Abstract
Nonalcoholic fatty liver disease (NAFLD) is associated with increased cardiovascular and liver-related mortality. NAFLD is characterized by both triglyceride and free cholesterol (FC) accumulation without a corresponding increment in cholesterol esters. The aim of this study was to evaluate the expression of cholesterol metabolic genes in NAFLD and relate these to disease phenotype. NAFLD was associated with increased SREBP-2 maturation, HMG CoA reductase (HMGCR) expression and decreased phosphorylation of HMGCR. Cholesterol synthesis was increased as measured by the circulating desmosterol:cholesterol ratio. miR-34a, a microRNA increased in NAFLD, inhibited sirtuin-1 with downstream dephosphorylation of AMP kinase and HMGCR. Cholesterol ester hydrolase was increased while ACAT-2 remained unchanged. LDL receptor expression was significantly decreased and similar in NAFLD subjects on or off statins. HMGCR expression was correlated with FC, histologic severity of NAFLD and LDL-cholesterol. These data demonstrate dysregulated cholesterol metabolism in NAFLD which may contribute to disease severity and cardiovascular risks.
Comment
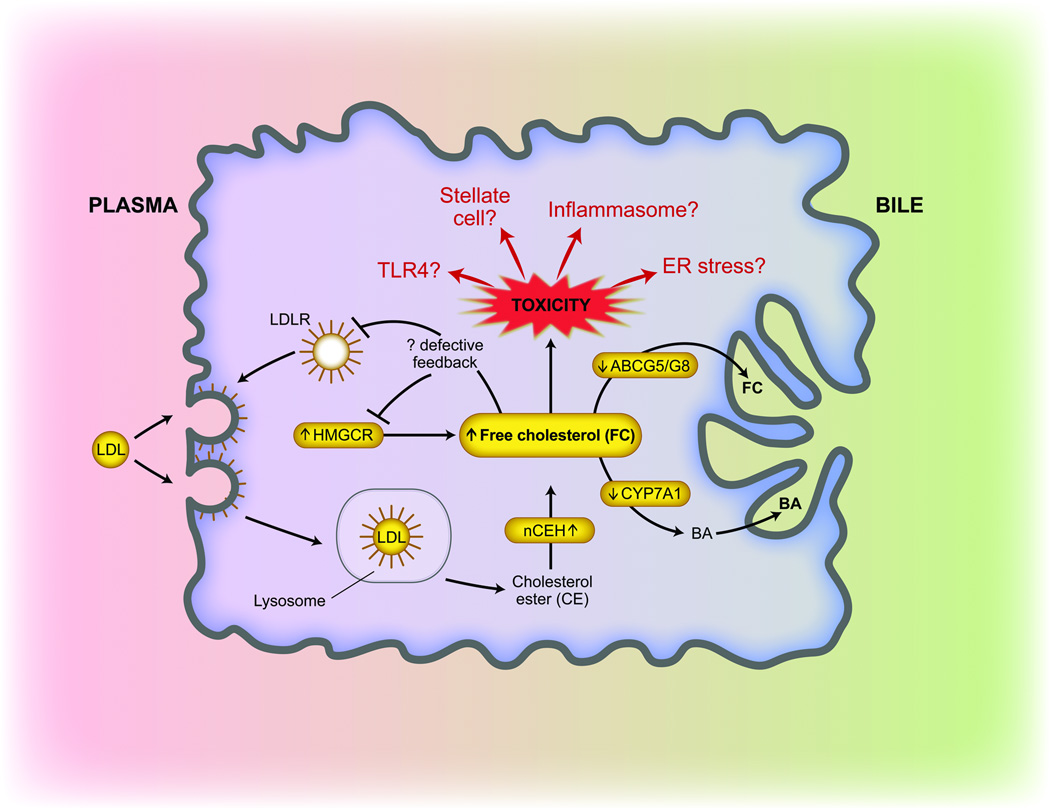
Non-alcoholic fatty liver disease (NAFLD) is characterized by hepatic lipid accumulation in the absence of significant alcohol consumption. Risk factors for NAFLD mirror those for cardiovascular disease and include obesity, insulin resistance, hypertension and dyslipidemia. Within the spectrum of NAFLD, histologic evidence of inflammation and hepatocyte ballooning defines a subset of patients with non-alcoholic steatohepatitis (NASH) who are at increased risk for progression of their disease, including the development of hepatic fibrosis, cirrhosis, liver failure and hepatocellular cancer. Approximately 20% of adults in the United States are affected by NAFLD (1, 2) and approximately 3–5% exhibit NASH, with higher rates observed with obesity or other components of the metabolic syndrome (outlined above) (3). A variety of mechanisms have been implicated in the development of NAFLD and its progression to NASH but the molecular, genetic and biochemical pathways remain incompletely understood, despite considerable efforts towards understanding the role of hepatic fatty acid and triglyceride metabolism (2). In order to advance understanding of the role of neutral lipid mediators in the pathogenesis of NAFLD and NASH, renewed attention has been directed to the role of cholesterol, and more specifically, hepatic free cholesterol (FC) (Figure 1). Comparative hepatic and serum lipidomic analysis of normal and NAFLD samples revealed increased hepatic FC and other lipids in NASH (4, 5). Epidemiologic studies have also implicated an association of dietary cholesterol—but not total fat consumption—with risk of liver cancer-related death and hospitalization (6). The role of dietary cholesterol has also been investigated in murine models of NAFLD. For example, a cholate and cholesterol-enriched high fat, atherogenic diet promoted both oxidative stress and experimental NASH (7) while increased hepatic free cholesterol was observed in association with a transition from NAFLD to NASH in obese, hyperinsulinemic mice (8). Other work, using both genetic and nutritional models of hepatic steatosis, implicated mitochondrial free cholesterol loading (but not fatty acids or triglycerides) in sensitizing mice to TNF and Fas induced steatohepatitis (9). These studies (among others) provide a foundation for exploration of the role of hepatic cholesterol metabolism in human subjects with NAFLD.
Figure 1.
The study of Sanyal and colleagues sought to dissect parameters of cholesterol metabolism in NAFLD and NASH (10). The study design yielded extensive metadata, incorporating clinical characteristics, histology, gene expression profiling including mRNA and protein analysis as well as functional biochemical data. The authors studied four groups of patients: lean normal, obese normal, NAFLD, and NASH patients. The key findings include increased expression of HMC CoA reductase (HMGCR) (the rate-limiting enzyme in cholesterol synthesis) in individuals with NAFLD compared to control and control-obese subjects. In addition, the findings revealed a significant positive correlation between hepatic HMGCR and free cholesterol content and, importantly, demonstrated a predictive relationship between HMGCR and ballooning and NAFLD activity score. There was an increase in nuclear SREBP-2, which may have accounted for the increased HMGCR expression. miR-34a, previously shown to be overexpressed in NASH (11), was shown here to be associated with decreased SIRT-1 expression and decreased phosphorylation (and increased activity) of HMGCR suggesting that miR-34a overexpression may contribute to the overall dysregulation of HMGCR activity in NASH patients. The consequences of these expression changes were functionally supported by an increase in serum desmosterol:sitosterol ratio, again indicating increased hepatic cholesterol synthesis in NASH. Dysregulated hepatic expression of genes related to cholesterol catabolism and efflux were also observed in patients with NAFLD and NASH. Cholesterol ester hydrolase (nCEH) expression was increased, contributing to the increased free cholesterol. There was also decreased expression of CYP7A1 and CYP27A protein, raising the question of whether cholesterol catabolism to bile acid was altered. Finally, there was decreased expression of two candidate cholesterol efflux genes, ABCG1 and ABCG8 in NAFLD patients but no change was observed in ABCA1 expression. It is possible that FC conversion to LXR agonists in NASH patients acted to maintain expression of these cholesterol transporters.
These findings together emphasize the importance of homeostatic regulation of hepatic cholesterol metabolism in the development and progression of NAFLD and NASH and suggest important new avenues for further investigation (Figure 1). There is a need for more detailed biochemical interrogation of the intracellular metabolic pathways that promote hepatic FC accumulation in the face of decreased LDLR expression in NAFLD patients, despite the predicted inverse relationship with circulating LDL. In addition, there was paradoxically no augmentation in LDLR expression in statin-treated versus untreated NASH patients. These data raise the possibility that patients with NAFLD respond differently to statin therapy compared with controls, and that impaired augmentation of the LDLR expression might limit the hypolipidemic effect of statins. These data warrant further study as individuals with NAFLD suffer elevated cardiovascular complications (12) and benefit from effective hypolipidemic therapy (13). In addition, the mechanism by which SREBP-2 maturation is promoted in patients with NASH despite increased intracellular cholesterol remains to be elucidated. The in vitro observation that increased miR-34a expression in Huh-7 cells increased the active form of HMGCR suggests the possibility of therapeutic targeting. It is important to note, however, that miR-34a was shown to act as a tumor suppressor via regulation of SIRT1 and p53 signaling in human colon cancer cells (14). Any potential miR-34a/SIRT1 directed interventions must carefully weigh broader effects on cellular signaling pathways. It will also be of interest to understand the importance of hepatocyte versus stellate cell cholesterol metabolism, in view of recent findings showing augmented TLR4 signaling and hepatic fibrosis with stellate cell cholesterol loading (15). Disruptions in ER stress signaling pathways cause dysregulation of lipid homeostatic mechanisms in NAFLD (16, 17), but whether this plays a role in dysregulated cholesterol homeostasis in human NAFLD, and the mechanisms by which disordered hepatic FC may modulate ER stress pathways, either as a cause or a consequence of altered lipid metabolism warrant further investigation. Finally, it is worth noting that cholesterol crystals activate the NLRPI inflammasome in macrophages and induce IL-1β expression (18, 19). Thus it remains possible that free cholesterol may directly promote cell damage.
The availability of human liver biopsy specimens for molecular genetic, biochemical, and histologic analysis should allow investigation of some of the above questions, and the authors are to be commended for developing the resources that allow these studies to occur. Ultimately, these and other studies may allow improved NAFLD therapy to decrease morbidity in the large number of individuals at risk for its attendant complications in the coming years.
This is a commentary on article Min HK, Kapoor A, Fuchs M, Mirshahi F, Zhou H, Maher J, Kellum J, Warnick R, Contos MJ, Sanyal AJ. Increased hepatic synthesis and dysregulation of cholesterol metabolism is associated with the severity of nonalcoholic fatty liver disease. Cell Metab. 2012;15(5):665-74.
Footnotes
Commentary on:
Min H-K, Kapoor A, Fuchs M, Mirshahi F, Zhou H, Maher J, et al Increased Hepatic Synthesis and Dysregulation of Cholesterol Metabolism Is Associated with the Severity of Nonalcoholic Fatty Liver Disease. Cell Metabolism 2012; 15: 665-674. (Reprinted with permission).
REFERENCES
- 1.Marcos A, Fisher RA, Ham JM, Olzinski AT, Shiffman ML, Sanyal AJ, Luketic VA, et al. Selection and outcome of living donors for adult to adult right lobe transplantation. Transplantation. 2000;69:2410–2415. doi: 10.1097/00007890-200006150-00034. [DOI] [PubMed] [Google Scholar]
- 2.Cohen JC, Horton JD, Hobbs HH. Human fatty liver disease: old questions and new insights. Science. 2011;332:1519–1523. doi: 10.1126/science.1204265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, et al. The diagnosis and management of non-alcoholic fatty liver disease: Practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005–2023. doi: 10.1002/hep.25762. [DOI] [PubMed] [Google Scholar]
- 4.Puri P, Baillie RA, Wiest MM, Mirshahi F, Choudhury J, Cheung O, Sargeant C, et al. A lipidomic analysis of nonalcoholic fatty liver disease. Hepatology. 2007;46:1081–1090. doi: 10.1002/hep.21763. [DOI] [PubMed] [Google Scholar]
- 5.Puri P, Wiest MM, Cheung O, Mirshahi F, Sargeant C, Min HK, Contos MJ, et al. The plasma lipidomic signature of nonalcoholic steatohepatitis. Hepatology. 2009;50:1827–1838. doi: 10.1002/hep.23229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ioannou GN, Morrow OB, Connole ML, Lee SP. Association between dietary nutrient composition and the incidence of cirrhosis or liver cancer in the United States population. Hepatology. 2009;50:175–184. doi: 10.1002/hep.22941. [DOI] [PubMed] [Google Scholar]
- 7.Matsuzawa N, Takamura T, Kurita S, Misu H, Ota T, Ando H, Yokoyama M, et al. Lipid-induced oxidative stress causes steatohepatitis in mice fed an atherogenic diet. Hepatology. 2007;46:1392–1403. doi: 10.1002/hep.21874. [DOI] [PubMed] [Google Scholar]
- 8.Van Rooyen DM, Larter CZ, Haigh WG, Yeh MM, Ioannou G, Kuver R, Lee SP, et al. Hepatic free cholesterol accumulates in obese, diabetic mice and causes nonalcoholic steatohepatitis. Gastroenterology. 2011;141:1393–1403. doi: 10.1053/j.gastro.2011.06.040. 1403 e1391-1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mari M, Caballero F, Colell A, Morales A, Caballeria J, Fernandez A, Enrich C, et al. Mitochondrial free cholesterol loading sensitizes to TNF- and Fas-mediated steatohepatitis. Cell Metab. 2006;4:185–198. doi: 10.1016/j.cmet.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 10.Min HK, Kapoor A, Fuchs M, Mirshahi F, Zhou H, Maher J, Kellum J, et al. Increased hepatic synthesis and dysregulation of cholesterol metabolism is associated with the severity of nonalcoholic fatty liver disease. Cell Metab. 2012;15:665–674. doi: 10.1016/j.cmet.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheung O, Puri P, Eicken C, Contos MJ, Mirshahi F, Maher JW, Kellum JM, et al. Nonalcoholic steatohepatitis is associated with altered hepatic MicroRNA expression. Hepatology. 2008;48:1810–1820. doi: 10.1002/hep.22569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Targher G, Day CP, Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med. 2010;363:1341–1350. doi: 10.1056/NEJMra0912063. [DOI] [PubMed] [Google Scholar]
- 13.Athyros VG, Tziomalos K, Gossios TD, Griva T, Anagnostis P, Kargiotis K, Pagourelias ED, et al. Safety and efficacy of long-term statin treatment for cardiovascular events in patients with coronary heart disease and abnormal liver tests in the Greek Atorvastatin and Coronary Heart Disease Evaluation (GREACE) Study: a post-hoc analysis. Lancet. 2010;376:1916–1922. doi: 10.1016/S0140-6736(10)61272-X. [DOI] [PubMed] [Google Scholar]
- 14.Yamakuchi M, Ferlito M, Lowenstein CJ. miR-34a repression of SIRT1 regulates apoptosis. Proc Natl Acad Sci U S A. 2008;105:13421–13426. doi: 10.1073/pnas.0801613105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Teratani T, Tomita K, Suzuki T, Oshikawa T, Yokoyama H, Shimamura K, Tominaga S, et al. A high-cholesterol diet exacerbates liver fibrosis in mice via accumulation of free cholesterol in hepatic stellate cells. Gastroenterology. 2012;142:152–164. doi: 10.1053/j.gastro.2011.09.049. e110. [DOI] [PubMed] [Google Scholar]
- 16.Rutkowski DT, Wu J, Back SH, Callaghan MU, Ferris SP, Iqbal J, Clark R, et al. UPR pathways combine to prevent hepatic steatosis caused by ER stress-mediated suppression of transcriptional master regulators. Dev Cell. 2008;15:829–840. doi: 10.1016/j.devcel.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee AH, Glimcher LH. Intersection of the unfolded protein response and hepatic lipid metabolism. Cell Mol Life Sci. 2009;66:2835–2850. doi: 10.1007/s00018-009-0049-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chu J, Thomas LM, Watkins SC, Franchi L, Nunez G, Salter RD. Cholesterol-dependent cytolysins induce rapid release of mature IL-1beta from murine macrophages in a NLRP3 inflammasome and cathepsin B-dependent manner. J Leukoc Biol. 2009;86:1227–1238. doi: 10.1189/jlb.0309164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rajamaki K, Lappalainen J, Oorni K, Valimaki E, Matikainen S, Kovanen PT, Eklund KK. Cholesterol crystals activate the NLRP3 inflammasome in human macrophages: a novel link between cholesterol metabolism and inflammation. PLoS One. 2010;5:e11765. doi: 10.1371/journal.pone.0011765. [DOI] [PMC free article] [PubMed] [Google Scholar]