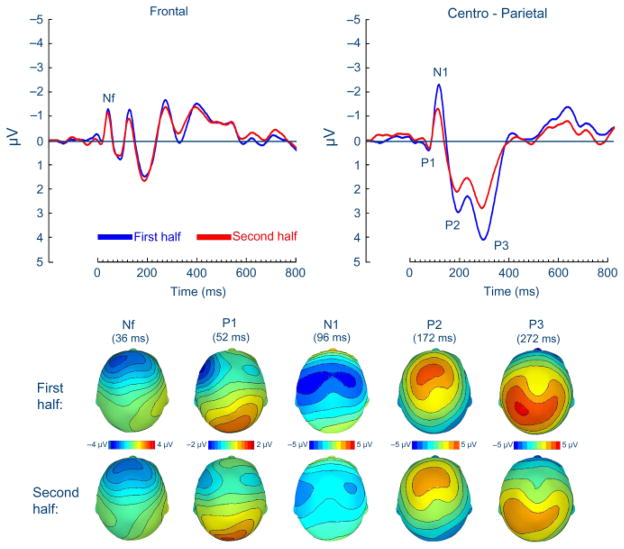
The number of presented inspiratory occlusions showed no difference between the second (158.1 ± 39.0) and first halves of the experiment (162.3 ± 40.0), t(13) = 1.00, n.s. As illustrated in Figure 1, the commonly observed RREP components Nf, P1, N1, P2, and P3 were obtained at similar scalp localizations for the second and first halves of the experiment (see Table 2). No differences between the second and first halves of the experiment were observed for the magnitudes of the early RREP components Nf and P1, t(13) = −0.10, n.s., and t(13) = 0.03, n.s. (Figure 1, Table 2). However, the subsequent RREP components N1, P2, and P3 showed significant magnitude reductions in the second half compared to the first half of the experiment, t(13) = −6.17, p<.001; t(13) = 2.29, p<.05, and t(13) = 3.23, p<.01 (Figure 1, Table 2).
Figure 1.
Group means for the respiratory-related evoked potential (upper panel) and related scalp topographies at their peak latencies (lower panel) at frontal and centro-parietal regions for the first and the second halves of the experiment.
Table 2.
Mean (SD) Amplitudes (in Microvolts) of the Respiratory-Related Evoked Potential (RREP) during the First versus the Second Half of the Experiment
| RREP | First half | Second half |
|---|---|---|
| Nf (frontal) | −1.41 (0.87) | −1.39 (1.12) |
| P1 (centro-parietal) | 0.66 (1.06) | 0.65 (1.02) |
| N1 (centro-lateral) | −3.18 (1.97) | −1.78 (1.81)*** |
| P2 (central) | 4.13 (4.64) | 3.28 (4.06)* |
| P3 (centro-parietal) | 3.85 (2.35) | 2.67 (2.19)** |
p<.05;
p<.01;
p<.001 (paired t tests).
Respiratory Motor Drive
No difference was observed in P0.1, suggesting a similar central respiratory motor output, for the second (−1.45 ± 0.70 cmH2O) and first experimental halves (−1.41 ± 0.59 cmH2O), t(13) = 0.72, n.s.
Discussion
The present study examined habituation effects in the subjective perception and neural processing of respiratory sensations by comparing subjective and neural responses to short inspiratory occlusions presented early and late in the same experiment. The results show reduced subjective reports of respiratory perception when occlusions were presented later as compared to earlier during the experiment. Most importantly, this was paralleled by magnitude reductions in the RREP components N1, P2, and P3 during late compared to early periods of the experiment, whereas no such differences were observed for the earlier RREP components Nf and P1. Other potentially confounding variables such as number of presented occlusions, attentional focus to the respiratory stimulation, and central respiratory motor output (P0.1) showed no differences between the early and late periods of the experiment. These findings suggest that habituation in the neural processing of repeatedly experienced respiratory sensations can underlie parallel habituation effects in the subjective perception of these sensations. This neural habituation effect occurs at processing stages of respiratory sensations (starting ~100 ms after stimulus onset) that are already related to higher-order cognitive processes, but not at stages exclusively associated with their early, sensory processing.
The present findings suggest a neural mechanism that might explain recent findings showing habituation in subjective reports of respiratory sensations after repeated or chronic respiratory stimulation (Bloch-Salisbury et al., 1996; Carrieri-Kohlman et al., 2001; Li et al., 2006; Wan et al., 2009). For example, repeated hypercapnic rebreathing challenges in healthy individuals in the studies by Wan and colleagues (Li et al., 2006; Wan et al., 2009) resulted in decreased ratings for perceived dyspnea over trials. Similar habituation effects were observed by Bloch-Salisbury and coworkers (1996), who induced chronically elevated PaCO2 levels in ventilator-dependent patients over several days by increasing inspired CO2 levels. When intermittently challenged with additional increases of inspired CO2, patients perceived less dyspnea during days with chronically elevated PaCO2 levels compared to those days with normal baseline PaCO2 levels. It should be noted, however, that habituation effects are not respiration specific, but are also found for other sensory modalities, including olfactory, auditory, or painful stimuli (Bingel, Schoell, Herken, Büchel, & May, 2007; Bradley, Lang, & Cuthbert, 1993; Dalton, 2000). Therefore, habituation per se is a general adaptive process saving neural resources for other, more relevant tasks when repeated sensory information becomes redundant and loses its significance.
However, on the basis of earlier findings that showed reduced perception of dyspnea to be associated with delayed treatment, including near-fatal asthma attacks (Barreiro et al., 2004; Feldman et al., 2007; Kifle et al., 1997; Kikuchi et al., 1994; Magadle et al., 2002), habituation effects in the underlying neural processing of respiratory perception might constitute a potential risk factor in asthma. It can be assumed that repeated or sometimes chronic experience of dyspnea in the course of disease leads to reduced neural processing and, thus, reduced subjective perception of acute asthma symptoms. Indeed, a recent fMRI study demonstrated that longer disease duration in asthma patients was correlated with reduced brain activations during acute resistive load induced dyspnea, which was interpreted as an habituation effect (von Leupoldt et al., 2009). The present study supports this interpretation with improved methodology by demonstrating habituation effects in the neural processing and subjective perception of respiratory sensations when these sensations are repeatedly induced over a longer time period in a well-controlled experimental setting. Our results are, therefore, converging with the few earlier studies using respiratory-related evoked potentials, which similarly emphasized the potential role of reduced neural processing of respiratory sensations in different groups of patients with pediatric and adult asthma (Davenport et al., 2000; Fauroux et al., 2007; Webster & Colrain, 2002).
When interpreting the present results, some limitations should be kept in mind. Besides the rather small sample size, only healthy individuals were examined. Moreover, our findings of habituation to experimentally induced respiratory sensations might not fully compare to the pathological characteristics of respiratory sensations found in asthma. Therefore, future studies will be important in determining whether patients with asthma or other respiratory diseases show comparable habituation effects in the neural processing of respiratory sensations. In this regard, it will be interesting to examine habituation effects related to treatment outcomes and whether specific subgroups of patients are specifically vulnerable to these effects, for example, show more rapid or pronounced habituation.
In summary, the present study demonstrates that reduced subjective reports of respiratory perception after repeated respiratory stimulation are paralleled by magnitude reductions in RREP components N1, P2, and P3, but not Nf and P1. Thus, habituation in the neural processing of respiratory sensations seems to underlie habituation effects in the subjective perception of respiratory sensations. Future studies are required to examine whether patients with respiratory diseases such as asthma show comparable neural responses and whether these are related to reduced perception of respiratory symptoms or negative treatment outcomes.
Acknowledgments
This study was supported in part by a stipend (Heisenberg-Stipendium, DFG LE 1843/9-1) from the German Research Society (Deutsche Forschungsgemeinschaft, DFG) to Andreas von Leupoldt and by a grant from the National Institute of Mental Health (P50MH72850) to Peter J. Lang. The authors thank Andreas Keil for his valuable support of the present study.
References
- American Thoracic Society. Dyspnea: Mechanisms, assessment, and management: A consensus statement. American Journal of Respiratory and Critical Care Medicine. 1999;159:321–340. doi: 10.1164/ajrccm.159.1.ats898. [DOI] [PubMed] [Google Scholar]
- Banzett RB, Dempsey JA, O’Donnell DE, Wamboldt MZ. Symptom perception and respiratory sensation in asthma. American Journal of Respiratory and Critical Care Medicine. 2000;162:1178–1182. doi: 10.1164/ajrccm.162.3.9909112. [DOI] [PubMed] [Google Scholar]
- Barreiro E, Gea J, Sanjuas C, Marcos R, Broquetas J, Milic-Emili J. Dyspnoea at rest and at the end of different exercises in patients with near-fatal asthma. European Respiratory Journal. 2004;24:219–225. doi: 10.1183/09031936.04.00074703. [DOI] [PubMed] [Google Scholar]
- Bingel U, Schoell E, Herken W, Büchel C, May A. Habituation to painful stimulation involves the antinociceptive system. Pain. 2007;131:21–30. doi: 10.1016/j.pain.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Bloch-Salisbury E, Shea SA, Brown R, Evans K, Banzett RB. Air hunger induced by acute increase in PCO2 adapts to chronic elevation of PCO2 in ventilated humans. Journal of Applied Physiology. 1996;81:949–956. doi: 10.1152/jappl.1996.81.2.949. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ, Cuthbert BN. Emotion, novelty, and the startle reflex: Habituation in humans. Behavioral Neuroscience. 1993;107:970–980. doi: 10.1037//0735-7044.107.6.970. [DOI] [PubMed] [Google Scholar]
- Carrieri-Kohlman V, Gormley JM, Eiser S, Demir-Deviren S, Nguyen H, Paul SM, et al. Dyspnea and the affective response during exercise training in obstructive pulmonary disease. Nursing Research. 2001;50:136–146. doi: 10.1097/00006199-200105000-00002. [DOI] [PubMed] [Google Scholar]
- Chan PS, Davenport PW. Respiratory-related evoked potential measures of cerebral cortical respiratory information processing. Biological Psychology. 2010;84:4–12. doi: 10.1016/j.biopsycho.2010.02.009. [DOI] [PubMed] [Google Scholar]
- Dalton P. Psychophysical and behavioral characteristics of olfactory adaptation. Chemical Senses. 2000;25:487–492. doi: 10.1093/chemse/25.4.487. [DOI] [PubMed] [Google Scholar]
- Davenport PW, Chan PY, Zhang W, Chou YL. Detection threshold for inspiratory resistive loads and respiratory- related evoked potentials. Journal of Applied Physiology. 2007;102:276–285. doi: 10.1152/japplphysiol.01436.2005. [DOI] [PubMed] [Google Scholar]
- Davenport PW, Cruz M, Stecenko AA, Kifle Y. Respiratory- related evoked potentials in children with life-threatening asthma. American Journal of Respiratory and Critical CareMedicine. 2000;161:1830–1835. doi: 10.1164/ajrccm.161.6.9903077. [DOI] [PubMed] [Google Scholar]
- Davenport PW, Friedman WA, Thompson FJ, Franzen O. Respiratory-related cortical potentials evoked by inspiratory occlusion in humans. Journal of Applied Physiology. 1986;60:1843–1848. doi: 10.1152/jappl.1986.60.6.1843. [DOI] [PubMed] [Google Scholar]
- Fauroux B, Renault F, Boelle PY, Donzel-Raynaud C, Nicot F, Clément A, et al. Impaired cortical processing of inspiratory loads in children with chronic respiratory defects. Respiratory Research. 2007;8:61. doi: 10.1186/1465-9921-8-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman JM, McQuaid EL, Klein RB, Kopel SJ, Nassau JH, Mitchell DK, et al. Symptom perception and functional morbidity across a 1-year follow-up in pediatric asthma. Pediatric Pulmonology. 2007;42:339–347. doi: 10.1002/ppul.20584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferree TC, Luu P, Russell GS, Tucker DM. Scalp electrode impedance, infection risk, and EEG data quality. Clinical Neurophysiology. 2001;112:536–544. doi: 10.1016/s1388-2457(00)00533-2. [DOI] [PubMed] [Google Scholar]
- Global Initiative for Asthma (GINA) Global Strategy for Asthma Management and Prevention. 2008 Available from: http://www.ginasthma.org.
- Harver A, Squires N, Bloch-Salisbury E, Katkin E. Eventrelated potentials to airway occlusion in young and old subjects. Psychophysiology. 1995;32:121–129. doi: 10.1111/j.1469-8986.1995.tb03303.x. [DOI] [PubMed] [Google Scholar]
- Kifle Y, Seng V, Davenport PW. Magnitude estimation of inspiratory resistive loads in children with life-threatening asthma. American Journal of Respiratory and Critical Care Medicine. 1997;156:1530–1535. doi: 10.1164/ajrccm.156.5.9703011. [DOI] [PubMed] [Google Scholar]
- Kikuchi Y, Okabe S, Tamura G, Hida W, Homma M, Shirato K, Takishima T. Chemosensitivity and perception of dyspnea in patients with a history of near-fatal asthma. New England Journal of Medicine. 1994;330:1329–1334. doi: 10.1056/NEJM199405123301901. [DOI] [PubMed] [Google Scholar]
- Li W, Daems E, Van de Woestijne KP, Van Diest I, Gallego J, De Peuter S, et al. Air hunger and ventilation in response to hypercapnia: Effects of repetition and anxiety. Physiology and Behaviour. 2006;88:47–54. doi: 10.1016/j.physbeh.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Logie SL, Colrain IM, Webster KE. Source localisation of the early components of the respiratory-related evoked potential. Brain Topography. 1998;11:153–164. doi: 10.1023/a:1022210723257. [DOI] [PubMed] [Google Scholar]
- Magadle R, Berar-Yanay N, Weiner P. The risk of hospitalization and near-fatal and fatal asthma in relation to the perception of dyspnea. Chest. 2002;121:329–333. doi: 10.1378/chest.121.2.329. [DOI] [PubMed] [Google Scholar]
- Miller MR, Crapo R, Hankinson J, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. European Respiratory Journal. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- von Leupoldt A, Keil A, Chan PY, Bradley MM, Lang PJ, Davenport PW. Cortical sources of the respiratory-related evoked potential. Respiratory Physiology & Neurobiology. 2010;170:198–201. doi: 10.1016/j.resp.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Leupoldt A, Sommer T, Kegat S, Eippert F, Baumann HJ, Klose H, et al. Down-regulation of insular cortex responses to dyspnea and pain in asthma. American Journal of Respiratory and Critical Care Medicine. 2009;180:232–238. doi: 10.1164/rccm.200902-0300OC. [DOI] [PubMed] [Google Scholar]
- von Leupoldt A, Vovk A, Bradley MM, Keil A, Lang PJ, Davenport PW. The impact of emotion on respiratory-related evoked potentials. Psychophysiology. 2010;47:579–586. doi: 10.1111/j.1469-8986.2009.00956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan L, Van Diest I, De Peuter S, Bogaerts K, Van den Bergh O. Repeated breathlessness experiences induced by hypercapnia: Differential effects on intensity and unpleasantness. Chest. 2009;135:455–461. doi: 10.1378/chest.08-1226. [DOI] [PubMed] [Google Scholar]
- Webster KE, Colrain IM. The respiratory-related evoked potential: Effects of attention and occlusion duration. Psychophysiology. 2000;37:310–318. [PubMed] [Google Scholar]
- Webster KE, Colrain IM. P3-specific amplitude reductions to respiratory and auditory stimuli in subjects with asthma. American Journal of Respiratory and Critical Care Medicine. 2002;166:47–52. doi: 10.1164/rccm.2012006. [DOI] [PubMed] [Google Scholar]
- Whitelaw WA, Derenne JP. Airway occlusion pressure. Journal of Applied Physiology. 1993;74:1475–1483. doi: 10.1152/jappl.1993.74.4.1475. [DOI] [PubMed] [Google Scholar]