Abstract
Purpose
Cervical tumor response on posttherapy 2[18F]fluoro-2-deoxy-D-glucose-positron emission tomography (FDG-PET) is predictive of survival outcome. The purpose of this study was to use gene expression profiling to identify pathways associated with tumor metabolic response.
Experimental Design
This was a prospective tissue collection study for gene expression profiling of 62 pretreatment biopsies from patients with advanced cervical cancer. Patients were treated with definitive radiation. Fifty-three patients received concurrent chemotherapy. All patients underwent a pretreatment and a 3-month posttherapy FDG-PET/computed tomography (CT). Tumor RNA was harvested from fresh frozen tissue and hybridized to Affymetrix U133Plus2 GeneChips. Gene set enrichment analysis (GSEA) was used to identify signaling pathways associated with tumor metabolic response. Immunohistochemistry and in vitro FDG uptake assays were used to confirm our results.
Results
There were 40 biopsies from patients with a complete metabolic response (PET-negative group) and 22 biopsies from patients with incomplete metabolic response (PET-positive group). The 3-year cause-specific survival estimates were 98% for the PET-negative group and 39% for the PET-positive group (P < 0.0001). GSEA identified alterations in expression of genes associated with the PI3K/Akt signaling pathway in patients with a positive follow-up PET. Immunohistochemistry using a tissue microarray of 174 pretreatment biopsies confirmed p-Akt as a biomarker for poor prognosis in cervical cancer. The phosphoinositide 3-kinase (PI3K) inhibitor LY294002 inhibited FDG uptake in vitro in cervical cancer cell lines.
Conclusions
Activation of the PI3K/Akt pathway is associated with incomplete metabolic response in cervical cancer. Targeted inhibition of PI3K/Akt may improve response to chemoradiation.
Introduction
Cervical cancer ranks among the top 3 cancer diagnoses in women worldwide and is a leading cause of cancer death in developing countries. In the United States in 2011, 12,710 new diagnoses and 4,290 cancer deaths are expected (1). Patients who present with locally advanced carcinoma of the cervix are treated with definitive chemoradiation therapy. Most commonly, single-agent cisplatin is given once weekly for 6 cycles concurrently with radiation. Expected 5-year overall survival for patients with locally advanced cervical carcinoma treated in this manner is 70% to 80% (2, 3).
Therapeutic response, as determined by posttherapy 2[18F]fluoro-2-deoxy-D-glucose-positron emission tomography (FDG-PET) and more recently FDG-PET/computed tomography (CT), has been shown to be predictive of progression-free and overall survival outcomes (4–6). In a prospective data collection study at our institution, 3-year cause-specific survival was 100% and 51% for patients with a complete versus a partial metabolic response on 3-month posttherapy FDG-PET (P < 0.001). Corresponding 3-year progression-free survivals were 78% and 35% (P < 0.0001), respectively. Multivariate analysis showed that metabolic response was more predictive of treatment outcome than all known pretreatment related factors, including Federation Internationale des Gynaecologistes et Obstetristes (FIGO) stage and lymph node status. Posttherapy FDG-PET may, therefore, be used as an immediately available surrogate biomarker for overall response to therapy.
Microarray analysis of tissue biopsy specimens has been widely implemented as a high-throughput method for the detection of altered gene expression. With respect to cervical carcinoma, gene expression profiling has been used in several small studies to identify genes associated with poor outcome after treatment (7–11). More recently, Lando and colleagues analyzed gene dosage alterations in 97 patients with cervical cancer by array comparative genomic hybridization (aCGH; ref. 12). Their analysis identified losses in 3 chromosomal regions (3p, 13q, and 21q) that were associated with poor outcome after chemoradiotherapy in cervical cancer. Integration of the aCGH data with gene expression data identified 4 candidate genes associated with poor prognosis after chemoradiation treatment (RYBP, GBE1, FAM48A, and MED4). Gene ontology analysis of their results implicated several biologic processes in chemoradiation response in cervical cancer including protein translation, carbohydrate metabolism, apoptosis, and chromosomal maintenance.
Very few studies have explored the molecular mechanisms that regulate treatment response in cervical cancer, and no studies have specifically examined the signaling pathways that influence cervical tumor metabolic response. The objective of the current study was to use gene expression profiling as a discovery tool to identify signaling pathways associated with incomplete metabolic response after chemoradiation therapy in cervical cancer.
Materials and Methods
Patients
The study population included 62 patients prospectively enrolled into a tumor-banking protocol at the time of diagnosis of cervical cancer (March 1998 through December 2006). Eligibility criteria for tumor banking were as follows: (i) patients had a pathologic diagnosis of invasive carcinoma of the cervix; (ii) patient age was greater than or equal to 18 years; (iii) tumor of patient had adequate tissue to obtain a 1-cm biopsy; and (iv) patients were scheduled to undergo radiation therapy with or without chemotherapy in the Department of Radiation Oncology at Washington University School of Medicine (St. Louis, MO). As part of the tumor banking protocol, tumor biopsies were obtained and frozen prior to the initiation of therapy. As part of standard practice at our institution, both pretreatment and posttreatment FDG-PET/CT imaging studies were obtained. Approval from the institutional Human Research Protection Office was obtained for this study and all patients signed informed consent.
Over the time period from 1998 to 2006, 131 patients contributed specimens to the tumor bank. After pathologic review, 86 specimens were found to have more than 25% tumor content and minimal contaminating normal cells (blood, lymphocytes, or cervical stromal cells). Gene chip hybridization was carried out on 70 specimens with the sufficient RNA yield and quality for hybridization. Prior to data analysis, 8 samples were excluded because of additional data from pathologic review (3), absence of 3-month follow-up PET data (3), or duplicate biopsies from the same patient (2). Patient samples were divided into 2 data sets for GeneChip hybridization and data analysis (see GeneExpression Profiling later). Clinical characteristics between the 2 groups were similar (Table 1). The test and validation sets consisted of 20 and 42 pretreatment cervical biopsies, respectively. Radiotherapy was conducted in all patients and consisted of external irradiation and intracavitary brachytherapy. Concurrent chemotherapy (weekly cycles of 40 mg/m2 cisplatin) was administered to 53 patients.
Table 1.
Clinical characteristics
| Test set (n = 20) | Validation set (n = 42) | |
|---|---|---|
| Age at Dx | 54 (38–75) | 50 (27–78) |
| Clinical stage | ||
| I | 4 | 15 |
| II | 12 | 12 |
| III | 3 | 14 |
| IV | 1 | 1 |
| PET + lymph node | ||
| None | 9 | 14 |
| Pelvic | 9 | 22 |
| PALN | 2 | 4 |
| SCV | 0 | 2 |
| Chemotherapy | ||
| Yes | 18 | 35 |
| No | 2 | 7 |
| Metabolic response | ||
| PET negative | ||
| Complete metabolic response | 14 | 26 |
| PET positive | ||
| Partial metabolic response | 4 | 10 |
| Progressive disease | 2 | 6 |
Abbreviations: PALN, para-aortic; SCV, supraclavicular.
Clinical follow-up
Clinical follow-up was conducted 6 weeks after the completion of therapy. Posttherapy FDG-PET was carried out 3 months after the completion of therapy. Patients were followed clinically thereafter according to institutional guidelines. Median follow-up time for patients alive at the time of last follow-up was 68 months (range: 15–134 months). At the time of last follow-up, 3 patients were alive with cervical cancer and 18 patients had died because of cervical cancer. Three patients died because of intercurrent illness, and 2 patients died because of toxicity. The remaining 36 patients had no evidence of disease.
PET imaging
Before November 2002, FDG-PET was carried out using a conventional PET scanner and interpreted as previously described (13). Thereafter, all FDG-PET studies were conducted with a hybrid PET/CT scanner using methods described by Wright and colleagues (14). For patients in this study, 24 were imaged with conventional PET and 38 were imaged with PET/CT. PET studies were deferred until the blood glucose concentration was less than 200 mg/dL. PET/CT images were interpreted in a standard clinical fashion, both separately and in a fused mode. Standardized uptake value (SUVmax) was determined for primary tumors from the pretreatment FDG-PET as previously described (15). Three-month follow-up PET results were reviewed and compared with the pretreatment PET study as previously described (6). There were 40 pretreatment biopsies from patients with no residual FDG uptake on the 3-month posttherapy PET (PET-negative group). The PET-positive group consisted of 22 pretreatment biopsies from patients with residual or progressive disease on the 3-month post-therapy PET. Within the PET-positive group, there were 14 pretreatment biopsies from patients with persistent FDG uptake on the 3-month posttherapy PET in sites of abnormal FDG uptake on the pretreatment study (partial metabolic response) and 8 pretreatment biopsies from patients with new sites of abnormal FDG uptake on the 3-month posttherapy PET (progressive disease).
Statistical analysis
Survival and tumor recurrence were measured from the completion of treatment. The Kaplan–Meier (product limit) method was used to derive estimates of survival based on total sample size. StatView version 5.0.1 software (SAS Institute) was used for the analysis. P less than 0.05 was set as the threshold for significance for all study outcomes. Tests of equivalence of estimates of survival were carried out by the generalized Wilcoxon log-rank test. A paired t test was used to compare the results of p-Akt staining to pretreatment cervix tumor SUVmax.
Gene expression profiling
Pretreatment tumor biopsies were frozen at the time of collection. Frozen sections were histologically reviewed for documentation of invasive cancer; only biopsies with more than 25% tumor were included in this study. Tumor RNA was harvested from fresh frozen tissue with TRIzol reagent (Invitrogen) as described (16). RNA samples were then labeled and hybridized to Affymetrix Human Genome U133 Plus 2.0 expression microarrays (Affymetrix) using standard protocols from the Laboratory for Clinical Genomics, Bethesda, MD (16, 17). To carry out interarray comparisons, the raw scan data from each microarray were scaled to a target intensity of 1,500 with the Affymetrix GCOS 1.2 (MAS 5) statistical algorithm (http://www.affymetrix.com).
Basic microarray data visualization, data filtering, and hierarchical clustering were carried out using the Spotfire DecisionSite for Functional Genomics as described previously (16). Gene set enrichment analysis (GSEA; http://www.broad.mit.edu/gsea) identified signaling pathways associated with tumor metabolic response. Depending on sample size, phenotype or gene set permutation analysis with ratio-of-classes or signal-to-noise gene ranking was carried out, as recommended by the program authors.
Immunohistochemistry
To generate a validation set for our gene expression data, a tissue microarray (TMA) was constructed from 174 archived paraffin-embedded pretreatment cervical cancer biopsies. Approval for construction of the TMA using archived specimens was obtained from the Washington University Human Research Protection Office. A waiver of informed consent was obtained. Briefly, slides were reviewed by a gynecologic pathology specialist (P.C. Huettner). The tumors were histologically typed as squamous cell carcinoma (n = 149), adenocarcinoma (n = 10), or other (n = 5). Areas containing invasive carcinoma were indicated on the glass slides by marking them with a dotting pen. Paired 1-mm punches were then taken from the corresponding regions of the paraffin blocks and placed into TMA blocks. Unstained 4-μm sections from these blocks were used for p-Akt staining. The TMA slides were dewaxed in xylene and rehydrated using graded alcohols. The slides were immersed in 100 μmol/L citrate buffer and boiled for 10 minutes in a pressure cooker to retrieve antigen. Endogenous peroxidase was blocked by applying 0.3% hydrogen peroxide for 20 minutes. Sections were subsequently blocked in 5% goat serum in TBS/Tween-20. The rabbit monoclonal anti-human p-Akt 473 antibody (736E11; Cell Signaling Technology; 1:50) was used as a primary antibody overnight at 4°C. Goat anti-rabbit (sc2040; Santa Cruz) was used as a secondary antibody. Slides were developed in diaminobenzidine, dehydrated, and mounted. Slides were scored for p-Akt intensity by 2 independent observers who were blinded to corresponding clinical data (0, negative; 1, weak; 2, intermediate; and 3, strong). One hundred and sixty-four of 174 (93%) of the biopsies had interpretable p-Akt immunohistochemistry results. The remaining 10 were uninterpretable due to artifact or detachment of the biopsy from the glass slide during processing.
Western blotting
Cervix cell lines SW756, Caski, C33A, C41, Me180, HeLa, HT-3, and SiHa were grown in standard media supplemented with FBS to 70% to 80% confluence. Cells were washed twice with PBS and extracted in lysis buffer containing 1% NaF, 0.5% Na3VO4, and protease inhibitor. Equal amounts of proteins from each sample were added in loading buffer and were heated for 3 minutes at 100°C. Samples were loaded on 10% SDS-PAGE gel for running 2 hours and were then transferred onto Bio-Rad nitrocellulose membranes (Bio-Rad). After blocking for 1 hour with 5% nonfat dry milk, blots were probed overnight at 4°C with primary antibodies against p-AktSer473 (1:1,000; Cell Signaling Technology), total Akt (1:1,000; Cell Signaling Technology), or β-actin (1:10,000; Sigma). β-Actin was used as the internal control. Blots were probed with horseradish peroxidase (HRP)-conjugated anti-rabbit or anti-mouse polyclonal IgG secondary antibodies for 1 hour at room temperature. After incubation in chemiluminescent substrate (ECL Western blotting detection reagents; Amersham/GE Healthcare) for 1 minute, blots were exposed on Classic Film BX (MIDSCI).
FDG uptake assays
Cervix cell lines SiHa, ME180, and C33A were grown in media supplemented with FBS to 70% to 80% confluence. Thirty minutes prior to adding radiolabeled glucose, media were changed to glucose-free Dulbecco’s Modified Eagle’s Medium (DMEM) + 10% FBS. Cells were incubated for 1 hour at 37°C in each of the following conditions: 20 μCi FDG alone, 20 μCi FDG +5 mmol/L glucose and 50 μmol/L cytochalasin B, 20 μCi FDG and 100 μmol/L LY294002 (Cell Signaling Technology). Cells were rinsed in cold PBS, harvested in 500 μL of 1% SDS + 10 mmol/L Na borate and counted on a gamma counter.
Results
Metabolic response and survival outcome
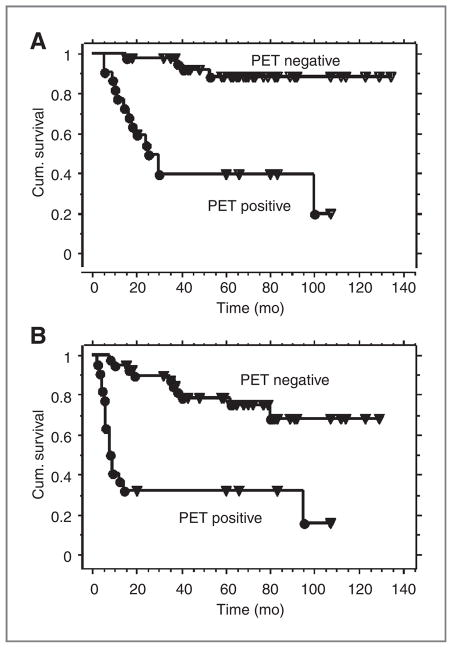
There were 40 pretreatment biopsies from patients with no evidence of disease on the 3-month posttherapy PET (PET-negative group) and 22 pretreatment biopsies from patients with residual or progressive disease on the 3-month posttherapy PET (PET-positive group). The 3-year cause-specific survival estimates were 98% for the PET-negative group and 39% for the PET-positive group (Fig. 1A). The corresponding 3-year progression-free survival estimates were 84% and 32%, respectively (Fig. 1B).
Figure 1.
A, Kaplan–Meier curves for cause-specific survival for the 62 patient data set. There were 40 pretreatment biopsies from patients with no evidence of disease on the 3-month posttherapy PET (PET-negative group) and 22 pretreatment biopsies from patients with residual or progressive disease on the 3-month posttherapy PET (PET-positive group). B, Kaplan–Meier curves for progression-free survival for the 62 patient data set.
Gene expression profiling
We examined global gene expression in cervical tumors as a means to identify signaling pathways whose up- or down-regulation correlated with tumor metabolic response, as shown by 3-month posttherapy FDG-PET/CT. The patient population consisted of 62 women diagnosed with cervical cancer; see Table 1 for clinical characteristics. Tissue from tumor biopsies was collected and fresh frozen for subsequent mRNA isolation, quality assessment, and performance of Affymetrix U133+2 gene expression microarrays, as described previously (16). Because tumors were banked and subjected to microarray analysis in 2 discrete time frames, during which interim there was a change in the Affymetrix RNA target preparation method, the data were divided into 2 groups, a training (20 tumors) and a test or validation set (42 tumors) based on these time frames. Clinical characteristics were similar between the 2 groups (Table 1).
The specific goal of the gene expression analysis was the identification of dysregulated signaling pathways associated with tumor metabolic response. GSEA (http://www.broad.mit.edu/gsea), a powerful bioinformatics tool that assesses whether an a priori defined set of genes (e.g., those in a common signaling pathway) shows statistically significant, concordant differences between 2 biologic states (i.e., phenotypes). Therefore, we used GSEA to test whether the expression of known oncogenic or metabolic pathways in cervical tumors was significantly correlated with metabolic response measured by 3-month PET results. A literature search was conducted to identify signaling pathways previously implicated in therapeutic response for cervical cancer. The results of this literature search identified Ras pathway, hypoxia/HIF1/VEGF, NF-κB/immune modulators, PI3K/PTEN/Akt/mTOR, and epidermal growth factor receptor (EGFR) as possible targets. In addition, all available pathways related to glucose metabolism were tested (GLUTs/glycolysis/glucose metabolism). All pathways analyzed showed a subset of upregulated genes in PET-positive tumors compared with PET-negative tumors. However, overlapping pathways involving phosphoinositide 3-kinase (PI3K), Akt, and PTEN signaling showed strikingly significant upregulation of the entire pathway [false discovery rate (FDR) Q values < 0.05]. These expression differences are exemplified by the KEGG endometrial cancer signaling pathway (http://www.genome.jp/kegg/pathway/hsa/hsa05213.html), which includes PI3K, Akt, and PTEN signaling. This pathway showed significant enrichment in PET-positive versus PET-negative tumors (FDR Q value = 0.006; Fig. 2). Genes with higher expression in PET-positive tumors have higher enrichment scores and are plotted on the left portion of the graph, whereas those with lower expression in PET-positive tumors have lower enrichment scores and are plotted on the right portion of the graph. The net effect is a sigmoidal curve. The bottom portion of the plot shows the value of the ranking metric moving down the list of ranked genes. A positive ranking metric indicates that a gene is correlated with the PET-positive phenotype. The table in Fig. 2 enumerates the genes in the pathway for which a majority of probe sets were significantly enriched and upregulated in PET-positive versus PET-negative tumors. Note that members of the Akt, PI3K, and PTEN pathways are significantly enriched.
Figure 2.
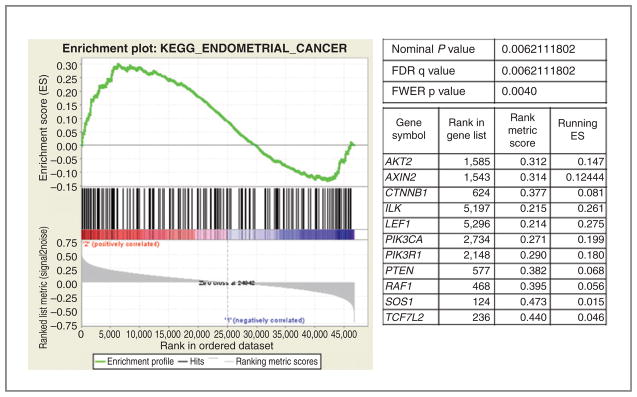
GSEA enrichment plot of KEGG endometrial cancer pathway genes in PET+ versus PET− tumors. Genes in the KEGG endometrial cancer signaling pathway showed significant enrichment in PET+ versus PET− tumors (FDR q value = 0.006). The top portion of the figure plots the enrichment scores (ES) for each gene, whereas the bottom portion of the plot shows the value of the ranking metric moving down the list of ranked genes. The table enumerates the genes in the pathway for which a majority of probe sets were significantly enriched and upregulated in PET-positive versus PET-negative tumors. FDR q value (FDR, false discovery rate); FWER p value (FWER, family wise-error rate).
Immunohistochemistry
To test whether Akt pathway activation is associated with survival outcome in human cervical cancers at the protein level, we carried out p-Akt immunohistochemistry using a commercially available anti-pS473 antibody and a TMA of 174 archived paraffin-embedded pretreatment cervical cancer biopsies. Two independent observers blinded to the clinical outcome and metabolic response data interpreted the results. The majority of the biopsies (88%) showed some p-Akt signal. Within this group, variation in intensity of p-Akt staining was observed: 68% had weak staining, 26% medium staining, and 6% strong staining (Fig. 3).
Figure 3.
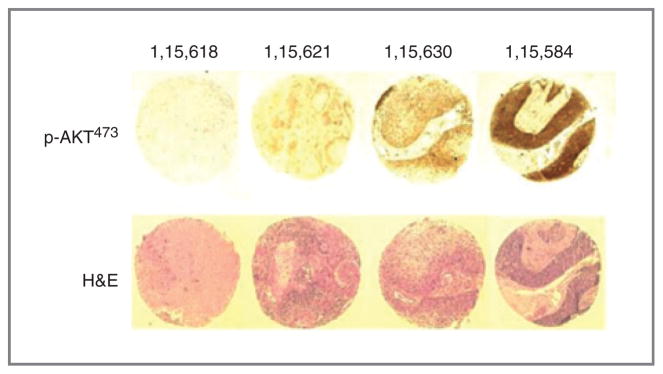
Immunohistochemical staining of the TMA from human cervical cancers. Top, example of p-AKT437 staining from different cases: negative (case number: 1,15,618), weak (1,15,621), medium (1,15,630), and strong (1,15,584). Bottom, hematoxylin and eosin (H&E) staining.
The results of p-Akt immunohistochemistry were compared with clinical outcome and risk factors for recurrence, including both pre- and posttreatment FDG-PET scan results. A paired t test was carried out to test the association between p-Akt staining and FDG uptake on the pretreatment FDG-PET (as assessed by maximum standardized uptake value/SUVmax). The results showed a statistically significant association between p-Akt staining intensity and increased SUVmax on the pretreatment PET, implying that activation of Akt in cervical tumors is associated with increased glucose uptake in vivo (mean difference: −10.9, P < 0.0001, DF 113).
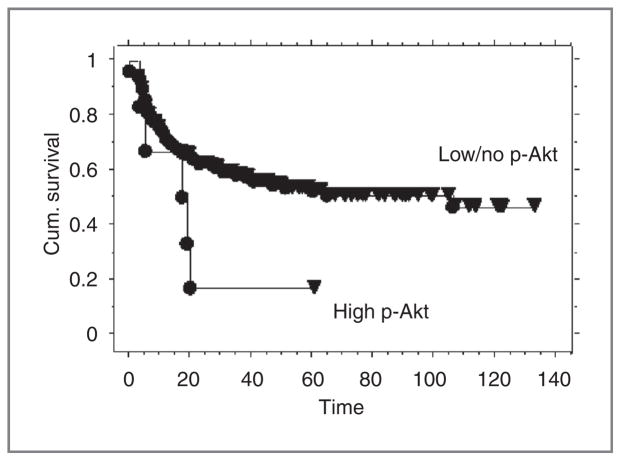
There was an association with high p-Akt signal by immunohistochemistry and decreased progression-free survival in patients with squamous cell carcinoma (Fig. 4). Five of 6 (83%) patients with high p-Akt expression have had a recurrence with a mean time to recurrence of 12.8 months. In the low/no p-Akt expression group, 64 of 143 (45%) patients have had a recurrence with a mean time to recurrence of 16.1 months.
Figure 4.
Kaplan–Meier curves for progression-free survival in patients with squamous cell carcinoma and high Akt expression versus low/no Akt expression. Pretreatment biopsies positive for squamous cell carcinoma were tested for p-Akt by immunohistochemistry as described in Fig. 2. Results are shown for 149 patients with squamous cell histology and high p-Akt immunohistochemistry (n = 6) versus squamous cell histology and low/no Akt expression (n = 143; P = 0.53).
Glucose uptake assays
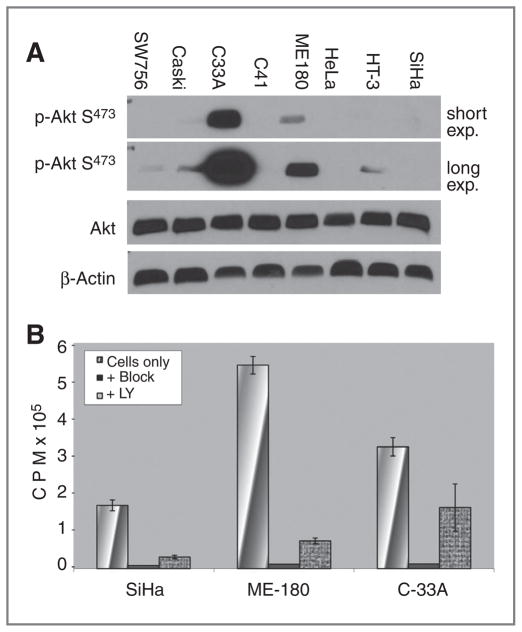
To test whether the PI3K/Akt pathway regulates glucose uptake in cervical cancer cells, we began by carrying out Western blot analysis for p-Akt using 8 different cervical cancer cell lines. These results show a range of baseline p-Akt expression in cervical cancer cell lines (Fig. 5A), with C33A and ME180 cells having relative high baseline expression of p-Akt versus SiHa cells which had little to no baseline expression of p-Akt. We hypothesized that inhibition of the PI3K/Akt pathway may affect glucose uptake and this was tested by conducting in vitro FDG uptake assays in the presence and absence of the PI3K inhibitor, LY294002. These results show that inhibition of PI3K decreases glucose uptake in cervical cell lines in vitro, although the effect was still present in cell lines that did not overexpress p-Akt (Fig. 5B).
Figure 5.
A, Western blotting for total Akt and p-Akt from 8 cervical cancer cell lines. Cervix cell lines SW756, Caski, C33A, C41, Me180, HeLa, HT-3, and SiHa were grown in standard media supplemented with FBS to 70% to 80% confluence. Cells were washed and extracted in lysis buffer containing 1% NaF, 0.5% Na3VO4, and protease inhibitor. Blots were probed with primary antibodies against p-AktSer473 (1:1,000; Cell Signaling Technology), total Akt (1:1,000, Cell Signaling Technology), or β-actin (1:10,000, Sigma). β-Actin was used as the internal control. B, glucose uptake assays for cervical cancer cell lines. Cervix cell lines SiHa, ME180, and C33A were grown in media supplemented with FBS to 70% to 80% confluence. Thirty minutes before adding radiolabeled glucose, media were changed to glucose-free DMEM + 10% FBS. Cells were incubated for 1 hour at 37°C in each of the following conditions: 20 μCi FDG alone, 20 μCi FDG + 5 mmol/L glucose and 50 μmol/L cytochalasin B, 20 μCi FDG and 100 μmol/L LY294002 (LY; Cell Signaling Technology). Cells were rinsed in cold PBS, harvested in 500 μL of 1% SDS + 10 mmol/L Na borate and counted on a gamma counter.
Discussion
Our previous clinical data showed that patients with cervical cancer with incomplete metabolic response after chemoradiation have poor survival outcome (6). In the current study, we used GSEA and 62 fresh frozen pre-treatment cervical cancer biopsies to identify signaling pathways that are associated with posttreatment metabolic response in cervical cancer. GSEA identified reproducible alterations in expression of genes from the PI3K/Akt signaling pathway in patients with incomplete metabolic response. To confirm these results at the protein level, we tested 174 archived paraffin-embedded specimens for p-Akt expression using immuohistochemistry. Our results show that pretreatment p-Akt expression is common in cervical cancer (88% p-Akt positive). In the subset of patients with squamous cell histology, increased expression of p-Akt was associated with decreased progression-free survival outcome after chemoradiation. In addition, increased expression of p-Akt was associated with increased glucose uptake on the pretreatment FDG-PET, suggesting that p-Akt expression is associated with tumor glucose uptake in vivo.
The PI3K/Akt signaling pathway is known to be dysregulated in several cancer sites, most notably endometrium where loss of PTEN and mutations of PIK3CA have been reported. For cervical cancer, a detailed analysis of the PI3K/Akt pathway using human tumor samples is lacking; however, the cumulative results of several small studies suggest that the PI3K/Akt pathway is activated in cervical cancer. In a Norwegian study of 46 paraffin-embedded specimens with no associated clinical outcome data, p-Akt staining was positive in 39 (85%) of the samples (18). In a Korean study of 27 patients (9 with recurrence and 18 without), expression of p-Akt was associated with local recurrence after primary radiotherapy (19). Our immunohistochemistry results with 174 patient samples are consistent with these preliminary studies and validate an association between pretreatment p-Akt expression and decreased survival outcome after standard chemoradiation.
Previous studies have shown that amplification of the long arm of chromosome 3 is an early event in cervical cancer tumorigenesis, a region which includes the PIK3CA gene (3q26.3). In the Norwegian study, PI3KCA gene copy number was determined by quantitative real-time PCR and was estimated to be 3 or more in 28 of 40 cases (18). A second group recently reported increased messenger RNA levels for PIK3CA in 12 cervical cancer specimens known to have amplification of 3q compared with normal cervix controls (20). These results are compelling and suggest that chromosomal amplification of the PIK3CA gene may be one mechanism of Akt activation in cervical cancer specimens. However, in a recent study of cervical cancers by integrated aCGH and gene expression analysis, although overexpression of several other genes in the 3q26 region was found to correlate with amplification, an association between overexpression of the PIK3CA gene and amplification of this chromosomal region was not found (H. Lyng, personal communication; ref. 12). Additional mechanisms may exist for activation of Akt in cervical cancer and this warrants further study. In a recent study from MD Anderson Cancer Center (Houston, TX), PIK3CA mutations were identified in 2 of 15 (13%) of patients with cervical cancer (21). Experiments are ongoing in our laboratory to determine the mechanism of Akt activation in human cervical tumors.
Our results suggest that dysregulation of the PI3K/Akt pathway is associated with persistent or in some cases new metabolically active tumor after local radiotherapy. It is possible that activation of Akt is a marker of radiotherapy resistance in cervical cancer, and indeed, an association between Akt pathway activation and radiation resistance has been documented in other tumor sites (22–25). Using the PI3K inhibitor LY294002, Lee and colleagues reported that pretreatment of cervical cancer cell lines increased radiation sensitivity in vitro (26). Additional study will be needed to determine whether up front treatment with PI3K or Akt inhibitors will radiosensitize cervical tumors in vivo.
Alternatively, or perhaps in combination, activation of Akt may be a marker of altered glucose metabolism in cervical cancer. In our study, increased expression of p-Akt was associated with increased glucose uptake on the pretreatment FDG-PET, suggesting that p-Akt expression is associated with tumor glucose uptake in vivo.
To follow-up on this result, we tested a series of cervical cancer cell lines for p-Akt expression and FDG uptake in vitro. When the PI3K inhibitor LY294002 was used, we observed a decrease in FDG uptake; however, this effect was also present in cell lines that did not overexpress p-Akt in the baseline study. It should be noted that our Western blot analysis for baseline p-Akt were conducted in the presence of 25 mmol/L glucose, and there are reports that Akt is activated when cells are exposed to glucose or during glucose uptake (27, 28). We have observed robust endogenous Akt activation in C33A compared with SiHa and ME180 cells. We determined that LY294002, a known PI3K inhibitor had a greater effect in SiHa and ME180 than C33A cells. This observation suggests that while LY294002 inhibited Akt-mediated glucose uptake in SiHa and ME180, where the endogenous Akt levels are low, its effect in C33A is limited by preexisting high endogenous p-Akt expression and may require a higher dose of LY294002 for the same effect. It is possible that in those cells with no basal Akt activation, presence of glucose may lead to Akt activation and subsequent glucose uptake and that this effect is significantly reduced when PI3K is inhibited. Experiments are ongoing in our laboratory to follow-up on these results.
The limitations of this study are those of any gene expression profiling analysis, including the fact that mRNA expression change does not necessarily equate with functional changes to signaling pathways. However, we mitigated this limitation by using an orthogonal method, namely immunohistochemistry assay of proteins in the Akt pathway, in a large validation set of tumor specimens, and found very similar results. Furthermore, we showed functional evidence of the role of the Akt pathway by showing that the PI3K inhibitor LY294002 inhibited FDG uptake in cervical cancer cell lines.
In summary, we identified alterations in expression of genes from the PI3K/Akt pathway that are associated with metabolic response in cervical cancer (n = 62). These results were validated using immunohistochemistry for p-Akt and a TMA (n = 174). Pretreatment phosphorylation of Akt is common in cervical cancer specimens (88%), and overexpression of p-Akt is associated with decreased survival outcome after radiotherapy. Expression of p-Akt is associated with increased FDG uptake in cervical tumors in vivo. Inhibition of PI3K decreases FDG uptake in vitro.
Translational Relevance.
Metabolic response [as determined by posttherapy 2[18F]fluoro-2-deoxy-D-glucose-positron emission tomography (FDG-PET) scanning] is predictive of survival outcome after chemoradiation in cervical cancer. The objective of this study was to use gene expression profiling as a discovery tool to identify signaling pathways associated with cervical tumor metabolic response. Using gene set enrichment analysis (GSEA), we identified alterations in expression of genes from the PI3K/Akt pathway (n = 62) that were associated with incomplete metabolic response to chemoradiation. These results were validated using immunohistochemistry for p-Akt and a pretreatment tumor biopsy microarray (n = 174). Pretreatment phosphorylation of Akt was common in our data set (88%), and overexpression of p-Akt was associated with decreased survival outcome after radiotherapy. Expression of p-Akt was associated with increased pretreatment FDG uptake in cervical tumors in vivo and inhibition of phosphoinositide 3-kinase (PI3K) decreased FDG uptake in vitro. These results suggest that targeted inhibition of the PI3K/Akt pathway may improve treatment outcome in cervical cancer.
Acknowledgments
The authors thank Mike Zahner and Rebecca Andrews for technical assistance and Alvin J. Siteman Cancer Center at Washington University School of Medicine and Barnes-Jewish Hospital in St. Louis, MO, for the use of the Molecular and Genomics Analysis Core, which provided Gene Chip hybridization services.
Grant Support
This work was supported in part by NCI R01 CA095713 (J.S. Rader), the Susan G. Komen Foundation (Q. Yang), NCI NIH CA129440 (Q. Yang), and by the Amercian Cancer Society IRG-58-010-54 (J.K. Schwarz). The Siteman Cancer Center is supported by an NCI Cancer Center Support Grant #P30 CA91842.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–36. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 2.Morris M, Eifel PJ, Lu J, Grigsby PW, Levenback C, Stevens RE, et al. Pelvic radiation with concurrent chemotherapy compared with pelvic and para-aortic radiation for high-risk cervical cancer. N Engl J Med. 1999;340:1137–43. doi: 10.1056/NEJM199904153401501. [DOI] [PubMed] [Google Scholar]
- 3.Eifel PJ, Winter K, Morris M, Levenback C, Grigsby PW, Cooper J, et al. Pelvic irradiation with concurrent chemotherapy versus pelvic and para-aortic irradiation for high-risk cervical cancer: an update of radiation therapy oncology group trial (RTOG) 90-01. J Clin Oncol. 2004;22:872–80. doi: 10.1200/JCO.2004.07.197. [DOI] [PubMed] [Google Scholar]
- 4.Grigsby PW, Siegel BA, Dehdashti F. Lymph node staging by positron emission tomography in patients with carcinoma of the cervix. J Clin Oncol. 2001;19:3745–9. doi: 10.1200/JCO.2001.19.17.3745. [DOI] [PubMed] [Google Scholar]
- 5.Schwarz JK, Grigsby PW, Dehdashti F, Delbeke D. The role of 18F-FDG PET in assessing therapy response in cancer of the cervix and ovaries. J Nucl Med. 2009;50 (Suppl 1):64S–73S. doi: 10.2967/jnumed.108.057257. [DOI] [PubMed] [Google Scholar]
- 6.Schwarz JK, Siegel BA, Dehdashti F, Grigsby PW. Association of posttherapy positron emission tomography with tumor response and survival in cervical carcinoma. JAMA. 2007;298:2289–95. doi: 10.1001/jama.298.19.2289. [DOI] [PubMed] [Google Scholar]
- 7.Klopp AH, Eifel PJ. Gene expression profiling in cervical cancer: state of the art and future directions. Cancer J. 2006;12:170–4. doi: 10.1097/00130404-200605000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Kitahara O, Katagiri T, Tsunoda T, Harima Y, Nakamura Y. Classification of sensitivity or resistance of cervical cancers to ionizing radiation according to expression profiles of 62 genes selected by cDNA microarray analysis. Neoplasia. 2002;4:295–303. doi: 10.1038/sj.neo.7900251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wong YF, Sahota DS, Cheung TH, Lo KW, Yim SF, Chung TK, et al. Gene expression pattern associated with radiotherapy sensitivity in cervical cancer. Cancer J. 2006;12:189–93. doi: 10.1097/00130404-200605000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Harima Y, Togashi A, Horikoshi K, Imamura M, Sougawa M, Sawada S, et al. Prediction of outcome of advanced cervical cancer to thermo-radiotherapy according to expression profiles of 35 genes selected by cDNA microarray analysis. Int J Radiat Oncol Biol Phys. 2004;60:237–48. doi: 10.1016/j.ijrobp.2004.02.047. [DOI] [PubMed] [Google Scholar]
- 11.Harima Y, Ikeda K, Utsunomiya K, Shiga T, Komemushi A, Kojima H, et al. Identification of genes associated with progression and metastasis of advanced cervical cancers after radiotherapy by cDNA microarray analysis. Int J Radiat Oncol Biol Phys. 2009;75:1232–9. doi: 10.1016/j.ijrobp.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 12.Lando M, Holden M, Bergersen LC, Svendsrud DH, Stokke T, Sundfor K, et al. Gene dosage, expression, and ontology analysis identifies driver genes in the carcinogenesis and chemoradioresistance of cervical cancer. PLoS Genet. 2009;5:e1000719. doi: 10.1371/journal.pgen.1000719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grigsby PW, Siegel BA, Dehdashti F, Mutch DG. Posttherapy surveillance monitoring of cervical cancer by FDG-PET. Int J Radiat Oncol Biol Phys. 2003;55:907–13. doi: 10.1016/s0360-3016(02)04287-6. [DOI] [PubMed] [Google Scholar]
- 14.Wright JD, Dehdashti F, Herzog TJ, Mutch DG, Huettner PC, Rader JS, et al. Preoperative lymph node staging of early-stage cervical carcinoma by [18F]-fluoro-2-deoxy-D-glucose-positron emission tomography. Cancer. 2005;104:2484–91. doi: 10.1002/cncr.21527. [DOI] [PubMed] [Google Scholar]
- 15.Kidd EA, Siegel BA, Dehdashti F, Grigsby PW. The standardized uptake value for F-18 fluorodeoxyglucose is a sensitive predictive biomarker for cervical cancer treatment response and survival. Cancer. 2007;110:1738–44. doi: 10.1002/cncr.22974. [DOI] [PubMed] [Google Scholar]
- 16.Payton JE, Grieselhuber NR, Chang LW, Murakami M, Geiss GK, Link DC, et al. High throughput digital quantification of mRNA abundance in primary human acute myeloid leukemia samples. J Clin Invest. 2009;119:1714–26. doi: 10.1172/JCI38248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Available from: http://pathology.wustl.edu/research/cores/lcg/index.php.
- 18.Bertelsen BI, Steine SJ, Sandvei R, Molven A, Laerum OD. Molecular analysis of the PI3K-AKT pathway in uterine cervical neoplasia: frequent PIK3CA amplification and AKT phosphorylation. Int J Cancer. 2006;118:1877–83. doi: 10.1002/ijc.21461. [DOI] [PubMed] [Google Scholar]
- 19.Kim TJ, Lee JW, Song SY, Choi JJ, Choi CH, Kim BG, et al. Increased expression of pAKT is associated with radiation resistance in cervical cancer. Br J Cancer. 2006;94:1678–82. doi: 10.1038/sj.bjc.6603180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Henken FE, Banerjee NS, Snijders PJ, Meijer CJ, De-Castro Arce J, Rosl F, et al. PIK3CA-mediated PI3-kinase signalling is essential for HPV-induced transformation in vitro. Mol Cancer. 2011;10:71. doi: 10.1186/1476-4598-10-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Janku F, Tsimberidou AM, Garrido-Laguna I, Wang X, Luthra R, Hong DS, et al. PIK3CA mutations in patients with advanced cancers treated with PI3K/AKT/mTOR axis inhibitors. Mol Cancer Ther. 2011;10:558–65. doi: 10.1158/1535-7163.MCT-10-0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schuurbiers OC, Kaanders JH, van der Heijden HF, Dekhuijzen RP, Oyen WJ, Bussink J. The PI3-K/AKT-pathway and radiation resistance mechanisms in non-small cell lung cancer. J Thorac Oncol. 2009;4:761–7. doi: 10.1097/JTO.0b013e3181a1084f. [DOI] [PubMed] [Google Scholar]
- 23.Chakravarti A, Zhai G, Suzuki Y, Sarkesh S, Black PM, Muzikansky A, et al. The prognostic significance of phosphatidylinositol 3-kinase pathway activation in human gliomas. J Clin Oncol. 2004;22:1926–33. doi: 10.1200/JCO.2004.07.193. [DOI] [PubMed] [Google Scholar]
- 24.Jiang Z, Pore N, Cerniglia GJ, Mick R, Georgescu MM, Bernhard EJ, et al. Phosphatase and tensin homologue deficiency in glioblastoma confers resistance to radiation and temozolomide that is reversed by the protease inhibitor nelfinavir. Cancer Res. 2007;67:4467–73. doi: 10.1158/0008-5472.CAN-06-3398. [DOI] [PubMed] [Google Scholar]
- 25.Gupta AK, McKenna WG, Weber CN, Feldman MD, Goldsmith JD, Mick R, et al. Local recurrence in head and neck cancer: relationship to radiation resistance and signal transduction. Clin Cancer Res. 2002;8:885–92. [PubMed] [Google Scholar]
- 26.Lee CM, Fuhrman CB, Planelles V, Peltier MR, Gaffney DK, Soisson AP, et al. Phosphatidylinositol 3-kinase inhibition by LY294002 radio-sensitizes human cervical cancer cell lines. Clin Cancer Res. 2006;12:250–6. doi: 10.1158/1078-0432.CCR-05-1084. [DOI] [PubMed] [Google Scholar]
- 27.Ou YH, Torres M, Ram R, Formstecher E, Roland C, Cheng T, et al. TBK1 directly engages Akt/PKB survival signaling to support oncogenic transformation. Mol Cell. 2011;41:458–70. doi: 10.1016/j.molcel.2011.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krusenstjerna-Hafstrom T, Madsen M, Vendelbo MH, Pedersen SB, Christiansen JS, Moller N, et al. Insulin and GH signaling in human skeletal muscle in vivo following exogenous GH exposure: impact of an oral glucose load. PLoS One. 2011;6:e19392. doi: 10.1371/journal.pone.0019392. [DOI] [PMC free article] [PubMed] [Google Scholar]