Abstract
BACKGROUND
Because low grade serous carcinoma of the ovary is relatively chemo resistant disease, this study evaluated Selumetinib (AZD6244), an inhibitor of mitogen-activated protein kinase kinase (MEK-1/2), and explored associations between RAS, and RAF family mutations with clinical outcome.
METHODS
Women with recurrent low-grade serous ovarian or peritoneal carcinoma were eligible and received Selumetinib at 100 mg. orally b.i.d. until progression or toxicity were enrolled in Gynecologic Oncology protocol 239(NCT00551070). This trial has been completed and we are reporting the results. The primary endpoint of this trial was to examine tumor response rate to Selumetinib. The study used all-treated patients to determine response rate and overall survival.
FINDINGS
Fifty-two patients were enrolled over two years. Eight patients (15.4%) had complete (1) or partial (7) responses, and 34 (65%) had stable disease. There were no treatment-related deaths. There were three observed grade 4 toxicities and 46 grade 3 toxicities that occurred in more than one patient. Observed grade 4 toxicities were cardiac (1), pain (1), and pulmonary (1). Grade 3 toxicities that occurred included gastrointestinal (13), dermatologic (9), and metabolic (7).
CONCLUSIONS
Selumetinib is well tolerated, and is active in the treatment of recurrent low-grade serous carcinoma. In exploratory analyses, response to Selumetinib did not appear to be related to RAS/RAF mutational status. The 63% disease control is encouraging and worthy of further evaluation of MEK inhibitors in this population. This study was supported by National Cancer Institute grants to the Gynecologic Oncology Group.
Keywords: Selumetinib, cancer, serous, ovarian, low-grade
INTRODUCTION
Low-grade serous carcinoma (LGSC) of the ovary is a unique tumor that is distinguished from high-grade serous carcinoma (HGSC) by differences in its pathological features, associated molecular changes, and its natural clinical course, Table 1.1–4 Serous ovarian tumors of low malignant potential (LMP), and low-grade serous carcinomas of the ovary have a higher frequency of KRAS and BRAF mutations, a higher frequency of expression of active mitogen-activated protein kinase (MAPK), and a lower frequency of p53 mutations than do high-grade serous carcinomas1,2,5–8 In addition, gene expression profiles of LGSC and serous tumors of LMP are similar and very distinct from the expression profiles of HGSCs.6,7,9
TABLE 1.
Characteristics of Low Grade and High Grade Serous Carcinomas
| Low Grade | High Grade | |
|---|---|---|
| Age | 56 | 63 |
| Mean OS (months) | 99 | 57 |
| RR to platinum/taxane | 2%–4% | 80% |
| KRAS,BRAF+ | 30%–50% | 2%–3% |
| P53+ | 55% | 25% |
| BRCA+ | 4% | 22% |
Clinical observations that appear to link serous tumors of LMP and LGSC and contrast them to HGSCs are equally compelling.10 These patients are diagnosed at a younger age and have a longer overall survival (OS) compared to women with HGSC. LGSC is relatively chemo resistant, not only to upfront agents, but also in the setting of recurrent disease.11,12 The response rates (RR) for both platinum resistant and sensitive disease only approach 2%–4%, respectively.12 It therefore is important to continue the search for active targeted agents for these tumors, capitalizing on our better understanding of the molecular orgins of the tumors.
Selumetinib (AZD6244, ARRY-142886) is a potent, selective, orally-available, and non-ATP competitive small molecule inhibitor of the mitogen-activated protein (MAP) kinase, mitogen-activated protein kinase kinase (MEK-1/2).13 Given the high frequency of mutational alterations in the MAPK pathway found in low-grade serous ovarian cancers, molecular inhibitors of pathway activation could offer a targeted strategy to control tumor growth. Based on this preliminary information, the Gynecologic Oncology Group (GOG) proposed to test Selumetinib in a phase II clinical trial for patients with recurrent low-grade serous carcinoma of the ovary or peritoneum. The primary objective of this trial was to examine tumor RR to Selumetinib.
MATERIALS and METHODS
Study Design
This open-label phase II study was approved by the local institutional review board at each participating institution within the GOG. Eligible participants included women with an initial primary diagnoses of serous borderline, low-grade serous ovarian or peritoneal carcinoma that had biopsy proven recurrent LGSC (invasive micropapillary serous carcinoma, or invasive grade I serous carcinoma) confirmed by blinded pathologic review of recurrent tumor. Eligible women were at least 18 years of age, had a GOG performance status of 0, 1, or 2, had disease measurable by physical exam or medical imaging according to GOG Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1, and had adequate hematologic, renal, hepatic, pulmonary, and cardiac function with no active infections. Diagnosis of LGSC was confirmed prospectively by pathologic review of recurrent tissue only prior to enrollment in protocol. This pathologic review was conducted by a subcommittee of five GOG pathologists. Women were ineligible if they had a concomitant or prior malignancy (other than a non melanoma skin cancer) within the preceding five years, had prior chemotherapy within the past four weeks, or had a history of prior MEK inhibitor use. Patients provided written informed consent consistent with federal, state, and local requirements and gave authorization permitting the release of personal health information.
Assessments
Patients were assessed prior to each cycle of treatment. Radiographic disease measurements were required every other cycle using standard RECISTv 1.1 response criteria.14 OS was defined as length of time from date of study entry to death, or the date of last contact. Progression-free survival (PFS) was defined as the period from study entry until disease progression, death, or the last date of contact. Patients who received any drug were evaluable for efficacy and toxicity.
Treatment
Treatment consisted of Selumetinib hydrogen sulfate administered at a dose of 50 mg. twice daily (b.i.d.), approximately 12 hours apart. Four weeks constituted a cycle. A cycle of therapy was not administered unless the absolute neutrophil count was ≥1500 and platelets were ≥100,000 ul. Creatinine was required to be <1·5X institutional upper limit of normal (ULN). Bilirubin was required to be ≤1·5× ULN (Common Terminology Criteria for Adverse Events - CTCAE v3.0 grade 1). Serum glutamic-oxalocetic transaminase (SGOT) and alkaline phosphatase were required to be ≤2·5 x ULN (CTCAE v3.0 grade 1). Sensory and motor neuropathy for each patient was required to be < grade 2.
Selumetinib dose adjustments were based on the hematologic, dermatologic, and gastrointestinal (diarrhea, transaminases) toxicity. Treatment delays of up to 21 days were permitted. Subjects who failed to recover adequate counts within a three-week delay were removed from study.
Study Endpoints
The primary objectives were to: examine the tumor RR of patients on Selumetinib, examine the acute toxicity of Selumetinib using CTCAE version 3.0, and define the pharmacokinetic profile for Selumetinib, at a dose of 50 mg. twice daily (b.i.d.), approximately 12 hours apart. Response was determined as best response at any time The secondary objectives were to: examine the acute and chronic toxicity of Selumetinib using the 21 major categories of the CTCAE version 3.0.1.22, examine the dose and number of courses of Selumetinib given, and estimate the PFS, and OS of women receiving Selumetinib. The translational research objectives were to: examine DNA isolation with sequencing of BRAF, and KRAS mutation analysis and explore their relationship with tumor response in patients treated with Selumetinib. In post hoc data exploration, we examined CA-125 responses (at least a 50% reduction from baseline CA-125) in patients with baseline CA-125 >=2×ULN.
Mutational Analysis of KRAS and BRAF Genes
Formalin-fixed, paraffin-embedded tissue samples from 40 patients enrolled into the study were obtained. Genomic DNA was purified from the tumor component, with 34 patients having sufficient DNA, 20 ng, for mutational analysis. All specimens used were reviewed for tumor/stromal content and were greater than 50% tumor cells. The molecular platform utilized in this study has a sensitivity of approximately 90% for FFPE. However, if significant tumor heterogeneity exists and the mutation containing cells account for less than 10% of the specimen it is possible that a mutation was not detected. The ovarian tumors were analyzed for a codon 599 mutation in BRAF, and codon 12 and 13 mutations in KRAS. Analysis of the 1796T/A status in BRAF was performed using a polymerase chain reaction (PCR)-based restriction fragment length polymorphism (RFLP) technique or direct sequencing. For RFLP method, the BRAF PCR product of exon 15, which contains nucleotide position 1796, was digested with TspR1 (New England Biolabs, Inc., Beverly, MA) at 65°C for 3 hours. The PCR products were electrophoresed on a 10% polyacrylamide gel and were also sequenced to validate the RFLP results. KRAS mutational status at codon 12 or 13 was analyzed either by digital PCR or direct sequencing.
Mass-spectrometric genotyping
Genomic DNA from all tumor samples was purified and subjected to phi29 polymerase multiple strand-displacement whole-genome amplification. After quantification and dilution of genome-amplified DNA, multiplexed PCR was performed in 5-ml volumes containing 0·1 units of Taq polymerase, 5 ng of genome-amplified genomic DNA, 2·5 pmol of each PCR primer and 2·5 mmol of dNTP. Thermocycling was at 95 1C for 15 minutes followed by 45 cycles of 95 1C for 20 s, 56 1C for 30 s and 72 1C for 30s. Unincorporated dNTPs were deactivated using 0·3 U of shrimp alkaline phosphatase, and primer extension was carried out using 5·4 pmol of each primer extension probe, 50 mmol of the appropriate dNTP/ddNTP combination and 0·5 units of Thermosequenase DNA polymerase. Reactions were cycled at 94 1C for 2 minutes, followed by 40 cycles of 94 1C for 5 s, 50 1C for 5 s and 72 1C for 5 s. After the addition of a cation exchange resin to remove residual salt from the reactions, 7 nl of the purified primer extension reaction was loaded onto a matrix pad (3-hydroxypicoloinic acid) of a SpectroCHIP (Sequenom). SpectroCHIPs were analyzed using a Bruker Biflex III matrix-assisted laser desorption/ionization–time of flight (MALDI-TOF) mass spectrometer (SpectroREADER, Sequenom). Mutation calls for each sample were determined using the default settings of MassArray Typer 3.4 Analyzer (Sequenom). Successful genotyping assays were defined as those in which 75% of all genotyping calls were obtained.
Statistical Analysis
We tested the null hypothesis (H0) that the objective RR is 10% or less against the alternative (H1) that it is greater than 10% assuming the true RR for the agent is 25%. The study used a flexible, 2-stage accrual design that allowed stopping early for lack treatment activity.23 During the first stage of accrual, with 27 eligible patients, ≥4 patients were required to have a complete (CR) or partial response (PR) in order to open the second phase of accrual. At the end of the second stage, with 52 eligible patients, the regimen would be considered active if at ≥8 patients had a PR or CR. If the true response rate were 10%, the average probability of designating the treatment as active would be limited to 10%; on the other hand, if the true response rate were 25%, the probability of correctly classifying the treatment as active would be 90%. There were no historical data available on what the response rate is for treatments in this patient population; therefore a threshold of 10% for the null was used as indicator of no activity. Secondary and exploratory analyses were conducted to assess associations between patient demographics, clinical outcomes, and biological characteristics. The purpose was to characterize and screen in a hypothesis generating fashion. Tests with p-values less than 0.05 were deemed suggestive, and expected relationships with p-values between 5% and 10% could be noted as a trend. Fisher’s Exact test was used to compare response rates by mutational status.. Survival curves were generated using the Kaplan-Meier method. SAS® version 9.3 was used for all statistical analyses. This study is registered with ClinicalTrials.gov, NCT00551070. The funding source, The National Cancer Institute, provided the study drug, Selumetinib, used in this study. The corresponding author had full access to all of the data and the final responsibility to submit for publication.
RESULTS
Patient Characteristics
Fifty-two patients were accrued between December 17, 2007 and November 23, 2009, and the data were locked on May 16, 2011. All 52 patients enrolled were eligible, treated, and evaluable. The patient characteristics are presented in Table 2. Median age was 51 years (range 24–77). The majority of patients were white, had GOG performance status of 0, and ovarian site of disease. Thirty (57%) patients had received three or more prior chemotherapeutic regimens; of the 126 total regimens received, 82 (65%) contained platinum, including 20 patients who received two platinum regimens, and 5 who received three platinum regimens. Only one of the 126 regimens was a horomonal therapy (Lupron).
TABLE 2.
Patient Characteristics in Entire Cohort and Mutation (BRAF, KRAS,) Subset
| Characteristic | Category | In Entire Cohort (N=52) | In Mutation Subset (N=34) |
|---|---|---|---|
| Age | 20–29 | 3 (5.8) | 1 (2.9) |
| 30–39 | 11 (21.2) | 7 (20.6) | |
| 40–49 | 8 (15.4) | 6 (17.6) | |
| 50–59 | 17 (32.7) | 11 (32.4) | |
| 60–69 | 9 (17.3) | 8 (23.5) | |
| 70–79 | 4 (7.7) | 1 (2.9) | |
| Race | Unspecified | 2 (3.8) | 0 (0.0) |
| Asian | 2 (3.8) | 0 (0.0) | |
| African American | 2 (3.8) | 2 (5.9) | |
| White | 46 (88.5) | 32 (94.1) | |
| Ethnicity | Hispanic or Latino | 7 (13.5) | 3 (8.8) |
| Non-Hispanic | 42 (80.8) | 29 (85.3) | |
| Unknown/Not specified | 3 (5.8) | 2 (5.9) | |
| GOG Performance Status | 0 | 32 (61.5) | 23 (67.6) |
| 1 | 20 (38.5) | 11 (32.4) | |
| Site of Disease | Ovary | 47 (90.4) | 30 (88.2) |
| Peritoneum | 5 (9.6) | 4 (11.8) | |
| Cell Type | Serous Adenocarcinoma | 52 (100.0) | 34 (100.0) |
| Number of Previous | 1 | 10 (19.2) | 6 (17.6%) |
| Regimens | 2 | 12 (23.1) | 8 (23.5%) |
| 3 | 28 (53.8) | 19 (55.9%) | |
| 4 | 2 (3.8) | 1 (2.9%) | |
| Prior Radiation | No | 44 (84.6) | 29 (85.3) |
| Yes | 8 (15.4) | 5 (14.7) | |
| Prior Immunotherapy | No | 50 (96.2) | 33 (97.1) |
| Yes | 2 (3.8) | 1 (2.9) | |
| Prior Surgery | No | 3 (5.8) | 2 (5.9) |
| Yes | 49 (94.2) | 32 (94.1) |
Clinical Outcomes
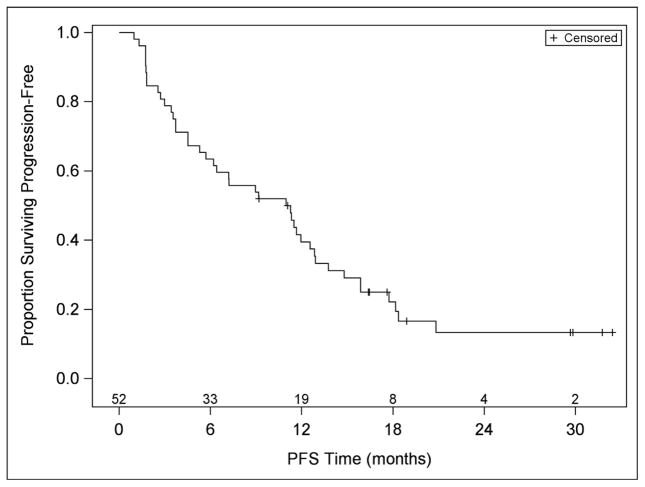
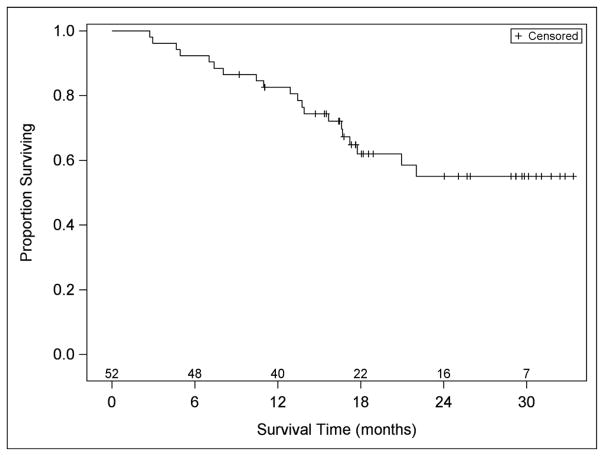
Twenty-seven patients were accrued and eligible in stage 1 of the study: four patients had partial responses, response was determined as best response at any time. At the end of the study, with 52 evaluable patients, eight patients (15.4%) had a partial or complete response (90% confidence interval [CI]: 7.9%, 26.1%) (Table 3). For patients who had partial or complete responses, the median (25th – 75th percentiles) time to response was 4.8 (2.7 – 8.9) months, and the duration of response was 10.5 (8.2 - not estimable due to three censored patients). The median PFS was 11 months (first and third quartiles were 3·6 and 15·9 months, respectively) (Table 3, Figure 1). Thirty-three (63%) patients experienced a PFS >6 months duration. Median overall survival has not been reached; the Kaplan-Meier estimate (95% CI) of the percentage of people surviving at least 24 months is 55% (40%, 71%). The median (25th and 75th percentiles) follow-up in patients who have not died is 21 (17–30) months. The median number of cycles received was 4·5, and 33% (17/52) of patients received at least 12 cycles of treatment. Of the 438 cycles received, 30 (7%) were delayed: 12 due to protocol-related AEs, 5 due to non-protocol illnesses, 5 due to personal reasons, and 8 due to scheduling. In post hoc data exploration, of 31 patients with CA-125 >=2 x ULN, 6 patients (19%) CA-125 responses; three of the 31 had tumor responses, and 1 had both.
TABLE 3.
Response Rate and Progression Free Survival
| Characteristic | Category | No. of Cases | % of Cases |
|---|---|---|---|
| Response | Complete Response | 1 | 1.9 |
| Partial Response | 7 | 13.5 | |
| Stable Disease | 34 | 65.4 | |
| Increasing Disease | 8 | 15.4 | |
| Indeterminate | 2 | 3.8 | |
| Cycles of Treatment | 1 | 7 | 13.5 |
| 2 | 8 | 15.4 | |
| 3 | 6 | 11.5 | |
| 4 | 5 | 9.6 | |
| 5 | 1 | 1.9 | |
| 6 | 2 | 3.8 | |
| 7 | 2 | 3.8 | |
| 10 | 4 | 7.7 | |
| 12 | 2 | 3.8 | |
| 13 | 1 | 1.9 | |
| 14 | 3 | 5.8 | |
| 15 | 2 | 3.8 | |
| >15† | 9 | 17.3 | |
| Alive‡ | Without progression | 10 | 19.2 |
| With progression | 22 | 42.3 | |
| Dead‡ | From disease | 17 | 32.7 |
| From neither Rx nor disease | 1 | 1.9 | |
| From undetermined cause | 1 | 1.9 | |
| Pending | 1 | 1.9 |
Three patients remain on study therapy (after receiving 20, 33, and 35 cycles).
As of database lock, May 16, 2011.
Figure 1.
Progression Free Survival and Overall Survival of study
Toxicity
There were no treatment-related deaths (Table 4). Observed grade 4 toxicities were cardiac (1), pain (1), and pulmonary (1). Grade 3 toxicities that occurred in more than one patient included gastrointestinal (13), dermatologic (9), metabolic (7), fatigue (6), anemia (4), pain (4), constitutional (3), and cardiac (2). Twenty-two (42%) of the 52 patients had dose reductions. Thirteen (25%) of the 52 patients came off study due to toxicity. No patients died due to treatment.
TABLE 4.
Adverse Events
| Grade | |||||||
|---|---|---|---|---|---|---|---|
| Adverse Event | 0 | 1 | 2 | 3 | 4 | 5 | Total |
| Leukopenia | 42 | 7 | 3 | 0 | 0 | 0 | 52 |
| Thrombocytopenia | 46 | 5 | 1 | 0 | 0 | 0 | 52 |
| Neutropenia | 43 | 6 | 2 | 1 | 0 | 0 | 52 |
| Anemia | 28 | 14 | 6 | 4 | 0 | 0 | 52 |
| Allergy/Immunology | 51 | 0 | 0 | 1 | 0 | 0 | 52 |
| Cardiac | 45 | 2 | 2 | 2 | 1 | 0 | 52 |
| Coagulation | 51 | 1 | 0 | 0 | 0 | 0 | 52 |
| Constitutional | 12 | 14 | 23 | 3 | 0 | 0 | 52 |
| Dermatologic | 14 | 12 | 17 | 9 | 0 | 0 | 52 |
| Gastrointestinal | 2 | 21 | 16 | 13 | 0 | 0 | 52 |
| Genitourinary/Renal | 51 | 1 | 0 | 0 | 0 | 0 | 52 |
| Hemorrhage | 46 | 5 | 0 | 1 | 0 | 0 | 52 |
| Infection | 49 | 1 | 2 | 0 | 0 | 0 | 52 |
| Lymphatics | 27 | 17 | 8 | 0 | 0 | 0 | 52 |
| Metabolic | 17 | 24 | 4 | 7 | 0 | 0 | 52 |
| Musculoskeletal | 51 | 1 | 0 | 0 | 0 | 0 | 52 |
| Neurosensory | 40 | 12 | 0 | 0 | 0 | 0 | 52 |
| Other Neurological | 41 | 9 | 2 | 0 | 0 | 0 | 52 |
| Ocular/Visual | 47 | 4 | 1 | 0 | 0 | 0 | 52 |
| Pain | 22 | 13 | 12 | 4 | 1 | 0 | 52 |
| Pulmonary | 43 | 8 | 0 | 0 | 1 | 0 | 52 |
| Sexual/Reproductive | 51 | 1 | 0 | 0 | 0 | 0 | 52 |
| Vascular | 51 | 0 | 0 | 1 | 0 | 0 | 52 |
| Fatigue | 11 | 14 | 21 | 6 | 0 | 0 | 52 |
Mutational Analysis
Forty of the 52 patients provided FFPE tumor, and, of these, 34 had sufficient DNA for the BRAF, and KRAS mutation assays to be performed (Table 2). Of the 34 patients who had the mutation analyses done: 28 (82%) were tissue from primary tumor, 2 (6%) were tissue from metastatic tumor, and 4 (12%) were tissue from recurrent/persistent tumor.. Clinical characteristics for patients in the mutation subset are also presented in Table 1. Two of 34 (6%) patients had BRAF mutations, 14/34 (41%) had KRAS mutations, seven of 34 (21%) had other RAS mutations, and 12/34 (35%) had none of these mutations (Table 5A). Tumor response by mutation status is shown (Table 5B). There were no statistically significant differences in the proportion of patients with complete or partial responses (CR or PR) for any mutation.
TABLE 5.
| A: Type of Mutation
| |
|---|---|
| Presence of activating mutation | N=34 |
| n (%) | |
| No | 12 (35%) |
| KRAS/BRAF | |
| BRAF | 2 (6%) |
| KRAS | 14 (41%) |
| B: Tumor Response (CR, PR) by BRAF, KRAS, Mutation
| ||||
|---|---|---|---|---|
| Response (CR,PR) | ||||
| N | No n (%) |
Yes n (%) |
p-value† | |
| Total | ||||
| 34 | 27 (79.4%) | 7 (20.6%) | ||
| BRAF Mutation | ||||
| No | 32 | 25 (78.1%) | 7 (21.9%) | 1.000 |
| Yes | 2 | 2 (100.0%) | 0 (0.0%) | |
| KRAS Mutation | ||||
| No | 20 | 15 (75.0%) | 5 (25.0%) | 0.672 |
| Yes | 14 | 12 (85.7%) | 2 (14.3%) | |
| BRAF or KRAS Mutation | ||||
| No | 18 | 13 (72.2%) | 5 (27.8%) | 0.405 |
| Yes | 16 | 14 (87.5%) | 2 (12.5%) | |
p-value from Fisher’s Exact test.
Note: Percentages are row percentages.
DISCUSSION
Selumetinib is well tolerated, and is active in the treatment of recurrent low-grade serous carcinoma. The median PFS was 11 months, with PFS >6 months in 63% of patients. In exploratory analyses, response to Selumetinib did not appear to be related to RAS/RAF mutational status. These results are appealing because LGSC have been increasingly recognized as more chemoresistant than HGSC, with the majority of patients (88%) having positive second-look surgery.10,12,15–17. Persistent disease after primary chemotherapy was the only factor associated with shorter OS time.10 Primary peritoneal carcinoma (PPC) is histologically indistinguishable from epithelial ovarian cancer, and has similar clinical characteristics, patterns of spread, response to treatment, and survival rates.16,18 Low-grade serous PPC also appears to have similar clinical characteristics as low-grade serous ovarian cancer. At the completion of primary treatment consisting of maximal surgical cytoreductive effort followed by adjuvant chemotherapy, 66·7% of low-grade serous PPC patients were noted to have persistent or progressive disease. The 5-year PFS was 16%, yet the five-year OS was 69%.16 In the recurrent setting, the chemo resistance of LGSC is even more profound. An evaluation of 58 patients from the University of Texas MD Anderson Cancer Center with recurrent LGSC who received 108 separate chemotherapy regimens (“patient-regimens”), revealed four responses—(one complete and three partial); for an overall RR (ORR) of 3·7% (11). The median time to progression was 29 weeks.12 This is in stark contrast with data from previous reports of ovarian cancer trials, which predominantly involved patients with HGSC.19,20 In platinum-sensitive disease HGSC patients treated with a taxane-platinum drug combination, RRs ranged from 66% to 90%, with median PFS durations of nine to 19 months.21,22 In phase III studies of women with platinum-resistant/refractory disease, reports on several chemotherapeutic agents produced RRs in the range of 15%-30%.12,23,24 PFS durations generally ranged from 2–6 months. These cytotoxic chemotherapies carry associated toxicity. Twenty nine percent of patients experienced grade 3–4 toxicity. 12,23,24 Thirty eight percent of patients receiving gemcitabine, 71% of topotecan patients, and 19% of pegylated liposomal doxyrubicin patients experienced grade 3 or 4 toxicities. The majority of grade 3–4 toxicities were hematologic for topotecan and gemcitabine 77% and 38% respectively and palmar-plantar erythrodysesthesia and stomatitis 22% and 9%, respectively, for pegylated liposomal doxyrubicin (PLD). Fatigue (grade 2, 3, or 4) also is higher with gemcitabine and topotecan compared with PLD. This compares to the 6% G4 toxicity overall, with one(2%) G3-4 hematologic toxicity observed in the current study. There was a 25% grade 3 gastrointestinal toxicity and 17% grade 3 dermatologic toxicity observed however these were tolerable and manageable.
The chemoresistant nature of recurrent LGSC to cytotoxic chemotherapy makes effective alternative molecular therapies of paramount importance. The poor observed RR of recurrent LGSC patients in the MD Anderson cohort was in the most favorable setting, 53% of patients having received only one prior cycle of chemotherapy. While there was no control group specifically in the current trial and cross trial comparisons can be fraught with statistical imprecision, in the current trial the majority of patients (57%) had three or more prior chemotherapy regimens compared to 25% in the MD Anderson cohort.12 Despite this heavily pretreated condition, the disease stability rate of 65% and median PFS of 11 months is substantially improved over that observed with the MD Anderson cohort of 60% and 7.3 months, respectively. The activity of Selumetinib in recurrent LGSCs confirms the importance of targeting the MAPK pathway in this subset of patients.
At the time this study was planned, a high frequency of BRAF and KRAS mutations in LGSCs had been observed, and our hypothesis that MAPK inhibition would be a potentially active therapeutic intervention was based on this observation. Our results suggest that Selumetinib is an active agent, but not necessarily because of BRAF or KRAS mutational activation per se, as determined by limited archival specimen analysis. Given the current regulatory trends and recommendations for clinical trial design that are moving in a more restrictive direction this was an important and potentially provocative decision. The decision to allow patients with and without BRAF or KRAS mutational activation proved to be appropriate. Unless the evidence for limiting eligible patient populations is compelling, trial design must allow the hypothesis to be adequately tested clinically. A limitation of our correlative study is that the concordance of BRAF or KRAS mutational activation between primary and recurrent/metastatic disease has not been adequately studied. Additionally, if significant tumor heterogeneity exists and the mutation containing cells account for less than 10% of the specimen it is possible that a mutation was not detected.
Given the robust activation of the MAPK pathway in LGSCs, molecular targeting of this pathway provides a logical treatment option for patients with this disease. Additionally, both the angiogenesis pathway and the IGF/insulin axis are attractive molecular targets also worth exploring in LGSC in combination with MAPK inhibitors. In the current study Selumetinib exhibits considerable activity in recurrent low-grade serous tumors. The 15% RR is 4X that observed for cytotoxic chemotherapy in the setting of recurrent low-grade serous tumors. The regimen also has considerable less toxicity when compared to cytotoxic regimens with a 6% incidence of grade 4 toxicities which is substantially less than the 18%–71% rate observed for cytotoxic agents. Additionally, Selumetinib displayed a robust disease stability rate with a median PFS of 11 months, and 63% of patients experiencing a PFS >6 months duration. These results warrant further evaluation of inhibitors of the MAPK pathway in LGSCs.
Research in Context.
A systematic review was conducted as a part of planning this trial. The NCI Pub Med database was searched with mesh terms: serous ovarian cancer, MAPK, and mutation rate. Only studies conducted or evaluating low-grade serous carcinomas were included. This review revealed evidence of alterations in the MAPK pathway being prominent in LGSC when compared to high grade ovarian cancers. The relative chemorefractory nature of LGSC was confirmed. This highlighted a disease which was systemically progressive without any current effective cytotoxic chemotherapy for recurrent progressive disease that did not harbor increased toxicity with minimal clinical gains. The present trial confirms the activity of the MAPK in low-grade serous ovarian cancer and the potential for MEK inhibitors in treating this malignancy.
Acknowledgments
This study was supported by National Cancer Institute grants to the Gynecologic Oncology Group Administrative Office (CA 27469) and the Gynecologic Oncology Group Statistical Office (CA 37517). The trial was sponsored by the Cancer Therapy Evaluation Program of the NCI. The following Gynecologic Oncology Group member institutions participated in the primary treatment studies: University of Mississippi Medical Center, University of California Medical Center at Irvine, The Cleveland Clinic Foundation, Washington University School of Medicine, Memorial Sloan-Kettering Cancer Center, Columbus Cancer Council, MD Anderson Cancer Center, Fox Chase Cancer Center, University of Oklahoma, University of Chicago, Case Western Reserve University, Women and Infants Hospital and Community Clinical Oncology Program.
Footnotes
DMG had funding provided by NCI (CTEP) and Gynecologic Oncology Group. All other co-authors have no conflicts of interest to declare.
CONFLICTS OF INTEREST The authors have no conflicts of interest to declare.
AUTHOR CONTRIBUTIONS
JF was responsible for trial development, trial PI, and manuscript writing. WEB was responsible for study design, data analysis, data interpretion and writing of the manuscript. VV provided manuscript approval. HAL oversaw the specimen collection/distribution and translational research component of GOG-0239. HAL also assisted with translational research data analysis, interpretion and writing of the manuscript. RLC participated in writing/review of the manuscript, data collection/interpretation, and patient enrollment. MM provided data and manuscript review. RSM provided manuscript approval. SDY provided manuscript approval. DM contributed patients to the trial, edited and reviewed the manuscript. WHR contributed to study design, pathology review/data collection, data interpretation and manuscript review. MJB provided design of trial, monitoring of trial, performance of translational research endpoints, and writing of manuscript. DMG provided literature search, study design, data collection, patient accrual, data analysis, data interpretation and writing of manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Singer G, Oldt R, 3rd, Cohen Y, et al. Mutations in BRAF and KRAS characterize the development of low-grade ovarian serous carcinoma. J Natl Cancer Inst. 2003;95:484–6. doi: 10.1093/jnci/95.6.484. [DOI] [PubMed] [Google Scholar]
- 2.Singer G, Kurman RJ, Chang HW, et al. Diverse tumorigenic pathways in ovarian serous carcinoma. Am J Pathol. 2002;160:1223–8. doi: 10.1016/s0002-9440(10)62549-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diaz-Padilla I, Malpica AL, Minig L, et al. Ovarian low-grade serous carcinoma: a comprehensive update. Gynecol Oncol. 2012;126:279–85. doi: 10.1016/j.ygyno.2012.04.029. [DOI] [PubMed] [Google Scholar]
- 4.Alsop K, Fereday S, Meldrum C, et al. BRCA mutation frequency and patterns of treatment response in BRCA mutation-positive women with ovarian cancer: a report from the Australian Ovarian Cancer Study Group. J Clin Oncol. 2012;30:2654–63. doi: 10.1200/JCO.2011.39.8545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singer G, Stohr R, Cope L, et al. Patterns of p53 mutations separate ovarian serous borderline tumors and low- and high-grade carcinomas and provide support for a new model of ovarian carcinogenesis: a mutational analysis with immunohistochemical correlation. Am J Surg Pathol. 2005;29:218–24. doi: 10.1097/01.pas.0000146025.91953.8d. [DOI] [PubMed] [Google Scholar]
- 6.Bonome T, Lee JY, Park DC, et al. Expression profiling of serous low malignant potential, low-grade, and high-grade tumors of the ovary. Cancer Res. 2005;65:10602–12. doi: 10.1158/0008-5472.CAN-05-2240. [DOI] [PubMed] [Google Scholar]
- 7.Jazaeri AA, Yee CJ, Sotiriou C, et al. Gene expression profiles of BRCA1-linked, BRCA2-linked, and sporadic ovarian cancers. J Natl Cancer Inst. 2002;94:990–1000. doi: 10.1093/jnci/94.13.990. [DOI] [PubMed] [Google Scholar]
- 8.Wong KK, Tsang YT, Deavers MT, et al. BRAF mutation is rare in advanced-stage low-grade ovarian serous carcinomas. AmJ Pathol. 2010;177:1611–7. doi: 10.2353/ajpath.2010.100212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meinhold-Heerlein I, Bauerschlag D, Hilpert F, et al. Molecular and prognostic distinction between serous ovarian carcinomas of varying grade and malignant potential. Oncogene. 2005;24:1053–65. doi: 10.1038/sj.onc.1208298. [DOI] [PubMed] [Google Scholar]
- 10.Gershenson DM, Sun CC, Lu KH, et al. Clinical behavior of stage II–IV low-grade serous carcinoma of the ovary. Obstet Gynecol. 2006;108:361–8. doi: 10.1097/01.AOG.0000227787.24587.d1. [DOI] [PubMed] [Google Scholar]
- 11.Schmeler KM, Sun CC, Bodurka DC, et al. Neoadjuvant chemotherapy for low-grade serous carcinoma of the ovary or peritoneum. Gynecol Oncol. 2008;108:510–4. doi: 10.1016/j.ygyno.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 12.Gershenson DM, Sun CC, Bodurka D, et al. Recurrent low-grade serous ovarian carcinoma is relatively chemoresistant. Gynecol Oncol. 2009;114:48–52. doi: 10.1016/j.ygyno.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 13.Friday BYC, Sminth P, Adejei A. A potential role for the modulation of a negative feedback loop between Erk and Raf mediating sensitivity to the MEK inhibitor AZD6244 (ARRY-142886) in human lung cancer cell lines. Proc Am Assoc Cancer Res. 2006;47:A4868. [Google Scholar]
- 14.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 15.Shvartsman HS, Sun CC, Bodurka DC, et al. Comparison of the clinical behavior of newly diagnosed stages II-IV low-grade serous carcinoma of the ovary with that of serous ovarian tumors of low malignant potential that recur as low-grade serous carcinoma. Gynecol Oncol. 2007;105:625–9. doi: 10.1016/j.ygyno.2007.01.030. [DOI] [PubMed] [Google Scholar]
- 16.Schmeler KM, Sun CC, Malpica A, et al. Low-grade serous primary peritoneal carcinoma. Gynecol Oncol. 2011;121:482–617. doi: 10.1016/j.ygyno.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 17.Schlumbrecht MP, Sun CC, Wong KN, et al. Clinicodemographic factors influencing outcomes in patients with low-grade serous ovarian carcinoma. Cancer. 2011;116:3741–9. doi: 10.1002/cncr.25929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Killackey MA, Davis AR. Papillary serous carcinoma of the peritoneal surface: matched-case comparison with papillary serous ovarian carcinoma. Gynecol Oncol. 1993;51:171–4. doi: 10.1006/gyno.1993.1267. [DOI] [PubMed] [Google Scholar]
- 19.Gore ME, Fryatt I, Wiltshaw E, et al. Treatment of relapsed carcinoma of the ovary with cisplatin or carboplatin following initial treatment with these compounds. Gynecol Oncol. 1990;36:207–11. doi: 10.1016/0090-8258(90)90174-j. [DOI] [PubMed] [Google Scholar]
- 20.Markman M, Rothman R, Hakes T, et al. Second-line platinum therapy in patients with ovarian cancer previously treated with cisplatin. J Clin Oncol. 1991;9:389–93. doi: 10.1200/JCO.1991.9.3.389. [DOI] [PubMed] [Google Scholar]
- 21.Parmar MK, Ledermann JA, Colombo N, et al. Paclitaxel plus platinum-based chemotherapy versus conventional platinum-based chemotherapy in women with relapsed ovarian cancer: the ICON4/AGO-OVAR-2. 2 trial. Lancet. 2003;361:2099–106. doi: 10.1016/s0140-6736(03)13718-x. [DOI] [PubMed] [Google Scholar]
- 22.Rose PG, Blessing JA, Mayer AR, et al. Prolonged oral etoposide as second-line therapy for platinum-resistant and platinum-sensitive ovarian carcinoma: a Gynecologic Oncology Group study. J Clin Oncol. 1998;16:405–10. doi: 10.1200/JCO.1998.16.2.405. [DOI] [PubMed] [Google Scholar]
- 23.Gordon AN, Fleagle JT, Guthrie D, et al. Recurrent epithelial ovarian carcinoma: a randomized phase III study of pegylated liposomal doxorubicin versus topotecan. J Clin Oncol. 2001;19:3312–22. doi: 10.1200/JCO.2001.19.14.3312. [DOI] [PubMed] [Google Scholar]
- 24.Mutch DG, Orlando M, Goss T, et al. Randomized phase III trial of gemcitabine compared with pegylated liposomal doxorubicin in patients with platinum-resistant ovarian cancer. Clin Oncol. 2007;25:2811–8. doi: 10.1200/JCO.2006.09.6735. [DOI] [PubMed] [Google Scholar]