Abstract
Understanding the etiopathological processes of Alzheimer’s disease (AD) in the preclinical and early clinical stages will be important in developing new therapeutic targets and biomarkers. There is growing consensus that nonamyloid targets will be necessary to reverse or slow AD progression. Lipidomic, metabolomic and targeted approaches have identified pathways and products of sphingolipid metabolism that are altered early in the course of AD and contribute to the neuropathological alterations associated with AD, including amyloid-β production, tau formation and neurodegeneration. In this article, we briefly review the current literature on the role of sphingolipids in the underlying pathophysiology of AD, and then discuss the current state of translating these findings to clinical populations and the potential utility of plasma sphingolipids as diagnostic and/or prognostic indicators of AD.
Keywords: Alzheimer’s disease, amyloid, biomarker, blood, ceramide, lipid, neurodegeneration, sphingolipid, tau
An estimated 35.6 million people worldwide are currently living with dementia, of which 60–70% are diagnosed with Alzheimer’s disease (AD) [1]. As the number of AD cases is expected to double every 20 years, the public health burden threatens to explode in the middle of this century unless disease-modifying therapies are found to treat, or slow, the progression of the disease. Much of the escalation of the prevalence of dementia will be due to increases in low and middle income countries. In 2010, approximately 57.7% of people with dementia lived in low and middle income countries; this will increase to 70.5% by 2050 [1]. There are currently no disease-modifying therapies for AD. As evidenced by the many failed treatment trials for AD to date, there appears to be no treatment benefit in the fully symptomatic stage of the disease. One explanation for this lack of efficacy is that treatments may be administered too late in the disease process. In addition, virtually all AD treatment trials to date have focused on antiamyloid therapies. An alternative hypothesis is that AD can be caused or induced by nonamyloid factors including calcium dysregulation, altered cell signaling, oxidative stress, inflammation and lipid perturbations, so other therapeutic approaches may be necessary [2].
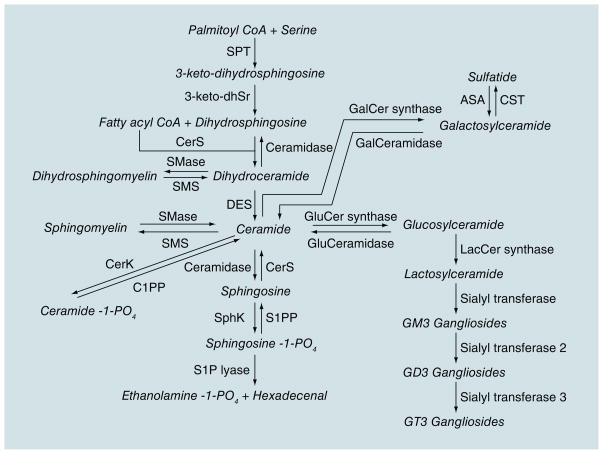
Lipids play an important role in the structure of neuronal cell membranes, directly affecting the solubility and fluidity of the membrane. The homeostasis of membrane lipids in neurons and myelin is a key component in preventing loss of synaptic plasticity, cell death and, ultimately, substantial neurodegeneration [3,4]. Sphingolipids are a class of lipids derived from the aliphatic amino alcohol sphingosine (Figure 1). This class of lipids makes up approximately one third of the content of eukaryotic cell membranes and is highly enriched in the CNS, especially in myelin where the proportion of sphingolipids is more than half the total lipid content. In addition to important structural roles, sphingolipid metabolites function as second messengers that modulate critical signaling functions for inter- and intracellular events. In the brain, the proper balance of sphingolipids is essential for normal neuronal function [5], as evidenced by a number of severe brain disorders that are the result of deficiencies in enzymes that control sphingolipid metabolism. For example, Niemann–Pick disease (type I) involves a deficiency in sphingomyelinase (an enzyme that catalyzes the hydrolysis of sphingomyelin) and mutations in the gene coding for glucocerebrosidase, the enzyme that breaks down glucosylceramide into glucose and ceramide, is a common genetic factor identified in Parkinson’s disease. While these neurological disorders are the result of gross disruptions in sphingolipid metabolism, recent discoveries from a number of laboratories suggest that more subtle changes in sphingolipid balance may be intimately involved in a number of neurodegenerative diseases [6–11].
Figure 1. Sphingolipid metabolism.
Products are indicated in italics.
Lipidomics, metabolomics and targeted approaches have identified pathways and products of sphingolipid metabolism that are altered early in the course of AD and contribute to the neuropathological alterations associated with AD, including amyloid-β production, tau formation and neurodegeneration [5]. While most studies to date have demonstrated a relationship between sphingolipids and AD pathology in preclinical models, recent studies have begun to translate these findings to humans. The present review will first provide a brief summary of sphingolipid metabolism and the evidence linking sphingolipids to Alzheimer pathology at the basic scientific level in animals. Next, the current state of the translation of these findings to clinical and epidemiological populations will be reviewed. Lastly, the potential clinical utility and limitations of blood-based sphingolipids as biomarkers of AD progression will be discussed.
Sphingolipid metabolism
Sphingolipid metabolism is a dynamic process that modulates the formation of a number of bioactive metabolites including multiple ceramides, ceramide-1-phosphate, sphingosine-1-phosphate (S1P) and glycosphingolipids. Sphingomyelins are largely found in the outer leaf of the cell membrane or lipoprotein complexes [12] and contribute to the asymmetric shape and marked curvature of membranes. An early event in apoptosis is the loss of this membrane symmetry [8]. There is strong evidence that cholesterol and sphingomyelin preferentially interact with each other in neuronal membranes [13]. This interaction has a direct effect on the structure and permeability of the cell membrane and has been implicated in ‘raft’ domains, which are important in cellular processes and second messenger systems [14]. The processing of APP by β- and γ-secretases occurs in these rafts. Thus, the lipid membrane content can exacerbate AD pathology.
Sphingomyelin can be rapidly hydrolyzed to ceramide through the actions of a family of sphingomyelinases. Ceramides are second messengers that regulate cell differentiation, proliferation and apoptosis by activating signaling cascades and promoting free radical generation [15]. While ceramides are important for cell survival and are essential for injury-induced cytokine production, at high levels ceramides inhibit cell division and induce cellular dysfunction and apoptosis [16]. Ceramides also activate protein phosphatases and kinases, enzymes involved in stress signaling cascades [17].
Ceramidase catalyzes the deacylation of ceramide to produce a free fatty acid and sphingosine. Sphingosine has been shown to inhibit protein kinase C and can also affect the activity of specific kinases [17]. A metabolite of sphingosine is S1P. Ceramides and sphingosines are usually associated with negative effects on cell growth and survival, while S1P has the opposite effect. Thus, the dynamic balance between the concentrations of these bioactive metabolites helps determine cell function and fate.
Gangliosides, an abundant, heterogeneous family of glycosphingolipids in the brain, are important components of cellular membranes and also affect neuronal plasticity and cell survival. These lipids play key roles in signal transduction, synaptic function, neurite outgrowth and neuronal regeneration, and are concentrated in lipid rafts (see [18] for review). Abundant evidence suggests that the amount and composition of some gangliosides change with age [19,20]. For instance, the incorporation rate of GM1 into myelin and hippocampal GD1A levels clearly decrease with advancing age [20]. As increasing age is the strongest risk factor for sporadic AD and the number of amyloid plaques also increases with age, changes in ganglioside composition could be a key trigger in inducing or increasing Alzheimer pathology.
Cell & animal studies suggest sphingolipids contribute to Alzheimer’s pathology
Sphingolipids & amyloid-β
Several lines of evidence suggest both direct and indirect associations between ceramides and amyloid-β levels [21], the hallmark of AD pathology. First, exposure of cultured neurons to amyloid-β1– 42 directly increases ceramide levels and decreases sphingomyelin levels by activating neutral sphingomyelinase [22–24]; inhibiting this increase protects neurons from amyloid-β-induced cell death [8]. Second, amyloid-β1–42 indirectly increases ceramides through an oxidative stress-mediated mechanism [8,21,25]; the increased ceramides then increase inflammatory and reactive oxygen species, further enhancing the pathology in a self-sustaining way. Last, ceramides may regulate amyloid-β1–42 production by modulating β-, and potentially γ-secretase activity [26,27].
Gangliosides are thought to directly contribute to amyloid burden in AD (see [18,28] for more in-depth reviews). Several studies have shown that amyloid-β adopts an altered conformation by binding to GM1, one of the major brain gangliosides, to form GM1-bound amyloid-β. GM1-bound amyloid-β has a strong tendency to form large amyloid-β assemblies and has altered immunoreactivity, thus acting as a seed for the assembly of soluble amyloid-β in the AD brain [29,30]. In addition to the direct contribution to amyloid burden in AD, gangliosides may also help to explain why amyloid-β deposits in the brain in an area-specific manner, leading to the formation of amyloid angiopathy and senile plaques. GM3 and GM1 are selectively expressed on vascular smooth muscle cells and presynaptic neuronal membranes [31,32]. Thus, area-specific amyloid deposition, such as amyloid angiopathy and senile plaques, may be attributed to the regional expression of particular ganglioside species [33]. These findings have yet to be translated to clinical studies.
In addition to ceramides and gangliosides, sphingomyelin and sphingosine-1-phosphate may also contribute to amyloid-β levels. Sphingomyelin levels are significantly perturbed in the brains of mice with human PS1 mutations compared with controls [34]. In addition, sphingosine-1-phosphate has been hypothesized to bind to β-secretase and to affect the proteolytic processing of the enzyme and subsequent amyloid-β production [35].
Sphingolipids & tau
While most studies to date have focused on the associations between sphingolipids and amyloid-β, recent studies have also linked sphingolipids to tau phosphorylation. PP2A is the major brain enzyme that regulates tau phosphorylation [36,37]. While the exact mechanism is unknown, several studies have established that ceramides modulate PP2A activity [38–40] and this association may be specific for the ceramide with the 18-carbon acyl chain length (C18) [40]. Given the association between ceramide and tau, it is interesting to speculate that the positive feedback loop between ceramides and amyloid-β further increases ceramide levels and thereafter leads to tau hyperphosphorylation [41], supporting the current hypothesis that amyloid-β pathology is deposited prior to neurofibrillary tangles. However, many individuals have tau phosphorylation and neurodegeneration without concomitant amyloid deposition. Further research is needed to understand how additional pathological factors, such as α-synuclein, can also induce ceramide production and lead to tau phosphorylation.
Sphingolipids & neurodegeneration
Previous reviews have highlighted the role of ceramides in apoptosis [42]. Briefly, stress signals including cytokines (e.g., TNF-α, IL-1 and fas ligand), nerve growth factor, nitric oxide, reactive oxygen species, cytotoxic agents (e.g., chemotherapeutic agents), environmental stress, injury and infections can lead to increased levels of ceramide through the activation of sphingomyelinases, or through the de novo synthesis of ceramide [43]. Inflammation and insulin resistance are specifically known to increase ceramide production, which then further increase ceramides in a positive feedback loop [44]. While the exact mechanisms by which these increased ceramides then induce apoptosis are unclear, modification of the PI3K–Akt pathway has been repeatedly observed. The PI3K–Akt pathway is associated with survival responses in that it inhibits the expression of proapoptotic factors (i.e., Bad, Bax, GSK-3 and P53) and activates antiapoptotic proteins (i.e., Bcl-2, Bcl-XL) [42]. PTEN is a critical inhibitor of the PI3K–Akt pathway. Importantly, ceramide-induced translocation of PTEN to the cell membrane inhibits the PI3K–Akt pathway, resulting in increased BAD mobilization to the mitochondria and subsequent release of additional pro-apoptotic mediators [42,44]. Ceramide-induced deactivation of the PI3K/Akt pathway also increases GSK-3B and tau phosphorylation [45].
Ceramides mediate the relationship between amyloid-β & neurodegeneration
While the exact mechanisms are unknown, studies have suggested ceramides mediate the relationship between amyloid-β and neurodegeneration. Amyloid-β increased ceramide levels in cultured neurons and induced death approximately 12–24 h later [8]. However, reducing this ceramide increase by savaging free radicals or by inhibiting the synthesis of ceramide, greatly reduced neuronal death [8,46]. These observations have recently been confirmed in mouse models of AD. Significant increases in long-chain ceramides occur very early in the cortex of the APPSL–PS1Ki mouse model, prior to neuronal loss [47]. The APPSL–PS1Ki mouse model is differentiated from other AD mouse models in its drastic neuronal loss, in part due to N-terminal truncated amyloid-β variants. Notably, in other mouse models, including APPSL, APPSL–PS1M146L and APPsw, in which there is not extensive early neuronal loss, ceramides were not found to be increased in the cortex or hippocampus [48,49]. Thus, similar to the discussion with tau above, amyloid-β-induced ceramide increases can lead to subsequent neurodegeneration. The development of therapeutic means of reducing the ceramide accumulation could delay or prevent the subsequent neurodegeneration and tau accumulation that correlate with the clinical symptoms of AD.
Notably, as mentioned above, some sphingo-lipids exert opposing effects on cell death and survival. While both ceramide and sphingosine are proapoptotic, sphingosine-1-phosphate is primarily antiapoptotic. Thus, the dynamic balance between these lipids can determine a cell’s fate [50]. While this balance has been examined in cardiovascular diseases and cancer, little research has been conducted in AD and other neurodegenerative diseases at the basic scientific level, in animals or in the clinic.
Postmortem & cerebrospinal fluid sphingolipids in AD
The vast majority of research to date examining the role of sphingolipids in the pathophysiology of AD has been at the basic scientific level and has only been applied to animals. More recently, studies are attempting to translate these findings to humans by examining postmortem tissue, cerebrospinal fluid (CSF) and peripheral sphingolipid levels. Studies examining brain sphingolipids in AD patients, other neurological diseases and controls were previously reviewed [51] and are updated and briefly discussed here (see Table 1 for the updated summary of findings from published studies) [8,46,52–57]. While multiple postmortem studies have examined sphingolipid levels and gene expression patterns of enzymes in the sphingolipid metabolism pathway, the results are difficult to compare given the study-specific differences in sphingolipids and brain regions examined, use of gray or white matter (or both), and the clinical and pathological severity of the AD brains (i.e., mild, moderate or severe AD).
Table 1.
Postmortem human studies examining sphingolipid alterations in Alzheimer’s disease.
| Author (year) | Brain regions and sample size | Findings | Ref. |
|---|---|---|---|
| Pettegrew et al. (2001) | Gray matter from MFG, MTG, inferior parietal lobule, occipital cortex and cerebellar cortex: 45 AD, 11 NC | ↑SM in AD vs NC when all brain regions were combined or individually in CRB and inferior parietal cortex, but not in occipital, superior/middle frontal or superior temporal cortices. Levels of SM positively correlated with the number of Aβ plaques, but not with neurofibrillary tangles | [52] |
| Han et al. (2002) | Gray and white matter from MFG, MTG, inferior parietal lobe, CA1 portion of hippocampus and subiculum, entorhinal cortex, CRB: 5; CDR = 0–3; CDR = 0.5–4; CDR = 1–6; CDR = 2–4; CDR = 3 | ↓Sulfatide in gray and white matter of frontal, temporal and parietal lobes, and CRB, in CDR = 0.5–3 vs CDR = 0. Sulfatide mass did not increase with increasing AD severity from CDR = 0.5 to CDR = 3. ↑Ceramide in both gray and white matter of all brain regions in CDR = 0.5–3 vs CDR = 0 brains. Ceramide mass of CDR = 2 or 3 ↓compared with CDR = 0.5 or 1, but still higher than CDR = 0. No differences in SM or GalC mass or GalC sulfotransferase activity by disease severity | [53] |
| Cutler et al. (2004) | Whole tissue from MFG and CRB: 7 AD, 7 NC. Membrane raft preparations of frontal cortex: 2 NC, 5 mild AD, 8 moderate AD, 12 severe AD | ↑Ceramide C24:0 and galactoceramide C24:0 and ↓SM C24:0 in MFG of AD vs NC. No differences in the CRB. ↑Ceramide C18:0 and C24:0 from AD vs NC. Both ceramides ↑ with disease severity. No differences in GalC, sulfatide or SM | [8] |
| Huang et al. (2004) | Gray matter from frontotemporal areas of 10 AD and 10 NC | ↑AC in AD vs NC. Acid ceramidase colocalized in cell bodies of neurons with neurofibrillary tangles but not AB plaques | [54] |
| Katsel et al. (2007) | Brain tissue from frontal, parietal, temporal and occipital areas; cingulate, caudate, hippocampus and putamen: 19; CDR = 0–16; CDR = 0.5–18; CDR = 1–16; CDR = 2–20; CDR = 0–28; CDR = 4–5 | Temporal and frontal cortices had greatest number of transcripts with altered expression. Several genes within sphingolipid pathway up- or downregulated at specific disease stages vs CDR = 0. Enzymes controlling longchain ceramide (C22:0 and C24:0) synthesis upregulated early in disease process (CDR = 0.5) and glucosylceramide downregulated. No alteration in gene expression for enzymes that control SM and glycosphingolipid turnover into ceramides | [55] |
| He et al. (2010) | Gray matter from frontotemporal area: 9 AD, 6 NC. Divided samples into soluble (cytosolic) and membrane fractions | ↑Membrane, not cytosolic ASM and AC activity in AD vs NC. No difference in NSM activity. ↑Ceramide and sphingosine and ↓SM and S1P levels in soluble (cytosolic) fractions. Positive correlation between ASM activity and AB and PHF-1. Negative correlation between S1P levels and AB and PHF-1 | [46] |
| Marks et al. (2008) | Frontal cortex: 18 AD, 11 NC | ↓GCS activity in AD vs NC. Reduction in GCS activity strongly correlated with degrees of atrophy on the CERAD scale in AD | [58] |
| Bandaru et al. (2009) | Gray and white matter from MFG, MTG and CRB. 30 AD (15 APOE ε4 and 15 APOE ε3). 26 NC (6 APOE ε4 and 20 APOE ε3) | AD vs NC comparison: ↑ SM C16:0, C18:0, C22:0 and C24:0, ceramide C18:0, C24:0 and steraoyl in gray matter; ↓ceramide C16:0, C22:0, C24:1, steraoyl and sulfatide in white matter of MFG. No differences in MTG. APOE ε4 vs ε3: among AD, ε4 carriers had ↓SM C22:0 and C24:0 and ↑ceramide C18:0, C24:1 and sulfatide in gray matter, and ↑ ceramide C22:0 in white matter of the MFG. No differences in the MTG or CRB by ε4 status. Lipid levels in NC did not vary by APOE ε4 allele status in any brain region | [56] |
| Filippov et al. (2012) | Frontal cortex. Ceramide levels in 19 AD, 6 AD plus other neuropathology (AD plus NP), 9 NP only, 6 NC. Gene expression of ASM, NSM2, UGCG, GalC in 8 AD, 4 NP and 3 NC | ↑Total ceramide levels in all disease groups (AD, AD plus NP and NP) vs NC. No differences in ceramides between AD, AD plus NP, and NP groups. Gene expression was not perturbed similarly within each group. However, in general, ASM and NSM2, and GalC were upregulated in both AD and NP, compared with NC and UGCG, which were downregulated | [57] |
| Chan et al. (2012) | Gray matter from PFC, ERC, and CRB in 10 AD and 10 NC | PFC: ↑Ceramides, glucosylceramide and GalC in AD vs NC. ↑Longchain SM (22:1 and 26:1) and ↓SMC20:0 in AD vs NC ERC: ↑In all SM levels AD vs NC. No differences in ceramide levels. No group differences in any lipid in the CRB |
[81] |
AB: Amyloid-β; AD: Alzheimer’s disease; CERAD: Consortium to Establish a Registry for Alzheimer’s Disease; CDR: Clinical Dementia Rating Scale; CRB: Cerebellum; ERC: Entorhinal cortex; MFG: Middle frontal gyrus; MTG: Middle temporal gyrus; NC: Normal control; PFC: Prefrontal cortex; SM: Sphingomyelin.
Overall, ceramide levels were typically found to be elevated in most brain regions of AD patients compared with nonpathological age-matched controls [8,46,53,56,57]. Similarly, gene expression of enzymes contributing to elevated ceramide levels are upregulated in AD patients relative to controls [55,57,58]. Levels appear to vary by disease severity, with ceramide levels or gene expression patterns increasing at the earliest stages of the disease and decreasing at moderate-to-severe stages [53,55]. The specificity of elevated brain ceramides to AD pathology is uncertain. One study reported that high acid sphingomyelinase activity, leading to high ceramides, positively correlated with levels of both amyloid-β and phosphorylated tau [46]. However, another study did not find differences in sphingolipid levels when comparing the brains of AD patients to other neuropathologies (i.e., Lewy bodies and tauopathy, fronto-temporal lobar degeneration) [57]. Thus, it is unlikely elevated brain ceramides will be specific to AD pathology, especially given the strong role of ceramides in global neurodegenerative processes.
In contrast to the relative consistency among studies examining postmortem ceramides, findings from studies of sphingomyelins vary. Sphingomyelin levels have been reported to be increased [52,56] or decreased [8,46] in AD versus control brains. A reason for these discrepancies could be the study variations in disease severity. The sphingolipid pathway is quite dynamic and it is possible that there are shifts in the pathway with elevations of specific lipids depending on the pathology present and degree of neurodegeneration.
The use of postmortem tissue samples is important when identifying a biomarker for AD because a definite diagnosis of AD requires autopsy documentation of pathology. However, in vivo studies, such as those using CSF or peripheral measures, are necessary to determine whether biomarkers can be used as indicators or predictors of disease progression. While several postmortem studies have examined sphingolipids levels in AD patients, few studies have examined CSF sphingolipid levels. One study reported that CSF sulfatides were decreased in cognitively normal individuals compared with those with “incipient dementia of the Alzheimer type” [59]. Another study found that CSF ceramide levels were higher in AD patients compared with those with other neurological diseases (cervical spondylosis, tension-type headache, metabolic encephalopathy or infarct) [60]. However, there was not a significant difference in levels between AD and amyotrophic lateral sclerosis patients, suggesting this may be a general response to neurodegeneration. More recently, two studies examined CSF levels of sphingomyelin [61,62]. The first reported higher sphingomyelin levels in probable AD cases relative to cognitively normal controls [61]. The second study extended these findings to examine whether CSF sphingomyelins varied by disease severity [62]. In this study, sphingomyelin levels were elevated in prodromal AD patients, but not mild or moderate AD. Notably, no study to date has examined the correlation between CSF sphingolipids and CSF amyloid-β or tau levels.
Peripheral sphingolipids: risk & progression of AD
A blood-based biomarker would be superior to more costly and invasive CSF and neuroimaging measures, and a blood draw would be more feasible and acceptable if repeated measures are needed for tracking disease progression or therapeutic response. Blood-based biomarkers would also be more readily accessible in developing countries, which is critical since it has been estimated that 70% of AD cases will be in low- to middle-income countries by 2050 [1]. However, the development of a blood-based biomarkers for neurodegenerative diseases is difficult due to the limited passage of many molecules through the blood–brain barrier, and the difficulty in directly linking peripheral markers and brain processes. An ongoing hypothesis is that peripheral changes over the course of AD may reflect a systemic metabolic signature of the disease [63]. While results examining several plasma or serum markers for AD have been published, few have shown consistent results across studies. For example, as illustrated in a recent systematic review and meta-analysis, both high and low plasma amyloid-β1–42 levels have been found to increase the risk of AD, or there is no association [64]. There is also a high degree of variation in plasma amyloid-β levels within and across studies. More recently, studies have begun to examine peripheral sphingolipid levels and clinical outcomes across a range of AD severities, with promising results for ceramides.
The initial peripheral sphingolipid studies examined levels of gangliosides and the activity of corresponding lysosomal enzymes (e.g., β-galactosidase). Both ganglioside levels and enzyme activity were elevated in the leukocytes [65] and fibroblasts [66,67] of AD patients relative to age-matched cognitively normal controls. However, while mean levels differed, there was significant overlap between groups, suggesting these lipids may not have the sensitivity and specificity to be acceptable diagnostic biomarkers for AD. Longitudinal studies have also not been conducted to determine whether gangliosides could be useful as predictive biomarkers of cognitive decline or progression.
Subsequent peripheral sphingolipid studies have primarily focused on ceramides and sphingomyelins in the blood. The relationship between peripheral ceramides, sphingomyelins and cognitive impairment was first examined in a pilot study of 100 women, aged 70–79 years at baseline, enrolled in WHAS II [10]. The women, all cognitively normal at baseline, were followed up to six times over 9 years. Cross-sectionally, lower levels of serum ceramides and sphingomyelin were associated with memory impairment, but longitudinally high ceramide levels predicted memory impairment. In fact, none of the women with blood levels of ceramide d18:1–C22:0 in the lowest tertile, and only one woman with blood levels of ceramides d18:1–C16:0 and d18:1–C24:0 in the lowest tertile developed memory impairment over 9 years of follow-up. All WHAS II participants were subsequently adjudicated for dementia and dementia type, and the relationship between serum sphingolipids and incident dementia (all-cause and AD) has now been examined. Higher baseline serum ceramides were associated with an increased risk of all-cause dementia and AD [68]. Notably, these relationships were stronger with AD than with all-cause dementia. Compared with the lowest tertile, the middle and highest tertiles of ceramide d18:1–C16:0 were associated with a tenfold (95% CI: 1.2–85.1) and 7.6-fold increased risk of AD (95% CI: 0.9–62.1), respectively. The highest tertiles of ceramide d18:1–C24:0 (hazard ratio: 5.1, 95% CI: 1.1–23.6) and lactosylceramide (hazard ratio: 9.8, 95% CI: 1.2–80.1) were also associated with risk of AD. Total and HDL cholesterol and triglycerides were not associated with risk of developing dementia or AD. These results suggest that the association between peripheral ceramides and incident AD is not due to a general lipid effect, but is specific to sphingolipids.
While the above studies suggested that high peripheral ceramides were associated with an increased risk of memory impairment and AD, it was not clear if peripheral sphingolipids could be utilized as diagnostic markers for AD or if they were associated with brain pathology. A study using clinically well-characterized (including neuroimaging) cognitively normal individuals and persons with amnestic mild cognitive impairment (MCI) and AD attempted to address these questions [69]. Cross-sectionally, plasma ceramide d18:1–C22:0 and d18:1–C24:0 levels were significantly perturbed in patients with amnestic MCI compared with both normal control and AD, replicating the previous study’s cross-sectional results. However, as with gangliosides, there was much overlap between groups, indicating blood ceramides will not be useful diagnostic markers for AD. Longitudinally, over 1 year of follow-up, high plasma ceramide levels, particularly d18:1–C22:0 and d18:1–C24:0, predicted cognitive decline and hippocampal volume loss among amnestic MCI patients, again suggesting the predictive value of peripheral ceramides. Furthermore, the results also suggest a relationship with hippocampal atrophy, a cardinal feature of AD, thereby somewhat validating the use of peripheral sphingolipid measures. In line with the hypothesis that peripheral sphingolipids could be predictors of cognitive decline, a study of AD patients, with a follow-up of 2.4 years on average, also reported that high plasma ceramide and low sphingomyelin levels were associated with a faster rate of cognitive decline [70].
The above studies all used targeted mass spectrometry techniques to quantitatively measure individual sphingolipid species in the blood. Using a multidimensional mass spectrometry-based shotgun lipidomics approach, Han et al. measured over 800 lipid species in the plasma of AD cases and cognitively normal controls [71]. Of the lipids examined, specific species of sphingomyelins and ceramides, and the sphingomyelin–ceramide ratio, differed between AD cases and cognitively normal controls. Thus, these results suggest that the peripheral sphingolipid findings are robust across different populations and methods.
Steps for further research & validation
The above-described results are consistent and promising, and suggest the potential utility of peripheral sphingolipids as biomarkers of cognitive progression at all stages of AD (i.e., cognitively normal individuals, amnestic MCI and AD). However, while the longitudinal nature of the peripheral ceramide studies suggests that changes in blood ceramide levels precedes cognitive changes, clinical trials and direct experimental evidence in humans identifying a causal association have not yet been published. Furthermore, with the exception of the one study examining peripheral ceramides and hippocampal volume loss [69], no studies have examined peripheral measures of sphingolipids in relation to CSF amyloid-β or tau levels, or other neuroimaging modalities. Additional research is necessary to determine whether sphingolipid metabolites could be therapeutic targets for the prevention or treatment of AD. It is possible that plasma ceramides could be useful as predictors of cognitive progression for the purposes of clinical trial enrichment. If AD drugs that target the sphingolipid pathway are developed, peripheral sphingolipids could also be utilized as biomarkers of therapeutic response. However, in addition to further clarifying the pathological associations between peripheral sphingolipids and AD pathology, additional research and validation is needed to develop plasma sphingolipids into clinically useful biomarkers that can be used at the population level. First, normal ranges of plasma sphingolipid levels and factors that cause normal variation in these levels (e.g., age, sex and ethnicity) need to be determined and validated using high-throughput methodologies. While an important study examining the genetic determinants of circulating sphingolipid concentrations was conducted in European populations, standards were not run for each individual lipid species to obtain specific plasma concentrations [72]. Identifying normal ranges and within-individual variability will not only be important for examining peripheral sphingolipids in AD and other neurodegenerative diseases, but will also help further sphingolipid research and its clinical application in other diseases such as cancer, diabetes, atherosclerosis and hypertension. Second, in vivo relationships between plasma sphingolipids and Alzheimer pathology need to be assessed using CSF and/or neuroimaging markers of amyloid-β and tau. This will be important to establish whether perturbations in these lipids are specific to AD pathophysiology (or tauopathies if the only relationship is with tau). While it is attractive to identify an AD-specific marker that leads to new disease-specific therapeutic opportunities, the identification of a peripheral neurodegenerative biomarker that can predict rate of progression would also be extremely valuable.
Finally, it is important to understand the mechanisms by which peripheral sphingolipids are associated with brain processes. It has been elegantly shown that toxic ceramides generated in one tissue can exert negative effects on other tissues, including the brain [44]. However, no published study to date has directly assessed the correlation between plasma and CSF sphingolipids in cognitively normal individuals or persons with amnestic MCI or AD. While CSF may not be directly indicative of neuronal processes, it is often used as an indirect measure of brain metabolism. A complexity of examining correlations in sphingolipid levels between the brain and periphery is that some enzymes in the sphingolipid pathway have tissue-specific expression levels, resulting in the differential distribution of specific carbon chain lengths. For example, ceramides with 18 carbons are the most abundant in the brain, whereas ceramides with 22 and 24 carbons are most abundant in the blood. Thus, it may be beneficial to examine the correlations between total ceramide levels and specific chain lengths.
Recent studies with synthetic ceramides of short chain lengths (C2, C5 or C6) suggest that these lipids can cross the blood–brain barrier [73,74]. However, it remains uncertain as to the penetrability of long chain ceramides and other sphingolipids. If there is a strong correlation between peripheral and CSF ceramide levels, peripheral ceramides can be utilized as a direct measure of brain ceramides. However, if a direct correlation is not found, this does not mean that blood ceramides will not be helpful in the prognosis of AD. As ceramides and other sphingolipids can act as second messengers, it is likely these lipids will affect other toxic compounds that cross the blood–brain barrier [44]. Thus, peripheral sphingolipids indirectly affect brain processes. Moreover, several studies have suggested that sphingolipids are critical factors in the development of cardiovascular diseases and insulin resistance [75–79]. All of these vascular outcomes are known to increase the risk of AD and also affect the rate of disease progression after an AD diagnosis [80]. Thus, there are several mechanisms by which peripheral sphingolipids can directly or indirectly increase the risk of AD and affect the rate of disease progression [51].
Conclusion
In the brain, the proper balance of sphingolipids is essential to sustain neuronal function. Lipidomics, metabolomics and targeted approaches suggest products of sphingolipid metabolism, especially ceramides, are altered early in the course of AD and contribute to the neuropathological alterations associated with AD, including amyloid-β production, tau formation and neurodegeneration. While most research to date has been at the basic scientific level in animals, recent studies are translating these findings to clinical populations to determine whether sphingolipid metabolites can be useful treatment targets and biomarkers for AD. Notably, multiple studies of different populations and methodologies suggest that elevated blood ceramides are associated with an increased risk of AD and progression of cognitive decline. These results suggest that plasma ceramides could be used as biomarkers of AD progression and for the purposes of trial enrichment, but not as a diagnostic biomarker. With the ongoing development of drugs targeting the sphingolipid pathway, plasma sphingolipids could also be utilized as indicators of therapeutic response. While basic scientific studies have linked sphingolipids to AD pathology, clinical studies have not examined the associations between CSF or plasma sphingolipids, and CSF or neuroimaging biomarkers of AD. Studies which do this are needed to determine whether sphingolipids will be specific to AD or are more general markers of neurodegenerative processes. Additional research is also needed to determine the direct and indirect effects of peripheral sphingolipids on brain processes.
Future perspective
While basic scientific research in animals has shown sphingolipids increase amyloid-β, tau and neurodegeneration, these findings need to be extended to human samples using well-conducted experimental study designs. In parallel, continued development of high-throughput sphingolipid assays is necessary to develop plasma sphingolipids into clinically useful biomarkers of AD progression. These assays will also be important to expand clinical research in other areas including cardiovascular diseases, diabetes and cancer. The further development of plasma sphingolipids into biomarkers for AD progression will have the advantages of being superior to more costly and invasive CSF and neuroimaging measures, more feasible and acceptable for repeated measures, and more readily accessible in developing countries.
Executive summary.
Sphingolipid metabolism
Sphingolipids make up approximately one third of the content of eukaryotic cell membranes and are highly enriched in the CNS.
In addition to important structural roles, sphingolipid metabolites function as second messengers that modulate critical signaling functions for both inter- and intra-cellular events.
Sphingolipid metabolism is a dynamic process that modulates the formation of a number of bioactive metabolites including ceramide, ceramide-1-phosphate, sphingosine, S1P and glycosphingolipids.
Cell & animal studies suggest sphingolipids contribute to Alzheimer’s pathology
Cellular and animal studies have found both direct and indirect links between sphingolipids, mainly ceramides, amyloid-β, tau phosphorylation and neurodegeneration.
Postmortem & cerebrospinal fluid sphingolipids in Alzheimer’s disease
Ceramide levels are elevated, and gene expression of enzymes contributing to ceramide levels are upregulated, in brain regions of Alzheimer’s disease (AD) patients relative to nonpathological age-matched controls, and vary by disease severity. Studies examining brain sphingomyelins in AD patients and controls have been inconsistent.
Cerebrospinal fluid sphingolipids including ceramides, sphingomyelins and sulfatides are elevated in AD brains compared with nonpathological controls, and vary by disease severity.
Peripheral sphingolipids: risk & progression of AD
-
Elevated blood ceramide levels have been found to predict:
Cognitive impairment and AD among cognitively normal individuals
Memory decline and hippocampal volume loss among amnestic mild cognitive impairment patients
Faster rates of cognitive decline among AD patients
Steps for further research & validation
Further development of high-throughput methods is needed to develop clinically useful sphingolipid assays.
Studies assessing the relationship between both cerebrospinal fluid and peripheral sphingolipids, and cerebrospinal fluid amyloid-β and tau, and neuroimaging measures are needed. The studies will help determine whether sphingolipid perturbations and biomarkers will be specific to AD or more global markers of neurodegeneration.
Additional research is also needed to assess both the direct and indirect mechanisms by which peripheral sphinoglipids can affect brain processes.
Experimental designs in humans are needed to confirm mechanistic associations between peripheral and brain sphingolipids, and Alzheimer’s pathology.
Footnotes
For reprint orders, please contact: reprints@futuremedicine.com
Financial & competing interests disclosure
This work was supported by a grant from the National Institute on Aging (U01 AG37526). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Alzheimer’s Disease International. World Alzheimer Report 2009 Executive Summary. London, UK: 2009. [Google Scholar]
- 2.Pimplikar SW, Nixon RA, Robakis NK, Shen J, Tsai LH. Amyloid-independent mechanisms in Alzheimer’s disease pathogenesis. J Neurosci. 2010;30(45):14946–14954. doi: 10.1523/JNEUROSCI.4305-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mielke MM, Lyketsos CG. Lipids and the pathogenesis of Alzheimer’s disease: is there a link? Int Rev Psychiatry. 2006;18(2):173–186. doi: 10.1080/09540260600583007. [DOI] [PubMed] [Google Scholar]
- 4.Lütjohann D, Meichsner S, Pettersson H. Lipids in Alzheimer’s disease and their potential for therapy. Clin Lipidol. 2012;7(1):65–78. [Google Scholar]
- 5.Haughey NJ, Bandaru VV, Bae M, Mattson MP. Roles for dysfunctional sphingolipid metabolism in Alzheimer’s disease neuropathogenesis. Biochim Biophys Acta. 2010;1801(8):878–886. doi: 10.1016/j.bbalip.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.France-Lanord V, Brugg B, Michel PP, Agid Y, Ruberg M. Mitochondrial free radical signal in ceramide-dependent apoptosis: a putative mechanism for neuronal death in Parkinson’s disease. J Neurochem. 1997;69(4):1612–1621. doi: 10.1046/j.1471-4159.1997.69041612.x. [DOI] [PubMed] [Google Scholar]
- 7.Cutler RG, Pedersen WA, Camandola S, Rothstein JD, Mattson MP. Evidence that accumulation of ceramides and cholesterol esters mediates oxidative stress-induced death of motor neurons in amyotrophic lateral sclerosis. Ann Neurol. 2002;52(4):448–457. doi: 10.1002/ana.10312. [DOI] [PubMed] [Google Scholar]
- 8▪▪.Cutler RG, Kelly J, Storie K, et al. Involvement of oxidative stress-induced abnormalities in ceramide and cholesterol metabolism in brain aging and Alzheimer’s disease. Proc Natl Acad Sci USA. 2004;101(7):2070–2075. doi: 10.1073/pnas.0305799101. Describes how ceramides may mediate the relationship between amyloid-β deposition and neurodegeneration. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haughey NJ, Cutler RG, Tamara A, et al. Perturbation of sphingolipid metabolism and ceramide production in HIV-dementia. Ann Neurol. 2004;55(2):257–267. doi: 10.1002/ana.10828. [DOI] [PubMed] [Google Scholar]
- 10.Mielke MM, Bandaru VV, Haughey NJ, Rabins PV, Lyketsos CG, Carlson MC. Serum sphingomyelins and ceramides are early predictors of memory impairment. Neurobiol Aging. 2010;31(1):17–24. doi: 10.1016/j.neurobiolaging.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mielke MM, Bandaru VV, McArthur JC, Chu M, Haughey NJ. Disturbance in cerebral spinal fluid sphingolipid content is associated with memory impairment in subjects infected with the human immunodeficiency virus. J Neurovirol. 2010;16(6):445–456. doi: 10.3109/13550284.2010.525599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Merrill AH, Jr, Jones DD. An update of the enzymology and regulation of sphingomyelin metabolism. Biochim Biophys Acta. 1990;1044(1):1–12. doi: 10.1016/0005-2760(90)90211-f. [DOI] [PubMed] [Google Scholar]
- 13.Slotte JP. Cholesterol-sphingomyelin interactions in cells – effects on lipid metabolism. Subcell Biochem. 1997;28:277–293. [PubMed] [Google Scholar]
- 14.Brown DA, London E. Structure and function of sphingolipid- and cholesterol-rich membrane rafts. J Biol Chem. 2000;275(23):17221–17224. doi: 10.1074/jbc.R000005200. [DOI] [PubMed] [Google Scholar]
- 15.Andrieu-Abadie N, Gouaze V, Salvayre R, Levade T. Ceramide in apoptosis signaling: relationship with oxidative stress. Free Radic Biol Med. 2001;31(6):717–728. doi: 10.1016/s0891-5849(01)00655-4. [DOI] [PubMed] [Google Scholar]
- 16.Goodman Y, Mattson MP. Ceramide protects hippocampal neurons against excitotoxic and oxidative insults, and amyloid β-peptide toxicity. J Neurochem. 1996;66(2):869–872. doi: 10.1046/j.1471-4159.1996.66020869.x. [DOI] [PubMed] [Google Scholar]
- 17.Hannun YA. Functions of ceramide in coordinating cellular responses to stress. Science. 1996;274(5294):1855–1859. doi: 10.1126/science.274.5294.1855. [DOI] [PubMed] [Google Scholar]
- 18.Ariga T, McDonald MP, Yu RK. Role of ganglioside metabolism in the pathogenesis of Alzheimer’s disease – a review. J Lipid Res. 2008;49(6):1157–1175. doi: 10.1194/jlr.R800007-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ohsawa T. Changes of mouse brain gangliosides during aging from young adult until senescence. Mech Ageing Dev. 1989;50(2):169–177. doi: 10.1016/0047-6374(89)90012-2. [DOI] [PubMed] [Google Scholar]
- 20.Svennerholm L, Bostrom K, Jungbjer B, Olsson L. Membrane lipids of adult human brain: lipid composition of frontal and temporal lobe in subjects of age 20 to 100 years. J Neurochem. 1994;63(5):1802–1811. doi: 10.1046/j.1471-4159.1994.63051802.x. [DOI] [PubMed] [Google Scholar]
- 21▪▪.Mattson MP, Cutler RG, Jo DG. Alzheimer peptides perturb lipid-regulating enzymes. Nat Cell Biol. 2005;7(11):1045–1047. doi: 10.1038/ncb1105-1045. Effectively summarizes the relationships between ceramides and amyloid-β. [DOI] [PubMed] [Google Scholar]
- 22.Jana A, Pahan K. Human immunodeficiency virus type 1 gp120 induces apoptosis in human primary neurons through redox-regulated activation of neutral sphingomyelinase. J Neurosci. 2004;24(43):9531–9540. doi: 10.1523/JNEUROSCI.3085-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee JT, Xu J, Lee JM, et al. Amyloid-β peptide induces oligodendrocyte death by activating the neutral sphingomyelinase–ceramide pathway. J Cell Biol. 2004;164(1):123–131. doi: 10.1083/jcb.200307017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grimm MO, Grimm HS, Patzold AJ, et al. Regulation of cholesterol and sphingomyelin metabolism by amyloid-β and presenilin. Nat Cell Biol. 2005;7(11):1118–1123. doi: 10.1038/ncb1313. [DOI] [PubMed] [Google Scholar]
- 25.Gulbins E, Kolesnick R. Raft ceramide in molecular medicine. Oncogene. 2003;22(45):7070–7077. doi: 10.1038/sj.onc.1207146. [DOI] [PubMed] [Google Scholar]
- 26.Puglielli L, Ellis BC, Saunders AJ, Kovacs DM. Ceramide stabilizes β-site amyloid precursor protein cleaving enzyme 1 and promotes amyloid β-peptide biogenesis. J Biol Chem. 2003;278(22):19777–19783. doi: 10.1074/jbc.M300466200. [DOI] [PubMed] [Google Scholar]
- 27.Kalvodova L, Kahya N, Schwille P, et al. Lipids as modulators of proteolytic activity of BACE: involvement of cholesterol, glycosphingolipids, and anionic phospholipids in vitro. J Biol Chem. 2005;280(44):36815–36823. doi: 10.1074/jbc.M504484200. [DOI] [PubMed] [Google Scholar]
- 28.Yanagisawa K. Role of gangliosides in Alzheimer’s disease. Biochim Biophys Acta. 2007;1768(8):1943–1951. doi: 10.1016/j.bbamem.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 29.Yanagisawa K, Odaka A, Suzuki N, Ihara Y. GM1 ganglioside-bound amyloid β-protein (A β): a possible form of preamyloid in Alzheimer’s disease. Nat Med. 1995;1(10):1062–1066. doi: 10.1038/nm1095-1062. [DOI] [PubMed] [Google Scholar]
- 30.Hayashi H, Kimura N, Yamaguchi H, et al. A seed for Alzheimer amyloid in the brain. J Neurosci. 2004;24(20):4894–4902. doi: 10.1523/JNEUROSCI.0861-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamamoto N, Hirabayashi Y, Amari M, et al. Assembly of hereditary amyloid β-protein variants in the presence of favorable gangliosides. FEBS Lett. 2005;579(10):2185–2190. doi: 10.1016/j.febslet.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 32.Yamamoto N, Matsubara E, Maeda S, et al. A ganglioside-induced toxic soluble Aβ assembly. Its enhanced formation from Aβ bearing the Arctic mutation. J Biol Chem. 2007;282(4):2646–2655. doi: 10.1074/jbc.M606202200. [DOI] [PubMed] [Google Scholar]
- 33.Oikawa N, Yamaguchi H, Ogino K, et al. Gangliosides determine the amyloid pathology of Alzheimer’s disease. Neuroreport. 2009;20(12):1043–1046. doi: 10.1097/WNR.0b013e32832e4b9d. [DOI] [PubMed] [Google Scholar]
- 34.Eckert GP, Muller WE. Presenilin 1 modifies lipid raft composition of neuronal membranes. Biochem Biophys Res Commun. 2009;382(4):673–677. doi: 10.1016/j.bbrc.2009.03.070. [DOI] [PubMed] [Google Scholar]
- 35.Takasugi N, Sasaki T, Suzuki K, et al. BACE1 activity is modulated by cell-associated sphingosine-1-phosphate. J Neurosci. 2011;31(18):6850–6857. doi: 10.1523/JNEUROSCI.6467-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goedert M, Jakes R, Qi Z, Wang JH, Cohen P. Protein phosphatase 2A is the major enzyme in brain that dephosphorylates tau protein phosphorylated by proline-directed protein kinases or cyclic AMP-dependent protein kinase. J Neurochem. 1995;65(6):2804–2807. doi: 10.1046/j.1471-4159.1995.65062804.x. [DOI] [PubMed] [Google Scholar]
- 37.Gong CX, Grundke-Iqbal I, Iqbal K. Dephosphorylation of Alzheimer’s disease abnormally phosphorylated tau by protein phosphatase-2A. Neuroscience. 1994;61(4):765–772. doi: 10.1016/0306-4522(94)90400-6. [DOI] [PubMed] [Google Scholar]
- 38.Chalfant CE, Kishikawa K, Mumby MC, Kamibayashi C, Bielawska A, Hannun YA. Long chain ceramides activate protein phosphatase-1 and protein phosphatase-2A. Activation is stereospecific and regulated by phosphatidic acid. J Biol Chem. 1999;274(29):20313–20317. doi: 10.1074/jbc.274.29.20313. [DOI] [PubMed] [Google Scholar]
- 39.Dobrowsky RT, Kamibayashi C, Mumby MC, Hannun YA. Ceramide activates heterotrimeric protein phosphatase 2A. J Biol Chem. 1993;268(21):15523–15530. [PubMed] [Google Scholar]
- 40▪.Mukhopadhyay A, Saddoughi SA, Song P, et al. Direct interaction between the inhibitor 2 and ceramide via sphingolipid-protein binding is involved in the regulation of protein phosphatase 2A activity and signaling. FASEB J. 2009;23(3):751–763. doi: 10.1096/fj.08-120550. Describes a mechanism by which ceramide may regulate tau phosphorylation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oddo S, Caccamo A, Tran L, et al. Temporal profile of amyloid-β (Aβ) oligomerization in an in vivo model of Alzheimer disease. A link between Aβ and tau pathology. J Biol Chem. 2006;281(3):1599–1604. doi: 10.1074/jbc.M507892200. [DOI] [PubMed] [Google Scholar]
- 42.Arboleda G, Morales LC, Benitez B, Arboleda H. Regulation of ceramide-induced neuronal death: cell metabolism meets neurodegeneration. Brain Res Rev. 2009;59(2):333–346. doi: 10.1016/j.brainresrev.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 43.Hannun YA, Luberto C. Ceramide in the eukaryotic stress response. Trends Cell Biol. 2000;10(2):73–80. doi: 10.1016/s0962-8924(99)01694-3. [DOI] [PubMed] [Google Scholar]
- 44.de la Monte SM. Triangulated mal-signaling in Alzheimer’s disease: roles of neurotoxic ceramides, ER stress, and insulin resistance reviewed. J Alzheimers Dis. 2012;30:S231–S249. doi: 10.3233/JAD-2012-111727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang Z, Zhao R, Qi J, Wen S, Tang Y, Wang D. Inhibition of glycogen synthase kinase-3β by Angelica sinensis extract decreases β-amyloid-induced neurotoxicity and tau phosphorylation in cultured cortical neurons. J Neurosci Res. 2011;89(3):437–447. doi: 10.1002/jnr.22563. [DOI] [PubMed] [Google Scholar]
- 46.He X, Huang Y, Li B, Gong CX, Schuchman EH. Deregulation of sphingolipid metabolism in Alzheimer’s disease. Neurobiol Aging. 2010;31(3):398–408. doi: 10.1016/j.neurobiolaging.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barrier L, Ingrand S, Fauconneau B, Page G. Gender-dependent accumulation of ceramides in the cerebral cortex of the APP(SL)/PS1Ki mouse model of Alzheimer’s disease. Neurobiol Aging. 2010;31(11):1843–1853. doi: 10.1016/j.neurobiolaging.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 48.Barrier L, Fauconneau B, Noel A, Ingrand S. Ceramide and related-sphingolipid levels are not altered in disease-associated brain regions of APP and APP/PS1 mouse models of Alzheimer’s disease: relationship with the lack of neurodegeneration? Int J Alzheimers Dis. 2011:920958. doi: 10.4061/2011/920958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cheng H, Zhou Y, Holtzman DM, Han X. Apolipoprotein E mediates sulfatide depletion in animal models of Alzheimer’s disease. Neurobiol Aging. 2010;31(7):1188–1196. doi: 10.1016/j.neurobiolaging.2008.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cuvillier O, Pirianov G, Kleuser B, et al. Suppression of ceramide-mediated programmed cell death by sphingosine-1-phosphate. Nature. 1996;381(6585):800–803. doi: 10.1038/381800a0. [DOI] [PubMed] [Google Scholar]
- 51.Mielke MM, Lyketsos CG. Alterations of the sphingolipid pathway in Alzheimer’s disease: new biomarkers and treatment targets? Neuromolecular Med. 2010;12(4):331–340. doi: 10.1007/s12017-010-8121-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pettegrew JW, Panchalingam K, Hamilton RL, McClure RJ. Brain membrane phospholipid alterations in Alzheimer’s disease. Neurochem Res. 2001;26(7):771–782. doi: 10.1023/a:1011603916962. [DOI] [PubMed] [Google Scholar]
- 53▪.Han X, Holtzman DM, McKeel DW, Jr, Kelley J, Morris JC. Substantial sulfatide deficiency and ceramide elevation in very early Alzheimer’s disease: potential role in disease pathogenesis. J Neurochem. 2002;82(4):809–818. doi: 10.1046/j.1471-4159.2002.00997.x. Describes, for the first time, the perturbations of sulfatides and ceramides in postmortem brains of early Alzheimer’s disease cases. [DOI] [PubMed] [Google Scholar]
- 54.Huang Y, Tanimukai H, Liu F, Iqbal K, Grundke-Iqbal I, Gong CX. Elevation of the level and activity of acid ceramidase in Alzheimer’s disease brain. Eur J Neurosci. 2004;20(12):3489–3497. doi: 10.1111/j.1460-9568.2004.03852.x. [DOI] [PubMed] [Google Scholar]
- 55.Katsel P, Li C, Haroutunian V. Gene expression alterations in the sphingolipid metabolism pathways during progression of dementia and Alzheimer’s disease: a shift toward ceramide accumulation at the earliest recognizable stages of Alzheimer’s disease? Neurochem Res. 2007;32(4–5):845–856. doi: 10.1007/s11064-007-9297-x. [DOI] [PubMed] [Google Scholar]
- 56.Bandaru VV, Troncoso J, Wheeler D, et al. ApoE4 disrupts sterol and sphingolipid metabolism in Alzheimer’s but not normal brain. Neurobiol Aging. 2009;30(4):591–599. doi: 10.1016/j.neurobiolaging.2007.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Filippov V, Song MA, Zhang K, et al. Increased ceramide in brains with Alzheimer’s and other neurodegenerative diseases. J Alzheimers Dis. 2012;29(3):537–547. doi: 10.3233/JAD-2011-111202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Marks N, Berg MJ, Saito M. Glucosylceramide synthase decrease in frontal cortex of Alzheimer brain correlates with abnormal increase in endogenous ceramides: consequences to morphology and viability on enzyme suppression in cultured primary neurons. Brain Res. 2008;1191:136–147. doi: 10.1016/j.brainres.2007.10.066. [DOI] [PubMed] [Google Scholar]
- 59.Han X, Fagan AM, Cheng H, Morris JC, Xiong C, Holtzman DM. Cerebrospinal fluid sulfatide is decreased in subjects with incipient dementia. Ann Neurol. 2003;54(1):115–119. doi: 10.1002/ana.10618. [DOI] [PubMed] [Google Scholar]
- 60.Satoi H, Tomimoto H, Ohtani R, et al. Astroglial expression of ceramide in Alzheimer’s disease brains: a role during neuronal apoptosis. Neuroscience. 2005;130(3):657–666. doi: 10.1016/j.neuroscience.2004.08.056. [DOI] [PubMed] [Google Scholar]
- 61.Kosicek M, Kirsch S, Bene R, et al. Nano-HPLC-MS analysis of phospholipids in cerebrospinal fluid of Alzheimer’s disease patients – a pilot study. Anal Bioanal Chem. 2010;398(7–8):2929–2937. doi: 10.1007/s00216-010-4273-8. [DOI] [PubMed] [Google Scholar]
- 62.Kosicek M, Zetterberg H, Andreasen N, Peter-Katalinic J, Hecimovic S. Elevated cerebrospinal fluid sphingomyelin levels in prodromal Alzheimer’s disease. Neurosci Lett. 2012;516(2):302–305. doi: 10.1016/j.neulet.2012.04.019. [DOI] [PubMed] [Google Scholar]
- 63.Mattila KM, Frey H. Two-dimensional analysis of qualitative and quantitative changes in blood cell proteins in Alzheimer’s disease: search for extraneuronal markers. Appl Theor Electrophor. 1995;4(4):189–196. [PubMed] [Google Scholar]
- 64.Koyama A, Okereke O, Yang T, Blacker D, Selkoe DJ, Grodstein F. Plasma Amyloid-β as a predictor of dementia and cognitive decline: a systematic review and meta-analysis. Arch Neurol. 2012;69(7):824–831. doi: 10.1001/archneurol.2011.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kalanj-Bognar S, Rundek T, Furac I, Demarin V, Cosovic C. Leukocyte lysosomal enzymes in Alzheimer’s disease and Down’s syndrome. J Gerontol A Biol Sci Med Sci. 2002;57(1):B16–B21. doi: 10.1093/gerona/57.1.b16. [DOI] [PubMed] [Google Scholar]
- 66.Emiliani C, Urbanelli L, Racanicchi L, et al. Up-regulation of glycohydrolases in Alzheimer’s Disease fibroblasts correlates with Ras activation. J Biol Chem. 2003;278(40):38453–38460. doi: 10.1074/jbc.M303030200. [DOI] [PubMed] [Google Scholar]
- 67.Pitto M, Raimondo F, Zoia C, Brighina L, Ferrarese C, Masserini M. Enhanced GM1 ganglioside catabolism in cultured fibroblasts from Alzheimer patients. Neurobiol Aging. 2005;26(6):833–838. doi: 10.1016/j.neurobiolaging.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 68.Mielke MM, Bandaru VVR, Xia J, et al. Serum ceramides increase the risk of Alzheimer disease: the Women’s Health and Aging Study II. Neurology. 2012;79:633–641. doi: 10.1212/WNL.0b013e318264e380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mielke MM, Haughey NJ, Ratnam Bandaru VV, et al. Plasma ceramides are altered in mild cognitive impairment and predict cognitive decline and hippocampal volume loss. Alzheimer’s Dement. 2010;6(5):378–385. doi: 10.1016/j.jalz.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mielke MM, Haughey NJ, Bandaru VV, et al. Plasma sphingomyelins are associated with cognitive progression in Alzheimer’s disease. J Alzheimers Dis. 2011;27(2):259–269. doi: 10.3233/JAD-2011-110405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Han X, Rozen S, Boyle SH, et al. Metabolomics in early Alzheimer’s disease: identification of altered plasma sphingolipidome using shotgun lipidomics. PLoS ONE. 2011;6(7):e21643. doi: 10.1371/journal.pone.0021643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72▪.Hicks AA, Pramstaller PP, Johansson A, et al. Genetic determinants of circulating sphingolipid concentrations in European populations. PLoS Genet. 2009;5(10):e1000672. doi: 10.1371/journal.pgen.1000672. Describes the genetic variants that influence levels of circulating sphingolipids in the population. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73▪.de la Monte SM, Longato L, Tong M, Wands JR. Insulin resistance and neurodegeneration: roles of obesity, type 2 diabetes mellitus and non-alcoholic steatohepatitis. Curr Opin Investig Drugs. 2009;10(10):1049–1060. Describes potential mechanisms by which peripheral ceramides affect brain pathology. [PMC free article] [PubMed] [Google Scholar]
- 74.de la Monte SM, Tong M, Nguyen V, Setshedi M, Longato L, Wands JR. Ceramide-mediated insulin resistance and impairment of cognitive-motor functions. J Alzheimers Dis. 2010;21(3):967–984. doi: 10.3233/JAD-2010-091726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ichi I, Nakahara K, Miyashita Y, et al. Association of ceramides in human plasma with risk factors of atherosclerosis. Lipids. 2006;41(9):859–863. doi: 10.1007/s11745-006-5041-6. [DOI] [PubMed] [Google Scholar]
- 76.Nelson JC, Jiang XC, Tabas I, Tall A, Shea S. Plasma sphingomyelin and subclinical atherosclerosis: findings from the multi-ethnic study of atherosclerosis. Am J Epidemiol. 2006;163(10):903–912. doi: 10.1093/aje/kwj140. [DOI] [PubMed] [Google Scholar]
- 77.Summers SA. Ceramides in insulin resistance and lipotoxicity. Prog Lipid Res. 2006;45(1):42–72. doi: 10.1016/j.plipres.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 78.Holland WL, Summers SA. Sphingolipids, insulin resistance, and metabolic disease: new insights from in vivo manipulation of sphingolipid metabolism. Endocr Rev. 2008;29(4):381–402. doi: 10.1210/er.2007-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Haus JM, Kashyap SR, Kasumov T, et al. Plasma ceramides are elevated in obese subjects with type 2 diabetes and correlate with the severity of insulin resistance. Diabetes. 2009;58(2):337–343. doi: 10.2337/db08-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mielke MM, Rosenberg PB, Tschanz J, et al. Vascular factors predict rate of progression in Alzheimer disease. Neurology. 2007;69(19):1850–1858. doi: 10.1212/01.wnl.0000279520.59792.fe. [DOI] [PubMed] [Google Scholar]
- 81.Chan RB, Oliveira TG, Cortes EP, et al. Comparative lipidomic analysis of mouse and human brain with Alzheimer disease. J Biol Chem. 2012;287(4):2678–2688. doi: 10.1074/jbc.M111.274142. [DOI] [PMC free article] [PubMed] [Google Scholar]