Abstract
Heroin and morphine exposure can cause physical dependence, with symptoms manifesting during their withdrawal. Inter-individual differences in symptom frequency during morphine withdrawal are a common finding that, in rodents, is demonstrably attributable to genotype. However, it is not known whether inter-individual differences characterize heroin withdrawal, and whether such variation can be similarly influenced by genotype. Therefore, we injected mice of ten inbred strains with acute and chronic heroin doses and compared their jumping frequencies, a common index of withdrawal magnitude, during naloxone-precipitated withdrawal. The data revealed significant strain frequency differences (range after acute and chronic heroin injection: 0-104 and 0-142 jumps, respectively) and substantial heritability (h2 = 0.94 to 0.96), indicating that genetic variance is associated with heroin withdrawal. The rank order of strain sensitivity for acute and chronic heroin withdrawal jumping, and for the current heroin and previous morphine strain data, were significantly correlated (r = 0.75-0.94), indicating their genetic and, ultimately, physiological commonality. These data suggest that the genetic liability to heroin dependence remains constant across a period of heroin intake, and that heroin and morphine dependence may benefit from common treatment strategies.
Keywords: Heroin, morphine, dependence, withdrawal, naloxone, inbred, heritability
1. Introduction
Opioids such as heroin and morphine remain widely abused drugs with a high physical dependence liability (Pouletty, 2002; Tarabar and Nelson, 2003). Manifested during a withdrawal syndrome comprised of behavioral and physiological signs, dependence is commonly assumed to result from chronic opioid use but in fact can be induced after even an acute opioid injection (Blasig et al., 1973; Kest et al., 2001, 2002; Klein et al., 2007; Smits, 1975). In humans, the aversive character of opioid withdrawal symptoms in addicted populations is regarded as a causative factor in their continued use and abuse of opioids and is thought to engender their dangerous drug seeking behavior (Jasinski, 1977; Kenny et al., 2006).
The severity of opioid dependence in human populations has been reported to be subject to inter-individual variation. That is, whereas even minimal prior opioid exposure can cause dependence in some individuals, others are resistant to dependence despite chronic intake (Ikeda et al., 2005; Pasternak, 2004). These findings may be attributable to variation in genotype. Indeed, variability in naloxone-precipitated withdrawal (NPW) magnitude in rodent subjects has been demonstrated to be associated with genetic variability (Han et al., 2004; Ikeda et al., 2005; Kest et al., 2002; Liang et al., 2006). For example, rat and mouse strains differ in both their sensitivity to undergo withdrawal per se and in the incidence of several withdrawal symptoms (Brase et al., 1977; Hoffman et al., 1998; Liang et al., 2006). Substantial inter-strain variability is also reported for NPW jumping frequency (Brase et al. 1977; Kest et al., 2002; Liang et al., 2006; Suzuki et al., 1991), a sensitive and reliable index of opioid withdrawal magnitude (El-Kadi and Sharif, 1994, Kest et al., 2001; Klein et al., 2007; Saelens et al., 1971; Smits, 1975). For example, substantial heritability estimates were obtained in a survey of 11 inbred (isogenic) mouse strains rendered dependent to morphine after both acute and chronic morphine treatment, and strain mean jumping frequency differences as great as 100-fold were observed in the most divergent responders (Kest et al., 2002). In addition to demonstrating a genetic contribution to response variability, strain surveys also identify strains with the most divergent responses to serve as progenitors in subsequent linkage analysis studies to identify genetic regions, and ultimately genes, contributing to genetic variability. This approach, referred to as quantitative trait locus (QTL) mapping, has already been successfully employed for several traits related to drug and alcohol abuse, including morphine dependence (Kest et al., 2004).
Despite the substantial abuse of heroin, there are no studies assessing the possible contribution of genotype to physical dependence induced by heroin. Since heroin is rapidly converted to morphine in vivo (Inturrisi et al. 1983) and both drugs preferentially bind and activate G opioid receptors (Selley et al., 2001; Watson et al., 1996), heroin and morphine were logically thought to have a highly similar, if not identical, pharmacological profile. It is however becoming increasingly recognized that substantial mechanistic differences underlie heroin and morphine analgesia (Ikeda et al., 1999; Rossi et al. 1995, 1996) tolerance (Gilbert et al., 2004; Lange et al., 1980; Rossi et al., 1996), and, more recently, dependence (Bao et al., 2007; Klein et al., 2007). For example, δ1 opioid receptor blockade attenuates NPW jumping frequencies in mice subject to acute and chronic heroin treatment, but not those identically treated with equieffective doses of morphine (Klein et al., 2007). Thus, extrapolating from one of these drugs to the other is done somewhat tenuously.
To determine whether, like for morphine, the magnitude of heroin dependence is also dependent on genotype, we compared NPW jumping frequencies of ten inbred mouse strains injected with heroin. Although acute as well as chronic opioid administration induces dependence (McLemore et al., 1997; Wiley and Downs, 1979), they do so by recruiting similar - but not identical – physiological substrates. This has recently been shown for heroin dependence as well (Klein et al., 2007). Thus, separate groups of mice from each strain were subjected to either acute or chronic heroin injection protocols. The demonstration of a genetic correlation between two heritable traits among isogenic strains can be used as evidence of the existence of pleiotropic genes with a common influence on both traits (Hegmann and Possidente, 1981). If the traits have genes in common, then one can strongly infer their common physiology. Thus, NPW jumping frequencies obtained after acute and chronic heroin treatment were correlated. In addition, the inbred mouse strains tested here were previously rendered dependent to morphine and surveyed for NPW jumping using protocols virtually identical to those utilized in the present study (Kest et al., 2002). Thus, the strain data obtained here also afforded the opportunity to assess the genetic correlation between heroin and morphine dependence as well.
2. Materials and methods
2.1 Subjects
Male mice of the following inbred strains were obtained from The Jackson Laboratory (Bar Harbor, ME) and subject to acute and chronic heroin treatment: 129P3/J (n: acute, 6; chronic, 8), A/J (n: acute, 8; chronic, 8), AKR/J (n: acute, 8; chronic, 8), BALB/c/J (n: acute, 8; chronic, 11), C3H/He/J (n: acute, 8; chronic, 8), C57BL/6/J (n: acute, 8; chronic, 8), CBA/J (n: acute, 8; chronic, 8), DBA/2/J (n: acute, 8; chronic, 8), SJL/J (n: acute, 8; chronic, 8), and SWR/J (n: acute, 8; chronic, 8). Additional mice (n = 6 / strain) served in control groups. All mice were housed four to a cage with same strain mates in the College of Staten Island Animal Facility. Mice were allowed free access to food (Purina chow) and water in a temperature-controlled (22°C) environment maintained on a 12:12 h light/dark cycle (lights on at 07:00 h). All testing was performed following an acclimation period of at least 1 week after arrival and at 7-9 weeks of age. All experimental protocols were approved by the College of Staten Island Institutional Animal Care and Use Committee for the use of animal subjects.
2.2 Heroin treatment
Acute dependence was induced by a single subcutaneous 50 mg/kg heroin injection followed by a single naloxone dose (50 mg/kg) 2 h later. In the chronic dependence condition, heroin was injected t.i.d. (09:00 h, 13:00 h and 17:00 h) for three days using a dosing schedule of 5, 10, and 20 mg/kg on Days 1, 2, and 3, respectively. On Day 4, a final 20 mg/kg heroin dose was injected, followed by a single naloxone dose (50 mg/kg) 1 h later. Control mice (n = 6/strain) were similarly injected with naloxone but were previously treated with saline instead of heroin. The different heroin and naloxone doses, as well as heroin-naloxone intervals, used here to study acute and chronic heroin withdrawal have been previously shown to yield maximal NPW jumping frequencies in CD-1 mice following acute and chronic heroin treatment, respectively (Klein et al., 2007).
2.3 Drugs
Both heroin hydrochloride, generously supplied by the Research Resources program of the National Institute on Drug Abuse (Rockville, MD), and naloxone hydrochloride(Sigma-Aldrich, St. Louis, MO) were dissolved in 0.9% physiological saline. Both drugs were injected via the subcutaneous route in a volume of 10 ml/kg.
2.4 Naloxone-precipitated withdrawal
Immediately after naloxone injection, heroin and saline-treated control subjects were placed into individual Plexiglas observation cylinders (25 × 11 cm), and the frequency of jumps for each subject was tallied over the next 15 min. The jumping response - defined as the simultaneous removal of all four paws from the horizontal surface - is a reliable and sensitive index of opioid withdrawal intensity and the most widely used (El-Kadi and Sharif, 1994; Kest et al., 2001; Miyamoto and Takemori, 1993; Ritzmann, 1981; Smits, 1975; Saelens et al., 1971; Way et al., 1969). Furthermore, only jumping frequency has been shown to have a positive dose-response relationship with acute and chronic heroin withdrawal (Klein et al., 2007). Thus, although symptoms such as diarrhea, ptosis, wet-dog shakes, and lacrimation were occasionally observed in the present study, they were excluded from analysis. Mean jump frequency per 15 min was used as the measure of dependence for each strain.
2.5 Data analysis
Jumping frequencies obtained in the acute and chronic dependence paradigms were analyzed separately using a two-way (strain X condition) ANOVA. Control and heroin treated mice were compared post-hoc within-strain only using Scheffe's test. Narrow-sense trait heritability was determined by comparing the between-strain variance to the total variance. Since animals are isogenic (i.e., genetically identical) within individual inbred strains, between-strain variance provides a measure of additive genetic (“allelic”) variation (VA), whereas within-strain variance (“error variance”) represents environmental variability (VE). An estimate of narrow-sense heritability (h2) for each trait was obtained using the formula: h2 = VA/(VA + VE) (Falconer and Mackay, 1996). Since these ten strains were initially chosen randomly, these values are likely accurate estimates (Hegmann and Possidente, 1981). To assess genetic correlation between acute and chronic heroin dependence, strains were also ranked from smallest to highest according to jumping frequencies and subject to Spearman's rank statistic (rs), ensuring that correlation estimates were not unduly influenced by extreme scoring strains. Acute and chronic heroin data were also correlated in the identical manner with acute and chronic morphine dependence data, respectively (Kest et al., 2002). All correlations were subject to Bonferroni correction for multiple comparisons. For all statistical tests, the criterion for significance was p ≤ 0.05.
3. Results
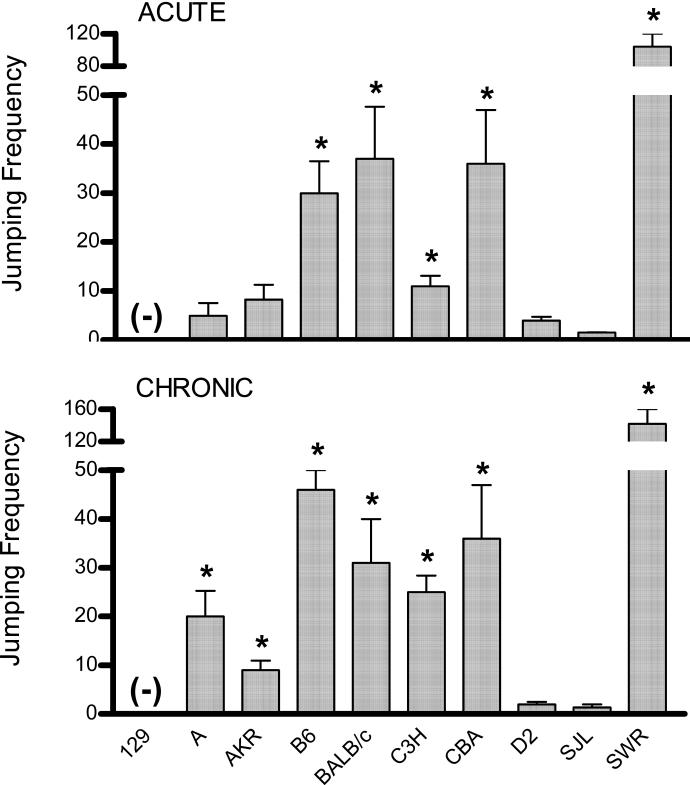
Analysis of NPW jumping frequencies in inbred mice subject to heroin or saline (i.e. control) injection revealed significant main effects of strain (acute: F(9,118) = 43.2, chronic: F(9,123) = 18.7; both p < 0.001), treatment (acute: F(1,118) = 151.8, chronic: F(1,123) = 80.9; both p < 0.001), and their interaction (acute: F(9,118) = 31.4, chronic: F(9,123) = 14.5; both p < 0.001). As illustrated in Figure 1, the range of mean frequency values obtained after both heroin treatment protocols was considerably broad. Specifically, whereas 129P3 mice did not respond after any heroin treatment, jumping frequencies as high as 104 and 142 were observed in SWR mice after acute and chronic heroin treatment, respectively. There were no significant strain differences in control mice (data not shown) nor were seizures evident in heroin treated subjects.
Figure 1. Withdrawal jumping frequencies in 10 inbred mouse strains following acute and chronic heroin treatment.
Mice undergoing acute heroin treatment (top figure) were injected once with heroin (50 mg/kg) and withdrawal precipitated 2 h later. Chronically treated mice (bottom figure) were injected repeatedly (t.i.d.) with heroin doses of 5, 10, and 20 mg/kg on Days 1, 2, and 3, respectively. Withdrawal was precipitated 1 h after a final 20 mg/kg heroin dose on Day 4. Withdrawal was precipitated by injecting a single naloxone dose (50 mg/kg). Jumping frequencies were tallied for the next 15 min and are presented as strain mean values. Except for 129P3 mice subject to acute heroin treatment (n = 6), each strain was comprised of a minimum of 8 subjects per each treatment condition. (-) Indicates no response; * indicates a significant difference relative to within-strain controls.
Narrow-sense heritability of acute and chronic heroin dependence was estimated as h2 = 0.98 and h2 = 0.96, respectively. These estimates are likely biased upward because of the extreme outlier status of the SWR strain. Even if this strain is excluded, however, heritability estimates remain high, at h2 = 0.96 and 0.94, respectively.
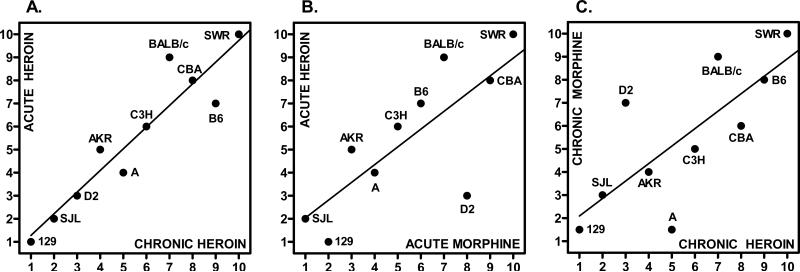
The scatterplot matrix in Figure 2 shows the regression of strain ranks for mean jumping frequencies after acute and chronic heroin and morphine treatment. The visual impressions of a correlation between responses are statistically confirmed by the rs correlation coefficients provided in Table 1. All pairwise correlation coefficients were significant after Bonferroni correction for multiple comparisons, and indicate a high degree of genetic correlation.
Figure 2. Genetic correlation of acute and chronic heroin and morphine dependence.
Symbols represent 10 inbred mouse strains arranged by their rank order of naloxone-precipitated withdrawal jumping frequency (1, lowest frequency; 10, highest frequency). Except for 129P3 mice subject to acute heroin treatment (n = 6), each strain was comprised of a minimum of 8 subjects per each treatment condition. As indicated by the labels appearing on the inside of the x and y axis, data are for the correlation between acute and chronic heroin injection (A), acute heroin and acute morphine injection (B), and between chronic morphine and chronic heroin injection (C). The corresponding Spearman rank coefficients (rs = 0.75-0.94; Table 1) indicate a significant correlation for each pairwise comparison. Some strain names are abbreviated as follows: 129, 129P3; B6, C57BL/6; C3H, C3H/He; D2, DBA/2.
Table 1.
Spearman's rank correlation coefficients comparing the mean jumping frequencies of acute and chronic heroin dependence, acute heroin and morphine dependence and chronic heroin and morphine dependence in inbred strain of mice.
| Acute Heroin | Chronic Heroin | |
|---|---|---|
| Chronic Heroin | 0.94** | -- |
| Acute Morphine | 0.77* | -- |
| Chronic Morphine | -- | 0.75* |
Mice in the Acute Heroin group were injected once (50 mg/kg) whereas those in the Chronic Heroin group were injected t.i.d. for three days (5, 10, and 20 mg/kg on Days 1, 2, and 3, respectively) and received a final single injection (20 mg/kg) on Day 4. Withdrawal in both cases was elicited by a single naloxone injection (50 mg/kg) at the completion of heroin treatment. Morphine data were obtained from Kest et al. (2002). All correlation coefficients were significant
P < 0.05
P< 0.01
after Bonferroni correction for multiple comparisons.
4. Discussion
4.1 Genetic variability
The present study surveyed NPW jumping frequencies in ten inbred mouse strains after acute and chronic heroin treatment. In mice, jumping frequency is the most widely used measure of opioid withdrawal and is a sensitive and reliable index of heroin and morphine withdrawal magnitude (El-Kadi and Sharif, 1994; Kest et al., 2001; Klein et al., 2007; Saelens et al., 1971; Smits, 1975). All strains considered, jumping frequency was greater following chronic relative to acute heroin administration, consistent with previous quantitative comparisons of opioid dependence after acute and chronic opioid treatment in various species including humans (Bickel et al., 1988; Eisenberg, 1982; Heishman et al., 1989; Jasinski, 1977; Kest et al., 2001; Martin and Eades, 1964). More relevant to the aims of this study, the data revealed an array of jumping frequency values and yielded substantial h2 estimates (acute: 0.96; chronic: 0.94) even when an extreme responding strain (i.e., SWR) was excluded from analysis. Since all the mice in the present study were subject to identical handling/treatment protocols, the data demonstrate that the magnitude of acute and chronic heroin dependence is associated with genetic variability and is subject to substantial heritability. Identical conclusions have been reported with regards to morphine dependence in mice (Kest et al., 2002). The actual genes contributing to the strain differences in heroin dependence may be any one or more of those whose proteins regulate the pharmacodynamic (opioid receptors and their intracellular messengers) and/or pharmacokinetic (enzymes that regulate heroin metabolism) effects of heroin, as well as those genes that specifically underlie opioid dependence. Furthermore, the genetic basis for these strain differences will almost certainly vary with the two particular strains being compared. That is, it is highly unlikely that a single mechanism will completely account for the distribution of strain mean jumping frequencies reported here. Therefore, even if the background information of every strain tested here were at hand, it would be beyond the scope of the present report (and likely erroneous) to speculate which possible substrates underlie frequency differences for each pairwise comparison. The identity of genes contributing to heroin dependence and withdrawal symptoms thus remain to be uncovered by subsequent QTL mapping. We are however intrigued by the finding that whereas heroin analgesia in some strains is mediated by the μ-opiate receptor subtype, it is mediated by the δ-opiate receptor subtype in others (Rady et al., 1991, 1994, 2000). These data indicate that genetic factors contribute to the opioid receptor selectivity of heroin in mice. Since dependence caused by μ-opiate receptor activity is more severe than that caused by δ-opiate receptor activity (Cowan et al., 1988; Maldonado et al., 1990), it is possible that differences in withdrawal magnitude between some strains may result from differences in heroin opiate receptor type selectivity. This is an interesting possibility that warrants study.
4.2 Genetic correlations
By using a sufficient number of randomly-chosen strains, the present study design should provide a valid estimate of the genetic correlation of acute and chronic dependence traits (Hegmann and Possidente, 1981). To ensure that correlation estimates were not unduly influenced by extreme scoring strains such as 129P3 and SWR, values subject to correlation were analyzed using Spearman's rank statistic. Based on these considerations, the covariation of naloxone-precipitated jumping among strains after acute and chronic heroin injection (r = 0.94) suggests the contribution of common genes and common (although not necessarily identical) physiological substrates. To date, we have indeed found a highly similar, but not identical, contribution of various opioid and excitatory amino acid receptors to heroin dependence (Klein et al., 2007). The remarkably high degree of genetic correlation between acute and chronic heroin dependence provides for the following additional implications. First, it attests to the resiliency of the putative mechanistic relationship between acute and chronic heroin dependence despite the inherent differences in treatment duration (single injection vs. 3 daily injections for three days, respectively) and cumulative heroin dosing (50 mg/kg and 125 mg/kg, respectively). Second, it engenders confidence in our assessment of the inter-strain variability in dependence after chronic heroin injection since the acute heroin treatment protocol is not exposed to the potentially confounding contribution of strain differences in contextual learning which has clearly been shown to affect dependence liability (Azorlosa et al., 1994; Deffner-Rappold et al., 1996). Third and lastly, the obtained significant correlation implies that NPW jumping frequencies after a single heroin exposure very likely reflect the development of physical dependence and are not artifactual motor responses (Ritzmann, 1981).
The data also reveal a strong genetic correlation between acute heroin and acute morphine dependence, and between chronic heroin and chronic morphine dependence. Heroin is rapidly converted to 6-monoacetylmorphine (6-MAM) and then morphine in vivo (Inturrisi et al., 1983). Thus, it is possible that the impressive strain rank order correlation coefficients for withdrawal jumping frequencies after heroin and morphine treatment actually resulted from morphine activity in each case. A role for morphine in dependence caused by chronic heroin injection is an intriguing possibility since withdrawal is not precipitated soon after heroin administration and there is ample time over the several treatment days for the conversion of heroin to morphine and an increase in central morphine levels. This supposition must however be tempered with the caveat that, despite the conversion of heroin to morphine, the effects of chronic heroin treatment are not simply mediated by morphine. For example, chronic administration of these two opioids cause different neurochemical changes in brain μ-opiate receptor function (Bolger et al., 1988) and opioid dependence that is differentially regulated by hippocampal function (Bao et al., 2007) and δ-opiate receptors (Klein et al., 2007; Suzuki et al., 1997). The possibility that morphine mediates dependence after acute systemic heroin injection is similarly tenuous since the heroin-naloxone interval utilized (2 h) may not be of sufficient duration for morphine levels to appreciably accumulate. Specifically, 6-MAM levels are significantly increased while morphine levels are still relatively low during the first hour after heroin injection (Way et al., 1960, 1965). By the time morphine levels are increased relative to 6-MAM heroin injection 2 h after heroin injection, mice are already undergoing NPW. Furthermore, while is not known how, or even if, 6-MAM induces dependence, it is interesting to note that the pharmacological profile of 6-MAM in the production of analgesia is distinct from that of morphine but identical to that of heroin (Brown et al., 1997; Rady et al., 1994, 1991, 2000; Schuller et al., 1999). Further work is needed to address the possible contribution of 6-MAM and morphine to dependence following acute and chronic heroin treatment. Nevertheless, the present data suggest that heroin and morphine dependence liability are genetically identical. Collectively, the present data correlation data indicate that the genetic liability to heroin dependence is independent of the magnitude or duration of heroin exposure, and that the same treatment strategy could be equally effective throughout the course of heroin intake. Furthermore, the impressive genetic association between heroin and morphine dependence suggests that highly similar treatments protocols might be effective in both morphine and heroin dependence. Indeed, selective blockade of δ2 opioid receptors as well as the NMDA and AMPA subtypes of excitatory amino acid receptors attenuates NPW jumping frequencies in mice rendered opioid dependent after both acute and chronic treatment with heroin or morphine (Kest et al., 1996; Klein et al., 2007; Suzuki et al., 1997; McLemore et al., 1997, Trujillo and Akil, 1991).
4.3 Dependence- resistant and sensitive strains
The present data identifies 129P3 mice as being absolutely refractory to jumping during heroin withdrawal. As noted above, only jumping frequency alone among withdrawal measures has been shown to have a positive dose-response relationship with withdrawal from opioids (El-Kadi and Sharif, 1994; Kest et al., 2001; Marshall and Weinstock, 1971; Smits, 1975) including heroin (Klein et al., 2007). Although 129P3 mice may thus be regarded as a genetic model of insensitivity to opioid dependence, it is certainly possible that evidence of dependence in this strain will be manifest when monitoring other NPW symptoms. In contrast to 129P3 mice, SWR mice were much more sensitive to NPW from heroin than any other strain, with jumping frequencies ~3-fold greater than the strain with next highest tally after either acute or chronic heroin injection. Since this is the first report of heroin withdrawal in these two strains, we are unable to compare and validate the responses obtained here with those of other studies. However, their responses during heroin withdrawal are similar to those observed in a survey of acute and chronic morphine dependence in 11 inbred strains (Kest et al., 2002). Specifically, 129P3 NPW jumping frequencies were also minimal (<7 jumps/15 min) and among the lowest of all other strains, whereas the response frequency in SWR mice were at minimum 2- to 2.5- fold greater than any other strain.
Among inbred strains, the SWR mouse has not been the particular focus of many studies. Consequently, there are few if any descriptions in this strain of physiological systems, particularly those with relevance to dependence, which might differ from other mouse strains that might afford insight into their relatively increased sensitivity to heroin and morphine dependence. We do, however, believe that some comment regarding the dependence resistance of 129P3 mice is possible by referring to neurobiological characterizations of 129S6 mice (previously referred to as 129/SvEv), another 129 substrain. Such a comparison may be of heuristic value since they are highly related (although not identical; Simpson et al., 1997) genetically. There is evidence that 129S6 mice are deficient in GM1 ganglioside-regulated excitatory opioid function (Crain and Shen, 2000). GM1 ganglioside has been shown to block the translocation and activation of protein kinase C from cytosol to neuronal membranes, a biochemical pathway critical in the development of opioid dependence (Crain and Shen, 2000; Mayer et al. 1995). In another study, 129S6 mice were shown to possess deficiencies in the NMDA excitatory amino acid receptor system, and/or the biochemical cascade activating nitric oxide synthase consequent to its activation (Kolesnikov et al. 1998). The importance of the NMDA/ nitric oxide signaling system in dependence to opioids such as morphine (Elliott et al., 1995; Herman et al., 1995) and heroin (Klein et al., 2007) has been clearly demonstrated as well. Further studies will determine if the compromised GM1 ganglioside and NMDA/nitric oxide signaling in the 129S6 mouse contribute to the ability of 129P3 mice to resist heroin dependence. However, even if this signaling deficiency were demonstrated in 129P3 mice and determined to underlie their resistance to heroin dependence, the genetic basis of this deficiency may not be identical to those contributing to the genetic variability between this inbred strain and any other. Those genes may be identified in future gene mapping studies.
Acknowledgements
Supported by the Center for Developmental Neuroscience at the College of Staten Island (AJ, BK), a PSC/CUNY grant (BK), NIDA grant DA001457 (CEI), and NIDA center grant DA005130 (CEI).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Azorlosa JL, Hartley NE, Deffner-Rappold C. Context-specific morphine tolerance and withdrawal: The effects of interdose interval. Psychobiology. 1994;22:304–311. [Google Scholar]
- Bao G, Kang L, Li H, Li Y, Pu L, Xia P, Ma L, Pei G. Morphine and Heroin Differentially Modulate In Vivo Hippocampal LTP in Opiate-Dependent Rat. Neuropsychopharmacology. 2007;32:1738–49. doi: 10.1038/sj.npp.1301308. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Stitzer ML, Liebson IA, Bigelow GE. Acute physical dependence in man: effects of naloxone after brief morphine exposure. J Pharmacol Exp Ther. 1988;244:126–32. [PubMed] [Google Scholar]
- Blasig J, Herz A, Reinhold K, Zieglgansberger S. Development of physical dependence on morphine in respect to time and dosage and quantification of the precipitated withdrawal syndrome in rats. Psychopharmacologia. 1973;33:19–38. doi: 10.1007/BF00428791. [DOI] [PubMed] [Google Scholar]
- Bolger GT, Skolnick P, Rice KC, Weissman BA. Differential regulation of mu-opiate receptors in heroin- and morphine-dependent rats. FEBS Lett. 1988;234:22–6. doi: 10.1016/0014-5793(88)81294-8. [DOI] [PubMed] [Google Scholar]
- Brase DA, Loh HH, Way EL. Comparison of the effects of morphine on locomotor activity, analgesia and primary and protracted physical dependence in six mouse strains. J Pharmacol Exp Ther. 1977;201:368–74. [PubMed] [Google Scholar]
- Brown GP, Yang K, King MA, Rossi GC, Leventhal L, Chang A, Pasternak GP. 3-Methoxynaltrexone, a selective heroin/morphine-6β-glucuronide antagonist. FEBS Lett. 1997;412:35–38. doi: 10.1016/s0014-5793(97)00710-2. [DOI] [PubMed] [Google Scholar]
- Cowan A, Zhu XZ, Mosberg HI, Omnaas JR, Porreca F. Direct dependence studies in rats with agents selective for different types of opioid receptor. J Pharmacol Exp Ther. 1988;246:950–5. [PubMed] [Google Scholar]
- Crain SM, Shen K. Enhanced analgesic potency and reduced tolerance of morphine in 129/SvEv mice: evidence for a deficiency in GM1 ganglioside-regulated excitatory opioid receptor functions. Brain Res. 2000;856:227–35. doi: 10.1016/s0006-8993(99)02446-4. [DOI] [PubMed] [Google Scholar]
- Deffner-Rappold C, Azorlosa JL, Baker JD. Acquisition and extinction of context-specific withdrawal. Psychobiology. 1996;24:219–226. [Google Scholar]
- Eisenberg RM. Further studies on the acute dependence produced by morphine in opiate naive rats. Life Sci. 1982;31:1531–40. doi: 10.1016/0024-3205(82)90043-1. [DOI] [PubMed] [Google Scholar]
- Elliott K, Kest B, Man A, Kao B, Inturrisi CE. N-methyl-D-aspartate (NMDA) receptors, mu and kappa opioid tolerance, and perspectives on new analgesic drug development. Neuropsychopharmacology. 1995;13:347–56. doi: 10.1016/0893-133X(95)00083-P. [DOI] [PubMed] [Google Scholar]
- El-Kadi AO, Sharif SI. The influence of various experimental conditions on the expression of naloxone-induced withdrawal symptoms in mice. Gen Pharmacol. 1994;25:1505–1510. doi: 10.1016/0306-3623(94)90181-3. [DOI] [PubMed] [Google Scholar]
- Gilbert AK, Hosztafi S, Mahurter L, Pasternak GW. Pharmacological characterization of dihydromorphine, 6-acetyldihydromorphine and dihydroheroin analgesia and their differentiation from morphine. Eur J Pharmacol. 2004;492:123–30. doi: 10.1016/j.ejphar.2004.03.050. [DOI] [PubMed] [Google Scholar]
- Han W, Ide S, Sora I, Yamamoto H, Ikeda K. A possible genetic mechanism underlying individual and interstrain differences in opioid actions: focus on the mu opioid receptor gene. Ann N Y Acad Sci. 2004;1025:370–5. doi: 10.1196/annals.1307.045. [DOI] [PubMed] [Google Scholar]
- Hegmann JP, Possidente B. Estimating genetic correlations from inbred strains. Behav Genet. 1981;11:103–14. doi: 10.1007/BF01065621. [DOI] [PubMed] [Google Scholar]
- Heishman SJ, Stitzer ML, Bigelow GE, Liebson IA. Acute opioid physical dependence in humans: effect of varying the morphine-naloxone interval I. J Pharmacol Exp Ther. 1989;250:485–91. [PubMed] [Google Scholar]
- Herman BH, Vocci F, Bridge P. The effects of NMDA receptor antagonists and nitric oxide synthase inhibitors on opioid tolerance and withdrawal. Medication development issues for opiate addiction. Neuropsychopharmacology. 1995;13:269–93. doi: 10.1016/0893-133X(95)00140-9. [DOI] [PubMed] [Google Scholar]
- Hoffmann O, Plesan A, Wiesenfeld-Hallin Z. Genetic differences in morphine sensitivity, tolerance and withdrawal in rats. Brain Res. 1998;806:232–7. doi: 10.1016/s0006-8993(98)00768-9. [DOI] [PubMed] [Google Scholar]
- Ikeda K, Ide S, Han W, Hayashida M, Uhl GR, Sora I. How individual sensitivity to opiates can be predicted by gene analyses. Trends Pharmacol Sci. 2005;26:311–7. doi: 10.1016/j.tips.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Inturrisi CE, Schultz M, Shin S, Umans JG, Angel L, Simon EJ. Evidence from opiate binding studies that heroin acts through its metabolites. Life Sci. 1983;33:773–6. doi: 10.1016/0024-3205(83)90616-1. [DOI] [PubMed] [Google Scholar]
- Jasinski DR. Assessment of the abuse potentiality of morphine like drugs (methods used in man). In: Martin WR, editor. Handbook of Experimental Pharmacology. Springer-Verlag; Berlin: 1977. pp. 197–258. [Google Scholar]
- Kenny PJ, Chen SA, Kitamura O, Markou A, Koob GF. Conditioned withdrawal drives heroin consumption and decreases reward sensitivity. J Neurosci. 2006;26:5894–900. doi: 10.1523/JNEUROSCI.0740-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kest B, Lee CE, McLemore GL, Inturrisi CE. An antisense oligodeoxynucleotide to the delta opioid receptor (DOR-1) inhibits morphine tolerance and acute dependence in mice. Brain Res Bull. 1996;39:185–8. doi: 10.1016/0361-9230(95)02092-6. [DOI] [PubMed] [Google Scholar]
- Kest B, Palmese CA, Hopkins E, Adler M, Juni A, Mogil JS. Naloxone-precipitated withdrawal jumping in 11 inbred mouse strains: evidence for common genetic mechanisms in acute and chronic morphine physical dependence. Neuroscience. 2002;115:463–469. doi: 10.1016/s0306-4522(02)00458-x. [DOI] [PubMed] [Google Scholar]
- Kest B, Palmese C, Hopkins E, Juni A, Adler M. Assessment of acute and chronic morphine dependence in male and female mice. Pharmacol Biochem Behav. 2001;70:149–56. doi: 10.1016/s0091-3057(01)00600-1. [DOI] [PubMed] [Google Scholar]
- Kest B, Palmese CA, Juni A, Chesler EJ, Mogil JS. Mapping of a quantitative trait locus for morphine withdrawal severity. Mamm Genome. 2004;15:610–7. doi: 10.1007/s00335-004-2367-3. [DOI] [PubMed] [Google Scholar]
- Klein G, Juni A, Inturrisi CE, Kest B. Acute and chronic heroin dependence in mice: contribution of opioid and excitatory amino acid receptors. Eur J Pharmacol. 2007 doi: 10.1016/j.ejphar.2008.02.035. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolesnikov Y, Jain S, Wilson R, Pasternak GW. Lack of morphine and enkephalin tolerance in 129/SvEv mice: evidence for a NMDA receptor defect. J Pharmacol Exp Ther. 1998;284:455–9. [PubMed] [Google Scholar]
- Lange DG, Roerig SC, Fujimoto JM, Wang RI. Absence of cross-tolerance to heroin in morphine-tolerant mice. Science. 1980;208:72–4. doi: 10.1126/science.7361110. [DOI] [PubMed] [Google Scholar]
- Liang DY, Guo T, Liao G, Kingery WS, Peltz G, Clark JD. Chronic pain and genetic background interact and influence opioid analgesia, tolerance, and physical dependence. Pain. 2006;121:232–40. doi: 10.1016/j.pain.2005.12.026. [DOI] [PubMed] [Google Scholar]
- Maldonado R, Feger J, Fournie-Zaluski MC, Roques BP. Differences in physical dependence induced by selective mu or delta opioid agonists and by endogenous enkephalins protected by peptidase inhibitors. Brain Res. 1990;520:247–54. doi: 10.1016/0006-8993(90)91712-p. [DOI] [PubMed] [Google Scholar]
- Marshall I, Weinstock M. Quantitative method for assessing one symptom of the withdrawal syndrome in mice after chronic morphine administration. Nature. 1971;234:223–4. doi: 10.1038/234223a0. [DOI] [PubMed] [Google Scholar]
- Martin WR, Eades CG. A comparison between acute and chronic physical dependence in the chronic spinal dog. J Pharmacol Exp Ther. 1964;146:385–94. [PubMed] [Google Scholar]
- Mayer DJ, Mao J, Price DD. The development of morphine tolerance and dependence is associated with translocation of protein kinase C. Pain. 1995;61:365–74. doi: 10.1016/0304-3959(95)00023-L. [DOI] [PubMed] [Google Scholar]
- McLemore GL, Kest B, Inturrisi CE. The effects of LY293558, an AMPA receptor antagonist, on acute and chronic morphine dependence. Brain Res. 1997;778:120–126. doi: 10.1016/s0006-8993(97)00985-2. [DOI] [PubMed] [Google Scholar]
- Miyamoto Y, Takemori AE. Relative involvement of supraspinal and spinal mu opioid receptors in morphine dependence in mice. Life Sci. 1993;52:1039–44. doi: 10.1016/0024-3205(93)90196-a. [DOI] [PubMed] [Google Scholar]
- Pasternak GW. Multiple opiate receptors: déjà vu all over again. Neuropharmacol. 2004;47:312–323. doi: 10.1016/j.neuropharm.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Pouletty P. Drug addictions: towards socially accepted and medically treatable diseases. Nat Rev Drug Discov. 2002;1:731–6. doi: 10.1038/nrd896. [DOI] [PubMed] [Google Scholar]
- Rady JJ, Aksu F, Fujimoto JM. The heroin metabolite, 6-monoacetylmorphine, activates delta opioid receptors to produce antinociception in Swiss-Webster mice. J Pharmacol Exp Ther. 1994;268:1222–31. [PubMed] [Google Scholar]
- Rady JJ, Holmes BB, Portoghese PS, Fujimoto JM. Morphine tolerance in mice changes response of heroin from mu to delta opioid receptors. Proc Soc Exp Biol Med. 2000;224:93–101. doi: 10.1046/j.1525-1373.2000.22406.x. [DOI] [PubMed] [Google Scholar]
- Rady JJ, Roerig SC, Fujimoto JM. Heroin acts on different opioid receptors than morphine in Swiss Webster and ICR mice to produce antinociception. J Pharmacol Exp Ther. 1991;256:448–57. [PubMed] [Google Scholar]
- Ritzmann RF. Opiate dependence following acute injections of morphine and naloxone: the assessment of various withdrawal signs. Pharmacol Biochem Behav. 1981;14:575–7. doi: 10.1016/0091-3057(81)90320-8. [DOI] [PubMed] [Google Scholar]
- Rossi GC, Brown GP, Leventhal L, Pasternak GW. Novel receptor mechanisms for heroin and morphine-6β-glucuronide analgesia. Neurosc Lett. 1996;216:1–4. doi: 10.1016/0304-3940(96)12976-1. [DOI] [PubMed] [Google Scholar]
- Saelens JK, Granat FR, Sawyer WK. The mouse jumping test--a simple screening method to estimate the physical dependence capacity of analgesics. Arch Int Pharmacodyn Ther. 1971;190:213–218. [PubMed] [Google Scholar]
- Schuller AG, King MA, Zhang J, Bolan E, Pan YX, Morgan DJ, Chang A, Czick ME, Unterwald EM, Pasternak GW, Pintar JE. Retention of heroin and morphine–6–glucuronide analgesia in a new line of mice lacking exon 1 of MOR–1. Nat Neurosci. 1999;2:151–156. doi: 10.1038/5706. [DOI] [PubMed] [Google Scholar]
- Selley DE, Cao CC, Sexton T, Schwegel JA, Martin TJ, Childers SR. mu Opioid receptor-mediated G-protein activation by heroin metabolites: evidence for greater efficacy of 6-monoacetylmorphine compared with morphine. Biochem Pharmacol. 2001;62:447–55. doi: 10.1016/s0006-2952(01)00689-x. [DOI] [PubMed] [Google Scholar]
- Simpson EM, Linder CC, Sargent EE, Davisson MT, Mobraaten LE, Sharp JJ. Genetic variation among 129 substrains and its importance for targeted mutagenesis in mice. Nat Genet. 1997;16:19–27. doi: 10.1038/ng0597-19. [DOI] [PubMed] [Google Scholar]
- Smits SE. Quantitation of physical dependence in mice by naloxone-precipitated jumping after a single dose of morphine. Res Commun Chem Pathol Pharmacol. 1975;10:651–61. [PubMed] [Google Scholar]
- Suzuki T, Otani K, Misawa M. Differential sensitivity to physical dependence on morphine and codeine in three inbred strains of mice. Jpn J Pharmacol. 1991;57:455–62. doi: 10.1254/jjp.57.455. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Tsuji M, Mori T, Misawa M, Nagase H. Involvement of delta 1 and delta 2 opioid receptor subtypes in the development of physical dependence on morphine in mice. Pharmacol Biochem Behav. 1997;57:293–299. doi: 10.1016/s0091-3057(96)00319-x. [DOI] [PubMed] [Google Scholar]
- Tarabar AF, Nelson LS. The resurgence and abuse of heroin by children in the United States. Curr Opin Pediatr. 2003;15:210–5. doi: 10.1097/00008480-200304000-00013. [DOI] [PubMed] [Google Scholar]
- Trujillo KA, Akil H. Inhibition of morphine tolerance and dependence by the NMDA receptor antagonist MK-801. Science. 1991;251:85–7. doi: 10.1126/science.1824728. [DOI] [PubMed] [Google Scholar]
- Watson B, Meng F, Akil H. A chimeric analysis of the opioid receptor domains critical for the binding selectivity of mu opioid ligands. Neurobiol Dis. 1996;3:87–96. doi: 10.1006/nbdi.1996.0009. [DOI] [PubMed] [Google Scholar]
- Way EL, Kemp JW, Young JM, Grassetti DR. The pharmacologic effects of heroin in relationship to its rate of biotransformation. J Pharmacol Exp Ther. 1960;129:144–54. [PubMed] [Google Scholar]
- Way EL, Loh HH, Shen FH. Simultaneous quantitative assessment of morphine tolerance and physical dependence. J Pharmacol Exp Ther. 1969;167:1–8. [PubMed] [Google Scholar]
- Way EL, Young MJ, Kemp JW. Metabolism of Heroin and its Pharmacologic Implications. Bull Narcotics. 1965;17:25–33. [Google Scholar]
- Wiley JN, Downs DA. Naloxone-precipitated jumping in mice pretreated with acute injections of opioids. Life Sci. 1979;25:797–801. doi: 10.1016/0024-3205(79)90525-3. [DOI] [PubMed] [Google Scholar]