Abstract
Protein palmitoylation, by modulating the dynamic interaction between protein and cellular membrane, is involved in a wide range of biological processes, including protein trafficking, sorting, sub-membrane partitioning, protein-protein interaction and cell signaling. To explore the role of protein palmitoylation in adipocytes, we have performed proteomic analysis of palmitoylated proteins in adipose tissue and 3T3-L1 adipocytes and identified more than 800 putative palmitoylated proteins. These include various transporters, enzymes required for lipid and glucose metabolism, regulators of protein trafficking and signaling molecules. Of note, key proteins involved in membrane translocation of the glucose-transporter Glut4 including IRAP, Munc18c, AS160 and Glut4, and signaling proteins in the JAK-STAT pathway including JAK1 and 2, STAT1, 3 and 5A and SHP2 in JAK-STAT, were palmitoylated in cultured adipocytes and primary adipose tissue. Further characterization showed that palmitoylation of Glut4 and IRAP was altered in obesity, and palmitoylation of JAK1 played a regulatory role in JAK1 intracellular localization. Overall, our studies provide evidence to suggest a novel and potentially regulatory role for protein palmitoylation in adipocyte function.
Keywords: palmitoylation, adipocyte, insulin, Glut4, JAK, STAT
Introduction
Protein S-acylation is a post-translational lipid modification through which a fatty acid moiety is attached onto the cysteine residues.1 Since protein-S-acylation is almost exclusively through the attachment of palmitic acid, a 16-carbon saturated fatty acid to the cysteine residues, protein S-acylation is generally referred as protein S-palmitoylation, or simply palmitoylation. Lipid modification equips the protein with a strong hydrophobic moiety serving as an anchor to facilitate interaction of the modified protein with cellular membranes.2,3 In eukaryotes, the interaction between protein and membrane is directly involved in protein trafficking, sorting, subcellular domain partitioning, protein-protein interaction and cell signaling. Thus, by modulating the interaction between protein and membrane, lipid modification of proteins is likely to play a role in cellular function. Three types of protein lipid modification exist in eukaryotes including myristoylation, isopenylation/farnesylation and palmitoylation.4 Among these, palmitoylation is the most common and the only one that is reversible.5 Correspondingly, protein palmitoylation is considered as the prevalent lipid modification that can mediate a dynamic interaction between protein and cellular membrane and, thereby, subcellular trafficking and cell signaling (for a review, see ref. 6).
Adipose tissue is an energy reservoir and an active endocrine organ. As an energy reservoir, adipose tissue actively transports glucose and fatty acids from blood for storage as lipids. Glucose transport into the adipocyte is mediated by insulin-responsive Glut4 membrane translocation and is essential for the regulation of blood glucose levels.7 Both clinical and animal model studies have demonstrated that impaired Glut4 membrane translocation represents a primary defect of insulin action in type II diabetic individuals.8 As an endocrine organ, adipose tissues secrete many different adipokines,9 which modulate peripheral insulin sensitivity.10,11 Adipose tissue also includes other cell types including preadipocytes, immune infiltrating cells and endothelial cells. Adipokines, such as leptin, and other paracrine secretory products, including IL-6, LIF, IFN-γ and PRL, actively contribute to the functionality of adipocytes, mainly by activating the JAK-STAT pathway, to mediate downstream effects via STA1, STAT3 or STAT5 (for a review, see ref. 12).
Glut4 membrane translocation, adipokine signaling and lipid production in adipocytes all require protein trafficking and sorting, leading us to hypothesize that protein palmitoylation may play an essential role in these processes. At present, the knowledge regarding protein palmitoylation in adipocyte is very limited. To begin to explore the role of protein palmitoylation in adipocytes, we have performed a proteomic analysis of adipocyte S-acylated proteins in adipose tissue and 3T3-L1 adipocytes and isolated more than 800 putative palmitoylated proteins. Overall, our results argue that protein palmitoylation is involved in a wide range of adipocyte activities, including Glut4 membrane trafficking and JAK-STAT signaling, which modulates insulin signaling and adipocyte differentiation.12
Results
Thiopropyl captivation (TPC) of S-acylated protein assay
To analyze the palmitoylated proteins, we used the thiopropyl captivation (TPC) of S-acylated proteins derived from RAC assay.13 This protocol is outlined in Figure 1A. Briefly, total cell lysates or cellular fractions were first incubated with methylmethanethiosulfonate (MMTS) to block free cysteine residues. Next, the proteins were precipitated with acetone and resuspended into a binding buffer supplemented with hydroxylamine and thiopropyl sepharose. In this step, Hydroxylamine hydrolyzes thioester bonds to free cysteine residues from acylation, which is promptly captured by thiopropyl sepharose through formation of a disulfide bond between newly freed cysteine residues and thiopropyl group. Once the proteins were captured onto thiopropyl sepharose, the unbound proteins were removed and the bound proteins were released for further analysis (e.g., western blot or mass spectrometry).
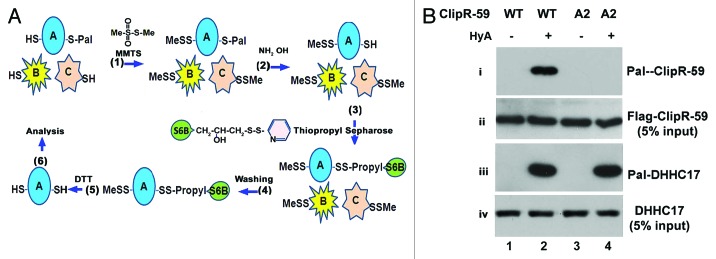
Figure 1. A thiopropyl capture S-acylation protein assay. (A) (1) The cell lysates, or factions, in a blocking buffer are incubated with MMTS (0.1%) to block free cysteine residues and precipitated with 70% acetone. (2–3) The precipitated proteins are resuspended into a binding buffer that consists of hydroxylamine (HyA) or NaCl (served as a control) and thiopropyl sepharose 6M and incubated for 2–4 h (a 10% of reaction mixture is removed as the input at this step). (4) The beads are washed to remove no-binding proteins. (5) The beads are treated with DTT to release the protein from thiopropyl sepharose 6M. (6) The purified proteins are ready for further analysis, i.e., western blot, proteosomics. SH, free cysteine; Pal-S, palmitoylated cysteine residue; S6B, Sepharose 6B. (B) Analysis of palmitoylation of ClipR-59 and DHHC17, respectively with the total cell lysates from HEK293 cells lysates expressing Flag-tagged, wild-type and palmitoylation-defective C2A2 ClipR-59, respectively. HyA, hydroxylamine; –, treated with NaCl; +, treated with hydroxylamine.
To assess whether TPC assay is suitable to analyze palmitoylated proteins, we applied this assay to total lysates of HEK293 cells transiently transfected with FLAG-tagged wild-type ClipR-59, which has been shown to be modified by palmitoylation at two conserved cysteine residues at 534 and 535.14 Palmitoylation-defective ClipR-59 cysteine-alanine mutant (C2A2-ClipR-59) of CLIPR-59 was used as a negative control. As shown in Figure 1B, wild-type was captured by thiopropyl beads while C2A2-ClipR-59 mutant was not. Failure to capture C2A2-ClipR-59 was not because of the activity of Thiopropyl beads as DHHC17, an endogenous palmitoylated protein was captured in the lysates from the cells expressing either wild-type (lane 2, panel 3) or palmitoylation defective (lane 4, panel 3) ClipR-59. Taken together, we conclude that TPC is a reliable assay for analyzing palmitoylated proteins.
Identification of palmitoylated proteins in adipocytes and adipose tissue
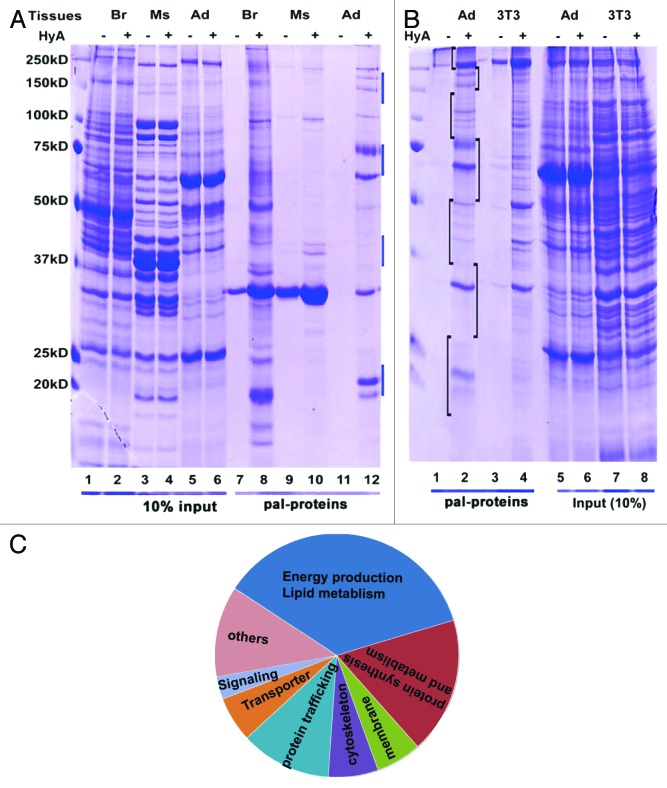
After demonstrating that TPC assay is an effective method to isolate cellular palmitoylated proteins, we next assessed the status of protein palmitoylation in adipose, brain and muscle, respectively. Shown in Figure 2A, each of these tissues showed palmitoylation of multiple proteins. Protein palmitoylation was initially described in brain,15,16 which also showed the highest abundance of palmitoylated proteins. Compared with muscle, both brain and adipose tissue showed high palmitoylation of proteins, perhaps a reflection of the high lipid content of both brain and adipose. Moreover, palmitoylation of a set of proteins was specifically observed in adipose tissue (compare lanes 10 and 12, the bars on the right highlight the adipose tissue-specific palmitoylated proteins).
Figure 2. Analyzing palmitoylated proteins from adipocytes and adipose tissue. (A) Palmitoylated proteins from mouse epididymal fat pad, brain and muscles. The total membrane proteins were extracted from indicated tissues and subjected to TCA assay. The isolated proteins were separated on SDS PAGE and stained with Coomassie Blue. All of tissues are from 2-month-old mice. HA, hydroxylamine; Pal-proteins, palmitoylated protein. Br, brain; Ms, skeletal muscle; Ad, adipose tissue; Pal-protein, potential palmitoylated proteins. (B) Thiopropyl Captivation assay of epididymal fat pad (ad) and 3T3-L1 adipocytes (3T3). (C) The distribution of isolated palmitoylated proteins identified by mass spectrometry from adipocytes and 3T3-L1 adipocytes.
To examine the role of palmitoylation in adipocytes, we next isolated the total palmitoylated proteins from epididymal fat pads and 3T3-L1 adipocytes using the TPC assay. Isolated proteins were separated by SDS-PAGE and the different regions of gels were excised for mass-spectrometric (MS) analysis based on the range of molecular weights (MW) (indicated in Fig. 2B, lane 2). Following MS, putative palmitoylated proteins were identified based on three criteria: (1) at least three unique peptides of the MS spectrum match the protein, (2) the identified protein falls within the correct MW range and (3) MS spectra identify the protein from both adipose tissue and 3T3-L1 adipocytes. Based on these criteria, a total of 856 putative palmitoylated proteins were identified (Table S1). These include many known palmitoylated proteins including Flotillin,17 huntingtin,18 Ras,19 G-proteins,20-22 SNAP23,23 CD151,24 CD35,25 NCAM,26 sortilin,27 PI4KIIα,28 Tubulin29 and membrane palmitoylated proteins 6 and 7, further indicating the effectiveness of TPC assay employed to isolate palmitoylated proteins.
The identified palmitoylated proteins are functionally highly diverse. Based on their established functions, about one-third are the metabolic enzymes of lipid metabolism and energy production; one-third are the factors that are involved in protein metabolism including protein translation and degradation, about 15% are the cytoskeletal, and membrane proteins and about one-tenth are the proteins involved in protein trafficking, including Rab GTPase, various transporter and vesicle trafficking factors (Fig. 2C; Table S1). Taken together, these data imply that protein palmitoylation is involved in a wide range of adipocyte functions.
Palmitoylated proteins in Glut4 vesicle trafficking
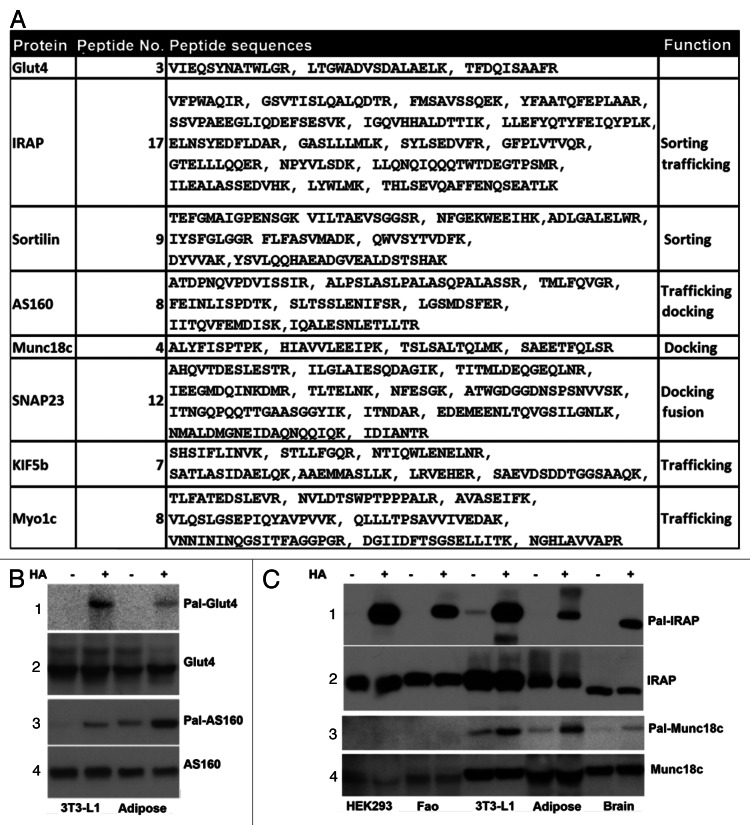
A list of palmitoylated proteins that have established roles in Glut4 membrane translocation is presented in Figure 3A. Among these proteins, SNAP23,23 sortilin,27 PI4KIIα28 and Flotillin17 are known to be palmitoylated whereas, Glut4, IRAP, Munc18c, AS160, RAB14, KIF5B and Myo1c are novel targets for palmitoylation (for a review about the functions of these proteins in Glut4 membrane trafficking, see refs. 30 and 31). Because Glut4 is the center of insulin-dependent Glut4 vesicle membrane trafficking and IRAP, Munc18c and AS160 play different regulatory roles in Glut4 membrane trafficking, we assessed their presence in Thiopropyl beads using western blots. As presented in Figure 3B and C, we observed that each of these proteins were associated with Thiopropyl beads following hydroxylamine treatment, but not under control (NaCl) conditions in adipocytes and adipose tissue (top panels). These data suggest that IRAP, Munc18c and AS160 are most likely palmitoylated in both adipose tissue and 3T3-L1 adipocytes.
Figure 3. The palmitoylated proteins in Glut4 membrane translocation. (A) A subset of proteins in Glut4 membrane translocation identified by Thiopropyl capture (TPC) assay and mass spectrometry (MS) in epididymal fat pad and 3T3-L1 adipocytes. The number of peptides and their corresponding sequences from MS were shown. (B) Western blot analysis of TPC assay of epididymal fat pad, 3T3-L1 adipocytes with anti-Glut4 (1) and anti-AS160 antibodies (3). The input levels of each protein is shown in panels 2 and 4. (C) Western blot analysis of TPC assay of epididymal fat pad, 3T3-L1 adipocytes, HEK293 cells and Fao cells with anti-IRAP (1) and anti-Munc18c antibodies (3). The input levels of each protein are shown in panels 2 and 4, with indicated antibodies.
Glut4 is specifically expressed in adipocytes where Munc18c and IRPA are widely expressed. To determine whether Munc18C and IRAP are also palmitoylated in other cell or tissue types, total cellular lysates from HEK293 cells, hepatoma Fao cells and brain were subjected to TPC and western blot assays. We observed that both proteins were associated with Thiopropyl beads in HEK293 cells, rat hepatoma Fao cells and brain, indicating that these proteins are palmitoylated in a wide variety of cell types and tissues.
Glut4 and IRAP are major cargo proteins for Glut4 vesicles. To further validate that both proteins are palmitoylated, we next performed 17-octadecynoic acid metabolic labeling and Click Chemistry, an assay that labels cellular proteins in HEK293T cells that transiently express either Flag-tagged Glut4 or HA-tagged IRAP. As a control, the cells were labeled in parallel with palmitic acid. Shown in Figure 4A, both Flag-tagged Glut4 (panel 1) and HA-tagged IRAP (panel 3) were detected in 17-ODCA labeled cells, but not in cells treated with palmitic acid (compare lanes 1 and 2, respectively), demonstrating that both Glut4 and IRAP can be palmitoylated in vivo.
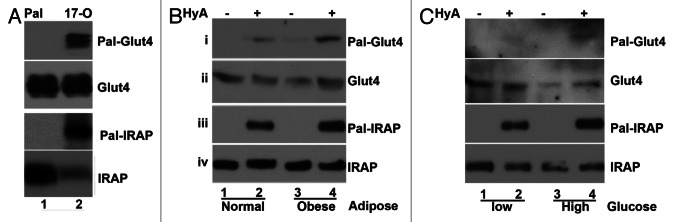
Figure 4. The status of Glut4 and IRAP palmitoylation under obesity. (A) Metabolic labeling and Click-Chemistry analysis HEK293T cells that transiently transfected with indicated expression vectors. Twenty-four hours post-transfection, the cells were metabolic labeled with either palmitic acid or 17-OCDA for overnight. The total cell lysates were prepared for Click Chemistry and western blot with indicated antibodies. (B) The adipose tissues from normal or obesity mice (8 week under high-calorie diet) were subjected to TPC assay. The isolated proteins were analyzed on western blot with anti-Glut4 (1) and anti-IRAP (3) antibodies, respectively. The input of each protein was presented in panels 2 and 4. (C) TPC assay of 3T3-L1 adipocytes that were cultured in with low glucose (2.5 mM) or high glucose (22.5 mM) overnight. Panels 1 and 3 are palmitoylated Glut4 and IRAP. Panels 2 and 4 are the 5% input of each protein.
Glut4 membrane translocation is essential for regulation of blood glucose level. Impaired Glut4 membrane translocation is the primary cause of hyperglycemia, associated with obesity and type II diabetes. We were interested in knowing the palmitoylation status of Glut4 and IRAP in adipose tissue in obesity. Toward this goal, the palmitoylation status of Glut4 and IRAP in the adipose tissue from 4-month-old diet-induced obese mice was examined. Shown in Figure 4B, the palmitoylation of both Glut4 and IRAP was increased (panels 1 and 3, compare lanes 2 and 4).
Next, we examined the palmitoylation status of Glut4 and IRAP in 3T3-L1 adipocytes that were cultured either in low glucose (2.5 mM) or high glucose (22.5 mM) medium. Presented in 4C, the level of Glut4 and IRAP palmitoylation was elevated when 3T3-L1 adipocytes were cultured in high glucose medium (compare lanes 2 and 4, panel 1 and 3, respectively). At present, the reasons and mechanisms resulting in glucose-dependent alteration of Glut4 and IRAP palmitoylation are not clear. Regardless, these results would argue that palmitoylation of these proteins might play a role in Glut4 membrane trafficking.
Palmitoylated proteins in signaling pathways
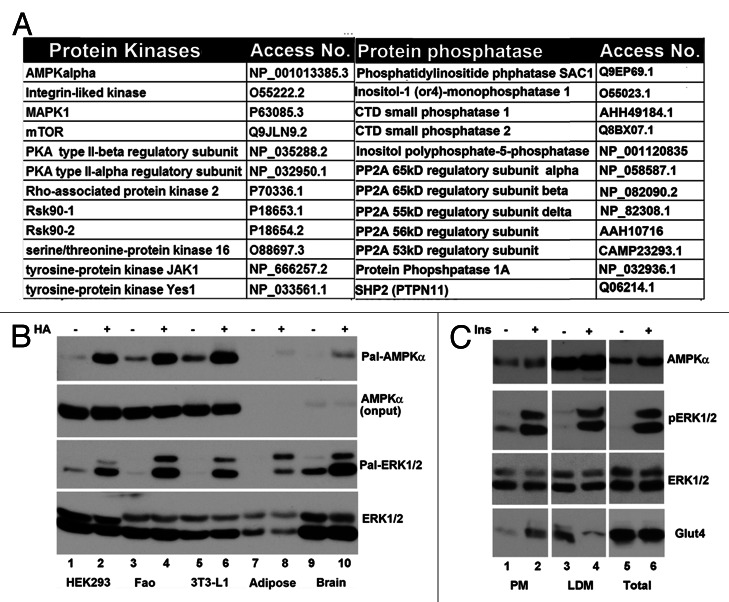
A partial list of well-studied protein serine kinases and phosphatases that are involved in cell signaling are presented in Figure 5A. These include Ser/Thr kinases AMPKα, integrin-linked kinase (ILK1), MAPK1 (ERK2), mTOR, PKA (regulatory subunit), Rsk90 and STK16, tyrosine kinases JAK1 and Yes1, protein phosphatases SHP2, PP2A (regulatory subunits) and PP1B. Among them, Ras32 and STK1633 are known to be palmitoylated. Since none of these proteins are adipocyte-specific, we selectively assessed the association of AMPKα and MAPK1 (p42ERK2) in membrane fraction using TPC assay. Shown in Figure 5B, we observed that AMPKα and both ERK1 and 2 were captured by thiopropyl beads under Hydroxylamine treatment. In agreement with these results, both AMPKα and ERK are metabolically labeled in cells treated with 17-octadecynoic acid, strongly indicating that these proteins are palmitoylated.
Figure 5. Palmitoylated protein kinases and phosphatases in signal transduction pathway. (A) A subset of kinases and phosphatases identified through Thiopropyl capture (TPC) assay and mass spectrometry (MS) in epididymal fat pad and 3T3-L1 adipocytes. The number of peptides and their corresponding sequences from MS were shown. (B) Western blot analysis of TPC assay of epididymal fat pad, 3T3-L1 adipocytes, HEK293 cells and Fao cells with anti AMPK1a (1) and anti-ERK (3). The input level of each protein is shown in panels 2 and 4, respectively. (C) Western blot analysis of AMPKα and MAPK1 in adipocyte subcellular fractions. (1) AMPKa in different fractions, (2) phospho-ERK and (3) total ERK in different fractions and (4) Glut4 in different fractions.
Palmitoylation of AMPKα and MAPK1 suggests that both proteins would be associated with membranes. To examine this, PM (plasma membrane) and LDM (low-density microsome) fractions isolated from 3T3-L1 adipocytes treated with or without insulin, were probed with anti-AMPKα and MAPK1-specific antibodies by western blotting. Presented in Figure 5C, both AMPK1α and ERK1/2 were found in PM and LDM, arguing that both proteins are associated with cellular membranes, which is consistent with the potential palmitoylation of these proteins.
Palmitoylation in JAK-STAT pathway
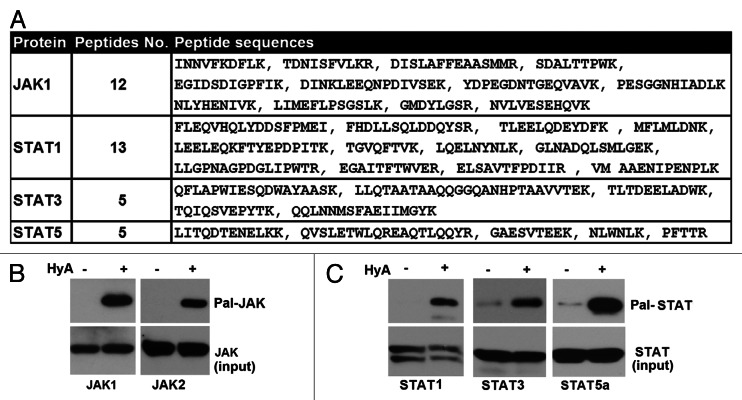
Activated by a variety of cytokines and hormones, the JAK-STAT pathway has been implicated in adipocyte differentiation, body energy metabolism and the development of insulin resistance (for a review, see ref. 34). Mass-spectrometric analysis indicated the potential palmitoylation of four proteins of the JAK-STAT pathway including JAK1, STAT1, STAT3 and STAT5A (Fig. 6A). JAKs are a family of tyrosine kinases including JAK1, JAK2, JAK3 and Tyk2. Both JAK1 and JAK2 are expressed in adipocytes. Therefore, we first assessed the possibility that both JAK1 and JAK2 are palmitoylated in adipocytes. Shown in Figure 6B, both JAK1 and JAK2 were captured by thiopropyl beads under hydroxylamine treatment (compare lanes 1 and 2 each top panel). In the same experiments, we also examined the association of STAT1, STAT3 and STAT5a with thiopropyl beads and found that each of the three STAT proteins were associated with thiopropyl beads under hydroxylamine treatment but not in control (NaCl) (Fig. 6C, compare lanes 1 and 2 each top panel). Thus, these data argue that both JAKs and STATs are potentially palmitoylated in adipose cells.
Figure 6. Palmitoylated proteins in JAK-STAT pathway. (A) JAK and STAT identified by Thiopropyl capture (TPC) assay and mass spectrometry (MS) in epididymal fat pad and 3T3-L1 adipocytes. The number of peptides and their corresponding sequences from MS were shown. (B) Western blot analysis of TPC assay of epididymal fat pad and 3T3-L1 adipocytes with individual anti-JAK antibodies. (C) Western blot analysis of TPC assay of epididymal fat pad and 3T3-L1 adipocytes with individual anti-JAK antibodies.
Based on the palmitoylation prediction program (www.csspalm.biocuckoo.org), two cysteine residue positions, 541 and C542, in JAK1 that are predicted to be palmitoylated are conserved through JAK family kinases (Fig. 7A). To determine whether Cys541 and 542 are indeed palmitoylated, we substituted these cysteine residues with serine in JAK1 (Cys541/542S JAK1) and examined the palmitoylation status of Cys541/542 JAK1 with TPC assay using transiently transfected HEK293T cells. As seen in Figure 7B, cysteine to serine substitutions in JAK1 (C541/542S JAK1) were sufficient to completely abolish palmitoylation of JAK1 (compare lanes 2 and 4, top panel), clearly identifying cysteine residues at 541 and 542 in JAK1 are palmitoylated.
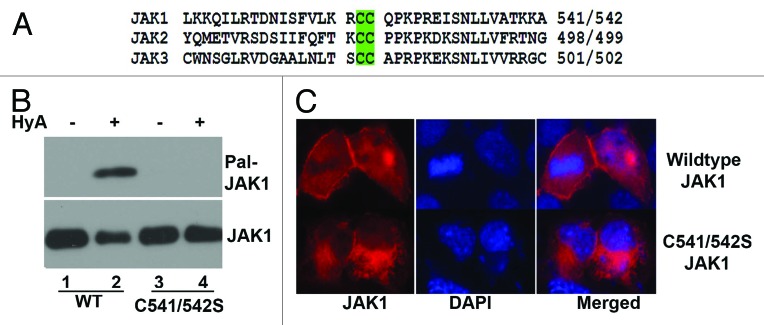
Figure 7. Characterization of JAK palmitoylation. (A) The schematic presentation of JAK peptide that contains palmitoylated cysteine residues. (B) TPC assay of Flag-tagged, wild-type and palmitoylation defective JAK1 (C541/542S JAK1) transiently expressed in HEK293 cells. (1) Palmitoylated JAK1; (2) the input level of JAK1. (C) Immunostaining of COS-7 cells transient transfected with Flag-tagged, wild-type or C541/542S JAK1 with anti-Flag antibody following Cy3 conjugated goat anti-mouse IG antibodies. The blue is the cell nuclei stained with DAPI.
JAKs are generally bound to the plasma membrane. The known localization of JAKs, along with the known role of palmitoylation in modulating protein-membrane interactions, prompted us to examine whether palmitoylation of Cys541/542 facilitates JAK1 membrane association. When expressed in COS-7 cells, wild-type JAK1 (Fig. 7C) exhibited clear membrane association (top panel), but substitution of cysteine residues in JAK1 (C541/542SJAK1) markedly altered JAK1 membrane association (bottom panel). Taken all together, these data suggest that palmitoylation of JAK1 modulates JAK1 membrane association.
Discussion
Protein palmitoylation has been implicated in a wide range of biological processes including protein trafficking, membrane signaling and membrane trafficking. Our interest in the role of posttranslational modifications, and their regulatory role in metabolic signaling, prompted us to inquire whether palmitoylation is a prominent modification of proteins expressed in adipocytes. These cells were chosen because of their obvious role in lipid storage and glucose homeostasis.
Toward this goal, we performed proteomic analysis of total palmitoylated proteins from both primary adipose tissue and from 3T3-L1 adipocytes. From these studies, we identified upwards of 800 putative palmitoylated proteins that are expressed in primary adipose tissue and cultured adipocytes. Among the palmitoylated proteins, we observed a high representation of various transporters, regulators of vesicular trafficking and signaling molecules that likely participate in a wide array of cellular processes including signaling, membrane translocation, cytoskeleton protein network, transport, secretory function, lipid, protein and energy metabolism. Taken together, palmitoylation appears to be involved in a wide array of adipocyte functions and significantly contribute toward glucose disposal and insulin action.
Given that a large number of palmitoylated proteins were isolated from adipose tissue, we focused on a distinct set of novel palmitoylated proteins that are related to glucose homeostasis and cell signaling. First, we verified that Glut4, IRAP, Munc18c and AS160 were represented in spectra obtained from TPC isolated palmitoylated proteins in both cultured adipocytes and adipose tissue. We have also validated palmitoylation of both Glut4 and IRAP using 17-OCDA metabolic labeling and Click Chemistry in adipose tissue. More importantly, palmitoylation of both proteins was found to be elevated in obesity. Insulin-dependent Glut4 membrane translocation constitutes a central mechanism for glucose uptake and disposal in both muscle and adipose tissue. Although Glut4 is the central player in the insulin-dependent vesicular uptake of glucose across the plasma membrane, IRAP is a major cargo protein in Glut4 containing insulin-responsive vesicles (GIRV). IRAP is not only involved in the sorting of GIRV, but also modulates GIRV trafficking.31 Munc18c is a membrane t-SNARE-associated protein and modulates GIRV membrane docking and fusion.35 AS160 is the major Akt substrate that modulates GIRV membrane docking.36,37 Identification of these proteins as palmitoylated proteins strongly suggests that protein palmitoylation plays an essential role for insulin-dependent, Glut4-mediated vesicular uptake of glucose. While the specific mechanisms that induce these changes remain unknown, importance of protein palmitoylation is highlighted by its potential role in glucose transport and its modulation in adipose tissue of obese insulin-resistant mice.
In addition to proteins required for glucose transport, we assessed the palmitoylation of several kinases including, ERK1/2 and AMPKα. Cellular compartmentalization of ERK1/2 and other kinases is consistent with the palmitoylation of these kinases. For AMPK, palmitoyation may have a more specific and defined role. AMPK is a heterotrimer that consists of three subunits: α, β and γ, which are differentially distributed in cellular compartments.38 Of the three subunits, AMPKβ is myristoylated, which, in turn, regulates membrane association and subsequent activation by upstream kinases.39 Thus, myristoylation serves to prime the activation of AMPKβ. Palmitoylation of AMPKα implies that there are two distinct lipid modifications in AMPK complex. Therefore, it is tempting to speculate that palmitoylation of α and myrystoylation of β may together recruit AMPK to the plasma membrane. As an energy sensor, AMPK modulates lipid metabolism. It is noteworthy several AMPK substrates, including acetyl-CoA carboxylase α (ACACA, ACC) and malonyl-CoA decarboxylase (MLYCD, MCD), are membrane-associated enzymes,40 and activation of AMPK leads to AMPK intracellular partitioning.39 Thus, it is plausible that palmitoylation of AMPK modulates compartmentalization of AMPK signaling to differentially phosphorylate its substrates.
Finally, we also examined palmitoylation of JAK1 kinase and its downstream effector STAT proteins. Based on their association with thiopropyl beads, our results suggested palmitoylation of JAK1, JAK2, STAT1, STAT3 and STAT5. Furthermore, we mapped JAK1 palmitoylation to Cys541 and 542, which, in turn, regulated the membrane localization of JAK1.
It is well-established that upon simulation, JAK1 kinase undergoes autophosphorylation, which, in turn, recruits and phosphorylates STAT proteins thus enabling nuclear translocation and transcriptional activation of STAT proteins. JAK kinase-dependent phosphorylation of STAT proteins occurs on or proximal membrane and positioning JAK and STAT at the membrane is required for activation of JAK-STAT signal transduction pathway.41 JAK is targeted to the cognate receptor and plasma membrane via the FERM domain.41-44 JAK1 also requires an additional perhaps the SH2 domain for membrane recruitment. The localization of Cys541 and 542 to the SH2 domain in JAK1 would suggest that palmitoylation of SH2-like domain may constitute the second JAK1 membrane targeting signal. Since both JAK and STAT have been shown to be associated membrane microdomains,45,46 it is highly probable that palmitoylation of JAK and STAT may be instrumental for this targeting event. JAK-STAT signal pathway is involved in a wide range of biological processes. In adipocytes, this pathway modulates adipocyte differentiation and energy metabolism.34 Taken together, palmitoylation of the three sets of proteins discussed here, may regulate various aspects of adipocyte biology.
In this report, we mainly analyzed adipocyte protein palmitoylation in a qualitative way. It is noted that quantitative analysis of protein palmitoylation can also be achieved in TPC assay. Thiopropyl beads capture palmitoylation proteins quantitatively via formation of disulfide cross-linkage. In addition, unlike other modification studies, e.g, phosphorylation, the level of modified protein and that of total cellular protein are determined with different reagents i.e., anti-phospho and anti-non-phospho antibodies, the level of palmitoylated protein and that of total cellular protein are determined with same antibody in TPC assay. In this regard, it is possible to determine the relative level of palmitoylated form by comparing the ratio of thiopropyl bead captured protein and input. For example, in this report, we consistently found that the ratio of palmitoylated IRAP and total cellular IRAP is high, whereas, that of Mun18c is low (see Fig. 4). This will indicate that the cellular level of palmitoylated IRAP is high, whereas, that of Munc18c is low. The reason for that is varied. But it could be that IRAP palmitoylation is more stable than that of Munc18c. Since palmitoylation is reversible, by which protein trafficking is regulated, the lower level of palmitoylation may reflect the notion that the modified protein is constantly shuttling.
To date, proteomic analysis of total protein palmitoylation has been performed in neurons,47 T cells,48 platelets,49 macrophages33 and prostate cancer cells.50 Upon comparing the palmitoylated proteins isolated from adipocytes with those from other cells, we find that enzymes regulating lipid and energy metabolism are unique to the adipocyte, again underscoring an important, though poorly understood, role for palmitoylation in regulating adipocyte biology. A better understanding of palmitoylation in adipocyte biology is likely to have long-ranging implications for developing new strategies in the treatment of obesity and diabetes.
Materials and Methods
Reagents
Hydroxylamine HCl (159417), 3-isobutyl-1-methylxanthine (IBMX) (I5879), dexamethasone (D4902), methylmethanethiosulfonate (MMTS) (64306), insulin (I6624), palmitic acid (P0500), anti-Flag (F3165), anti-DHHC17 (SAB2500508), HRP-conjugated anti-HA (H6533) and anti-munc18c (SAB1406498), anti-Glut4 antibodies (G4173), were from Sigma. Biotin-Azide (B10184) was from Invitrogen. Anti-AS160 antibody (ABS54) was from Millipore. All commonly used chemicals were from Thermo Scientific. Thiopropyl Sepharose 6B (17-0420-01) was from GE Healthcare Life Science. Anti-AMPK, anti-JAK (9945S), anti-STAT (9939S) and anti-Akt anti-phospho-Akt antibodies (8200S) were from Cell Signaling. 17-octadecynoic acid (90270) is from Cayman.
Plasmid construction
Mouse IRAP (MMM1013-9201983) and JAK1 (MMM1013-7513113) cDNA were purchased from Openbiosystems. Human Glut4 cDNA was the gift of Dr G.I. Bell of University of Iowa.51 To generate the tagged peptide, the primers corresponding to each cDNA were amplified by PCR and cloned into pcDNA-Flag or pcDNA-HA expression vectors. The mutation of putative palmitoylation sites in JAK1 was generated through site-directed mutagenesis by PCR. The primers used are IRAP: forward: GGGGATCCATGGAGTCCTTTACC; reverse: GGGAGCTCTACAGCCACTGGGAG. Glut4: forward: GGGAATTC ATGCCGTCGGGCTTCC; reverse: GGTCTAGATCAGTCGTTCTCATCTG. JAK1: forward: GGGAATTCATGCAGTATCTAAATAT; reverse: GGTCTAGATTATTTTAAAAGTGCTTC. For site-directed mutagenesis, the primers used are: forward: CTTTGTGCTGAAACGATCCTCTCAGCCTAAGCCTCGAG; reverse: CTCGAGGCTTAGGCTGAGAGGATCGTTTC AGCACAAAG.
Cell culture and transient transfection
HEK293 cells were cultured in DMEM (11995073, Life Technologies) supplemented with 10% FBS (26140079, Life Technologies) and 1× antibiotic-antimycotic (15240112, Life Technologies). 3T3-L1 preadipocytes (CL-183, ATCC) were cultured in DMEM supplemented with 10% bovine serum and 1× antibiotic-antimycotic. The differentiation of 3T3-L1 adipocytes has been described. The transient transfections were performed with lipofectamine 2000 (11668019, Life Technologies) according to manufacturer’s protocol.
Animals
The normal (380056) and obese (380050) C57B/6 mice were purchased from Jackson Laboratory. The obese mice were fed a high calorie diet (60% kcal fat) for 8 weeks. The detailed information about these mice can be found at www.jaxmice.jax.org/diomice/index.html.
Isolation and characterization of palmitoylated proteins
The procedure for isolation of total palmitoylated proteins were outlined in Figure 1A. Briefly, total cell or tissue homogenates in cell lysates buffer (10 mM HEPES, 10 mM NaCl, pH 7.6) were spun at 500 g for 5 min to remove nuclei. Then, the supernatants were centrifuged at 175 kg for 60 min. The pellets (cell membranes including plasma membrane, high-density microsomes and low-density microsome) were resuspended into blocking buffer (100 mM HEPES, 1 mM EDTA, 2.5% SDS) supplemented with 0.1% MMTS and incubated at 42°C for 15 min. Then 2 vol of acetone was added into above reaction mixture and incubated at −20°C for 20 min. After washed with 70% cold acetone, the pellet was resuspended into capturing buffer (100 mM HEPES, 1 mM EDTA, 1.0% SDS). Then, water-swollen thiopropyl sepharose 6B was added. Then, the sample was divided into two equal parts. To one part, hydroxylamine Cl (pH = 7.5) was added to a final concentration of 0.2 M. To the other part, an equal amount of NaCl (control) was added. After 3 h incubation at room temperature, the beads were washed with capturing buffer. After washing, the beads were incubated with 50 mM DTT. Thirty minutes later, the beads were spun and supernatant was saved for SDS-PAGE (authors will provide more detailed protocol if requested). The mass spectrometry was performed in Harvard Taplin MS Core facility.
17-octadecynoic acid metabolic labeling and Click Chemistry
The 17-ODCA metabolic labeling and Click Chemistry was performed as described.47 Briefly, HEKT 3T3 cells were transiently transfected with the expression vectors that express the tagged target peptides (Flag-Glut4, and HA-IRAP in this study). Twenty-four hours post-transfection, the cells were metabolically labeled with 50 uM of 17-ODCA or palmitic acid (served as a control) for overnight. Then, the total cell lysates were prepared for Click Chemistry. After the biotinylated proteins were purified via streptavidin-agarose (20347, Thomas Scientific), the purified proteins were analyzed on western blot with corresponding antibodies.
Western blot
After the indicated treatments as described in the figure legends, cells were washed twice with PBS and lysed with cell lysis buffer (20 mM Tris pH 7.6, 150 mM NaCl, 0.5 mM EDTA, 0.5 mM DTT, 10 mM, 1% Triton X-100 or 1% NP-40, 10% glycerol, protease and phosphatase inhibitors). Equal amounts of protein (20–30 ug) were subjected to SDS-PAGE electrophoresis and transferred to polyvinylidene fluoride membrane (Biorad). The membranes were incubated with each primary antibody, followed by incubation with a horseradish peroxidase-conjugated secondary antibody (Biorad). The protein bands were visualized using the ECL detection system (Pierces).
Subcellular fractionation assay
3T3-L1 adipocytes with or without insulin treatment were suspended into HES I buffer (0.25 M sucrose, 20 mm Tris pH 7.6, 1 mM EDTA, plus a protease-inhibitor mixture). The cells were homogenized by passing a 23 gauge needle 10 times, and then the homogenates were centrifuged at 19 kg for 20 min. To isolate membrane fraction, the resultant pellets from the 19 kg centrifugation were layered on HES II buffer (1.12 M sucrose, 20 mM Tris, pH 7.6, 1 mM EDTA) and centrifuged at 100 kg for 60 min. The resulted pellets were designated as nuclear and mitochondria fraction. The plasma membrane layers were removed from the sucrose cushion and suspended into HES I buffer and centrifuged at 41 kg for 20 min. The resultant pellets were plasma membrane (PM). To isolate microsomes, the resultant supernatant from the 19 kg centrifugation was centrifuged at 175 kg for 75 min and the pellets were collected as low-density microsomes (LDM). The supernatant from the 175 kg centrifugation was saved and designated as cytosol.
Cell imaging
Transfected COS-7 cells were fixed in 3.7% paraformaldehyde. The fixed cells were permeablized with 0.2% Triton X100 for 3 min. Then, the cells were incubated with indicated antibodies in 3% BSA for 1–2 h followed by Cy3 conjugated goat-anti-mouse IgG antibodies. The cells were mounted on glass-cover slides. The fluorescence imaging was captured with confocal microscopy (Olympus).
Supplementary Material
Acknowledgments
The author is indebted to Dr G.I. Bell for the gift of human Glut4 cDNA. This work was supported by NIH grant RO1 DK084319 and R56 DK084319 to K.D. K.D. was the recipient of The American Diabetes Association Career Development Award.
Glossary
Abbreviations:
- TPC
thiopropyl captivation
- AS160
Akt substrate 160 kD
- STAT
signal transducers and activators of transcription
- IRAP
insulin responsive amino peptidase
- SHP2
SH2-containing phosphatase 2
- Munc18c
mammalian homolog of unc-18
- PM
plasma membranes
- LDM
low density microsome(s)
- SNAP23
soluble NSF attachment protein 23 kD
- NCAM
neural cell adhesion molecule/CD56
- PI4K
phosphotidylinositol 4-kinases II
- KIF5B
kinesin family member 5B
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/adipocyte/article/22117
References
- 1.Mumby SM. Reversible palmitoylation of signaling proteins. Curr Opin Cell Biol. 1997;9:148–54. doi: 10.1016/S0955-0674(97)80056-7. [DOI] [PubMed] [Google Scholar]
- 2.Hu JS, James G, Olson EN. Protein fatty acylation: a novel mechanism for association of proteins with membranes and its role in transmembrane regulatory pathways. Biofactors. 1988;1:219–26. [PubMed] [Google Scholar]
- 3.Resh MD. Fatty acylation of proteins: new insights into membrane targeting of myristoylated and palmitoylated proteins. Biochim Biophys Acta. 1999;1451:1–16. doi: 10.1016/S0167-4889(99)00075-0. [DOI] [PubMed] [Google Scholar]
- 4.Aicart-Ramos C, Valero RA, Rodriguez-Crespo I. Protein palmitoylation and subcellular trafficking. Biochim Biophys Acta. 2011;1808:2981–94. doi: 10.1016/j.bbamem.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 5.Iwanaga T, Tsutsumi R, Noritake J, Fukata Y, Fukata M. Dynamic protein palmitoylation in cellular signaling. Prog Lipid Res. 2009;48:117–27. doi: 10.1016/j.plipres.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 6.Smotrys JE, Linder ME. Palmitoylation of intracellular signaling proteins: regulation and function. Annu Rev Biochem. 2004;73:559–87. doi: 10.1146/annurev.biochem.73.011303.073954. [DOI] [PubMed] [Google Scholar]
- 7.Nandi A, Kitamura Y, Kahn CR, Accili D. Mouse models of insulin resistance. Physiol Rev. 2004;84:623–47. doi: 10.1152/physrev.00032.2003. [DOI] [PubMed] [Google Scholar]
- 8.Savage DB, Petersen KF, Shulman GI. Disordered lipid metabolism and the pathogenesis of insulin resistance. Physiol Rev. 2007;87:507–20. doi: 10.1152/physrev.00024.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galic S, Oakhill JS, Steinberg GR. Adipose tissue as an endocrine organ. Mol Cell Endocrinol. 2010;316:129–39. doi: 10.1016/j.mce.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 10.Lihn AS, Pedersen SB, Richelsen B. Adiponectin: action, regulation and association to insulin sensitivity. Obes Rev. 2005;6:13–21. doi: 10.1111/j.1467-789X.2005.00159.x. [DOI] [PubMed] [Google Scholar]
- 11.Rabe K, Lehrke M, Parhofer KG, Broedl UC. Adipokines and insulin resistance. Mol Med. 2008;14:741–51. doi: 10.2119/2008-00058.Rabe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Richard AJ, Stephens JM. Emerging roles of JAK-STAT signaling pathways in adipocytes. Trends Endocrinol Metab. 2011;22:325–32. doi: 10.1016/j.tem.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Forrester MT, Hess DT, Thompson JW, Hultman R, Moseley MA, Stamler JS, et al. Site-specific analysis of protein S-acylation by resin-assisted capture. J Lipid Res. 2011;52:393–8. doi: 10.1194/jlr.D011106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lallemand-Breitenbach V, Quesnoit M, Braun V, El Marjou A, Poüs C, Goud B, et al. CLIPR-59 is a lipid raft-associated protein containing a cytoskeleton-associated protein glycine-rich domain (CAP-Gly) that perturbs microtubule dynamics. J Biol Chem. 2004;279:41168–78. doi: 10.1074/jbc.M406482200. [DOI] [PubMed] [Google Scholar]
- 15.Folch J, Lees M. Proteolipides, a new type of tissue lipoproteins; their isolation from brain. J Biol Chem. 1951;191:807–17. [PubMed] [Google Scholar]
- 16.Dalva MB. Neuronal activity moves protein palmitoylation into the synapse. J Cell Biol. 2009;186:7–9. doi: 10.1083/jcb.200906101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Y, Martin BR, Cravatt BF, Hofmann SL. DHHC5 protein palmitoylates flotillin-2 and is rapidly degraded on induction of neuronal differentiation in cultured cells. J Biol Chem. 2012;287:523–30. doi: 10.1074/jbc.M111.306183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang K, Yanai A, Kang R, Arstikaitis P, Singaraja RR, Metzler M, et al. Huntingtin-interacting protein HIP14 is a palmitoyl transferase involved in palmitoylation and trafficking of multiple neuronal proteins. Neuron. 2004;44:977–86. doi: 10.1016/j.neuron.2004.11.027. [DOI] [PubMed] [Google Scholar]
- 19.Dudler T, Gelb MH. Palmitoylation of Ha-Ras facilitates membrane binding, activation of downstream effectors, and meiotic maturation in Xenopus oocytes. J Biol Chem. 1996;271:11541–7. doi: 10.1074/jbc.271.19.11541. [DOI] [PubMed] [Google Scholar]
- 20.Casey PJ. Lipid modifications of G proteins. Curr Opin Cell Biol. 1994;6:219–25. doi: 10.1016/0955-0674(94)90139-2. [DOI] [PubMed] [Google Scholar]
- 21.Osterhout JL, Waheed AA, Hiol A, Ward RJ, Davey PC, Nini L, et al. Palmitoylation regulates regulator of G-protein signaling (RGS) 16 function. II. Palmitoylation of a cysteine residue in the RGS box is critical for RGS16 GTPase accelerating activity and regulation of Gi-coupled signalling. J Biol Chem. 2003;278:19309–16. doi: 10.1074/jbc.M210124200. [DOI] [PubMed] [Google Scholar]
- 22.Milligan G, Grassie MA, Wise A, MacEwan DJ, Magee AI, Parenti M. G-protein palmitoylation: regulation and functional significance. Biochem Soc Trans. 1995;23:583–7. doi: 10.1042/bst0230583. [DOI] [PubMed] [Google Scholar]
- 23.Greaves J, Gorleku OA, Salaun C, Chamberlain LH. Palmitoylation of the SNAP25 protein family: specificity and regulation by DHHC palmitoyl transferases. J Biol Chem. 2010;285:24629–38. doi: 10.1074/jbc.M110.119289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Charrin S, Manié S, Oualid M, Billard M, Boucheix C, Rubinstein E. Differential stability of tetraspanin/tetraspanin interactions: role of palmitoylation. FEBS Lett. 2002;516:139–44. doi: 10.1016/S0014-5793(02)02522-X. [DOI] [PubMed] [Google Scholar]
- 25.Jochen A, Hays J. Purification of the major substrate for palmitoylation in rat adipocytes: N-terminal homology with CD36 and evidence for cell surface acylation. J Lipid Res. 1993;34:1783–92. [PubMed] [Google Scholar]
- 26.Kleene R, Mzoughi M, Joshi G, Kalus I, Bormann U, Schulze C, et al. NCAM-induced neurite outgrowth depends on binding of calmodulin to NCAM and on nuclear import of NCAM and fak fragments. J Neurosci. 2010;30:10784–98. doi: 10.1523/JNEUROSCI.0297-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCormick PJ, Dumaresq-Doiron K, Pluviose AS, Pichette V, Tosato G, Lefrancois S. Palmitoylation controls recycling in lysosomal sorting and trafficking. Traffic. 2008;9:1984–97. doi: 10.1111/j.1600-0854.2008.00814.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barylko B, Mao YS, Wlodarski P, Jung G, Binns DD, Sun HQ, et al. Palmitoylation controls the catalytic activity and subcellular distribution of phosphatidylinositol 4-kinase IIalpha. J Biol Chem. 2009;284:9994–10003. doi: 10.1074/jbc.M900724200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zambito AM, Wolff J. Palmitoylation of tubulin. Biochem Biophys Res Commun. 1997;239:650–4. doi: 10.1006/bbrc.1997.7525. [DOI] [PubMed] [Google Scholar]
- 30.Rowland AF, Fazakerley DJ, James DE. Mapping insulin/GLUT4 circuitry. Traffic. 2011;12:672–81. doi: 10.1111/j.1600-0854.2011.01178.x. [DOI] [PubMed] [Google Scholar]
- 31.Kandror KV, Pilch PF. The sugar is sIRVed: sorting Glut4 and its fellow travelers. Traffic. 2011;12:665–71. doi: 10.1111/j.1600-0854.2011.01175.x. [DOI] [PubMed] [Google Scholar]
- 32.Apolloni A, Prior IA, Lindsay M, Parton RG, Hancock JF. H-ras but not K-ras traffics to the plasma membrane through the exocytic pathway. Mol Cell Biol. 2000;20:2475–87. doi: 10.1128/MCB.20.7.2475-2487.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berson AE, Young C, Morrison SL, Fujii GH, Sheung J, Wu B, et al. Identification and characterization of a myristylated and palmitylated serine/threonine protein kinase. Biochem Biophys Res Commun. 1999;259:533–8. doi: 10.1006/bbrc.1999.0811. [DOI] [PubMed] [Google Scholar]
- 34.Richard AJ, Stephens JM. Emerging roles of JAK-STAT signaling pathways in adipocytes. Trends Endocrinol Metab. 2011;22:325–32. doi: 10.1016/j.tem.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.James DE. MUNC-ing around with insulin action. J Clin Invest. 2005;115:219–21. doi: 10.1172/JCI24158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Watson RT, Pessin JE. Bridging the GAP between insulin signaling and GLUT4 translocation. Trends Biochem Sci. 2006;31:215–22. doi: 10.1016/j.tibs.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 37.Bai L, Wang Y, Fan J, Chen Y, Ji W, Qu A, et al. Dissecting multiple steps of GLUT4 trafficking and identifying the sites of insulin action. Cell Metab. 2007;5:47–57. doi: 10.1016/j.cmet.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 38.Warden SM, Richardson C, O’Donnell J, Jr., Stapleton D, Kemp BE, Witters LA. Post-translational modifications of the beta-1 subunit of AMP-activated protein kinase affect enzyme activity and cellular localization. Biochem J. 2001;354:275–83. doi: 10.1042/0264-6021:3540275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oakhill JS, Chen ZP, Scott JW, Steel R, Castelli LA, Ling N, et al. β-Subunit myristoylation is the gatekeeper for initiating metabolic stress sensing by AMP-activated protein kinase (AMPK) Proc Natl Acad Sci USA. 2010;107:19237–41. doi: 10.1073/pnas.1009705107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kusunoki J, Kanatani A, Moller DE. Modulation of fatty acid metabolism as a potential approach to the treatment of obesity and the metabolic syndrome. Endocrine. 2006;29:91–100. doi: 10.1385/ENDO:29:1:91. [DOI] [PubMed] [Google Scholar]
- 41.Behrmann I, Smyczek T, Heinrich PC, Schmitz-Van de Leur H, Komyod W, Giese B, et al. Janus kinase (Jak) subcellular localization revisited: the exclusive membrane localization of endogenous Janus kinase 1 by cytokine receptor interaction uncovers the Jak.receptor complex to be equivalent to a receptor tyrosine kinase. J Biol Chem. 2004;279:35486–93. doi: 10.1074/jbc.M404202200. [DOI] [PubMed] [Google Scholar]
- 42.Tong W, Sulahian R, Gross AW, Hendon N, Lodish HF, Huang LJ. The membrane-proximal region of the thrombopoietin receptor confers its high surface expression by JAK2-dependent and -independent mechanisms. J Biol Chem. 2006;281:38930–40. doi: 10.1074/jbc.M607524200. [DOI] [PubMed] [Google Scholar]
- 43.Radtke S, Jörissen A, de Leur HS, Heinrich PC, Behrmann I. Three dileucine-like motifs within the interbox1/2 region of the human oncostatin M receptor prevent efficient surface expression in the absence of an associated Janus kinase. J Biol Chem. 2006;281:4024–34. doi: 10.1074/jbc.M511779200. [DOI] [PubMed] [Google Scholar]
- 44.Ragimbeau J, Dondi E, Alcover A, Eid P, Uzé G, Pellegrini S. The tyrosine kinase Tyk2 controls IFNAR1 cell surface expression. EMBO J. 2003;22:537–47. doi: 10.1093/emboj/cdg038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sehgal PB, Guo GG, Shah M, Kumar V, Patel K. Cytokine signaling: STATS in plasma membrane rafts. J Biol Chem. 2002;277:12067–74. doi: 10.1074/jbc.M200018200. [DOI] [PubMed] [Google Scholar]
- 46.Kim J, Adam RM, Solomon KR, Freeman MR. Involvement of cholesterol-rich lipid rafts in interleukin-6-induced neuroendocrine differentiation of LNCaP prostate cancer cells. Endocrinology. 2004;145:613–9. doi: 10.1210/en.2003-0772. [DOI] [PubMed] [Google Scholar]
- 47.Martin BR, Cravatt BF. Large-scale profiling of protein palmitoylation in mammalian cells. Nat Methods. 2009;6:135–8. doi: 10.1038/nmeth.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yap MC, Kostiuk MA, Martin DD, Perinpanayagam MA, Hak PG, Siddam A, et al. Rapid and selective detection of fatty acylated proteins using omega-alkynyl-fatty acids and click chemistry. J Lipid Res. 2010;51:1566–80. doi: 10.1194/jlr.D002790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dowal L, Yang W, Freeman MR, Steen H, Flaumenhaft R. Proteomic analysis of palmitoylated platelet proteins. Blood. 2011;118:e62–73. doi: 10.1182/blood-2011-05-353078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Martin BR, Wang C, Adibekian A, Tully SE, Cravatt BF. Global profiling of dynamic protein palmitoylation. Nat Methods. 2012;9:84–9. doi: 10.1038/nmeth.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fukumoto H, Kayano T, Buse JB, Edwards Y, Pilch PF, Bell GI, et al. Cloning and characterization of the major insulin-responsive glucose transporter expressed in human skeletal muscle and other insulin-responsive tissues. J Biol Chem. 1989;264:7776–9. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.