Abstract
The effects of the selective peroxisome proliferator activated receptor-gamma (PPAR-γ) inhibitor GW9662 on phenylbutyrate (PB)-induced NMR-detectable lipid metabolites was investigated on DU145 prostate cancer cells. DU145 cells were perfused with 10 mM PB in the presence or absence of 1 µM of GW9662 and the results monitored by 31P and diffusion-weighted 1H NMR spectroscopy. GW9662 completely reversed PB-induced NMR-visible lipid and total choline accumulation in 1H spectra and glycerophosphocholine and β-NTP in 31P spectra. In addition, pre-incubation with GW9662 significantly reduced PB-induced caspase-3 activation, reversed the G1 block as measured by flow cytometry, and otherwise had little effect on cell survival as measured by MTT assay. These results suggest that the NMR visible lipid accumulation and apoptosis induced by PB treatment occurs through a mechanism that is mediated by PPAR-γ.
Keywords: Phenylbutyrate, NMR, Differentiation therapy, Peroxisome proliferator activating receptor gamma, Apoptosis, NMR-visible lipids, Glycerophosphocholine
INTRODUCTION
Differentiating agents are chemotherapeutic agents that can cause either reversion of the malignant phenotype or the triggering of apoptosis. Phenylacetate (PA) and phenylbutyrate (PB) are differentiating agents that induce G1 cell cycle arrest, cytostasis, differentiation and apoptosis in a variety of cell types, including prostate adenocarcinomas (1–3). Clinical trials have shown that PB treatment leads to improvements in prognostic indicators, disease stabilization and improvements of hematological parameters in advanced solid tumors and hematological malignancies (4–6). The mechanism of PA- and PB-induced differentiation is presently under investigation. PB has been shown to have coordinate effects on lipid metabolism including the inhibition of the cholesterol biosynthetic pathway (7,8), inhibition of protein prenylation and histone deacetylase (9), and the induction of peroxisome proliferator-activated receptors (PPARs) alpha and gamma (10,11).
PPARs are ligand-activated transcription factors, implicated in growth control and differentiation (11,12). PPAR-α regulates fatty acid metabolism and is highly expressed in liver, kidney and intestine. PPAR-γ exists in three isoforms (PPAR-γ1-γ3). PPAR-γ2 is expressed primarily in adipose tissue and is a potent regulator of adipocyte differentiation (13). Since one manifestation of adipocyte differentiation is increased uptake and synthesis of triglycerides, we hypothesized that PPAR-γ antagonists may modulate PB-induced effects on cell cycle and lipid metabolism.
GW9662 (2-chloro-5-nitro-N-phenylbenzamide) is an irreversible antagonist of PPAR-γ, identified in a competition-binding assay against the human ligand-binding domain. It has been shown to bind to PPAR-γ with an IC50 in the nanomolar range while exhibiting a 10 and 600-fold less potency in binding to PPAR-α and PPAR-δ, respectively. Incubation of GW9662 with PPAR-γ has shown covalent modification at Cys285, as determined by mass spectroscopic studies of the PPAR-γ binding domain. The antagonistic activity of GW9662 has been determined in cell-based reporter assays, and confirmed in adipocyte differentiation assays (14).
We have previously shown that the response of DU145 prostate cancer cells to PA or PB can be detected as increases in lipid metabolite levels using nuclear magnetic resonance spectroscopy (NMR) (1). Increases in neutral lipids and total choline (tCho) in 1H MR spectra, and glycerophosphocholine (GPC) in 31P MR spectra were accompanied by increased markers of differentiation, including lipid droplet accumulation and G1 cell cycle arrest. The effects of PB were greater than those of PA, showing higher elevations in MR-visible lipid metabolites, but only PB-treatment led to apoptosis. Further, we showed that this response could be modulated by specific inhibition of lipid metabolic pathways. Inhibition of cholesterol biosynthesis with the HMG-CoA reductase inhibitor lovastatin increased PB-induced NMR-visible tCho and GPC, reversing late markers of apoptosis with no significant effect on neutral lipids or cell cycle arrest (3). On the basis of these studies, we decided to examine the effects of the PPAR-γ inhibitor GW9662 on the lipid metabolite levels induced by PB in DU145 prostate cancer cells using 1H and 31P MR spectroscopy. The long-term goals of these studies are to decipher the mechanisms underlying changes in lipid metabolites that can be measured non-invasively, to improve the efficacy of clinical magnetic resonance spectroscopy in the detection of the response to differentiation therapy and in monitoring tumor apoptosis.
MATERIALS AND METHODS
DU145 cells
DU145 human prostate adenocarcinoma cells were cultured in Eagle's minimum essential medium (EMEM) supplemented with 10% fetal bovine serum (FBS) (Sigma, St. Louis, MO), penicillin-streptomycin (100 units / ml penicillin, 70 µM streptomycin) and 2 mM glutamine (Invitrogen, Carlsbad, CA) (1). Cells were routinely cultured in 150 cm2 tissue culture flasks in a humidified atmosphere of 5% CO2 in air at 37°C.
Drugs
Phenylbutyrate was obtained from Scandinavian Formulas (Sellersville, PA). GW9662 was obtained from Sigma-Aldrich (St. Louis, MO). PB was dissolved in culture medium to a stock solution of 100 mM. GW9662 (25 mg) was dissolved in DMSO (Fisher, Molecular Biology Grade) to a stock solution of 9×10−3 M.
NMR Measurements
Cell perfusion procedures including the apparatus used for these experiments has been described in detail elsewhere (1,3). Briefly, DU145 cells were seeded on Biosilon microcarrier beads (Nunc, Denmark) in Petri dishes at a concentration of 3.0×106 cells per ml. After 48 h incubation, 3.5 ml of microcarriers containing 3 – 4×107 cells were transferred to a 10 mm NMR tube and perfused with culture medium (1.5 ml/min) through a teflon tube inserted to the bottom of the NMR tube (1). The medium temperature was regulated using a heated water bath, water jacketed tubing and the Varian temperature control system, and oxygenation maintained by pumping 95% O2 / 5% CO2 through a lung containing silicon tubing with high gas permeability.
Cells were perfused for at least one hour in the magnet during which time baseline data was collected. PB and/or GW9662 were added to the medium reservoir to a final concentration of 10 mM and 1 µM and the cells were perfused for 16 h during which time NMR data was collected.
NMR spectra were acquired on a Varian INOVA 9.4 T NMR spectrometer (Palo Alto, CA) using a 10 mm-multinuclear probe (Doty Scientific, Columbia, SC). Three data sets were alternately acquired in one-hour blocks, a diffusion-weighted (DW) proton spectrum with and without CHESS water suppression and a 31P NMR spectrum (1). For the DW pulse sequence the following acquisition parameters were used: spectral width, 4,000 Hz; repetition time (TR), 2 s; data size, 2 K; number of transients, 256 (8 in the absence of water suppression); echo time (TE), 21 ms; mixing time (TM), 89 ms; time between diffusion gradients (∆), 100 ms; duration of diffusion gradient (δ), 3 ms; diffusion gradient strength (Gdiff), 18 G/cm (b=2.1×109 s/m2). For 31P NMR spectra, 2000 transients were acquired with a 60° rf pulse, TR = 1 s; a data size of 2 K, and a spectral width of 7,000 Hz. A line broadening of 10 and 15 Hz was applied to 1H and 31P spectra respectively before Fourier transformation and 31P spectra for four consecutive time points were summed to improve signal to noise. Phosphorous chemical shifts were expressed relative to an internal reference of α-NTP at -10.0 ppm, which corresponds to a chemical shift of phosphoric acid at 0 ppm.
Resonance intensities were fit using the AMARES subroutine in the jMRUI NMR spectral analysis software package (15,16). Peak areas were scaled to the intracellular water resonance at the same time point obtained from DW spectra collected without water suppression and reported as changes relative to time zero. At least three separate experiments were performed for each condition and the data reported as the mean ± standard error.
Caspase-3 Assay
Caspase 3 activity was measured using the Invitrogen EnzChek Caspase-3 Assay Kit #1 (Invitrogen, Carlsbad, CA). DU145 cells (1×106) were seeded in 100 mm tissue culture plates. At 24 hours, the cells were pre-treated with 1 µM GW9662 followed by the addition of 10 mM PB 1 hour later. Control cultures received an equivalent volume of PBS. After 16 h treatment, the cells were washed once with PBS, scraped from the plate, centrifuged and resuspended in 50 µl lysis buffer (10 mM Tris, pH 7.5, 100 mM NaCl, 1 mM EDTA in 0.01% Triton X-100). The cells were frozen in a dry ice ethanol bath, thawed, centrifuged and an aliquot of the supernatant removed for a protein assay (Bradford, BioRad, Hercules, CA). A solution containing 10 µg of total protein was constituted to a total volume of 90 µL in lysis buffer in a 96-well plate and 90 µL of 20 µM z-DEVD-AMC in reaction buffer (20 mM PIPES, pH 7.4, 4 mM EDTA, 0.2% CHAPS, 10 mM DTT) was added. The plate was covered for 4 hours at room temperature and the fluorescence read on a Gemini XPS fluorescent plate reader (λex = 342 nm, λem= 441 nm).
MTT Assay
DU145 cells were seeded in 24-well plates at a concentration of 1×104 cells/ml per well. After 48 h, cells were pre-treated for 1 h with1 µM GW9662 (or PBS for controls) followed by addition of varying concentrations of PB. After 16 h incubation, cells were washed twice with Hank’s balanced salt solution (HBSS) with calcium and magnesium followed by treatment with 5 mg/ml MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; Sigma-Aldrich, St. Louis, MO) in HBSS for 15 min at 37°C. The resulting precipitate was dissolved in 200 µl DMSO (Fisher Scientific, Molecular Biology Grade) and the absorbance read on a Bio-Tek ELX808 micro plate reader at 540 nm.
Cell Cycle Analysis
Cells were seeded in 6-well tissue culture plates at a concentration of 3.75×105 cells/ml. At 24 h, cells were treated with GW9662 or PBS followed one hour later by treatment with PB as described above and incubated for 16 h. Washed and trypsinized cells were resuspended in 0.5 ml of 100 mg/ml propidium iodide in 1% Triton X-100/0.9 % NaCl and incubated for 1 h at 4°C with 50 µl of 50 µg/ml RNase. Each condition was examined in triplicate on two different days. Samples were analyzed on a Becton-Dickinson FACScan flow cytometer. A minimum of 1×104 events was collected and DNA histograms were constructed using the Mod Fit LT (Verity Software House, Topsham, ME). Statistical significance was determined using a two-sided Student’s t-test assuming equal variances for the two treatment groups.
RESULTS
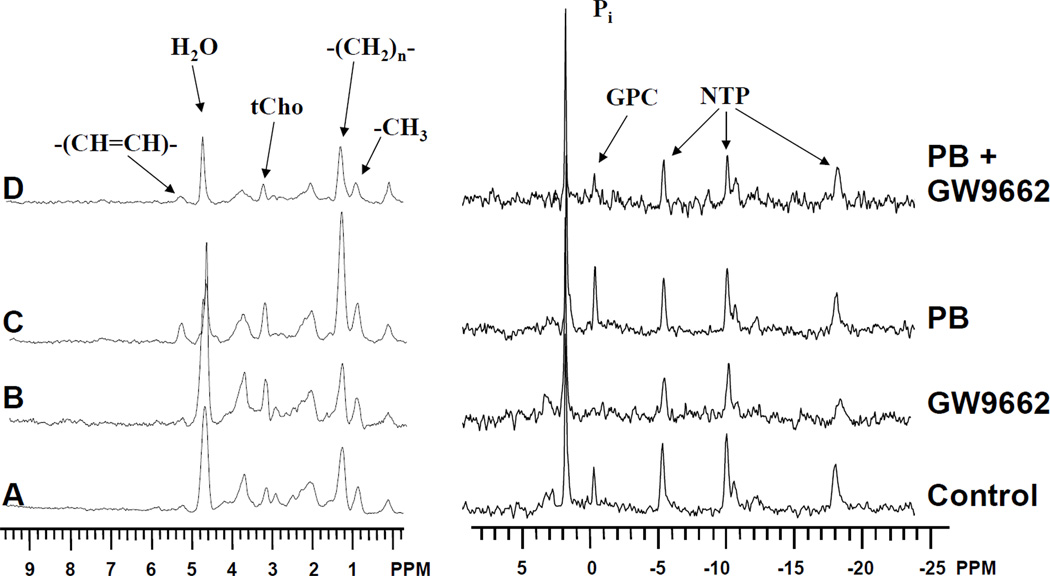
Proton and phosphorous MR spectra of DU145 cells are displayed on the left and right panels of Figure 1 respectively. Spectra are shown of cells perfused for 16 h with culture medium (Figure 1A), 1 µM GW9662 (Figure 1B), 10 mM PB (Figure 1C) and 10 mM PB with 1 h pre-perfusion with 1 µM GW9662 (Figure 1D). As previously shown (1,3), perfusion with 10 mM PB led to the time-dependent increase of a number of resonances associated with alterations in lipid metabolism (Figure 1C). In 1H spectra, this includes the 1.3 ppm and 0.9 ppm resonances arising predominantly from the methylene and terminal methyl groups of fatty acyl chains in neutral lipids and the olefinic 5.3 ppm resonance from unsaturated fatty acids. Alterations in phospholipid metabolism were also evident as increases in the total choline (tCho) trimethyl resonance at 3.2 ppm in 1H spectra, and a corresponding increase in GPC at 0.5 ppm in 31P spectra. Increases in these resonances were also observed in control cells perfused with culture medium alone, although these changes were of much smaller magnitude (Figure 1A). A 1-hour pre-perfusion with culture medium containing 1µM GW9662 was able to attenuate the increases in GPC and lipid resonances in both control (Figure 1B) and PB-treated (Figure 1D) cultures.
Figure 1.
Diffusion-weighted 1H NMR spectra (left traces) and 31P NMR spectra (right traces) of perfused DU145 prostate cancer cells treated with A) control at 16 h B) 1 µM GW9662 for 16 h; C) 10 mM PB for 16 h; D) 1 µM GW9662 and 10 mM PB for 16 h. The 0 ppm peak in 1H spectra results from the microcarrier beads used as a growth support for the cells. Additional peak assignments are as shown.
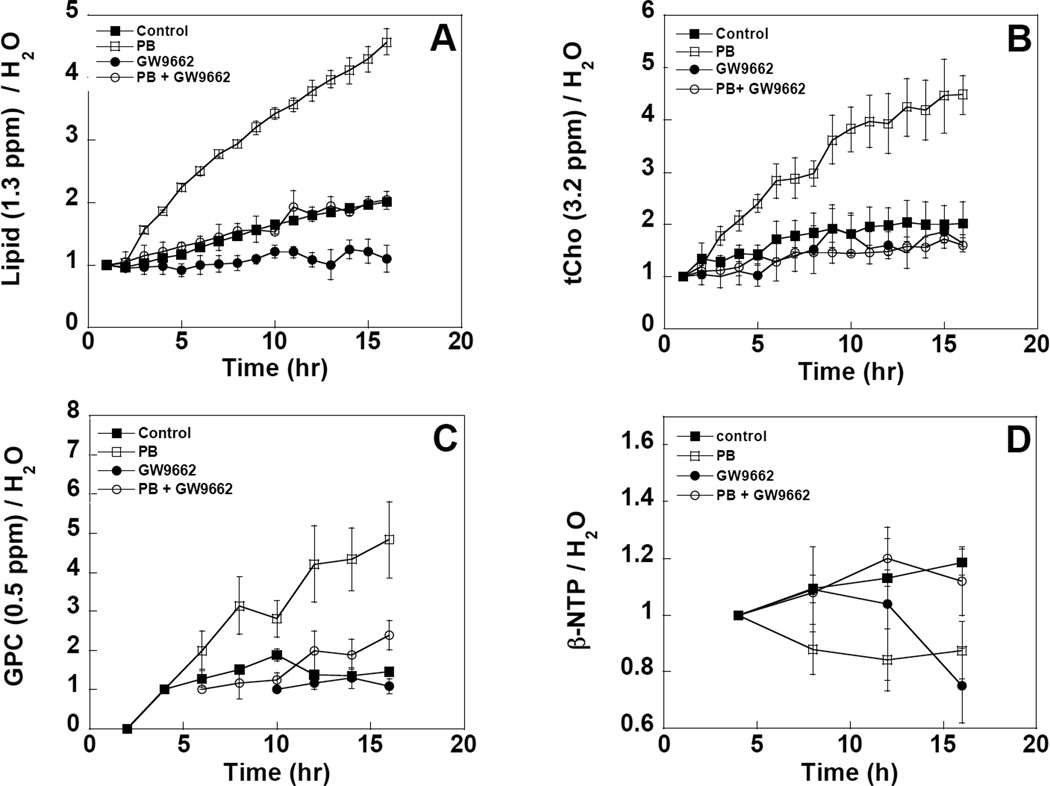
The changes in lipid resonance intensity as a function of time are displayed in Figure 2. Previously reported data from PB-treated and untreated control cells are also plotted on the same graphs for comparison purposes(1). The four panels show the time-dependent changes in two 1H resonances, the 1.3 ppm methylene (Figure 2A) and the 3.2 ppm tCho peaks (Figure 2B), and two 31P resonances, the 0.5 ppm GPC peak (Figure 2C) and the β-NTP peak at -18 ppm (Figure 2D). The data in Figures 2A to C all show a similar trend. The time dependent increase in methylene, tCho and GPC resonances induced by PB could be reduced to control levels by pre-perfusion with 1 µM GW9662. Even in the absence of PB, GW9662 was able to attenuate the 1.3 ppm methylene and the 0.5 ppm GPC resonances to a level below that observed in cultures perfused with medium alone. These data indicate that GW9662 affects triglyceride and phospholipid metabolism in both control and PB-treated cells. Pre-perfusion with GW9662 was able to reverse the NTP loss associated with PB treatment, returning NTP to control levels (Figure 2D). However, treatment with GW9662 alone led to a 25% decrease in NTP at longer times, indicating some possible toxicity associated with this compound at this concentration.
Figure 2.
Time dependent changes in the area of the A) methylene resonance at 1.3 ppm; B) the tCho resonance at 3.2 ppm; C) the GPC resonance at 0.5 ppm and D) the β-NTP resonance at −18 ppm of perfused DU145 cells as measured by 1H and 31P NMR. Changes in resonance areas were scaled to the intracellular water resonance at the analogous time point and are reported as changes relative to time zero. Open circles indicate cells treated with 1 µM GW9662 and 10 mM PB, filled circles indicate control cells treated with GW9662 alone, open squares indicate cells treated with 10 mM PB alone, filled squares indicate untreated control cells.
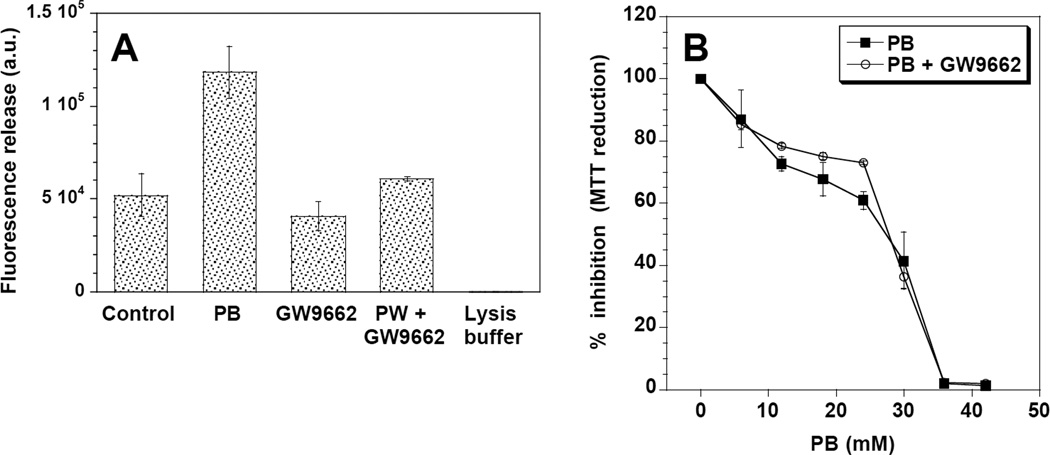
Since the increase in intracellular triglycerides has been associated with PB-induced apoptosis (1,3), we measured the effect of GW9662 on caspase-3 activation and MTT reduction. Treatment with 10 mM PB for 16 h induced a significant increase in caspase-3 activity (p < 0.05), that was reversed by pre-incubation with GW9662 (Figure 3A). No significant changes were induced by treatment with GW9662 alone. The effects of GW9662 on MTT reduction were subtler. Figure 3B shows that PB-treated cells displayed a concentration-dependent decrease in MTT staining, with an IC50 of approximately 30 mM. The addition of 1 µM GW9662 appeared to have a protective effect in cells treated with 10 – 25 mM PB, increasing percent viability by up to 13%, but had no effect at higher concentrations.
Figure 3.
Caspase-3 activation (a) and MTT dye reduction (b) in DU145 cells. Caspase-3 activation was significantly higher in DU145 cells treated with PB alone relative to untreated controls (*; p< 0.05), GW9662 only treated cells, and PB + GW9662 treated cells. In the MTT assay, no significant difference was observed in the cytotoxicity of cells treated with PB alone or PB + 1 µM GW9662.
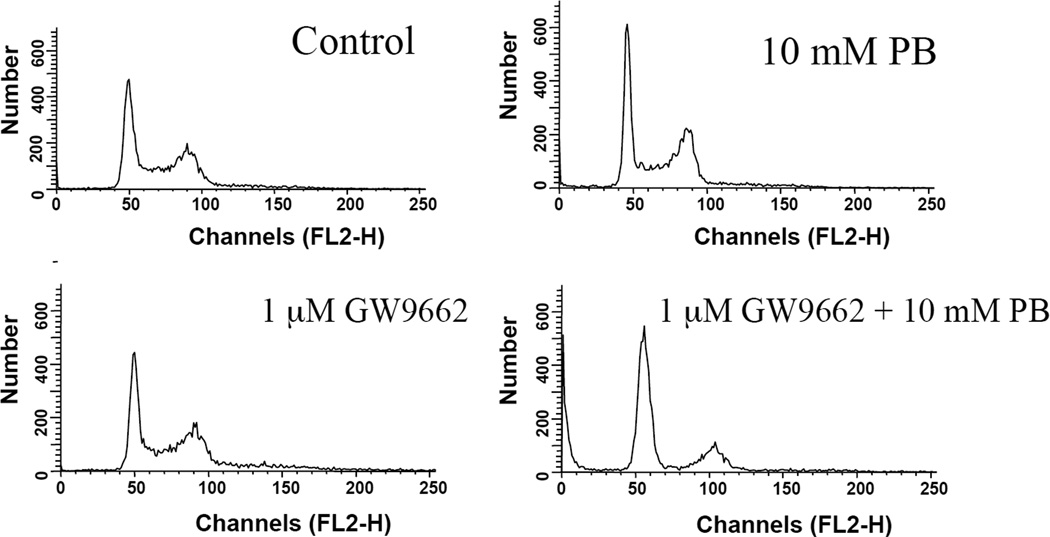
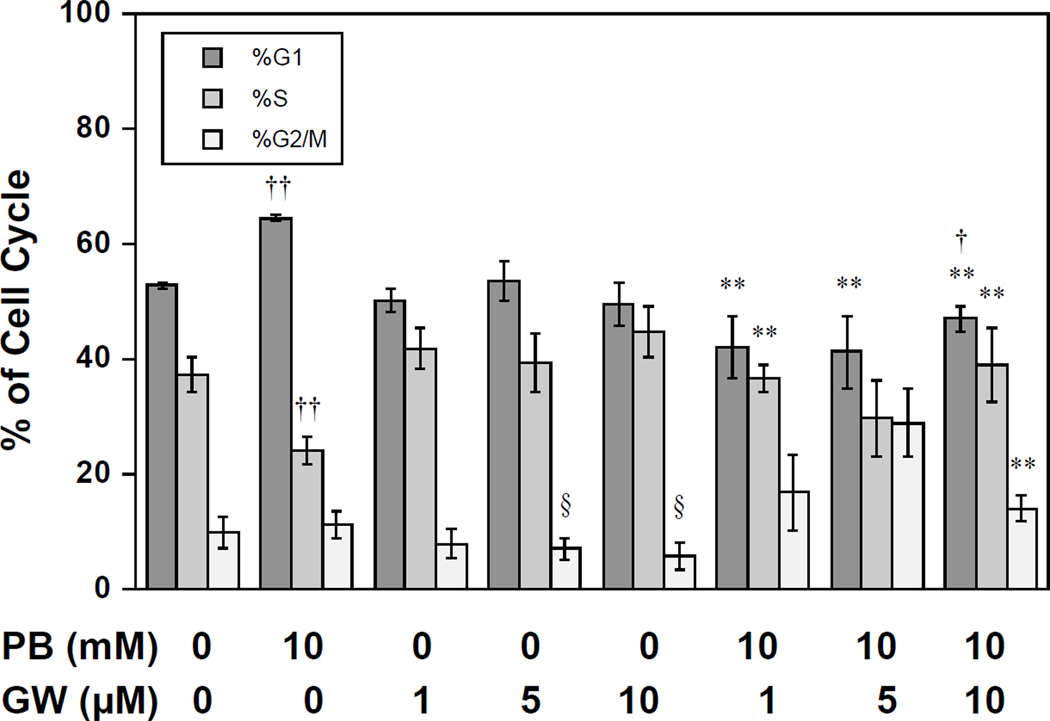
Finally, we investigated the effects of GW9662 on PB-induced cell cycle arrest. Figure 4 shows DNA histograms for 16 h treatment and Figure 5 shows calculated cell cycle percentages for a series of drug-inhibitor combinations. Incubation with only GW9662 (1 to 10 µM) had no significant effect on cell cycle distribution. As expected, treatment with PB led to a G1 arrest with a significant increase in G1-fraction and decrease in S relative to control cells (p < 0.01, t-test, Figure 5). Pre-incubation with GW9662 reversed the G1 block induced by PB-treatment, displaying a cell cycle distribution that was not significantly different from control cells, except for a decrease in G1 fraction at 10 µM GW9662 (p < 0.05, Figure 5). The pre-incubation of PB-treated cultures with 5 or 10 µM GW9662 also caused a significant increase in G2/M relative to cultures treated with the equivalent concentration of GW9662 alone (p < 0.05, Figure 5).
Figure 4.
Flow cytometric DNA histograms showing cell cycle distribution in DU145 cells treated with PB in the presence or absence of GW9662
Figure 5.
Cell cycle distribution of DU145 cells treated with PB and 0, 5 and 10 µM of GW9662. Significance is indicated by †: significantly different from control, p< 0.05; ††: significantly different from control; p< 0.01; *: significantly different from PB-treated, p< 0.05; **: significantly different from PB-treated, p< 0.01; §: significantly different from PB-treated at equivalent GW9662 concentration.
DISCUSSION
In this study, the effects of the irreversible PPAR-γ antagonist GW9662 were investigated on PB-treated DU145 prostate cells. Pretreatment with GW9662 was able to prevent or reverse PB-induced changes in MR-visible metabolite levels including increases in mobile lipids, tCho and GPC, as well as the loss of NTP. Moreover, GW9662 pretreatment was also able to reduce indicators of PB-induced cellular growth arrest and toxicity including the G1 arrest, induction of caspase-3 and, to some extent, cell death as measured by the MTT assay. These results indicate that PPAR-γ activation is an early event in PB-induced toxicity and differentiation.
Many PPAR-γ agonists have been shown to cause apoptosis or differentiation in cancer cell lines (17–19). Moreover, induction of apoptosis can be attenuated by pre-treatment with PPAR-γ antagonists such as GW9662 (20,21). The data presented here indicate that apoptosis induced by the differentiating agent phenylbutyrate is mediated by PPAR-γ and demonstrates that PB may effectively act as a PPAR-γ agonist. Phenylbutyrate activates PPAR in astrocytes, and binds to the peroxisome proliferator-activated receptors alpha and gamma (22). PA and PB have previously been shown to upregulate PPAR-γ, and to a lesser extent PPAR-α (11). PB has also been shown to enhance growth inhibition in adenocarcinomas induced by the PPAR-γ ligand ciglitazone (23). Recent evidence shows that valproic acid, a histone deacetylase inhibitor with functional and structural similarities to PB also induces PPAR-γ activation (24). It is interesting to note that one paper reports that GW9662 has no effect on the abilities of PB to induce cytotoxicity or the formation of lipid droplets in T47D breast cancer cells (25). However, these investigators used the inhibitor at 5–20 fold higher concentrations than in the current study, and observed inhibition of proliferation accompanied by decreased DNA synthesis. We observed no significant changes in S-phase with GW9662 treatment. (26). However, our observation that treatment with GW9662 alone caused NTP depletion at longer time periods demonstrates the potential toxicity of this compound at higher concentrations and longer exposure time.
A number of papers have demonstrated the increase in NMR-visible neutral lipids and lipid droplets in cells exposed to chemotherapeutic agents (1,3,27,28) and many have correlated these increases with apoptosis in vitro (29–31) and in vivo (32). Our previous work has shown that while the induction in intracellular triglyceride synthesis leading to mobile lipid formation and apoptosis are common pathways initiated by chemotherapeutic agents, the observed changes in lipid metabolism are not necessarily causal for apoptosis to occur. For example, treatment with either of the differentiating agents PA or PB led to increases in mobile lipid, tCho and GPC in DU145 cells, but only PB caused apoptosis (1). Moreover, attempted inhibition of the effects of PB via the HMG-CoA reductase pathway by treatment with lovastatin led to further increases in GPC, while mobile lipids and other measures of cell death remained static (cell cycle arrest, proliferation, caspase-3 activity) or were reversed (NTP depletion, TUNEL staining) (3). A number of studies have shown that downstream inhibition of triglyceride formation does not alter the induction of apoptosis or overall levels of cell death. Treatment with Triascin C, an inhibitor of long-chain acyl CoA synthase was unable to affect levels of Fas-induced apoptosis (33). Chlorpromazine, an inhibitor of lysosomal metabolism and autophagic lipid processing reduced intracellular triglycerides and mobile lipid resonances but had no effect on cytotoxicity induced by the antimitochondrial agent tetraphenylphosphonium chloride (34). These results suggest that lipid accumulations occur via a parallel pathway with apoptosis, but further studies are required to determine their actual role in the process of cell death. Certainly, the concept of lipoapoptosis, apoptosis induced by lipid overload, has been recognized as a source of cell death in non-adipose tissues (35). In this case, the PPAR-γ regulated shunting of fatty acids into cytoplasmic triglycerides would function as part of the stress response, removing toxic lipid metabolites and partially slowing passage through apoptosis. This phenomenon has also been proposed as a mechanism for delaying apoptosis in neutrophils (36).
Our data indicate that GW9662 could prevent GPC formation in both PB-treated and control cells. GPC is formed from the abundant membrane phospholipid, phosphatidylcholine, in a two-step enzymatic reaction involving phospholipase A2 and lysophospholipase. Although GPC levels increase in human mammary cells undergoing malignant transformation (37), phosphocholine (PC) levels in the same cells increase dramatically, such that the relative ratio of GPC/PC decreases significantly. This decrease in GPC/PC ratio has been associated with increased malignancy and can be reversed by treatment of human mammary cells with the non-specific cyclooxygenase (COX) inhibitor indomethacin (38). COX converts the arachidonic acid released from phospholipids by PLA2 into prostaglandin H2 (PGH2) as the first step in eicosanoid synthesis for the production of prostaglandins. The fact that prostaglandin J2, a natural ligand for PPAR-γ, is produced downstream from PGH2 indicates that PPAR-γ and PLA2 metabolism may be closely linked.
In fact, a number of recent studies have demonstrated the regulation of PPAR-γ by PLA2 in a variety of tissue types. Not only can overexpression of cPLA2 lead to significant increases in PPAR-γ mediated gene transcription in HepG2 cells (39) and in human airway epithelial cells (40), but direct treatment with sPLA2-I also increases PPAR-γ expression in a rat uterine cell line (41). PPAR-γ expression can be reduced by the pharmacological inhibition of cPLA2 in Hep-G2 cells (39) and in macrophages and macrophage cell lines (42) or by RNA silencing of iPLA2β or iPLA2γ in differentiating NIH-3T3 L1 cells (43).
Conversely, it is worth noting that the agonistic and antagonistic modulation of PPAR-γ can also influence PLA2 activity. For example, PPAR-γ agonists induce activation of PPAR-γ, PLA2 and COX-2 in rat heart that can be inhibited by GW9662 (44). Furthermore, PPAR-γ agonists, such as rosiglitazone, lead to increased expression of COX-2 in monocytes (45) and a GW9662-reversible prostaglandin release in rat aortic vascular smooth muscle cells (46). Finally, treatment of A549 xenografts with the docetaxel and the COX-2 inhibitor celecoxib significantly induced apoptosis and inhibited tumor growth while having mixed effects on protein expression: enhanced PPAR-γ, decreased cPLA2 and no effect on COX-2 (47). Taken together, these studies indicate a complex relationship between PPAR-γ expression and PLA2 activity. Our observation of PB-induced increases and GW9662-induced reductions in GPC levels is consistent with a mechanism whereby activation of PPAR-γ is responsible for increased PLA2 activity. Further studies are needed to determine which PLA2 isoforms are responsible for the increases in mobile lipids and GPC by differentiating agents, and what feedback mechanisms are involved in their regulation. Since these metabolite levels can easily be observed in human tumors and animal models using in vivo MR spectroscopy, the ability to dissect the relevant pathways in their regulation are important steps towards understanding tumor biochemistry and in the functional use of non-invasive spectroscopic methods in tumor detection and monitoring of therapy.
ACKNOWLEDGEMENTS
This study was supported by NIH R01 CA114347 and the SAIR Program (R24-CA83105) at the University of Pennsylvania. MM wishes to thank the MMRRCC Regional Resource training grant (T32-HL-07614) for a postdoctoral fellowship. The authors wish to acknowledge the University of Pennsylvania Small Animal Imaging Facility (SAIF). jMRUI software was provided by the participants of EU network programs: Human Capital and Mobility, CHRX-CT94-0432; Training and Mobility of Researchers, ERB-FMRX-CT970160.
ABBREVIATIONS
- CHAPS
3[(3-cholamidopropyl)dimethylammonio]-propanesulfonic acid
- CHESS
chemical shift selective (water suppression)
- COX
cyclooxygenase
- DTT
dithiothreitol
- DW
diffusion-weighted
- EDTA
ethylenediaminetetraacetic acid
- EMEM
Eagle's minimum essential medium
- FA
fatty acid
- FACS
fluorescence activated cell sorter
- FBS
fetal bovine serum
- GPC
sn-glycero-3-phosphocholine
- GW9662
2-chloro-5-nitro-N-phenylbenzamide
- HBSS
Hank’s balanced salt solution
- HMG-CoA
hydroxymethylglutaryl coenzyme A
- IC50
Half-maximal inhibitory concentration
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- NTP
nucleoside triphosphate
- PA
phenylacetate
- PB
phenylbutyrate
- PBS
phosphate buffered saline
- PGH2
prostaglandin H2
- PIPES
piperazine-1,4-bis(2-ethanesulfonic acid)
- PPAR
peroxisome proliferator activated receptor
- rf
radiofrequency
- tCho
total choline
- TE
echo time
- TR
repetition time
- TM
mixing time
- TUNEL
terminal deoxynucleotidyl dUTP nick end labeling
REFERENCES
- 1.Milkevitch M, Shim H, Pilatus U, Pickup S, Wehrle JP, Samid D, Poptani H, Glickson JD, Delikatny EJ. Increases in NMR visible lipid and glycerophosphocholine during phenylbutyrate-induced apoptosis in human prostate cancer cells. Biochimica Biophysica Acta. 2005;1734:1–12. doi: 10.1016/j.bbalip.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 2.Samid D, Shack S, Myers CE. Selective growth arrest and phenotypic reversion of prostate cancer cells in vitro by nontoxic pharmacological concentrations of phenylacetate. Journal of Clinical Investigation. 1993;91(5):2288–2295. doi: 10.1172/JCI116457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Milkevitch M, Jeitner TM, Beardsley N, Delikatny EJ. Lovastatin enhances phenylbutyrate-induced MR-visible glycerophosphocholine but not apoptosis in DU145 prostate cells. Biochimica Biophysica Acta. 2007;1771:1531–1539. doi: 10.1016/j.bbalip.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 4.Gilbert J, Baker SD, Bowling MK, Grochow L, Figg WD, Zabelina Y, Donehower RC, Carducci MA. A phase I dose escalation and bioavailability study of oral sodium phenylbutyrate in patients with refractory solid tumor malignancies. Clinical Cancer Research. 2001;7(8):2292–2300. [PubMed] [Google Scholar]
- 5.Gore SD, Weng LJ, Figg WD, Zhai S, Donehower RC, Dover G, Grever MR, Griffin C, Grochow LB, Hawkins A, Burks K, Zabelena Y, Miller CB. Impact of prolonged infusions of the putative differentiating agent sodium phenylbutyrate on myelodysplastic syndromes and acute myeloid leukemia. Clinical Cancer Research. 2002;8(4):963–970. [PubMed] [Google Scholar]
- 6.Camacho LH, Olson J, Tong WP, Young CW, Spriggs DR, Malkin MG. Phase I dose escalation clinical trial of phenylbutyrate sodium administered twice daily to patients with advanced solid tumors. Invest New Drugs. 2007 Apr;25(2):131–138. doi: 10.1007/s10637-006-9017-4. [DOI] [PubMed] [Google Scholar]
- 7.Castillo M, Martinez-Cayuela M, Zafra MF, Garcia-Peregrin E. Effect of phenylalanine derivatives on the main regulatory enzymes of hepatic cholesterogenesis. Molecular & Cellular Biochemistry. 1991;105(1):21–25. doi: 10.1007/BF00230371. [DOI] [PubMed] [Google Scholar]
- 8.Prasanna P, Thibault A, Liu L, Samid D. Lipid metabolism as a target for brain cancer therapy: synergistic activity of lovastatin and sodium phenylacetate against human glioma cells. Journal of Neurochemistry. 1996;66(2):710–716. doi: 10.1046/j.1471-4159.1996.66020710.x. [DOI] [PubMed] [Google Scholar]
- 9.Davis T, Kennedy C, Chiew YE, Clarke CL, deFazio A. Histone deacetylase inhibitors decrease proliferation and modulate cell cycle gene expression in normal mammary epithelial cells. Clinical Cancer Research. 2000;6(11):4334–4342. [PubMed] [Google Scholar]
- 10.Pineau T, Hudgins WR, Liu L, Chen LC, Sher T, Gonzalez FJ, Samid D. Activation of a human peroxisome proliferator-activated receptor by the antitumor agent phenylacetate and its analogs. Biochemical Pharmacology. 1996;52(4):659–667. doi: 10.1016/0006-2952(96)00340-1. [DOI] [PubMed] [Google Scholar]
- 11.Samid D, Wells M, Greene ME, Shen W, Palmer CN, Thibault A. Peroxisome proliferator-activated receptor gamma as a novel target in cancer therapy: binding and activation by an aromatic fatty acid with clinical antitumor activity. Clinical Cancer Research. 2000;6(3):933–941. [PubMed] [Google Scholar]
- 12.Feige JN, Gelman L, Michalik L, Desvergne B, Wahli W. From molecular action to physiological outputs: PPAR are nuclear receptors at the crossroads of key cellular functions. Progress in Lipid Research. 2006;45:120–159. doi: 10.1016/j.plipres.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 13.Corton JC, Anderson SP, Stauber A. Central role of peroxisome proliferator-activated receptors in the actions of peroxisome proliferators. Annual Review of Pharmacology & Toxicology. 2000;40:491–518. doi: 10.1146/annurev.pharmtox.40.1.491. [DOI] [PubMed] [Google Scholar]
- 14.Leesnitzer LM, Parks DJ, Bledsoe RK, Cobb JE, Collins JL, Consler TG, Davis RG, Hull-Ryde EA, Lenhard JM, Patel L, Plunket KD, Shenk JL, Stimmel JB, Therapontos C, Willson TM, Blanchard SG. Functional consequences of cysteine modification in the ligand binding sites of peroxisome proliferator activated receptors by GW9662. Biochemistry. 2002 May 28;41(21):6640–6650. doi: 10.1021/bi0159581. [DOI] [PubMed] [Google Scholar]
- 15.Naressi A, Couturier C, Devos JM, Janssen M, Mangeat C, de Beer R, Graveron-Demilly D. Java-based graphical user interface for the MRUI quantitation package. MAGMA. 2001;12:141–152. doi: 10.1007/BF02668096. [DOI] [PubMed] [Google Scholar]
- 16.Vanhamme L, van den Boogaart A, Van Huffel S. Improved method for accurate and efficient quantification of MRS data with use of prior knowledge. Journal of Magnetic Resonance. 1997;129:35–43. doi: 10.1006/jmre.1997.1244. [DOI] [PubMed] [Google Scholar]
- 17.Ray DM, Bernstein SH, Phipps RP. Human multiple myeloma cells express peroxisome proliferator-activated receptor gamma and undergo apoptosis upon exposure to PPARgamma ligands. Clin Immunol. 2004 Nov;113(2):203–213. doi: 10.1016/j.clim.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 18.Han S, Wada RK, Sidell N. Differentiation of human neuroblastoma by phenylacetate is mediated by peroxisome proliferator-activated receptor gamma. Cancer Research. 2001;61(10):3998–4002. [PubMed] [Google Scholar]
- 19.Nagata D, Yoshihiro H, Nakanishi M, Naruyama H, Okada S, Ando R, Tozawa K, Kohri K. Peroxisome proliferator-activated receptor-gamma and growth inhibition by its ligands in prostate cancer. Cancer Detect Prev. 2008;32(3):259–266. doi: 10.1016/j.cdp.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 20.Au-Yeung KK, Liu PL, Chan C, Wu WY, Lee SS, Ko JK. Herbal isoprenols induce apoptosis in human colon cancer cells through transcriptional activation of PPARgamma. Cancer Invest. 2008 Aug;26(7):708–717. doi: 10.1080/07357900801898656. [DOI] [PubMed] [Google Scholar]
- 21.Magenta G, Borenstein X, Rolando R, Jasnis MA. Rosiglitazone inhibits metastasis development of a murine mammary tumor cell line LMM3. BMC Cancer. 2008;8:47. doi: 10.1186/1471-2407-8-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu N, Qiang W, Kuang X, Thuillier P, Lynn WS, Wong PK. The peroxisome proliferator phenylbutyric acid (PBA) protects astrocytes from ts1 MoMuLV-induced oxidative cell death. J Neurovirol. 2002 Aug;8(4):318–325. doi: 10.1080/13550280290100699. [DOI] [PubMed] [Google Scholar]
- 23.Chang TH, Szabo E. Enhanced growth inhibition by combination differentiation therapy with ligands of peroxisome proliferator-activated receptor-gamma and inhibitors of histone deacetylase in adenocarcinoma of the lung. Clin Cancer Res. 2002 Apr;8(4):1206–1212. [PubMed] [Google Scholar]
- 24.Angelucci A, Muzi P, Cristiano L, Millimaggi D, Cimini A, Dolo V, Miano R, Vicentini C, Ceru MP, Bologna M. Neuroendocrine transdifferentiation induced by VPA is mediated by PPARgamma activation and confers resistance to antiblastic therapy in prostate carcinoma. Prostate. 2008 May 1;68(6):588–598. doi: 10.1002/pros.20708. [DOI] [PubMed] [Google Scholar]
- 25.Lea MA, Sura M, Desbordes C. Inhibition of cell proliferation by potential peroxisome proliferator-activated receptor (PPAR) gamma agonists and antagonists. Anticancer Research. 2004;24(5A):2765–2771. [PubMed] [Google Scholar]
- 26.Jeitner TM, Delikatny EJ, Bartier WA, Capper HR, Hunt NH. Inhibition of drug naive and resistant leukaemia cell proliferation by low molecular weight thiols. Biochem. Pharmacol. 1998;55:793–802. doi: 10.1016/s0006-2952(97)00575-3. [DOI] [PubMed] [Google Scholar]
- 27.Delikatny EJ, Roman SK, Hancock R, Jeitner TM, Lander CM, Rideout DC, Mountford CE. Tetraphenylphosphonium chloride induced MR-visible lipid accumulation in a malignant human breast cell line. International Journal of Cancer. 1996b;67:72–79. doi: 10.1002/(SICI)1097-0215(19960703)67:1<72::AID-IJC13>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 28.Delikatny EJ, Cooper WA, Brammah S, Sathasivam N, Rideout DC. Nuclear magnetic resonance-visible lipid induced by cationic lipophilic chemotherapeutic agents are accompanied by increased lipid droplet formation and damaged mitochondria. Cancer Research. 2002;62:1394–1400. [PubMed] [Google Scholar]
- 29.Al-Saffar NM, Titley JC, Robertson D, Clarke PA, Jackson LE, Leach MO, Ronen SM. Apoptosis is associated with triacylglycerol accumulation in Jurkat T-cells. British Journal of Cancer. 2002;86(6):963–970. doi: 10.1038/sj.bjc.6600188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bezabeh T, Mowat MRA, Jarolim L, Greenberg AH, Smith ICP. Detection of drug-induced apoptosis and necrosis in human cervical carcinoma cells using 1H NMR spectroscopy. Cell Death and Differentiation. 2001;8:219–224. doi: 10.1038/sj.cdd.4400802. [DOI] [PubMed] [Google Scholar]
- 31.Di Vito M, Lenti L, Knijn A, Iorio E, D'Agostino F, Molinari A, Calcabrini A, Stringaro A, Meschini S, Arancia G, Bozzi A, Strom R, Podo F. 1H NMR-visible mobile lipid domains correlate with cytoplasmic lipid bodies in apoptotic T-lymphoblastoid cells. Biochimica et Biophysica Acta. 2001;1530(1):47–66. doi: 10.1016/s1388-1981(00)00165-7. [DOI] [PubMed] [Google Scholar]
- 32.Hakumäki JM, Poptani H, Sandmair AM, Ylä-Herttuala S, Kauppinen RA. 1H MRS detects polyunsaturated fatty acid accumulation during gene therapy of glioma: Implications for the in vivo detection of apoptosis. Nature Medicine. 1999;5:1323–1327. doi: 10.1038/15279. [DOI] [PubMed] [Google Scholar]
- 33.Iorio E, Di Vito M, Spadaro F, Ramoni C, Lococo E, Carnevale R, Lenti L, Strom R, Podo F. Triacsin C inhibits the formation of 1H NMR-visible mobile lipids and lipid bodies in HuT 78 apoptotic cells. Biochimica et Biophysica Acta. 2003;1634(1–2):1–14. doi: 10.1016/j.bbalip.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 34.Sathasivam N, Brammah S, Wright LC, Delikatny EJ. Inhibition of tetraphenylphosphonium-induced NMR-visible lipid accumulation in human breast cells by chlorpromazine. Biochimica et Biophysica Acta. 2003;1633:149–160. doi: 10.1016/s1388-1981(03)00121-5. [DOI] [PubMed] [Google Scholar]
- 35.Unger RH, Orci L. Lipoapoptosis: its mechanism and its diseases. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids. 2002 Dec 30;1585(2–3):202–212. doi: 10.1016/s1388-1981(02)00342-6. [DOI] [PubMed] [Google Scholar]
- 36.Wright LC, Groot Obbink KL, Delikatny EJ, Santangelo RT, Sorrell TC. The origin of 1H NMR-visible triacylglycerol in human neutrophils: high fatty acid environments result in preferential sequestration of palmitic acid into plasma membrane triacylglycerol. European Journal of Biochemistry. 2000;267:68–78. doi: 10.1046/j.1432-1327.2000.00955.x. [DOI] [PubMed] [Google Scholar]
- 37.Aboagye EO, Bhujwalla ZM. Malignant transformation alters membrane choline phospholipid metabolism of human mammary epithelial cells. Cancer Research. 1999;59(1):80–84. [PubMed] [Google Scholar]
- 38.Glunde K, Ackerstaff E, Natarajan K, Artemov D, Bhujwalla ZM. Real-time changes in 1H and 31P NMR spectra of malignant human mammary epithelial cells during treatment with the anti-inflammatory agent indomethacin. Magnetic Resonance in Medicine. 2002;48(5):819–825. doi: 10.1002/mrm.10295. [DOI] [PubMed] [Google Scholar]
- 39.Han C, Demetris AJ, Michalopoulos G, Shelhamer JH, Wu T. 85-kDa cPLA(2) plays a critical role in PPAR-mediated gene transcription in human hepatoma cells. Am J Physiol Gastrointest Liver Physiol. 2002 Apr;282(4):G586–G597. doi: 10.1152/ajpgi.00305.2001. [DOI] [PubMed] [Google Scholar]
- 40.Pawliczak R, Han C, Huang XL, Demetris AJ, Shelhamer JH, Wu T. 85-kDa cytosolic phospholipase A2 mediates peroxisome proliferator-activated receptor gamma activation in human lung epithelial cells. J Biol Chem. 2002 Sep 6;277(36):33153–33163. doi: 10.1074/jbc.M200246200. [DOI] [PubMed] [Google Scholar]
- 41.Specty O, Pageaux JF, Dauca M, Lagarde M, Laugier C, Fayard JM. Control of cell proliferation via transduction of sPLA(2)-I activity and possible PPAR activation at the nuclear level. FEBS Lett. 2001 Feb 9;490(1–2):88–92. doi: 10.1016/s0014-5793(00)02414-5. [DOI] [PubMed] [Google Scholar]
- 42.Knowles HJ, te Poele RH, Workman P, Harris AL. Niacin induces PPARgamma expression and transcriptional activation in macrophages via HM74 and HM74a-mediated induction of prostaglandin synthesis pathways. Biochem Pharmacol. 2006 Feb 28;71(5):646–656. doi: 10.1016/j.bcp.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 43.Su X, Mancuso DJ, Bickel PE, Jenkins CM, Gross RW. Small interfering RNA knockdown of calcium-independent phospholipases A2 beta or gamma inhibits the hormone-induced differentiation of 3T3-L1 preadipocytes. J Biol Chem. 2004 May 21;279(21):21740–21748. doi: 10.1074/jbc.M314166200. [DOI] [PubMed] [Google Scholar]
- 44.Ye Y, Nishi SP, Manickavasagam S, Lin Y, Huang MH, Perez-Polo JR, Uretsky BF, Birnbaum Y. Activation of peroxisome proliferator-activated receptor-gamma (PPAR-gamma) by atorvastatin is mediated by 15-deoxy-delta-12,14-PGJ2. Prostaglandins Other Lipid Mediat. 2007 Aug;84(1–2):43–53. doi: 10.1016/j.prostaglandins.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 45.Pontsler AV, St Hilaire A, Marathe GK, Zimmerman GA, McIntyre TM. Cyclooxygenase-2 is induced in monocytes by peroxisome proliferator activated receptor gamma and oxidized alkyl phospholipids from oxidized low density lipoprotein. J Biol Chem. 2002 Apr 12;277(15):13029–13036. doi: 10.1074/jbc.M109546200. [DOI] [PubMed] [Google Scholar]
- 46.Bishop-Bailey D, Warner TD. PPARgamma ligands induce prostaglandin production in vascular smooth muscle cells: indomethacin acts as a peroxisome proliferator-activated receptor-gamma antagonist. Faseb J. 2003 Oct;17(13):1925–1927. doi: 10.1096/fj.02-1075fje. [DOI] [PubMed] [Google Scholar]
- 47.Shaik MS, Chatterjee A, Jackson T, Singh M. Enhancement of antitumor activity of docetaxel by celecoxib in lung tumors. Int J Cancer. 2006 Jan 15;118(2):396–404. doi: 10.1002/ijc.21325. [DOI] [PMC free article] [PubMed] [Google Scholar]