Abstract
Organ transplantation is now a well-established procedure for the treatment of end-stage organ failure due to various causes, but is a victim of its own success in that there is a growing disparity in numbers between the donor organ pool available for transplantation and the patients eligible for such a procedure; hence, an alternative solution to the limited donor organ pool is both desirable and necessary. Tissue engineering is an interdisciplinary field that applies the principles of engineering and life sciences toward the development of functional replacement tissues for clinical use. A recent innovation in tissue and organ engineering is the technique of whole-organ decellularization, which allows the production of complex three-dimensional extracellular matrix (ECM) bioscaffolds of the entire organ with preservation of the intrinsic vascular network. These bioscaffolds can then be recellularized to create potentially functional organ constructs as a regenerative medicine strategy for organ replacement. We review the current applications and methods in using xenogeneic whole-organ ECM scaffolds to create potentially functional bioartificial organ constructs for surgical implantation, and present a comparison of specific trends within this new and developing technique.
Introduction
Organ transplantation is now a well-established procedure for the treatment of end-stage organ failure due to various causes, but is a victim of its own success in that there is a growing disparity in numbers between the donor organ pool available for transplantation and the patients eligible for such a procedure, with a high mortality rate in those who are on the waiting list. While there is ongoing debate to address this issue with strategies, both within a wider social or legislative context (e.g., so-called opt-out or presumed consent organ donation1) and others more specifically applicable to certain groups (e.g., living kidney donation), these strategies can be either controversial or not without additional clinical risk (e.g., to the living donor). An alternative solution to the limited donor organ pool is both desirable and necessary.
Tissue engineering is an interdisciplinary field that applies the principles of engineering and life sciences toward the development of functional replacement tissues for clinical use. Many developments within this field employ the seeding and cultivation of cells onto acellular scaffolds; however, control and utilization of the types of cell, scaffold material, interactions between the two, and the optimal conditions for the regeneration of functional tissue replacement are complex and vary from the nano- to macroscale.2 One group of biological scaffold materials that are already commonly in use for a variety of reconstructive surgical applications is that derived from the extracellular matrix (ECM).3 The ECM is secreted by the resident cells of each tissue and organ, and scaffolds derived from this material are produced by the process of decellularization of the specific tissue.4
A recent innovation in decellularization is the generation of whole-organ ECM scaffolds.5–9 This is a new technique in the application of decellularization agents (e.g., detergents) by using antegrade or retrograde perfusion of the inherent vascular network within the organ. This technique has led to the production of so-called bioartificial scaffolds10,11 that preserve the three-dimensional (3D) ECM structure of an organ in its entirety.10–27 This complete 3D structure gives site-specific architecture and composition, which control function at a local level within the organ. This scaffold can then be subsequently repopulated with cells using the same perfusion technique to produce organ regeneration to a functional degree. This type of scaffold combines the natural advantages and properties of the ECM in promoting and regulating cell proliferation and differentiation, the organizational and architectural complexity of the whole organ, as well as the supportive vascular network for the provision of oxygen and nutrients required for 3D tissue metabolism. This work has been carried out with preliminary success in animal studies of the heart,10 the lungs,11,14,21 and the liver,17 and the decellularized whole-organ scaffold has also been produced in the kidney,18–20 as well other tissues such as small bowel26 and skeletal muscle.27
We review the current applications and methods in using xenogeneic whole-organ ECM scaffolds to create potentially functional bioartificial organ constructs for surgical implantation, and compare specific trends within this new and developing technique to give a concise overview of existing experimental work across all the relevant organ systems as a tool for researchers.
The ECM
The ECM is the naturally occurring scaffold material secreted and manufactured by the resident cells of each tissue and organ. The complex 3D organization of the ECM and its components are dictated by the tissue from which the ECM is derived and can be considered specific to that tissue or organ28–30; however, individual components are common throughout most tissues such as collagen, laminin, fibronectin, and hyaluronic acid. The structural and functional molecules of the ECM are in a state of dynamic equilibrium within the surrounding microenvironment,31 and also provide the means by which cells communicate with each other and the external environment.32,33 By definition, the ECM possesses the ideal characteristics of the tissue-engineered scaffold or biomaterial (in addition to the functions already described), being biocompatible, a supportive medium for blood vessels, nerves, and lymphatics and for the diffusion of nutrients from the blood, as well as being able to undergo constructive remodeling and degradation within the body's own systems.34–36
The Properties of ECM
Bioinductive properties of ECM
The ability of the ECM scaffold to facilitate and integrate cellular and external environmental cues is the consequence of its bioinductive properties, allowing the constructive remodeling of tissue after the in vivo implantation of ECM scaffolds.37–39 This remodeling cannot be solely attributed to characteristics such as viscoelastic behavior, biomechanical properties, or host cell attachment through collagen, fibronectin, and laminin ligands within the ECM. Growth factors such as vascular endothelial growth factor,40 basic fibroblast growth factor,41,42 and transforming growth factor-beta44,45 are responsible for crucial events of remodeling such as angiogenesis, abundant host cell infiltration, mitogenesis, and deposition and organization of new host ECM. These can survive tissue processing and terminal sterilization41,43,44 to exert an effect on tissue remodeling and are released during the degradation of the ECM scaffold.40–45 Indeed, the degradation process itself, which is mediated by enzymatic and cellular processes, may be considered as a mechanism for controlled release of the ECM constituent molecules. The process of degradation and growth factor release continues until the scaffold is completely degraded. Degradation products of the molecules that constituted the ECM may mediate a subsequent series of remodeling events. These subsequent events include the release of cryptic peptides that initiate and sustain the recruitment of circulating, bone marrow-derived undifferentiated progenitor cells that actively participate in long-term tissue remodeling,46,47 the generation of antimicrobial peptides that protect the remodeling site from pathogens,48–53 other peptides that modulate angiogenesis, and the recruitment of endothelial cells over periods up to 6–8 weeks.54
Biomechanical properties of ECM
The mechanical properties of the ECM can be predominantly understood from the combination of its collagen fiber architecture and kinematics. The tissue from which an ECM scaffold is harvested will define its structural characteristics (such as fiber size, orientation, and alignment) and mechanical properties. For instance, small intestinal submucosa (SIS)-derived ECM has been shown to have a preferred fiber alignment in a spiral arrangement along the longitudinal axis of the small intestine,40,55,56 and it is likely that this structural arrangement facilitates dilation and retraction of the small intestine during peristalsis and transport of intraluminal contents. An understanding of the collagen fiber structure from each organ is important to closely match the scaffold mechanical properties to those of the intended target organ.
Degradation and constructive remodeling of ECM
The bioinductive properties of ECM scaffolds depend crucially on the efficient and effective degradation of the scaffold material and facilitate the constructive remodeling of injured tissue. The species of origin, tissue of origin, and processing techniques during the production of the ECM scaffold can differ markedly and factors such as method of decellularization, use of chemical crosslinking agents, and means of sterilization can affect the degradability and host response to the scaffold material.57 Quantitative studies of 14C-labeled SIS used in augmentation cystoplasty58 and Achilles tendon reconstruction59 procedures have shown that 50% or more of the ECM scaffold is degraded and removed from the implantation site by 28 days. The degradation products are excreted via the urine with no recycling to other tissues. Furthermore, the replacement of the degraded SIS with functional (and similar to normal) host tissue occurred without the loss-of-function, for example, bladder or tendon rupture suggests a rapid infiltration and/or proliferation of functional host cells at the remodeling site with deposition of new ECM. In these two models of degradation analysis, there was also the critical involvement of biomechanical factors such as physiological bladder filling and emptying38 or progressive weight bearing postoperatively in the tendon repair model.34 Environmental factors may also play an important role in determining in situ regeneration or transformation of implanted tissues, such as that seen in the formation of host-derived tracheal tissue within a long segment of allogenic aortic graft transplanted as a potential conduit for tracheal replacement.60,61 Hence, understanding of the additional factors that modulate remodeling is also essential to utilize ECM scaffolds effectively.
Host immune response to xenogeneic ECM
Most ECM scaffold biomaterials and commercially available surgical implants are of xenogeneic origin, such as a porcine or bovine source with a few types from human allogeneic tissue.3 Nonhuman biomaterials have been used in humans for many years without evidence of adverse host immunological outcomes, for example, porcine heart valve replacements and porcine and bovine insulin for the treatment of diabetes mellitus. Xeno- and allogeneic cellular antigens (e.g., cell membrane Gal epitope) are by definition recognized as foreign by the host and can induce a hyperacute rejection response after organ xenotranplantation; however, components of the ECM are generally conserved among species and tolerated well. With the SIS ECM, complement activation or cell-mediated rejection response does not occur even with the small amounts of Gal epitope that are present,62,63 and this could be further prevented with either use of transgenic gal knockout animals for tissue harvesting or treatment of harvested ECM with galactosidase.
In terms of the cell-mediated immune response, the Th1 lymphocyte phenotype is associated with macrophage activation, complement fixation, and CD8+ cytotoxic cell differentiation, and activation of the Th1 pathway is implicated in transplant rejection.64,65 In contrast, activation of the Th2 lymphocyte pathway does not lead to these events, differs markedly both in the profile of cytokines and antibody isotypes produced, and is hence associated with transplant acceptance.66,67 Implantation studies of the SIS ECM in mice have shown that a Th2 type immune response occurs, similar to that elicited by syngeneic muscle tissue.68,69
The likely innate immune-mediated inflammatory response to ECM materials and bioscaffolds also plays an important role in their degradation in situ and subsequent constructive remodeling process, and this can be demonstrated by the macrophage phenotype response. Macrophages are phenotypically and functionally plastic, but can be broadly characterized into a polarized M1/M2 phenotype classification, which forms a parallel to the Th1/Th2 response in that M1 macrophages are associated with a proinflammatory and M2 with a constructive remodeling response.70 The constructive remodeling process can be correlated with the ability of the implanted material to direct the macrophage phenotype, and the ECM has been shown to promote the switch from M1 to M2 after implantation.71,72 However, the use of chemical crosslinking agents that may interfere with the macrophage-mediated breakdown of the ECM material inhibits the beneficial M2 phenotype and leads to chronic inflammation and fibrosis.73
Hence, it can be seen that the overall host immune response for any particular ECM material will be variable and dependent on the diversity of tissue sources and processing methods used in its preparation.
Decellularization and Processing of ECM Scaffolds
Because of the complexity of the 3D organization and composition of all the structural and functional molecules of the ECM that have not yet been fully characterized (and for each type of tissue), it is not currently possible to synthesize this biomaterial in the laboratory. Hence, ECM scaffolds are produced by the process of decellularization of naturally derived tissues, and this can be achieved by a variety of agents and techniques.3 The complexity and length of the decellularization protocol are correlated with the degree of structural and biological conservation required for the postprocessed tissue, especially for composite tissues and whole organs. Any agent or method will cause some disruption of the ECM composition and ultrastructure, and minimization of these effects is desirable as complete avoidance is not yet feasible. While it is not possible to remove 100% of cellular material from the ECM, it is possible to quantitatively assay cell components such as DNA, mitochondria, and membrane phospholipids. Residual cellular and nuclear material may contribute to cytocompatibility problems in vitro and adverse host responses in vivo.74–77 It is also necessary to sterilize ECM scaffolds before in vitro use or implantation, as well as the removal of pyrogens (such as endotoxins and intact viral and bacterial DNA) that may be present.
Biological scaffolds may be sterilized by simple treatments such as incubation with acids or solvents, or other treatments such as ethylene oxide exposure, gamma-radiation, and electron beam irradiation. While the simple methods can lack penetration or may damage key ECM components,78 the latter ones can affect ECM ultrastructure and mechanical properties,79–81 and newer methods such as supercritical carbon dioxide requires further investigation.82 Hence, this necessary process is another factor that can critically influence the properties of the bioscaffold, and must be taken into account during the wider-scale standardization and quality assurance of such ECM products before clinical translation.
Whole-Organ Decellularization: Perfusion–Decellularization
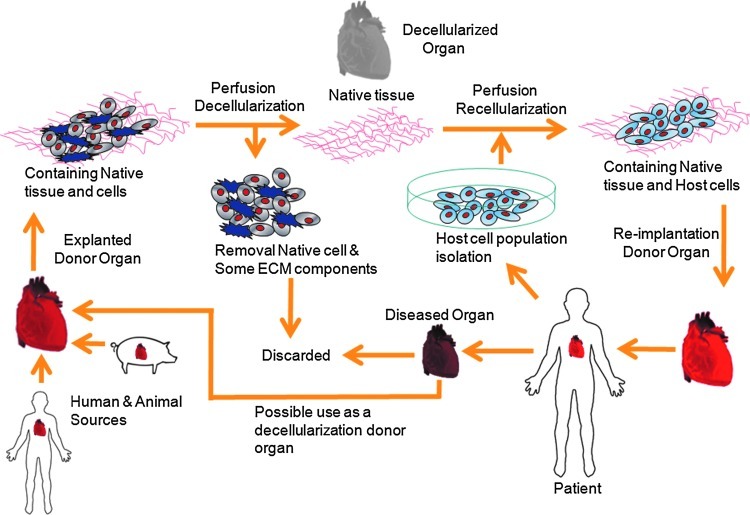
This is a relatively new technique that uses anterograde or retrograde perfusion via the intrinsic vascular network as a means of applying the decellularizing agent while largely preserving the 3D architecture of the organ from which the ECM has been isolated. It is the only technique to allow decellularization of thick 3D tissue sections or complete organs in a way that was not previously possible. Since the vascular network exists to minimize the diffusion distance for oxygen to cells, this is a particularly efficient way of delivering decellularizing agents throughout the tissue and transporting the cellular material from the tissue. The vascular network remains intact even after full decellularization has been achieved, hence offering a route for the efficient delivery and penetration of cells and nutrients into the ECM scaffold during the process of recellularization to create a functional organ construct (see Fig. 1 for the schematic diagram of the life cycle of whole-organ decellularization and potential clinical applications).
FIG. 1.
Schematic diagram of the life cycle of whole-organ decellularization and potential clinical applications. Color images available online at www.liebertpub.com/teb
An Overview of Whole-Organ Decellularization Work
The heart
The first report in which whole-organ decellularization was successfully performed was on the heart.10 The aorta of a rat heart was cannulated to allow retrograde coronary perfusion with heparinized phosphate-buffered saline with adenosine, 1% sodium dodecyl sulfate (SDS), and 1% Triton X-100 in sequence alternating with rinsing with deionized water between steps. This produced a decellularized scaffold that was structurally similar to the heart, but had a translucent white appearance throughout (Fig. 2). Reimplantation of the acellular heart scaffold onto the aorta demonstrated an intact and patent vascular network (Fig. 3). Recellularization of the heart scaffold with cardiomyocytes under electrophysiological stimulation formed a construct capable of muscular contraction.
FIG. 2.
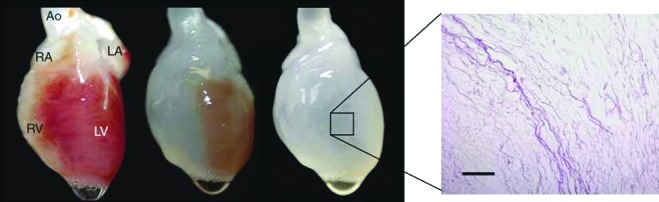
The heart in stages of increasing decellularization over time with 1% sodium dodecyl sulfate as the decellularizing agent, with the end result of a translucent appearing heart-like scaffold structure. The panel on the right shows histological evidence (H&E staining) of decellularization. Reproduced from [8]. Scale bar=200 μm. Color images available online at www.liebertpub.com/teb
FIG. 3.
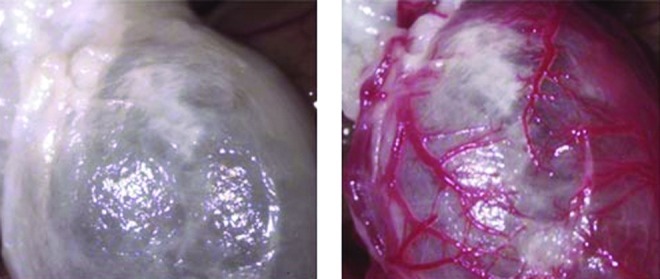
Coronary perfusion and evidence of intact coronary vasculature (right) after orthotopic implantation of the decellularized heart scaffold (left) and unclamping of the aorta. Reproduced from [8]. Color images available online at www.liebertpub.com/teb
The lungs
A number of groups have investigated the use of perfusion decellularization in the lungs,11,13–15,21 which contain two accessible compartments separated by a short diffusion distance, that is, the vascular and the airway systems, by using varying protocols of vascular perfusion alone,11,21 or in combination with endotracheal instillation,14,15 or perfusion of the airway compartment.13 The decellularizing agents used were also far more varied from protocol to protocol, from SDS at different concentrations11,13,21 to 8 mM CHAPS,14 to a combination of Triton X-100/deoxycholate/DNAse/bleach.15 All groups demonstrated preservation of the major components of the ECM and the micro- and macroarchitecture of the lung. Recellularization was achieved with all the reports, although Petersen et al. mimicked physiological conditions within the bioreactor by ventilation of the airway compartment at negative pressure at 1 breath/min, and maintained pulmonary artery pressure at 20 mmHg or below. Orthotopic implantation of these tissue-engineered lung constructs demonstrated active gas exchange occurring via sampling of pulmonary arterial and venous blood. However, results in this study and Ott et al. showed vascular leakage and pulmonary edema after a few hours of implantation, indicating damage to the microvasculature from the decellularization process. In a follow-up study to Ott et al., Song et al. demonstrated further optimization of graft preservation and oxygenative function postimplantation for as long as 7 days.21 Recellularization was performed with mixtures of whole-lung cell isolates in four cases,11,14,15,21 but one group used a homogeneous mouse embryonic stem cell population,13 which showed that the matrix was capable of promoting site-appropriate differentiation without any other specific differentiation cues.
The liver
Liver decellularization has been performed by antegrade perfusion via the portal vein with SDS and Triton X-100, either alone or in combination,16,17,23,24 although another study used trypsin/EGTA,25 and Baptista et al. used Triton X-100 in combination with ammonium hydroxide.18,22 Decellularization was demonstrated with histology and evidence of DNA removal, and retention of key ECM constituents with preservation of microvasculature and ECM ultrastructure. Hepatocytes were seeded onto the acellular liver scaffold via portal vein perfusion and were shown to be functional in four studies17,22,24,25 by showing evidence of synthesis of lactate dehydrogenase, albumin, and urea after heterotopic implantation. In particular, two studies attempt to scale-up this approach to further approximate human-scale engineered organ constructs by use of ferret22 and porcine24 livers, and both studies seeded the bioscaffolds with human liver cells, which were shown to be compatible on these xenogeneic scaffolds.
The kidney
Several groups have decellularized whole kidneys by the perfusion method with preservation of the vascular network and complete cell removal.18–20 Decellularizing agents vary from 1% Triton or SDS18,19 to 3% Triton with DNase and additional 4% SDS.20 The preservation and presence of ECM constituents such as laminin and collagen IV were also demonstrated, as well as collagen I, fibronectin, and heparin sulfate in a separate study, which did not employ perfusion.83 The latter study juxtaposed fetal cells in fresh renal explants with the ECM scaffolds by layering fetal kidney tissue against the ECM, which supported developmental renal phenotypes in these cells after they migrated into the ECM. In the study by Ross et al., decellularized rat kidneys scaffolds were reseeded with murine embryonic stem cells, and the renal ECM was shown to support the renal differentiation of the embryonic stem cells.
Other tissues/organs
The technique of whole-organ decellularization is also expanding into other tissues and organs such as the small bowel and skeletal muscle, especially as it has the potential to allow the development of a viable replacement in the absence of any current clinical alternative, for example, intestinal failure and large-muscle defects due to trauma. Totonelli et al. have demonstrated the application of this technique to small bowel26 and shown the viability of amniotic fetal stem cells seeded onto the decellularized scaffold and its angiogenic properties, although perfusion recellularization was not performed. Perniconi et al. have decellularized whole skeletal muscles derived from rats by an incubation method, and further have implanted the acellular scaffolds in vivo within an equivalent muscle defect to show infiltration of inflammatory and stem cells within the scaffolds.27
Common trends seen within published protocols
The main parameters of interest for the perfusion decellularization and recellularization protocols are compared and summarized in Tables 1a–d and 2a and b, respectively.
Table 1a.
Summary of Species and Strain Used for Decellularization
| Organ | Authors | Species (strain) |
|---|---|---|
| Heart | Ott et al., 2008 | Rat (Fischer 344) |
| Heart | Wainwright et al., 2010 | Pig |
| Lung | Ott et al., 2010 | Rat (Sprague Dawley) |
| Lung | Petersen et al., 2010 | Rat (Fischer 344) |
| Lung | Cortiella et al., 2010 | Rat |
| Lung | Price et al., 2010 | Mouse (C57BL/6) |
| Lung | Song et al., 2011 | Rat (Sprague-Dawley) |
| Liver | Uygun et al., 2010 | Rat (Lewis) |
| Liver | Shupe et al., 2010 | Rat (Fischer 344) |
| Liver | Soto-Gutierrez et al., 2011 | Rat (Sprague-Dawley) |
| Liver | Baptista et al., 2011 | Ferret |
| Liver | De Kock et al., 2011 | Rat (Sprague-Dawley) |
| Liver | Barakat et al., 2011 | Pig (Yorkshire) |
| Kidney | Ross et al., 2009 | Rat (Sprague-Dawley) |
| Kidney | Liu et al., 2009 | Rat (Wistar) |
| Liver, Kidney, Pancreas, Small bowel | Baptista et al., 2009 | Ferret liver Pig kidney/pancreas/small bowel |
| Small bowel | Totonelli et al., 2012 | Rat (Sprague-Dawley) |
| Skeletal muscle | Perniconi et al., 2012 | Mouse (BALB/C) |
Table 1d.
Summary of Decellularized Whole-Organ Scaffold Characterization and Techniques
| Organ | Authors | Histology/IHC | EM | Quantitative assay for DNA/ECM components | Vascular characterization | Mechanical testing |
|---|---|---|---|---|---|---|
| Heart | Ott et al., 2008 | +/+ | + | - | Corrosion casting + implantation | + |
| Heart | Wainwright et al., 2010 | +/+ | + | + | - | + |
| Lung | Ott et al., 2010 | +/− | + | - | - | + |
| Lung | Petersen et al., 2010 | +/+ | + | - | Micro-CT | + |
| Lung | Cortiella et al., 2010 | −/− (IF) | - | (Gel electrophoresis for DNA) | - | - |
| Lung | Song et al., 2011 | - | - | - | - | - |
| Lung | Price et al., 2010 | +/+ | + | + | - | + |
| Liver | Uygun et al., 2010 | +/+ | + | + | Corrosion casting | - |
| Liver | Shupe et al., 2010 | +/+ | - | - | - | |
| Liver | Soto-Gutierrez et al., 2011 | +/+ | + | (Gel electrophoresis for DNA) | Corrosion casting | - |
| Liver | Baptista et al., 2011 | +/+ | + | + | Fluoroscopy + confocal microscopy | - |
| Liver | De Kock et al., 2011 | +/+ | + | - | Corrosion casting | - |
| Liver | Barakat et al., 2011 | +/+ | - | - | Corrosion casting + fluoroscopy | |
| Kidney | Ross et al., 2009 | +/+ | + | - | - | - |
| Kidney | Liu et al., 2009 | +/− (IF) | + | - | - | - |
| Liver, Kidney, Pancreas, Small bowel | Baptista et al., 2009 | +/− | - | - | Fluoroscopy | - |
| Small bowel | Totonelli et al., 2012 | +/+ | + | + | - | + |
| Skeletal muscle | Perniconi et al., 2012 | +/+ | - | - | - | - |
IHC, immunohistochemistry; EM, electron microscopy; IF, immunofluorescence; ECM, extracellular matrix.
Table 2a.
Summary of Conditions Used for Whole-Organ Scaffold Recellularization
| Organ | Authors | Cell population(s) | Cell numbers (million) | Seeding route (bolus injection/perfusion/other) | Perfusion flow rate/pressure |
|---|---|---|---|---|---|
| Heart | Ott et al., 2008 | Rat cardiocytes | 50–75 | Intramural injection | 6 mL/min |
| Lung | Ott et al., 2010 | Human carcinomatous alveolar cells | 91.25±31.72 | Tracheal perfusion | 10–15 mm Hg |
| Lung | Petersen et al., 2010 | Rat lung epithelial cells | 100 | Airway injection | 1–5 mL/min |
| Lung | Cortiella et al., 2010 | Murine ESCs | 2 | Tracheal injection | (Nonperfusion) |
| Lung | Price et al., 2010 | Murine fetal lung cells | 3 | Infusion in culture medium | (Nonperfusion) |
| Lung | Song et al., 2011 | Rat fetal lung cells | Not stated | Tracheal perfusion | 10–15 mm Hg |
| Liver | Uygun et al., 2010 | Rat hepatocytes | 50 | Bolus intravascular injection×4 | Not stated |
| Liver | Shupe et al., 2010 | Rat liver progenitor cells | 1 | Inferior vena caval injection | Not perfused or cultured |
| Liver | Soto-Gutierrez et al., 2011 | Murine hepatocytes | 10–50 | Intraparenchymal injection vs. steady perfusion vs. bolus injection | 2 mL/min |
| Liver | Baptista et al., 2011 | Human fetal liver cells | 70 | Portal vein perfusion | 3 mL/min |
| Liver | Barakat et al., 2011 | Human fetal stellate cells + human fetal hepatocytes | 350+1000 | Portal vein perfusion | 90 mL/min |
| Kidney |
Ross et al., 2009 |
Murine ESCs |
2 |
Arterial + ureteric injection |
(Nonperfusion) |
| Liver, Kidney, Pancreas, Small bowel | Baptista et al., 2009 | Human HepG2 cells | 30 | Intravascular injection | 6 mL/min |
ESC, embryonic stem cell.
Table 2b.
Summary of Recellularized Organ Construct Characterization and Techniques
| Organ | Authors | Histology/IF | TUNEL | EM | Functional assessment | In vivo implantation |
|---|---|---|---|---|---|---|
| Heart | Ott et al., 2008 | +/+ | + | + | LV pressure monitoring + live video (contractility) | − |
| Lung | Ott et al., 2010 | +/+ | − | + | Dynamic lung fn tests, blood gases, live video | + |
| Lung | Petersen et al., 2010 | +/+ | + | + | Compliance testing, blood gases | + |
| Lung | Cortiella et al., 2010 | −/+ | + | − | − | − |
| Lung | Price et al., 2010 | −/+ | − | − | − | − |
| Liver | Uygun et al., 2010 | +/+ | + | + | LDH/urea/albumin assay RT-PCR of enzymes |
+ |
| Liver | Shupe et al., 2010 | +/− | − | − | − | − |
| Liver | Soto-Gutierrez et al., 2011 | +/+ | − | − | Albumin/cytochrome/ammonia assay | − |
| Liver | Baptista et al., 2011 | +/+ | + | − | Urea/albumin/prostacylin assays Platelet deposition studies |
− |
| Liver | Barakat et al., 2011 | +/−(IHC) | + | − | Urea/albumin/lactate assays | + |
| Kidney | Ross et al., 2009 | +/−(IHC) | − | − | − | − |
| Liver, Kidney, Pancreas, Small bowel | Baptista et al., 2009 | +/−(IHC) | − | − | − | − |
LV, left ventricle; LDH, lactate dehydrogenase; RT-PCR, reverse transcriptase–polymerase chain reaction.
Table 1a shows the different animal species and strains used, with the most common being rat, although there is an intentional move toward larger species to scale-up the technique to approach more human-sized organ constructs.
Table 1b displays the most common decellularizing agents used, with the predominance of SDS whether alone or in combination with Triton X-100. When another agent is quoted, there is a far wider disparity between protocols that almost vary from article to article (7 out of 18 in total). There are also other marked differences in protocols such as the additional use of physical methods (freezing) and whether DNAse or RNAse is also employed in combination with the main decellularizing agent (full details in Appendix Tables A1 and A2).
Table 1b.
Summary of Decellularization Agents Used
| Organ | Authors | SDS | SDS + Triton | Other |
|---|---|---|---|---|
| Heart | Ott et al., 2008 | (+) | + | − |
| Heart | Wainwright et al., 2010 | − | − | + |
| Lung | Ott et al., 2010 | (+) | + | − |
| Lung | Petersen et al., 2010 | − | − | + |
| Lung | Cortiella et al., 2010 | + | − | − |
| Lung | Price et al., 2010 | − | − | + |
| Lung | Song et al., 2011 | (+) | + | − |
| Liver | Uygun et al., 2010 | (+) | + | − |
| Liver | Shupe et al., 2010 | (+) | + | − |
| Liver | Soto-Gutierrez et al., 2011 | − | − | + |
| Liver | Baptista et al., 2011 | − | − | + |
| Liver | De Kock et al., 2011 | (+) | + | − |
| Liver | Barakat et al., 2011 | + | − | − |
| Kidney | Ross et al., 2009 | (+) | + | − |
| Kidney | Liu et al., 2009 | (+) | + | − |
| Liver, Kidney, Pancreas, Small bowel | Baptista et al., 2009 | − | − | + |
| Small bowel | Totonelli et al., 2012 | − | − | + |
| Skeletal muscle | Perniconi et al., 2012 | + | − | − |
SDS, sodium dodecyl sulfate.
Table 1c summarizes the decellularization pump setup parameters, that is, flow rate (or pressure) and duration of decellularization (for the entire protocol, including periods of nonperfusion if relevant). While flow rates vary widely depending on the animal species, generally the rates adopted tend to be low and subphysiological vascular flow rates, while flow pressures tend to mimic physiological in situ ones. The total duration of the decellularization protocol gives an indication of how widely varying the protocols can be from group to group, whether in terms of the additional steps/methods used, for example, freezing, storage phases within the decellularization process, and the flow rates and concentrations applied with even the same decellularizing agents. The most marked contrast may be seen between De Kock et al. and Uygun et al. in work on the liver bioscaffold (1–2 h vs. up to 5 days), especially as the former quote directly from the latter as a baseline for developing their more streamlined protocol.
Table 1c.
Summary of Conditions Used for Perfusion Decellularization
| Organ | Authors | Flow rate | Perfusion pressure | Duration of decellularization (perfusion) |
|---|---|---|---|---|
| Heart | Ott et al., 2008 | - | 77.5 mm Hg | 12–13 h |
| Heart | Wainwright et al., 2010 | 1.3L/min | - | 6–7 h |
| Lung | Ott et al., 2010 | - | 30 mm Hg | 2–3 h |
| Lung | Petersen et al., 2010 | - | <20 mm Hg | (Total 500 mL perfused) |
| Lung | Cortiella et al., 2010 | N/A | N/A | N/A |
| Lung | Price et al., 2010 | N/A | N/A | N/A |
| Lung | Song et al., 2011 | - | 80 cm H2O | 2–3 h |
| Liver | Uygun et al., 2010 | 1 mL/min | - | 5 days |
| Liver | Shupe et al., 2010 | 5 mL/min | - | (Total 300 mL×4 perfused) |
| Liver | Soto-Gutierrez et al., 2011 | 8 mL/min | - | 20–26 h |
| Liver | Baptista et al., 2011 | 5 mL/min | - | (Total 40×+ 50×volume of the liver) |
| Liver | De Kock et al., 2011 | 30 mL/min | - | 1–2 h |
| Liver | Barakat et al., 2011 | - | 80 mm Hg | 2–3 days |
| Kidney | Ross et al., 2009 | - | 100 mm Hg | (Not stated) |
| Kidney | Liu et al., 2009 | - | 100 cm H2O | 12–13 h |
| Liver, Kidney, Pancreas, Small bowel | Baptista et al., 2009 | 10–60 mL/min | - | 24 h |
| Small bowel | Totonelli et al., 2012 | 0.6 mL/h | - | 31 h (per DET cycle) |
| Skeletal muscle | Perniconi et al., 2012 | N/A | N/A | 24–48 h |
Table 1d summarizes the characterization of the decellularized organ scaffold and the techniques used; there is a common panel of tests to demonstrate a variety of important structural properties preserved in the ECM that are necessary for both the effectiveness of the bioscaffold for subsequent recellularization and the decellularization process itself. As can be seen, groups vary in how much of this panel of tests are completed. Not shown are any functional tests of ECM bioactivity, as this is limited so far to single growth factor assays and in two groups only.23,25
Table 2a summarizes the four main parameters in the recellularization protocols: cell population, cell numbers, route of seeding, and perfusion rate. The bioreactor conditions tend to be similar, that is, incubator conditions, and duration of continuous culture is on average 7 days (with one group moving onto a longer period21), and hence these parameters have been omitted from the table. Full details can be found in Appendix Table A2. Cell populations used have been commonly the mixed population-native cells isolated from fetal or neonatal organs as the initial proof of concept, but work is now moving onto human cell populations and stem cell populations to demonstrate biocompatibility and the bioinductive properties of the ECM scaffold. Cell numbers are by necessity high, for example, 50–100 million on rat-derived scaffolds, but exponentially higher on larger species; however, this is usually still only 10%–20% of normal physiological cell numbers. The route of seeding can depend on the physiological compartments available (other than the vascular), for example, the airway, and also direct injection is possible, but most researchers utilize the vascular route. There has been some comparison between a continuously perfused seeding process and a bolus intravascular injection, with some evidence that the latter affords higher engraftment.17
Table 2b displays those techniques for characterization of the recellularized organ constructs (where applicable), and these are usually a combination of histology-based techniques and functional testing specific to the organ/tissue in question.
Summary
In terms of decellularization protocols, these vary widely in many aspects, for example, strain/species of animal used, decellularization agents and other parameters (plus postdecellularization sterilization) to achieve the whole-organ ECM scaffold required for recellularization. On one hand, this is an indication of the universal applicability of the principle of perfusion decellularization to all animal tissues, and while variations in the optimal protocol can also be attributed to differences in the nature of each type of organ/tissue and its specific structure and composition, it also appears that a complete and systematic rationale for determining the optimal perfusion decellularization process has not yet been achieved. Some studies have started to formally assess achieved of decellularization protocols, for example, scaffold properties with varying number of decellularization cycles,26 while only one study reports direct optimization from a baseline protocol taken from another study.23 Characterization of the structural components of the ECM has yielded similar methods and results across most of the groups, but only two groups have attempted to assess the presence of the (presumed) bioinductive molecules present in the ECM with a quantification assay of two specific growth factors.23,25 This is an area that requires further investigation in all the organs/tissues of relevance, as regulatory and standardization issues (e.g., quality control and release criteria) are fundamental to the clinical translation and commercialization of these ECM products. ECM products are known to vary from batch to batch (even animal to animal), and hence standardization of basic parameters (species/strain, gender, age, and weight) needs to be maintained to achieve meaningful comparison in outcomes, or from group to group. These donor factors, and others relating to the use of biological agents within certain protocols, may require a degree of dynamicity within the decellularization process, but currently the extreme disparity between some protocols suggests the need for a more evidence-based approach. Preoptimization and standard operating procedures are even more critical when it is likely that postprocessing testing of the ECM bioscaffold (and the recellularized construct) is likely to be limited in scope in those cases destined for implantation. Minimum standardized biological requirements within whole-organ ECM scaffolds (e.g., threshold DNA levels and structural integrity growth factor levels/activity) should also be established to maintain biological quality and clinical safety.
Regarding the recellularization process, most groups use a similar anatomical approach to the vascular perfusion setup for circulation of medium during continuous culture and also use appropriate physiological adjuncts to help stimulate constructive remodeling (e.g., in the heart and lung models). Most groups have investigated the use of a mixed population of organ-specific cells isolated from neonatal or fetal animals to seed the acellular ECM scaffold as proof of concept and also to achieve the full extent of cellular variety and density required to populate a whole-organ scaffold. Several groups have also attempted reseeding with embryonic stem cells, which have demonstrated proliferation and differentiation specific to organ-specific bioinductive cues within the ECM scaffold. In addition, human cell populations have also been seeded onto the xenogeneic scaffolds to demonstrate biocompatibility. Again, there are quite widely varying parameters for the recellularization protocol between groups in terms of cell numbers and culture conditions, and this is an area where further systematic optimization is required. A physiological degree of cellular engraftment has not yet been achieved with any group, partly because of the very large cell numbers required. Finally, functional assessment of recellularized constructs depends on the degree of recellularization achieved and varies from organ to organ and whether the graft is suitable for implantation in vivo. Generally, only very short-term viability and implantation in vivo have been achieved so far (e.g., 6 h), but one study has expanded this into a 7-day implantation period after improving graft preservation.21
Appendix Tables A1 and A2 summarize further details of the decellularization and recellularization protocols used in the work published from the groups above.
Future Work and Considerations: Surgical Implantation of Bioartificial Organ Constructs
It has been shown so far that whole-organ decellularization can yield whole-organ ECM bioscaffolds capable of supporting recellularization on a complex 3D level and sustaining appropriate cellular and tissue functionality. These bioartificial organ constructs also allow relatively easy surgical manipulation, especially as they preserve the intact vascular pedicle for direct anastomosis at the orthotopic implantation site. The intact and inherent vasculature permits direct perfusion of the tissue and maintains provision of nutrients and oxygen to the implanted graft, in an exact parallel to the transplant procedure. As such, this technique could provide an ideal shortcut to bioengineering an organ replacement that is structurally and histologically similar to the original organ in question, uses established surgical procedures for implantation, is able to potentially support cellular function to the same degree, is biologically compatible, and comes from a widely available source (e.g., porcine or other xenogeneic organs).7
Currently, there are limitations to all the tissue-engineered organ constructs produced so far in terms of functionality and viability in vivo, varying from achieving sufficient or comparable to normal cellular density, achieving full cellular and tissue differentiation, and assessment of long-term viability, functionality, and constructive remodeling of organ grafts implanted in vivo. It would also be necessary to expand these techniques on larger-animal organs, which are closer in size and functionality to human ones. These are issues to be addressed and optimized before work can proceed to the clinical stage, in conjunction with the regulatory issues that guide cell choice (such as those as by the U.S. Food and Drug Administration regarding the use of autologous human cells applied in a nonhomologous location, or the use of allogeneic cells in virtually all cases), as well as a common language for reporting outcomes in trials.
In addition, it would be important to investigate the possibility of organ regeneration from an alternative cell source such as a stem cell population. Possibilities for this may be embryonic stem cells as has been tried in the lung or mesenchymal stem cells, which can differentiate into functional nephrons after injection into the nephrogenic site of developing rat embryos,84 and are furthermore involved in the repair and recovery of renal function after acute renal injury.85,86 If eventually autologous cells such as mesenchymal stem cells from the recipient alone could be used to create a functional organ graft, there could be significant clinical implications in overcoming the immune barrier and problem of rejection, with further great potential benefits to the patient.
Appendix Table A1.
Summary of the Decellularization Protocols Used in the Literature
| |
Decellularization protocol |
||||||
|---|---|---|---|---|---|---|---|
| Organ | Authors |
Tissue harvesting (species/strain/age, explantation details) |
Perfusion type | Agent(s) + duration | Flow rate/pressure | Sterilization | Characterization of decellularised scaffold |
| Heart | Ott et al., 2008 | 12-week-old Fischer 344 rats Systemic heparinization |
Retrograde coronary perfusion via ascending aorta | Heparinized PBS + 10 μM adenosine for 15 mins + 1% SDS for 12 h + 1% Triton X-100 for 30 mins | 77.4 mm Hg coronary arterial pressure | PBS + 100 μ/mL pen-strep for 124 h | Histology + immunofluorescence of ECM–collagen I + III, laminin, fibronectin Aortic valve–Evans blue perfusion SEM/TEM of ECM architecture Vascular corrosion resin casting + heterotopic transplantation Mechanical (stress-strain/tangential modulus) testing |
| Heart | Wainwright et al., 2010 | Adult pigs (strain not specified) | Retrograde coronary perfusion via ascending aorta | Frozen at −80°C for 16 h then thawed at room temperature (RT) + hypotonic type 1 water for 15 mins + 0.02% trypsin/0.05% EDTA/0.05% NaN3 for 2 h + 3% Triton X-100/0.05% EDTA/0.05% NaN3 for 2 h + 4% deoxycholic acid for 2 h | 1–1.3 L/min | 0.1% peracetic acid/4% ethanol for 4 h at 1.7 L/min | Histology + IHC/immunofluorescence of ECM–DAPI, elastin, collagens (I, III, IV), GAGs DNA/GAG/elastin quantitative assays SEM of ECM architecture Mechanical (ball burst) testing |
| Lung | Ott et al., 2010 | 12-week-old male Sprague-Dawley rats Systemic heparinization |
Retrograde pulmonary arterial perfusion | Heparinized PBS for 15 mins + 0.1% SDS for 120 mins + 1% Triton-X100 for 10 mins | 30 mm Hg pulmonary arterial pressure | PBS + 100u/mL pen-strep for 72 h | Histology of ECM–collagen, proteoglycans, elastin + Morphometry and stereology of alveoli TEM of ECM alveolar architecture Dynamic lung testing–compliance + vital capacity |
| Lung | Petersen et al., 2010 | 3-month-old Fischer 344 rats Intraperitoneal heparin |
Antegrade perfusion via right ventricle | PBS + 50 μ/mL + 1 μg/mL SNP + 8 mM CHAPS/1M NaCl/25 mM EDTA in PBS until total 500 mL of fluid perfused + 90 μ/mL benzonase | <20 mm Hg pulmonary arterial pressure | PBS + 10% pen-strep (duration not stated) | Histology + immunofluorescence of ECM–elastin, collagen I + IV, laminin, fibronectin DNA/collagen/GAG/elastin quantitative assays SEM/TEM of ECM architecture Micro-CT of vascular + alveolar architecture Mechanical (stress–strain/compliance) testing |
| Lung | Cortiella et al., 2010 | Rats (age/strain not specified) | Mechanical agitation within bioreactor + initial freezing process | Frozen at −70°C for then thawed at 40°C /flash frozen×4 times + 1% SDS with continuous circulation for 5 weeks + DNAse/RNAse | (2.5 rpm rotational speed within bioreactor) | PBS + pen-strep + amphotericin for 24 h | Immunofluorescence—DAPI, MHC-1 Gel electrophoresis of DNA content |
| Lung | Price et al., 2010 | 2–3-month-old C57BL/6 female mice | Injection via right ventricle + trachea | 0.1% Triton X-100 2% sodium deoxycholate DNase Repeated injection/incubation steps—not continuous perfusion |
N/A | All solutions sterile with added pen-strep | Histology + immunofluorescence of ECM–collagen, elastin, DAPI, laminin Collagen/GAG/laminin/elastin quantitative assays SEM of ECM architecture Pulmonary function tests |
| Lung | Song et al., 2011 | Male Sprague-Dawley rats (260–280 g) Systemic heparinization |
Antegrade pulmonary arterial perfusion | Heparinized PBS for 15 mins + 0.1% SDS for 120 mins + 1% Triton-X100 for 10 mins | 80 cm H2O | PBS + 100u/mL pen-strep for 72 h | (Not performed) |
| Liver | Uygun et al., 2010 | Female adult (150–200 g) Lewis rats Intracardiac heparin |
Antegrade perfusion via portal vein | Initial 20 mL 0.9% saline then frozen at −80°C for 4 h then thawed at 4°C + PBS at 1 mL/min overnight + 0.01% SDS for 72 h + 0.1% SDS for 24 h + 1% SDS for 24 h + 1% Triton-X100 for 30 mins | 1 mL/min | PBS + 0.1% peracetic acid for 3 h | Histology + immunofluorescence of ECM–collagen I + IV, laminin, fibronectin DNA/collagen/GAG quantitative assays SEM of ECM architecture Vascular corrosion resin casting |
| Liver | Shupe et al., 2010 | Adult Fischer 344 rats | Retrograde perfusion via inferior vena cava | Initial 100 mL PBS + 1%, 2%, 3% Triton X-100 in sequence until 300 mL each perfused + 0.1% SDS until 300 mL perfused | 5 mL/min | 300 mL PBS | Histology + immunofluorescence of ECM–collagen IV, laminin, DAPI |
| Liver | Soto-Gutierrez et al., 2011 | Male adult (250–300 g) Sprague-Dawley rats Systemic heparinization |
Retrograde perfusion via inferior vena cava | Initial 5–10 mL PBS/heparin, and then frozen at −80°C for 24 h then thawed at RT + 0.02% trypsin/0.05% EGTA for 2 h + 3% Triton X-100/0.05% EGTA for 18–24 h | 8 mL/min | 2MRad gamma irradiation | Histology + immunohistochemistry (IHC) of ECM–collagen IV, laminin, fibronectin, DAPI Gel electrophoresis of DNA content SEM/TEM of ECM architecture Vascular corrosion resin casting |
| Liver | Baptista et al., 2011 | Ferret (age/strain/gender not specified) | Antegrade perfusion via portal vein | Initial dH2O (40×volume of liver) + 1% Triton X-100/0.1% NH4OH (50×volume of liver) | 5 mL/min | Not stated | IHC + Western blotting—collagen I/III/IV, laminin, fibronectin DNA/collagen/GAG quantitative assays SEM of ECM architecture Fluoroscopy + confocal microscopy of vasculature |
| Liver | De Kock et al., 2011 | Male adult (250–300 g) Sprague-Dawley rats Systemic heparinization |
Antegrade perfusion via portal vein | Initial Krebs-Henseleit buffer for 15 mins + 1% Triton X-100 for 30 mins + 1% SDS for 30 mins | 30 mL/min | N/A | Histology + IHC of ECM–collagen I + IV, laminin, fibronectin, VEGF SEM of ECM architecture Vascular corrosion resin casting |
| Liver | Barakat et al., 2011 | Yorkshire swine (25–35 kg) | Antegrade perfusion via portal vein | Initial 6–10 L dH2O + 20 L 0.25% SDS (store in 0.25% SDS for 48 h) + 40 L 0.5% SDS over 2–3 h + 20 L dH2O + 10% formalin + 40 L PBS | 80 mm Hg | 10% formalin | Histology + IHC of ECM–collagen I + IV, laminin, fibronectin Vascular corrosion resin casting + fluoroscopy |
| Kidney | Ross et al., 2009 | Male adult (250–300 g) Sprague-Dawley rats Systemic heparinization |
Antegrade perfusion via renal artery | Initially saline + SNP + 3% Triton-X100, DNAse + 3% Triton-X100 + 4% SDS (durations not stated) |
100 mm Hg | Not stated | Histology + IHC of ECM–collagen IV, laminin SEM of ECM architecture |
| Kidney | Liu et al., 2009 | 12-week (180–220 g) Wistar rats | Antegrade perfusion via renal artery | Initial PBS/heparin for 15 mins + 1%SDS for 12 h + 1% Triton-X100 for 30 mins | 100 cm H2O | PBS + pen-strep for 48 h | Histology + immunofluorescence of ECM–DAPI SEM of ECM architecture |
| Liver, Kidney, Pancreas, Small bowel | Baptista et al., 2009 | Ferret liver Pig kidney/pancreas/small bowel |
Not stated | 1% Triton X-100/0.1% Ammonium hydroxide for 24 h or until translucent | 10–60 mL/min | Not stated | Histology Fluoroscopy of vasculature |
| Small bowel | Totonelli et al., 2012 | Adult (320–350 g) Sprague-Dawley rats | Antegrade perfusion via superior mesenteric artery | Detergent–enzymatic treatment (DET): Deionized water for 24 h + 0.4% sodium deoxycholate for 4 h + 2000 kU DNAse for 3 h (1–4 cycles) |
0.6 mL/h | N/A | Histology + IHC of ECM–MHC-II, SMA, vimentin, MNF116 DNA/collagen/GAG quantitative assays SEM/TEM of ECM architecture Mechanical testing—stress/strain |
| Skeletal muscle | Perniconi et al., 2012 | Adult BALB/C mice | (Incubation/rotation method) | 1% SDS for 24 or 48 h | (N/A) | PBS washes | Histology + immunofluorescence of ECM–actin, laminin, fibronectin |
PBS, phosphate-buffered saline; ECM, extracellular matrix; SDS, sodium dodecyl sulfate; SNP, sodium nitroprusside; SEM, scanning electron microscopy; TEM, transmission electron microscopy; N/A, not applicable; GAG, glycosaminoglycan; VEGF, vascular endothelial growth factor.
Appendix Table A2.
Summary of the Recellularization Protocols Used in the Literature
| |
Recellularization protocol |
||||
|---|---|---|---|---|---|
| Organ | Authors | Bioreactor setup/special conditions (in addition to vascular perfusion) | Cell population/numbers + reseeding | Culture conditions | Characterization of organ construct and functionality |
| Heart | Ott et al., 2008 | Cannulation of left atrium and ascending aorta–pulsatile distension of left ventricle + compliance loop attached to ascending aorta to reproduce physiological preload, after-load and intraventricular pressure. Coordinated electrical stimulation at 5–20 V via electrodes |
50–75 million rat neonatal cardiocytes isolated and seeded via intramural injection + 2 million rat aortic endothelial cells via perfusion | Culture medium (CM) perfused at atrial flow rate (FR) 20 mL/min, coronary FR 6 mL/min 37°C 5% CO2 incubator Duration of culture: 4–8 days |
Histology (engraftment) + immunofluorescence—TUNEL, α-actin, MHC, vWF, connexin-43 TEM of ultra-architecture Perfusion with CMFDA to demonstrate endothelialization LV pressure monitoring + live video to demonstrate contractile activity |
| Heart | Wainwright et al., 2010 | (Nonperfusion culture system: lyophilized sheets of cardiac ECM used) | 500000 cells/cm2 white leghorn chicken embryonic cardiomyocytes isolated | 37°C 5% CO2 incubator Duration of culture: 4 days |
Immunofluoresence—α-actinin + β-tubulin to identify cardiomyocytes |
| Lung | Ott et al., 2010 | Perfusion system through pulmonary artery, left atrium, and trachea; pulmonary vein drained to equilibration chamber Trachea connected to gas ventilation chamber. Alternating air and fluid (culture medium) perfusion |
91.25±31.72 million carcinomatous human alveolar basal epithelial cells by gravity perfusion through trachea + 66.57±18.22 human umbilical cord venous endothelial cells (HUVECs) by gravity perfusion of pulmonary artery and vein; 308.57±146.9 million rat fetal lung cells isolated + HUVECs seeded via same method | CM perfused at 10–15 mm Hg constant pressure with negative pressure ventilation 37°C 5% CO2 incubator Duration of culture: 5–9 days |
Histology (engraftment) + immunofluorescence—caveolin-1, T1α, Ttf1, vimentin, pro-SPC TEM of ultra-architecture Immunoblotting–SP-A, SP-C Morphometry + stereology of lung architecture In vitro function testing–blood gas values, dynamic lung function tests Orthotopic transplantation into syngeneic male rats–fluoroscopy and live video, blood gas values |
| Lung | Petersen et al., 2010 | Pulmonary artery cannulation for perfusion loop Breathing loop/negative pressure ventilation via trachea cannulation + syringe pump |
100 million rat neonatal lung epithelial cells isolated and injected into airway compartment + 30 million lung vascular endothelial cells via artery | CM perfused at 1–5 mL/min with negative pressure ventilation at 1 breath/min 37°C 5% CO2 incubator Duration of culture: 4–8 days |
Histology (engraftment, TUNEL) + immunofluorescence—CD-31, CCSP, pro-SPC, aquaporin-5, α-actin, Cy-14. TEM of ultra-architecture Stress-strain + compliance testing Orthotopic transplantation into syngeneic male Fischer 344 rats + blood gas values tested to demonstrate gas exchange |
| Lung | Cortiella et al., 2010 | (Nonperfusion bioreactor: rotating chamber system) | 2 million murine embryonic stem cells (ESCs) injected into trachea (I million cells into each side/lung scaffold) | CM circulated by pump action at speed of 2 rpm 37°C 5% CO2 incubator Duration of culture: 14–21 days |
Immunofluorescence—DAPI, live/dead staining, TUNEL, cytokeratin 18, CD31, pro-SPC, laminin, collagen IV |
| Lung | Price et al., 2010 | (Nonperfusion bioreactor: scaffolds suspended in CM flasks) Tracheal cannulation for ventilation loop |
3 million fetal lung cells isolated from day E17 gestation B6 mice infused into CM | Mechanical ventilation at 180 breaths/min (300 μL volume) 37°C 5% CO2 incubator Duration of culture: 7 days |
Immunofluorescence—DAPI, cytokeratin 18, CD31, pro-SPC, aquaporin-5, CCSP, CD45, CD11b, vimentin |
| Lung | Song et al., 2011 | Perfusion system through pulmonary artery, left atrium, and trachea; pulmonary vein drained to equilibration chamber Trachea connected to gas ventilation chamber Alternating air and fluid (culture medium) perfusion |
Rat fetal lung cells + HUVECs (numbers not stated) | CM perfused at 1–5 mL/min with negative pressure ventilation at 1 breath/min 37°C 5% CO2 incubator Duration of culture: 7–10 days |
Histology + IHC–TTF-1, pro-SPC, CC10, CD31, vimentin Morphometry + stereology of lung architecture In vitro function testing—blood gas values, dynamic lung function tests Orthotopic transplantation into syngeneic male rats up to 14 days–fluoroscopy and live video, blood gas values |
| Liver | Uygun et al., 2010 | Portal vein cannulation for perfusion loop | 50 million rat hepatocytes isolated and seeded via bolus intravascular injection×4 + 40 million cardiac endothelial cells via perfusion | Perfusion FR not stated 37°C 5% CO2 incubator Duration of culture: 1–5 days |
Histology (engraftment) + immunofluorescence—TUNEL, albumin, G6pc, Ugt1a LDH release quantification Urea + albumin synthesis quantification RT-PCR of drug metabolism enzymes Heterotopic transplantation into syngeneic rats + fresh blood perfusion model to test viability and hepatocyte function |
| Liver | Shupe et al., 2010 | Inferior vena cava (IVC) cannulation, but no continuous perfusion culture performed | 1 million rat liver progenitor cells WB344 via IVC injection | - | Histology |
| Liver | Soto-Gutierrez et al., 2011 | Portal vein cannulation for perfusion loop | 10–50 million murine adult hepatocytes injected directly into parenchyma vs. perfusion in CM at 2 mL/min vs. perfused in multiple boluses | Perfusion FR 2 mL/min 37°C 5% CO2 incubator Duration of culture: 7 days |
Histology (engraftment) + immunofluorescence—DAPI, albumin, Ki67 Albumin synthesis/cytochrome activity/ammonia metabolism quantification assays |
| Liver | Baptista et al., 2011 | Portal vein cannulation for perfusion loop | 70 million human fetal liver cells + 30 million HUVECs coinfusion via portal vein | Perfusion FR 3 mL/min for seeding×16 h; Perfusion FR 0.5 mL/min 37°C 5% CO2 incubator Duration of culture: 7 days |
Histology + immunofluorescence—TUNEL, Ki-67 Urea/albumin/prostacylin synthesis quantification Platelet deposition studies |
| Liver | De Kock et al., 2011 | N/A | N/A | N/A | N/A |
| Liver | Barakat et al., 2011 | Posterior segments only (x3) Portal vein cannulation for perfusion loop |
350 million human fetal stellate cells + 1 billion human fetal hepatocytes via portal vein | Perfusion FR 90 mL/min 37°C 5% CO2 incubator Duration of culture: 3–13 days |
Histology + IHC–CK-18, CK-19, AFP, CYP3A4, TUNEL, Ki-67 Urea/albumin/lactate synthesis quantification Orthotopic transplantation - histology |
| Kidney | Ross et al., 2009 | Renal artery cannulation for perfusion loop, also cannulation of ureter | 2 million murine pluripotent ES cells injected via artery or ureter | Sections of seeded graft cultured for 3–10 days 37°C 5% CO2 incubator Perfusion of whole organ at 120/80 mm Hg for 6–10 days |
Histology (engraftment + morphology) + IHC—pancytokeratin, Pax-2, Ksp-cadherin, KI-67 RT-PCR of Pax-2 + Ksp-cadherin expression |
| Kidney | Liu et al., 2009 | (N/A) | (N/A) | (N/A) | (N/A) |
| Liver, Kidney, Pancreas, Small bowel | Baptista et al., 2009 | Recellularization performed on ferret liver via portal vein cannulation and perfusion | 30 million human HepG2 cells + 30 million mouse endothelial cells injected intravascularly | Perfusion FR 6 mL/min 37°C 5% CO2 incubator Duration of culture: 7 days |
Histology IHC–albumin, vWF, KI-67 |
| Small bowel | Totonelli et al., 2012 | N/A | N/A | N/A | N/A |
| Skeletal muscle | Perniconi et al., 2012 | N/A | N/A | N/A | N/A |
RT-PCR, reverse transcriptase–polymerase chain reaction; LDH, lactate dehydrogenase.
Acknowledgments
With warm thanks to Prof Molly M Stevens for her advice and guidance with the text. In addition, M He is supported by the Royal College of Surgeons of England Research Fellowship program; A Callanan is supported by the Irish Research Council for Science, Engineering and Technology (IRCSET), and Marie Curie International Mobility Fellowship cofund grant (PD/2010/INSP/1948).
Disclosure Statement
No competing financial interests exist.
References
- 1.Bird S.M. Harris J. Time to move to presumed consent for organ donation. BMJ. 2010;340:2188. doi: 10.1136/bmj.c2188. [DOI] [PubMed] [Google Scholar]
- 2.Place E.S. Evans N.D. Stevens M.M. Complexity in biomaterials for tissue engineering. Nat Mater. 2009;8:457. doi: 10.1038/nmat2441. [DOI] [PubMed] [Google Scholar]
- 3.Crapo P.M. Gilbert T.W. Badylak S.F. An overview of tissue and whole organ decellularization processes. Biomaterials. 2011;32:32. doi: 10.1016/j.biomaterials.2011.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Badylak S.F. The extracellular matrix as a biologic scaffold material. Biomaterials. 2007;28:3587. doi: 10.1016/j.biomaterials.2007.04.043. [DOI] [PubMed] [Google Scholar]
- 5.Badylak S.F. Taylor D. Uygun K. Whole-organ tissue engineering: decellularization and recellularization of three-dimensional matrix scaffolds. Annu Rev Biomed Eng. 2011;13:27. doi: 10.1146/annurev-bioeng-071910-124743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Badylak S.F. Weiss D. J. Caplan A. Macchiarini P. Engineered whole organs and complex tissues. Lancet. 2012;379:943. doi: 10.1016/S0140-6736(12)60073-7. [DOI] [PubMed] [Google Scholar]
- 7.Taylor D.A. From stem cells and cadaveric matrix to engineered organs. Curr Opin Biotech. 2009;20:598. doi: 10.1016/j.copbio.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 8.Song J.J. Ott H.C. Organ engineering based on decellularized matrix scaffolds. Trends Mol Med. 2011;17:424. doi: 10.1016/j.molmed.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 9.Gilbert T.W. Strategies for tissue and organ decellularization. J Cell Biochem. 2012;113:2217. doi: 10.1002/jcb.24130. [DOI] [PubMed] [Google Scholar]
- 10.Ott H.C., et al. Perfusion-decellularized matrix: using nature's platform to engineer a bioartificial heart. Nat Med. 2008;14:213. doi: 10.1038/nm1684. [DOI] [PubMed] [Google Scholar]
- 11.Ott H.C., et al. Regeneration and orthotopic transplantation of a bioartificial lung. Nat Med. 2010;16:927. doi: 10.1038/nm.2193. [DOI] [PubMed] [Google Scholar]
- 12.Wainwright J.M., et al. Preparation of cardiac extracellular matrix from an intact porcine heart. Tissue Eng Part C Methods. 2010;16:525. doi: 10.1089/ten.tec.2009.0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cortiella J., et al. Influence of acellular natural lung matrix on murine embryonic stem cell differentiation and tissue formation. Tissue Eng Part A. 2010;16:2565. doi: 10.1089/ten.tea.2009.0730. [DOI] [PubMed] [Google Scholar]
- 14.Petersen T.H., et al. Tissue-engineered lungs for in vivo implantation. Science. 2010;329:538. doi: 10.1126/science.1189345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Price A.P., et al. Development of a decellularized lung bioreactor system for bioengineering the lung: the matrix reloaded. Tissue Eng Part A. 2010;16:2581. doi: 10.1089/ten.tea.2009.0659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shupe T., et al. Method for the decellularization of intact rat liver. Organogenesis. 2010;6:134. doi: 10.4161/org.6.2.11546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uygun B.E., et al. Organ reengineering through development of transplantable recellularized liver graft using decellularized liver matrix. Nat Med. 2010;16:814. doi: 10.1038/nm.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baptista P.M., et al. Whole organ decellularization - a tool for bioscaffold fabrication and organ bioengineering. Conf Proc IEEE Eng Med Biol Soc. 2009;2009:6526. doi: 10.1109/IEMBS.2009.5333145. [DOI] [PubMed] [Google Scholar]
- 19.Liu C.X., et al. [Preparation of whole-kidney acellular matrix in rats by perfusion] Nan Fang Yi Ke Da Xue Xue Bao. 2009;29:979. [PubMed] [Google Scholar]
- 20.Ross E.A., et al. Embryonic stem cells proliferate and differentiate when seeded into kidney scaffolds. J Am Soc Nephrol. 2009;20:2338. doi: 10.1681/ASN.2008111196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song J.J. Kim S.S. Liu Z. Madsen J.C. Mathisen D.J. Vacanti J.P. Ott H.C. Enhanced in vivo function of bioartificial lungs in rats. Ann Thorac Surg. 2011;92:998. doi: 10.1016/j.athoracsur.2011.05.018. [DOI] [PubMed] [Google Scholar]
- 22.Baptista P.M. Siddiqui M.M. Lozier G. Rodriguez S.R. Atala A. Soker S. The use of whole organ decellularization for the generation of a whole liver organoid. Hepatology. 2011;53:604. doi: 10.1002/hep.24067. [DOI] [PubMed] [Google Scholar]
- 23.De Kock J. Ceelen L. De Spiegelaere W. Casteleyn C. Claes P. Vanhaecke T. Rogiers V. Simple and quick method for whole-liver decellularization: a novel in vitro three-dimensional bio-engineering tool? Arch Toxicol. 2011;85:607. doi: 10.1007/s00204-011-0706-1. [DOI] [PubMed] [Google Scholar]
- 24.Barakat O. Abbasi S. Rodriguez G. Rios J. Wood R.P. Ozaki C. Holley L.S. Gauthier P.K. Use of decellularized porcine liver for engineering humanized liver organ. J Surg Res. 2012;173:e11. doi: 10.1016/j.jss.2011.09.033. [DOI] [PubMed] [Google Scholar]
- 25.Soto-Gutierrez A. Zhang L. Medberry C. Fukumitsu K. Faulk D. Jiang H. Reing J. Gramignoli R. Komori J. Ross M. Nagaya M. Lagasse E. Stolz D. Strom S.C. Fox I.J. Badylak S.F. A whole organ regenerative medicine approach for liver replacement. Tissue Eng Part C Methods. 2011;17:677. doi: 10.1089/ten.tec.2010.0698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Totonelli G. Maghsoudlou P. Garriboli M. Riegler J. Orlando G. Burns A.J. Sebire N.J. Smith V.V. Fishman J.M. Ghionzoli M. Turmaine M. Birchall M.A. Atala A. Soker S. Lythgoe M.F. Seifalian A. Pierro A. Eaton S. De Coppi P. A rat decellularized small bowel scaffold that preserves villus crypt architecture for intestinal regeneration. Biomaterials. 2012;33:3401. doi: 10.1016/j.biomaterials.2012.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perniconi B. Costa A. Aulino P. Teodori L. Adamo S. Coletti D. The pro-myogenic environment provided by whole organ scale acellular scaffolds from skeletal muscle. Biomaterials. 2011;32:7870. doi: 10.1016/j.biomaterials.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 28.Gilbert T.W., et al. Collagen fiber alignment and biaxial mechanical behavior of porcine urinary bladder derived extracellular matrix. Biomaterials. 2008;29:4775. doi: 10.1016/j.biomaterials.2008.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sacks M.S. Gloeckner D.C. Quantification of the fiber architecture and biaxial mechanical behavior of porcine intestinal submucosa. J Biomed Mater Res. 1999;46:1. doi: 10.1002/(sici)1097-4636(199907)46:1<1::aid-jbm1>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 30.Brown B., et al. The basement membrane component of biologic scaffolds derived from extracellular matrix. Tissue Eng. 2006;12:519. doi: 10.1089/ten.2006.12.519. [DOI] [PubMed] [Google Scholar]
- 31.Bissell M.J. Aggeler J. Dynamic reciprocity: how do extracellular matrix and hormones direct gene expression? Prog Clin Biol Res. 1987;249:251. [PubMed] [Google Scholar]
- 32.Kleinman H.K. Philp D. Hoffman M.P. Role of the extracellular matrix in morphogenesis. Curr Opin Biotechnol. 2003;14:526. doi: 10.1016/j.copbio.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 33.Rosso F., et al. From cell-ECM interactions to tissue engineering. J Cell Physiol. 2004;199:174. doi: 10.1002/jcp.10471. [DOI] [PubMed] [Google Scholar]
- 34.Gilbert T.W., et al. Degradation and remodeling of small intestinal submucosa in canine Achilles tendon repair. J Bone Joint Surg Am. 2007;89:621. doi: 10.2106/JBJS.E.00742. [DOI] [PubMed] [Google Scholar]
- 35.Badylak S., et al. Resorbable bioscaffold for esophageal repair in a dog model. J Pediatr Surg. 2000;35:1097. doi: 10.1053/jpsu.2000.7834. [DOI] [PubMed] [Google Scholar]
- 36.Ritchey M.L. Ribbeck M. Successful use of tunica vaginalis grafts for treatment of severe penile chordee in children. J Urol. 2003;170(4 Pt 2):1574. doi: 10.1097/01.ju.0000083694.44384.39. discussion 1576. [DOI] [PubMed] [Google Scholar]
- 37.Badylak S.F., et al. The use of xenogeneic small intestinal submucosa as a biomaterial for Achilles tendon repair in a dog model. J Biomed Mater Res. 1995;29:977. doi: 10.1002/jbm.820290809. [DOI] [PubMed] [Google Scholar]
- 38.Boruch A.V., et al. Constructive remodeling of biologic scaffolds is dependent on early exposure to physiologic bladder filling in a canine partial cystectomy model. J Surg Res. 2010;161:217. doi: 10.1016/j.jss.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 39.Badylak S.F., et al. Biologic scaffolds for constructive tissue remodeling. Biomaterials. 2011;32:316. doi: 10.1016/j.biomaterials.2010.09.018. [DOI] [PubMed] [Google Scholar]
- 40.Hodde J.P., et al. Vascular endothelial growth factor in porcine derived extracellular matrix. Endothelium. 2001;8:11. doi: 10.3109/10623320109063154. [DOI] [PubMed] [Google Scholar]
- 41.Hodde J.P. Ernst D.M. Hiles M.C. An investigation of the long-term bioactivity of endogenous growth factor in OASIS Wound Matrix. J Wound Care. 2005;14:23. doi: 10.12968/jowc.2005.14.1.26721. [DOI] [PubMed] [Google Scholar]
- 42.Voytik-Harbin S.L., et al. Identification of extractable growth factors from small intestinal submucosa. J Cell Biochem. 1997;67:478. [PubMed] [Google Scholar]
- 43.McDevitt C.A. Wildey G.M. Cutrone R.M. Transforming growth factor-beta1 in a sterilized tissue derived from the pig small intestine submucosa. J Biomed Mater Res A. 2003;67:637. doi: 10.1002/jbm.a.10144. [DOI] [PubMed] [Google Scholar]
- 44.Hodde J.P., et al. Retention of endothelial cell adherence to porcine-derived extracellular matrix after disinfection and sterilization. Tissue Eng. 2002;8:225. doi: 10.1089/107632702753724996. [DOI] [PubMed] [Google Scholar]
- 45.Hodde J., et al. Fibronectin peptides mediate HMEC adhesion to porcine-derived extracellular matrix. Biomaterials. 2002;23:1841. doi: 10.1016/s0142-9612(01)00310-6. [DOI] [PubMed] [Google Scholar]
- 46.Badylak S.F., et al. Marrow-derived cells populate scaffolds composed of xenogeneic extracellular matrix. Exp Hematol. 2001;29:1310. doi: 10.1016/s0301-472x(01)00729-9. [DOI] [PubMed] [Google Scholar]
- 47.Zantop T., et al. Extracellular matrix scaffolds are repopulated by bone marrow-derived cells in a mouse model of achilles tendon reconstruction. J Orthop Res. 2006;24:1299. doi: 10.1002/jor.20071. [DOI] [PubMed] [Google Scholar]
- 48.Badylak S.F., et al. Comparison of the resistance to infection of intestinal submucosa arterial autografts versus polytetrafluoroethylene arterial prostheses in a dog model. J Vasc Surg. 1994;19:465. doi: 10.1016/s0741-5214(94)70073-7. [DOI] [PubMed] [Google Scholar]
- 49.Badylak S.F., et al. Host protection against deliberate bacterial contamination of an extracellular matrix bioscaffold versus Dacron mesh in a dog model of orthopedic soft tissue repair. J Biomed Mater Res B Appl Biomater. 2003;67:648. doi: 10.1002/jbm.b.10062. [DOI] [PubMed] [Google Scholar]
- 50.Brennan E.P., et al. Antibacterial activity within degradation products of biological scaffolds composed of extracellular matrix. Tissue Eng. 2006;12:2949. doi: 10.1089/ten.2006.12.2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Medberry C.J., et al. Resistance to Infection of Five Different Materials in a Rat Body Wall Model. J Surg Res. 2012;173:38. doi: 10.1016/j.jss.2010.08.035. [DOI] [PubMed] [Google Scholar]
- 52.Sarikaya A., et al. Antimicrobial activity associated with extracellular matrices. Tissue Eng. 2002;8(1):63. doi: 10.1089/107632702753503063. [DOI] [PubMed] [Google Scholar]
- 53.Malmsten M. Davoudi M. Schmidtchen A. Bacterial killing by heparin-binding peptides from PRELP and thrombospondin. Matrix Biol. 2006;25:294. doi: 10.1016/j.matbio.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 54.Li F., et al. Low-molecular-weight peptides derived from extracellular matrix as chemoattractants for primary endothelial cells. Endothelium. 2004;11:199. doi: 10.1080/10623320490512390. [DOI] [PubMed] [Google Scholar]
- 55.Orberg J. Baer E. Hiltner A. Organization of collagen fibers in the intestine. Connect Tissue Res. 1983;11:285. doi: 10.3109/03008208309004861. [DOI] [PubMed] [Google Scholar]
- 56.Orberg J.W. Klein L. Hiltner A. Scanning electron microscopy of collagen fibers in intestine. Connect Tissue Res. 1982;9:187. doi: 10.3109/03008208209160260. [DOI] [PubMed] [Google Scholar]
- 57.Badylak S.F. Freytes D.O. Gilbert T.W. Extracellular matrix as a biological scaffold material: Structure and function. Acta Biomaterialia. 2009;5:1. doi: 10.1016/j.actbio.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 58.Record R.D., et al. In vivo degradation of 14C-labeled small intestinal submucosa (SIS) when used for urinary bladder repair. Biomaterials. 2001;22:2653. doi: 10.1016/s0142-9612(01)00007-2. [DOI] [PubMed] [Google Scholar]
- 59.Gilbert T.W. Stewart-Akers A.M. Badylak S.F. A quantitative method for evaluating the degradation of biologic scaffold materials. Biomaterials. 2007;28:147. doi: 10.1016/j.biomaterials.2006.08.022. [DOI] [PubMed] [Google Scholar]
- 60.Martinod E., et al. Tracheal regeneration following tracheal replacement with an allogenic aorta. Ann Thorac Surg. 2005;79:942. doi: 10.1016/j.athoracsur.2004.08.035. [DOI] [PubMed] [Google Scholar]
- 61.Makris D., et al. Tracheal replacement with cryopreserved allogenic aorta. Chest. 2010;137:60. doi: 10.1378/chest.09-1275. [DOI] [PubMed] [Google Scholar]
- 62.McPherson T.B., et al. Galalpha(1,3)Gal epitope in porcine small intestinal submucosa. Tissue Eng. 2000;6:233. doi: 10.1089/10763270050044416. [DOI] [PubMed] [Google Scholar]
- 63.Raeder R.H., et al. Natural anti-galactose alpha1,3 galactose antibodies delay, but do not prevent the acceptance of extracellular matrix xenografts. Transpl Immunol. 2002;10:15. doi: 10.1016/s0966-3274(01)00044-2. [DOI] [PubMed] [Google Scholar]
- 64.Zhai Y. Ghobrial R.M. Kupiec-Weglinski J.W. Th1 and Th2 cytokines in organ transplantation: paradigm lost? Crit Rev Immunol. 1999;19:155. [PubMed] [Google Scholar]
- 65.Chen N. Gao Q. Field E.H. Prevention of Th1 response is critical for tolerance. Transplantation. 1996;61:1076. doi: 10.1097/00007890-199604150-00016. [DOI] [PubMed] [Google Scholar]
- 66.Bach F.H., et al. Accomodation of vascularised xenografts: expression of “protective genes” by donor endothelial cells in a host Th2 cytokine environment. Nat Med. 1997;3:196. doi: 10.1038/nm0297-196. [DOI] [PubMed] [Google Scholar]
- 67.Piccotti J.R., et al. Are Th2 helper T lymphocytes beneficial, deleterious or irrelevant in promoting allograft survival? Transplantation. 1997;63:619. doi: 10.1097/00007890-199703150-00001. [DOI] [PubMed] [Google Scholar]
- 68.Allman A.J, et al. Xenogeneic extracellular matrix grafts elicit a TH2-restricted immune response. Transplantation. 2001;71:1631. doi: 10.1097/00007890-200106150-00024. [DOI] [PubMed] [Google Scholar]
- 69.Allman A.J., et al. The Th2-restricted immune response to xenogeneic small intestinal submucosa does not influence systemic protective immunity to viral and bacterial pathogens. Tissue Eng. 2002;8:53. doi: 10.1089/107632702753503054. [DOI] [PubMed] [Google Scholar]
- 70.Brown B.N., et al. Macrophage polarization: an opportunity for improved outcomes in biomaterials and regenerative medicine. Biomaterials. 2012;33:3792. doi: 10.1016/j.biomaterials.2012.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Badylak S.F., et al. Macrophage phenotype as a determinant of biologic scaffold remodeling. Tissue Eng Part A. 2008;14:1835. doi: 10.1089/ten.tea.2007.0264. [DOI] [PubMed] [Google Scholar]
- 72.Brown B.N, et al. Macrophage phenotype as a predictor of constructive remodeling following the implantation of biologically derived surgical mesh materials. Acta Biomater. 2011;8:978. doi: 10.1016/j.actbio.2011.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Valentin J.E., et al. Macrophage participation in the degradation and remodeling of extracellular matrix scaffolds. Tissue Eng Part A. 2009;15:1687. doi: 10.1089/ten.tea.2008.0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nagata S. Hanayama R. Kawane K. Autoimmunity and the clearance of dead cells. Cell. 2010;140:619. doi: 10.1016/j.cell.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 75.Brown B.N., et al. Macrophage phenotype and remodelling outcomes in response to biologic scaffolds with and without a cellular component. Biomaterials. 2009;30:1482. doi: 10.1016/j.biomaterials.2008.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang Q., et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464:104. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zheng M.H., et al. Porcine small intestine submucosa (SIS) is not an acellular collagenous matrix and contains porcine DNA: possible implications in human implantation. J Biomed Mater Res B Appl Biomater. 2005;73:61. doi: 10.1002/jbm.b.30170. [DOI] [PubMed] [Google Scholar]
- 78.Gorschewsky O., et al. Quantitative analysis of biochemical characteristics of bone-patellar tendon-bone allografts. Biomed Mater Eng. 2005;15:403. [PubMed] [Google Scholar]
- 79.Rosario D.J., et al. Decellularization and sterilization of porcine urinary bladder matrix for tissue engineering in the lower urinary tract. Regen Med. 2008;3:145. doi: 10.2217/17460751.3.2.145. [DOI] [PubMed] [Google Scholar]
- 80.Freytes D.O. Stoner R.M. Badylak S.F. Uniaxial and biaxial properties of sterilized porcine urinary bladder matrix scaffolds. J Biomed Mater Res B Appl Biomater. 2008;84:408. doi: 10.1002/jbm.b.30885. [DOI] [PubMed] [Google Scholar]
- 81.Sun W.Q. Leung P. Calorimetric study of extracellular tissue matrix degradation and instability after gamma irradiation. Acta Biomater. 2008;4:817. doi: 10.1016/j.actbio.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 82.Qiu Q.Q., et al. Inactivation of bacterial spores and viruses in biological material using supercritical carbon dioxide with sterilant. J Biomed Mater Res B Appl Biomater. 2009;91:572. doi: 10.1002/jbm.b.31431. [DOI] [PubMed] [Google Scholar]
- 83.Nakayama K.H., et al. Decellularized rhesus monkey kidney as a three-dimensional scaffold for renal tissue engineering. Tissue Eng Part A. 2010;16:2207. doi: 10.1089/ten.tea.2009.0602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yokoo T., et al. Human mesenchymal stem cells in rodent whole embryo culture are reprogrammed to contribute to kidney tissues. Proc Natl Acad Sci U S A. 2005;102:3296. doi: 10.1073/pnas.0406878102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Morigi M., et al. Life-sparing effect of human cord blood mesenchymal stem cells in experimental acute kidney injury. Stem Cells. 2010;28:513. doi: 10.1002/stem.293. [DOI] [PubMed] [Google Scholar]
- 86.Morigi M., et al. Mesenchymal stem cells are renotropic, helping to repair the kidney and improve function in acute renal failure. J Am Soc Nephrol. 2004;15:1794. doi: 10.1097/01.asn.0000128974.07460.34. [DOI] [PubMed] [Google Scholar]