Abstract
An insufficient cellular immune response seems to be critical for the immunopathogenesis of chronic hepatitis B virus infection. We have previously demonstrated no differences of T-lymphocyte subsets in blood between inactive hepatitis B s antigen (HBsAg) carriers and patients with HBeAg-negative chronic active hepatitis B. This study investigated the peripheral blood cytokine profile in patients with HBeAg-negative chronic active hepatitis B infection (Group A, n = 21) and inactive HBsAg carriers (Group B, n = 13). Serum cytokines [interferon (IFN)-γ, tumor necrosis factor-α, interleukin (IL)-1b, IL-4, IL-12, IL-10, IL-2, IL-5, IL-8] were analyzed by using flow cytometry. Patients with chronic active disease presented with significantly decreased levels of IFN-γ and IL-10 compared to inactive carriers (P = 0.048 and P = 0.008, respectively). In HBeAg-negative chronic active hepatitis B patients, a significant negative correlation of IFN-γ levels with serum hepatitis B viral load was noted (P = 0.021). In conclusion, patients with HBeAg-negative chronic active hepatitis B and HBsAg inactive carriers display a different cytokine profile. Decreased Th1 response observed in patients with chronic active hepatitis B could be implicated in the persistence of virus replication and ongoing progression of liver disease.
Keywords: Cytokines, Hepatitis B, Flow cytometry, Immunoreactive fibronectin-γ
TO THE EDITOR
Chronic hepatitis B (CHB) is a highly heterogeneous disease regarding the levels of virus replication, liver disease activity and humoral responses. Hepatitis B virus (HBV) is not directly cytopathic for the infected cells and the host immune response, mainly the T-cell-mediated, plays a pivotal role in the immunopathogenesis of hepatitis B[1,2]. To date, there are only limited studies examining the immune responses in hepatitis B e antigen (HBeAg)-negative CHB, which is the main type of CHB in Greece and other Mediterranean countries. In a recent study, investigating T-lymphocyte subsets in peripheral blood and liver tissue of patients with HBeAg-negative CHB, we demonstrated evidence of an insufficient cellular immune response that might be critical for the ineffective virus clearance and liver damage in CHB[3]. However, no differences in T-lymphocyte subsets in blood were detected between inactive HBsAg carriers and patients with HBeAg-negative chronic active hepatitis B. Therefore, the question of how most HBsAg patients are able to maintain a low replication level and mild liver inflammation (inactive carriers), while a number of them develop chronic active hepatitis with an enhanced HBV replication level and severe liver damage, remains unanswered. The present study was focused on this specific question, investigating the produced cytokine profiles in patients with HBeAg (-) chronic active HBV infection and chronic inactive HBsAg carriers.
The study enrolled twenty-one patients with positive serum HBsAg for at least 6 mo, positive serum HBV DNA with high viral load (> 20 000 copies/mL), measured at least twice in a period of 12 mo, and aminotransferase levels higher than twice the upper normal limits, who were not currently treated nor had ever been treated with any antiviral agent (HBeAg-negative chronic hepatitis B - Group A) and thirteen patients with positive serum HBsAg for at least 6 mo, undetectable HBV DNA and normal serum aminotransferase levels (inactive carriers - Group B). All HBV infected patients were HBeAg negative, anti-Hbe positive and anti-HDV negative. Exclusion criteria were the presence of decompensated cirrhosis, HBV/HBV co-infection, alcohol abuse, human immunodeficiency virus or human T-cell lymphoma virus infection, any immunosuppressive treatment and other liver diseases, such as drug hepatotoxicity, α-1 antitrypsin deficiency, Wilson’s disease, hemochromatosis, autoimmune hepatitis and liver cancer. Liver biopsies were obtained only from the patients with positive serum HBV DNA and elevated liver enzymes and were indicative of chronic active hepatitis B infection. In each biopsy, several histological features were assessed and finally, the hepatitis activity index was applied and the architectural grade recorded[4].
Blood tests were obtained before the initiation of any kind of treatment and analyzed for serum aminotransferases with an automatic Olympus AU 640 system (Olympus, Rungis, France), whilst serum HBV DNA load was assessed with the real time fluorescent quantitative polymerase chain reaction method (real time PCR), with a lower limit of detection of about 1000 viral genome copies/mL. Serum cytokine levels [interferon (IFN)-γ, tumor necrosis factor-α, interleukin (IL)-1b, IL-4, IL-12, IL-10, IL-2, IL-5, IL-8] were evaluated by using a commercially available Flow Cytomix Human Basic Kit Assay (Bender MedSystems, Vienna, Austria), following the manufacturer’s instructions. Quantitation measurements were performed by flow cytometer instrument FC 500 and accompanying CXP Software (Beckman Coulter, Calif, United States). Flow Cytomix Pro 2.3 Software was used to perform calculations (Bender MedSystems). Standard curves for each cytokine were generated with manufacturer-supplied reference analyte (pg/mL concentrations). Statistical analyses were performed using the Mann-Whitney U test since data was not normally distributed (Shapiro-Wilk Test). Correlation between paired variables in patients with HBeAg (-) chronic active hepatitis B was estimated by a non-parametric Spearman correlation test. In all cases, a P-value of less than 0.05 was considered as significant.
Patients’ characteristics, serum aminotransferases and HBV DNA levels are shown in Table 1. Inactive HBV carriers (Group B) had significantly increased production of IFN-γ and IL-10 cytokines compared with HBeAg-negative chronic active hepatitis B patients (Group A) (P = 0.048 and P = 0.008, respectively, Table 2). In HBeAg-negative chronic active hepatitis B patients, a significant negative correlation between serum HBV viral load and IFN-γ production was noted (Figure 1), whilst no correlation existed between IFN-γ and alanine amiotransferase levels.
Table 1.
Characteristics of hepatitis B e antigen--negative chronic active hepatitis B patients and asymptomatic hepatitis B virus carriers
| Parameters | Group A (n = 21) | Group B (n = 13) | P value |
| Males (%) | 12/21 (57) | 7/13 (54) | NS |
| Age (yr) | 44 (19-60) | 39.5 (32-47) | NS |
| HBV DNA (c/mL) | 1.25×106 (0.04×106-3×106) | < 1000 | N/A |
| ALT (IU/L) | 90 (73-108) | 32 (22-39) | < 0.001 |
| AST (IU/L) | 85 (69-102) | 28 (17-39) | < 0.001 |
| Histopathology | N/A | ||
| HAI score | |||
| Category A | 2 (1-4) | ||
| Category B | 0 (0-1) | ||
| Category C | 2 (0-3) | ||
| Category D | 3 (1-4) | ||
| Total score | 7 (4-13) | ||
| Stage | 2 |
Data expressed as median (min-max), upper limit of normal for both aminotransferases: 40 IU/L. Group A: Hepatitis B e antigen--negative chronic active hepatitis B patients; Group B: Asymptomatic hepatitis B virus carriers. NS: Non-significant; NA: Not applicable; HBV: Hepatitis B virus; ALT: Alanine amiotransferase; AST: Aspartate aminotransferase; HAI: Hepatitis activity index.
Table 2.
Cytokine levels in hepatitis B e antigen-negative chronic active hepatitis B patients and asymptomatic hepatitis B virus carriers
| Cytokines (pg/mL) | Group A (n = 21) | Group B (n = 13) | P value |
| IFN-γ | 0.3 (0-17.5) | 18.3 (0-5137) | 0.048 |
| TNF-α | 101.3 (0-462.2) | 202.6 (0-1044) | NS |
| IL-1b | 98.5 (0-25196) | 61.0 (0-11149) | NS |
| IL-4 | 285.4 (0-10885) | 4.9 (0-4313) | NS |
| IL-12 | 154.6 (0-39042) | 172.6 (0-10618) | NS |
| IL-10 | 0 (0-41.4) | 18.6 (0-14393) | 0.008 |
| IL-8 | 314.8 (51.1-32592) | 383.0 (67.8-14075) | NS |
| IL-5 | 78.4 (0-515.5) | 198.0 (0-853.7) | NS |
| IL-2 | 158.9 (28.2-126842) | 146.4 (29.5-163730) | NS |
Data expressed as median (min-max). Group A: Hepatitis B e antigen-negative chronic active hepatitis B patients; Group B: Asymptomatic HBV carriers. NS: Non-significant; IFN-γ: Interferon-γ; TNF-α: Tumor necrosis factor-α; IL: Interleukin.
Figure 1.
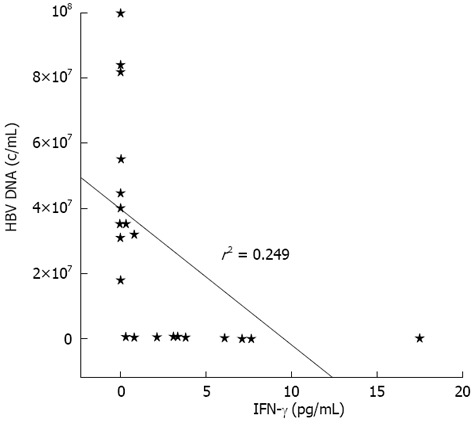
Correlation between serum-interferon-γ levels and viral load in patients with hepatitis B e antigen (-) chronic active hepatitis B. IFN-γ: Interferon-γ; HBV: Hepatitis B virus.
This study demonstrates that patients with HBeAg negative chronic hepatitis B display a different cytokine profile depending on the degree of viremia and liver inflammation. A potential limitation of the present study is the relatively small number of patients included. According to our results, HBsAg inactive carriers displayed a strong production of IFN-γ (Th1 type immune response) and IL-10 (Th2 type immune response) in peripheral blood compared to patients with HBeAg-negative chronic active hepatitis B. Both Th1 and Th2 T-cells mediate humoral and cellular immunity able to neutralize HBV by antibodies and inhibit HBV replication through cytokines[5]. Therefore, we can speculate that HBsAg inactive carriers suppress HBV replication through their capability to produce the Th1 type antiviral cytokine IFN-γ. In support of this theory, we demonstrated a negative correlation between IFN-γ and the levels of viremia in chronic active hepatitis B patients; however, the other side of the coin might be that the continuing presence of viral load in serum could induce an impairment of IFN-γ[6-8]. Normal aminotransferases levels in HBsAg inactive carriers might indicate that IFN-γ promotes viral clearance through non-cytolytic mechanism(s)[9-11]. Alternatively, it could be explained by a counterbalancing effect of the observed increased production of IL-10 (Th2 type cytokine) on the excessive Th1 action, although IL-10 exerts a regulatory effect on Th2 type response as well[9-11].
In conclusion, this study demonstrates that T-cell immunity is functionally impaired in chronic active hepatitis B patients. In addition, an inverse correlation was shown between the increase of one of the major determinants of Th1 response (IFN-γ cytokine) and the decline of HBV load in blood samples of patients with chronic active hepatitis B. These findings suggest that impaired immunity could be associated with the persistence of HBV load and the elevation of serum aminotransferases in patients with active disease. On this basis, we are tempted to speculate that not only drugs with antiviral potency but also immunomodulating agents that can restore T cell function might be effective for a successful treatment of chronic HBV infection.
Footnotes
P- Reviewer Imazeki F S- Editor Zhai HH L- Editor Roemmele A E- Editor Yan JL
References
- 1.Ferrari C, Penna A, Bertoletti A, Valli A, Antoni AD, Giuberti T, Cavalli A, Petit MA, Fiaccadori F. Cellular immune response to hepatitis B virus-encoded antigens in acute and chronic hepatitis B virus infection. J Immunol. 1990;145:3442–3449. [PubMed] [Google Scholar]
- 2.Chisari FV, Ferrari C. Hepatitis B virus immunopathogenesis. Annu Rev Immunol. 1995;13:29–60. doi: 10.1146/annurev.iy.13.040195.000333. [DOI] [PubMed] [Google Scholar]
- 3.Dimitropoulou D, Karakantza M, Tsamandas AC, Mouzaki A, Theodorou G, Gogos CA. T-lymphocyte subsets in peripheral blood and liver tissue of patients with chronic hepatitis B and C. In Vivo. 2011;25:833–840. [PubMed] [Google Scholar]
- 4.Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, Denk H, Desmet V, Korb G, MacSween RN. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696–699. doi: 10.1016/0168-8278(95)80226-6. [DOI] [PubMed] [Google Scholar]
- 5.Guidotti LG. The role of cytotoxic T cells and cytokines in the control of hepatitis B virus infection. Vaccine. 2002;20 Suppl 4:A80–A82. doi: 10.1016/s0264-410x(02)00392-4. [DOI] [PubMed] [Google Scholar]
- 6.Bertoletti A, Gehring AJ. The immune response during hepatitis B virus infection. J Gen Virol. 2006;87:1439–1449. doi: 10.1099/vir.0.81920-0. [DOI] [PubMed] [Google Scholar]
- 7.Baumert TF, Thimme R, von Weizsäcker F. Pathogenesis of hepatitis B virus infection. World J Gastroenterol. 2007;13:82–90. doi: 10.3748/wjg.v13.i1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lai CL, Yuen MF. The natural history of chronic hepatitis B. J Viral Hepat. 2007;14 Suppl 1:6–10. doi: 10.1111/j.1365-2893.2007.00909.x. [DOI] [PubMed] [Google Scholar]
- 9.Bertoletti A, Maini M, Williams R. Role of hepatitis B virus specific cytotoxic T cells in liver damage and viral control. Antiviral Res. 2003;60:61–66. doi: 10.1016/j.antiviral.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 10.Guidotti LG, Ando K, Hobbs MV, Ishikawa T, Runkel L, Schreiber RD, Chisari FV. Cytotoxic T lymphocytes inhibit hepatitis B virus gene expression by a noncytolytic mechanism in transgenic mice. Proc Natl Acad Sci USA. 1994;91:3764–3768. doi: 10.1073/pnas.91.9.3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guidotti LG, Rochford R, Chung J, Shapiro M, Purcell R, Chisari FV. Viral clearance without destruction of infected cells during acute HBV infection. Science. 1999;284:825–829. doi: 10.1126/science.284.5415.825. [DOI] [PubMed] [Google Scholar]


