Abstract
Background
Postpartum depression is associated with reduced breastfeeding duration. We previously hypothesized that shared neuroendocrine mechanisms underlie this association. We sought to measure the association between maternal mood and neuroendocrine response to breastfeeding.
Methods
We conducted a longitudinal cohort study of women recruited during pregnancy who intended to breastfeed. Baseline depression and anxiety history were assessed with a structured clinical interview. We measured mood symptoms using validated psychometric instruments, and we quantified affect and neuroendocrine responses to breastfeeding during laboratory visits at 2 and 8 weeks postpartum.
Results
We recruited 52 women who intended to breastfeed, among whom 47 completed 8-week follow-up. Duration and intensity of breastfeeding through 8 weeks were similar among mothers with lower versus higher anxiety and depression scores. In the third trimester, oxytocin was inversely correlated with Edinburgh Postnatal Depression Scale (EPDS) score (p=0.03). We did not find differences in neuroendocrine profile during breastfeeding at 2 weeks postpartum. Among the 39 women who breastfed at 8 weeks postpartum, oxytocin area under the curve during breastfeeding was inversely correlated with maternal EPDS and STAI-State and STAI-Trait anxiety scores (all p≤0.01). Higher anxiety and depression scores was further associated with lower oxytocin (group p<0.05) during feeding. During feeding at both visits, higher anxiety and depression scores were also associated with more negative affect: mothers reported feeling less happy and more depressed, overwhelmed, and stressed during feeding than women with lower scores.
Conclusion
Symptoms of depression and anxiety were associated with differences in oxytocin response and affect during breastfeeding.
Introduction
In the first weeks after childbirth, mother and infant navigate a complex transition from the physiology of pregnancy to the early postpartum period. Key challenges for the mother include adjusting to loss of placental hormones, establishing lactation, and coping with the stressor of caring for a new infant. Difficulties with this transition may present clinically as postpartum depression (PPD) and failed lactation.1
Both depression and curtailed breastfeeding confer substantial morbidity for mother and child. Postpartum depression is associated with impaired parenting behavior, diminished maternal-infant attachment, and, in extreme cases, maternal suicide and infanticide.2–4 Prominent anxiety symptoms are a distinguishing feature of postpartum depression, and many women seeking treatment have primary complaints of ruminating and obsessive thoughts.5,6 Never breastfeeding or early weaning is associated with increased infant risk of infectious morbidity and chronic disease, as well as increased maternal risk of breast and ovarian cancer, type 2 diabetes, hypertension, and myocardial infarction.7–10
Observational studies have reported associations between postpartum depression and both early weaning and negative breastfeeding experiences.11–15 However, the mechanisms underlying these associations remain to be determined. Several neuroendocrine mechanisms, including gonadal steroids, oxytocin, prolactin, the hypothalamic pituitary adrenal axis, and the thyroid, are implicated in both maternal mood and breastfeeding physiology, as we have previously reviewed.1 We hypothesized that maternal postpartum mood symptoms and unplanned early weaning share a common pathogenesis that involves alterations in neuroendocrine function. Identifying such shared mechanisms may lead to new therapeutic options for reducing the morbidity of both postpartum depression and early weaning.
No studies to our knowledge have measured maternal neuroendocrine response to breastfeeding among mothers with and without mood symptoms. Defining such differences may both elucidate physiologic changes underlying perinatal mood disorders and identify novel targets for prevention and treatment. We hypothesized that maternal depression and anxiety symptoms would be associated with shorter breastfeeding duration and with differences in affect and neuroendocrine response to infant feeding. To test this hypothesis, we recruited a prospective cohort of women in the third trimester of pregnancy who intended to breastfeed, and we measured neuroendocrine response and affect during feeding sessions at 2 and 8 weeks postpartum.
Materials and Methods
Study participants were recruited from prenatal obstetric and perinatal psychiatry clinics at University of North Carolina (UNC) Hospitals from February 2010 to February 2011. Women in the third trimester of a singleton pregnancy who intended to breastfeed for at least 3 months were invited to participate. We limited our enrollment to women intending to breastfeed at least 3 months so that any weaning during the study period would be unplanned. We planned to recruit 25 women with current or past depression or anxiety and 25 women with no such history, anticipating that 10% of the women without a history of mood disorders would develop postpartum depression based on a conservative estimate of prevalence of PPD.16 Assuming 28 women with postnatal symptoms and 22 without symptoms, this sample size provided 80% power to detect a 0.82 standard deviation (SD) difference in continuous measures between symptomatic and asymptomatic women. We excluded women with (1) diagnosis of Axis I disorders other than unipolar depression or anxiety disorders, (2) use of tobacco or illicit substances, (3) prenatal diagnosis of congenital anomaly likely to interfere with breastfeeding, (4) maternal endocrine disorder associated with low milk supply, including thyroid disorder and pregestational diabetes, or (5) chronic medication that is contraindicated during lactation. The UNC Institutional Review Board approved the study, and all participants provided written informed consent.
Women were enrolled in the third trimester of pregnancy and were assessed by a board-certified psychiatrist with extensive training in administration of structured research interviews (SMB) using the depression and anxiety disorder modules of the Structured Clinical Interview Non-Patient version (SCID-NP).17 In addition, participants provided a morning non-fasting blood sample. At enrollment in the third trimester and at 2- and 8-weeks postpartum, mothers completed the Edinburgh Postnatal Depression Scale (EPDS)18 and the Spielberger State and Trait Anxiety Inventories (STAI).19
Edinburgh postnatal depression scale
The EPDS was developed specifically for assessing postpartum depression and relies much less than standard depression screens on somatic, or physical, questions.20 It also has multiple questions that specifically assess for anxiety symptoms.21 The EPDS is a widely validated instrument commonly used internationally to assess postpartum depression. The 10-item EPDS is a self-report screening scale and the response format is frequency-based. A cutoff of score of ≥12 on the EPDS has been consistently shown to be correlated with a clinical diagnosis of major depressive disorder, when compared with a structured clinical interview.20 EPDS scores of 10–12 have been associated with an accurate diagnosis of minor depressive disorder. Multiple reports in the literature have documented that the EPDS demonstrates good sensitivity and specificity in identifying women suffering from perinatal depression.16 In our analysis, we used a cutoff score of ≥10 as a positive screen.
Spielberger State and Trait Anxiety Inventory
We quantified anxiety symptoms using the Spielberger STAI.19 For the trait inventory, participants rate how they generally feel, using a four-level Likert scale ranging from “almost never” to “almost always” to rate statements such as “I feel satisfied with myself,” “I have disturbing thoughts,” and “I make decisions easily.” For the state inventory, participants rate their feelings at this moment, using a four-level Likert scale ranging from “not at all” to “very much so” to rate 20 statements such as “I feel calm,” “I feel nervous,” or “I feed content.” The trait inventory provides a stable measure of anxiety, whereas the state inventory captures perceived stress “right now” (Alpha=0.90 to 0.94 and 0.89 to 0.92, respectively). Because we sought to measure current maternal anxiety, we used the state inventory to quantify anxiety symptoms in our study population. The Spielberger STAI has been validated in for use in perinatal populations.22 Population normative data shows that the median score for both state and trait anxiety among women ages 19–39 is 34.19
Breastfeeding intensity
Breastfeeding intensity was assessed at 2 and 8 weeks postpartum with a 1-week infant feeding recall and was defined as the percentage of all milk feedings that were breast milk.23 Mothers were asked to report how many times in the last day they had fed their infant breast milk, formula, or other foods. If they had not fed the food in the past day, they were asked how many times they had fed the food in the past week. Breastfeeding intensity was calculated as [number of breast milk feedings per week / (number of breast milk feedings+formula feedings per week)]. For example, if a mother breastfed 8 times a day and fed her infant formula twice in the past week, her breastfeeding intensity was calculated as (breastfed 8 times a day×7 days) / [(breastfed 8 times a day×7 days)+(formula fed 2 times)]=56 / (56+2)=breastfeeding intensity of 96.5%. All participants had access to lactation support through the North Carolina Women's Hospital Breastfeeding WarmLine.
Observed feeding session
At 2 and 8 weeks postpartum, mothers brought their infants to the Mother-Infant Biobehavioral Laboratory for an afternoon observed feeding session beginning at 1:00 p.m. An antecubital i.v. was placed in the mother's arm to enable multiple blood draws at predetermined intervals. After a 10-minute habituation period and 10 minutes of baseline rest, a venous blood sample was obtained. Mothers chose to breastfeed, express milk using an electronic pump, or both breast- and bottle-feed at each study visit, as best reflected their usual feeding routine. We used this approach so that we could quantify the neuroendocrine profile of a typical feeding session for each mother-infant dyad. During the 2-week visit, 46 mothers expressed milk or breastfed, and during the 8-week visit, 39 mothers expressed milk or breastfed. One mother used an electronic pump to express milk during both the 2-week and 8-week visit. A second mother breastfed and then supplemented with a bottle at the 2-week visit. Two mothers were taking fenugreek, an herbal galactogogue used to increase milk supply, at both the 2- and 8-week visits. One mother was using hormonal contraception at the 8-week visit.
Samples were obtained at the onset of minutes 1, 4, 7, and 10 of the feeding session. Ten minutes after the feeding ended, a resting blood sample was obtained. At the end of baseline rest, at minute 10 of feeding, and after 10 minutes of post-feeding rest, mothers rated their affect on visual analog scales in 5 areas: stressed, worried, nervous, or tense; happy, relaxed, comfortable, or satisfied; irritated, annoyed, ‘pissed off,’ or furious; depressed, sad, down or unhappy; and overwhelmed, unable to control things, or discouraged (0=not at all; 9=extremely).
Neuroendocrine marker assessment
Because oxytocin release is pulsatile, we measured levels at baseline, at 1, 3, 7, and 10 minutes of feeding, and after a post-feeding rest. Other markers were measured at baseline only (estradiol, progesterone), at baseline, minute 10, and after feeding (prolactin) or at baseline and after feeding (cortisol, corticotropin releasing factor [CRF], free T4, and total T4). Each venous blood sample was collected into pre-chilled vacutainer tubes, immediately cold-centrifuged, aliquoted into pre-chilled cryotubes, and stored at –80°C for later endocrine assays. The level of oxytocin in EDTA-treated plasma, with aprotinin added to prevent peptide degradation, was determined by enzyme immunoassay (EIA) with extraction (Enzo Life Sciences) as per previously described methods.24 The level of detection was 1.1 pg/mL, with intra- and inter-assay CV of 4.6 and 8.7 respectively. We used commercial EIA to measure T4, free T4, estradiol, progesterone, and cortisol, commercial radioimmunoassay (RIA) to measure prolactin (MP Biomedicals), and high sensitivity EIA to measure CRF (Bachem).
Statistical analysis
Characteristics of the study population were measured using chi-square tests or Fisher's exact tests for categorical variables and analysis of variance for continuous variables. To determine whether maternal depression or anxiety history was associated with subsequent breastfeeding, we used Cox proportional hazards regression to model the association between prenatal depression or anxiety, assessed by diagnostic interview at enrollment, mood symptoms at each study time point, and timing of introduction of formula and of weaning altogether. We reported median values and interquartile ranges for breastfeeding intensity because this measure was not normally distributed, and we used the Kruskal-Wallis test to determine whether prenatal history of depression or anxiety or higher versus lower depression or anxiety symptoms were associated with breastfeeding intensity at the 2- and 8-week visits.
To quantify associations between maternal mood and neuroendocrine profile, we measured Spearman correlations between EPDS, STAI State and Trait anxiety scales, and neuroendocrine markers. We included all participants for correlations between mood measures and baseline neuroendocrine markers, and we included those mothers who breastfed or pumped during each study visit for correlations between mood measures and post-feeding measures. Because oxytocin levels vary due to pulsatile release, a composite variable was created for oxytocin, using area under the curve (AUC)25 to reflect overall oxytocin across the feeding session. We used repeated measures analysis to quantify the association between subclinical anxiety and/or depression and profiles of oxytocin, prolactin, cortisol, CRF, T4, and free T4 among mothers who breastfed or pumped at the postpartum study visits, adjusting for days postpartum and parity (0 or 1+). Residuals were tested for normality, and, if non-normal, neuroendocrine markers were log transformed.
To measure associations between higher versus lower maternal symptoms and affect during feeding, we initially used repeated measures analysis. However, residuals were not normally distributed, and due to the large number of scores of 0, results were not amenable to transformation. We therefore compared median affect scores and interquartile ranges, using Wilcoxon rank sum tests to compare affect at baseline, minute 10 of feeding, and post-feeding rest.
Our study population included women with and without mood symptoms who were taking antidepressants. To test whether current antidepressant use modified associations between mood symptoms and neuroendocrine profile, we tested for interactions between current medication use and subclinical depression and/or anxiety using a cross-product term. For all models, p values<0.05 were considered statistically significant.
Results
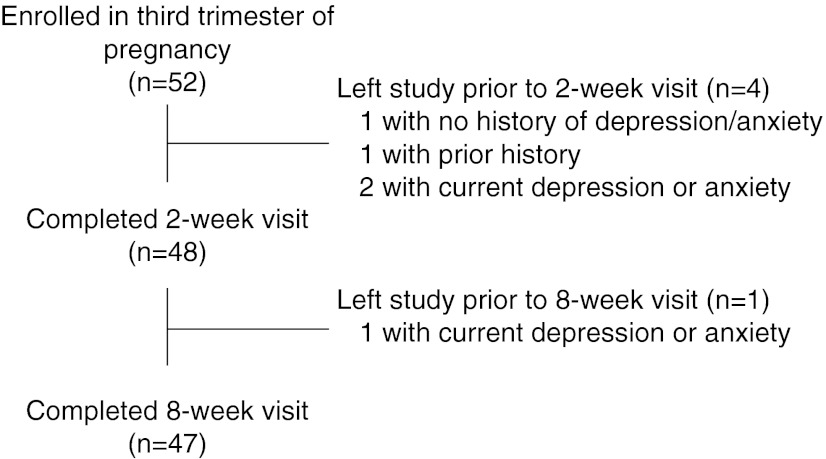
We recruited 52 women during the third trimester. Of these, 4 participants dropped out of the study before the 2-week postpartum visit. An additional participant dropped out before the 8-week visit, leaving 47 women for analysis who completed all three visits (Fig. 1). Among those who did not complete the 2-week visit, 1 had no prior history of depression or anxiety, 1 had a past history, and 2 were currently depressed or anxious at enrollment. One additional participant who was currently depressed or anxious at enrollment did not attend the 8-week visit.
FIG. 1.
Study participant flow chart.
Among those who completed the study, 23 had no history of depression or anxiety, 16 had a lifetime history, and 8 were currently depressed or anxious based on diagnostic interview at enrollment. The three groups were similar in age, race/ethnicity, income, and education (Table 1). Women with no depression or anxiety history were more likely to be primiparous (58.3% vs. 41.7%; chi square p=0.07). At baseline and during follow-up, women with past or current depression or anxiety were more likely to meet criteria for mild depression, defined as EPDS ≥10; and higher anxiety, defined as STAI ≥34; and for Fisher's exact p<0.05 for mild depression prenatally and at 8 weeks; and for higher anxiety symptoms at all three time points. At the final study visit, 54% (13/24) women with a history of depression or anxiety reported higher depression and/or anxiety symptoms, compared with 4% (1/23) of women without a prenatal history of depression or anxiety (p<0.01).
Table 1.
Characteristics of Study Population by Depression/Anxiety History Assessed at Enrollment in the Third Trimester
| Never depression or anxiety* | Lifetime depression or anxiety* | Current depression or anxiety* | All* | |
|---|---|---|---|---|
| n | 23 | 16 | 8 | 47 |
| Age, mean (SD) | 31.3 (5.2) | 32.0 (5.2) | 31.8 (2.3) | 31.6 (4.8) |
| Race | ||||
| White | 19 (82.6) | 13 (81.3) | 7 (87.5) | 39 (83.0) |
| Black or African American | 3 (13.0) | 3 (18.8) | 1 (12.5) | 7 (14.9) |
| Asian | 1 (4.3) | 0 (0.0) | 0 (0.0) | 1 (2.1) |
| Education | ||||
| < 4-yr college graduate | 4 (17.4) | 3 (18.8) | 1 (12.5) | 8 (17.0) |
| Graduated 4-yr college | 4 (17.4) | 6 (37.5) | 4 (50.0) | 14 (29.8) |
| Post graduate | 15 (65.2) | 7 (43.8) | 3 (37.5) | 25 (53.2) |
| Body mass index | ||||
| <25 | 7 (30.4) | 5 (31.3) | 3 (37.5) | 15 (31.9) |
| 25 to <30 | 11 (47.8) | 6 (37.5) | 4 (50.0) | 21 (44.7) |
| 30+ | 5 (21.7) | 5 (31.3) | 1 (12.5) | 11 (23.4) |
| Income | ||||
| Less than $40,000 | 6 (27.3) | 2 (12.5) | 2 (25.0) | 10 (21.7) |
| $40000–59999 | 4 (18.2) | 2 (12.5) | 1 (12.5) | 7 (15.2) |
| $60,000–99,999 | 6 (27.3) | 6 (37.5) | 3 (37.5) | 15 (32.6) |
| $100,000 or above | 6 (27.3) | 6 (37.5) | 2 (25.0) | 14 (30.4) |
| Primiparous | 16 (69.6) | 7 (43.8) | 3 (37.5) | 26 (55.3) |
| Mode of delivery | ||||
| Spontaneous vaginal birth | 17 (85.0) | 9 (69.2) | 7 (87.5) | 33 (80.5) |
| Vacuum | 0 (0.0) | 2 (15.4) | 1 (12.5) | 3 (7.3) |
| C-section | 3 (15.0) | 2 (15.4) | 0 (0.0) | 5 (12.2) |
| Depression symptoms (EPDS ≥10) | ||||
| Prenatal | 0 (0.0) | 1 (6.3) | 2 (25.0) | 3 (6.4) |
| 2 weeks postpartum | 2 (8.7) | 5 (31.3) | 1 (12.5) | 8 (17.0) |
| 8 weeks postpartum | 0 (0.0) | 2 (12.5) | 2 (25.0) | 4 (8.5) |
| Higher anxiety symptoms (STAI-State ≥34) | ||||
| Prenatal | 3 (13.0) | 4 (25.0) | 7 (87.5) | 14 (29.8) |
| 2 weeks postpartum | 2 (8.7) | 7 (43.8) | 6 (75.0) | 15 (31.9) |
| 8 weeks postpartum | 1 (4.3) | 8 (50.0) | 4 (50.0) | 13 (27.7) |
n (%) unless otherwise specified.
SD, standard deviation; EPDS, Edinburgh Postnatal Depression Scale; STAI, State and Trait Anxiety Inventories.
At the 2-week visit, all mothers were still breastfeeding, but 2 mothers chose to feed their infants from a bottle during the study visit, leaving 46 women who breastfed or pumped during the study visit. At the 8-week visit, 42 mothers were still breastfeeding, but 2 mothers chose to formula-feed during the study visit and one mother chose to hold her baby without feeding, leaving 39 women who breastfed or pumped during the study visit. Among the 39 women who breastfed during the 8-week visit, 15 had subclinical anxiety or depression at 2 weeks, and 11 had subclinical symptoms at 8 weeks. Six had subclinical symptoms at 2 weeks that resolved by 8 weeks, and 2 developed subclinical symptoms between the 2- and 8-week visits.
Breastfeeding intensity was high in our study population. Similar proportions of subclinically symptomatic (71%) and asymptomatic (73%) women received assistance from a lactation consultant in the first two weeks postpartum (Fisher's exact p=1.0). At 2 weeks, 33 of 48 (68.8%) of mothers were exclusively breastfeeding, 15 of 48 (31.2%) were supplementing with formula, and no mothers had stopped breastfeeding altogether. At 8 weeks, 33 of 47 (70.2%) of mothers were exclusively breastfeeding, 9 of 47 (19.2%) were supplementing, and 5 of 47 (10.6%) had stopped breastfeeding. The median breastfeeding intensity was 100% (IQR 94.8%–100%) at 2 weeks and 100% (IQR 93.3%–100%) at 8 weeks. We found no association between maternal prenatal depression/anxiety history or symptoms and concurrent breastfeeding intensity or exclusivity at 2 or 8 weeks postpartum. Women who were exclusively breastfeeding at 2 weeks were more likely to report subclinically symptomatic anxiety at 8 weeks (12/32, 37.5%, vs. 1/15, 6.7%; Fisher's exact p=0.04). In Cox proportional hazards models, we found no association between psychiatric history and timing of introduction of formula or of weaning altogether.
Patterns of association between neuroendocrine markers and mood differed by study visit. At the prenatal visit (N=52), oxytocin was inversely correlated with EPDS score (Spearman R=–0.30, p=0.03). We found no association between prenatal depression, indexed by EPDS, or anxiety, indexed by STAI, and cortisol, CRF, estradiol, progesterone, prolactin, FT4, or total T4.
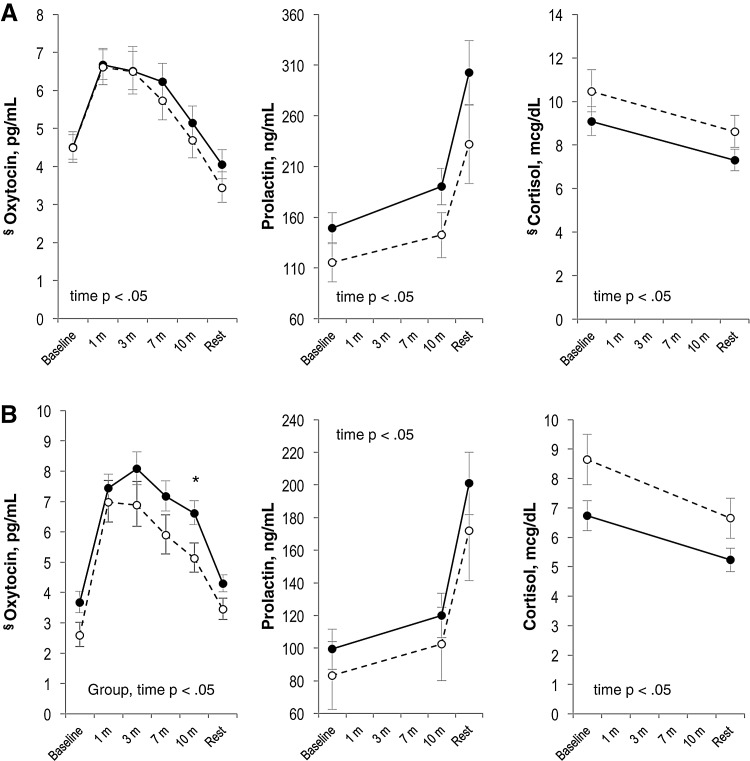
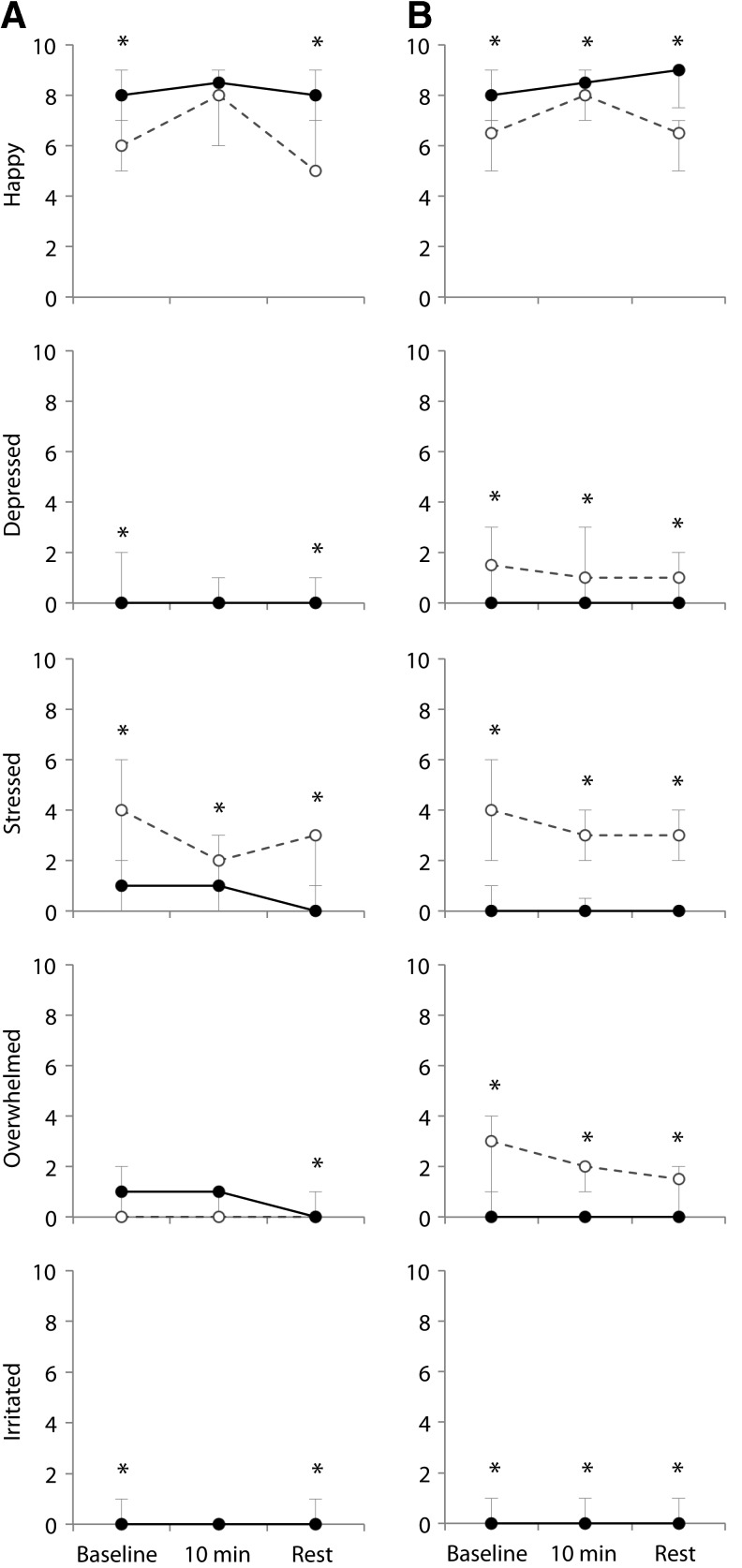
At the 2-week visit, we found associations between maternal mood, baseline oxytocin, and maternal affect during feeding. Among the 48 women who presented for the 2-week visit, baseline oxytocin was inversely correlated with EPDS score (Spearman R=–0.33, p=0.03). We found no correlations between maternal EPDS or STAI score and baseline cortisol, CRF, estradiol, progesterone, prolactin, FT4, or total T4. In repeated measures analysis among mothers who expressed milk or breastfed at the 2-week visit (Fig. 3A, N=46), we found no differences in oxytocin profile during feeding among mothers with higher depression/anxiety symptoms (EPDS ≥10 and/or STAI ≥34) versus the profile among mothers with lower symptoms. Mothers with higher symptoms reported feeling more depressed, overwhelmed, and stressed during feeding than mothers with lower symptoms (p<0.05 for all comparison, Fig. 4A). In addition, we observed several non-statistically significant patterns that may merit exploration in future studies. Among mothers with higher symptoms, we found lower prolactin and higher cortisol levels than among mothers with lower symptoms. When we compared CRF trajectories during feeding, we found a decrease in CRF among lower-symptom mothers and an increase among higher-symptom mothers (group×time, p=0.09).
FIG. 3.
Maternal neuroendocrine profile during breastfeeding sessions at 2 weeks postpartum (A) and 8 weeks postpartum (B), among mothers with higher (dashed line) versus lower (solid line) mood symptoms, defined as EPDS ≥10 and/or Spielberger state anxiety score ≥34. Least square means±standard error in models adjusting for primiparity and infant age at study visit. Group, time, and group×time p values reported for repeated measures analysis with unstructured covariance. At 2 weeks: n=17 with higher symptoms, 29 with lower symptoms. At 8 weeks: n=11 with higher symptoms, 28 with lower symptoms. *Simple effect p value for group<0.05 at this time point. §Geometric means presented because residuals in untransformed analyses were not normally distributed.
FIG. 4.
Median and interquartile range for maternal affect during breastfeeding sessions at 2 weeks (A) and 8 weeks (B) postpartum, among mothers with higher (dashed line) versus lower (solid line) mood symptoms, defined as EPDS ≥10 and/or Spielberger state anxiety score ≥34. At 2 weeks: n=17 with higher symptoms, 28 with lower symptoms. At 8 weeks: n=11 with higher symptoms, 28 with lower. *Wilcoxon p value for group<0.05 at this time point.
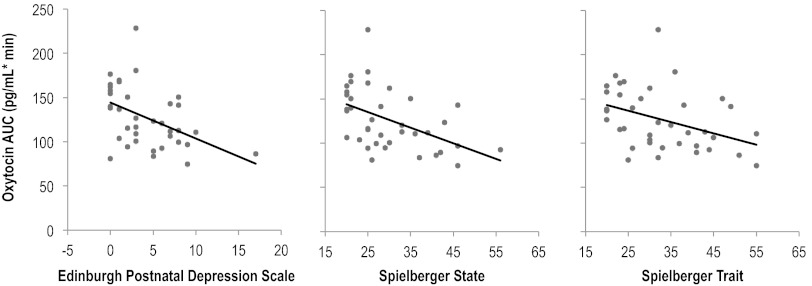
At the 8-week visit, we found statistically significant associations between maternal mood and oxytocin, total T4, and affect during feeding. Among the 47 women who attended the 8-week visit, total T4 was inversely correlated with EPDS (Spearman R=−0.34; p=0.03). Among the 39 women who breastfed or expressed milk during the visit, EPDS and STAI anxiety scores were inversely correlated with oxytocin measures during and after feeding (Table 2) and with oxytocin area under the curve (Fig. 2; Spearman R for EPDS, STAI of −0.48, −0.53, and −0.44, respectively, all p<0.01). The correlation between EPDS and oxytocin area under the curve remained with exclusion of an outlier with an EPDS of 17 (Spearman R=–0.35, p=0.02). We further found that total T4 before and after feeding was inversely correlated with EPDS score at 8 weeks (Spearman R=–0.41, p=0.01 before and R=−0.36, p=0.03 after). We found no significant correlations between maternal mood measures and baseline cortisol, CRF, estradiol, progesterone, prolactin, or free T4 (Spearman p>0.05 for all correlations).
Table 2.
Correlations Between Maternal Depression/Anxiety and Oxytocin During Feeding at 8 Weeks Postpartum, Among Participants Breastfeeding or Pumping During the Study Visit
| EPDS* | STAI-State* | STAI-Trait* | |
|---|---|---|---|
| Oxytocin baseline | −0.22 | −0.44 | −0.22 |
| 0.18 | 0.01 | 0.17 | |
| Feeding minute 1 | −0.12 | −0.33 | −0.20 |
| 0.48 | 0.04 | 0.23 | |
| Feeding minute 4 | −0.27 | −0.38 | −0.23 |
| 0.10 | 0.02 | 0.17 | |
| Feeding minute 7 | −0.50 | −0.50 | −0.44 |
| <0.01 | <0.01 | 0.01 | |
| Feeding minute 10 | −0.36 | −0.38 | −0.37 |
| 0.03 | 0.02 | 0.02 | |
| Oxytocin after post-feed rest | −0.45 | −0.53 | −0.40 |
| 0.01 | <0.01 | 0.01 | |
| Oxytocin AUC | −0.48 | −0.53 | −0.44 |
| <0.01 | <0.01 | <0.01 |
Spearman R, p value. Spearman R coefficients with p<0.05 are shown in bold.
N=39.
AUC, area under curve.
FIG. 2.
Correlation between maternal mood at 8 weeks postpartum and oxytocin area under the curve during breastfeeding (n=39). Spearman R: EPDS, R=–0.48, p<0.01; STAI-State, R=–0.53, p<0.01; STAI-Trait, R=–0.44, p<0.01.
In repeated measures analyses among women who were breastfeeding or pumping during the study visit (Fig. 3B, N=39), we found lower oxytocin levels during and after feeding among higher-symptom mothers compared with lower-symptom mothers (group p<0.05). Consistent with the 2-week visit, we further found that mothers with higher mood symptoms reported feeling less happy and more depressed, overwhelmed, and stressed than mothers with lower mood symptoms (Fig. 4B, group p<0.05 for all comparisons).
In addition, we found potentially interesting, but statistically non-significant, differences in prolactin, cortisol, and CRF trajectory in repeated measures analysis. As at the 2-week visit, higher-symptom mothers had lower prolactin and higher cortisol levels. We again found differences in CRF trajectory, although at 8 weeks, CRF increased during feeding among lower-symptom mothers and decreased among higher-symptom mothers.
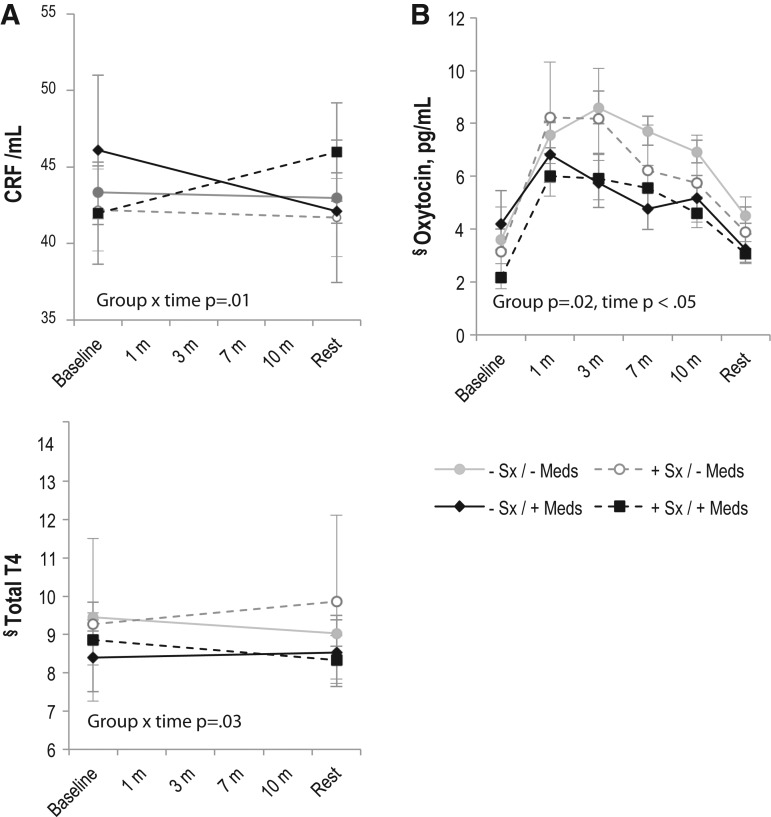
When we tested for interactions between maternal antidepressant use, higher versus lower symptoms and neuroendocrine markers, we found interactions at 2 weeks for both CRF and Total T4 and at 8 weeks for oxytocin (Fig. 5). At 2 weeks, CRF levels decreased during breastfeeding for women with lower symptoms, regardless of medication use (n=26, no medications; n=3, with medications), and for women with higher symptoms who were not taking medication (n=10). CRF levels increased during feeding for women who were taking medication but had higher symptoms (n=7). Total T4 levels decreased during feeding for lower-symptom women who were not taking medication and for higher-symptom women who were taking medication but increased for lower-symptom women who were taking medication and for higher-symptom women who were not taking medication. At the 8-week visit, we found the lowest oxytocin levels among women who had higher symptoms despite taking medications (n=6), followed by lower-symptom women taking medication (n=4), higher-symptom women not on medication (n=5), and finally, lower-symptom women not taking medication (n=24). Higher symptoms of depression/anxiety and antidepressant medications were similarly associated with oxytocin area under the curve at 8 weeks (mean oxytocin pg/mL-minutes [SD] for each: lower symptoms/no antidepressants: 139.9 [34.7]; higher symptoms/no antidepressants: 111.7 [23.1]; lower symptoms/antidepressants: 108.5 [11.1]; higher symptoms/antidepressants: 100.6 [30.0], ANOVA p=0.02).
FIG. 5.
Maternal neuroendocrine profile during breastfeeding sessions at 2 weeks (A) and 8 weeks (B) postpartum, among mothers with higher versus lower mood symptoms (+Sx vs. − Sx), defined as EPDS ≥10 and/or Spielberger state anxiety score ≥34, and with (+) or without (–) concurrent antidepressant medication (Meds) use. Least square means±standard error in models adjusting for primiparity and infant age at study visit. Group, time, and group×time p values reported for repeated measures analysis with unstructured covariance. At 2 weeks: n=26 lower Sx / − Meds; n=3 higher Sx / − Meds; n=10 higher Sx / − Meds; n=10 higher Sx /+Meds. At 8 weeks: n=24 lower Sx / − Meds; n=4 higher Sx / − Meds; n=5 higher Sx / − Meds; n=6 higher Sx /+Meds. §Geometric means presented because residuals in untransformed analyses were not normally distributed.
Discussion
In a longitudinal study of women intending to breastfeed, we found differences in oxytocin profile and affect during feeding among women with lower vs. higher mood symptoms. Consistent with our hypothesis, higher depression and anxiety symptoms were correlated with lower levels of oxytocin. We further found that depression scores at 8 weeks were correlated with lower total T4. Contrary to our hypothesis, we did not find differences in duration or exclusive or any breastfeeding by maternal depression/anxiety history, nor did we find statistically significant associations between mood history or symptoms and gonadal steroids, cortisol, CRF, prolactin, or free T4.
These findings confirm and extend earlier work relating maternal mood to breastfeeding experience and neuroendocrine markers during pregnancy and early postpartum. We have previously reported that not liking breastfeeding in the first 2 weeks was associated with depressive symptoms, defined as EPDS >12 at 2 months postpartum, in the Infant Feeding Practices Study II.15 Poor maternal mental/emotional health has also been associated with reduced odds of exclusive breastfeeding at 6 months,26 and anxiety state prior to hospital discharge predicted lower exclusive breastfeeding at 3 months among first-time mothers.27 Furthermore, in animal models, chronic maternal stress is associated with reduced mothering behavior and pup growth.28 Contrary to assertions that breastfeeding prevents postpartum depression,29 we found that exclusive breastfeeding at 2 weeks was associated with increased mood symptoms at 8 weeks in our sample.
Other authors have reported associations between lactogenic hormones and maternal mood. Skrundz et al. recently reported an inverse association between oxytocin levels during pregnancy and EPDS score ≥10 at 2 weeks postpartum.30 Nissen et al. measured the association between personality and neuroendocrine markers in the early postpartum period and found that, among women who gave birth by cesarean section, anxiety was inversely correlated with basal oxytocin and prolactin levels.31 Ours is the first study to our knowledge to measure oxytocin response to the physiologic trigger of breastfeeding among mothers with lower vs. higher mood symptoms. Our findings associating lower oxytocin with higher anxiety and depression suggest that oxytocin dysregulation may contribute to perinatal mood disorders. Further studies are needed to determine whether maternal mood is similarly associated with differences in oxytocin response to other mother–infant interactions.
Our finding of decreased total T4 among women with higher EPDS scores is also consistent with earlier studies. In a longitudinal study, lower mean total T4 and free T4 levels in late pregnancy predicted more severe postpartum depression ratings.32
In our sample, prolactin levels were lower among higher-symptom mothers, although this difference was not statistically significant. Earlier work showed an inverse correlation between alpha-adrenergic activity and prolactin area under the curve during milk expression among mothers of preterm infants,33 as well as inverse correlation between prolactin and norepinephrine AUC during infant interaction in mothers of term infants tested between 2 and 6 months postpartum.34 Other authors have found that both obesity35 and family history of alcoholism36 are associated with blunted prolactin response to suckling.
We also found a non-significant interaction between higher maternal symptoms and CRF trajectory during breastfeeding. This association merits exploration in future studies, given that dysregulated cortisol and hypothalamic pituitary adrenal (HPA) axis reactivity are among the most robust biological findings in major depression39. In a longitudinal study (n=17), Magiakou et al.38 reported a diminished adrenocorticotropic hormone (ACTH) response to ovine corticotropin-releasing hormone (CRH) among women with postpartum depressive symptoms, compared with euthymic mothers. Jolley et al.39 reported higher ACTH and lower cortisol following exercise stress among depressed (n=9) versus non-depressed (n=13) mothers at 12 weeks postpartum. These findings suggest that HPA axis recovery following parturition is delayed in women with mood symptoms.
We found interactions between higher maternal symptoms, medication use, and neuroendocrine markers at 2 weeks (total T4 and CRF) and 8 weeks (oxytocin). Because women with more severe disease are more likely to be treated, an observational study such as ours cannot separate the effect of treatment from the severity of underlying disease. However, antidepressants are known to modulate HPA axis function, which may explain the association between medication use and altered CRF trajectory at 2 weeks.40 This is the first study to our knowledge to measure associations between medication use and oxytocin levels during breastfeeding. Animal studies suggest that citalopram,41 but not fluoxetine,42 is associated with increases in oxytocin. We found that both higher symptoms and antidepressant treatment were associated with lower oxytocin levels during feeding, suggesting that both underlying pathophysiology and antidepressant use are associated with changes in oxytocin release. Our finding that the women who had higher symptoms despite treatment had the lowest oxytocin levels suggests that more severe illness is associated with greater derangements of oxytocin physiology, underscoring the importance of close follow-up, both for management of mood symptoms and lactation support. Given the small number of women in medication and higher-symptom subgroups, further studies will be needed to validate these findings.
This is the first study to our knowledge to measure associations between maternal mood and neuroendocrine markers in established breastfeeding. Strengths of our study include our prospective assessment of psychiatric history and breastfeeding intention prior to birth and our use of standardized scales to measure maternal affect and infant feeding patterns. We further limited our study population to women intending to breastfeed at least three months, ensuring that any weaning that occurred during the study period was unplanned, and therefore more likely to reflect breastfeeding difficulties than personal preference. However, our study also has several limitations, and our findings must be interpreted in the context of the study design. Our sample size was based on the assumption that all mothers in the high-risk group would have perinatal mood symptoms, but our study population had only mild symptoms during follow-up. Using a subclinical threshold, only 54% of high-risk women were symptomatic at 8 weeks, reducing our power to detect differences between symptomatic and asymptomatic women. However, despite the mild symptoms among our participants, we found differences in oxytocin trajectories between more and less symptomatic women. Multiple testing is also a concern. We measured correlations with multiple markers at three time points. Given the exploratory nature of study, we did not adjust for multiple comparisons, and our findings should therefore be viewed as preliminary. Our sample size did not allow us to quantify the impact of maternal use of galactogogues, pumping, and mixed feeding on our results. Mothers who are experiencing breastfeeding problems may adopt these strategies to compensate for an underlying neuroendocrine deficit, or use of these strategies may affect neuroendocrine profiles. Future crossover design studies comparing within-subject profiles during pumping and breastfeeding and with and without galactogogues may be able to disentangle these issues.
Other authors have found that depressive symptoms in the early postpartum period are associated with shorter breastfeeding duration.11,12 We did not find an association between maternal psychiatric history or higher mood symptoms and breastfeeding discontinuation in our sample. However, several factors limited our ability to evaluate this association. Our sample size was small, we limited enrollment to women intending to breastfeed, and follow-up ended at 8 weeks postpartum. Longer follow-up may have revealed differences in breastfeeding duration between more symptomatic and less symptomatic groups. Furthermore, fewer women than we anticipated experienced perinatal depression symptoms. Moreover, many women in our cohort had previously successfully breastfed. It is possible that with longer follow-up, we would have found differences in breastfeeding duration or intensity. The socioeconomic profile of our participants also differed from the general population: more than half reported household incomes>$60,000 per year and post-graduate education. The resources available to these women may have enabled them to continue to breastfeed despite symptoms of depression or anxiety. Further studies enrolling a more diverse population of women are needed to address these issues.
In conclusion, in a prospective cohort study, we found that higher symptoms of depression and anxiety were associated with reduced oxytocin response to breastfeeding at 8 weeks postpartum. These results support our hypothesis that maternal mood symptoms are associated with changes in neuroendocrine response to lactation. Such differences may both predispose women to postpartum depression and interfere with successful breastfeeding. Further research defining the intersection of perinatal mood disorders and neuroendocrine responses to breastfeeding may lead to novel strategies to reduce maternal mood symptoms and enable women to breastfeed successfully, thereby improving health outcomes across two generations.
Acknowledgments
The authors wish to thank the mothers and infants who participated in the study. In addition, we wish to thank Dr. Robert Hamer for statistical advice.
This manuscript was supported by National Institutes of Health (NIH) 5K12HD050113-04 (to AMS), K01DA019949-01A1 (to KG), P01DA022446 (KG), and K23MH085165 (to SMB) and by UL1RR025747 from the National Center for Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosure Statement
Dr. Meltzer-Brody receives research funding from Astra Zeneca. The authors have no other conflicts of interest to declare.
References
- 1.Stuebe AM. Grewen K. Pedersen CA. Propper C. Meltzer-Brody S. Failed lactation and perinatal depression: common problems with shared neuroendocrine mechanisms? J Womens Health (Larchmt) 2012;21:264–272. doi: 10.1089/jwh.2011.3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murray L. Stein A. The effects of postnatal depression on the infant. Baillieres Clin Obstet Gynaecol. 1989;3:921–933. doi: 10.1016/s0950-3552(89)80072-0. [DOI] [PubMed] [Google Scholar]
- 3.Marmorstein NR. Malone SM. Iacono WG. Psychiatric disorders among offspring of depressed mothers: associations with paternal psychopathology. Am J Psychiatry. 2004;161:1588–1594. doi: 10.1176/appi.ajp.161.9.1588. [DOI] [PubMed] [Google Scholar]
- 4.Flynn HA. Davis M. Marcus SM. Cunningham R. Blow FC. Rates of maternal depression in pediatric emergency department and relationship to child service utilization. Gen Hosp Psychiatry. 2004;26:316–322. doi: 10.1016/j.genhosppsych.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 5.Chaudron LH. Nirodi N. The obsessive-compulsive spectrum in the perinatal period: a prospective pilot study. Arch Womens Ment Health. 2010;13:403–410. doi: 10.1007/s00737-010-0154-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abramowitz JS. Meltzer-Brody S. Leserman J, et al. Obsessional thoughts and compulsive behaviors in a sample of women with postpartum mood symptoms. Arch Womens Ment Health. 2010;13:523–530. doi: 10.1007/s00737-010-0172-4. [DOI] [PubMed] [Google Scholar]
- 7.Ip S. Chung M. Raman G, et al. Breastfeeding and Maternal and Infant Health Outcomes in Developed Countries. Evid Rep Technol Assess. 2007;153:1–186. [PMC free article] [PubMed] [Google Scholar]
- 8.Stuebe A. The risks of not breastfeeding for mothers and infants. Rev Obstet Gynecol. 2009;2:222–231. [PMC free article] [PubMed] [Google Scholar]
- 9.Stuebe AM. Schwarz EB. Grewen K, et al. Duration of lactation and incidence of maternal hypertension: a longitudinal cohort study. Am J Epidemiol. 2011;174:1147–1158. doi: 10.1093/aje/kwr227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwarz EB. Ray RM. Stuebe AM, et al. Duration of lactation and risk factors for maternal cardiovascular disease. Obstet Gynecol. 2009;113:974–982. doi: 10.1097/01.AOG.0000346884.67796.ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dennis C-L. McQueen K. The relationship between infant-feeding outcomes and postpartum depression: a qualitative systematic review. Pediatrics. 2009;123:e736–751. doi: 10.1542/peds.2008-1629. [DOI] [PubMed] [Google Scholar]
- 12.Taveras EM. Capra AM. Braveman PA. Jensvold NG. Escobar GJ. Lieu TA. Clinician Support and Psychosocial Risk Factors Associated With Breastfeeding Discontinuation. Pediatrics. 2003;112:108–115. doi: 10.1542/peds.112.1.108. [DOI] [PubMed] [Google Scholar]
- 13.Chaudron LH. Klein MH. Remington P. Palta M. Allen C. Essex MJ. Predictors, prodromes and incidence of postpartum depression. J Psychosom Obstet Gynaecol. 2001;22:103–112. doi: 10.3109/01674820109049960. [DOI] [PubMed] [Google Scholar]
- 14.Dennis CL. The breastfeeding self-efficacy scale: psychometric assessment of the short form. J Obstet Gynecol Neonatal Nurs. 2003;32:734–744. doi: 10.1177/0884217503258459. [DOI] [PubMed] [Google Scholar]
- 15.Watkins S. Meltzer-Brody S. Zolnoun D. Stuebe A. Early breastfeeding experiences and postpartum depression. Obstet Gynecol. 2011;118:214–221. doi: 10.1097/AOG.0b013e3182260a2d. [DOI] [PubMed] [Google Scholar]
- 16.Gaynes BN. Gavin N. Meltzer-Brody S, et al. Perinatal depression: prevalence, screening accuracy, and screening outcomes. Evid Rep Technol Assess (Summ) 2005;119:1–8. doi: 10.1037/e439372005-001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.First MB. Spitzer RL. Gibbon M. Williams JBW. New York: Biometrics Research, New York State Psychiatric Institute; 2002. Structured clinical interview for DSM-IV-TR Axis I disorders, research version, non-patient edition (SCID-I/NP) [Google Scholar]
- 18.Cox JL. Holden JM. Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150:782–786. doi: 10.1192/bjp.150.6.782. [DOI] [PubMed] [Google Scholar]
- 19.Spielberger C. Palo Alto, CA: Consulting Psychologists Press; 1983. Manual for the State-Trait Anxiety Inventory. [Google Scholar]
- 20.Cox JL. Holden JM. Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150:782–786. doi: 10.1192/bjp.150.6.782. [DOI] [PubMed] [Google Scholar]
- 21.Brouwers EP. van Baar AL. Pop VJ. Does the Edinburgh Postnatal Depression Scale measure anxiety? J Psychosom Res. 2001;51:659–663. doi: 10.1016/s0022-3999(01)00245-8. [DOI] [PubMed] [Google Scholar]
- 22.Meades R. Ayers S. Anxiety measures validated in perinatal populations: a systematic review. J Affect Disord. 2011;133:1–15. doi: 10.1016/j.jad.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 23.Li R. Fein SB. Grummer-Strawn LM. Association of breastfeeding intensity and bottle-emptying behaviors at early infancy with infants' risk for excess weight at late infancy. Pediatrics. 2008;122:S77–84. doi: 10.1542/peds.2008-1315j. [DOI] [PubMed] [Google Scholar]
- 24.Grewen KM. Davenport RE. Light KC. An investigation of plasma and salivary oxytocin responses in breast- and formula-feeding mothers of infants. Psychophysiol. 2010;47:625–632. doi: 10.1111/j.1469-8986.2009.00968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pruessner JC. Kirschbaum C. Meinlschmid G. Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28:916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- 26.Jones JR. Kogan MD. Singh GK. Dee DL. Grummer-Strawn LM. Factors associated with exclusive breastfeeding in the United States. Pediatrics. 2011;128:1117–1125. doi: 10.1542/peds.2011-0841. [DOI] [PubMed] [Google Scholar]
- 27.Zanardo V. Gasparetto S. Giustardi A, et al. Impact of anxiety in the puerperium on breast-feeding outcomes: role of parity. J Pediatr Gastroenterol Nutr. 2009;49:631–634. doi: 10.1097/MPG.0b013e31819e6446. [DOI] [PubMed] [Google Scholar]
- 28.Nephew BC. Bridges RS. Effects of chronic social stress during lactation on maternal behavior and growth in rats. Stress. 2011;14:677–684. doi: 10.3109/10253890.2011.605487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gallup GG., Jr. Nathan Pipitone R. Carrone KJ. Leadholm KL. Bottle feeding simulates child loss: postpartum depression and evolutionary medicine. Med Hypotheses. 2010;74:174–176. doi: 10.1016/j.mehy.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 30.Skrundz M. Bolten M. Nast I. Hellhammer DH. Meinlschmidt G. Plasma oxytocin concentration during pregnancy is associated with development of postpartum depression. Neuropsychopharmacology. 2011;36:1886–1893. doi: 10.1038/npp.2011.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nissen E. Gustavsson P. Widstrom AM. Uvnas-Moberg K. Oxytocin, prolactin, milk production and their relationship with personality traits in women after vaginal delivery or Cesarean section. J Psychosom Obstet Gynaecol. 1998;19:49–58. doi: 10.3109/01674829809044221. [DOI] [PubMed] [Google Scholar]
- 32.Pedersen CA. Johnson JL. Silva S, et al. Antenatal thyroid correlates of postpartum depression. Psychoneuroendocrinology. 2007;32:235–245. doi: 10.1016/j.psyneuen.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 33.Chatterton RT., Jr. Hill PD. Aldag JC. Hodges KR. Belknap SM. Zinaman MJ. Relation of plasma oxytocin and prolactin concentrations to milk production in mothers of preterm infants: influence of stress. J Clin Endocrinol Metab. 2000;85:3661–3668. doi: 10.1210/jcem.85.10.6912. [DOI] [PubMed] [Google Scholar]
- 34.Grewen KM. Light KC. Plasma oxytocin is related to lower cardiovascular and sympathetic reactivity to stress. Biol Psychol. 2011;87:340–349. doi: 10.1016/j.biopsycho.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rasmussen KM. Kjolhede CL. Prepregnant overweight and obesity diminish the prolactin response to suckling in the first week postpartum. Pediatrics. 2004;113:e465–471. doi: 10.1542/peds.113.5.e465. [DOI] [PubMed] [Google Scholar]
- 36.Mennella JA. Pepino MY. Breastfeeding and prolactin levels in lactating women with a family history of alcoholism. Pediatrics. 2010;125:e1162–1170. doi: 10.1542/peds.2009-3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gold PW. Gabry KE. Yasuda MR. Chrousos GP. Divergent endocrine abnormalities in melancholic and atypical depression: clinical and pathophysiologic implications. Endocrinol Metab Clin North Am. 2002;31:37–62. doi: 10.1016/s0889-8529(01)00022-6. vi. [DOI] [PubMed] [Google Scholar]
- 38.Magiakou MA. Mastorakos G. Rabin D. Dubbert B. Gold PW. Chrousos GP. Hypothalamic corticotropin-releasing hormone suppression during the postpartum period: implications for the increase in psychiatric manifestations at this time. J Clin Endocrinol Metab. 1996;81:1912–1917. doi: 10.1210/jcem.81.5.8626857. [DOI] [PubMed] [Google Scholar]
- 39.Jolley SN. Elmore S. Barnard KE. Carr DB. Dysregulation of the hypothalamic-pituitary-adrenal axis in postpartum depression. Biol Res Nurs. 2007;8:210–222. doi: 10.1177/1099800406294598. [DOI] [PubMed] [Google Scholar]
- 40.Raison CL. Miller AH. When not enough is too much: the role of insufficient glucocorticoid signaling in the pathophysiology of stress-related disorders. Am J Psychiatry. 2003;160:1554–1565. doi: 10.1176/appi.ajp.160.9.1554. [DOI] [PubMed] [Google Scholar]
- 41.Uvnas-Moberg K. Bjokstrand E. Hillegaart V. Ahlenius S. Oxytocin as a possible mediator of SSRI-induced antidepressant effects. Psychopharmacology (Berl) 1999;142:95–101. doi: 10.1007/s002130050867. [DOI] [PubMed] [Google Scholar]
- 42.Marar IE. Amico JA. Vasopressin, oxytocin, corticotrophin-releasing factor, and sodium responses during fluoxetine administration in the rat. Endocrine. 1998;8:13–18. doi: 10.1385/ENDO:8:1:13. [DOI] [PubMed] [Google Scholar]