Abstract
Incomplete spinal cord injury (SCI) can result in profound impairments in volitional strength and reflex excitability, which contribute to loss of function. Human and animal models suggest that disruption of endogenous monoaminergic input, particularly serotonin (5-HT), from supraspinal centers contributes to this impaired motor function following SCI. In the present study, we investigated the effects of 5-HT medications on motor function in individuals with chronic (>1 year) SCI. Clinical measures of strength, spasticity/spasms, and walking ability were assessed in 12 individuals with chronic incomplete SCI following acute administration of either 8 mg cyproheptadine, a 5-HT antagonist, or 10 mg escitalopram, a selective 5-HT reuptake inhibitor (SSRI), in a double-blinded, randomized, crossover fashion. Results indicated that 5-HT medications modulated both volitional and reflexive behaviors with little change in walking performance; 5-HT antagonist medications depressed clinical measures of strength and spasticity/spasms, whereas SSRIs augmented both strength and spasticity/spasms. These changes are consistent with the dysregulation of 5-HT sensitive spinal neurons following SCI. This understanding may augment clinicians' awareness of the motor consequences of 5-HT medications.
Key words: 5-HT, SCI, spasm, spasticity, strength
Introduction
Incomplete spinal cord injury (SCI) can result in profound impairments in motor control and limitations in function. Two prominent, functionally relevant impairments include weakness, defined as deficits in force generation during maximal voluntary effort contractions and hyperactive reflexes, which encompass spasticity, defined as velocity-dependent increases in stretch reflex excitability and spasms, defined here as sustained, involuntary muscle activation. A variety of pharmacological, physical and/or surgical interventions have been directed toward improving function thorough amelioration of these impairments, although their relative efficacy is unclear.
The mechanisms underlying spasticity and spasms following SCI are multifaceted, and include changes in both afferent pathways and spinal circuits. One mechanism underlying spastic motor behaviors that has gained considerable support is the role of altered spinal motoneuron excitability following SCI. Under healthy conditions, synaptic depolarization of the motoneuron is influenced by persistent inward (Ca2+ and Na+) currents (PICs), which amplify and sustain membrane depolarization. Motoneuron PIC activation, and the resulting motor output, is strongly influenced by spinal concentrations of endogenous neuromodulators, in particular serotonin (5-HT).1 The SCI disease progression results in structural changes to these 5-HT receptors in the chronic stages post-injury, allowing for PIC activation with minimal residual descending 5-HT.2–4 Dysregulation of PIC activation contributes to the presence of both spasticity and spasms; accordingly, medications that block 5-HT activity may decrease spasticity/spasms,3 whereas medications that augment 5-HT bioavailability may increase spasticity/spasms.5
Volitional motor output may also be dependent on motoneuron PIC activity. Under healthy conditions, volitional motor tasks produce variable PIC amplification through the activity-dependent release of 5-HT.6,7 Following incomplete SCI, the loss of descending corticospinal pathways contributes to decreased force generating capacity, whereas residual 5-HT inputs from brainstem centers may still modulate motor output. For example, PIC activation may help explain the observations of increased time to task failure during sustained motor activity and increases in peak volitional torque generation during maximal effort contractions in patients with incomplete SCI.8–10 Further, medications that decrease 5-HT may decrease volitional strength and functional performance following SCI, 3,11 whereas preliminary data suggest that medications thataugment 5-HT may increase strength.12,13
The current study assesses acute alterations in clinical measures of volitional strength, spasticity/spasms, and locomotor function following single dose administration of 5-HT medications in subjects with incomplete SCI. The primary hypotheses were that administration of a 5-HT antagonist (cyproheptadine) would depress volitional and reflexive motor function, whereas an agent that augmented 5-HT bioavailability, such as a selective 5-HT reuptake inhibitor (SSRI; escitalopram), would increase volitional and reflex function. Understanding how these medications affect motor output following SCI may have an important role in clinical decision making, particularly as a large number of individuals with SCI utilize medications that modulate synaptic 5-HT activity.
Methods
Individuals with chronic motor incomplete SCI were recruited to participate in a double-blinded, randomized crossover design study to assess the acute effects of over-encapsulated, oral administration of a 5-HT antagonist (8 mg cyproheptadine, Periactin, Merck Inc.) and an SSRI (10 mg escitalopram oxalate, Lexapro, Forest Pharmaceuticals Inc.) on clinical assessments of motor activity. The dosage of medications was chosen based on previous published and unpublished data.3,13 Both medications cross the blood–brain barrier and act upon 5-HT, among other, synapses in the central nervous system. Cyproheptadine acts upon the postsynaptic neuron and blocks ligand-mediated and constitutive activity of the 5-HT receptor. Escitalopram acts upon presynaptic 5-HT reuptake mechanisms to increase the duration/concentration of endogenous 5-HT in the synaptic cleft. All subjects underwent a 14 day washout period for antidepressant, antispastic, and all other medications with known interactions with the study agents. All procedures were approved by the Northwestern University Institutional Review Board and all subjects provided informed written consent.
Clinical examinations of strength, reflexes, and locomotor function were conducted prior to and 4.5 h following administration of either agent, corresponding to peak plasma concentration of study medication. Testing took approximately 30 min and the order of strength, reflexes, and locomotor testing was consistent during each session. A minimum of 7 days separated the two testing conditions. The clinical examinations have been described in detail previously with abbreviated descriptions provided here.12 The Lower Extremity Motor Score (LEMS) is a standard clinical evaluation of strength of five lower extremity myotomes (L2–S1; hip flexion, knee extension, ankle dorsiflexion, great toe extension, ankle plantarflexion) bilaterally. Strength is scored on a 0–5 scale, ranging from “no muscle contraction” to “normal amount of resistance to examiners efforts” and summed bilaterally for a total possible score of 50. Strength data were also separated by stronger versus weaker limb, and by stronger (≥3) versus weaker (<3) muscle groups.
Lower extremity reflex behaviors were assessed using both the modified Ashworth assessment (mAsh) and the Spinal Cord Assessment Tools for Spastic Reflexes (SCATS). The mAsh assesses resistance to rapid joint movement applied by the experimenter to the passive subject. This evaluation is used to assess spasticity of bilateral knee extensors and flexors using a 0–5 scale ranging from “no increase in muscle tone” to “complete rigidity.” Scores were summed bilaterally for a total possible score of 20, with individual assessments also analyzed separately. The SCATS is a clinical evaluation of three types of lower extremity spasms (flexor spasms, extensor spasms, plantarflexor clonus). Each spasm is graded on a 0–3 scale based upon the amplitude or duration of reflex activity. Data were summed bilaterally for a total score of 18, with individual assessments also analyzed separately.
Walking function was assessed using an instrumented walking platform (GaitMatII ®, Equitest, Chalfont, PA). Walking data were collected during walking over two to three trials at the patients fastest-possible velocity, with assistive devices and lower extremity bracing below the knee as needed. Primary outcomes included velocity, step length, and cadence.
Potential differences in clinical measures from baseline values for either medication were assessed using a Wilcoxon signed rank test; when non-significant changes were observed, baseline measures were averaged. Reliability between baseline testing was assessed using an intraclass correlation coefficient (ICC) (2,1) model and the variability was described using the median of the absolute difference between testing sessions. A Friedman's test was used to assess changes between baseline, 5-HT antagonist, and SSRI conditions. A post-hoc Wilcoxon signed rank test was used to determine specific differences. Within-subject change scores for each medication were calculated and analyzed in a similar manner. Potential correlations among variables were assessed using Spearman rank test. Significance was set at α=0.05 for all tests and data in the text are presented as median (25th–75th percentile).
Results
Twelve individuals (10 male) with history of traumatic motor incomplete SCI participated in this study. Subjects had a median age of 50.5 (42.5–60.5) years and median duration of injury of 93 (63–235) months. Two subjects had high cervical injuries (C1–C4), seven subjects had low cervical injuries (C4–C8), and three subjects had thoracic injuries (T1–T10); using the American Spinal Injury Association (ASIA) Impairment Scale (AIS), five subjects were classified as AIS C and seven subjects were classified as AIS D.
No differences were observed in baseline clinical measures of strength, reflexes, or walking function between testing days (all p>0.05). Baseline clinical measures showed moderate to good reliability with ICC scores ranging from 0.73 to 0.86. The median of the absolute difference between baseline testing sessions was 3.5 (1.5–7.0), 1.5 (0.5–2.0), and 1.0 (0.0– 2.0) for LEMS, SCATS, and mAsh values. Baseline walking measures showed excellent reliability with ICC scores ranging from 0.97 to 0.99. The median of the absolute difference between baseline testing was negligible for speed and step length (<0.03 m/sec and <0.02 m), whereas cadence was 2.6 (1.2–5.5) steps/min.
Modulation of volitional and reflexive motor output
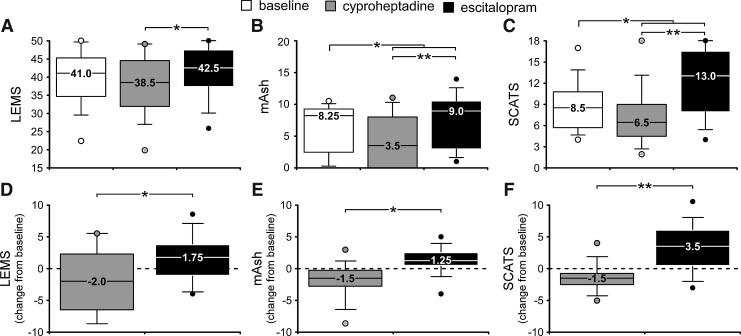
Participants had a median baseline LEMS score of 41 (34.75–45.25); for reflex behaviors, median baseline mAsh scores were 8.25 (2.5–9.25) and SCATS scores were 8.5 (5.75–10.75) (Fig. 1A–C). There was no significant correlation between baseline LEMS and SCATS values (ρ=0.00, p=0.99), although the correlation between SCATS and mAsh values approached significance (ρ=0.54, p=0.07). A significant positive correlation between baseline LEMS and mAsh values was observed, however (ρ=0.65,p<0.05).
FIG. 1.
Assessments of volitional and reflexive motor behaviors following administration of serotonergic medication in patients with motor incomplete spinal cord injury (SCI). (A–C) Median Lower Extremity Motor Score (LEMS), modified Ashworth assessment (mAsh) and Spinal Cord Assessment Tools for Spastic Reflexes (SCATS) scores are shown during baseline conditions and following either serotonin (5-HT)-agonist (8 mg cyproheptadine) or a selective 5-HT reuptake inhibitor (SSRI) (10 mg escitalopram). (D–F) A median change of 1.25–3.5 is observed when individual change scores are assessed. Dashed line indicates no change from baseline and is provided for visual reference. *p<0.05; **p<0.01.
Median LEMS decreased to 38.5 (32–44.5) following 5-HT antagonist administration, and increased to 42.5 (37.5–47.5) following SSRI administration. No clear trends in LEMS change scores for individual myotomes were observed (p>0.05), nor did clear trends emerge when unilateral LEMS scores were grouped by weaker and stronger sides (p>0.05). Categorizing LEMS change scores for individual myotomes as either weaker (<3) and stronger (≥3) revealed significant differences between study medications (p<0.01 and p<0.05 respectively).
For reflex measures, mAsh values decreased to 3.5 (0.0–8.0) and SCATS values decreased to 6.5 (4.75–8.5) with 5-HT antagonist administration. Conversely, mAsh values increased to 9 (3.5– 10.25) and SCATS scores increased to 13 (8.0–16.25) following SSRI administration. Individual change scores in these values followed a similar trend (Fig. 1D–F). Change scores in individual mAsh assessments demonstrated significant differences between medications for both knee flexor and knee extensor spasticity (both p<0.05). Change scores for individual SCATS assessments demonstrated significance for extensor spasms and plantarflexor clonus (both p<0.05), and flexor spasms approached significance (p=0.06).
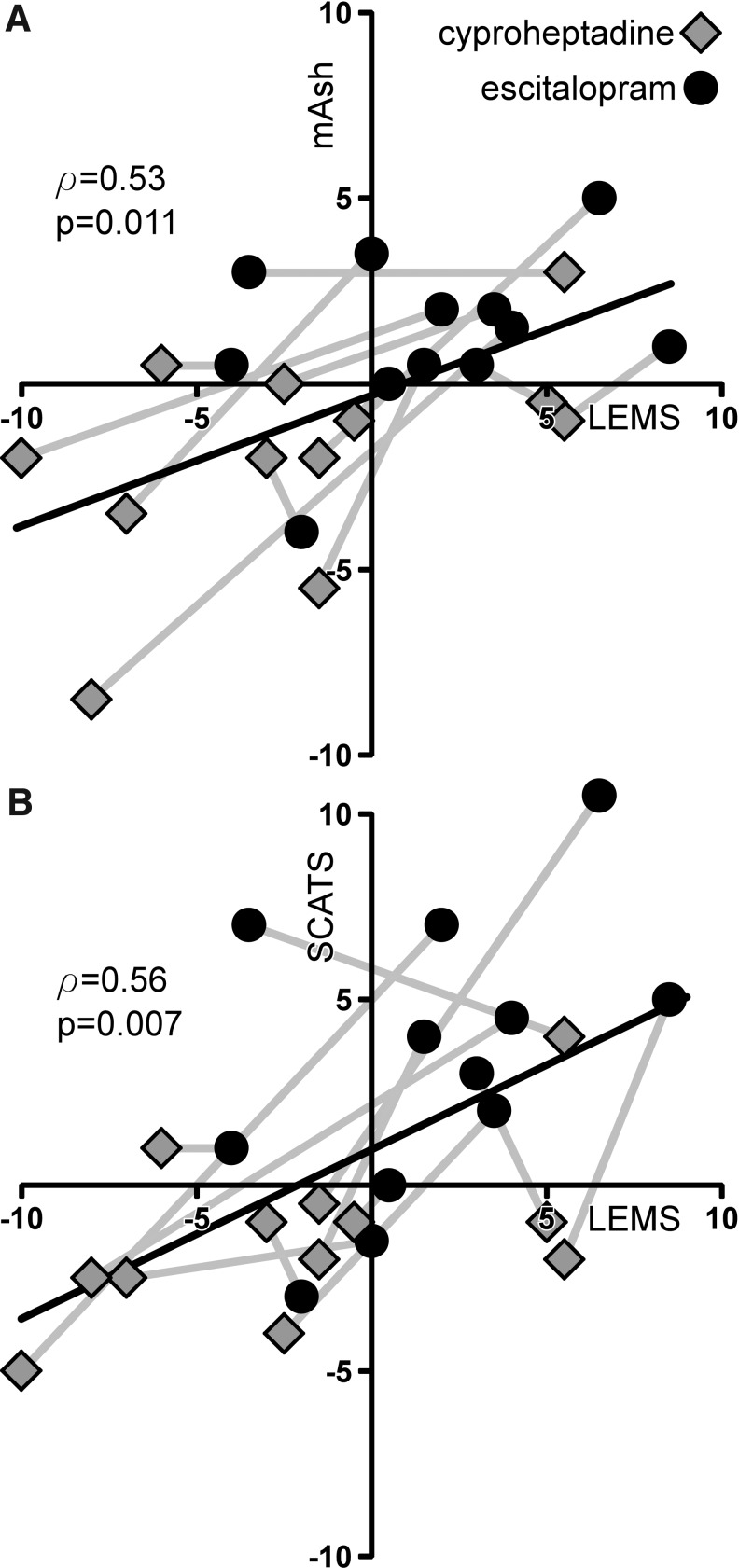
Changes in strength and spastic motor behaviors described previously appeared to covary with the different 5-HT medications. Significant correlations between both LEMS and mAsh change scores (ρ=0.53, p=0.01, Fig. 2A), and LEMS and SCATS change scores were observed (ρ=0.54, p<0.01, Fig. 2B).
FIG. 2.
Correlation between volitional and reflexive motor behaviors following acute administration of serotonergic medication in patients with motor incomplete spinal cord injury (SCI). Change in Lower Extremity Motor Score (LEMS) values are positively correlated with changes in both (A) modified Ashworth assessment (mAsh) and (B) Spinal Cord Assessment Tools for Spastic Reflexes (SCATS) values. Gray lines link the subjects' data for each of the two medications.
Modulation of walking performance
For measures of walking performance, the median baseline walking speed was 0.71 m/sec (0.49–0.90), with a median stride length of 0.55 m (0.47–0.59) and cadence of 75 steps/min (66–93). Following 5-HT antagonists, median gait speed decreased significantly to 0.66 m/sec (0.36–0.82) (p<0.05) and were mediated by reduced stride length (0.53 m [0.39–.60]; p<0.05), but not cadence (73 steps/min [64–91]). In contrast, SSRIs resulted in nonsignificant decreases in walking speed (0.63 m/s [0.44–0.77]), stride length (0.51 m [0.44–0.57]), and cadence (71 steps/min [66–87]). There were no significant correlations between changes in gait speed and changes in clinical measures of strength or spastic motor behaviors (all p>0.05).
Discussion
In the present investigation, single-dose administration of medications that modulate 5-HT bioavailability demonstrated divergent trends in motor function in subjects with motor incomplete SCI. Namely, 5-HT antagonists decreased clinical measures of both strength and spasticity/spasms, whereas administration of SSRIs augmented both behaviors. Acute administration of either medication appears to be ineffective at improving locomotor function.
Serotonergic modulation of motor output
The finding that volitional strength and spastic motor behaviors covary following different SSRI medication is of primary interest in the current study. In addition, significant correlations in baseline clinical measures of strength and spasticity (mAsh), but not spasms (SCATS), were observed. Although difficulties exist in distinguishing voluntary versus involuntary contractions with clinical measures, the combined findings are consistent with the hypothesis that common neural pathways underlie selected voluntary and involuntary behaviors. Potential mechanisms underlying such changes include modulation of passive and active (PIC) properties of spinal moto- and interneurons, in addition to separate modulatory effects on afferent and descending pathways. The coupling of reflex and volitional motor output may also be seen during quantitative assessments, as reflex activity in individuals with SCI can strongly influence static and dynamic volitional behaviors.10
Changes in strength and reflex activity were relatively consistent following either medication, with few outliers from different subjects on separate testing days, (see Fig. 2). However, a rather large increase in SCATS values was observed following SSRI medication (Fig. 1F). This behavior may be related to the time-dependent assessment of extensor and clonic spasms in the SCATS, and suggests that increased persistence of spasms with SSRIs may account for the large differences. Despite this observation, the general correlations between medication-induced changes in strength and spasticity/spasms were observed.
Walking performance decreased following administration of either 5-HT medications, although only significantly following 5-HT antagonists. These findings contrast with previous investigations that demonstrate improved walking ability following longer-duration cyproheptidine administration in patients with incomplete SCI.14, 15 This decline may have been the result of recruitment of relatively higher functioning patients in the present study, in which one subject presented with a LEMS of 50. However, all patients demonstrated substantial gait impairments at baseline, with little potential for ceiling effects. Mechanisms underlying this immediate decline in speed following either medication are unclear, as both strength and reflex activity contribute to locomotor function. A likely contributing factor was use of altered neuromuscular control strategies following either medication, with limited time allowed for subjects to adapt to the agents. As such, subjects may have chosen to walk at slower, more stable speeds. Long-term application of these medications may allow adaptation to the changes in neuromuscular excitability.
Clinical implications
Despite significant, immediate changes in motor impairments following either 5-HT agent, an important clinical implication is the lack of substantial positive improvements in functional walking performance. Although the use of SSRIs to improve motor function following neurological injury has been suggested,16 animal and human studies suggest combined pharmacological and physical (training) interventions to maximize therapeutic benefits.17,18 However more data are required, as recent studies also suggest that use of SSRIs for mental health impairments may contribute to poorer rehabilitation outcomes.19
Whereas the motor consequences of these agents are of primary interest, the use of SSRIs in SCI is common,20,21 and the resultant side effects may not be well understood. As such, patients may be prescribed antispastic agents to counteract the observed increase in spasticity with SSRIs. Use of multiple medications is common in SCI, and may increase risk of drug interactions in addition to a host of adverse effects.22–24 Use of medications to treat side effects of other medications could be avoided if SSRI-induced increases in spasticity/spasms were accounted for through coordinated, interdisciplinary medical management.
Conclusion
Acute administration of 5-HT medications modulates both volitional and reflexive behaviors in humans with chronic incomplete SCI. 5-HT antagonists will decrease clinical measures of strength and spasticity/spasms whereas SSRIs will increase clinical measures of strength and spasticity/spasms. These data suggest that 5-HT may have actions on pathways common to both voluntary and involuntary force generation. Although further work is underway to elucidate the precise physiological mechanisms underlying the changes observed, the understanding that serotonergic medications alter motor function following SCI may have immediate clinical implications and can increase the clinicians' awareness of motor consequences of medications that alter the bioavailability of 5-HT.
Acknowledgments
Funding for the present work was provided through a University of Illinois at Chicago (UIC) doctoral scholarship and Foundation for Physical Therapy Scholarship to Dr. Thompson and National Institutes of Health (NIH)/National Institute of Child Health and Human Development (NICHD) R21-HD046876 and the Craig H. Neilsen Foundation (grant # 36830) to Dr. Hornby.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Heckman C.J. Lee R.H. Brownstone R.M. Hyperexcitable dendrites in motoneurons and their neuromodulatory control during motor behavior. Trends Neurosci. 2003;26:688–695. doi: 10.1016/j.tins.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 2.Gorassini M.A. Knash M.E. Harvey P.J. Bennett D.J. Yang J.F. Role of motoneurons in the generation of muscle spasms after spinal cord injury. Brain. 2004;127:2247–2258. doi: 10.1093/brain/awh243. [DOI] [PubMed] [Google Scholar]
- 3.Murray K.C. Nakae A. Stephens M.J. Rank M. D'Amico J. Harvey P.J. Li X. Harris R.L. Ballou E.W. Anelli R. Heckman C.J. Mashimo T. Vavrek R. Sanelli L. Gorassini M.A. Bennett D.J. Fouad K. Recovery of motoneuron and locomotor function after spinal cord injury depends on constitutive activity in 5-HT2C receptors. Nat. Med. 2010;16:694–700. doi: 10.1038/nm.2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bennett D.J. Gorassini M. Fouad K. Sanelli L. Han Y. Cheng J. Spasticity in rats with sacral spinal cord injury. J Neurotrauma. 1999;16:69–84. doi: 10.1089/neu.1999.16.69. [DOI] [PubMed] [Google Scholar]
- 5.Stolp–Smith K.A. Wainberg M.C. Antidepressant exacerbation of spasticity. Arch. Phys. Med. Rehabil. 1999;80:339–342. doi: 10.1016/s0003-9993(99)90148-x. [DOI] [PubMed] [Google Scholar]
- 6.Gerin C. Becquet D. Privat A. Direct evidence for the link between monoaminergic descending pathways and motor activity I. A study with microdialysis probes implanted in the ventral funiculus of the spinal cord. Brain Res. 1995;704:191–201. doi: 10.1016/0006-8993(95)01111-0. [DOI] [PubMed] [Google Scholar]
- 7.Veasey S.C. Fornal C.A. Metzler C.W. Jacobs B.L. Response of serotonergic caudal raphe neurons in relation to specific motor activities in freely moving cats. J. Neurosci. 1995;15:5346–5359. doi: 10.1523/JNEUROSCI.15-07-05346.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomas C.K. del Valle A. The role of motor unit rate modulation versus recruitment in repeated submaximal voluntary contractions performed by control and spinal cord injured subjects. J. Electromyogr. Kinesiol. 2001;11:217–229. doi: 10.1016/s1050-6411(00)00055-9. [DOI] [PubMed] [Google Scholar]
- 9.Hornby T.G. Lewek M.D. Thompson C.K. Heitz R. Repeated maximal volitional effort contractions in human spinal cord injury: Initial torque increases and reduced fatigue. Neurorehabil. Neural Repair. 2009;23:928–938. doi: 10.1177/1545968309336147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thompson C.K. Lewek M.D. Jayaraman A. Hornby T.G. Central excitability contributes to supramaximal volitional contractions in human incomplete spinal cord injury. J. Physiol. 2011;589:3739–3752. doi: 10.1113/jphysiol.2011.212233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barbeau H. Richards C.L. Bedard P.J. Action of cyproheptadine in spastic paraparetic patients. J. Neurol. Neurosurg. Psychiatry. 1982;45:923–926. doi: 10.1136/jnnp.45.10.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thompson C.K. Jayaraman A. Kinnaird C. Hornby T.G. Methods to quantify pharmacologically induced alterations in motor function in human incomplete. sci. J. Vis. Exp. 2011 Apr 18;(50):2148. doi: 10.3791/2148. pii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hornby T.G. Schmit B.D. Theiss R.D. Serotonergic modulation of motor function in human spinal cord injury. 2006 Neuroscience Meeting Planner; Atlanta, GA: Society for Neuroscience; 2006. 2006. Program No. 146.20. [Google Scholar]
- 14.Wainberg M. Barbeau H. Gauthier S. The effects of cyproheptadine on locomotion and on spasticity in patients with spinal cord injuries. J. Neurol. Neurosurg. Psychiatry. 1990;53:754–763. doi: 10.1136/jnnp.53.9.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Norman K.E. Pepin A. Barbeau H. Effects of drugs on walking after spinal cord injury. Spinal Cord. 1998;36:699–715. doi: 10.1038/sj.sc.3100674. [DOI] [PubMed] [Google Scholar]
- 16.Dam M. Tonin P. De Boni A. Pizzolato G. Casson S. Ermani M. Freo U. Piron L. Battistin L. Effects of fluoxetine and maprotiline on functional recovery in poststroke hemiplegic patients undergoing rehabilitation therapy. Stroke. 1996;27:1211–1214. doi: 10.1161/01.str.27.7.1211. [DOI] [PubMed] [Google Scholar]
- 17.Pariente J. Loubinoux I. Carel C. Albucher J.F. Leger A. Manelfe C. Rascol O. Chollet F. Fluoxetine modulates motor performance and cerebral activation of patients recovering from stroke. Ann. Neurol. 2001;50:718–729. doi: 10.1002/ana.1257. [DOI] [PubMed] [Google Scholar]
- 18.Chollet F. Tardy J. Albucher J.F. Thalamas C. Berard E. Lamy C. Bejot Y. Deltour S. Jaillard A. Niclot P. Guillon B. Moulin T. Marque P. Pariente J. Arnaud C. Loubinoux I. Fluoxetine for motor recovery after acute ischaemic stroke (flame): A randomised placebo–controlled trial. Lancet Neurol. 2011;10:123–130. doi: 10.1016/S1474-4422(10)70314-8. [DOI] [PubMed] [Google Scholar]
- 19.Weeks D.L. Greer C.L. Bray B.S. Schwartz C.R. White J.R., Jr. Association of antidepressant medication therapy with inpatient rehabilitation outcomes for stroke, traumatic brain injury, or traumatic spinal cord injury. Arch. Phys. Med. Rehabil. 2011;92:683–695. doi: 10.1016/j.apmr.2010.12.026. [DOI] [PubMed] [Google Scholar]
- 20.Kemp B.J. Krause J.S. Depression and life satisfaction among people ageing with post-polio and spinal cord injury. Disabil. Rehabil. 1999;21:241–249. doi: 10.1080/096382899297666. [DOI] [PubMed] [Google Scholar]
- 21.Smith B.M. Weaver F.M. Ullrich P.M. Prevalence of depression diagnoses and use of antidepressant medications by veterans with spinal cord injury. Am. J. Phys. Med. Rehabil. 2007;86:662–671. doi: 10.1097/PHM.0b013e318114cb6d. [DOI] [PubMed] [Google Scholar]
- 22.Krause J.S. Zhai Y. Saunders L.L. Carter R.E. Risk of mortality after spinal cord injury: An 8-year prospective study. Arch. Phys. Med. Rehabil. 2009;90:1708–1715. doi: 10.1016/j.apmr.2009.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kohout R.K. Saunders L.L. Krause J.S. The relationship between prescription medication use and ability to ambulate distances after spinal cord injury. Arch. Phys. Med. Rehabil. 2011;92:1246–1249. doi: 10.1016/j.apmr.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krause J.S. Risk for subsequent injuries after spinal cord injury: A 10-year longitudinal analysis. Arch. Phys. Med. Rehabil. 2010;91:1741–1746. doi: 10.1016/j.apmr.2010.07.219. [DOI] [PMC free article] [PubMed] [Google Scholar]