Abstract
Alterations in the airway epithelium have been associated with the development of asthma in elite athletes and in subjects that are susceptible to exercise-induced bronchoconstriction (EIB). The syndrome of EIB refers to acute airflow obstruction that is triggered by a period of physical exertion. Asthmatics who are susceptible to EIB have increased levels of cysteinyl leukotrienes (CysLTs, i.e., LTs C4, D4, and E4) in induced sputum and exhaled breath condensate, and greater shedding of epithelial cells into the airway lumen. Exercise challenge in individuals susceptible to this disorder initiates a sustained increase in CysLTs in the airways, and secreted mucin release and smooth muscle constriction, which may be mediated in part through activation of sensory nerves. We have identified a secreted phospholipase A2 (sPLA2) with increased levels in the airways of patients with EIB called sPLA2 group X (sPLA2-X). We have found that sPLA2-X is strongly expressed in the airway epithelium in asthma. Further, we discovered that transglutaminase 2 (TGM2) is expressed at increased levels in asthma and serves as a regulator of sPLA2-X. Finally, we demonstrated that sPLA2-X acts on target cells such as eosinophils to initiate cellular eicosanoid synthesis. Collectively, these studies identify a novel mechanism linking the airway epithelium to the production of inflammatory eicosanoids by leukocytes.
Introduction
Asthma can be viewed as a group of related phenotypes with significant heterogeneity in both the underlying genetic and environmental determinants as well as in the clinical manifestations of the disease (1). A prominent manifestation of asthma is exercise-induced bronchoconstriction (EIB), a syndrome where a brief period of exercise or increase in ventilation triggers airflow obstruction that lasts 30 to 90 minutes in the absence of treatment (2). We have conducted a number of studies that examine the immunological basis of EIB, and mediator release in the airways following exercise challenge. Here we review the underlying immunopathology that leads to EIB, and the nature of mediator release in the airways following exercise challenge. These studies serve as the foundation of our work examining the regulation of mediator formation in the airways, particularly the identification of sPLA2-X as an important regulator of eicosanoid formation (3–6), the discovery that transglutaminase 2 (TGM2) is expressed at increased levels in asthma and serves as a regulator of secreted phospholipase A2 group X (sPLA2-X) (7), and that sPLA2-X acts on target cells such as eosinophils to initiate cellular eicosanoid synthesis (8). Collectively, these studies identify a novel mechanism linking the airway epithelium to the production of inflammatory eicosanoids by leukocytes in the airways.
Clinical Implications
The importance of EIB within the spectrum of asthma is due to the impact of the exercise-related symptoms and airflow obstruction. Exercise-related asthma symptoms are associated with reduced health-related quality of life in children (9), and exercise challenge can serve as a stimulus for severe bronchoconstriction (10). Hyperpolarized helium images of regional ventilation following exercise challenge demonstrate closure or near closure of segmental airways of the lungs during EIB (11). Bronchoconstriction induced by exercise can be life-threatening as demonstrated by a population-based study that found 61 of 263 sports-related fatalities in young adults were caused by asthma exacerbation during exercise (12). Two recent large cohort studies with long-term longitudinal follow-up demonstrated that exercise-induced wheeze prior to the age of 5 (13) and airway hyperresponsiveness (AHR) to cold dry air hyperpnea in childhood (14) are among the strongest predictors of persistent asthma in adulthood.
Immunopathology of Asthma with Exercise-induced Bronchoconstriction (EIB)
Cross sectional studies in adults suggest that EIB is a discrete phenotype with distinct pathophysiology that is most strongly related to other aspects of indirect AHR (15). Whether or not this phenotype is a durable clinical phenotype awaits further longitudinal epidemiological studies. A modest size cross-sectional study established a prevalence of EIB of 46% out of 164 asthmatic children who were not using any daily controller therapy, and found that the prevalence of EIB was increased in the children whose asthma was more severe (16). The severity of EIB is generally not associated with the baseline forced expiratory volume in one second (FEV1) (2, 16, 17), and is only weakly associated with the degree of direct AHR (18, 19).
An inflammatory basis of EIB is suggested by an increase in the fraction of exhaled nitric oxide (FENO) among asthmatics who are susceptible to EIB (20), especially in subjects with atopy (21). In a comparison that we conducted between two groups of asthmatics, one with EIB and the other without EIB, we found that the concentration and number of columnar epithelial cells in induced sputum was much higher in asthmatics with EIB, suggesting that the epithelium is disrupted, and epithelial cells are shed into the airway lumen in this disorder (2). We found that the concentration of cysteinyl leukotrienes (CysLTs, LTs C4, D4 and E4) are increased in induced sputum of adults with EIB (2), and another group found that the levels of CysLTs are increase in exhaled breath condensate of children with EIB (17). Eicosanoids such as LTs and prostaglandins (PG)s are formed from arachidonic acid (AA) that is released by the hydrolysis of the sn-2 position of membrane phospholipids by a family of phospholipase A2 (PLA2) enzymes. Although AA and many of the products of AA readily move across the cell membrane, the formation of inflammatory eicosanoids such as CysLTs and PGD2 is largely restricted to myeloid cells, especially mast cells and eosinophils that contain LTC4 synthase and mast cells that contain PGD2 synthase (22). The connection between epithelial shedding and increased production of inflammatory lipid mediators has led us to consider mechanisms involving the epithelial regulation of leukocyte mediator production and function. One aspect of this relationship between the epithelium and leukocytes is that PGE2, an eicosanoid produced in large quantities by the epithelium that inhibits EIB (23) is consistently decreased in relation to the generation of CysLTs in subjects with EIB (2).
We found that the number of eosinophils was increased overall in subjects with EIB, but sputum eosinophilia per se does not appear to be required for EIB (2). In line with these observations the magnitude and onset of the suppression of EIB in response to high dose but not low dose inhaled corticosteroid (ICS) therapy was associated with the degree of sputum eosinophilia (24). Subjects with EIB who did not have sputum eosinophilia were less likely to have an improvement in EIB during ICS therapy compared to those with evidence of sputum eosinophilia (24). We also found in a genome-wide expression study of airway cells of patients with EIB relative to patients with asthma that did not have EIB, that the expression of the mast cell genes tryptase and carboxypeptidase A3 (CPA3) were significantly increased in the EIB positive group (7). These data are consistent with the recent findings of a unique intraepithelial mast cell phenotype in asthma notable for the high expression of tryptase and CPA3, but low expression of chymase that was described particularly in the Th2 high molecular phenotype (25, 26). Collectively these studies indicate that patients with EIB represent a group of subjects with prominent cellular inflammation and epithelial shedding into the airway lumen in association with increased production of inflammatory eicosanoids.
Inflammatory mediator release in the airways during EIB
Studies conducted in our lab and others indicate that exercise challenge initiates the release of inflammatory mediators into the airways in asthmatics with EIB, but the precise mechanism that initiates these events remains an area of controversy (27). Under most conditions, heat and water are transferred out of the airways during exercise as the inspired air is equilibrated to the temperature and humidity of the lower airways. The amount of water transferred out of the airways during exercise is strongly associated with the severity of bronchoconstriction following exercise challenge. It is likely that water transfer from the airways during exercise serves as a stimulus to the epithelium and leukocytes residing within the epithelium to initiate the release of mediators.
Studies from our laboratory and others indicate that there is a sustained increase in CysLTs and other bronchoconstrictive eicosanoids such as PGD2 in the airways following exercise challenge to induce EIB (28, 29). The levels of CysLTs are elevated at 30 min, 1 hour, and 6 hours after exercise challenge in asthmatics with EIB (28, 29). It is clear from pharmacological inhibitor studies blocking either 5-lipoxygenase (5-LO) or the CysLT1 receptor that CysLTs, especially LTD4 plays a pathological role in this disorder (28, 30–33). However, the inhibition of EIB by CysLT inhibitors alone is incomplete, implicating other bronchoconstrictive eicosanoids and/or the reduction in bronchoprotective mediators such as PGE2 in the pathogenesis of EIB. Fish oil supplementation, high in n-3 polyunsaturated fatty acids (PUFA) that inhibit synthesis of 2-series PGs and the 4-series LTs, inhibits EIB and the increase in both CysLTs and PGD2 in the airways (34), and the mast cell product 9α, 11β-PGF2 in the urine following exercise challenge (35). The epithelium itself may play a key role in the regulation and production of inflammatory mediators. Following exercise challenge the level of PGE2 declines in the airways of asthmatics with EIB (28), altering the balance of bronchoconstrictive to bronchoprotective mediators favoring bronchoconstriction in the period following exercise challenge (3). A unifying explanation for these findings is that the epithelium triggers the production of inflammatory eicosanoids by leukocytes that are in close contact, and that there is shunting of epithelial-derived AA away from the epithelium and towards the production of inflammatory eicosanoids by adjacent leukocytes. Inflammatory cells co-cultured with epithelial cells in vitro have increased synthesis of leukocyte-derived eicosanoids (36). Under the influence of interleukin-13 (IL-13) in vitro, the epithelium has reduced capacity for PGE2 synthesis through a reduction in the synthetic enzymes cyclooxygenase-2 (COX-2) and PGE synthase 1 (37). The epithelium itself may also serve directly as an important source of inflammatory mediators such as the eicosanoid 15S-Hydroxyeicosatetranoic Acid (15S HETE) that is increased in the airways of patients with EIB after exercise challenge (3). Studies in asthma have found that the key enzyme in the 15S HETE synthetic pathway, 15-Lipoxigenase-1, has increased expression in the airway epithelium of patients with asthma (38, 39). These findings suggest that alterations in the airway epithelium may serve to regulate the production of inflammatory eicosanoids.
Mast cells and eosinophils are strongly implicated as the cellular sources of CysLTs and other eicosanoids in EIB. The eosinophil product eosinophilic cationic protein (ECP) is released into the airways following challenge, and the amount of ECP release varies with the severity of the EIB under different experimental conditions (29). Following exercise challenge, histamine and the mast cell protease tryptase are released into the airways, and inhibition of EIB with a CysLT1 receptor inhibitor along with an antihistamine reduced the amount of histamine released after exercise challenge (28). In an analogous situation using manitol challenge, pharmacological inhibitors indicate that histamine is responsible for bronchoconstriction early after challenge, while the release of CysLTs is responsible for sustained bronchoconstriction (40).
Sensory Nerve Involvement in EIB
The production of eicosanoids such as CysLTs in the airway may initiate bronchoconstriction in part through the activation of sensory airway nerves. Sensory nerves release neurokinins when activated through a process call retrograde axonal transmission leading to bronchoconstriction and mucus release. Sensory nerves may be activated directly by osmotic stimuli, but several eicosanoids can significantly alter the activation threshold of these nerves. In a guinea pig model of hyperpnea-induced bronchoconstriction (HIB), either a 5-LO inhibitor or a CysLT1 antagonist inhibited HIB and the release of neurokinins, while a neurokinin 2 receptor antagonist inhibited HIB, but not the release of leukotrienes, suggesting that leukotrienes are involved in the release of neurokinins that cause bronchoconstriction (41). Similarly in a dog model, a combination neurokinin 1 and 2 receptor antagonist inhibited HIB and the generation of LTs that are known in this model to cause HIB (42). We demonstrated that mucin 5AC (MUC5AC), the predominant gel-forming mucin of goblet cells is released into the airways during EIB and is associated with the levels of CysLTs in the airways (43). Further, the levels of neurokinin A and CysLTs in these individuals post-exercise challenge are correlated, suggesting that CysLTs mediate the activation of sensory nerves and mucus release during EIB in humans (43).
Identification of secreted PLA2 group X (sPLA2-X) in the airway epithelium as a potential regulator of eicosanoid production
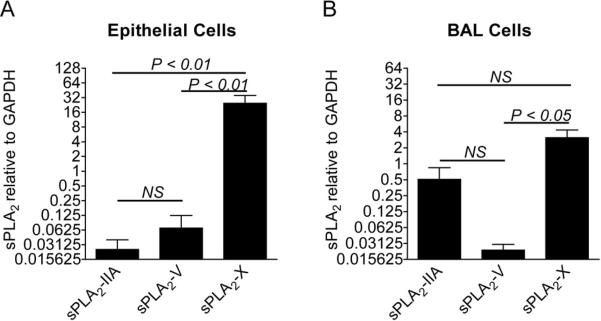
The first rate-limiting step in the formation of the CysLTs and other eicosanoids is the release of arachidonic acid (AA) from membrane phospholipids that is regulated by the PLA2 enzymes. It is clear from many studies that cytosolic PLA2 (cPLA2α) has a major function in efficient eicosanoid synthesis, evidenced by the marked reduction in eicosanoid production when the gene is knocked out in a murine model of asthma (44). However, in the presence of cPLA2α, several secreted PLA2s (sPLA2)s have been shown to significantly increase AA release over cPLA2α alone, and may preferentially direct eicosanoid production toward LT synthesis (45). Although the identities of specific sPLA2s were not characterized, increased sPLA2 activity was identified in nasal lavage fluid and in bronchoalveolar lavage (BAL) fluid following allergen challenge (46–48). To determine the identities of the sPLA2s in human airways, we examined induced sputum samples from asthmatics with EIB as well as a non-asthmatic control group and found that sPLA2 groups X and XIIA predominate at the level of gene expression (3). Immunocytochemistry indicated that groups X and XIIA are primarily present in columnar epithelial cells and bronchial macrophages. Of the mammalian sPLA2s, groups V and X have generated the most interest because of their capacity to initiate cellular eicosanoid synthesis (49), particularly sPLA2 group X (sPLA2-X) since it is the most potent of the sPLA2s at releasing AA from membrane phospholipids. Because sPLA2-X is able to hydrolyze phosphatidylcholine-rich vesicles at a rate comparable with its action on anionic phospholipids, sPLA2-X releases AA when added exogenously to the phosphatidylcholine-rich extracellular plasma membrane of mammalian cells. In murine models of asthma, genetic deficiency of either sPLA2-V or sPLA2-X attenuates the development of allergen-induced inflammation, mucus release, and AHR (6, 50), as does inhibition of human sPLA2-X expressed in a transgenic mouse model (5). In our initial study we found that, following exercise challenge in asthmatics with EIB, there were increases in sPLA2-X protein in induced sputum supernatant and the percentage of epithelial cells immunostaining for sPLA2-X, suggesting that activation or release of sPLA2-X may be involved in the generation of eicosanoids following exercise challenge (3). In subsequent work to better understand the identities of the sPLA2s in human airways, we have found that sPLA2-X and sPLA2-IIA are the predominant sPLA2s in human BAL fluid both in subjects with and without asthma (4). In the airway epithelium, the expression of sPLA2-X predominated (Figure 1), while both sPLA2-X and sPLA2-IIA were expressed in BAL cells (4). The levels of sPLA2-X in BAL fluid were increased in asthma, particularly in severe asthma and correlated with lung function and eicosanoid formation in the airways. In contrast, although sPLA2-X2-IIA was elevated in asthma, it was not associated with lung function, cellular inflammation or eicosanoid levels (4). Taken together, these results suggest a prominent role of sPLA2-X in asthma as a regulator of cellular inflammation and eicosanoid formation. Studies are currently underway to better understand the distribution of sPLA2-X expression within the epithelium of patients with asthma and non-asthmatic subjects. It is notable that in the murine model, the expression of sPLA2-X co-localizes to cells expressing MUC5AC suggesting prominent expression in secretory cells such as goblet cells (6).
Figure 1. Expression of the sPLA2 enzymes in the airway epithelium and BAL cells.
The gene expression of sPLA2-IIA, sPLA2-V, and sPLA2-X was assessed in epithelial brushings and BAL fluid from asthmatic and non-asthmatic subjects. The expression of sPLA2-X was significantly higher than the expression of either sPLA2-V or sPLA2-IIA in the epithelium (A). There was no difference in the expression of sPLA2-IIA and sPLA2-V in the epithelium. The expression of sPLA2-X in BAL cells was significantly higher than sPLA2-V, but no different than sPLA2-IIA (B). There was no statistically significant difference between the expression of sPLA2 groups IIA and V in BAL cells (Adapted from reference 4).
Transglutaminase 2 (TGM2) is increased in the airways of patients with asthma and regulates the activity of sPLA2-X
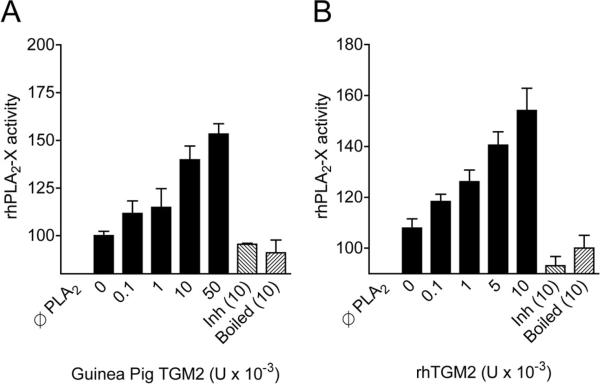
In our comparison of genes expressed in airway cells, we found that the expression of TGM2 is increased in asthmatics with EIB relative to asthmatics without EIB, and that TGM2 is markedly increased in either asthma group relative to non-asthmatic controls (7). Immunostaining for TGM2 in endobronchial biopsies from patients with asthma demonstrated TGM2 throughout the airway epithelium. In addition, primary epithelial cells proliferating in culture contain high amounts of TGM2. Although TGM2 has been implicated in a number of inflammatory diseases, our study was the first to clearly implicate TGM2 in asthma. Of interest is that TGM2 is upregulated by retinoic acid in transformed airway epithelial cells (51). The TGM2 gene is located on chromosome 20q11.2–12 near a cluster of genes related to epithelial barrier function in close proximity to a region linked to both atopic dermatitis and asthma (52). Two of the other differentially expressed genes in our study, secretory leukocyte peptidase inhibitor (SLPI) and cystatin 1 (CST1), are also located in this region of chromosome 20. TGM2 is a calcium-dependent enzyme that modifies protein structure through the transfer of an acyl group from glutamine to lysine or free amines resulting in a new inter- or intra-molecular amidic cross-link (53). TGM2 is also known to activate the transcription factor NFκB , which induces expression of pro-inflammatory cytokines (54). Using an in vitro assay of PLA2 activity, we found that recombinant human TGM2 enzymatically modifies sPLA2-X leading to a substantial increase in the PLA2 activity of the enzyme, suggesting that one of the mechanisms of TGM2 action in asthma is regulation of eicosanoid and lysophospholipid synthesis (Figure 2). In a prior investigation, dual inhibitors of TGM2 and sPLA2 reduced ocular inflammation in a rabbit model of allergen-induced conjunctivitis (53). It is now clear from more recent animal models that TGM2 is induced in the airways of mice sensitized and challenged with ovalbumin in the presence of adjuvant (55), as well as in mouse models of PMA-induced atopic dermatitis and IgE-dependent passive cutaneous anaphylaxis (56). In one study, a peptide that inhibits both TGM2 and PLA2 reduced allergen-induced airway inflammation and eicosanoid formation, but the specific role of TGM2 remains to be fully elucidated (55). In the studies of TGM2 in cutaneous anaphylaxis and atopic dermatitis, a chemical inhibitor of TGM2 partially inhibited PMA-induced dermatitis and IgE-dependent cutaneous anaphylaxis (55).
Figure 2. In vitro increase in secreted PLA2 group X activity by TGM2.
Pre-incubation of recombinant human sPLA2-X with purified TGM2 from guinea pig liver (A) or with recombinant human TGM2 (B) causes an increase in the PLA2 activity of the sPLA2-X enzyme. Denaturing the TGM2 with heat (boiled) or inhibiting the activity of the enzyme by saturating the enzyme with N-carbobenzoxy-Gln-Gly (Inh) demonstrate that the in vitro findings are due to the enzymatic activity of TGM2 (Adapted from reference 7).
Secreted PLA2 group X (sPLA2-X) initiates eicosanoid production by human eosinophils
Eosinophils have been implicated as a significant source of CysLTs involved in the pathogenesis of EIB based on increased levels of eosinophils in association with both high levels of CysLTs (2) and the severity of EIB (24) as well as evidence of activation of eosinophils in the airways following exercise challenge (29). The critical enzyme in CysLT formation, LTC4 synthase, is predominantly present in mast cells and eosinophils in the airways in subjects with asthma (22), and eosinophils are the predominant source of LTC4 synthase in aspirin exacerbated respiratory disease (AERD)(57). Following allergen challenge, the amount of CysLT formation in the airways is associated with the eosinophil count, further implicating eosinophils as a major source of CysLT production (58).
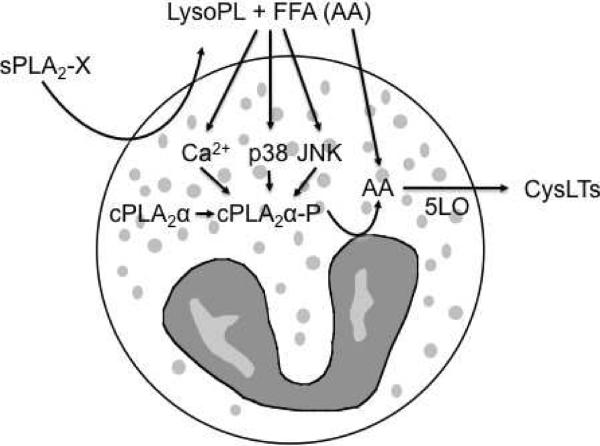
Based on the identification of sPLA2-X in the airways of patients with EIB and the evidence that eosinophils are a major source of CysLTs, we examined the ability of sPLA2-X to efficiently activate CysLT formation by human eosinophils (8). It is well known that group IVA cytosolic PLA2 (i.e. cPLA2α) plays a major role in endogenous CysLT synthesis in myeloid cells such as eosinophils (59, 60); however, it is reported that sPLA2 group V (sPLA2-V) initiates CysLT synthesis by human eosinophils in the absence of cPLA2α activation (61, 62). Although sPLA2-V and sPLA2-X both have high capacity to initiate cellular eicosanoid synthesis (49), sPLA2-V has been difficult to identify in the airways of patients with asthma (4) or EIB (3). To examine the role of sPLA2-X in eosinophil CysLT synthesis, we used recombinant human sPLA2-X to activate human eosinophils isolated from donors with a physician diagnosis of asthma and/or allergy. Exogenous sPLA2-X rapidly caused release of a large portion of labeled AA and CysLT synthesis that was related to the amount of sPLA2-X added exogenously to eosinophils. A specific, active site-directed inhibitor of sPLA2-X inhibited both CysLT synthesis and AA release indicating that sPLA2-X was responsible for the AA release and CysLT synthesis. In addition to AA release, sPLA2-X caused marked lysophospholipid release from eosinophils including LysoPC species known to induce a Ca2+ flux in eosinophils. Other lysophospholipid species enriched in AA in human eosinophils, including phosphatidylcholine (PC), phosphatidylinositol (PI), phosphatidylethanolamine (PE) (63), and plasmenyl PC and PE species (64), were released by the addition of sPLA2-X. Although it is clear that sPLA2-X serves as a major source of AA and lysophospholipids, the mechanism of CysLT formation is more complex. We found that selective inhibitors of cPLA2α suppressed CysLT formation mediated by sPLA2-X suggesting that sPLA2-X or a product of sPLA2-X activates cPLA2α. Activation of cPLA2α involves an intracellular calcium flux and phosphorylation of a serine residue by MAP kinases. Treatment with sPLA2-X initiated Ser505 -phosphorylation of cPLA2α and an intracellular Ca2+ flux in eosinophils, as well as translocation of cPLA2 and 5-LO to focal locations in the cytoplasm and in the perinuclear space. CysLT formation in response to sPLA2-X was attenuated by pharmacological inhibition of p38 and JNK MAP kinases; further LysoPC initiated CysLT formation that was similarly dependent upon p38 and JNK MAP kinases. Despite the finding of cPLA2α involvement, it was also apparent that AA release by sPLA2-X may still contribute to additional CysLT synthesis since the addition of sPLA2-X to eosinophils during fMLP-mediated CysLT synthesis further increased CysLT synthesis during these conditions of strong cPLA2α activation. Thus, we have demonstrated that sPLA2 CysLT synthesis in eosinophils through AA and lysophospholipid release through a mechanism involving cPLA2α and resulting in the amplification of CysLT synthesis in cells that are actively synthesizing CysLTs induced by another stimulus (Figure 3). These result imply that sPLA2-X mediated activation of eosinophils in the airways may be an important source of CysLTs in asthma.
Figure 3. Schematic representation of sPLA2-X-mediated CysLT synthesis by eosinophils.
sPLA2-X causes the release of lysophospholipids (LysoPL) and free fatty acids (FFA), including arachidonic acid (AA) from membrane phospholipids species. sPLA2-X causes CysLT synthesis that is dependent upon cPLA2α a and initiates a Ca2+ flux and cPLA2α phosphorylation in eosinophils. We found that the sPLA2-X causes a Ca2+ flux and that sPLA2-X- and LysoPC-induced CysLT synthesis could be inhibited by p38 and JNK inhibitors but not by a MEK 1/2 inhibitor. Free AA released by sPLA2-X may contribute to additional CysLT synthesis based on the observation that the addition of sPLA2-X to eosinophils treated with fMLP leads to additional CysLT synthesis (Adapted from reference 8).
Conclusions
We have found that EIB is a distinct syndrome in asthma that is related to indirect AHR, and is notable for increased production of CysLTs and shedding of epithelial cells into the airway lumen. Exercise challenge serves as a stimulus to the airway epithelium and adjacent leukocytes resulting in sustained CysLT and PGD2 release in association with smooth muscle contraction and the release of MUC5AC that may be the consequence of sensory nerve activation. Based on several lines of evidence, mast cells and eosinophils serve as the principal sources of inflammatory eicosanoids in this disorder. Our work in this area led to the identification of sPLA2-X that is strongly expressed in the airway epithelium and is released into the airway lumen in asthma. In a genome-wide expression study, we identified increased TGM2 and demonstrated that TGM2 serves as a regulator of sPLA2-X. Finally, we demonstrated that sPLA2-X acts on target cells such as eosinophils to initiate cellular eicosanoid synthesis. Collectively, these studies identify a novel mechanism linking the airway epithelium to the production of inflammatory eicosanoids by leukocytes.
Acknowledgments
Supported by National Institutes of Health grant HL089215.
Abbreviations
- 5-LO
5-Lipoxygenase
- 15S-HETE
15S-Hydroxyeicosatetranoic Acid
- AA
Arachidonic acid
- AERD
Aspirin exacerbated respiratory disease
- AHR
Airway Hyperresponsiveness
- BAL
Bronchoalveolar Lavage
- cPLA2α
Cytosolic Phospholipase A2α
- CysLT
Cysteinyl Leukotrienes
- CST1
Cystatin 1
- ECP
Eosinophilic Cationic Protein
- EIB
Exercise-induced Bronchoconstriction
- FENO
Fraction of exhaled nitric oxide
- FEV1
Forced Expiratory Volume in One Second
- HIB
Hyperpnea-induced Bronchoconstriction
- ICS
Inhaled Corticosteroid
- IL-13
Interleukin-13
- LT
Leukotriene
- MUC5AC
Mucin 5AC
- PG
Prostaglandin
- PUFA
Polyunsaturated Fatty Acid
- PLA2
Phospholipase A2
- SLPI
Secretory leukocyte peptidase inhibitor
- sPLA2
Secreted Phospholipase A2
- TGM2
Transglutaminase 2
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Moore WC, Meyers DA, Wenzel SE, Teague WG, Li H, Li X, D'Agostino R, Jr., Castro M, Curran-Everett D, Fitzpatrick AM, et al. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am J Respir Crit Care Med. 2010;181:315–323. doi: 10.1164/rccm.200906-0896OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hallstrand TS, Moody MW, Aitken ML, Henderson WR., Jr. Airway immunopathology of asthma with exercise-induced bronchoconstriction. J Allergy Clin Immunol. 2005;116:586–593. doi: 10.1016/j.jaci.2005.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hallstrand TS, Chi EY, Singer AG, Gelb MH, Henderson WR., Jr. Secreted phospholipase A2 group X overexpression in asthma and bronchial hyperresponsiveness. Am JRespir Crit Care Med. 2007;176:1072–1078. doi: 10.1164/rccm.200707-1088OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hallstrand TS, Lai Y, Ni Z, Oslund RC, Henderson WR, Jr., Gelb MH, Wenzel SE. Relationship between levels of secreted phospholipase A groups IIA and X in the airways and asthma severity. Clin Exp Allergy. 2011;41:801–810. doi: 10.1111/j.1365-2222.2010.03676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hendersen WR, Jr., Oslund RC, Bollinger JG, Ye X, Tien YT, Xue J, Gelb MH. Blockade of human group X secreted phospholipase A2-induced airway inflammation and hyperresponsiveness in a mouse asthma model by a selective group X secreted phospholipase A2 inhibitor. J Biol Chem. 2011 doi: 10.1074/jbc.M111.235812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Henderson WR, Jr., Chi EY, Bollinger JG, Tien YT, Ye X, Castelli L, Rubtsov YP, Singer AG, Chiang GK, Nevalainen T, et al. Importance of group X-secreted phospholipase A2 in allergen-induced airway inflammation and remodeling in a mouse asthma model. J Exp Med. 2007;204:865–877. doi: 10.1084/jem.20070029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hallstrand TS, Wurfel MM, Lai Y, Ni Z, Gelb MH, Altemeier WA, Beyer RP, Aitken ML, Henderson WR. Transglutaminase 2, a novel regulator of eicosanoid production in asthma revealed by genome-wide expression profiling of distinct asthma phenotypes. PLoS One. 2010;5:e8583. doi: 10.1371/journal.pone.0008583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lai Y, Oslund RC, Bollinger JG, Henderson WR, Jr., Santana LF, Altemeier WA, Gelb MH, Hallstrand TS. Eosinophil cysteinyl leukotriene synthesis mediated by exogenous secreted phospholipase A2 group X. J Biol Chem. 2010;285:41491–41500. doi: 10.1074/jbc.M110.153338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hallstrand TS, Curtis JR, Aitken ML, Sullivan SD. Quality of life in adolescents with mild asthma. Pediatr Pulmonol. 2003;36:536–543. doi: 10.1002/ppul.10395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marotel C, Natali F, Heyraud JD, Vaylet F, L'Her P, Bonnet D, Allard P. Severe forms of effort-induced asthma. Allerg Immunol (Paris) 1989;21:61–64. [PubMed] [Google Scholar]
- 11.Samee S, Altes T, Powers P, de Lange EE, Knight-Scott J, Rakes G, Mugler JP, 3rd, Ciambotti JM, Alford BA, Brookeman JR, et al. Imaging the lungs in asthmatic patients by using hyperpolarized helium-3 magnetic resonance: assessment of response to methacholine and exercise challenge. J Allergy Clin Immunol. 2003;111:1205–1211. doi: 10.1067/mai.2003.1544. [DOI] [PubMed] [Google Scholar]
- 12.Becker JM, Rogers J, Rossini G, Mirchandani H, D'Alonzo GE., Jr. Asthma deaths during sports: report of a 7-year experience. J Allergy Clin Immunol. 2004;113:264–267. doi: 10.1016/j.jaci.2003.10.052. [DOI] [PubMed] [Google Scholar]
- 13.Frank PI, Morris JA, Hazell ML, Linehan MF, Frank TL. Long term prognosis in preschool children with wheeze: longitudinal postal questionnaire study 1993-2004. BMJ. 2008;336:1423–1426. doi: 10.1136/bmj.39568.623750.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stern DA, Morgan WJ, Halonen M, Wright AL, Martinez FD. Wheezing and bronchial hyper-responsiveness in early childhood as predictors of newly diagnosed asthma in early adulthood: a longitudinal birth-cohort study. Lancet. 2008;372:1058–1064. doi: 10.1016/S0140-6736(08)61447-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joos GF, O'Connor B, Anderson SD, Chung F, Cockcroft DW, Dahlen B, DiMaria G, Foresi A, Hargreave FE, Holgate ST, et al. Indirect airway challenges. Eur Respir J. 2003;21:1050–1068. doi: 10.1183/09031936.03.00008403. [DOI] [PubMed] [Google Scholar]
- 16.Cabral AL, Conceicao GM, Fonseca-Guedes CH, Martins MA. Exercise-induced bronchospasm in children: effects of asthma severity. Am J Respir Crit Care Med. 1999;159:1819–1823. doi: 10.1164/ajrccm.159.6.9805093. [DOI] [PubMed] [Google Scholar]
- 17.Carraro S, Corradi M, Zanconato S, Alinovi R, Pasquale MF, Zacchello F, Baraldi E. Exhaled breath condensate cysteinyl leukotrienes are increased in children with exercise-induced bronchoconstriction. J Allergy Clin Immunol. 2005;115:764–770. doi: 10.1016/j.jaci.2004.10.043. [DOI] [PubMed] [Google Scholar]
- 18.Freezer NJ, Croasdell H, Doull IJ, Holgate ST. Effect of regular inhaled beclomethasone on exercise and methacholine airway responses in school children with recurrent wheeze. Eur Respir J. 1995;8:1488–1493. [PubMed] [Google Scholar]
- 19.Holzer K, Anderson SD, Douglass J. Exercise in elite summer athletes: Challenges for diagnosis. J Allergy Clin Immunol. 2002;110:374–380. doi: 10.1067/mai.2002.127784. [DOI] [PubMed] [Google Scholar]
- 20.Scollo M, Zanconato S, Ongaro R, Zaramella C, Zacchello F, Baraldi E. Exhaled nitric oxide and exercise-induced bronchoconstriction in asthmatic children. Am J Respir Crit Care Med. 2000;161:1047–1050. doi: 10.1164/ajrccm.161.3.9905043. [DOI] [PubMed] [Google Scholar]
- 21.Malmberg LP, Pelkonen AS, Mattila PS, Hammaren-Malmi S, Makela MJ. Exhaled nitric oxide and exercise-induced bronchoconstriction in young wheezy children - interactions with atopy. Pediatr Allergy Immunol. 2009;20:673–678. doi: 10.1111/j.1399-3038.2009.00858.x. [DOI] [PubMed] [Google Scholar]
- 22.Cai Y, Bjermer L, Halstensen TS. Bronchial mast cells are the dominating LTC4S- expressing cells in aspirin-tolerant asthma. Am J Respir Cell Mol Biol. 2003;29:683–693. doi: 10.1165/rcmb.2002-0174OC. [DOI] [PubMed] [Google Scholar]
- 23.Melillo E, Woolley KL, Manning PJ, Watson RM, O'Byrne PM. Effect of inhaled PGE2 on exercise-induced bronchoconstriction in asthmatic subjects. Am J Respir Crit Care Med. 1994;149:1138–1141. doi: 10.1164/ajrccm.149.5.8173753. [DOI] [PubMed] [Google Scholar]
- 24.Duong M, Subbarao P, Adelroth E, Obminski G, Strinich T, Inman M, Pedersen S, O'Byrne PM. Sputum eosinophils and the response of exercise-induced bronchoconstriction to corticosteroid in asthma. Chest. 2008;133:404–411. doi: 10.1378/chest.07-2048. [DOI] [PubMed] [Google Scholar]
- 25.Dougherty RH, Sidhu SS, Raman K, Solon M, Solberg OD, Caughey GH, Woodruff PG, Fahy JV. Accumulation of intraepithelial mast cells with a unique protease phenotype in T(H)2-high asthma. J Allergy Clin Immunol. 2010;125:1046–1053. e1048. doi: 10.1016/j.jaci.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Woodruff PG, Boushey HA, Dolganov GM, Barker CS, Yang YH, Donnelly S, Ellwanger A, Sidhu SS, Dao-Pick TP, Pantoja C, et al. Genome-wide profiling identifies epithelial cell genes associated with asthma and with treatment response to corticosteroids. Proc Natl Acad Sci U S A. 2007;104:15858–15863. doi: 10.1073/pnas.0707413104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anderson SD, Kippelen P. Airway injury as a mechanism for exercise-induced bronchoconstriction in elite athletes. J Allergy Clin Immunol. 2008;122:225–235. doi: 10.1016/j.jaci.2008.05.001. quiz 236-227. [DOI] [PubMed] [Google Scholar]
- 28.Hallstrand TS, Moody MW, Wurfel MM, Schwartz LB, Henderson WR, Jr., Aitken ML. Inflammatory basis of exercise-induced bronchoconstriction. Am J Respir Crit Care Med. 2005;172:679–686. doi: 10.1164/rccm.200412-1667OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mickleborough TD, Lindley MR, Ray S. Dietary salt, airway inflammation, and diffusion capacity in exercise-induced asthma. Med Sci Sports Exerc. 2005;37:904–914. [PubMed] [Google Scholar]
- 30.Meltzer SS, Hasday JD, Cohn J, Bleecker ER. Inhibition of exercise-induced bronchospasm by zileuton: a 5-lipoxygenase inhibitor. Am J Respir Crit Care Med. 1996;153:931–935. doi: 10.1164/ajrccm.153.3.8630575. [DOI] [PubMed] [Google Scholar]
- 31.Melo RE, Sole D, Naspitz CK. Exercise-induced bronchoconstriction in children: montelukast attenuates the immediate-phase and late-phase responses. J Allergy Clin Immunol. 2003;111:301–307. doi: 10.1067/mai.2003.66. [DOI] [PubMed] [Google Scholar]
- 32.Pearlman DS, van Adelsberg J, Philip G, Tilles SA, Busse W, Hendeles L, Loeys T, Dass SB, Reiss TF. Onset and duration of protection against exercise-induced bronchoconstriction by a single oral dose of montelukast. Ann Allergy Asthma Immunol. 2006;97:98–104. doi: 10.1016/S1081-1206(10)61377-4. [DOI] [PubMed] [Google Scholar]
- 33.Philip G, Villaran C, Pearlman DS, Loeys T, Dass SB, Reiss TF. Protection againsx exercise-induced bronchoconstriction two hours after a single oral dose of montelukast. J Asthma. 2007;44:213–217. doi: 10.1080/02770900701209806. [DOI] [PubMed] [Google Scholar]
- 34.Mickleborough TD, Lindley MR, Ionescu AA, Fly AD. Protective effect of fish oil supplementation on exercise-induced bronchoconstriction in asthma. Chest. 2006;129:39–49. doi: 10.1378/chest.129.1.39. [DOI] [PubMed] [Google Scholar]
- 35.Mickleborough TD, Murray RL, Ionescu AA, Lindley MR. Fish oil supplementation reduces severity of exercise-induced bronchoconstriction in elite athletes. Am J Respir Crit Care Med. 2003;168:1181–1189. doi: 10.1164/rccm.200303-373OC. [DOI] [PubMed] [Google Scholar]
- 36.Wijewickrama GT, Kim JH, Kim YJ, Abraham A, Oh Y, Ananthanarayanan B, Kwatia M, Ackerman SJ, Cho W. Systematic evaluation of transcellular activities of secretory phospholipases A2. High activity of group V phospholipases A2 to induce eicosanoid biosynthesis in neighboring inflammatory cells. J Biol Chem. 2006;281:10935–10944. doi: 10.1074/jbc.M512657200. [DOI] [PubMed] [Google Scholar]
- 37.Trudeau J, Hu H, Chibana K, Chu HW, Westcott JY, Wenzel SE. Selective downregulation of prostaglandin E2-related pathways by the Th2 cytokine IL-13. J Allergy Clin Immunol. 2006;117:1446–1454. doi: 10.1016/j.jaci.2006.01.049. [DOI] [PubMed] [Google Scholar]
- 38.Bradding P, Redington AE, Djukanovic R, Conrad DJ, Holgate ST. 15-lipoxygenase immunoreactivity in normal and in asthmatic airways. Am J Respir Crit Care Med. 1995;151:1201–1204. doi: 10.1164/ajrccm/151.4.1201. [DOI] [PubMed] [Google Scholar]
- 39.Chu HW, Balzar S, Westcott JY, Trudeau JB, Sun Y, Conrad DJ, Wenzel SE. Expression and activation of 15-lipoxygenase pathway in severe asthma: relationship to eosinophilic phenotype and collagen deposition. Clin Exp Allergy. 2002;32:1558–1565. doi: 10.1046/j.1365-2222.2002.01477.x. [DOI] [PubMed] [Google Scholar]
- 40.Currie GP, Haggart K, Lee DK, Fowler SJ, Wilson AM, Brannan JD, Anderson SD, Lipworth BJ. Effects of mediator antagonism on mannitol and adenosine monophosphate challenges. Clin Exp Allergy. 2003;33:783–788. doi: 10.1046/j.1365-2222.2003.01688.x. [DOI] [PubMed] [Google Scholar]
- 41.Lai YL, Lee SP. Mediators in hyperpnea-induced bronchoconstriction of guinea pigs. Naunyn Schmiedebergs Arch Pharmacol. 1999;360:597–602. doi: 10.1007/s002109900090. [DOI] [PubMed] [Google Scholar]
- 42.Freed AN, McCulloch S, Meyers T, Suzuki R. Neurokinins modulate hyperventilation-induced bronchoconstriction in canine peripheral airways. Am J Respir Crit Care Med. 2003;167:1102–1108. doi: 10.1164/rccm.200201-055OC. [DOI] [PubMed] [Google Scholar]
- 43.Hallstrand TS, Debley JS, Farin FM, Henderson WR., Jr. Role of MUC5AC in the pathogenesis of exercise-induced bronchoconstriction. J Allergy Clin Immunol. 2007;119:1092–1098. doi: 10.1016/j.jaci.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Uozumi N, Kume K, Nagase T, Nakatani N, Ishii S, Tashiro F, Komagata Y, Maki K, Ikuta K, Ouchi Y, et al. Role of cytosolic phospholipase A2 in allergic response and parturition. Nature. 1997;390:618–622. doi: 10.1038/37622. [DOI] [PubMed] [Google Scholar]
- 45.Hallstrand TS, Henderson WR., Jr. An update on the role of leukotrienes in asthma. Curr Opin Allergy Clin Immunol. 2010;10:60–66. doi: 10.1097/ACI.0b013e32833489c3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bowton DL, Seeds MC, Fasano MB, Goldsmith B, Bass DA. Phospholipase A2 and arachidonate increase in bronchoalveolar lavage fluid after inhaled antigen challenge in asthmatics. Am J Respir Crit Care Med. 1997;155:421–425. doi: 10.1164/ajrccm.155.2.9032172. [DOI] [PubMed] [Google Scholar]
- 47.Chilton FH, Averill FJ, Hubbard WC, Fonteh AN, Triggiani M, Liu MC. Antigen-induced generation of lyso-phospholipids in human airways. J Exp Med. 1996;183:2235–2245. doi: 10.1084/jem.183.5.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stadel JM, Hoyle K, Naclerio RM, Roshak A, Chilton FH. Characterization of phospholipase A2 from human nasal lavage. Am J Respir Cell Mol Biol. 1994;11:108–113. doi: 10.1165/ajrcmb.11.1.8018333. [DOI] [PubMed] [Google Scholar]
- 49.Singer AG, Ghomashchi F, Le Calvez C, Bollinger J, Bezzine S, Rouault M, Sadilek M, Nguyen E, Lazdunski M, Lambeau G, et al. Interfacial kinetic and binding properties of the complete set of human and mouse groups I, II, V, X, and XII secreted phospholipases A2. J Biol Chem. 2002;277:48535–48549. doi: 10.1074/jbc.M205855200. [DOI] [PubMed] [Google Scholar]
- 50.Munoz NM, Meliton AY, Arm JP, Bonventre JV, Cho W, Leff AR. Deletion of secretory group V phospholipase A2 attenuates cell migration and airway hyperresponsiveness in immunosensitized mice. J Immunol. 2007;179:4800–4807. doi: 10.4049/jimmunol.179.7.4800. [DOI] [PubMed] [Google Scholar]
- 51.Ma Y, Koza-Taylor PH, DiMattia DA, Hames L, Fu H, Dragnev KH, Turi T, Beebe JS, Freemantle SJ, Dmitrovsky E. Microarray analysis uncovers retinoid targets in human bronchial epithelial cells. Oncogene. 2003;22:4924–4932. doi: 10.1038/sj.onc.1206728. [DOI] [PubMed] [Google Scholar]
- 52.Cookson W. The immunogenetics of asthma and eczema: a new focus on the epithelium. Nat Rev Immunol. 2004;4:978–988. doi: 10.1038/nri1500. [DOI] [PubMed] [Google Scholar]
- 53.Sohn J, Kim TI, Yoon YH, Kim JY, Kim SY. Novel transglutaminase inhibitors reverse the inflammation of allergic conjunctivitis. J Clin Invest. 2003;111:121–128. doi: 10.1172/JCI15937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee J, Kim YS, Choi DH, Bang MS, Han TR, Joh TH, Kim SY. Transglutaminase 2 induces nuclear factor-kappaB activation via a novel pathway in BV-2 microglia. J Biol Chem. 2004;279:53725–53735. doi: 10.1074/jbc.M407627200. [DOI] [PubMed] [Google Scholar]
- 55.Kim DY, Park BS, Hong GU, Lee BJ, Park JW, Kim SY, Ro JY. Anti-inflammatory effects of the R2 peptide, an inhibitor of transglutaminase 2, in a mouse model of allergic asthma, induced by ovalbumin. Br J Pharmacol. 2011;162:210–225. doi: 10.1111/j.1476-5381.2010.01033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim Y, Eom S, Kim K, Lee YS, Choe J, Hahn JH, Lee H, Kim YM, Ha KS, Ro JY, et al. Transglutaminase II interacts with rac1, regulates production of reactive oxygen species, expression of snail, secretion of Th2 cytokines and mediates in vitro and in vivo allergic inflammation. Mol Immunol. 2010;47:1010–1022. doi: 10.1016/j.molimm.2009.11.017. [DOI] [PubMed] [Google Scholar]
- 57.Cowburn AS, Sladek K, Soja J, Adamek L, Nizankowska E, Szczeklik A, Lam BK, Penrose JF, Austen FK, Holgate ST, et al. Overexpression of leukotriene C4 synthase in bronchial biopsies from patients with aspirin-intolerant asthma. J Clin Invest. 1998;101:834–846. doi: 10.1172/JCI620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Macfarlane AJ, Dworski R, Sheller JR, Pavord ID, Kay AB, Barnes NC. Sputum cysteinyl leukotrienes increase 24 hours after allergen inhalation in atopic asthmatics. Am J Respir Crit Care Med. 2000;161:1553–1558. doi: 10.1164/ajrccm.161.5.9906068. [DOI] [PubMed] [Google Scholar]
- 59.Evans JH, Spencer DM, Zweifach A, Leslie CC. Intracellular calcium signals regulating cytosolic phospholipase A2 translocation to internal membranes. J Biol Chem. 2001;276:30150–30160. doi: 10.1074/jbc.M100943200. [DOI] [PubMed] [Google Scholar]
- 60.Glover S, de Carvalho MS, Bayburt T, Jonas M, Chi E, Leslie CC, Gelb MH. Translocation of the 85-kDa phospholipase A2 from cytosol to the nuclear envelope in rat basophilic leukemia cells stimulated with calcium ionophore or IgE/antigen. J Biol Chem. 1995;270:15359–15367. doi: 10.1074/jbc.270.25.15359. [DOI] [PubMed] [Google Scholar]
- 61.Munoz NM, Kim YJ, Meliton AY, Kim KP, Han SK, Boetticher E, O'Leary E, Myou S, Zhu X, Bonventre JV, et al. Human group V phospholipase A2 induces group IVA phospholipase A2-independent cysteinyl leukotriene synthesis in human eosinophils. J Biol Chem. 2003;278:38813–38820. doi: 10.1074/jbc.M302476200. [DOI] [PubMed] [Google Scholar]
- 62.Munoz NM, Meliton AY, Lambertino A, Boetticher E, Learoyd J, Sultan F, Zhu X, Cho W, Leff AR. Transcellular secretion of group V phospholipase A2 from epithelium induces β2-integrin-mediated adhesion and synthesis of leukotriene C4 in eosinophils. J Immunol. 2006;177:574–582. doi: 10.4049/jimmunol.177.1.574. [DOI] [PubMed] [Google Scholar]
- 63.Chilton FH, Westcott JY, Zapp LM, Henson JE, Voelkel NF. Incorporation of arachidonic acid into lipids of the isolated perfused rat lung. J Appl Physiol. 1989;66:2763–2771. doi: 10.1152/jappl.1989.66.6.2763. [DOI] [PubMed] [Google Scholar]
- 64.Albert CJ, Thukkani AK, Heuertz RM, Slungaard A, Hazen SL, Ford DA. Eosinophil peroxidase-derived reactive brominating species target the vinyl ether bond of plasmalogens generating a novel chemoattractant, alpha-bromo fatty aldehyde. J Biol Chem. 2003;278:8942–8950. doi: 10.1074/jbc.m211634200. [DOI] [PubMed] [Google Scholar]