Abstract
Objective
To longitudinally assess the association between plasma viral load (PVL) and genital tract human immunodeficiency virus (GT HIV) RNA among HIV-1 infected women changing highly active antiretroviral therapy (HAART) because of detectable PVL on current treatment.
Methods
Women were eligible for the study if they had detectable PVL (defined as two consecutive samples with PVL>1000 copies/mL) and intended to change their current HAART regimen at the time of enrollment. Paired plasma and GT HIV-1 RNA were measured prospectively over 3 years. Longitudinal analyses examined rates of GT HIV-1 RNA shedding and the association with PVL.
Results
Sixteen women were followed for a median of 11 visits contributing a total of 205 study visits. At study enrollment, all had detectable PVL and 69% had detectable GT HIV-1 RNA. Half of the women changed to a new HAART regimen with ≥3 active antiretroviral drugs. The probability of having detectable PVL ≥30 days after changing HAART was 0.56 (95% CI: 0.37 to 0.74). Fourteen women (88%) had detectable PVL on a follow-up visit ≥30 or 60 days after changing HAART; and 12 women (75%) had detectable GT HIV-1 RNA on a follow-up visit ≥30 or 60 days after changing HAART. When PVL was undetectable, GT shedding occurred at 11% of visits, and when PVL was detectable, GT shedding occurred at 47% of visits.
Conclusions
Some treatment-experienced HIV-infected women continue to have detectable virus in both the plasma and GT following a change in HAART, highlighting the difficulty of viral suppression in this patient population.
Introduction
Virologic failure while on highly active antiretroviral therapy (HAART) remains a challenging issue for human immunodeficiency (HIV)-1–infected individuals with prior treatment experience.1 In clinical practice, the ability to achieve virologic suppression in both the genital tract (GT) and plasma can be compromised by limited drug options due to accumulating drug resistance, lack of adequate treatment adherence, and inappropriate drug choice.2 Among treatment-experienced HIV-infected individuals in care, almost half may harbor a drug-resistant virus,3 and the heterosexual and vertical transmission of drug-resistant virus has been documented.4–6
The sexual and vertical transmission of HIV results from the presence of the virus in the GT;7,8 however, plasma HIV-1 RNA levels have been used clinically as a surrogate measure for GT HIV-1 RNA to determine the risk of transmission.9 Studies have demonstrated that HAART suppresses viral replication not only in the blood and lymphoid tissues, but also in the male and female GT.10 GT HIV-1 RNA is correlated with plasma HIV-1 RNA (or plasma viral load, PVL),11 and the presence of plasma HIV-1 RNA is the strongest predictor of GT HIV-1 RNA detection.12 However, women receiving HAART may still be infectious at some time points due to GT shedding despite suppressed viremia in the plasma. A previous longitudinal study by our group found that over half of the women had genital HIV-1 RNA shedding.13 A cross-sectional study by Kovacs et al. also found HIV-1 RNA in the genital secretions of over half the women.14 Neely et al. found detectable HIV-1 RNA in the genital secretions in 15% of the women.15 Beyond HIV-1 RNA in the plasma, other factors have also been shown to impact HIV-1 RNA shedding in the genital tract, including ulcerative disease, exudate, inflammation-associated cellular changes, cervical ectopy, sexually transmitted infections, menstrual cycle, alcohol use, and hormonal contraception.16 A persistent question has been whether a subcompartment of the female genital tract (i.e., ectocervix, endocervix, or vagina) preferentially sheds virus.17 In a previous study, we found that in almost three-fourths of women, only a single subcompartment had evidence of viral shedding, suggesting sampling from only one subcompartment could fail to detect genital tract HIV-1 RNA.18
Data from HIV serodiscordant couples have repeatedly demonstrated the association between viral suppression and decreased HIV sexual transmission.19,20 In the era of HAART, HIV-infected partners in discordant relationships were substantially less likely to transmit HIV to their partners if they were on HAART,21 and populations with higher levels of HAART coverage had lower population viral loads and a lower number of HIV diagnoses.22,23 Recent data have shown that HAART can decrease the risk of HIV sexual transmission within serodiscordant couples by 96%.24,25
It is hypothesized that there are patients, representing a segment of the treatment-experienced HIV-infected population with limited effective HAART options, who may continue to have uncontrolled plasma and/or GT viral load and who may facilitate the spread of HIV. The objective of this study was to evaluate and describe patterns of GT HIV-1 RNA shedding in treatment-experienced women. PVL and GT HIV-1 RNA were longitudinally assessed among women changing HAART regimens because of detectable PVL on current HAART. Understanding patterns of GT HIV-1 shedding in treatment-experienced women with unsuppressed PVL and potential drug resistance has implications for both clinical management and for HIV secondary transmission.26,27
Methods
Study setting and patient population
Participants included HIV-1 infected women on HAART at the Miriam Hospital Immunology Center (Providence, RI) between September 2003 and September 2008. At the time of the current study, the Immunology Center provided care to 984 patients, of whom 37% were women, most (55%) below 44 years of age; 29% of patients identified as African-American and 18% as Latino/a. Women were recruited in the course of clinical care by their primary HIV physician provider. Eligibility criteria for this observational study included: (1) detectable PVL (defined as 2 consecutive PVL samples>1000 copies/mL), and (2) intention of the HIV clinician to change the current HAART regimen at the time of enrollment. Women were followed at 2 weeks following enrollment and then every 4 weeks until their PVL was undetectable (≤80 copies/mL). Once PVL became undetectable, study visits occurred every 3 months for 36 months. All participants were reimbursed at each study visit with a $25 cash voucher. The Miriam Hospital Institutional Review Board approved the study.
At enrollment, demographic information and medical and reproductive histories were collected; and women were tested for Neisseria gonorrheae and Chlamydia trachomatis by culture and Treponema pallidum and herpes simplex virus 2 (HSV2) by serology. At all study visits, including enrollment, women provided blood for PVL testing and underwent pelvic examinations for collection of GT secretions, testing for HIV-1 RNA, the presence of semen, and wet mount for Trichomonas vaginalis, bacterial vaginosis, and Candida infection. HSV-2 polymerase chain reaction (PCR) was done at follow-up visits on all cervicovaginal lavage samples. In addition, at each visit, a questionnaire was administered to collect information on self-reported HAART intake, last menstrual period, contraception use, vaginal sex with a male partner, and condom use. If a woman was diagnosed with a GT infection, she was treated and asked to come back two weeks after completing treatment to ensure resolution of the infection and for repeating PVL, GT HIV-1 RNA, and GT infection assessment. During follow up, only symptomatic women were tested for N. gonorrheae, C. trachomatis, and T. pallidum.
Laboratory measures
Screening PVL was quantitated by branched DNA (Siemens Corporation) with a lower limit of detection of 75 copies/mL. After enrollment into the study, PVL and GT HIV-1 RNA were quantitated by NucliSens® HIV-1 QT (bioMérieux, Inc.), with a lower-detection limit of 80 copies/mL for plasma. Samples were invalid when there was not adequate amplification of the internal calibrators. GT secretions were collected using Sno-strip filter paper from the endocervix, ectocervix, and vagina. After Sno-strip manufacture was discontinued in late 2005, Tear flo strips were substituted. For genital specimens the volume collected was 24 μL (3 Sno-Strips or 2 Tear flo per subcompartment), and the limit of detection of the test was 3300 copies/mL. Women who had a hysterectomy contributed only vaginal samples. A cervicovaginal lavage (CVL) using 10 mL of normal saline was also collected and processed for white blood cell counts using a Neubauer hemacytometer. The presence of seminal fluid in GT secretions was detected using the ABA card P30 test. Every attempt was made to procure a sample from each genital subcompartment (vagina, endocervix, and ectocervix); but at some visits, women did not have adequate secretions to be able to obtain a specimen from each genital subcompartment. In addition, every attempt was made to quantify the HIV-1 RNA from each sample, and in case of invalid viral load measurements, viral load quantification was repeated.
Clinical data
Clinical data, including CD4 cell count, antiretroviral (ARV) history, and reported ARV resistance mutations were collected from patient medical records by two study authors (KKV and SC). The nadir CD4 cell count was defined as the lowest documented cell count. CD4 cell count and genotyping were obtained at baseline and were determined as part of routine clinical management during the course of the study. Drug resistance mutations were defined according to the current International AIDS Society-USA mutation list.28 Predicted drug susceptibility was done with HIVDB scores (http://hivdb.stanford.edu), divided into five categories, namely susceptible, potential low-level resistance, low-level resistance, intermediate resistance, and high-level resistance. Active medications refer to ARVs to which the HIV had no major resistance. While we use the term HAART to refer to combination therapy with at least three ARVs from at least 2 drug classes, we employ the term ARV to refer to any single drug.
Statistical analysis
The probability of detectable PVL and of GT HIV-1 RNA during follow-up and at visits with undetectable and detectable PVL, and the odds of GT HIV-1 RNA shedding when PVL was detectable versus undetectable, were estimated using longitudinal models for binary outcomes (GT shedding yes/no) fitted using generalized estimating equations (GEE) with exchangeable within-subject correlation. Robust standard errors were used to calculate 95% confidence intervals (95% CI). The binary outcomes were detectable GT HIV-1 RNA in each genital subcompartment (endocervix, ectocervix, and vagina), and detectable GT HIV-1 RNA in at least one genital subcompartment (endocervix or ectocervix or vagina). Only visits ≥30 days following the change from the prior HAART regimen to the new regimen were analyzed. To ensure the results were not sensitive to the selection of 30 days, 60 days after changing HAART was also evaluated and any substantial differences described. For the odds of GT HIV-1 RNA shedding when PVL was detectable versus undetectable, both cross-sectional (between subject) and longitudinal (within subject) effects were estimated. To address potential confounding in the relationship between PVL and GT HIV-1 RNA detection, these models were adjusted for hysterectomy status (when applicable), CD4 cell count, and bacterial vaginosis (other GT infections were of low prevalence in the cohort). Although menstrual cycle phase may possibly affect GT HIV-1 RNA levels, with only 9 women with regular menstrual cycles, it was not possible to examine the relationship between menstrual phase and GT shedding in this dataset. All statistical analyses were conducted using R (version 2.9.0; Vienna, Austria, 2009). A p-value below 0.05 was considered statistically significant.
Results
A total of 16 women contributed 205 study visits with paired plasma and GT samples. There were a total 189 follow-up visits, the median number per woman was 11 (range 4–19 visits); 154 of these visits (81%) were ≥30 days, and 137 (72%) were ≥60 days after changing HAART. Table 1 presents the baseline characteristics of the women at study enrollment. The median age was 42 years (range 32 to 65). Three women had undergone a hysterectomy. The median period of ARV exposure was 6.9 years and the median period on HAART was 6.1 years. The median time on baseline HAART was 3.8 years (range: 0.3 to 8.9). About two-thirds of the women (63%) reported being sexually active in the prior 6 months, with 10/11 (91%) of them reporting condom use at their last sexual encounter. Four women (25%) were diagnosed with bacterial vaginosis and two women (13%) with T. vaginalis. All women were HSV-2 immunoglobin G antibody positive at baseline.
Table 1.
Demographic, Clinical, Treatment, and Behavioral Characteristics of Study Participants at Enrollment (N=16)
| Characteristic | Value* |
|---|---|
| Demographics | |
| Age (years) | 42 (32 to 65) |
| Race: | |
| Black/African American | 6/16 (38%) |
| Hispanic/Latina-Black | 2/16 (13%) |
| Hispanic/Latina-White | 4/16 (25%) |
| White/Non Hispanic | 4/16 (25%) |
| Clinical measures | |
| PVL (copies/mL) | 13,500 (1800 to 780,000) |
| CD4 cell count (cells/μL) | 224 (63 to 591) |
| Nadir CD4 cell count (cells/μL ) | 84 (10,389) |
| Treponema pallidum | 0/16 (0%) |
| Neisseria gonorrheae | 0/16 (0%) |
| Chlamydia trachomatis | 0/16 (0%) |
| Trichomonas vaginalis | 2/16 (13%) |
| Bacterial vaginosis | 4/16 (25%) |
| Candida albicans | 0/16 (0%) |
| Semen | 0/16 (0%) |
| Taking oral contraceptives | 2/16 (13%) |
| Hysterectomy | 3/16 (19%) |
| HIV treatment history | |
| Years of ARV exposure | 6.9 (2.9 to 13.9) |
| Years on HAART | 6.1 (2.9 to 10.5) |
| Years on current HAART regimen | 3.8 (0.3 to 8.9) |
| HIV sexual risk behaviors | |
| Sexually active in the last 6 months | 9/14 (64%) |
| Used a condom at last sexual encounter | 10/11 (91%) |
| Ever injected drugs | 6/16 (38%) |
| Ever have sex with an injection drug user | 5/16 (31%) |
| Ever have sex with an HIV positive man | 10/16 (63%) |
| Ever have sex for drugs or money | 4/16 (25%) |
Results presented as median (range) for continuous and as n/N (%) for categorical measures.
ARV, antiretroviral; HAART, highly active antiretroviral therapy; PVL, plasma viral load.
Fourteen women changed to their new HAART regimen at the time of study enrollment. However, two women changed regimens during follow up, one prior to her second follow-up visit on day 50 and one prior to her fourth follow-up visit on day 85. Almost all (94%) were sexually active at some point during the study. At visits during which women reported being sexually active, most women reported using condoms (85%). However, among those women who were sexually active, 31% reported not using a condom at least once during the study. On follow-up, 75% of women were diagnosed with bacterial vaginosis and 44% were diagnosed with candida vaginitis; one woman was diagnosed with trichomoniasis and two women tested PCR positive for HSV-2. Five women (31%) had semen detected in the GT.
Patient-level data
Table 2 presents the baseline and nadir CD4 cell counts, PVL, GT HIV-1 RNA by subcompartment (endocervix, ectocervix, and vagina), HAART regimen, and plasma drug resistance profiles with major nucleoside reverse transcriptase inhibitor (NRTI)/non-nucleoside reverse transcriptase inhibitor (NNRTI) and protease inhibitor (PI) mutations at enrollment. The ordering of rows in Table 2 was based on the number of active ARV agents in each woman's newly changed treatment regimen, separating women with ≥3 ARVs from those with ≤2 ARVs. Ten women (63%) were receiving an NNRTI-based HAART regimen and 5 (31%) were receiving a PI-based HAART regimen at enrollment. Women had relatively low CD4 cell counts (median 224 cells/μL), and the median nadir CD4 cell count was 84 cells/μL. All women had confirmed detectable PVL, ranging from 1800 copies/mL to as high as 780,000 copies/mL, and 11 women (69%) had detectable GT HIV-1 RNA, ranging from 3760 copies/mL to 112,000 copies/mL. Among the 13 women who did not have a hysterectomy, 2 women had GT HIV-1 RNA detectable in all 3 subcompartments. The most common NRTI/NNRTI mutations in the plasma viral population at the baseline visit were M184V (56%), K103N (44%), T215Y (31%), M41L (25%), L210W (25%), and L74V (25%). Six women also had available genotypes from the GT at baseline, and the detected mutations were concordant with plasma.
Table 2.
Current and Nadir CD4 Cell Count, Plasma Viral Load, Genital Tract HIV-1 RNA, Highly Active Antiretoviral Therapy Regimen, and Antiretroviral Resistance Profile at Baseline
| |
|
|
|
|
GT HIV-1 RNA by compartment (copies/mL) |
|
|
Drug resistance profile at baseline |
|
|||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient No. | Hyst. | Baseline CD4 (cells/μL) | Nadir CD4 (cells/μL) | PVL (copies/mL) | Endocervix | Ectocervix | Vagina | HAART regimen at baseline | New HAART regimen after changea | Major NRTI/NNRTI mutations | Major (and minor) PI mutations | Number of active drugs in new HAART regimen |
| Women with 3 or more active ARVs in HAART regimen after change | ||||||||||||
| 1 | No | 225 | 175 | 2800 | 4720 | ≤3300 | ≤3300 | 3TC, d4T, EFV | DDI3, TDF2, ATZ3 | M184V, T215Y/Y188L, | D30N, (L63P, A71V), N88D | 3 |
| 2b | No | 98 | 10 | 11,000 | 3760 | ≤3300 | 11,200 | AZT, 3TC, EFV | DDI1, TDF1, ATZ1 | M184V/K103N, P225H | (L63P) | 3 |
| 3b | No | 110 | 48 | 11,000 | 4240 | 46,400 | 18,400 | AZT, 3TC, NVP | ABC1, TDF1, LPV1 | M184V/K103N | – | 3 |
| 4 | No | 185 | 120 | 18,000 | 10,400 | ≤3300 | ≤3300 | AZT, 3TC, EFV | AZT1, TDF1, FTC5, LPV1 | M184V/L100I, K103N, | (L33I/V, M36I, L63P) | 3 |
| 5 | No | 591 | 389 | 27,000 | ≤3300 | ≤3300 | ≤3300 | 3TC, DDI, EFV | TDF1, FTC1, ATZ1 | K103N | (L63P, A71T) | 3 |
| 6c | No | 230 | 73 | 780,000 | 4880 | ≤3300 | 5200 | TDF, FTC, EFV | TDF1, FTC1, LPV1 | – | (L63P) | 3 |
| 7 | No | 237 | 46 | 150,000 | 104,0000 | 880,000 | NA | TDF, FTC, FPV | DDI1, ABC2, TPV1 | M184V | (L63P) | 3 |
| 8 | Yes | 214 | 138 | 10,000 | – | – | ≤3300 | DDI, TDF, FPV | AZT1, 3TC1, ABC1, DRV1 | – | (L10I, L63P, A71T, G73S), L90M | 4 |
| Women with 2 or fewer active ARVs in HAART regimen after change | ||||||||||||
| 9 | No | 108 | 31 | 16,000 | ≤3300 | ≤3300 | ≤3300 | 3TC, DDI, EFV | 3TC5, TDF1, ATZ1 | L74V, Y115F, M184V/L100I, K103N, P225H | (M36I, L63P) | 2 |
| 10b,c | No | 223 | 17 | 32,000 | 26,400 | NA | NA | d4T, TDF, EFV | d4T4, TDF2, ATZ1 | D67N, K70R, K219E | (L63P) | 2 |
| 11 | No | 416 | 79 | 2600 | ≤3300 | ≤3300 | 5360 | AZT, 3TC, ABC | EFV1, ATZ1 | M41L, D67N, M184V, L210W, T215Y | (M36I, L63P) | 2 |
| 12b,c | Yes | 230 | 88 | 21,000 | – | – | 13,000 | AZT, 3TC, NVF | DDI4, TDF3, EFV1 | M184V, L210W, T215Y | D30N, (L63P, A71T), N88D | 2 |
| 13b,c | No | 63 | 11 | 390,000 | 136,000 | 408,000 | 304,000 | 3TC, DDI, TDF, LPV | AZT4, 3TC5, TDF3, LPV5 | M41L, L74V, M184V, L210W, T215F/L100I, K103N, | (L10I), I154V, (L63P, A71V), V82A, I84V, L90M, (V11VI) | 1 |
| 14b,c | No | 353 | 265 | 4500 | ≤3300 | ≤3300 | ≤3300 | DDI, TDF, NVP | TDF4, FTC3, NVP5 | M41L, L74V, L210W, T215Y/V108I, Y181C, | – | 1 |
| 15 | No | 307 | 141 | 1800 | ≤3300 | ≤3300 | 112,0000 | 3TC, d4T, LPV | TDF1, FTC5, LPV4 | M184V/K103N | (L10F), M46I, I54V, I84V | 1 |
| 16c | Yes | 189 | 120 | 11,000 | – | – | ≤3300 | DDI, TDF, ATZ | TDF4, FTC1, ATZ5 | M41L, L74V, T215Y/ K103N, | (L10R, K20M, M36I), M46L, (L63P, G73S), V82T, L90M | 1 |
Regimens coded from 1 to 5 based on level of drug resistance using the baseline genotype: (1) susceptible, (2) potential low level resistance, (3) low-level resistance, (4) intermediate resistance, and (5) high-level resistance. Antiretrovirals with either intermediate or high baseline resistance were considered to not be active agents in the changed HAART regimen.
Refers to women who also had genital tract genotype profiles.
Refers to women who had follow-up genotype profiles.
NRTIs, nucleoside reverse transcriptase inhibitors; AZT, zidovudine; 3TC, lamivudine; DDI, didanosine; TDF, tenofovir; d4T, stavudine; FTC, emtricitabine; ABC, abacavir; NVF, nelfinavir; LPV, lopinavir; ATZ, atazanvir; FPV, fosamprenavir; DRV, darunavir; TPV, tipranivir; NNRTIs, non-nucleoside reverse transcriptase inhibitors; EFV, efavirenz; GT, genital tract; Hyst., hysterectomy status; PI, protease inhibitor.
Nine women changed from NNRTI-based HAART at enrollment to a PI-based regimen, and one woman on PI-based HAART changed to a NNRTI-based regimen; the remainder changed to another HAART regimen within the same drug class. Half of the women changed to new HAART regimens with ≥3 active ARVs, while the rest changed to regimens with ≤2 active ARVs. On follow-up, thirteen women (81%) had detectable GT HIV-1 RNA detected in at least one genital subcompartment; 67% of women had detectable GT HIV-1 RNA in the vagina, 77% in the ectocervix, and 85% in the endocervix.
Patterns of GT HIV-1 RNA shedding
The probability of having detectable PVL ≥30 days after changing HAART was 0.56 (95% CI: 0.37 to 0.74), with a similar estimate using data ≥60 days after changing HAART. Fourteen of 16 women (88%) had a detectable PVL on a follow-up visit ≥30 or 60 days after changing HAART; and 12 women (75%) had detectable GT HIV-1 RNA on a follow-up ≥30 or 60 days after changing HAART.
At visits ≥30 days after changing HAART regimens, the estimated overall probabilities of GT HIV-1 RNA shedding were between 21% and 25% of visits in each of the three genital subcompartments (Table 3); GT HIV-1 RNA shedding in at least one subcompartment was estimated to occur at 31% of visits (95% CI: 21% to 45%). When PVL was undetectable, shedding occurred in at least one of the three subcompartments at 11% of visits; when PVL was detectable, shedding occurred in at least one of three subcompartments at 47% of visits. The odds of shedding in each subcompartment and in at least one of the subcompartments was significantly increased when PVL was detectable. The between-participant (or cross-sectional) adjusted odds ratio (OR) of shedding ranged from 2.6 (95% CI: 0.80 to 8.7) for shedding in at least one subcompartment to 5.5 (95% CI: 1.4 to 22.7) for shedding in the vagina. The within-participant (or longitudinal) adjusted OR of shedding ranged from 11.0 (95% CI: 3.1 to 38.7) in at least one subcompartment to 32.2 (95% CI: 5.2 to 199.4) in the vagina. The results from models fitted utilizing visits ≥60 days after changing HAART had similar point estimates and considerable overlap of the confidence intervals (See Table 4).
Table 3.
Probability (95% CI) of Having HIV-1 RNA Detected in the Genital Tract over All Visits and Stratified When Plasma Viral Load Was Detectable and Undetectable, and the Between-Subject and Within-Subject Odds Ratios of Having Genital Tract Shedding When Plasma Viral Load is Detectable Versus Undetectable, at Visits ≥30 Days after Changing Highly Active Antiretroviral Therapy Regimens
| GT subcompartment | Overall probability of shedding | Overall probability of shedding with PVL ≤80 | Overall probability of shedding with PVL>80 | Between subject OR of shedding PVL>80 vs. PVL ≤80 at baselinea | Within-subject OR of shedding when PVL >80 vs. PVL ≤80 at follow-upb |
|---|---|---|---|---|---|
| Ectocervix | 0.25 (0.15, 0.39) | 0.05 (0.01, 0.28) | 0.41 (0.26, 0.59) | 4.8 (0.66, 35.4)3 | 17.0 (1.8, 158.5)c |
| Endocervix | 0.21 (0.12, 0.35) | 0.05 (0.02, 0.17) | 0.34 (0.19, 0.53) | 3.7 (0.66, 21.0)3 | 6.2 (1.03, 37.5)c |
| Vagina | 0.23 (0.13, 0.39) | 0.04 (0.01, 0.16) | 0.37 (0.22, 0.55) | 5.5 (1.4, 22.7)4 | 32.2 (5.2, 199.4)d |
| At least one subcompartment | 0.31 (0.21, 0.45) | 0.11 (0.03, 0.31) | 0.47 (0.35, 0.60) | 2.6 (0.80, 8.7)4 | 11.0 (3.1, 38.7)d |
Between-subject or cross-sectional effect: ratio of the odds of GT shedding when PVL>80 versus PVL ≤80 for women with PVL ≤80 at baseline compared to women with PVL>80 at baseline.
Within-subject or longitudinal effect: odds ratio of GT shedding for women who transition from PVL ≤80 at baseline to PVL>80 at follow-up.
Model adjusted for baseline CD4, change in CD4, baseline BV, change in BV from baseline.
Model adjusted for baseline CD4, change in CD4, baseline BV, change in BV from baseline, and hysterectomy status (yes/no).
OR, odds ratio; BV, bacterial vaginosis.
Table 4.
Probability (96% CI) of Having HIV-1 RNA Detected in the Genital Tract over All Visits and Stratified When Plasma Viral Load Was Detectable and Undetectable, and the Between-Subject and Within-Subject Odds Ratios of Having Genital Tract Shedding when Plasma Viral Load is Detectable Versus Undetectable, at Visits ≥60 Days after Changing Highly Active Antiretroviral Therapy Regimens
| GT subcompartment | Overall probability of shedding | Overall probability of shedding with PVL ≤80 | Overall probability of shedding with PVL>80 | Between subject OR of shedding PVL>80 vs. PVL ≤80 at baselinea | Within-subject OR of shedding when PVL>80 vs. PVL ≤80 at follow-upb |
|---|---|---|---|---|---|
| Ectocervix | 0.23 (0.14, 0.37) | 0.04 (0.01, 0.21) | 0.40 (0.25, 0.57) | 15.1 (1.5, 156.6)c | 20.1 (0.86, 466.4)c |
| Endocervix | 0.21 (0.11, 0.34) | 0.06 (0.02, 0.18) | 0.33 (0.18, 0.52) | 6.9 (0.53, 88.8)c | 4.8 (1.01, 22.9)c |
| Vagina | 0.22 (0.13, 0.37) | 0.02 (0.003, 0.10) | 0.37 (0.22, 0.54) | 9.3 (0.84, 101.5)d | 27.6 (2.9, 266.8)d |
| At least one subcompartment | 0.32 (0.21, 0.45) | 0.10 (0.03, 0.28) | 0.50 (0.38, 0.62) | 6.5 (2.6, 16.8)d | 6.5 (3.0, 14.1)d |
Between-subject or cross-sectional effect: ratio of the odds of GT shedding when PVL>80 vs. PVL ≤80 for women with PVL ≤80 at baseline compared to women with PVL>80 at baseline.
Within-subject or longitudinal effect: odds ratio of GT shedding for women who transition from PVL ≤80 at baseline to PVL>80 at follow-up.
Model adjusted for baseline CD4, change in CD4, baseline BV, and change in BV from baseline.
Model adjusted for hysterectomy status (yes/no), baseline CD4, change in CD4, baseline BV, and change in BV from baseline.
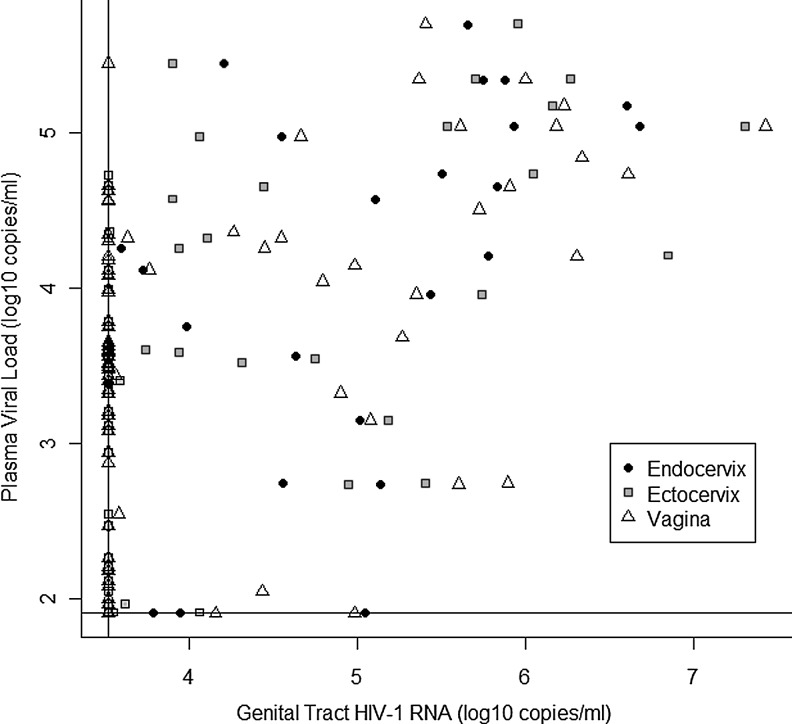
At visits after changing HAART (i.e., visits ≥30 days on new regimen), the higher the PVL, the higher the GT HIV-1 RNA; however, there were also instances in which women with undetectable PVL continued to have GT HIV-1 RNA shedding (see Fig. 1). The mean quantity of GT HIV-RNA when PVL was detectable was 238,200 copies/mL in the endocervix, 575,500 copies/mL in the ectocervix, and 556,700 copies/mL in the vagina. At visits ≥30 days after changing HAART, the mean quantity of GT HIV-1 RNA when PVL was undetectable was 5318 copies/mL in the endocervix, 3596 copies/mL in the ectocervix, and 4873 copies/mL in the vagina.
FIG. 1.
Scatterplot of plasma viral load log10(plasma viral load [PVL]) versus log10 (genital tract human immunodeficiency virus [GT HIV]-1 RNA) at visits ≥30 days after changing highly active antiretroviral therapy (HAART) regimens. Horizontal and vertical lines represent the lower limit of assay detection.
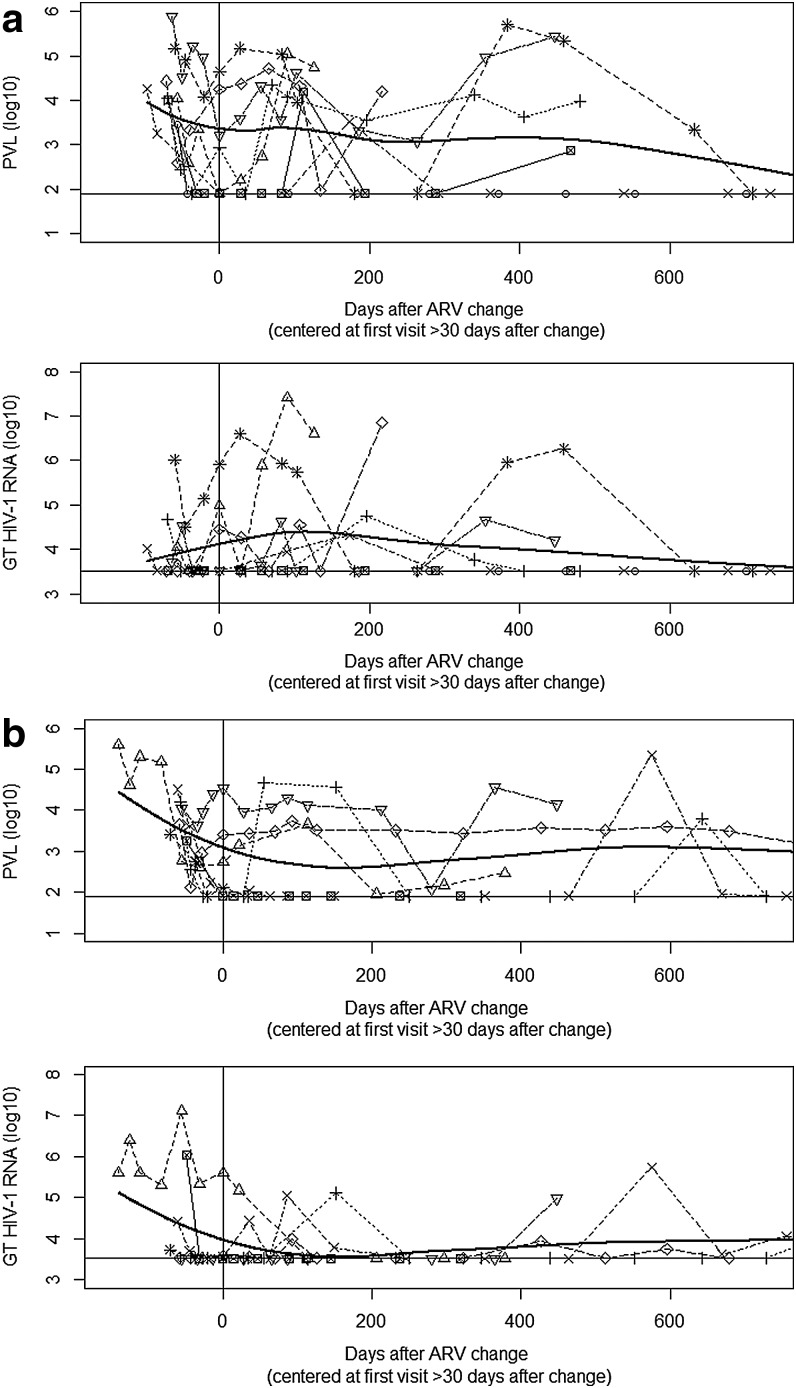
Figure 2a documents the longitudinal pattern of PVL and GT HIV-1 RNA at study visits following changing HAART among women on ≥3 active ARVs. In general, both PVL and GT HIV-1 RNA decreased slightly at the time of changing regimens but then remained detectable. Two out of 8 women on ≥3 active ARVs achieved sustained virological suppression in both the GT and plasma. When all women, regardless of number of active ARVs, were included, the longitudinal pattern of PVL and GT HIV-1 RNA was not markedly different. Figure 2b documents the longitudinal pattern of PVL and GT HIV-1 RNA at study visits following changing HAART among women on<3 active ARVs.
FIG. 2.
(a) Log10(PVL) and log10(GT HIV-1 RNA) at study visits following changing HAART regimens among women receiving ≥3 active antiretrovirals (ARVs). (b) Log10(PVL) and log10(GT HIV-1 RNA) at study visits following changing HAART regimens among women receiving<3 active ARVs. Horizontal lines denote the lower limit of detection of the assays. The vertical line denotes the first visit the participant was on the new changed HAART regimen for ≥30 days. The thick black curve represents a smooth polynomial curve fitted to the data using weighted least squares.
Seven women (44%) changed HAART more than once following enrollment. Six of these seven women underwent repeat genotyping from the plasma. All six women developed additional resistance mutations. Thirteen mutations were accumulated overall (on average 2 per patient), 9 NRTI/NNRTI- and 4 PI-associated. The most common additional NRTI/NNRTI mutation was M184V (3/6) and PI mutation was M36I (2/6). All but one of these women who developed additional mutations were receiving ≤2 active ARVs.
Discussion
The current study conducted among a clinic population of treatment-experienced HIV-1 infected women followed over time demonstrates that some of these women will continue to have detectable virus in both the plasma and GT, highlighting the difficulty of suppressing plasma and GT HIV-1 RNA in this group of patients. There have been few studies to prospectively assess GT HIV-1 RNA shedding among women changing HAART due to treatment failure. In clinical practice, using PVL as a surrogate measure for GT HIV-1 RNA, which is harder to obtain and quantify, and for which a commercial assay is not available, is a reasonable option. The findings of the current study highlight the complexities of effective treatment management for extensively HAART-experienced women. These difficulties include the need for an experienced HIV care provider for appropriate clinical decision making to optimally select ARVs from limited drug options, optimizing drug adherence, and maintaining patient engagement in clinical care.
Data from treatment-naïve women have shown that suppression of HIV-1 RNA in the GT can be rapid; however, genital shedding may still occur intermittently for a substantial proportion of women with well-controlled systemic HIV replication.29,30 Graham et al. documented that within a cohort of 20 ARV-naïve Kenyan women, half of the women had detectable HIV-1 RNA or proviral DNA in the GT at 4 weeks on HAART.30 Nagot et al. found that among 39 ARV-naïve women in Burkina Faso, only 2 women had persistently detectable genital HIV-1 RNA after 18 weeks on HAART, whereas almost half of the women (16/34) with consistently undetectable plasma HIV-1 RNA shed HIV-1 in the GT at least once between 18 and 28 weeks on HAART.29 The current study conducted among treatment-experienced women in the United States with substantially longer follow-up showed that the response to therapy measured by plasma and GT viral levels may be further attenuated compared to short-term outcomes among treatment-naïve women.
Though the detection of HIV-1 RNA in the GT was primarily driven by shedding in the plasma, when PVL was undetectable, shedding occurred in the GT at 11% of visits. In a previous study conducted among treatment-experienced women with undetectable virus in the plasma, over a third of the women demonstrated discordance between plasma and GT HIV-1 RNA. Other studies have similarly noted viral compartmentalization in the blood and genital tract.14,15,31,32 Though genital tract shedding is primarily driven by plasma viremia, clinicians may not be able to solely rely on HAART to eradicate the potential for the sexual and perinatal transmission of HIV.
In the current study, half of the women changed to HAART regimens with ≥3 active ARVs. However, PVL and GT HIV-1 RNA shedding did not substantially vary in these women compared to the overall cohort, suggesting that the lack of viral suppression in both the plasma and GT was likely not due to a lack of active ARVs. It is more likely that women may not have adequately adhered to their new HAART regimens. The ability of ARV drugs to reach the GT in concentrations adequate to inhibit local viral replication may be important to prevent the evolution of drug-resistant variants of HIV in the GT while on HAART. Data have shown that PIs and NNRTIs penetrate poorly into the GT relative to NRTIs.33 All but three women in the current study were changed to regimens containing tenofovir, which has been shown to reach high concentrations in genital secretions.2 The current study did not measure drug levels in the GT, and future studies are needed to assess the relationship between GT drug concentrations and viral rebound.
Beyond clinical management, these findings, which include the longitudinal assessment of GT HIV-1 RNA, have implications for HIV sexual transmission among treatment-experienced women. Recent data show that GT HIV-1 RNA predicts sexual transmission of HIV independently of PVL.10,24 Though most women in this study reported protected sex with condoms, almost all women were sexually active and almost one-third did not use a condom at some point during the study. Due to the presence of drug resistance in this population of women, there could be potential transmission of drug resistant virus.5,6
There were several limitations to note in the current study. The small sample size may limit the generalizability of our findings and our precision. Additional factors have also been identified that may impact GT HIV-1 RNA shedding, including alcohol use, cervical ectopy, and exudate, which were not assessed in the current study. We were able to collect menstrual data on only nine women who were premenopausal; however, since the current study was not designed to examine the effect of menstrual phase on GT HIV-1 RNA shedding and since GT shedding was primarily driven by plasma HIV-1 RNA in these patients, we were not able to assess the effect of menstrual cycle phase on GT HIV-1 RNA shedding. Since this was an observational study, treatment changes were driven by the decisions of the primary care provider. GT HIV-1 RNA was not assessed in real time to be used for clinical purposes. Data spanning a time period from 2003–2008 may not be representative of currently available HAART regimens. There are currently new classes of ARVs for HAART-experienced patients needing to change therapy due to treatment failure, such as integrase inhibitors (i.e., raltegravir), new NNRTIs (i.e., etravirine), and entry inhibitors (i.e., maraviroc). Sexual risk behaviors and treatment adherence utilized participant self-report, which can be susceptible to recall bias. ARV intake was assessed by asking women whether they were taking their ARVs, but did not utilize more detailed adherence measures. Only some women had available GT genotyping, but these results were concordant with plasma genotyping. Earlier data among HAART-experienced viremic patients have found that plasma genotyping provides accurate surrogate information about drug resistance in genital secretions and identifies the potential risk for transmission of drug resistant virus.34,35
Despite a relatively small sample of women, we did have a robust number of follow-up study visits over time. Additionally, we assessed PVL and GT HIV-1 RNA following both 30 and 60 days after changing HAART to account for a longer period of time to achieve treatment response after changing therapy. Future research is required to understand factors related to discordance between viral suppression and development of drug resistance in the plasma relative to the GT, particularly with the use of new classes of antiretroviral drugs. With increasing interest in a possible “cure” for HIV, understanding HIV compartmentalization, latency, GT drug levels and tissue penetration, and accumulation of resistant viruses will be important factors related to elimination of the HIV.
This study represents a subpopulation of treatment-experienced HIV-infected women who continue to fail HAART with detectable PVL and GT HIV-1 RNA following a change in treatment regimens most likely due to adherence problems. Hence, findings from this subpopulation of women may not be generalizable to the wider population of HIV-infected patients enrolled in care and seeking HAART. The findings may be applicable to clinic cohorts of treatment-experienced HIV-infected women receiving HAART but failing therapy; however, since the time of this study, treatment options have continued to improve. HAART-experienced women with accumulating drug resistance may be at risk of transmitting a resistant virus to their sex partners.
Author Disclosure
No competing financial interests exist.
Acknowledgments
This work was supported in part by National Institutes of Health (NIH) RO1 AI40350 K24 AI066884, Lifespan/Tufts/Brown Center for AIDS Research (P30AI42853), and Emory Center for AIDS Research (P30 AI050409). KK Venkatesh was also supported by a F-30 MD/PhD. Ruth Kirschtein National Research Service Award (NRSA) (grant number F30 MH079738-01A2).
KV, AKD, and SCU designed the study and wrote the manuscript. AKD with input from KV, RK, and SCU did the analyses. SCU, SC, and JK provided patient care and oversaw data collection. RK, JI, MPD, RD, and AC performed laboratory analyses and assisted in data interpretation. SCU, RK, RD, AKD, and AC provided oversight on analysis and manuscript writing.
References
- 1.Anderson A. Bartlett JA. Changing antiretroviral therapy in the setting of virologic relapse: review of the current literature. Curr HIV/AIDS Rep. 2006;3:79–85. doi: 10.1007/s11904-006-0022-1. [DOI] [PubMed] [Google Scholar]
- 2.Dumond JB. Yeh RF. Patterson KB, et al. Antiretroviral drug exposure in the female genital tract: implications for oral pre- and post-exposure prophylaxis. AIDS. 2007;21:1899–1907. doi: 10.1097/QAD.0b013e328270385a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.von Wyl V. Yerly S. Bürgisser P, et al. Swiss HIV cohort study: long-term trends of HIV type 1 drug resistance prevalence among antiretroviral treatment-experienced patients in Switzerland. Clin Infect Dis. 2009;48:979–987. doi: 10.1086/597352. [DOI] [PubMed] [Google Scholar]
- 4.Taylor S. Cane P. Workman J, et al. Identification of a transmission chain of HIV-1 drug resistant virus. AIDS Res Hum Retroviruses. 2003;19:353–361. doi: 10.1089/088922203765551700. [DOI] [PubMed] [Google Scholar]
- 5.Little S. Holte S. Routy JP, et al. Antiretroviral-drug resistance among patients recently infected with HIV. New Engl J Med. 2002;347:385–394. doi: 10.1056/NEJMoa013552. [DOI] [PubMed] [Google Scholar]
- 6.Grant R. Hecht FM. Warmerdam M, et al. Time trends in primary HIV-1 drug resistance among recently infected persons. JAMA. 2002;288:181–188. doi: 10.1001/jama.288.2.181. [DOI] [PubMed] [Google Scholar]
- 7.TC Wawer MJ. Sewankambo N, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. New Engl J Med. 2000;342:921–929. doi: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
- 8.Mofenson L. Lambert JS. Stiehm ER, et al. Risk factors for perinatal transmission of human immunodeficiency virus type 1 in women treated with zidovudine. Pediatric AIDS Clinical Trials Group Study 185 Team. New Engl J Med. 1999;341:385–393. doi: 10.1056/NEJM199908053410601. [DOI] [PubMed] [Google Scholar]
- 9.Kalichman S. Di Berto G. Eaton L. Human immunodeficiency virus viral load in blood plasma and semen: review and implications of empirical findings. Sex Trans Dis. 2008;35:55–60. doi: 10.1097/olq.0b013e318141fe9b. [DOI] [PubMed] [Google Scholar]
- 10.Anton P. Herold BC. HIV transmission: time for translational studies to bridge the gap. Sci Transl Med. 2011;3:77ps11. doi: 10.1126/scitranslmed.3002329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cu-Uvin S. Caliendo AM. Reinert S, et al. Effect of highly active antiretroviral therapy on cervicovaginal HIV-1 RNA. AIDS. 2000;14:415–421. doi: 10.1097/00002030-200003100-00015. [DOI] [PubMed] [Google Scholar]
- 12.Cu-Uvin S. Snyder B. Harwell JI, et al. Association between paired plasma and cervicovaginal lavage fluid HIV-1 RNA levels during 36 months. J Acquir Immune Defic Syndr. 2006;42:584–587. doi: 10.1097/01.qai.0000229997.52246.95. [DOI] [PubMed] [Google Scholar]
- 13.Cu-Uvin S. DeLong AK. Venkatesh KK, et al. Genital tract HIV-1 RNA shedding among women with below detectable plasma viral load. AIDS. 2010;24:2489–2497. doi: 10.1097/QAD.0b013e32833e5043. [DOI] [PubMed] [Google Scholar]
- 14.Kovacs A. Wasserman SS. Burns D, et al. DATRI study group; WIHS study group. Determinants of HIV-1 shedding in the genital tract of women. Lancet. 2001;358:1593–1601. doi: 10.1016/S0140-6736(01)06653-3. [DOI] [PubMed] [Google Scholar]
- 15.Neely MN. Benning L. Xu J, et al. Cervical shedding of HIV-1 RNA among women with low levels of viremia while receiving highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2007;44:38–42. doi: 10.1097/01.qai.0000248352.18007.1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Homans J. Christensen S. Stiller T, et al. Permissive and protective factors associated with presence, level, and longitudinal pattern of cervicovaginal HIV shedding. J Acquir Immune Defic Syndr. 2012;60:99–110. doi: 10.1097/QAI.0b013e31824aeaaa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Asin S. Eszterhas SK. Rollenhagen C. Heimberg AM. Howell AL. HIV type 1 infection in women: increased transcription of HIV type 1 in ectocervical tissue explants. J Infect Dis. 2009;200:965–972. doi: 10.1086/605412. [DOI] [PubMed] [Google Scholar]
- 18.Cu-Uvin S. DeLong AK. Venkatesh KK, et al. Genital tract HIV-1 RNA shedding among women with below detectable plasma viral load. AIDS. 2010;24:2489–2497. doi: 10.1097/QAD.0b013e32833e5043. [DOI] [PubMed] [Google Scholar]
- 19.Fideli U. Allen SA. Musonda R, et al. Virologic and immunologic determinants of heterosexual transmission of human immunodeficiency virus type 1 in Africa. AIDS Res Hum Retroviruses. 2001;17:901–910. doi: 10.1089/088922201750290023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tovanabutra S. Robison V. Wongtrakul J, et al. Male viral load and heterosexual transmission of HIV-1 subtype E in northern Thailand. J Acquir Immune Defic Syndr. 2002;29:275–283. doi: 10.1097/00126334-200203010-00008. [DOI] [PubMed] [Google Scholar]
- 21.Donnell D. Baeten JM. Kiarie J, et al. Partners in Prevention HSV/HIV Transmission Study Team. Heterosexual HIV-1 transmission after initiation of antiretroviral therapy: a prospective cohort analysis. Lancet. 2010;375:2092–2098. doi: 10.1016/S0140-6736(10)60705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Montaner J. Lima VD. Barrios R, et al. Association of highly active antiretroviral therapy coverage, population viral load, and yearly new HIV diagnoses in British Columbia, Canada: a population-based study. Lancet. 2010;376:532–539. doi: 10.1016/S0140-6736(10)60936-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Das M. Chu PL. Santos GM, et al. Decreases in community viral load are accompanied by reductions in new HIV infections in San Francisco. PLoS One. 2010;5:e11068. doi: 10.1371/journal.pone.0011068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baeten J. Kahle E. Lingappa JR, et al. Genital HIV-1 RNA predicts risk of heterosexual HIV-1 transmission. Sci Transl Med. 2011;3:77ra29. doi: 10.1126/scitranslmed.3001888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cohen MS. Chen YQ. McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cohen M. Gay C. Kashuba AD. Blower S. Paxton L. Narrative review: antiretroviral therapy to prevent the sexual transmission of HIV-1. Ann Intern Med. 2007;146:591–601. doi: 10.7326/0003-4819-146-8-200704170-00010. [DOI] [PubMed] [Google Scholar]
- 27.Kemal K. Burger H. Mayers D. Anastos K. Foley B. Kitchen C. Huggins P. Schroeder T. Picchio G. Back S. Gao W. Meyer WA., 3rd Weiser B. HIV-1 drug resistance in variants from the female genital tract and plasma. Journal of Infectious Disease. 2007;195(4):535–545. doi: 10.1086/510855. [DOI] [PubMed] [Google Scholar]
- 28.Johnson V. Brun-Vézinet F. Clotet B, et al. Update of the drug resistance mutations in HIV-1: December 2010. Top HIV Med. 2010;18:156–163. [PubMed] [Google Scholar]
- 29.Nagot N. Ouedraogo A. Weiss HA, et al. Yerelon Study Group. Longitudinal effect following initiation of highly active antiretroviral therapy on plasma and cervico-vaginal HIV-1 RNA among women in Burkina Faso. Sex Transm Infect. 2008;84:167–170. doi: 10.1136/sti.2007.027987. [DOI] [PubMed] [Google Scholar]
- 30.Graham S. Holte SE. Peshu NM, et al. Initiation of antiretroviral therapy leads to a rapid decline in cervical and vaginal HIV-1 shedding. AIDS. 2007;21:501–507. doi: 10.1097/QAD.0b013e32801424bd. [DOI] [PubMed] [Google Scholar]
- 31.Andreoletti L. Skrabal K. Perrin V. Chomont N. Saragosti S. Gresenguet G. Moret H. Jacques J. Longo Jde D. Matta M. Mammano F. Belec L. Genetic and phenotypic features of blood and genital viral populations of clinically asymptomatic and antiretroviral-treatment-naive clade a human immunodeficiency virus type 1-infected women. J Clin Microbiol. 2007;45:1838–1842. doi: 10.1128/JCM.00113-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chomont N. Hocini H. Grésenguet G, et al. Early archives of genetically-restricted proviral DNA in the female genital tract after heterosexual transmission of HIV-1. AIDS. 2007;21:153–162. doi: 10.1097/QAD.0b013e328011f94b. [DOI] [PubMed] [Google Scholar]
- 33.Kwara A. Delong A. Rezk N, et al. Antiretroviral drug concentrations and HIV RNA in the genital tract of HIV-infected women receiving long-term highly active antiretroviral therapy. Clin Infect Dis. 2008;46:719–725. doi: 10.1086/527387. [DOI] [PubMed] [Google Scholar]
- 34.Kantor R. Wantman M. Katzenstein D, et al. ACTG A5077 Study Team. Viral shedding and drug resistance in plasma and genital compartments among viremic, multi-drug-experienced HIV-infected men and women. Paper presented at 15th Conference on Retroviruses and Opportunistic Infections (CROI); Boston, MA. Feb;2008 . [Google Scholar]
- 35.Katzenstein D. Winters M. Fiscus S, et al. AIDS Clin Trials Group 5077. Drug resistance in plasma and genital compartments among viremic, multi-drug-experienced men and women. Paper presented at 13th Conference on Retroviruses and Opportunistic Infections (CROI); Denver, CO. Feb;2006 . [Google Scholar]