Abstract
Objective
Examined cognitive appraisals of interference and tolerance in the prediction of distress and self-reported disability among persons presenting for low vision rehabilitation.
Design
Cross-sectional; correlational and path analyses.
Methods
One-hundred and thirteen patients (mean age, 71 years; 52 men and 61 women) presenting for low vision rehabilitation at a university-based center for low vision rehabilitation participated in an initial clinical vision examination and completed several questionnaires to evaluate cognitive appraisals, emotional distress, and self-reported disability.
Results
Path analyses indicated that greater tolerance was associated with less interference imposed by vision loss. Greater tolerance was also associated with less emotional distress, and symptom severity (visual acuity) was associated with self-reported disability. Cognitive appraisals (tolerance and interference) indirectly influenced self-reported disability through emotional distress.
Conclusions
The data indicate that appraisals of personal ability to tolerate vision loss and the perceived interference of vision loss on goal-directed behavior and expected activities have greater influence on distress and are subsequently predictive of disability in comparison to objective symptoms (visual acuity). Implications for clinical interventions and further research are discussed.
Accumulating evidence indicates that there is considerable variation in the ways persons with visual impairments react and adapt in the wake of vision loss (e.g., Brennan, 2002; Dreer, Elliott, Fletcher, & Swanson, 2005; Schilling & Wahl, 2006; Teitelbaum & Copolillo, 2005). For example, it has been argued that a major loss does not necessarily lead to depression or distress for some (Kleinschmidt, 1999) and yet for others, persistent depression may continue for longer than normally expected (Ciechanowski, Katon, & Russo, 2000). Additional support for the influential role of intrapersonal factors underlying depression associated with vision impairment has also been demonstrated (Tolman, Hill, Kleinschmidt, & Gregg, 2005). In an effort to find explanations for the wide variations in reactions related to adjustment to disability, contemporary models of adjustment and coping have been informative (Dodds, Ferguson, Ng, Flannigan, Hawes, & Yates, 1994; Hayeems, Gellar, Finkelstein, & Faden, 2005).
One of the most widely accepted models, the transactional model of stress appraisal and coping, posits that cognitive appraisals of a stressor and coping strategies are important factors that can have an influence on adaptation outcomes (Lazarus & DeLongis, 1983). Individuals presumably appraise a stressful situation for threats to their well-being (primary appraisal) and to assess if their behavior can have an impact on the stressor (secondary appraisal). Thus, the central feature of this model is the subjective, cognitive appraisal of the situation-specific event (rather than an objectively defined stressor, i.e. severity of vision loss) and one’s coping ability; both of which are considered critical to successfully managing a stressful situation (Folkman & Lazarus, 1985; Oliver & Brough, 2002). Thus, from this viewpoint, stress is the culmination of the dynamic interplay between the individual and the environment.
Converging evidence indicates that phenomenological appraisals of stress, personal resources, and subjective meaning are predictive of personal adjustment following chronic disease and disability (Elliott & Harkins, 1991; Elliott & Harkins, 1992; Elliott, Kurylo, & Rivera, 2002; Harkins, Elliott, & Wan, 2006). Generally, this work has examined appraisal processes relevant to various health conditions that yield considerable information beyond the concepts of primary and secondary appraisals (Ptacek & Pierce, 2003). Chronic health conditions can disrupt self-regulatory behavior and outcome expectancies, and these disruptions can trigger a variety of affective responses (Orbell, Hagger, Val Brown, & Tidy, 2004; Williamson, 1998). These key findings are consistent with investigations into goal-directed routine behavior that is interrupted or nonrewarded for overlearned responses (Averill, 1982, 1983; Berkowitz, 1989) based on the notion that individuals utilize cognitive schema comprised of expected behaviors, goals, rewards, and reinforcers in ongoing interactions with the environment. Therefore, when events occur that are incongruent with these expectations, a person will automatically appraise the degree to which the event interferes with goal-directed behaviors and previously anticipated outcomes.
Individuals appraise the extent to which specific health conditions may interfere with their expected behaviors and goal-directed pursuits. They then appraise the degree to which they may be able to tolerate disruption to expected behaviors and goal-directed pursuits (Elliott & Harkins, 1992; Harkins et al., 2006). Individuals differ, however, in their perceived ability to tolerate disruptions to expected behaviors and goal-directed pursuits imposed by the stress between their personal needs and environmental reinforcers (Dawis & Lofquist, 1984). This may involve efforts to exert control over internal emotional states upon realizing that behavioral control over specific events or symptoms may not be possible (Heckenhausen & Schulz, 1995).
Cognitive appraisals have substantial effects on emotional distress associated with a chronic disease, and this association can be stronger than the effects on distress attributable to disease symptom severity (Harkins et al., 2006). However, we do not know the degree to which these cognitive appraisals predict functional disability, as assessed on self-report measures frequently used in clinical research protocols. It is possible that these reports may be directly influenced by the degree to which sight loss interferes with goal-directed pursuits (i.e., interference appraisals), as one’s appraisals may be based in part on the disruptions in daily activities that an individual experiences from the condition. We do not know if the relation of interference to vision related self-reported disability would remain significant once objective-indicators of symptom severity are taken into account. It is also possible that the degree to which an individual can tolerant these disruptions may contribute to the prediction of vision related disability, independent of the variance attributable to perceived interference (e.g., Harkins et al., 2006).
The application of these concepts related to the transactional model of adjustment have recently gained increasing attention in the vision literature (Boerner, Reinhardt, & Horowitz, 2000; Jackson & Taylor, 2000; Ringering & Amaral, 2000). However, in comparison to studies examining coping responses (i.e., Boerner, 2004; Brennan, Horowitz, Reinhardt, Cimarolli, Benn, & Leonard, 2001; Ryan, Anas, Beamer, & Bajorek, 2003), only a few empirical investigations of a person’s appraisal of the condition of vision loss or its concomitants have been documented in the vision literature. For instance, Rovner and Casten (2002) found that the relationship between visual acuity and depression is mediated by the loss of valued, discretionary activities. From our perspective, personal reactions are directly determined by the degree to which vision loss interferes with desired pursuits, and with the degree to which an individual can tolerate these disruptions (Harkins et al., 2006).
In the current study, we were interested in further examining the relation of appraisal processes and an objective indicator of symptom severity (i.e., visual acuity) to both vision related self-reported disability and emotional distress reported by persons presenting to low vision rehabilitation services. A significant minority of persons with vision loss experience problems with depression and distress (Casten, Rovner, & Edmonds, 2002; Horowitz & Reinhardt, 2000; Scott, Schein, Feuer, Folstein, & Bandeen-Roche, 2001), and distress is associated with impaired activities of daily living (Heyl & Wahl, 2001; Ryan et al., 2003). We argue that emotional distress associated with vision loss is directly influenced by cognitive appraisals of the loss. In contrast to other studies that have relied upon self-reported measures of the stress, we used an objective index to quantify actual dysfunction (e.g., visual acuity). We hypothesized that reports of greater interference from changes in vision on expected behavior would be significantly predictive of greater emotional distress and vision related self-reported disability, above and beyond objective indicators of vision impairment (e.g., visual acuity). We predicted that distress associated with vision loss would be determined more by subjective, cognitive appraisals than by objective visual impairment severity. Based on previous research (Harkins et al., 2006), we expected that greater tolerance would contribute to the prediction of lower distress; however, we were uncertain if tolerance would be associated with self-reported disability.
Methods
Participants
Participants completed information as part of their initial clinical evaluation in a university-based low vision rehabilitation clinic. The sample included 52 men (M age = 71.23, SD = 14.93) and 61 women (M age = 70.49, SD = 18.13) who participated in a low vision evaluation upon admission to an outpatient low vision rehabilitation program. Participants’ better eye distance visual acuity logMAR scores ranged from .0 to 1.6 (SD = .82) and 8 participants’ visual acuity was documented as “hand motion only” or “no light perception”. The sample averaged 13 years of formal education (SD = 2.92). Eight percent of the participants were employed (N = 9); 71.5% were retired (N = 80); 11.5% were disabled (N = 13); 3.5% were unemployed (N = 4); and 6.2% were missing data (N = 7). Twenty-seven percent were widowed (N = 31); 5% were divorced (N = 6); 50% were married (N = 56); 1% was separated (N = 1); 16% were single (N = 18); and 1% was missing data (N = 1). Macular degeneration was the most frequent primary referring diagnosis for the sample (57.5%, N = 65); approximately 4.4% (N = 5) were diagnosed with diabetic retinopathy as a primary diagnosis; 4.4% (N = 5) were diagnosed with glaucoma; 2.7% (N = 3) were diagnosed with cataracts; 27.4% (N = 31) were diagnosed with other various vision impairments (e.g., Stargardt disease, etc.); and 3.5% (N = 4) were given a diagnosis of vision impairment of unknown origin upon intake. One-hundred and two persons were Caucasian and 11 persons were African American.
A version of the original National Eye Institute-Visual Function Questionnaire (NEI-VFQ: Mangione et al., 1998) was mailed to each participant prior to their appointment date. Although participants were instructed to complete this measure prior to their appointment, some participants arrived with incomplete questionnaires and subsequently completed the modified version of the NEI-VFQ in the clinic. The majority of participants completed the questionnaire with the assistance of a family member. Following participants’ initial eye examinations, a doctoral level clinical psychologist met individually with participants and read all instructions and administered measures.
Measures
Cognitive Appraisal Variables
Two separate tactile analogue scales (TAS) (Dreer et al., 2005) were employed to evaluate appraisals of interference and tolerance. Each TAS was presented in the form of a 10mL syringe without an attached needle. The measure of tolerance was assessed by asking participants, “How difficult is it for you to tolerate your changes in vision?” The appraisal was anchored by the phrase “no difficulty” = 0 and at the other end “greatest difficulty imaginable” = 100, with responses reversed scored so that lower scores indicate greater difficulty tolerating vision changes. Participants rated the degree of interference with daily activities on an item that was phrased, “How much do your changes in vision prevent or interfere with what you want to do?” This scale was anchored at opposite ends by descriptors “no interference” = 0 and “complete interference” = 100. Higher scores indicate perceptions of greater interference. The TASs used in this study were adapted from visual analogue scales (VAS) that have been successfully used in previous studies of interference and tolerance appraisals of pain (Elliott & Harkins, 1992; Harkins, Price, & Braith., 1989), chronic stress (Elliott, Chartrand & Harkins, 1994), and urinary incontinence (Harkins et al., 2006). Although these appraisals are predictably associated with measure of depression and trait measures of negative affectivity, they maintain significantly and independently contribute to the prediction of distress reported by community-residing adults (Elliott, et al., 1994; Harkins, et al., 2006).
Vision Symptom Severity
Visual acuity was obtained by using the Early Treatment Diabetic Retinopathy Study charts (ETDRS). ETDRS ratings were then converted to the logMAR scale (Bailey & Lovie, 1976). For the purposes of this study, we used the logMAR visual acuity score for the better eye. Higher scores for visual acuity indicate greater vision impairment.
Emotional Distress
TASs were used to evaluate negative emotions specifically attributed to vision loss (Dreer et al., 2005). These scales were concerned with feelings which might be expected to be evoked by vision loss or by thinking about the condition; these included anger, fear, depression, anxiety, and frustration. Each TAS was presented in the form of a 10mL syringe without an attached needle. Each emotion TAS was anchored by the verbal descriptors “none at all” = 0 and “the most severe imaginable” = 100. Participants were instructed to indicate the extent of depression, anger, fear, frustration, and anxiety, by pulling the syringe tube between the anchors. Higher scores on each scale indicate greater negative affect. The five items were averaged to provide a single indicator of distress.
Internal consistency for the averaged index score of emotional distress associated with vision loss was acceptable (α = .77; N = 92). Similar alphas have been observed for a variation of this instrument (visual analogue scales; VAS) in research with older clinical samples (.89) (Harkins et al., 2006), undergraduate students (.70, .71) (Elliott, Sherwin, Harkins, & Marmarosh, 1995), and undergraduate women (.86) (Elliott & Harkins, 1992). Higher VAS emotional distress scores are positively and significantly related to negative affectivity (Harkins et al., 1989). These studies support the validity of an emotional distress average score as a meaningful measure of domain-specific distress associated with a health problem (Harkins et al., 1989).
To evaluate the validity of the syringe analogue measurement of these dimensions, a subsample of consecutively referred participants (N = 13) completed a second administration by using a large emotional “thermometer” developed for the clinic and this study. The thermometer was made with poster board and divided into ten equal intervals from 0 to 10 (most severe imaginable). All participants were able to correctly identify the figure as a thermometer with numbers. Participants were given the same instructions and asked to indicate on the thermometer the intensity of each emotion. Participants either pointed directly on the thermometer to denote their response or verbally gave a number to the examiner. The correlation between the average score from the syringe administration and the thermometer indicated a moderately high degree of reliability (r = .88, p < .001).
Vision Related Self-Reported Disability
An abbreviated version of the National Eye Institute-Visual Function Questionnaire (NEI-VFQ) (Mangione et al., 1998) was used to evaluate the impact of visual impairment on daily functioning. The scale was shortened by the fourth author (DF) to contain items that were considered clinically important for the vision rehabilitation team to develop individualized rehabilitation programs. This reduced the measure to 23 of the 51 original NEI-VFQ items (Massof & Fletcher, 2001) and 5 of the original 13 domains: Near Vision (7 items), Distance Vision (7 items), Social Functioning (3 items), Dependency (4 items), and Mental Health (2 items). Responses for 17 of the items include the following range: 1 = “no difficulty at all”, 2 = “a little difficulty”, 3 = “moderate difficulty”, 4 = “extreme difficulty”, 5 = “stopped doing this because of your eyesight”, and 6 = “stopped doing this for other reasons or not interested in doing this.” The other 10 items require frequency or level of agreement ratings. Similar to the NEI-VFQ, this version is scored by linearly transforming a respondent’s ratings for each item to values that range from 0 to 100. Scores are combined to produce a total impairment score (Parrish et al., 1997), with higher scores indicating greater vision-specific disability. For this study, the sum of the 23-items was used as a total vision related disability score. The Cronbach’s alpha coefficient for the measure was .94 and the corrected item-total correlations ranged from .44 to .79.
Statistical Analysis
Path analysis was used to evaluate the hypothesized relationships between the predictor variables (i.e., cognitive appraisals, vision symptom severity variables, emotional distress) and vision related self-reported disability as the main outcome or endogenous variable. Path analysis permits the examination of the indirect and direct relationships among the variables. The model evaluated the specific hypotheses concerning the causal relations of vision symptom severity (i.e., visual acuity), cognitive appraisals (i.e., tolerance and interference), and emotional distress on vision related self-reported disability (Figure 1). Path analysis permits the evaluation of how well the collected data fit the hypothesized model (Byrne, 1989). The Comparative Fit Index (CFI) (Bentler, 1990) was used to evaluate the model. The CFI ranges from 0 to 1, with a fit index greater than .90 indicating good fit between the collected data and proposed model (Hu & Bentler, 1999). The amount of error variance in the model is reflected in the root mean square error of approximation (RMSEA); values less than .05 are considered adequate (Browne & Cudeck, 1993).
Figure 1.
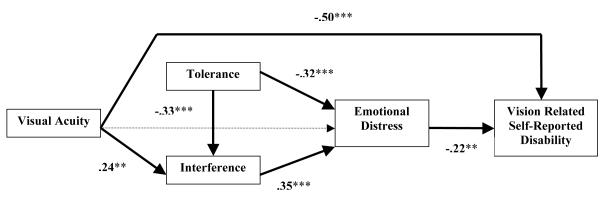
Path Diagram of the Model Tested to Evaluate the Impact of Symptom Severity (Acuity), Appraisals of Tolerance and Interference, and Emotional Distress on Vision Related Self-Reported Disability.
Note. Lower scores on tolerance indicate greater difficulty in the ability to tolerate vision related changes; higher scores for disability reflect fewer problems associated with vision-specific disability; higher scores for visual acuity indicate greater vision impairment.
Results
Table 1 displays the Pearson product-moment correlations among the predictor variables along with the means and standard deviations for each variable. Independent-samples t-tests revealed there were no statistically significant differences for gender on any of the major study variables.
Table 1.
Correlations of Emotional Distress, Cognitive Appraisal, Vision Related Self-Reported Disability, and Visual Acuity.
| Variable | 1 | 2 | 3 | 4 | 5 | M | SD |
|---|---|---|---|---|---|---|---|
| 1. Emotional Distress |
-- | -.44*** | .46*** | -.31** | .14 | 45.4 | 24.7 |
| 2. Tolerance | -- | -.34*** | .11 | -.02 | 58.1 | 27.4 | |
| 3. Interference | -- | -.37*** | .22* | 69.7 | 28.9 | ||
| 4. Disability | -- | -.53*** | 35.5 | 18.9 | |||
| 5. Visual Acuity | -- | 1.03 | .38 |
Note. Lower scores on tolerance indicate greater difficulty in the ability to tolerate vision related changes; higher scores for disability reflect fewer problems associated with vision-specific disability; higher scores for visual acuity indicate greater vision impairment.
p < .05
p < .01
p < .001
To test our full theoretical model of the relationships among distress, appraisal, disability, and vision symptom severity, we used maximum likelihood estimates of the path model shown in Figure 1. The model parameter estimation was implemented with the AMOS (Version 5.0) statistical software package. In this model, the criterion variable was vision related self-reported disability. The model assumes that the criterion is influenced by direct effects of both emotional distress and objective visual acuity. The model also assumes that emotional distress is directly influenced by the appraisal variables (interference and tolerance). Based on the results of Harkins et al (2006) we also included indirect effects of objective vision symptomatology (i.e., visual acuity) and tolerance on distress via the influence of these variables on appraisal of interference. Any missing values were imputed using Maximum Likelihood.
The model proved an acceptable fit to the data, X2(4) = 4.09, p = .39. The Comparative Fitness Index (CFI) was .99, and the RMSEA was .01. Figure 1 presents the standardized parameter estimates for the paths of the model. All parameter estimates were statistically significant at least at the .01 alpha level.
As indicated in Figure 1, both the direct effect of visual acuity (-.50, p < .01) and emotional distress (-.22, p < .01) on self-reported disability were statistically significant. Two-tailed significance tests for indirect effects were computed using bootstrapped standard errors (SE) for standardized effect estimates (based on 1000 samples). All hypothesized indirect effects were statistically significant. The indirect effect of visual acuity on distress (via perceived interference) was .08 (SE =.04, p < .05), and its indirect effect on disability (via interference and emotional distress) was −.02 (SE =.01, p < .05). Thus the total effect of visual acuity on self-reported disability was −.52 (SE =.08, p < .01). Appraisals of how interfering vision loss was on everyday living had a significant indirect effect on disability (via emotional distress) of −.08 (SE =.03, p < .01), and the degree to which an individual was able to tolerate vision loss had a significant indirect effect on disability through interference and emotional distress of .10 (SE = .03, p < .01).
Modifications of this model were assessed for exploratory purposes, but none provided reasonable fit to the data. When visual acuity was assumed to have a direct rather than indirect effect on emotional distress, the standardized path coefficient was .01, ns, the CFI was .58, and the RMSEA was .25. This finding that objective symptomatology had no direct effect on emotional distress is consistent with the results of Harkins et al (2006). When the direct path from visual acuity to vision related self-reported disability was removed from the model, the CFI was .66 and the RMSEA .23, indicating that objective visual acuity has both direct and indirect effects on disability.
Discussion
These results provide evidence for the role of subjective appraisal mechanisms for understanding emotional distress and disability associated with vision loss. In general, our findings illustrate that cognitive appraisals of the degree to which individuals can tolerate vision loss and how interfering vision loss is on everyday living are major factors associated with emotional distress. Distress subsequently predicts vision-related disability independent of actual impairment. Cognitive appraisals of interference and tolerance had significant indirect effects on disability. Severity of vision loss (e.g., acuity) was independently predictive of vision related disability. Thus, severity of vision impairment does not necessarily correspond with emotional distress following vision loss. Rather, other important psychosocial factors such as subjective appraisals may influence emotional reactions to chronic health conditions and may provide a more informative account of a person’s overall adjustment following vision loss.
From a theoretical standpoint, the findings support the component of the primary appraisal process detailed in the transactional model of stress appraisal and coping (Folkman & Moskowitz, 2004; Lazarus & Folkman, 1984). This activity is considered particularly important for understanding coping and adjustment in persons with chronic health conditions, generally (Elliott, Kurylo, & Rivera, 2002). While coping is relevant to understanding an individual’s active strategies in managing stressors, knowledge of the individual meaning of adaptation provides insight into an individual’s reactions to changing conditions and is not restricted to more active self-regulatory responses (Schilling & Whal, 2006). The current data suggest that those who perceive low vision as disruptive to expected behaviors, goals, and outcomes might be expected to have recurring difficulties with distress. Emotional adjustment may be related to the extent to which one cognitively and or behaviorally modifies expectations for daily activities, functional abilities, and desired outcomes (Williamson, 1998).
While the severity of vision loss was unrelated to the prediction of emotional distress this finding has been observed in other studies. An explanation for the lack of relationship may be that many persons with severe physical problems do not necessarily perceive their physical problems as difficult to tolerate or interfering, in other words as the primary source of stress in their life, and those who do are more distressed emotionally in general (Elliott et al., 2002); thus supporting the need for an understanding of the personal meaning of attached to vision loss as they relate to an individual’s previously anticipated goals and expectancies.
It is also important to note that severity of vision loss was unrelated to tolerance appraisals. It may be that other factors are operating to influence this cognitive activity. For example, an individual with significantly impaired visual acuity might not evaluate their situation involving vision loss as difficult to tolerate given the person has a strong social support network, financial status, and supportive work environment. In contrast, another person with the same degree of vision acuity impairment might perceive their situation as greatly difficult to tolerate as they might not have the confidence or resources to adaptively cope. These factors have been found to influence cognitive appraisal activity in the extant literature (Elliott et al., 2002).
The current findings have several important implications for clinical work in low vision rehabilitation. For example, evaluating a person’s appraisal of a chronic health condition (e.g., loss of vision) may be considered critical for identifying those who may be a risk for problems with emotional distress. Unfortunately, this type of assessment is not routinely conducted during low vision rehabilitation eye examinations. While distress may resolve in some persons without intervention, unmanaged and chronic distress may place a person at risk for depression and greater disability independent of vision loss. Thus, cognitive appraisal of interference and tolerance deserve attention during initial clinical eye examinations so that those who may be at risk are identified and referred for appropriate interventions. Administration of brief measures designed to evaluate appraisal processes and condition-specific distress such as the scales used in this study can be efficiently conducted in the clinic setting and quick to administer. Furthermore, the current findings should also be considered when designing rehabilitation programs for individuals. Rehabilitation outcomes might be enhanced when rehabilitation programs are tailored to the personal meaning and interpretation of vision loss on an individual’s expected goals. Eliciting an individual’s personal cognitive appraisals regarding vision loss might be an important initial step in low vision rehabilitation programs prior to working on specific aspects of important rehabilitation services (i.e., occupational therapy, training with devices).
Previous research indicates that cognitive appraisals of stress (Shewchuk, Elliott, MacNair, & Harkins, 1999) and of physical symptoms (Harkins, et al., 2006) are rather stable over time, but there is evidence that these appraisals may be amenable to cognitive-behavioral interventions. For example, interventions such as problem-solving therapy (PST) to change self-appraised problem-solving abilities is an empirically-supported intervention that may be useful in enhancing appraisal activity (Nezu, Felgoise, McClure, & Houts, 2003; Perri et al., 2001). Other studies examining alternative treatment interventions indicate that individuals can be taught to accept difficult thoughts, feelings, and bodily sensations without struggling with them, and focus on overt activities that contribute to important outcomes (i.e., Acceptance and Commitment Therapy; ACT) (Hayes, Strosahl, & Wilson, 1999). Therefore, it appears that some cognitive-behavioral interventions may offer some promise in working with personal appraisals of vision impairment. Generally, these approaches may be useful to: 1) minimize overall distress, 2) increase motivation for participation in rehabilitation efforts, 3) reevaluate goals and develop new goals and expectations, 4) identify alternative ways to tolerate and cope with stress, and 5) increase self-efficacy in regulating emotions. These types of cognitive-behavioral interventions are particularly needed in visually impaired populations (Lueck, 1997).
Several limitations related to this study exist. The sample was relatively homogeneous in age (primarily older adults), race (primarily Caucasian), and socioeconomic status (able to afford low vision rehabilitation services). While this type of sample is representative for our center given our current geographical location, future investigations are needed to determine whether these findings are generalizable to more demographically diverse samples. In addition, we captured persons seeking low vision rehabilitation services who may be distinctly different from those who do not seek services in terms of appraisal processes. Based on the literature demonstrating a relationship between appraisals and depression (i.e., Harkins et al., 2006; Tolman et al., 2005), one can speculate that appraisals may indeed impact motivation to seek low vision rehabilitation services. Further examination of appraisal processes in persons referred for low vision rehabilitation services who do not follow through and attend such services versus those who do not are warranted. Another limitation is related to the cross-sectional nature of the current study design. While a cross-sectional study can establish correlation and association, conclusions regarding causality cannot be determined. Prospective replications of the current findings and longitudinal studies would be beneficial. The study is limited to persons primarily who had central vision loss. Other factors that influence the person-environment dynamic of the transactional theory of coping should also be examined in conjunction with cognitive appraisals (i.e., personality, availability and type of services, social support).
While we attempted to expand upon previous research by using an “objective” measure (i.e., visual acuity), investigations using other visual objective measures should also be further examined (i.e., contrast sensitivity). Investigations focusing on individuals with positive appraisals or resilient attitudes are also needed in order to develop interventions that promote successful adjustment.
References
- Averill J. Anger and aggression: An essay on emotion. Springer-Verlag; New York: 1982. [Google Scholar]
- Averill J. Studies on anger and aggression. American Psychologist. 1983;8:1145–1160. doi: 10.1037//0003-066x.38.11.1145. [DOI] [PubMed] [Google Scholar]
- Bailey I, Lovie J. New design principles for visual acuity letter charts. American Journal of Optometry & Physiological Optics. 1976;53:740–745. doi: 10.1097/00006324-197611000-00006. [DOI] [PubMed] [Google Scholar]
- Bentler P. Comparitive fit indexes in structural models. Psychological Bulletin. 1990;107:238–246. doi: 10.1037/0033-2909.107.2.238. [DOI] [PubMed] [Google Scholar]
- Berkowitz L. Frustration-aggression hypothesis: Examination and reformulation. Psychological Bulletin. 1989;106:59–73. doi: 10.1037/0033-2909.106.1.59. [DOI] [PubMed] [Google Scholar]
- Boerner K. Adaptation to disability among middle-aged and older adults: The role assimilative and accommodative coping. The Journals of Gerontology. 2004;59B:P35–P42. doi: 10.1093/geronb/59.1.p35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boerner K, Reinhardt JP, Horowitz A. The effect of rehabilitation service use on coping patterns over time among older adults with age-related vision loss. Clinical Rehabilitation. 2006;20:478–487. doi: 10.1191/0269215506cr965oa. [DOI] [PubMed] [Google Scholar]
- Brennan M. Spirituality and psychosocial development in middle-age and older adults with vision loss. Journal of Adult Development. 2002;9:31–46. [Google Scholar]
- Brennan M, Horowitz A, Reinhardt JP, Cimarolli V, Benn DT, Leonard R. In their own words: Strategies developed by visually impaired elders to cope with vision loss. Journal of Gerontological Social Work. 2001;35:63–85. [Google Scholar]
- Browne M, Cudeck R. Alternative ways of assessing model fit. In: Bollen K, Long J, editors. Testing structural equation models. Sage; Newbury Park: 1993. pp. 133–162. [Google Scholar]
- Byrne B. A primer of LISEREL: Basic applications and programming for confirmatory factor analytic models. Springer-Verlag; New York: 1989. [Google Scholar]
- Casten R, Rovner BW, Edmonds S. The impact of depression in older adults with age-related macular degeneration. Journal of Visual Impairment and Blindness. 2002;96:399–406. [Google Scholar]
- Ciechanowski PS, Katon WJ, Russo JE. Impact of depressive symptoms on adherence, functions, and costs. Archives of Internal Medicine. 2000;160:3278–3285. doi: 10.1001/archinte.160.21.3278. [DOI] [PubMed] [Google Scholar]
- Dawis R, Lofquist L. A psychological theory of work adjustment. University of Minnesota Press; Minneapolis, MN: 1984. [Google Scholar]
- Dodds A, Ferguson E, Ng L, Flannigan H, Hawes G, Yates L. The concept of adjustment: A structural model. Journal of Visual Impairment and Blindness. 1994;88:487–497. [Google Scholar]
- Dreer LE, Elliott T, Fletcher D, Swanson M. Social problem-solving abilities and psychological adjustment of persons in low vision rehabilitation. Rehabilitation Psychology. 2005;50:232–238. [Google Scholar]
- Elliott T, Chartrand J, Harkins S. Negative affectivity, emotional distress, and the cognitive appraisal of occupational stress. Journal of Vocational Behavior. 1994;45:185–201. [Google Scholar]
- Elliott T, Harkins S. Psychosocial concomitants of persistent pain among persons with spinal cord injury. NeuroRehabilitation: An Interdisciplinary Journal. 1991;1:9–18. [Google Scholar]
- Elliott T, Harkins S. Emotional distress and the perceived interference of menstrual pain. Journal of Psychopathology and Behavioral Assessment. 1992;14:293–306. [Google Scholar]
- Elliott T, Kurylo M, Rivera P. Positive growth following an acquired physical disability. In: Snyder C, Lopez S, editors. Handbook of positive psychology. Oxford University Press; New York: 2002. pp. 687–699. [Google Scholar]
- Elliott T, Sherwin E, Harkins S, Marmarosh C. Self-appraised problem solving ability, affective states, and psychological distress. Journal of Counseling Psychology. 1995;42:105–115. [Google Scholar]
- Folkman S, Lazarus R. If it changes it must be a process: Study of emotion and coping during three stages of college examination. Journal of Personality and Social Psychology. 1985;48:150–170. doi: 10.1037//0022-3514.48.1.150. [DOI] [PubMed] [Google Scholar]
- Folkman S, Moskowitz JT. Coping: Pitfalls and promise. Annual Review of Psychology. 2004;55:745–774. doi: 10.1146/annurev.psych.55.090902.141456. [DOI] [PubMed] [Google Scholar]
- Harkins S, Elliott T, Wan T. Emotional distress and urinary incontinence among older women. Rehabilitation Psychology. 2006;51:346–355. [Google Scholar]
- Harkins S, Price D, Braith J. Effects of extroversion and neuroticism on experimental pain, clinical pain, and illness behavior. Pain. 1989;36:209–218. doi: 10.1016/0304-3959(89)90025-0. [DOI] [PubMed] [Google Scholar]
- Hayeems RZ, Gellar G, Finkelstein D, Faden RR. How patients experience progressive loss of visual function: A model of adjustment using qualitative methods. British Journal of Ophthalmology. 2005;89:615–620. doi: 10.1136/bjo.2003.036046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes SC, Strosahl KD, Wilson KG. Acceptance and commitment therapy: An experiential approach to behavior change. Guilford Press; New York: 1999. [Google Scholar]
- Heckenhausen J, Schulz R. A life-span theory of control. Psychological Review. 1995;102:284–304. doi: 10.1037/0033-295x.102.2.284. [DOI] [PubMed] [Google Scholar]
- Heyl V, Wahl HW. Psychosocial adaptation to age-related vision loss: A six-year perspective. Journal of Visual Impairment and Blindness. 2001;95:739–748. [Google Scholar]
- Horowitz A, Reinhardt J. Mental health issues in vision impairment. In: Silverstone B, Lang M, Rosenthal B, Faye E, editors. The Lighthouse handbook on vision impairment and vision rehabilitation. Vol. 2. Oxford University Press, Inc; New York: 2000. pp. 1089–1109. [Google Scholar]
- Hu LT, Bentler PM. Cut-off criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling. 1999;6:1–55. [Google Scholar]
- Jackson W, Taylor R. Visual impairment. In: Radnitz C, editor. Cognitive-behavioral therapy for persons with disabilities. Jason Aronson, Inc; Northvale, New Jersey: 2000. pp. 159–181. [Google Scholar]
- Kleinschmidt J. Older adults’ perspectives on their successful adjustment to vision loss. Journal of Visual Impairment & Blindness. 1999;93:69–81. [Google Scholar]
- Lazarus R, DeLongis A. Psychological stress and coping in aging. American Psychologist. 1983;38:245–254. doi: 10.1037//0003-066x.38.3.245. [DOI] [PubMed] [Google Scholar]
- Lazarus RS, Folkman S. Stress, appraisal, and coping. Springer; NY: 1984. [Google Scholar]
- Lueck A. The role of education and rehabilitation specialists in the comprehensive low vision care process. Journal of Visual Impairment and Blindness. 1997;91:423–434. [Google Scholar]
- Mangione CM, Lee P, Pitts J, Gutierrez P, Berry S, Hays RD. Psychometric properties of the National Eye Institute Visual Function Questionnaire (NEI-VFQ) Archives of Ophthalmology. 1998;116:1496–1504. doi: 10.1001/archopht.116.11.1496. [DOI] [PubMed] [Google Scholar]
- Massof R, Fletcher D. Evaluation of the NEI visual functioning questionnaire as an interval measure of visual ability in low vision. Vision Research. 2001;41:397–413. doi: 10.1016/s0042-6989(00)00249-2. [DOI] [PubMed] [Google Scholar]
- Nezu A, Felgoise S, McClure K, Houts P. Project Genesis: Assessing the efficacy of problem-solving therapy for distressed adult cancer patients. Journal of Consulting and Clinical Psychology. 2003;71:1036–1048. doi: 10.1037/0022-006X.71.6.1036. [DOI] [PubMed] [Google Scholar]
- Oliver J, Brough P. Cognitive appraisal, negative affectivity and psychological well-being. New Zealand Journal of Psychology. 2002;31:2–7. [Google Scholar]
- Orbell S, Hagger M, Brown V, Tidy J. Appraisal theory and emotional sequelae of first visit to colposcopy following an abnormal cervical screening result. British Journal of Health Psychology. 2004;9:533–555. doi: 10.1348/1359107042304560. [DOI] [PubMed] [Google Scholar]
- Parrish R, Gedde S, Scott I, Feuer W, Schiffman J, Mangione C. Visual function and quality of life among patients with glaucoma. Archives of Ophthalmology. 1997;115:1447–1455. doi: 10.1001/archopht.1997.01100160617016. [DOI] [PubMed] [Google Scholar]
- Perri M, Nezu A, McKelvey W, Shermer R, Renjilian D, Viegener B. Relapse prevention training and problem-solving therapy in the long-term management of obesity. Journal of Consulting and Clinical Psychology. 2001;69:722–726. [PubMed] [Google Scholar]
- Ptacek J, Pierce G. Issues in the study of stress and coping in rehabilitation settings. Rehabilitation Psychology. 2003;48:113–124. [Google Scholar]
- Ringering L, Amaral P. The role of psychosocial factors in adaptation to vision impairment and rehabilitation outcomes for adults and older adults. In: Silverstone B, Lange M, Rosenthal B, Faye E, editors. The Lighthouse handbook on vision impairment and vision rehabilitation. Vol. 1. Oxford University Press; New York: 2000. pp. 1029–1048. [Google Scholar]
- Rovner B, Casten R. Activity loss and depression in age-related macular degeneration. American Journal of Geriatric Psychiatry. 2002;10:305–310. [PubMed] [Google Scholar]
- Ryan E, Anas A, Beamer M, Bajorek S. Coping with age-related vision loss in everyday reading activities. Educational Gerontology. 2003;29:37–54. [Google Scholar]
- Schilling OK, Wahl HW. Modeling late-life adaptation in affective well-being under a severe chronic health condition: The case of age-related macular degeneration. Psychology and Aging. 2006;21:703–714. doi: 10.1037/0882-7974.21.4.703. [DOI] [PubMed] [Google Scholar]
- Scott I, Schein O, Feuer W, Folstein M, Bandeen-Roche K. Emotional distress in patients with retinal disease. American Journal of Ophthalmology. 2001;131:584–589. doi: 10.1016/s0002-9394(01)00832-7. [DOI] [PubMed] [Google Scholar]
- Shewchuk R, Elliott T, MacNair RR, Harkins S. Trait influences on stress appraisal and coping: An evaluation of alternative frameworks. Journal of Applied Social Psychology. 1999;29:685–704. [Google Scholar]
- Teitelbaum J, Copolillo A. Psychosocial issues in older adults’ adjustment to vision loss: Findings from qualitative interviews and focus groups. American Journal of Occupational Therapy. 2005;59:409–417. doi: 10.5014/ajot.59.4.409. [DOI] [PubMed] [Google Scholar]
- Tolman J, Hill RD, Kleinschmidt JJ, Gregg CH. Psychosocial adaptation to visual impairment and its relationship to depressive affect in older adults with age-related macular degeneration. The Gerontologist. 2005;45:747–753. doi: 10.1093/geront/45.6.747. [DOI] [PubMed] [Google Scholar]
- Williamson GM. The central role of restricted normal activities in adjustment to illness and disability: A model of depressed affect. Rehabilitation Psychology. 1998;43:327–347. [Google Scholar]


