Abstract
Autoimmunity is thought to contribute to poor neurological outcomes after spinal cord injury (SCI). There are few mechanism-based therapies, however, designed to reduce tissue damage and neurotoxicity after SCI because the molecular and cellular bases for SCI-induced autoimmunity are not completely understood. Recent groundbreaking studies in rodents indicate that B cells are responsible for SCI-induced autoimmunity. This novel paradigm, if confirmed in humans, could aid in the design of neuroprotective immunotherapies. The aim of this study was to investigate the molecular signaling pathways and mechanisms by which autoimmunity is induced after SCI, with the goal of identifying potential targets in therapies designed to reduce tissue damage and inflammation in the chronic phase of SCI. To that end, we performed an exploratory microarray analysis of peripheral blood mononuclear cells to identify differentially expressed genes in chronic SCI. We identified a gene network associated with lymphoid tissue structure and development that was composed of 29 distinct molecules and five protein complexes, including two cytokines, a proliferation-inducing ligand (APRIL) and B-cell–activating factor (BAFF), and one receptor, B-cell maturation antigen (BMCA) involved in B cell development, proliferation, activation, and survival. Real-time polymerase chain reaction analysis from ribonucleic acid samples confirmed upregulation of these three genes in SCI. To our knowledge, this is the first report that peripheral blood mononuclear cells produce increased levels of BAFF and APRIL in chronic SCI. This finding provides evidence of systemic regulation of SCI-autoimmunity via APRIL and BAFF mediated activation of B cells through BMCA and points toward these molecules as potential targets of therapies designed to reduce neuroinflammation after SCI.
Key words: autoimmunity, B cell, rehabilitation, spinal cord injury
Introduction
Recent evidence suggests that spinal cord injury (SCI) results in B cell dysfunction. SCI-induced immune depression syndrome1 has been reported immediately after SCI, while immune stimulation with subsequent systemic autoimmunity has been reported long-term after SCI.2,3 When the blood-spinal cord barrier is compromised by SCI, there is an exposure of central nervous system (CNS) antigens to secondary lymphoid tissues both locally within the cord and systemically. Lymphocytes with receptors specific to these antigens become activated and produce autoantibodies resulting in an autoimmune response.4,5 Autoantibodies specific to CNS antigens are present in mice after experimental SCI,6 and are thought to contribute to poor neurological function and impaired neurological recovery. B cell-deficient mice experience greater neurological and motor recovery after SCI compared with wild type mice, further underscoring the importance of B cell mediated autoimmunity in neurological outcomes after SCI.
Increases in activated B cells after experimental SCI in mice7 suggest that B cells mediate SCI-induced autoimmunity. The underlying molecular mechanisms involved in this process, however, remain poorly characterized. As a result, there are few mechanistic based therapies designed to reduce tissue damage and neurotoxicity after SCI or other CNS trauma.8 Thus, the aim of this exploratory study was to investigate the molecular signaling pathways by which autoimmunity is induced in the long term after SCI, with the goal of identifying potential targets in therapies designed to reduce tissue damage and inflammation in the chronic phase of SCI. To this end, we performed a genome-wide microarray analysis of circulating white blood cells from persons with chronic SCI. We hypothesized that the gene expression profiles would differ between subjects with and without SCI, and that any differentially expressed molecules or signaling pathways constitute potential mediators of SCI-induced autoimmunity. Identifying such mediators is the first step in developing standardized therapies designed to reduce the tissue damage and continued autoimmunity that has been observed in chronic SCI.
Methods
Subjects
We studied a convenience sample of 13 male subjects with chronic motor complete SCI and 7 male subjects without SCI. Subjects were recruited from our outpatient clinics or from our ongoing Boston SCI-Health Study.9 Subjects with SCI were eligible to participate if they were 18 years of age or older, 1 year or more after injury, were not ventilator dependent, and had no other neurological condition (multiple sclerosis, stroke, past polio). Subjects were not eligible to participate if they reported changes in their medical status or symptoms of systemic illness. Subjects without SCI were recruited from our outpatient clinics or by advertisement and were eligible to participate if they did not need an ambulatory aid, had no neurological conditions preventing independent walking, and had not previously received a diagnosis of osteoporosis. Subjects with active, full-thickness skin ulcers involving subcutaneous tissues (grade III) or muscle, bone, or supporting structures (grade IV) were not eligible to participate. Our Institutional Review Boards approved the study and all subjects gave informed consent.
SCI classification
American Spinal Injury Association Impairment Scale (AIS) A (no motor or sensory function below the neurological level of injury) or AIS B (no motor function below the neurological level of injury) SCI was confirmed by physical examination by a trained rater according to the AIS as described previously.9
Peripheral blood mononuclear cell isolation
For each subject, approximately 30 mL of peripheral blood were drawn into a heparinized tube and diluted at a ratio of 1:2 with phosphate-buffered saline (PBS) solution. The whole blood-PBS solution was layered onto one volume of Histopaque®-1077 (Sigma-Aldrich, St. Louis, MO). The layered solution was centrifuged, and white blood cells were isolated from the plasma-Histopaque interface.
Total ribonucleic acid (RNA) extraction and complementary deoxyribonucleic acid (cDNA) preparation
Total RNA was extracted from SCI and control peripheral blood mononuclear cells using the Qiagen RNeasy Mini Kit (Qiagen, Inc, Valencia, CA) according to the manufacturer's instructions. RNA concentration and integrity was assessed with the NanoDrop 8000 spectrophotometer (Fischer Scientific, Wilmington, DE). Two micrograms of RNA from each sample were reverse transcribed into cDNA using the QuantiTect Reverse Transcription Kit (Qiagen, Hilden, Germany).
Microarray analysis
Microarray analysis was performed by SeqWright, Inc. (Houston, TX). cDNA was hybridized to HG-U133A Plus 2.0 GeneChip oligonucleotide arrays (Affymetrix, Santa Clara, CA), each of which contains 54,675 sets of oligonucleotide probes targeting approximately 38,000 human genes. The GeneChip was then analyzed, the relative fluorescence of the chip region corresponding to each gene was determined, and the raw data were exported to CEL files for further analysis using the Partek Genomics Suite (PGS, Partek Inc., St. Louis, MO) and Ingenuity Pathway Analysis (IPA, Ingenuity Systems, Redwood City, CA). PGS was used to generate a list of 1453 genes from the raw CEL files that were significantly over- or underexpressed in SCI versus uninjured control subjects (p≤0.05). IPA software was used for further analysis of the genes identified by PGS, including the identification of differentially expressed genetic pathways. For each pathway identified, a significance score is calculated that represents the chance that the genes in the pathway are found there by chance. The significance score of a network is equal to the negative exponent of calculated p value of the network (ie, a significance score of 5 equals a p value of 1×10−5).10 Genes of interest identified in the microarray analysis were confirmed by quantative polymerase chain reaction (qPCR).
Quantitative real-time PCR (qPCR)
Peripheral blood mononuclear cell derived cDNA was used as a template for qPCR to confirm differential expression of selected genes of interest. Differential expression of B-cell maturation antigen (BCMA) (TNFRSF17), a proliferation-inducing ligand (APRIL) (TNFSF13), and B-cell–activating factor (BAFF) (TNFSF13B) was confirmed by qPCR using the following sequences: BCMA (NM_001192.2), sense 5′-TCCTTCCAGGCTGTTCTTTC-3′, anti-sense 5′-CATCGAAGTTGACAAGGTATGC-3′; APRIL (NM_001198622.1), sense 5′-GGTATCCCTGGCAGAGTCTC-3′, antisense 5′-CACATCACCTCTGTCACATCG-3′; BAFF (NM_001145645.2), sense 5′-GCAGACAGTGAAACACCAAC-3′, antisense 5′-AGAGGTACAGAGAAAGGGAGG-3′. Human β-actin (ACTB, NM_001101.3) was assayed as an endogenous control using the following sequences: sense: 5′-ACCGCGAGAAGATGACCCAG-3 and antisense 5′-GTACGGCCAGAGGCGTACAG-3. All reactions were performed in triplicate with an iCycler Optical Module system (Bio-Rad, Philadelphia, PA) in a 96-well PCR plate (ABgene, Epson, Surray County, UK) with a final reaction volume of 20 μL/well. Each reaction sample included Sybr-Green PCR Master Mix (Bio-Rad), 50 ng of template cDNA, and 0.25 μM of sense and antisense primer mix (Invitrogen Corporation, Carlsbad, CA). Optimized thermal cycling conditions were adjusted for each primer pair as follows: 95°C for 7 minutes followed by 45 cycles at 95°C for 10 seconds, then at 55°C (BCMA and β-actin), 57°C (BAFF), or 59°C (APRIL) for 15 seconds, 72°C for 15 seconds, and a final cycle at 95°C for 1 minute. All quantifications were normalized to β-actin using the cycle threshold (Ct) method.
Statistical analysis
To compare gene expression levels between samples from subjects with and without SCI, the relative expression of each gene was calculated using the comparative ΔΔCt method (fold of expression=2-(ΔΔCT±SD)). The Student t test was then used to analyze differences in gene expression between the two groups.
Results
Subject characteristics
Subject characteristics are presented in Table 1 (microarray analysis) and Table 2 (PCR confirmation). All participants were male and the majority white (12/13 with SCI, 5/7 without SCI). For those with SCI, the mechanism of injury was traumatic for 12/13 subjects. Three subjects had motor complete tetraplegia and nine subjects had motor complete paraplegia. All subjects with SCI used a wheelchair for mobility. The duration of injury ranged from 1.1 to 43.7 years.
Table 1.
Subject Characteristics: Microarray Analysis
| Groups | Subject | Age (y) | Injury level | Mechanism of injury | Injury duration (y) |
|---|---|---|---|---|---|
| SCI | |||||
| 1180 | 60.3 | T8 AIS A | Traumatic motor vehicle accident | 35.9 | |
| 6536 | 62.4 | T12 AIS A | Myelopathy | 20.8 | |
| 7087 | 46.2 | C5 AIS A | Traumatic fall | 31.2 | |
| 8800 | 31.9 | T3 AIS A | Traumatic gunshot injury | 18.0 | |
| 8919 | 35.1 | T8 AIS A | Traumatic motor vehicle accident | 16.5 | |
| 9847 | 67.1 | T11 AIS A | Traumatic motor vehicle accident | 43.7 | |
| No SCI | |||||
| 20007 | 68.7 | - | - | - | |
| 20025 | 55.6 | - | - | - | |
| 20029 | 62.5 | - | - | - | |
| 20039 | 60.6 | - | - | - | |
| 20372 | 50.7 | - | - | - | |
SCI=spinal cord injury; AIS=American Spinal Injury Association Impairment Scale.
Table 2.
Subject Characteristics: Quantitative Polymerase Chain Reaction Confirmation
| Groups | Subject | Age (y) | Injury level | Mechanism of injury | Injury duration (y) |
|---|---|---|---|---|---|
| SCI | |||||
| 6536 | 62.4 | T12 ASIA A | Myelopathy | 20.8 | |
| 8800 | 31.9 | T3 ASIA A | Traumatic gunshot injury | 18.0 | |
| 8919 | 35.1 | T8 ASIA A | Traumatic motor vehicle accident | 16.5 | |
| 3065 | 38.2 | T3 ASIA A | Traumatic surgical cord injury | 11.7 | |
| 4296 | 53.4 | T5 ASIA B | Traumatic surgical cord injury | 1.1 | |
| 1090 | 49.0 | T5 ASIA A | Traumatic fall | 4.7 | |
| 1099 | 54.0 | T7 ASIA A | Traumatic motor vehicle accident | 36.5 | |
| 1100 | 45.2 | C7 ASIA B | Traumatic motor vehicle accident | 4.0 | |
| 1101 | 24.5 | T8 ASIA A | Traumatic motor vehicle accident | 4.1 | |
| 1102 | 33.2 | T4 ASIA A | Traumatic gunshot injury | 6.0 | |
| No SCI | |||||
| 20025 | 55.6 | - | - | - | |
| 20029 | 62.5 | - | - | - | |
| 20039 | 60.6 | - | - | - | |
| 20372 | 50.7 | - | - | - | |
| 2001 | 18.0 | - | - | - | |
| 2002 | 27.5 | - | - | - | |
SCI=spinal cord injury; AIS=American Spinal Injury Association Impairment Scale.
Microarray and network analysis
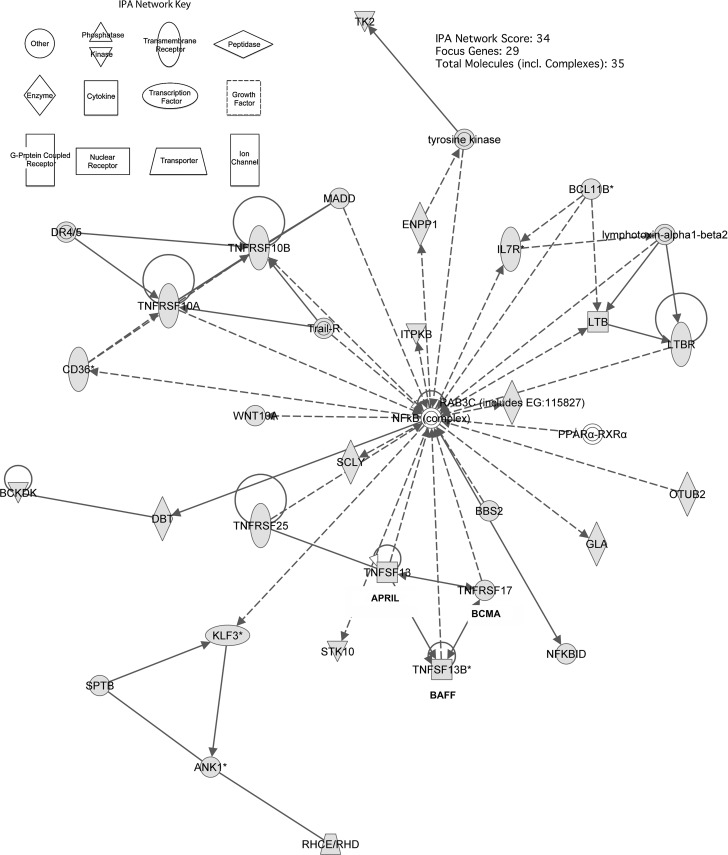
Microarray analysis revealed 1970 genes whose up- or down-regulation was statistically significant (p≤0.05). 1453 of those genes were identified by IPA software to be present in 25 statistically significant molecular pathways. A single network (Fig. 1), with associated network functions including “lymphoid tissue structure and development” (IPA score=34) and composed of 29 distinct molecules and five protein complexes (Table 3), was selected for further investigation because of the presence of numerous cytokines and receptor molecules related to B-cell development, activation, and proliferation. The central component of this network is the nuclear factor-kappaB (NF-κB) protein complex, a transcription factor complex involved in numerous signal transduction pathways. BCMA (TNFRSF17) APRIL (TNFSF13) and BAFF (TNFSF13B) were up-regulated by factors of 3.13 (p=0.004), 1.33 (p=0.03), and 1.23 (p=0.001), respectively, in subjects with SCI compared with uninjured controls.
FIG. 1.
Pathway analysis of peripheral blood mononuclear cell gene expression in subjects with chronic spinal cord injury (SCI) compared with subjects with no SCI. The network depicted is composed of 29 genes related to B-cell development, activation, and proliferation. The nuclear factor-kappaB (NF-κB) protein complex is the central component of this network. B-cell maturation antigen (BCMA), a proliferation-inducing ligand (APRIL), and B-cell–activating factor (BAFF) are highlighted.
Table 3.
Pathway Gene List
| Gene symbol | Gene name | Affymetrix probesSet ID | Fold change | p value | Family |
|---|---|---|---|---|---|
| ANK1 | ankyrin 1, erythrocytic | 205390_s_at | −1.475 | 2.371E-02 | Other |
| BBS2 | Bardet-Biedl syndrome 2 | 223227_at | −1.212 | 1.923E-02 | Other |
| BCKDK | branched chain ketoacid dehydrogenase kinase | 202030_at | 1.230 | 1.803E-02 | Kinase |
| BCL11B | B-cell CLL/lymphoma 11B (zinc finger protein) | 219528_s_at | −1.490 | 1.377E-02 | Other |
| CD36 | CD36 molecule (thrombospondin receptor) | 228766_at | 1.504 | 1.831E-02 | Transmembrane receptor |
| DBT | dihydrolipoamide branched chain transacylase E2 | 231919_at | −1.331 | 1.327E-02 | Enzyme |
| DR4/5 | complex | n/a | n/a | n/a | Group |
| ENPP1 | ectonucleotide pyrophosphatase/phosphodiesterase 1 | 205066_s_at | 1.117 | 4.180E-02 | Enzyme |
| GLA | galactosidase, alpha | 214430_at | 1.189 | 4.438E-02 | Enzyme |
| IL7R | interleukin 7 receptor | 205798_at | −1.574 | 3.039E-03 | Transmembrane receptor |
| ITPKB | inositol-trisphosphate 3-kinase B | 203723_at | −1.293 | 6.349E-03 | Kinase |
| KLF3 | Kruppel-like factor 3 (basic) | 225133_at | −1.150 | 1.416E-02 | Transcription regulator |
| LTB | lymphotoxin beta (TNF superfamily, member 3) | 207339_s_at | −1.365 | 1.018E-02 | Cytokine |
| LTBR | lymphotoxin beta receptor (TNFR superfamily, member 3) | 203005_at | 1.364 | 5.405E-03 | Transmembrane receptor |
| lymphotoxin-alpha1-beta2 | complex | n/a | n/a | n/a | Complex |
| MADD | MAP-kinase activating death domain | 210252_s_at | −1.137 | 1.787E-02 | Other |
| NFkB (complex) | complex | n/a | n/a | n/a | Complex |
| NFKBID | nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, delta | 241889_at | −1.316 | 3.737E-02 | Other |
| OTUB2 | OTU domain, ubiquitin aldehyde binding 2 | 222878_s_at | 1.099 | 4.855E-02 | Enzyme |
| PPAR_-RXR_ | complex | n/a | n/a | n/a | Complex |
| RAB3C (includes EG:115827) | RAB3C, member RAS oncogene family | 244170_at | −1.086 | 1.454E-02 | enzyme |
| RHCE/RHD | Rh blood group, D antigen | 215819_s_at | −1.389 | 3.200E-02 | Transporter |
| SCLY | selenocysteine lyase | 59705_at | 1.119 | 4.896E-02 | Enzyme |
| SPTB | spectrin, beta, erythrocytic | 214145_s_at | −1.193 | 1.134E-02 | Other |
| STK10 | serine/threonine kinase 10 | 228394_at | −1.198 | 2.931E-02 | Kinase |
| TK2 | thymidine kinase 2, mitochondrial | 204276_at | 1.245 | 1.057E-02 | Kinase |
| TNFRSF10A | tumor necrosis factor receptor superfamily, member 10a | 241371_at | −1.180 | 1.851E-02 | Transmembrane receptor |
| TNFRSF10B | tumor necrosis factor receptor superfamily, member 10b | 210405_x_at | 1.198 | 1.090E-02 | Transmembrane receptor |
| TNFRSF17 (BCMA) | tumor necrosis factor receptor superfamily, member 17 | 206641_at | 3.129 | 3.863E-03 | Transmembrane receptor |
| TNFRSF25 | tumor necrosis factor receptor superfamily, member 25 | 216042_at | −1.172 | 4.633E-02 | Transmembrane receptor |
| TNFSF13 (APRIL) | tumor necrosis factor (ligand) superfamily, member 13 | 210314_x_at | 1.329 | 2.769E-02 | Cytokine |
| TNFSF13B (BAFF) | tumor necrosis factor (ligand) superfamily, member 13b | 223501_at | 1.234 | 1.131E-03 | Cytokine |
| Trail-R | pathway | n/a | n/a | n/a | Group |
| tyrosine kinase | group | n/a | n/a | n/a | Group |
| WNT10A | wingless-type MMTV integration site family, member 10A | 223709_s_at | −1.322 | 1.154E-02 | Other |
Quantitative PCR
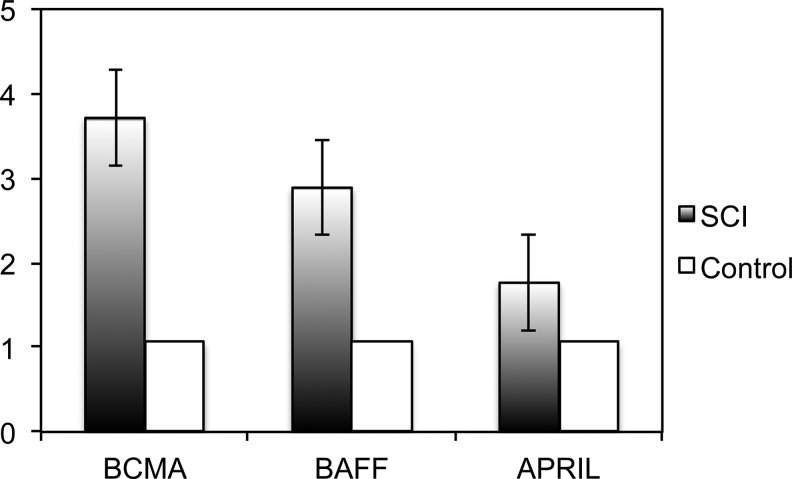
We found that BCMA (3.6 fold, p<0.05), APRIL (3.2 fold, p<0.05), and BAFF (2.0 fold, p<0.05) were all up-regulated in peripheral blood mononuclear cells from subjects with SCI versus the uninjured controls (Fig. 2).
FIG. 2.
B-cell maturation antigen (BCMA), a proliferation-inducing ligand (APRIL), and B-cell–activating factor (BAFF) are upregulated in chronic spinal cord injury (SCI). mRNA from peripheral blood mononuclear cells was subjected to quantitative polymerase chain reaction amplification analysis. BCMA, APRIL, and BAFF are significantly up-regulated in SCI, compared with uninjured controls (BCMA: 3.6 fold; APRIL: 3.2 fold, and BAFF: 2.0 fold; p<0.05).
Discussion
In this study, we performed microarray analysis of peripheral blood mononuclear cells to identify differentially expressed genes in chronic SCI. We identified 1453 genes participating in 25 distinct molecular pathways. One gene network, associated with lymphoid tissue structure and development, was composed of 29 distinct molecules and five protein complexes. Many of these genes encode cytokines and receptor molecules involved in B-cell development, proliferation, activation, and survival. We chose this pathway for further study because it contained three molecules (BMCA, APRIL, and BAFF) that have been previously reported in the development of other autoimmune conditions including systemic lupus erythematosus and multiple sclerosis.11 We further confirmed the up-regulation of BMCA, APRIL, and BAFF by qPCR analysis.
BCMA is a member of the tumor necrosis factor (TNF) receptor superfamily that has been shown to mediate B-cell survival and activation.12 Both BAFF and APRIL can bind BCMA and initiate signal transduction through NF-κB canonical and noncanonical pathways. Activation of NF-κB can, in turn, transcribe B-cell survival factors.13 APRIL and BAFF are members of the TNF ligand superfamily and have been shown to cause B-cell survival, proliferation, and differentiation into both antibody-secreting plasma cells and long-lived memory B cells.8 More importantly, APRIL and BAFF promote the formation of B-cell follicle structures. These molecules are present at high concentrations in the germinal centers of lymph follicles and coordinate many aspects of B-cell development and maturation.
BAFF, in particular, is implicated in regulation of self-tolerance. In mice, a reduction in BAFF levels decreased the number of self-reactive B cells in mice, while an increase in BAFF led to reconstitution of the self-reactive B-cell population.14 Transgenic mice overexpressing BAFF were shown to develop an SLE-like autoimmune disorder.15 Similarly, elevated circulating levels of BAFF have been reported in subjects with systemic lupus erythematosus, Sjögren's syndrome,16 and multiple sclerosis.17
It is known that SCI alters B-cell function both systemically and locally within the spinal cord lesion.2 As recently demonstrated, moderate thoracic SCI in a rodent model results in B-cell activation and pathogenic autoantibody production within both cerebral spinal fluid and the spinal cord. Injured mice lacking B cells demonstrated improved neurological function compared with mice with normal B cell function.6 The presence of follicle-like structures near the SCI lesion suggests that local factors within the cord direct the migration of activated B cells to the lesion. It is speculated that local production of BAFF and APRIL by microglia and astrocytes contribute to the establishment and maintenance of these follicle-like structures within the cord long term after SCI. To our knowledge, this is the first report that peripheral blood mononuclear cells produce increased levels of BAFF and APRIL in chronic SCI. This finding, albeit in a small sample, suggests systemic regulation of SCI-autoimmunity via APRIL and BAFF mediated activation of B cells through BMCA.
Autoimmunity can be both pathogenic and protective after neurological injury, depending on the characteristics of the antibodies (eg, isotypes, specificity, titer), the nature of the neurological injury (location, severity, and duration), and the interaction between lesion and antibodies (ie, location of antibody deposition relative to the site of injury).7,18,19 For instance, animal models of multiple sclerosis have demonstrated natural IgM autoantibodies that play a protective role enhancing remyelination and preventing neuronal apoptosis.20,21 Similarly, mice deficient in mature B cells demonstrated greater neuron survival after intravitreal glutamate injections.22 This response, however, may depend on other genetic factors, because opposite results were obtained in response to neurological injury in animals of different genetic backgrounds.23
A long-term protective autoimmune response has been speculated but not conclusively demonstrated after SCI. Axonal injury has been shown to stimulate a T-cell mediated protective response in rodents.24–26 There is also evidence in rodent models that SCI results in B-cell expansion with increased circulating IgG antibodies.2,27 These antibodies target auto-antigens (eg, myelin basic protein, actin, nuclear proteins).
The role of autoantibodies after SCI remains controversial. One group reported the presence of autoantibodies before injury in a rodent model of SCI and concluded that these antibodies are not neurotoxic.28 More recently, increased myelin basic protein antibody titers were found in 12 subjects with SCI (<10 years) compared with 18 healthy controls.29 The subjects with SCI also demonstrated increased T-cell proliferation against myelin basic protein. The authors speculate that this cellular and humoral response may not necessarily be pathological, because all subjects with SCI demonstrated stable neurological impairment over the course of the observation period despite the presence of autoantibodies. An alternative interpretation is that the ongoing presence of autoantibodies prevented improvement of neurological outcomes over the course of the observation period. There is also evidence to suggest that autoantibodies contribute to systemic autoimmune disease and aggravate secondary consequences such as dermatitis, renal dysfunction, and reproductive sterility.30
There are limitations to the current work that must be considered. This is a study of a relatively small sample of men. A larger study that includes women is needed to confirm our findings and assess for gender differences. In addition, subclinical infections may occur in SCI. While none of the participants in the current study were acutely ill, it is possible that our findings are influenced by the presence of subclinical infection. Despite these limitations, we offer evidence that after SCI, peripheral blood mononuclear cells overexpress BCMA, BAFF, and APRIL. Based on previous studies,7,22,29 we speculate that this promotes SCI-induced autoimmunity by allowing B cells to become activated by CNS debris and escape clonal deletion. These activated B cells secrete autoantibodies that contribute to tissue damage and neurotoxicity after SCI. It has been suggested the production of autoantibodies after SCI has widespread implications for health in chronic SCI, contributing to decreased lung function, decreased kidney function, low sperm count, joint pain, and dermatitis.4,30 Accordingly, BCMA, APRIL, and BAFF may be potential therapeutic targets to minimize tissue damage and neurotoxicity in chronic SCI.
Acknowledgments
This study received support from the National Institute of Child Health and Human Development [R21HD057030 and R21HD057030-02S1], the National Institute of Arthritis and Musculoskeletal and Skin Diseases [1R01AR059270-02], Department of Education, NIDRR H133N110010, the Office of Research and Development, Rehabilitation Research and Development [Merit Review Grant B6618R], and the Massachusetts Veterans Epidemiology Research and Information Center, Cooperative Studies Program, Department of Veterans Affairs.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Riegger T. Conrad S. Liu K. Schluesener H.J. Adibzahdeh M. Schwab J.M. Spinal cord injury-induced immune depression syndrome (SCI-IDS) Eur. J. Neurosci. 2007;25:1743–1747. doi: 10.1111/j.1460-9568.2007.05447.x. [DOI] [PubMed] [Google Scholar]
- 2.Ankeny D.P. Lucin K.M. Sanders V.M. McGaughy V.M. Popovich P.G. Spinal cord injury triggers systemic autoimmunity: evidence for chronic B lymphocyte activation and lupus-like autoantibody synthesis. J. Neurochem. 2006;99:1073–1087. doi: 10.1111/j.1471-4159.2006.04147.x. [DOI] [PubMed] [Google Scholar]
- 3.Hailer N.P. Immunosuppression after traumatic or ischemic CNS damage: it is neuroprotective and illuminates the role of microglial cells. Prog. Neurobiol. 2008;84:211–233. doi: 10.1016/j.pneurobio.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 4.Hayes K.C. Hull T.C. Delaney G.A. Potter P.J. Sequeira K.A. Campbell K. Popovich P.G. Elevated serum titers of proinflammatory cytokines and CNS autoantibodies in patients with chronic spinal cord injury. J. Neurotrauma. 2002;19:753–761. doi: 10.1089/08977150260139129. [DOI] [PubMed] [Google Scholar]
- 5.Kil K. Zang Y.C. Yang D. Markowski J. Fuoco G.S. Vendetti G.C. Rivera V.M. Zhang J.Z. T cell responses to myelin basic protein in patients with spinal cord injury and multiple sclerosis. J. Neuroimmunol. 1999;9:201–207. doi: 10.1016/s0165-5728(99)00057-0. [DOI] [PubMed] [Google Scholar]
- 6.Ankeney D.P. Guan Z. Popovich P.G. B cells produce pathogenic antibodies and impair recovery after spinal cord injury in mice. J. Clin. Invest. 2009;119:2990–2999. doi: 10.1172/JCI39780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ankeny D.P. Popovich P.G. B cells and autoantibodies: complex roles in CNS injury. Trends Immunol. 2010;31:332–338. doi: 10.1016/j.it.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dekaban G.A. Thawer S. Pathogenic antibodies are active participants in spinal cord injury. J. Clin. Invest. 2009;119:2881–2884. doi: 10.1172/JCI40839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morse L.R. Sudhakar S. Danilack V. Tun C. Lazzari A. Gagnon D.R. Garshick E. Battaglino R.A. Association between sclerostin and bone density in chronic spinal cord injury. J. Bone Miner. Res. 2012;27:352–359. doi: 10.1002/jbmr.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shanley T.P. Cvijanovich N. Lin R. Allen G.L. Thomas N.J. Doctor A. Kalyanaraman M. Tofil N.M. Penfil S. Monaco M. Odoms K. Barnes M. Sakthivel B. Aronow B.J. Wong H.R. Genome-level longitudinal expression of signaling pathways and gene networks in pediatric septic shock. Mol. Med. 2007;13:495–508. doi: 10.2119/2007-00065.Shanley. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mackay F. Silveira P.A. Brink R. B cells and the BAFF/APRIL axis: fast-forward on autoimmunity and signaling. Curr. Opin. Immunol. 2007;19:327–336. doi: 10.1016/j.coi.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 12.Rickert R.C. Jellusova J. Miletic A.V. Signaling by the tumor necrosis factor receptor superfamily in B-cell biology and disease. Immunol. Rev. 2011;244:115–133. doi: 10.1111/j.1600-065X.2011.01067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cancro M.P. D'Cruz D.P. Khamashta M.A. The role of B lymphocyte stimulator (BLyS) in systemic lupus erythematosus. J. Clin. Invest. 2009;119:1066–1073. doi: 10.1172/JCI38010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nikbakht N. Migone T.S. Ward C.P. Manser T. Cellular competition independent of BAFF/B lymphocyte stimulator results in low frequency of an autoreactive clonotype in mature polyclonal B cell compartments. J. Immunol. 2011;187:37–46. doi: 10.4049/jimmunol.1003924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mackay F. Woodcock S.A. Lawton P. Ambrose C. Baetscher M. Schneider P. Tschopp J. Browning J.L. Mice transgenic for BAFF develop lymphocytic disorders along with autoimmune manifestations. J. Exp. Med. 1999;190:1697–1710. doi: 10.1084/jem.190.11.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vadacca M. Margiotta D. Sambataro D. Buzzulini F. Lo Vullo M. Rigon A. Afeltra A. [BAFF/APRIL pathway in Sjögren syndrome and systemic lupus erythematosus: relationship with chronic inflammation and disease activity] (Ita) Reumatismo. 2010;62:259–265. doi: 10.4081/reumatismo.2010.259. [DOI] [PubMed] [Google Scholar]
- 17.Mackay F. Schneider P. Cracking the BAFF code. Nat. Rev. Immunol. 2009;9:491–502. doi: 10.1038/nri2572. [DOI] [PubMed] [Google Scholar]
- 18.Archelos J.J. Hartung H.P. Pathogenetic role of autoantibodies in neurological diseases. Trends Neurosci. 2000;23:317–327. doi: 10.1016/s0166-2236(00)01575-7. [DOI] [PubMed] [Google Scholar]
- 19.Popovich P.G. Longbrake E.E. Can the immune system be harnessed to repair the CNS? Nat. Rev. Neurosci. 2008;9:481–493. doi: 10.1038/nrn2398. [DOI] [PubMed] [Google Scholar]
- 20.Vargas M.E. Watanabe J. Singh S.J. Robinson W.H. Barres B.A. Endogenous antibodies promote rapid myelin clearance and effective axon regeneration after nerve injury. Proc. Natl. Acad. Sci. U. S. A. 2010;107:11993–11998. doi: 10.1073/pnas.1001948107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Warrington A.E. Rodriguez M. Method of identifying natural antibodies for remyelination. J. Clin. Immunol. 2010;30(Suppl 1):S50–S55. doi: 10.1007/s10875-010-9406-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schori H. Lantner F. Shachar I. Schwartz M. Severe immunodeficiency has opposite effects on neuronal survival in glutamate-susceptible and -resistant mice: adverse effect of B cells. J. Immunol. 2002;169:2861–2865. doi: 10.4049/jimmunol.169.6.2861. [DOI] [PubMed] [Google Scholar]
- 23.Schori H. Shechter R. Shachar I. Schwartz M. Genetic manipulation of CD74 in mouse strains of different backgrounds can result in opposite responses to central nervous system injury. J. Immunol. 2007;178:163–171. doi: 10.4049/jimmunol.178.1.163. [DOI] [PubMed] [Google Scholar]
- 24.Moalem G. Leibowitz-Amit R. Yoles E. Mor F. Cohen I.R. Schwartz M. Autoimmune T cells protect neurons from secondary degeneration after central nervous system axotomy. Nat. Med. 1999;5:49–55. doi: 10.1038/4734. [DOI] [PubMed] [Google Scholar]
- 25.Wang Y. Wang K. Chao R. Li J. Zhou L. Ma J. Yan J. Neuroprotective effect of vaccination with autoantigen-pulsed dendritic cells after spinal cord injury. J. Surg. Res. 2012;176:281–292. doi: 10.1016/j.jss.2011.06.066. [DOI] [PubMed] [Google Scholar]
- 26.Yoles E. Hauben E. Palgi O. Agranov E. Gothilf A. Cohen A. Kuchroo V. Cohen I.R. Weiner H. Schwartz M. Protective autoimmunity is a physiological response to CNS trauma. J. Neurosci. 2001;21:3740–3748. doi: 10.1523/JNEUROSCI.21-11-03740.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Popovich P.G. Stuckman S. Gienapp I.E. Whitacre C.C. Alterations in immune cell phenotype and function after experimental spinal cord injury. J. Neurotrauma. 2001;18:957–966. doi: 10.1089/089771501750451866. [DOI] [PubMed] [Google Scholar]
- 28.Ibarra A. Martinez S. Reyes J. Meza-Lucas A. Mandujano A. Grijalva I. Madrazo I. Correa D. Search for an IgG response against neural antigens in experimental spinal cord injury. Neuroscience. 2000;96:3–5. doi: 10.1016/s0306-4522(99)00541-2. [DOI] [PubMed] [Google Scholar]
- 29.Zajarias-Fainsod D. Carrillo-Ruiz J. Mestre H. Grijalva I. Madrazo I. Ibarra A. Autoreactivity against myelin basic protein in patients with chronic paraplegia. Eur. Spine J. 2012;21:964–970. doi: 10.1007/s00586-011-2060-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Davies A.L. Hayes K.C. Dekaban G.A. Clinical correlates of elevated serum concentrations of cytokines and autoantibodies in patients with spinal cord injury. Arch. Phys. Med. Rehabil. 2007;88:1384–1393. doi: 10.1016/j.apmr.2007.08.004. [DOI] [PubMed] [Google Scholar]