Abstract
Nerve-related complications have been frequently reported in dental procedures, and a very frequent type of occurrence involves the inferior alveolar nerve (IAN). The nerve injury in humans often results in persistent pain accompanied by allodynia and hyperalgesia. In this investigation, we used an experimental IAN injury in rats, which was induced by a Crile hemostatic clamp, to evaluate the effects of laser therapy on nerve repair. We also studied the nociceptive behavior (von Frey hair test) before and after the injury and the behavioral effects of treatment with laser therapy (emitting a wavelength of 904 nm, output power of 70 Wpk, a spot area of ∼0.1 cm2, frequency of 9500 Hz, pulse time 60 ns and an energy density of 6 J/cm2). As neurotrophins are essential for the process of nerve regeneration, we used immunoblotting techniques to preliminarily examine the effects of laser therapy on the expression of nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF). The injured animals treated with laser exhibited an improved nociceptive behavior. In irradiated animals, there was an enhanced expression of NGF (53%) and a decreased BDNF expression (40%) after laser therapy. These results indicate that BDNF plays a locally crucial role in pain-related behavior development after IAN injury, increasing after lesions (in parallel to the installation of pain behavior) and decreasing with laser therapy (in parallel to the improvement of pain behavior). On the other hand, NGF probably contributes to the repair of nerve tissue, in addition to improving the pain-related behavior.
Key words: BDNF, IAN, laser therapy, NGF, pain behavior
Introduction
Low-level laser therapy (LLLT) is commonly used for physical therapy purposes. Recently, a growing number of laboratory and clinical studies have shown that LLLT at extremely low energy doses (between 1 and 6 J/cm2), when administered through a particular emission mode (continuous or pulsed), is capable of eliciting significant biological effects.1 Therapeutic low-power laser, for example, accelerates wound healing,2,3 reduces pain symptoms, restores neural function after damage, improves bone healing and remodeling, normalizes hormonal function, stimulates endorphin release, and modulates the immune system.4,5
Over the past 10 years, clinical knowledge has been reported regarding the beneficial effects of LLLT in the treatment of nerve injury after dental procedures.6 Among the complications involving nerve damage in dentistry, the inferior alveolar nerve (IAN) is the most commonly injured nerve that could result from traumatic local anesthetic injections and dental treatment, because of the proximity of its course to the region of surgical extraction of third molar teeth. This is of great relevance to the dentist because IAN injury causes physiological and psychological disorders, involving disturbance of speech, eating, kissing, make-up application, shaving, and drinking,7 in addition to pain and frequent complete loss of sensation.8
Experimental studies have been developed to better understand the pathophysiological mechanisms of nerve regeneration and its complications. Nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF) belong to a still- growing family of neurotrophic molecules collectively called “neurotrophins,” which play an important role on survival, growth, and neural differentiation.9 NGF is involved in the development, maintenance, and regeneration of sensory and sympathetic nerves.10
The use of growth factors such as NGF has been studied for a long time in relation to neural injury. NGF can stimulate the response to nerve repair when delivered in particular ways.11 NGF is produced and released by target tissues and uptaken in a retrograde way to maintain neuronal survival. Topically applied NGF stimulates nerve regeneration and promotes function recovery in crushed rat sciatic nerves.12
BDNF is a potent survival factor, which has also been implicated in a variety of pathophysiological conditions.13 BDNF is mainly found in small to medium-sized neurons, predominantly those containing neuropeptides such as calcitonin gene-related peptide (CGRP).14 Usually, BDNF is included into dense core vesicles that undergo anterograde transport to presynaptic terminals,15 and can also act presynaptically to increase excitatory neurotransmitter release in the dorsal horn of the spinal cord.16 Many functions are assigned to BDNF, such as a mediator role in long-term potentiation (LTP) induction,17 and a signaling role in neuropathic pain development.18 Another important role for BDNF in adulthood appears to be as a central modulator of pain.19
The aim of this study was to verify if laser therapy could improve the pain-related behavior of rats after IAN injury. In addition, we attempted to identify the possible changes of NGF and BDNF levels in lesioned rats before and after laser therapy.
Methods
Animals
Male Wistar rats, weighing between 180 and 220 g (2 months old) were used in all experiments. Six animals were included in each experimental group. They were singly housed and maintained on a 12:12h light/dark cycle. The rats were adapted to the experimental environment for 3 days before the experiments started. All animals were tested during the light cycle at the same time of the day (9:00 a.m. to 12:00 a.m.). All procedures were approved by the Institutional Animal Care Committee of the University of São Paulo (protocol number 150 – book number 02/2010).
Surgical procedure
Inferior alveolar nerve injury
The right inferior alveolar nerve was crushed using a modification of the technique employed by Rehak, in which the animals were deeply anesthetized with ketamine (5 mg/100 g body weight, i.p.) and xylazine (1 mg/100 g body weight, i.p.), followed by the sterilization of skin around the inferior jaw with povidone-iodine. A vertical incision was made on a line between the corner of the mouth and the corner of the eye. The incision was placed midway between these points and was ∼1 cm long. Overlying the muscles, two nerves served as reference points. A clamp was placed directly beneath the uppermost of the two transverse nerves, and the extremity of the basal bone of the incisor tooth was gently palpated. Scissors were used to separate the muscle fibers. Clamps were placed in this area of separation to keep the muscle sections apart. Muscle fibers were scraped in the upper jaw to the incisor near the basal end. The IAN was then observed.20 The nerve was lifted from the canal and a Crile hemostatic clamp was applied to it.21 The second level of the rack was utilized for maintaining the nerve crush. The clamp was maintained closed in order to crush the nerve during 30 sec, to simulate the nerve crush situation that may occur during the extraction of third molar teeth. Muscle fibers were re-approximated, and the incision was sutured. A control group of false-operated animals (FOP), that is, without nerve crush, was also constituted.
The animals were returned to their cages and observed until the moment they woke up, and were then brought back to the animal facility.
Laser therapy
The animals were initially divided into four groups. Two of these groups were irradiated with laser GaAs (Gallium Arsenide, Laserpulse-Laser, Ibramed Brazil) emitting a wavelength of 904 nm, an output power of 70 Wpk, a spot area of ∼0.1 cm2, a frequency of 9500 Hz, a pulse time 60 ns, and an energy density of 6 J/cm2 (for complete parameters used in this work please see Table 1). Laser parameters used in this work were recommended by Jenkins and coworkers.22 Each session included the stimulation of five points, lasting 18 sec on each point. The other two groups did not undergo laser application, as follows: Group 1 consisted of animals that has sustained nerve damage and laser treatment; Group 2 consisted of sham animals with laser treatment; Group 3 consisted of operated animals, but without laser application; and Group 4 comprised a naive group used as control (without laser or surgery).
Table 1.
Specifications for Laser Parameters
| Device information | |
| Manufacturer | IBRAMED |
| Model identifier | LASERPULSE Diamond |
| Emitter type | GaAs |
| Irradiation parameters | |
| Center wavelength (nm) | 904 nm |
| Operating mode | Pulsed |
| Frequency (Hz) | 9500 Hz |
| Pulse on duration (sec) | 60 ns |
| Beam shape | Circular |
| Treatment parameters | |
| Beam spot size at target (cm2) | 0.1 cm2 |
| Exposure duration (sec) | 18 sec |
| Radiant exposure (J/cm2) | 6 J/cm2 |
| Radiant energy (J) | 6 J |
| Number of points irradiated | 5 |
| Area irradiated (cm2) | 0.5 cm2 |
| Application technique | Without skin contact |
| Number and frequency of treatment sessions | 10 sessions, performed every 2 days |
| Total radiant energy (J) | 30 J per session, 300 J over all sessions |
The treatment with the laser technique was initiated 2 days after the surgery for IAN injury or in sham animals. The laser treatment was performed every 2 days, totaling 10 sessions. After sterilization, the laser was placed on the skin surface directly above the course of the IAN. A point near the temporomandibular articulation and four points in the region of the inferior jaw body were irradiated, with a duration of 18 sec per point. Each point was irradiated with intervals of 30 sec, and each session had a total duration of 4 min.
Behavioral experiments
The development of neuropathic pain symptoms in the surface of the skin around the inferior jaw was evaluated by testing allodynia (Von Frey test) in response to a tactile stimulus applied to the surface of the skin around the inferior jaw, according to a modification of the method described by Chaplan and coworkers23 and Yonehara and coworkers.24
This assessment was conducted before surgery (baseline-BL), 2 days after surgery (to confirm pain behavior), and before each laser therapy session.
Testing was blind with regard to group designation. Briefly, a logarithmic series of five calibrated Semmes–Weinstein monofilaments (von Frey hair test, Stoelting, USA) was used. Each rat was accommodated in a plastic cage, and then von Frey filaments were applied to the surface of skin around the inferior right jaw, for a maximum of 10 sec. The threshold intensity of the stiffness stimulus required to elicit a response was determined by the animals' withdrawing or by their touching or scratching their facial regions after the von Frey filaments were applied. Range of log stiffness of the filaments was 2.83 (0.070g); 3.22 (0.160g); 3.61 (0.407g); 3.84 (0.692g); and 4.08 (1.202g). Baseline assessment was initiated with the 0.407g filament. In the event of withdrawal, the previous weaker, 0.160g monofilament was presented. In the absence of a positive response to the first stimulus, the next stronger monofilament was presented (0.692g). In the event of paw withdrawal, the same filament was again presented 60–90 sec later. When the animal showed a positive response in two consecutive trials with the same stiffness value, no further von Frey hairs were tested. Failure to respond to the strongest stimulus (1.202g) was considered to be the cutoff value. Response to the weakest stimulus (0.070g) was considered to be the lower cutoff value for that time point.23,24
Immunoblotting
Rats were killed by decapitation under isofluorane anesthesia and the crushed IAN was quickly removed and homogenized in an extraction buffer containing 100 mM Tris, pH 7.4, 10 mM ethylenediaminetetraacetic acid (EDTA), 2 mM phenylmethanesulfonylfluoride (PMSF), and 10 μg/mL aprotinin. After extraction, the homogenates were centrifuged at 12,000 rpm at 4°C for 20 min and the protein concentration of the supernatant was determined using the Bradford protein assay with albumin as a standard (Bio-Rad, Melville, NY).25 Samples containing 75 μg protein were loaded on a 12% acrylamide gel and electrotransferred to nitrocellulose membranes using a Bio-Rad miniature transfer apparatus for 1.5 h at 120 V. After transfer, the membranes were treated for 2 h at room temperature with a blocking solution containing 5% powdered milk, washed and incubated overnight at 4°C with anti-NGF (F30, 1:1000; Santa Cruz Biotechnology, Santa Cruz, CA)26 and BDNF antibodies (1:5000; Sigma, St. Louis, MO). The membranes were then washed and incubated for 2 h at room temperature with peroxidase-conjugated anti-rat (Zymed, San Francisco, CA) and anti-rabbit (GE Healthcare, San Francisco, CA) antibodies, diluted 1:5000. β-actin was used as an internal control (1:10,000, Sigma). The specifically bound antibody was visualized using a chemoluminescence kit (Amersham Biosciences, Piscataway, NJ). The blot was analyzed densitometrically using NIH-Scion Image 4.0.2 and quantified by optical densitometry of the developed autoradiographs (Scion Corporation, Release Beta 3b, National Institutes of Health, Bethesda, MD).
Statistical analysis
Results are presented as the mean±SEM. Statistical analyses of data were generated using GraphPAd Prism, version 4.02 (Graph-Pad Software Inc., San Diego, CA). Statistical comparison of more than two groups was performed using analysis of variance (ANOVA), followed by Bonferroni test. In all cases, p<0.05 was considered statistically significant.27
Results
Effects of laser therapy on mechanical allodynia induced by IAN injury
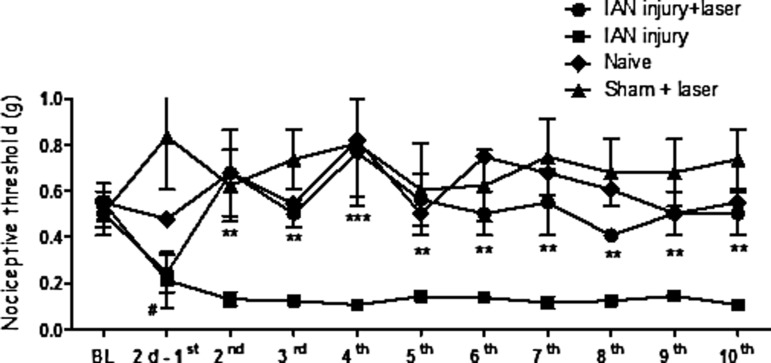
Rats subjected to IAN injury developed hypersensitivity to tactile stimulation (reduction of the withdrawal threshold to mechanical stimulus on the surface of skin around the inferior jaw), which started 2 days after the injury (Fig. 1). The baseline for all animals was ∼407g. After IAN injury, the threshold was significantly decreased to 0.070g. When the animals were subjected to the laser therapy technique, we observed a recovery of pain threshold to normal levels. These alterations were detected as early as day 2 and persisted at least until 1 month after surgery (Fig. 1). It is important to point out that all animals that received laser therapy sessions exhibit behavioral improvement.
FIG. 1.
Effect of laser therapy on pain threshold induced by inferior alveolar nerve (IAN) injury in rats. Pain threshold as measured by von Frey test, expressed in grams. Measurements were determined before (baseline- BL), 2 days after the lesion, at which time we also conducted the first session of laser therapy (2d-1st), and at different intervals after laser therapy sessions. The results represent the mean±SEM of six animals per group. **p<0.01 and ***p<0.001 for comparison with IAN injury group and #p<0.05 for comparison with BL.
No significant differences in pain thresholds were observed in sham-operated animals that received laser therapy sessions (taken as control, sham+laser) during the whole period analyzed. No significant differences of withdrawal threshold were observed on the side contralateral to the injury between measurements or at any time post-lesion (data not shown).
Effects of laser therapy on NGF and BDNF expression
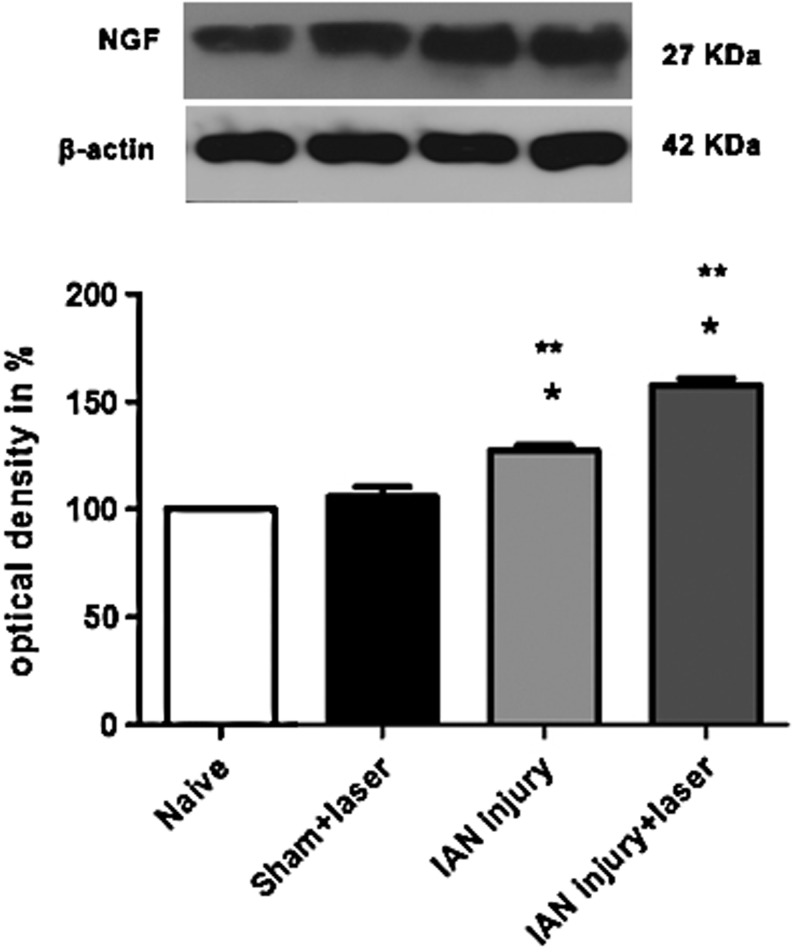
A single NGF-positive band was observed in IAN extracts from all groups analyzed. Our data show an increase of NGF levels on the side ipsilateral to the lesion in IAN-lesioned animals when compared with the same side from naive rats, taken as a control and designated as 100%. Densitometric analysis revealed that this NGF increase averaged 30% above the control. On the other hand, rats that received treatment with laser therapy (IAN injury+laser) had a larger increase of NGF expression (∼53% greater than the control) (Fig. 2). No statistical differences of NGF expression were observed between sham and naive animals.
FIG. 2.
Densitometric analysis of nerve growth factor (NGF) protein levels. Data are normalized in relation to data from naive rats, which were taken as 100%. Data are reported as mean±SEM of six animals per group. *p<0.05 compared with control naive group and **p<0.001 compared with sham+laser group.
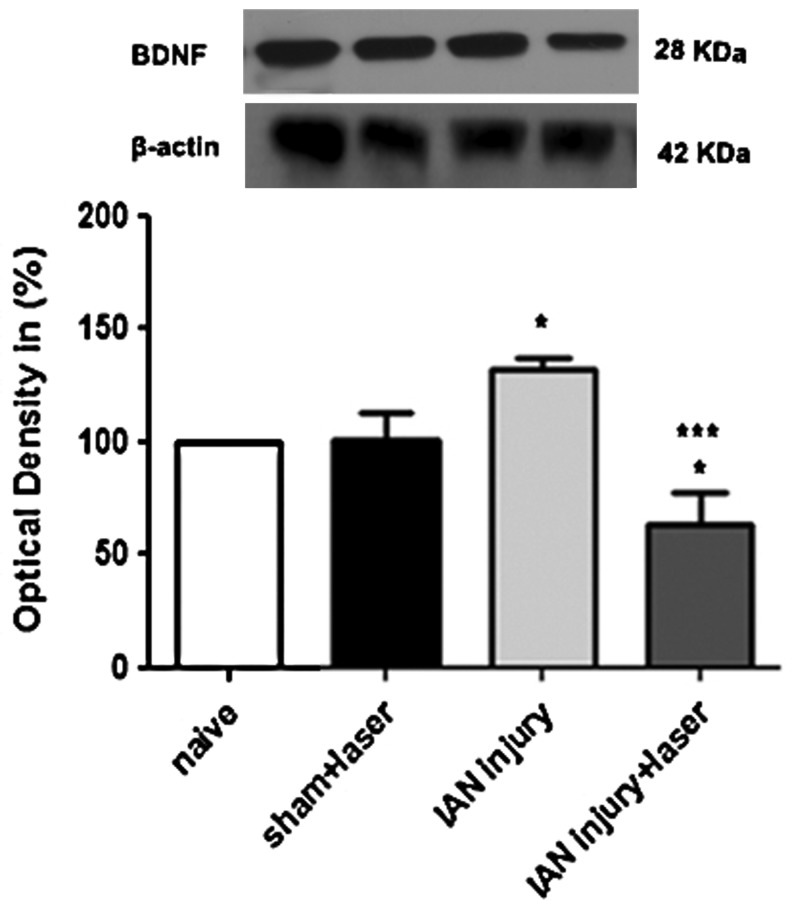
Our results also showed an increase of BDNF levels (∼30% greater than the control) after IAN injury when compared with naive animals. There was, however, a decrease (∼40% less than the control) after laser therapy treatment. No differences were observed between naive and sham animals, used as controls (Fig. 3).
FIG. 3.
Densitometric analysis of brain-derived neurotrophic factor (BDNF) protein levels. Data are normalized in relation to naïve rats; data taken as 100%. Data are reported as mean±SEM of six animals per group. *p<0.05 compared with control animals and ***p<0.001 compared with inferior alveolar nerve (IAN) injury group.
No differences were observed for β-actin between control and experimental groups at any time point (Figs. 2 and 3).
Discussion
The aim of our study was to evaluate the effects of laser therapy on pain-related behavioral improvement in IAN-injured rats, and to evaluate NGF and BDNF levels in the IAN after laser therapy.
Our behavioral results indicate that laser therapy played a role in the control and reversal of pain sensitivity, which occurred after induction of injury and remained until 20 days after lesion (time of the last session). Behavioral data confirmed the onset of neuropathic pain syndrome 2 days after IAN injury, as shown by reduced mechanical thresholds. Our behavioral data are in agreement with studies that show that injuries to the infraorbital nerve and the inferior alveolar nerve result in mechanical and thermal hypersensitivity.24,28–30 After laser therapy, we observed an improvement of pain behavior. These data are in agreement with data from in vitro and in vivo studies showing that laser therapy induces trophic-regenerative, anti-inflammatory and analgesic effects.31–34
Our immunoblotting results indicate that laser therapy modulates the expression of NGF in the IAN, increasing its expression in the group treated with laser therapy when compared with control, IAN-operated, and sham-operated animals. We demonstrated an increase of NGF in IAN-injured rats and an additional increase after laser therapy. This increased expression of NGF is in accordance with the current literature on NGF effects on the repair of nerve tissue.35 Neurotrophins can significantly increase the morphological and/or functional recovery of nerve injury.36 NGF signaling is also important for the trafficking and localization of mitochondria in neurons, presumably to fulfill higher demands for adenosine triphosphate (ATP) at the growth cone, initiating a signaling cascade that promotes cellular proliferation and cytoprotection. NGF also upregulates the metabolic activity of mitochondria.37 Exogenous administration of NGF in the terminal portion of nerves can protect the spinal ganglion neurons after peripheral nerve injury, and, in addition, NGF can promote myelination and axonal extension.38 However, exogenous NGF has a short half-life (2.4 min) and its activity is reduced by the action of local factors such as temperature and pH. Therefore, there has been a search for techniques that could produce a maximized effect of exogenous NGF.39 Within this context, the increased expression of endogenous NGF by the laser treatment observed here suggests that laser might be used as a therapeutic tool to assist in the repair of injured peripheral nerves, acting as an accelerator of the process.
Our immunoblotting results also showed that laser therapy modulates the expression of BDNF in the IAN, by reducing its expression in the group treated with laser therapy when compared with control, IAN-operated, and sham-operated animals. After nerve damage, BDNF from large diameter neurons is transported to the central terminals of primary afferents in the dorsal horn of the spinal cord.
BDNF can also modify the excitability of neurons and contribute to neuropathic sensations.40 Although the knowledge about the role of BDNF in the processing of pain sensation is far from complete, the role of BDNF in nociceptive pathways has been the subject of many studies.19,41–45 Several studies have reported that intrathecal injection of tropomyosin-related kinase B (trkB)-immunoglobulin G (IgG) and anti-BDNF prevents the development of thermal hyperalgesia and mechanical allodynia in neuropathic pain models.46 Allodynia induced by peripheral nerve injury can be inhibited by TrkB/Fc.47 Therefore, BDNF appears to be a signaling factor that may play a key role in neuropathic pain development.18 Studies showed that blocking BDNF activity with specific antibodies resulted in striking elimination of pain behavior in a neuropathic pain model.48 The increase of BDNF protein occurred here at time points that are compatible with a role in pain-related behaviors, suggesting that BDNF is involved in the nociceptive effects induced by IAN injury. Our data are in agreement with several studies showing that BDNF is involved in nociception.15,16,44 Interestingly, NGF can increase BDNF expression in chronically injured neurons, and this has been linked to increased analgesic sensations.49 BDNF expression is downregulated in small nociceptive neurons, predominantly described as expressing the NGF receptor. BDNF expression in injured sensory neurons leads to elevated interleukin-6 (IL-6) expression in neurons of the same size range as injured neurons that express BDNF. BDNF expression in medium to large injured neurons is largely IL-6 dependent.50,51 Therefore, as the LLLT irradiation was able to reduce the concentration of IL-6 both in vitro and in vivo,51,53 we suggest that the downregulation of BDNF could be mediated by a possible decrease of IL-6 after laser therapy. The reduction of BDNF levels by the laser treatment indicates, therefore, a positive effect of this therapy in neuropathic processes. Both neurotrophins (BDNF and NGF) have been implicated in exaggerated pain states such as inflammatory and neuropathic pain; they also regulate the survival, differentiation, and growth of neurons, both centrally and peripherally. However, NGF plays a prominent role not only in nociception, but also in nerve repair mechanisms.17 One intriguing, but largely unexplored, mechanism by which NGF may also generate and maintain hypersensitivity is by inducing aberrant sprouting and/or neuroma formation in response to tissue and/or nerve injury.54,55
The present results on the modulation of NGF and BDNF in the IAN after induction of injury and treatment with laser indicate that stimulation with laser acted positively in the control of pain sensitivity, which possibly occurred through downregulation of the expression of BDNF. In addition, as NGF has important roles in nerve repair,56,57 the upregulation of NGF after laser therapy may have accelerated the repair of nervous tissue observed in the present model.58,59 There is one study showing the level of NGF protein increased, whereas that of BDNF remained unchanged after model of hindpaw sensory restriction.60 This differential response of NGF and BDNF proteins to sensory restriction suggested different levels of gene regulation, that is, at pre-translational or post-translational states. Only a few studies have compared the effect of neural activity on neurotrophin mRNA and protein levels, and these works also report disparities between mRNA and protein levels.61–64
Conclusion
In summary, the present findings contribute to the understanding of the mechanisms involved in the therapeutic potential of laser therapy as a treatment for neuropathic pain induced by IAN injury. These results also suggest that NGF and BDNF levels could be influenced by laser therapy, implying that LLLT may be a useful procedure for neural tissue regeneration.
Acknowledgments
This study was supported by Fundação de Amparo a Pesquisa do Estado de São Paulo (FAPESP) (no. 11/22268-0; 10/20026-6) and International Association For Study of Pain (IASP) – 2009 IASP Early Career Grant and scan|design foundation by Inger and Jens Bruun.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Tunér J. Hode L. Laser Therapy. Clinical Practice and Scientific Background. Prima Books AB: Grängesberg; Sweden: 2002. p. 570. [Google Scholar]
- 2.Amorim J.C. de Sousa G.R. de Barros Silveira L. Prates R.A. Pinotti M. Ribeiro M.S. Clinical study of the gingiva healing after gingivectomy and low-level laser therapy. Photomed. Laser Surg. 2006;24:588–594. doi: 10.1089/pho.2006.24.588. [DOI] [PubMed] [Google Scholar]
- 3.Gal P. Mokry M. Vidinsky B. Kilik R. Depta F. Harakalova M. Longauer F. Mozes S. Sabo J. Effect of equal daily doses achieved by different power densities of low-level laser therapy at 635 nm on open skin wound healing in normal and corticosteroid-treated rats. Lasers Med. Sci. 2009;24:539–547. doi: 10.1007/s10103-008-0604-9. [DOI] [PubMed] [Google Scholar]
- 4.Rochkind S. Drory V. Alon M. Nissan M. Ouaknine G.E. Laser phototherapy (780 nm): a new modality in treatment of long-term incomplete peripheral nerve injury, a randomized double-blind placebo-controlled study. Photomed. Laser Surg. 2007;25:436–442. doi: 10.1089/pho.2007.2093. [DOI] [PubMed] [Google Scholar]
- 5.Miloro M. Halkias L.E. Mallery S. Travers S. Rashid R.G. Low-level laser effect on neural regeneration in Gore-Tex tubes. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2002;93:27–34. doi: 10.1067/moe.2002.119518. [DOI] [PubMed] [Google Scholar]
- 6.Hashmi J.T. Huang Y.Y. Sharma S.K. Kurup D.B. De Taboada L. Carroll J.D. Hamblin M.R. Effect of pulsing in low-level light therapy. Lasers Surg. Med. 2010;42:450–466. doi: 10.1002/lsm.20950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ziccardi V.B. Assael L.A. Atlas of the Oral and Maxillofacial Surgery Clinics of North America. Elsevier; Philadelpia, PA: 2001. Mechanisms of trigeminal nerve injuries; pp. 1–11. [PubMed] [Google Scholar]
- 8.Jerjes W. Swinson B. Moles D.R. El-Maaytah M. Banu B. Upile T. Kumar M. Al Khawalde M. Vourvachis M. Hadi H. Kumar S. Hopper C. Permanent sensory nerve impairment following third molar surgery: a prospective study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006;102:e1–7. doi: 10.1016/j.tripleo.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 9.Hanani M. Satellite glial cells in sensory ganglia: from form to function. Brain Res. Brain Res. Rev. 2005;48:457–476. doi: 10.1016/j.brainresrev.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 10.Wang L. Sun M. Jiang Y. Yang L. Lei D. Lu C. Zhao Y. Zhang P. Yang Y. Li J. Nerve growth factor and tyrosine kinase A in human salivary adenoid cystic carcinoma: expression patterns and effects on in vitro invasive behavior. J. Oral Maxillofac. Surg. 2006;64:636–641. doi: 10.1016/j.joms.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 11.Raghoebar G. M. Meijndert L. Kalk W.W. Vissink A. Morbidity of mandibular bone harvesting: a comparative study. Int. J. Oral Maxillofac. Implants. 2007;22:359–365. [PubMed] [Google Scholar]
- 12.Chen Z.W. Wang M.S. Effects of nerve growth factor on crushed sciatic nerve regeneration in rats. Microsurgery. 1995;16:547–551. doi: 10.1002/micr.1920160808. [DOI] [PubMed] [Google Scholar]
- 13.Almeida R.D. Manadas B.J. Melo C.V. Gomes J.R. Mendes C.S. Graos M.M. Carvalho R.F. Carvalho A.P. Duarte C.B. Neuroprotection by BDNF against glutamate-induced apoptotic cell death is mediated by ERK and PI3-kinase pathways. Cell Death Differ. 2005;12:1329–1343. doi: 10.1038/sj.cdd.4401662. [DOI] [PubMed] [Google Scholar]
- 14.Luo X.G. Rush R.A. Zhou X.F. Ultrastructural localization of brain-derived neurotrophic factor in rat primary sensory neurons. Neurosci. Res. 2001;39:377–384. doi: 10.1016/s0168-0102(00)00238-8. [DOI] [PubMed] [Google Scholar]
- 15.Merighi A. Salio C. Ghirri A. Lossi L. Ferrini F. Betelli C. Bardoni R. BDNF as a pain modulator. Prog. Neurobiol. 2008;85:297–317. doi: 10.1016/j.pneurobio.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 16.Wang X. Ratnam J. Zou B. England P.M. Basbaum A.I. TrkB signaling is required for both the induction and maintenance of tissue and nerve injury-induced persistent pain. J. Neurosci. 2009;29:5508–5515. doi: 10.1523/JNEUROSCI.4288-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pezet S. Malcangio M. McMahon S.B. BDNF: a neuromodulator in nociceptive pathways? Brain Res. Brain Res. Rev. 2002;40:240–249. doi: 10.1016/s0165-0173(02)00206-0. [DOI] [PubMed] [Google Scholar]
- 18.Geng S.J. Liao F.F. Dang W.H. Ding X. Liu X.D. Cai J. Han J.S. Wan Y. Xing G.G. Contribution of the spinal cord BDNF to the development of neuropathic pain by activation of the NR2B-containing NMDA receptors in rats with spinal nerve ligation. Exp. Neurol. 2010;222:256–266. doi: 10.1016/j.expneurol.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 19.Malcangio M. Lessmann V. A common thread for pain and memory synapses? Brain-derived neurotrophic factor and trkB receptors. Trends Pharmacol. Sci. 2003;24:116–121. doi: 10.1016/S0165-6147(03)00025-7. [DOI] [PubMed] [Google Scholar]
- 20.Rehak J.R. Course and resection of the inferior alveolar nerve in the albino rat. J Dent Res. 1963;42:1159–1168. doi: 10.1177/00220345630420051001. [DOI] [PubMed] [Google Scholar]
- 21.Camara C.N. Brito M.V. Silveira E.L. Silva D.S. Simoes V.R. Pontes R. W. Histological analysis of low-intensity laser therapy effects in peripheral nerve regeneration in Wistar rats. Acta Cir Bras. 2011;26:12–18. doi: 10.1590/s0102-86502011000100004. [DOI] [PubMed] [Google Scholar]
- 22.Jenkins P.A.C. Carroll J.D. How to report low–level laser therapy (LLLT)/ photomedicine dose and beam parameters in clinical and laboratory studies. Photomed. Laser Surg. 2011;29:785–787. doi: 10.1089/pho.2011.9895. [DOI] [PubMed] [Google Scholar]
- 23.Chaplan S.R. Bach F.W. Pogrel J.W. Chung J.M. Yaksh T.L. Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 24.Yonehara N. Kudo C. Kamisaki Y. Involvement of NMDA-nitric oxide pathways in the development of tactile hypersensitivity evoked by the loose-ligation of inferior alveolar nerves in rats. Brain Res. 2003;963:232–243. doi: 10.1016/s0006-8993(02)03983-5. [DOI] [PubMed] [Google Scholar]
- 25.Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 26.Wu C. Boustany L. Liang H. Brennan T.J. Nerve growth factor expression after plantar incision in the rat. Anesthesiology. 2007;107:128–135. doi: 10.1097/01.anes.0000267512.08619.bd. [DOI] [PubMed] [Google Scholar]
- 27.Snedecor G.W. Sokal R.R. Rohlf F.J. tatistical Methods Biometry. Owa State University Press; New York: 1946. p. 859. [Google Scholar]
- 28.Fried K. Bongenhielm U. Boissonade F.M. Robinson P.P. Nerve injury-induced pain in the trigeminal system. Neuroscientist. 2001;7:155–165. doi: 10.1177/107385840100700210. [DOI] [PubMed] [Google Scholar]
- 29.Nomura H. Ogawa A. Tashiro A. Morimoto T. Hu J.W. Iwata K. Induction of Fos protein-like immunoreactivity in the trigeminal spinal nucleus caudalis and upper cervical cord following noxious and non-noxious mechanical stimulation of the whisker pad of the rat with an inferior alveolar nerve transection. Pain. 2002;95:225–238. doi: 10.1016/S0304-3959(01)00403-1. [DOI] [PubMed] [Google Scholar]
- 30.Tsuboi Y. Takeda M. Tanimoto T. Ikeda M. Matsumoto S. Kitagawa J. Teramoto K. Simizu K. Yamazaki Y. Shima A. Ren K. Iwata K. Alteration of the second branch of the trigeminal nerve activity following inferior alveolar nerve transection in rats. Pain. 2004;111:323–334. doi: 10.1016/j.pain.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 31.Pereira A. N. Eduardo Cde P. Matson E. Marques M.M. Effect of low-power laser irradiation on cell growth and procollagen synthesis of cultured fibroblasts. Lasers Surg. Med. 2002;31:263–267. doi: 10.1002/lsm.10107. [DOI] [PubMed] [Google Scholar]
- 32.Anders J.J.T.B.R. Ilev I.K. Moges H. Longo L. Wu X. Waynant R.W. Light supports neurite outgrowth of human neural progenitor cells in vitro: the role of P2Y receptors. IEEE J. Sel. Top. Quantum Electron. 2008;14:118–125. [Google Scholar]
- 33.Shen C.C. Yang Y.C. Liu B.S. Large-area irradiated low-level laser effect in a biodegradable nerve guide conduit on neural regeneration of peripheral nerve injury in rats. Injury. 2011;42:803–813. doi: 10.1016/j.injury.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 34.Saygun I. Karacay S. Serdar M. Ural A.U. Sencimen M. Kurtis B. Effects of laser irradiation on the release of basic fibroblast growth factor (bFGF), insulin like growth factor-1 (IGF-1), and receptor of IGF-1 (IGFBP3) from gingival fibroblasts. Lasers Med. Sci. 2008;23:211–215. doi: 10.1007/s10103-007-0477-3. [DOI] [PubMed] [Google Scholar]
- 35.Lee A.C. Yu V.M. Lowe J.B., 3rd Brenner M.J. Hunter D.A. Mackinnon S.E. Sakiyama–Elbert S.E. Controlled release of nerve growth factor enhances sciatic nerve regeneration. Exp. Neurol. 2003;184:295–303. doi: 10.1016/s0014-4886(03)00258-9. [DOI] [PubMed] [Google Scholar]
- 36.Gravvanis A.I. Tsoutsos D.A. Tagaris G.A. Papalois A.E. Patralexis C.G. Iconomou T.G. Panayotou P.N. Ioannovich J.D. Beneficial effect of nerve growth factor-7S on peripheral nerve regeneration through inside-out vein grafts: an experimental study. Microsurgery. 2004;24:408–415. doi: 10.1002/micr.20055. [DOI] [PubMed] [Google Scholar]
- 37.Chada S.R. Hollenbeck P.J. Nerve growth factor signaling regulates motility and docking of axonal mitochondria. Curr. Biol. 2004;14:1272–1276. doi: 10.1016/j.cub.2004.07.027. [DOI] [PubMed] [Google Scholar]
- 38.Zochodne D.W. Cheng C. Neurotrophin and other grows factors in the regenerative milieu of proximal nerve stump tips. J. Anat. 2002;196:279–332. doi: 10.1046/j.1469-7580.2000.19620279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jubran M. Widenfalk J. Repair of peripheral nerve transections with fibrin sealant containing neurotrophic factors. Exp. Neurol. 2003;181:204–212. doi: 10.1016/s0014-4886(03)00041-4. [DOI] [PubMed] [Google Scholar]
- 40.Miletic G. Hanson E.N. Miletic V. Brain-derived neurotrophic factor-elicited or sciatic ligation-associated phosphorylation of cyclic AMP response element binding protein in the rat spinal dorsal horn is reduced by block of tyrosine kinase receptors. Neurosci. Lett. 2004;361:269–271. doi: 10.1016/j.neulet.2003.12.029. [DOI] [PubMed] [Google Scholar]
- 41.Bennett D.L. Neurotrophic factors: important regulators of nociceptive function. Neuroscientist. 2001;7:13–17. doi: 10.1177/107385840100700105. [DOI] [PubMed] [Google Scholar]
- 42.Binder D.K. Scharfman H.E. Brain-derived neurotrophic factor. Growth Factors. 2004;22:123–131. doi: 10.1080/08977190410001723308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chao M.V. Rajagopal R. Lee F.S. Neurotrophin signalling in health and disease. Clin. Sci. (Lond). 2006;110:167–173. doi: 10.1042/CS20050163. [DOI] [PubMed] [Google Scholar]
- 44.Pezet S. Malcangio M. Brain-derived neurotrophic factor as a drug target for CNS disorders. Expert Opin. Ther. Targets. 2004;8:391–399. doi: 10.1517/14728222.8.5.391. [DOI] [PubMed] [Google Scholar]
- 45.Pezet S. McMahon S.B. Neurotrophins: mediators and modulators of pain. Annu. Rev. Neurosci. 2006;29:507–538. doi: 10.1146/annurev.neuro.29.051605.112929. [DOI] [PubMed] [Google Scholar]
- 46.Yajima Y. Narita M. Usui A. Kaneko C. Miyatake M. Yamaguchi T. Tamaki H. Wachi H. Seyama Y. Suzuki T. Direct evidence for the involvement of brain-derived neurotrophic factor in the development of a neuropathic pain-like state in mice. J. Neurochem. 2005;93:584–594. doi: 10.1111/j.1471-4159.2005.03045.x. [DOI] [PubMed] [Google Scholar]
- 47.Coull J.A. Beggs S. Boudreau D. Boivin D. Tsuda M. Inoue K. Gravel C. Salter M.W. De Koninck Y. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature. 2005;438:1017–1021. doi: 10.1038/nature04223. [DOI] [PubMed] [Google Scholar]
- 48.Marcol W. Kotulska K. Larysz–Brysz M. Kowalik J.L. BDNF contributes to animal model neuropathic pain after peripheral nerve transection. Neurosurg, Rev. 2007;30:235–243. doi: 10.1007/s10143-007-0085-5. [DOI] [PubMed] [Google Scholar]
- 49.Fukuoka T. Kondo E. Dai Y. Hashimoto N. Noguchi K. Brain-derived neurotrophic factor increases in the uninjured dorsal root ganglion neurons in selective spinal nerve ligation model. J. Neurosci. 2001;21:4891–4900. doi: 10.1523/JNEUROSCI.21-13-04891.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Murphy P.G. Borthwick L.A. Altares M. Gauldie J. Kaplan D. Richardson P.M. Reciprocal actions of interleukin-6 and brain-derived neurotrophic factor on rat and mouse primary sensory neurons. Eur. J. Neurosci. 2000;12:1891–1899. doi: 10.1046/j.1460-9568.2000.00074.x. [DOI] [PubMed] [Google Scholar]
- 51.Shadiack A.M. Sun Y. Zigmond R.E. Nerve growth factor antiserum induces axotomy-like changes in neuropeptide expression in intact sympathetic and sensory neurons. J. Neurosci. 2001;21:363–371. doi: 10.1523/JNEUROSCI.21-02-00363.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Boschi E.S. Leite C.E. Saciura V.C. Caberlon E. Lunardelli A. Bitencourt S. Melo D.A. Oliveira J.R. Anti-Inflammatory effects of low-level laser therapy (660 nm) in the early phase in carrageenan-induced pleurisy in rat. Lasers Surg. Med. 2008;40:500–508. doi: 10.1002/lsm.20658. [DOI] [PubMed] [Google Scholar]
- 53.de Lima F.M. Villaverde A.B. Albertini R. Correa J.C. Carvalho R.L. Munin E. Araujo T. Silva J.A. Aimbire F. Dual effect of low-level laser therapy (LLLT) on the acute lung inflammation induced by intestinal ischemia and reperfusion: action on anti- and pro-inflammatory cytokines. Lasers Surg. Med. 2011;43:410–420. doi: 10.1002/lsm.21053. [DOI] [PubMed] [Google Scholar]
- 54.Diamond J. Foerster A. Holmes M. Coughlin M. Sensory nerves in adult rats regenerate and restore sensory function to the skin independently of endogenous NGF. J. Neurosci. 1992;12:1467–1476. doi: 10.1523/JNEUROSCI.12-04-01467.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kryger G.S. Kryger Z. Zhang F. Shelton D.L. Lineaweaver W.C. Buncke H.J. Nerve growth factor inhibition prevents traumatic neuroma formation in the rat. J. Hand Surg. Am. 2001;26:635–644. doi: 10.1053/jhsu.2001.26035. [DOI] [PubMed] [Google Scholar]
- 56.Shao Y. Ma H. Wu Y. Chen H. Zeng L. Li M. Long Z. Li Y. Yang H. Effect of nerve growth factor on changes of myelin basic protein and functional repair of peripheral nerve following sciatic nerve injury in rats. Chin. J. Traumatol. 2002;5:237–240. [PubMed] [Google Scholar]
- 57.Hayashida K. Clayton B.A. Johnson J.E. Eisenach J.C. Brain derived nerve growth factor induces spinal noradrenergic fiber sprouting and enhances clonidine analgesia following nerve injury in rats. Pain. 2008;136:348–355. doi: 10.1016/j.pain.2007.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kemp S.W. Walsh S.K. Midha R. Growth factor and stem cell enhanced conduits in peripheral nerve regeneration and repair. Neurol. Res. 2008;30:1030–1038. doi: 10.1179/174313208X362505. [DOI] [PubMed] [Google Scholar]
- 59.Cui Q. Actions of neurotrophic factors and their signaling pathways in neuronal survival and axonal regeneration. Mol, Neurobiol. 2006;33:155–179. doi: 10.1385/MN:33:2:155. [DOI] [PubMed] [Google Scholar]
- 60.Dupont E. Canu M.H. Stevens L. Falempin M. Effects of a 14-day period of hindpaw sensory restriction on mRNA and protein levels of NGF and BDNF in the hindpaw primary somatosensory cortex. Brain Res. Mol. Brain Res. 2005;133:78–86. doi: 10.1016/j.molbrainres.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 61.Humpel C. Wetmore C. Olson L. Regulation of brain-derived neurotrophic factor messenger RNA and protein at the cellular level in pentylenetetrazol-induced epileptic seizures. Neuroscience. 1993;53:909–918. doi: 10.1016/0306-4522(93)90476-v. [DOI] [PubMed] [Google Scholar]
- 62.Nanda S.A. Mack K.J. Seizures and sensory stimulation result in different patterns of brain derived neurotrophic factor protein expression in the barrel cortex and hippocampus. Brain Res. Mol. Brain Res. 2000;78:1–14. doi: 10.1016/s0169-328x(00)00054-1. [DOI] [PubMed] [Google Scholar]
- 63.Nawa H. Carnahan J. Gall C. BDNF protein measured by a novel enzyme immunoassay in normal brain and after seizure: partial disagreement with mRNA levels. Eur. J. Neurosci. 1995;7:1527–1535. doi: 10.1111/j.1460-9568.1995.tb01148.x. [DOI] [PubMed] [Google Scholar]
- 64.Pollock G.S. Vernon E. Forbes M.E. Yan Q. Ma Y.T. Hsieh T. Robichon R. Frost D.O. Johnson J.E. Effects of early visual experience and diurnal rhythms on BDNF mRNA and protein levels in the visual system, hippocampus, and cerebellum. J Neurosci. 2001;21:3923–3931. doi: 10.1523/JNEUROSCI.21-11-03923.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]