Abstract
Purpose of review
Several unique HIV-infected or HIV-resistant cohorts have been studied over the years to try and delineate the correlates of protection. Although several mechanisms have been put forward, studies aiming to integrate the different mechanisms into a comprehensive model are still lacking. Current systems biology approaches emphasize the importance of unifying independent datasets, provide tools that facilitate hypothesis formulation and testing, and direct us toward uncovering novel therapeutic targets by defining molecular networks perturbed during disease. This review will focus on the current findings that utilized systems biology techniques in order to identify correlates of protection from HIV disease progression and resistance to infection in unique cohorts of individuals as well as in nonhuman primate models of SIV infection.
Recent findings
Using systems biology technologies and data analysis tools, the studies described herein have found that pathways implicated in survival, cell cycling, inflammation, and oxidative stress work in unison to limit pathology caused by chronic immune activation. This situation favors the survival of effector lymphocytes and limits the dissemination of viral particles in HIV elite controllers, exposed-uninfected individuals, and natural hosts of SIV infection.
Summary
Systems and computational biology tools have clearly expanded our understanding of HIV pathogenesis by unifying independent observations and by giving us novel molecular targets to pursue. These molecular signatures have the potential to uncover correlates of protection in HIV disease and, in the era of personalized medicine, to determine predictive signatures of treatment efficacy and/or failure.
Keywords: elite controllers, exposed-uninfected, HIV, networks, pathways, resistance, systems biology
INTRODUCTION
After 30 years of HIV research, we still lack a clear understanding of many critical innate and adaptive mechanisms that influence disease course and responsiveness to therapy. Thus far, traditional immunobiology was reductionist in its approach whereby complex biological problems were deconstructed into their minimal parts. Systems biology is an interdisciplinary approach that systematically describes the complex interactions between all parts of a biological system, with a view toward elucidating new rules capable of predicting its behavior. In hypothesis-driven research, relationships are arrived at intuitively (a priori) and then tested, whereas systems biology finds relationships between independent sets of observations and models these data as complex networks. Recent advances in highthroughput technologies generate large amounts of data and utilize a combination of platforms such as genomics, transcriptomics, proteomics, and computational biology to interrogate systems through an iterative cycle of experimental analysis, modeling, and validation. This situation provides the opportunity to discover new aspects of immune responses to HIV that cannot be elucidated by an understanding of the individual parts alone.
A large number of groups have utilized systems biology approaches to understand HIV infection by assessing gene-expression profiles in cell lines or primary cells infected in vitro. Although an important direction in terms of understanding gene regulation within the infected cell, this approach does not address the complex interactions that occur within the infected individual, as infection of the host has consequences that are systemic and far-reaching beyond in-vitro examination of an isolated population. Because HIV pathogenesis is multifactorial, research efforts have shifted toward a systems approach aimed at identifying multiparametric signatures of protection, prevention, and treatment efficacy by unifying diverse sets of observations and defining inter-relationships between what was previously seen as unrelated. The goal is to identify key targets that will accelerate the development of better therapeutic interventions, preventive strategies, and vaccines.
SYSTEMS BIOLOGY: THE PATH TOWARD UNDERSTANDING HIV DISEASE PROGRESSION?
In order to identify and dissect the immune mechanisms responsible for protection from HIV disease progression, the most obvious place to look has been the rare individuals who naturally (in the absence of therapeutic intervention) control infection. Much of our understanding regarding viral evolution and immune control has come from the study of cohorts of elite controllers (ECs) who maintain undetectable viral loads for many years after seroconversion, long-term nonprogressors (LTNPs) and viremic nonprogressors (VNPs), who despite detectable viral loads, manage to maintain a functional CD4 T-cell compartment and remain asymptomatic for long periods of time in the absence of antiretroviral treatment [1]. Such individuals provide researchers with the opportunity to dissect an immune system that is exquisitely endowed with the capacity to maintain equilibrium between long-term control of viral replication, as in the case of ECs, and a functional immune system in the face of ongoing viral replication, as is the case in VNPs.
To date, numerous correlates associated with HIV disease progression have been identified: impaired function of innate and adaptive immune responses [2,3], polyclonal B-cell activation [4], increased T-cell turnover and decreased thymic output [5], decreased survival of central memory CD4 T cells [6], breakdown of mucosal barriers leading to microbial translocation and immune activation [7], increased frequencies of dysfunctional T cells with an activated phenotype and increased serum levels of proinflammatory cytokines and chemokines [8,9]. To better understand the host–pathogen inter-relationship and immune dysfunction and deregulation associated with disease progression at the genomic level, studies have looked at the transcriptional profile of whole peripheral blood mononuclear cells, HIV target cells (CD4, macrophage), immune effector cells [natural killer (NK) cells, B cells, and CD8 T cells], and mucosal tissues at portals of viral replication and entry, comparing responses in cohorts who experience variable disease outcomes [10].
One of the first systems biological analyses of natural protection in HIV infection investigated the gene-expression profiles of LTNPs in intestinal mucosal tissue, which is a major target of HIV-1 infection and CD4 T-cell depletion at early time points postinfection [11]. Sankaran et al. carried out a comparative microarray gene-expression analysis of jejunal biopsies from four antiretroviral therapy-naïve HIV-1 seropositive patients, four HIV-1 seronegative individuals, and three LTNPs. Overall, the mucosal gene-expression profiles from the high viral load (HVL) patients showed upregulation of 369 genes compared with HIV-negative donors, whereas the LTNP group demonstrated upregulation of 150 genes. Conversely, 411 genes were downregulated in LTNPs versus only 196 in HVLs when compared with HIV-negative controls. The authors then performed hierarchical clustering analysis to identify upregulation of genes involved in homeostasis, digestion, and the innate immune response in both the LTNP and HVL groups. A number of discriminating features of the geneexpression profiles between these two groups emerged. The expression of a wide array of immune response genes was induced in the gut mucosa of patients from both groups, such as the interferon pathway (IFITM2 and OAS2), cell surface receptor expression (PD-1, leukocyte immunoglobulin receptor, B2), and chemotaxis (Eotaxin, MCP-1). In contrast, a number of genes involved in lymphocyte activation and inflammation were found to be downregulated in LTNP and upregulated in HVL patients relative to HIV-negative controls. For example, RANTES, the major ligand for CCR5, was downregulated in two of three LTNPs and upregulated in three of four HVLs. The authors concluded that the profile of inflammatory gene expression in HVL patients was the result of high levels of viral replication in the gut-associated lymphoid tissue (GALT). A common expression pattern in both LTNP and HVL patients revealed downregulation of nutrient absorption and lipid metabolism-associated genes in the GALT, whereas those associated with amino acid metabolism were only downregulated in tissues isolated from HVL but not LTNP patients. The authors attributed the deregulation of metabolic gene expression and gastrointestinal pathology in both groups to HIV infection sequelae, even in the absence of viral replication. Furthermore, this study found that HVL samples displayed increased expression of genes related to cell cycling (CCNA2 and MCM4), growth, and cell adhesion that were not evident in the LTNP samples. Although this study outlined interesting trends in differential regulation of genes associated with metabolism, growth, cell cycle and trafficking, the most profound differences between the LTNP and HVL tissue profiles were clearly seen in the downregulation of genes with immune function in the LTNP group as compared with both HVLs and HIV-negative controls.
In 2007, Hyrcza et al. [12] utilized a systems biology approach to identify distinct transcriptional profiles in purified CD4+ and CD8+ T cells from patients at different clinical stages and rates of disease progression. Study participants included five early infected individuals, five chronically infected and progressing individuals, five LTNPs, and five HIV-negative controls all naïve to antiretroviral therapy. Microarray analysis was used to identify differentially expressed genes in pairwise group comparisons. Surprisingly, only four genes were differentially regulated between the early and chronic groups, and no genes showed differential regulation between the LTNP and HIV-negative group. The authors then combined groups to increase statistical power and found two unique gene-expression signatures, one characteristic of LTNP and HIV-negative T cells and the other associated with early and chronic HIV infection. From this analysis, it was shown that the number of differentially expressed genes was higher in CD8+ than CD4+ T cells, and downregulation of genes was only evident in the CD8+ subset. Upregulation of interferon-stimulated genes (ISGs) was apparent in early and chronic HIV-infected samples compared with LTNP and HIV-negative samples. This finding led the authors to evaluate whether use of an ISG signature alone would allow for unsupervised clustering of the CD4+ and CD8+ samples. Indeed, the selective use of an ISG gene signature allowed for hierarchical clustering of the early and chronic samples from those of LTNP and HIV-negative samples. These findings provide additional insight into the regulation and critical importance of the CD8 T-cell compartment in HIV pathogenesis.
The authors then looked at the expression of genes associated with T-cell differentiation using gene categories that discriminates the gene-expression profiles of thymocytes versus peripheral T cells [13]. Gene-expression data from early and chronic samples were enriched for a cluster of genes upregulated in single-positive thymocytes. Conversely, LTNP samples showed a gene-expression profile similar to circulating peripheral T cells. This finding is compatible with the higher turnover of T cells during progressive HIV infection and may be due partly to the augmented release of recent thymic emigrants as a compensatory mechanism to peripheral and mucosal CD4 T-cell loss [14]. Importantly, the families of genes that clustered with the different clinical categories were part of the apoptotic, cell cycling, and DNA replication pathways. This study uncovered a number of important mechanisms underlying natural protection against HIV infection. First, it demonstrated that gene-expression profiles from CD4+ and CD8+ T-cell populations were able to distinguish progressors from nonprogressors. Second, it demonstrated that LTNPs have a similar gene-expression profile in their peripheral blood to that of HIV-negative individuals. Third, this study identified the ISG signature in the peripheral blood as unique to early and chronically infected individuals versus LTNPs, and suggests that both early and chronically infected patients display a type I IFN chronic exposure signature.
Wu et al. [15▪▪] analyzed global gene-expression profiles in sorted CD4+ and CD8+ T cells from LTNPs and HIV-negative donors and compared these profiles with viremic and ART-treated aviremic patients. Gene-expression profiles from the purified cells were normalized and submitted to pairwise fold-change comparisons to identify differentially expressed genes. In contrast to the findings of Hyrcza et al., Wu et al. identified a small number of differentially regulated genes between LTNPs and HIV-negative samples. Analysis of gene ontology categories enriched in the viremic versus LTNP samples revealed that complement activation and C1qA/B/C complex were induced in CD4+ T cells, and catalytic activity and response to stimuli including the proteasome core complex were increased inCD8+ T cells. Gene set enrichment analysis (GSEA) revealed a large number of metabolic pathways that correlated with disease progression including the OXPHOS pathway for cellular energy production and the tricarboxylic acid (TCA) cycle. The authors suggest that metabolic pathway upregulation during HIV progression may be a compensatory mechanism in response to deregulation of mitochondrial function due to HIV infection and/or ART. GSEA also revealed a large number of immune-related pathways associated with progression such as cell cycle, apoptosis, cytotoxicity, complement activation, interleukin and interferon response, and cell adhesion. In CD4+ T cells, apoptotic genes such as TNFR1, BCL2, and BID were upregulated in the viremic versus LTNP samples. Although they found fewer pathways enriched in the T-cell compartments of LTNPs, genes associated with the ERK, JNK, and p38 branches of the MAPK pathway were found to be significantly upregulated in the LNTPs. Another selectively enriched pathway identified in T cells isolated from LTNPs, and regulated by the MAPK pathway, was the WNT-β-catenin pathway, important in promoting self-renewal. The authors proposed that activation of these signaling pathways in T cells could improve the efficacy of the antiviral response as well as promote survival of effector T-cell subsets.
SYSTEMS BIOLOGY APPROACHES IN NONHUMAN PRIMATE MODELS OF SIV INFECTION
A powerful model for studying HIV disease and for identifying potential correlates of natural protection is to compare the gene-expression profiles in pathogenic versus nonpathogenic SIV infection in natural host species. SIV infection in sooty mangabeys and African green monkeys (AGMs) is nonpathogenic in spite of high viral loads and persistent viral replication, whereas infection of rhesus macaques generally leads to chronic progressive disease. Distinguishing characteristics of the pathogenic model in rhesus macaques include apoptosis of lymphocytes and increased numbers of activated and proliferating T cells. In contrast, SIV-infected sooty mangabeys and AGMs do not suffer apoptotic loss of CD4+ T cells and demonstrate a lack of chronic immune activation in the face of persistent high levels of virus replication [16]. Recently, two groups carried out comparative longitudinal whole-genome expression studies of nonpathogenic (AGM and sooty mangabey) and pathogenic (rhesus macaque) SIV infection. Both studies found that the defining characteristic of nonpathogenic infection was the complete downregulation of immune activation following the initial phase of infection [17,18]. Transcriptional analysis of whole blood, lymph nodes, and/or purified CD4+ T cells from the susceptible versus nonsusceptible species identified that a robust antiviral immune response profile early after infection was common to both species. Despite similar profiles during acute infection, marked differences in gene expression were evident during the transition from acute to chronic phase infection. Jacquelin et al. showed that 28 days after infection, ISG expression in AGMs was significantly diminished returning to baseline levels in some animals. Importantly, an upregulation of a number of immuneregulatory genes in sooty mangabeys suggested a possible mechanism for attenuation of the immune response. Bosinger et al. also showed in AGMs and in nonpathogenically infected sooty mangabeys the induction of a number of immune-regulatory molecules such as ADAR (negative regulator of ISGs), IDO (immunoregulatory molecule induced in myeloid cells by IFN-γ), and TIM3 (immune inhibitory molecule). Collectively, these studies provided insight into the host mechanisms that limit susceptibility to virus infection, as well as the need to effectively regulate inflammatory responses in preventing HIV/SIV disease progression.
SYSTEMS BIOLOGY: THE PATH TO UNDERSTANDING HIV RESISTANCE?
Studying HIV pathogenesis in defined cohorts of individuals is one of a number of strategies used to guide the development of effective therapeutic or prophylactic HIV vaccines. Although elite controllers provide information on the natural control of HIV disease progression, HIV exposed-uninfected individuals provide important insight into the mechanisms underlying resistance to HIV infection. Exposed-uninfected individuals are a unique cohort of individuals highly exposed to HIV, while remaining uninfected and seronegative following sexual or parenteral exposure [19,20]. Maintenance of seronegativity despite virus exposure has been observed in commercial sex workers [21], sexual partners of HIV-infected persons [22,23], injection drug users [24], healthcare workers occupationally exposed to HIV contaminated body fluids [25], infants born to HIV-infected mothers [26], and hemophiliacs receiving contaminated blood products [27]. Thus far, despite long-standing efforts to unravel the correlates of protection in these individuals, a clear unifying explanation has not been forthcoming. Numerous studies have implicated components of the innate and adaptive immune system as well as genetic factors linked to resistance from infection. Studies have shown that CD8+ T cells [28,29], specific KIR haplotypes [30,31], polymorphisms in the IRF-1 gene, and DC-SIGN receptor expression [32] were all related to the exposed-uninfected phenotype. In addition, several anti-HIV factors have been identified in the mucosae such as the expression of neutralizing IgA, RANTES, SLP-1, and MIP1α and β [33,34].
Recent studies have applied systems biology approaches to try to identify networks and pathways responsible for protection from acquisition of virus [35]. A study by Misse et al. [36] used a comparative transcriptome and proteome analysis of peripheral blood T cells isolated from exposed-uninfected individuals and their HIV-infected sexual partner, and healthy controls. This study identified two genes that were highly upregulated in exposed-uninfected individuals versus their seropositive partner. Both IL-22 and Peroxiredoxin (PRDX2), described to play a role in innate defenses, were highly expressed in exposed-uninfected individuals. Both genes had anti-HIV activities: PRDX2 enhanced NK cell activity, whereas IL-22 induced the production of mucosal β-defensins 2, 3 and acute-phase serum amyloid A (A-SAA) which phosphorylates and reduces CCR5 surface expression, rendering memory CD4 T cells less susceptible to infection. The transcriptional profile of T cells isolated from exposed-uninfected individuals also showed an overexpression of antiapoptotic genes such as TNFRSF4 and DAD1 [36], which confers greater survival of effector T cells. Other reports have analyzed differences in the proteome of genital secretions in exposed-uninfected women. These studies identified the overexpression of serpin antiproteases (elafin/trappin-2) in the mucosae of HIV-resistant women, whereas inflammatory immune mediators such as complement were under-expressed [37,38▪▪].
NOVEL PATHWAYS IN ELITE CONTROL
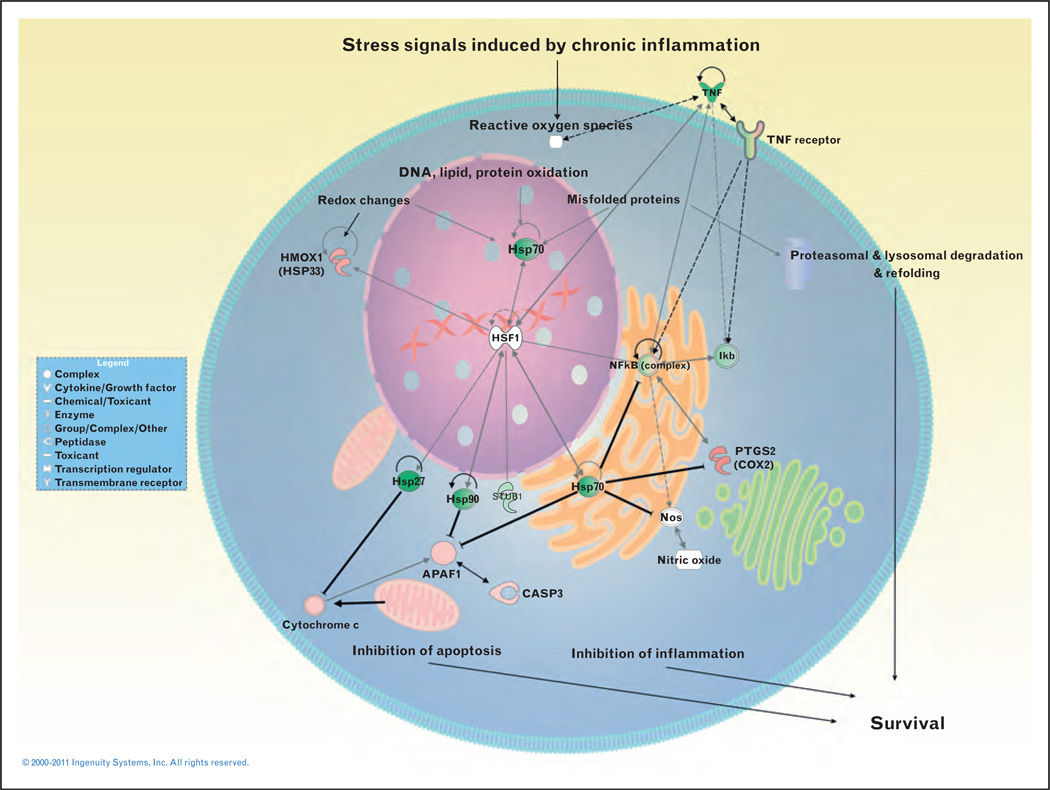
As previous system analyses were done on heterogeneous cell populations [39] and certain CD8+ T-cell specificities have been shown to be critical in control of persistent infection, our group analyzed the transcriptional profile of HIV tetramer-specific CD8+ T cells isolated during acute/early infection and chronic progressive HIV infection, from successfully treated individuals and from patients which naturally control viremia (elite controller). We found that 9014 genes were differentially modulated between HIV-specific CD8 T cells isolated from elite controllers versus chronically infected patients. Interestingly, 135 genes were able to classify and provide a statistically significant genetic signature for HIV-specific CD8+ T cells isolated from individuals in the different clinical categories. This classification was 100% accurate when analyzing the profile of acutely infected individuals compared to elite controllers. Strikingly, among the classifiers, genes involved in the heat shock response (HSP) were highly and significantly upregulated in elite controllers. These data suggest that CD8 T cells in elite controllers recruit molecular chaperones involved in response to cellular and oxidative stress that contribute to their survival and functional activity. GSEA confirmed this observation and showed that pathways involved in protein folding were significantly upregulated in this group as well as several targets of the heat shock transcription factor (HSF-1). We hypothesize from these findings that the HSP response regulates T-cell survival and function during HIV infection by controlling proinflammatory signals, detoxifying the cell from oxidized and misfolded proteins, inhibiting uncontrolled inflammation through regulation of NFκB, and by inhibiting proapoptotic mediators such as apaf-1, cytochrome C, Bax, and Bid (Fig. 1). These preliminary results illustrate the power of systems biology and its capacity to integrate and find relationship between various pathways and disease states.
FIGURE 1.
Network of proteins involved in the heat shock response, regulation of inflammation, apoptosis, and protein folding. Connectivity is based on analysis using the Ingenuity Biological Database. Gray lines indicate different gene interactions and dotted lines indicate indirect interactions. Bold black lines ending with the – symbol indicate inhibition. The coloring of the nodes is based on the fold change between elite controllers and chronically infected patients, with green colors indicating upregulated genes in HIV-specific CD8 T cells isolated from elite controllers.
CONCLUSION
Systems biology approaches are providing new clues into host–pathogen interactions and immune mechanisms involved in HIV disease progression, control, and resistance.
Understanding the contributions made by innate and adaptive immune defenses to prevent the acquisition of HIV and limit disease progression will be critical for developing novel approaches to HIV prophylaxis and therapy by validating new biomarkers predictive of disease outcome and treatment efficacy. The preliminary results highlighted by these studies emphasized the importance in identifying genomic and proteomic signatures that correlate with progressive and nonprogressive HIV disease. In addition, the results showed the importance of tissue-specific correlates whereby profiles identified in the periphery and in cellular subsets differed significantly. These studies clearly indicate how our understanding of HIV pathology has advanced and how we are aiming to integrate what we previously thought were irreconcilable differences.
KEY POINTS.
Systems biology is a multidisciplinary approach that aims to integrate independent observations.
Systems biology is providing insight into the mechanisms of control and resistance from infection.
Systems biology has uncovered novel pathways, such as the HSP response, involved in disease control.
Acknowledgements
The authors would like to thank Jeff Ahlers for reviewing the manuscript. This study is supported by funds from the U.S. National Institutes of Health (IDPIDA028871-01, AI076174-01A1) and the Bill and Melinda Gates Foundation (SEKALY06VIMC0, GH-HTR-05-02).
Footnotes
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue (pp. 87–88).
- 1.Deeks SG, Walker BD. Human immunodeficiency virus controllers: mechanisms of durable virus control in the absence of antiretroviral therapy. Immunity. 2007;27:406–416. doi: 10.1016/j.immuni.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 2.Alter G, Altfeld M. NK cell function in HIV-1 infection. Curr Mol Med. 2006;6:621–629. doi: 10.2174/156652406778195035. [DOI] [PubMed] [Google Scholar]
- 3.El-Far M, Halwani R, Said E, et al. T-cell exhaustion in HIV infection. Curr HIV/AIDS Rep. 2008;5:13–19. doi: 10.1007/s11904-008-0003-7. [DOI] [PubMed] [Google Scholar]
- 4.Lane HC, Masur H, Edgar LC, et al. Abnormalities of B-cell activation and immunoregulation in patients with the acquired immunodeficiency syndrome. N Engl J Med. 1983;309:453–458. doi: 10.1056/NEJM198308253090803. [DOI] [PubMed] [Google Scholar]
- 5.Douek DC, Betts MR, Hill BJ, et al. Evidence for increased T cell turnover and decreased thymic output in hiv infection. J Immunol. 2001;167:6663–6668. doi: 10.4049/jimmunol.167.11.6663. [DOI] [PubMed] [Google Scholar]
- 6.van Grevenynghe J, Procopio FA, He Z, et al. Transcription factor foxo3a controls the persistence of memory CD4+ T cells during HIV infection. Nat Med. 2008;14:266–274. doi: 10.1038/nm1728. [DOI] [PubMed] [Google Scholar]
- 7.Brenchley JM. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 8.Liu Z, Cumberland WG, Hultin LE, et al. CD8+ T-lymphocyte activation in HIV-1 disease reflects an aspect of pathogenesis distinct from viral burden and immunodeficiency. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;18:332–340. doi: 10.1097/00042560-199808010-00004. [DOI] [PubMed] [Google Scholar]
- 9.Deeks SG, Kitchen CM, Liu L, et al. Immune activation set point during early HIV infection predicts subsequent CD4+ T-cell changes independent of viral load. Blood. 2004;104:942–947. doi: 10.1182/blood-2003-09-3333. [DOI] [PubMed] [Google Scholar]
- 10.Giri MS, Nebozhyn M, Showe L, Montaner LJ. Microarray data on gene modulation by HIV-1 in immune cells: 2000–2006. J Leukoc Biol. 2006;80:1031–1043. doi: 10.1189/jlb.0306157. [DOI] [PubMed] [Google Scholar]
- 11.Sankaran S, Guadalupe M, Reay E, et al. Gut mucosal T cell responses and gene expression correlate with protection against disease in long-term HIV-1-infected nonprogressors. Proc Natl Acad Sci USA. 2005;102:9860–9865. doi: 10.1073/pnas.0503463102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hyrcza MD, Kovacs C, Loutfy M, et al. Distinct transcriptional profiles in ex vivo CD4+ and CD8+ T cells are established early in human immunodeficiency virus type 1 infection and are characterized by a chronic interferon response as well as extensive transcriptional changes in CD8+ T cells. J Virol. 2007;81:3477–3486. doi: 10.1128/JVI.01552-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee MS, Hanspers K, Barker CS, et al. Gene expression profiles during human CD4+ T cell differentiation. Int Immunol. 2004;16:1109–1124. doi: 10.1093/intimm/dxh112. [DOI] [PubMed] [Google Scholar]
- 14.Dion ML, Poulin JF, Bordi R, et al. HIV infection rapidly induces and maintains a substantial suppression of thymocyte proliferation. Immunity. 2004;21:757–768. doi: 10.1016/j.immuni.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 15. Wu JQ, Dwyer DE, Dyer WB, et al. Genome-wide analysis of primary CD4+ and CD8+ T cell transcriptomes shows evidence for a network of enriched pathways associated with HIV disease. Retrovirology. 2011;8:18–39. doi: 10.1186/1742-4690-8-18. This study identified a network of pathways functionally connected by mitochondria as a transcriptional signature of HIV disease progression. In addition, AKT, MAPK, and WNT pathways were closely related to HIV nonprogression.
- 16.Sodora DL, Allan JS, Apetrei C, et al. Toward an AIDS vaccine: lessons from natural simian immunodeficiency virus infections of African nonhuman primate hosts. Nat Med. 2009;15:861–865. doi: 10.1038/nm.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bosinger SE, Li Q, Gordon SN, et al. Global genomic analysis reveals rapid control of a robust innate response in SIV-infected sooty mangabeys. J Clin Invest. 2009;119:3556–3572. doi: 10.1172/JCI40115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jacquelin B, Mayau V, Targat B, et al. Nonpathogenic siv infection of African green monkeys induces a strong but rapidly controlled type I IFN response. J Clin Invest. 2009;119:3544–3555. doi: 10.1172/JCI40093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fowke KR, Nagelkerke NJ, Kimani J, et al. Resistance to HIV-1 infection among persistently seronegative prostitutes in Nairobi, Kenya. Lancet. 1996;348:1347–1351. doi: 10.1016/S0140-6736(95)12269-2. [DOI] [PubMed] [Google Scholar]
- 20.Rowland-Jones SL, McMichael A. Immune responses in HIV-exposed seronegatives: have they repelled the virus? Curr Opin Immunol. 1995;7:448–455. doi: 10.1016/0952-7915(95)80087-5. [DOI] [PubMed] [Google Scholar]
- 21.Rowland-Jones S, Sutton J, Ariyoshi K, et al. HIV-specific cytotoxic T-cells in HIV-exposed but uninfected Gambian women. Nat Med. 1995;1:59–64. doi: 10.1038/nm0195-59. [DOI] [PubMed] [Google Scholar]
- 22.Bernard NF, Yannakis CM, Lee JS, Tsoukas CM. Human immunodeficiency virus HIV-specific cytotoxic T lymphocyte activity in HIV-exposed seronegative persons. J Infect Dis. 1999;179:538–547. doi: 10.1086/314621. [DOI] [PubMed] [Google Scholar]
- 23.Makedonas G, Bruneau J, Alary M, et al. Comparison of HIV-specific CD8 T-cell responses among uninfected individuals exposed to HIV parenterally and mucosally. AIDS. 2005;19:251–259. [PubMed] [Google Scholar]
- 24.Beretta A, Weiss SH, Rappocciolo G, et al. Human immunodeficiency virus type 1 HIV-1 seronegative injection drug users at risk for HIV exposure have antibodies to HLA class I antigens and T cells specific for HIV envelope. J Infect Dis. 1996;173:472–476. doi: 10.1093/infdis/173.2.472. [DOI] [PubMed] [Google Scholar]
- 25.Clerici M, Levin JM, Kessler HA, et al. HIV-specific T-helper activity in seronegative healthcare workers exposed to contaminated blood. JAMA. 1994;271:42–46. [PubMed] [Google Scholar]
- 26.Rowland-Jones SL, Nixon DF, Aldhous MC, et al. HIV-specific cytotoxic T-cell activity in an HIV-exposed but uninfected infant. Lancet. 1993;341:860–861. doi: 10.1016/0140-6736(93)93063-7. [DOI] [PubMed] [Google Scholar]
- 27.Barretina J, Blanco J, Gutierrez A, et al. Evaluation of the putative role of c-c chemokines as protective factors of HIV-1 infection in seronegative hemophiliacs exposed to contaminated hemoderivatives. Transfusion. 2000;40:461–467. doi: 10.1046/j.1537-2995.2000.40040461.x. [DOI] [PubMed] [Google Scholar]
- 28.Makedonas G, Bruneau J, Lin H, et al. HIV-specific CD8 T cell activity in uninfected injection drug users is associated with maintenance of seronegativity. AIDS. 2002;16:1595–1602. doi: 10.1097/00002030-200208160-00004. [DOI] [PubMed] [Google Scholar]
- 29.Stranford SA, Skurnick J, Louria D, et al. Lack of infection in HIV-exposed individuals is associated with a strong CD8+ cell noncytotoxic anti-HIV response. Proc Natl Acad Sci USA. 1999;96:1030–1035. doi: 10.1073/pnas.96.3.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ravet S, Scott-Algara D, Bonnet E, et al. Distinctive NK-cell receptor repertoires sustain high-level constitutive NK-cell activation in HIV-exposed uninfected individuals. Blood. 2007;109:4296–4305. doi: 10.1182/blood-2006-08-040238. [DOI] [PubMed] [Google Scholar]
- 31.Bashirova AA, Thomas R, Carrington M. HLA/KIR restraint of HIV: surviving the fittest. Annu Rev Immunol. 2011;29:295–317. doi: 10.1146/annurev-immunol-031210-101332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wichukchinda N, Kitamura Y, Rojanawiwat A, et al. The polymorphisms in DC-SIGNR affect susceptibility to HIV type 1 infection. AIDS Res Hum Retroviruses. 2007;23:686–692. doi: 10.1089/aid.2006.0212. [DOI] [PubMed] [Google Scholar]
- 33.Iqbal SM, Ball TB, Kimani J, et al. Elevated T cell counts and RANTES expression in the genital mucosa of HIV-1-resistant Kenyan commercial sex workers. J Infect Dis. 2005;192:728–738. doi: 10.1086/432482. [DOI] [PubMed] [Google Scholar]
- 34.Hirbod T, Reichard C, Hasselrot K, et al. HIV-1 neutralizing activity is correlated with increased levels of chemokines in saliva of HIV-1-exposed uninfected individuals. Curr HIV Res. 2008;6:28–33. doi: 10.2174/157016208783571964. [DOI] [PubMed] [Google Scholar]
- 35.Burgener A, Sainsbury J, Plummer FA, Ball TB. Systems biology-based approaches to understand HIV-exposed uninfected women. Curr HIV/AIDS Rep. 2010;7:53–59. doi: 10.1007/s11904-010-0039-3. [DOI] [PubMed] [Google Scholar]
- 36.Misse D, Yssel H, Trabattoni D, et al. IL-22 participates in an innate anti-HIV-1 host-resistance network through acute-phase protein induction. J Immunol. 2007;178:407–415. doi: 10.4049/jimmunol.178.1.407. [DOI] [PubMed] [Google Scholar]
- 37.Burgener A, Boutilier J, Wachihi C, et al. Identification of differentially expressed proteins in the cervical mucosa of HIV-1-resistant sex workers. J Proteome Res. 2008;7:4446–4454. doi: 10.1021/pr800406r. [DOI] [PubMed] [Google Scholar]
- 38. Iqbal SM, Ball TB, Levinson P, et al. Elevated elafin/trappin-2 in the female genital tract is associated with protection against HIV acquisition. AIDS. 2009;23:1669–1677. doi: 10.1097/QAD.0b013e32832ea643. Using a proteomics approach, this study identified elafin/trappin-2 as a novel innate immune factor associated with resistance to HIV infection in a cohort of HIV-resistant Kenyan sex workers.
- 39.McLaren PJ, Mayne M, Rosser S, et al. Antigen-specific gene expression profiles of peripheral blood mononuclear cells do not reflect those of T-lymphocyte subsets. Clin Diagn Lab Immunol. 2004;11:977–982. doi: 10.1128/CDLI.11.5.977-982.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]