Abstract
The melanocortin 1 receptor (MC1R), a Gs protein-coupled receptor, plays an important role in human pigmentation. We investigated the regulation of expression and activity of the MC1R in primary human melanocyte cultures. Human beta defensin 3 (HBD3) acted as an antagonist for MC1R, inhibiting the α-melanocortin (α-MSH)-induced increase in the activities of adenylate cyclase, and tyrosinase, the rate-limiting enzyme for melanogenesis. α-Melanocortin and forskolin, which activate adenylate cyclase, and 12-o-tetradecanoyl phorbol 13-acetate, which activates PKC, increased, while exposure to ultraviolet radiation (UV) reduced, MC1R gene and membrane protein expression. Brief treatment with α-MSH resulted in MC1R desensitization, while continuous treatment up to 3 hours caused a steady rise in cAMP, suggesting receptor recycling. Pretreatment with agouti signaling protein or HBD3 prohibited responsiveness to α-MSH, but not forskolin, suggesting receptor desensitization by these antagonists. Melanocytes from different donors expressed different levels of the G-protein-coupled receptor kinases (GRK) 2, 3, 5, and 6, and β-arrestin 1. Therefore, in addition to MC1R genotype, regulation of MC1R expression and activity is expected to affect human pigmentation and the responses to UV.
Introduction
Skin and hair color are the outcome of synthesis of the dark brown pigment eumelanin, and the yellow-red pheomelanin by melanocytes, and skin pigmentation correlates directly with eumelanin content (Hennessy et al., 2005; Hauser et al., 2006). In mammals, including humans, eumelanin synthesis in melanocytes is regulated primarily via activation of the melanocortin 1 receptor (MC1R) by its agonist α-melanocortin (α-melanocyte stimulating hormone; α-MSH) (Geschwind et al., 1972; Hunt et al., 1995). Genetic studies in mice showed that loss of function mutations in mc1r result in a yellow coat color due to lack of eumelanin synthesis (Tamate and Takeuchi, 1984; Robbins et al., 1993). A similar coat color phenotype results from mutations that cause over expression of agouti, which encodes agouti signaling protein (ASIP), the physiological antagonist of the mc1r (Silvers, 1979; Yen et al., 1994; Abdel-Malek et al., 2001). This paradigm is recapitulated in humans. In human melanocytes, allelic variants of MC1R that result in loss of function of the receptor are strongly associated with red hair phenotype due to inhibition of eumelanin synthesis that is normally induced by α-MSH, (Box et al., 1997; Smith et al., 1998; Scott et al., 2002; Ringholm et al., 2004; Kadekaro et al., 2010). Treatment of human melanocytes with ASIP blocks the binding of α-MSH to its receptor, thus inhibiting eumelanin synthesis (Suzuki et al., 1997). Recently, a novel regulator of MC1R activity, CBD103, the canine homolog of human beta defensin 3 (HBD3), was discovered and found to bind to the MC1R with high affinity, and to regulate pigmentation in dogs and transgenic mice (Candille et al., 2007). The black coat color of HBD3 transgenic mice was attributed to competition with ASIP binding to the MC1R (Candille et al., 2007). The exact role of HBD3 in regulating human MC1R activity remained to be determined.
The MC1R gene is highly polymorphic, with at least 75 different allelic variants identified in different human populations (Garcia-Borron et al., 2005). Based on this property, the MC1R is considered an important determinant of the diversity of human pigmentation. This gene encodes a Gs protein-coupled receptor with seven transmembrane domains (Mountjoy et al., 1992). Some allelic variants of the MC1R affect skin and hair color by impairing binding of agonists to the MC1R, or inhibiting the activation of the agonist bound receptor. In particular, three variants, R151C, R160W and D294H, result in loss of function of the receptor due to lack of receptor signaling, and are strongly associated with red hair color (Scott et al., 2002; Kadekaro et al., 2010). Additionally, certain MC1R variants affect the desensitization of the receptor and its trafficking to the cell membrane (Beaumont et al., 2005; Sanchez-Mas et al., 2005; Sanchez-Laorden et al., 2006).
Generally, Gs protein-coupled receptors (GPCR) undergo homologous desensitization after stimulation with agonist. Desensitization results from phosphorylation of the receptors by the serine-threonine kinases, G-protein-coupled receptor kinases (GRKs), cAMP-dependent protein kinase A (PKA) or protein kinase C (PKC) (Bunemann and Hosey, 1999; Ferguson, 2001; Reiter and Lefkowitz, 2006; Premont and Gainetdinov, 2007). Receptor phosphorylation leads to recruitment of β-arrestin 1 and 2, which inhibit G protein coupling and serve as adaptors linking the receptors to the endocytic machinery. Previous studies on MC1R desensitization were carried out on human melanoma cells expressing endogenous MC1R, or cells (melanoma or HEK cells) transfected with human MC1R. Human melanocytes express relatively low numbers of MC1R on their surface (Donatien et al., 1992). Until now, it remained unknown whether the desensitization process plays a significant role in regulating MC1R when it is expressed at physiological levels in human melanocytes. The main goal of this study was to investigate the regulation of MC1R expression and activity by its physiological agonists and antagonists, and by UV. We demonstrate the effects of HBD3 and the regulation of MC1R expression and desensitization, which to our knowledge were not previously reported in primary human melanoctyes.
Results
To define the role of HBD3 in regulating MC1R signaling in primary human melanocytes, the effects of HBD3 on cAMP levels, proliferation, and tyrosinase activity in the absence or presence of α-MSH were determined and compared to those of the known MC1R physiological antagonist ASIP. Treatment of melanocytes with 1, 10 or 100 nM HBD3 had no effect on cAMP formation (Fig. 1a). When melanocytes were treated concomitantly with 1 nM α-MSH, and 1, 10, or 100 nM HBD3, only the highest concentration of HBD3 resulted in significant inhibition of the α-MSH- induced increase in cAMP levels. Based on these results, we used 100 nM HBD3 to demonstrate its antagonistic effects on the α-MSH induced-stimulation of cAMP formation, proliferation, and tyrosinase activity. As shown in Fig. 1b and c, concomitant treatment of a different melanocyte strain with 1 nM α-MSH and 100 nM HBD3 totally abrogated the effect of α-MSH on cAMP levels, and markedly inhibited its effect on proliferation and tyrosinase activity. Human beta defensin 3 did not reduce the forskolin-induced stimulation of cAMP production, suggesting that HBD3 does not directly inhibit adenylate cyclase (Fig. 1b). As we have previously reported, ASIP markedly abolished the stimulatory effects of α-MSH on cAMP levels, proliferation and tyrosinase activity (Fig. 1b, c; Suzuki et al., 1997). In this study, we used the fully MC1R active, cysteine-rich domain ASIP (80–132), with two mutations to enhance folding and stability (McNulty et al., 2005) (referred to throughout as ASIP). Treatment with 100 nM HBD3 had no effect, but significantly inhibited the stimulatory effects of 1 nm α-MSH on cell proliferation and tyrosinase activity (Fig. 1c).
Figure 1.
Effect of HBD3 on basal and α-MSH-induced cAMP levels, proliferation, and tyrosinase activity. (a) Melanocytes were treated with 0, 1 nM α-MSH, 1, 10 or 100 nM HBD3 ± 1 nM α-MSH for 45 minutes. (*)= significantly different from control at p<0.01, or (#) from α-MSH at p<0.05. In (b) melanocytes were treated with 100 nM HBD3 ±1 nM α-MSH or 1 μM forskolin, or 100 nM ASIP ± 1 nM α-MSH for 45 minutes. (*)= statistically different from control at p<0.001. (c) melanocytes were treated with the same concentrations of α-MSH and/or HDB3 or ASIP as in (b). (*)= significantly different from control at p<0.05, or (#) from α-MSH at p<0.001. Data represent mean percent of control +/− SEM. Triplicate experiments were performed.
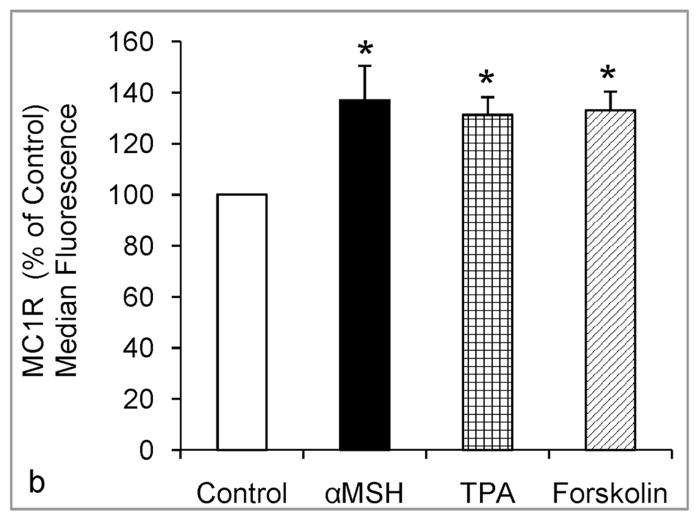
To investigate the transcriptional regulation of MC1R expression, we performed qRT-PCR on RNA isolated from melanocytes that were treated with 1 nM α-MSH, 1 μM forskolin, 100 nM HBD3 or ASIP, or irradiated with 75 or 105 mJ/cm2 UV (Fig. 2). Treatment with α-MSH increased the expression of MC1R after 8 hours. Forskolin also up regulated MC1R expression, suggesting that activation of the cAMP pathway is involved in transcriptional regulation of this gene. Neither HBD3 nor ASIP had any effect, while irradiation with UV resulted in marked reduction of MC1R expression. The effects of UV, α-MSH, forskolin, and TPA, were confirmed by immunostaining of the membrane bound MC1R in viable melanocytes followed by flow cytometric analysis (Fig. 3). We found that exposure to UV resulted in significant and dose-dependent reduction, which was evident 24 hours post irradiation, while α-MSH, forskolin or TPA significantly increased MC1R membrane expression 14 hours after treatment (Fig. 3).
Figure 2.
Regulation of MC1R gene expression by α-MSH, ASIP, HBD3 and UV. Melanocytes were maintained in medium lacking TPA and bovine pituitary extract overnight, then treated with 0, 1 nM α-MSH, 1 μM forskolin, 100 nM HBD3 or ASIP, or irradiated with 75 or 105 mJ/cm2 UV. Total RNA was isolated 8 hours after treatment, and equal amounts of RNA from each group were analyzed by qRT PCR. Similar results were obtained in 2 independent experiments using 2 different melanocyte strains. The data was normalized using GAPDH as a loading control and mean relative expression levels are presented +/− SEM.
Figure 3.
Regulation of cell surface expression of MC1R by α-MSH and UV, as determined by immunostaining for MC1R followed by flow cytometric analysis. (a) Melanocytes were irradiated with increasing doses of UV (0, 20, 50, 75, or 105 mJ/cm2), and immunostained for MC1R 24 hours after exposure. In (b) Melanocytes were treated with 0, 1nM α-MSH, 1 μM forskolin, or 5 ng/ml TPA for 14 hours. In (a) and (b), the data (percent of control +/− SEM) represent the combined results of 3 independent experiments. (*)= Statistically different from control at p<0.05.
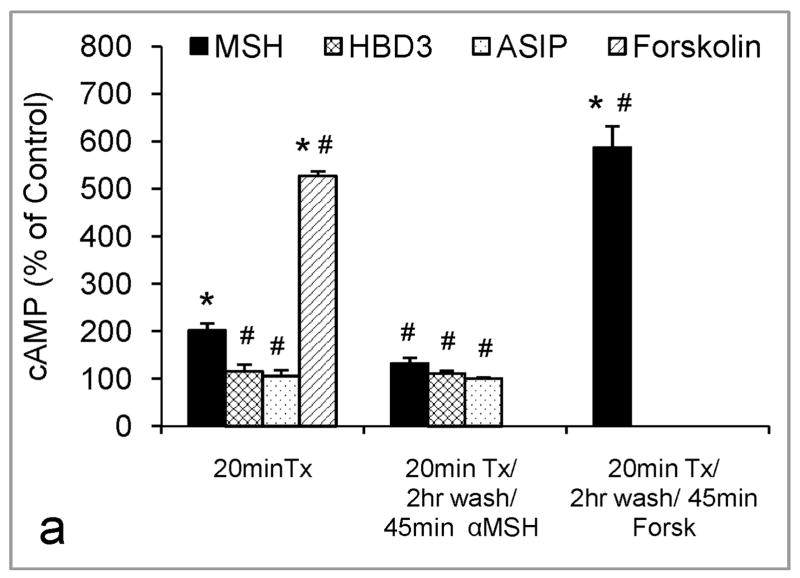
Generally GPCRs undergo desensitization upon prolonged or repeated exposure to their respective agonists. We found that the MC1R underwent desensitization after 20 minutes of treatment with 1 nM α-MSH (Fig. 4a). The inability of melanocytes to respond to retreatment with α-MSH with further increase in cAMP suggests homologous desensitization. Melanocytes could still respond to forskolin following brief treatment with α-MSH, indicating that adenylate cyclase could still be activated. Pretreatment of melanocytes with 1 nM HBD3 or ASIP for 20 minutes prevented melanocytes from responding to a challenge with 1 nM α-MSH (Fig. 4a). However, melanocytes pretreated with 100 nM HBD3 or ASIP responded to forskolin, suggesting that these antagonists affect the MC1R and not adenylate cyclase (Fig. 4b). Continuous treatment with α-MSH for 3 hours resulted in a greater increase in cAMP levels than brief treatment for 45 minutes, suggesting that although MC1R undergoes desensitization, the receptors might be rapidly recycled, and the agonist might enhance the trafficking of receptors to the cell surface (Fig. 4c). However, after 6 hours of continuous treatment with α-MSH, cAMP levels went down, presumably due to uncoupling of the receptor from Gs protein (Fig. 4c).
Figure 4.
Response to α-MSH after pretreatment with agonist or antagonists, and the role of PKA in MC1R desensitization. Data represent percent of control +/− SEM. In (a), In 10 independent experiments, melanocytes were pretreated with 0, 1 nM α-MSH, 1 nM ASIP or HBD3, or 1 μM forskolin, then challenged with α-MSH, as described in Materials and Methods. (*)= Statistically different from control, or (#) from α-MSH 20 minutes at p<0.001. In (b), melanocytes were pretreated with 100 nM ASIP or HBD3 then challenged with 1 μM forskolin. In (c), three melanocyte strains were treated for 45 minutes, 3 or 6 hours with 1 nM α-MSH. (*)= Statistically different from control, (+) from 45 minute α-MSH, or (#) from 3 hour α-MSH treatment at p<0.05. In (d), melanocytes were pretreated with 0, or 1 nM α-MSH ± 100 nM H-89. This experiment was repeated twice with similar findings. (*)= Statistically different from control or (#) from α-MSH 20 minutes at p<0.01.
Activation of PKA is thought to be a mechanism for GPCR desensitization. To investigate the potential role of PKA in this process, we compared MC1R desensitization in the presence or absence of the PKA inhibitor H-89. The results revealed that inhibition of PKA did not alleviate the α-MSH-induced MC1R desensitization, suggesting that this process is mediated by alternative mechanism(s), mainly by GRKs and β-arrestins (Fig. 4d).
Ten different melanocyte strains, each from a single donor, were utilized to compare the expression of GRK2, 3, 5, and 6, as well as β-arrestin 1, by Western blot analysis (Fig. 5). We found that all ten strains expressed relatively similar levels of GRK2 and β-arrestin 1, showed some variation in GRK3 levels, but differed tremendously in the expression of GRK5 and 6. These results were confirmed in additional nine melanocyte strains. The levels of GRKs did not correlate with the pigmentary phenotype of the donors (data not shown). Treatment with 1 or 10nM α-MSH, ASIP or HBD3 for 45, 60, or 90 minutes had variable effects on the levels of GRK2, 3, 5, and 6 in different melanocyte strains (data not shown). When increases in the levels of GRKs were observed, the kinetics and extent of the effects differed among the different strains tested, reflecting possible individual differences in desensitization.
Figure 5.
Comparison of basal levels of GRK 2, 3, 5, and 6 and β-arrestin 1 in a panel of cultured human melanocytes with different pigmentary phenotypes (C= lightly pigmented, B= darkly pigmented donor) and MC1R genotypes using Western blotting.
Discussion
The MC1R gene is a principle regulator of the diversity of human pigmentation and a melanoma susceptibility gene (Box et al., 1997; Smith et al., 1998; Palmer et al., 2000; Box et al. 2001; Kennedy et al., 2001; Garcia-Borron et al., 2005). We have demonstrated that this gene plays a central role in the UV response of human melanocytes (Im et al., 1998; Kadekaro, et al. 2005; Kadekaro et al., 2010). Elucidating how expression and activity of this receptor are regulated is pivotal for understanding its effects on melanocytes and the risk for melanoma. Unlike other GPCRs, the MC1R, MC3R, and MC4R have physiological agonists and antagonists, with α-MSH being the agonist for all 3 receptors, ASIP the antagonist for MC1R, and agouti related protein (AGRP) the antagonist for MC3R and MC4R (Ollmann et al., 1997; Suzuki et al., 1997). Beta defensin 3, best known for its antimicrobial activity and role in innate immunity (Harder et al., 2001), was recently found to bind to the MC1R and induce black coat color in domestic dogs and transgenic mice (Candille et al., 2007). We tested the effects of HBD3 on cultured human melanocytes and found that it inhibited the stimulatory effects of α-MSH on cAMP formation, tyrosinase activity, and proliferation (Fig. 1a, c). Previously it was shown that HBD3 alone had no effect on basal levels of cAMP in heterologous cells expressing the MC1R, but abrogated the effect of the potent melanocortin analog NDP-MSH on cAMP levels (Beaumont et al., 2011). HBD3 is synthesized by keratinocytes, and thus functions as a paracrine factor for melanocytes (Harder et al., 2001). Sawamura et al. reported that HBD3 in normal skin was mainly localized in the spinous and granular layers, however, Kesting et al. clearly showed HBD3 immunolocalization throughout the epidermis, with increased expression in wounded skin (Sawamura et al., 2005; Kesting et al., 2010). Comparison of HBD3 expression by a panel of primary human keratinocytes showed no correlation with the donor’s pigmentary phenotype (data not shown), suggesting that HBD3 is not a direct determinant of constitutive pigmentation. We found that HBD3 markedly inhibited α-MSH-induced, but not the forskolin-induced increase in cAMP levels, strongly suggesting that its inhibitory effect is mediated by blocking the MC1R (Fig. 1b). The results hereby presented identify a role for HBD3 as a physiological antagonist for the MC1R that can modulate the effects of α-MSH on human pigmentation and consequently the UV response.
Previously we reported results of Northern blot analysis showing that treatment of cultured human melanocytes by α-MSH increased, while exposure to UV reduced MC1R mRNA levels (Scott et al., 2002). Here, we employed qRT-PCR to further investigate the regulation of MC1R gene expression by its physiological agonists and antagonists and UV (Fig. 2). Results showed that treatment of melanocytes by α-MSH up regulated, while exposure to UV markedly down regulated, MC1R gene expression. As expected, treatment with ACTH had similar effects as α-MSH (data not shown). These findings are consistent with our microarray data showing reduction of MC1R expression by UV and increased expression by α-MSH (Kadekaro et al., 2010). Treatment with either ASIP or HBD3 had no effect on MC1R gene expression (Fig. 2). Previously we reported that low to moderate doses of UV had a transient inhibitory effect on MC1R mRNA levels (Scott et al., 2002). Accordingly, we expect that the inhibitory effect of the low doses of UV used in the current experiments (20–75 mJ/cm2 UV; Fig. 3a) to be short-lived. By immunostaining of viable melanocytes with MC1R–specific antibody followed by flow cytometric analysis, we verified that exposure to UV markedly decreased, while treatment with α-MSH, forskolin or TPA increased, MC1R cell surface expression (Fig. 3). The discrepancy between our results that UV reduced, and those previously reported by Funasaka et al. (Funasaka et al., 1998) that UV up regulated MC1R expression can possibly be attributed to different culture conditions of melanocytes, experimental protocols, and the UV source employed. Our results unequivocally demonstrate that MC1R expression is up regulated by its agonists, and suggest that activation of PKA and PKC are involved in this process.
The MC1R belongs to the large family of GPCR, which are activated by their cognate ligand that causes conformational changes of the receptor, leading to signaling via heterotrimeric G proteins (Bunemann and Hose, 1999; Premont and Gainetdinov, 2007). Activation of GPCR is turned off by the complex process of homologous desensitization. This process is initiated by GRKs that phosphorylate serine and threonine residues in the third intracellular loop or carboxy terminal domains of the activated GPCR (Ferguson 2001; Premont and Gainetdinov, 2007). Phosphorylated GPCR have a high affinity to members of the arrestin family, which bind to the receptors, uncouple them from heterotrimeric G proteins, and target them for internalization via clathrin-coated vesicles. It has been reported that MC1R expressed by human or mouse melanoma cells or by heterologous HEK cells undergo desensitization (Sanchez-Mas et al., 2005; Sanchez-Laorden et al., 2006). However, MC1R desensitization has not yet been investigated in human melanocytes, which express low numbers of the receptor (Donatien et al., 1992), and differ from HEK cells in GRK expression (data not shown). Additionally, human and mouse melanoma cells were found to express GRK2 and GRK6 only (Sanchez-Mas et al., 2005), while human melanocytes express GRK2, 3, 5, and 6 (Fig. 5). These differences underscore the importance of investigating MC1R desensitization by the endogenous cellular machinery in human melanocytes, as reported for other GPCR (Bunemann and Hosey, 1999; Premont and Gainetdinov, 2007).
Using 10 different melanocyte strains, we found that in all, MC1R underwent homologous desensitization (Fig. 4a). Brief pretreatment with agonist or the antagonists ASIP or HBD3 did not result in heterologous desensitization, as evidenced by the ability to respond to forskolin, indicating that adenylate cyclase was not desensitized (Fig. 4a, b). Continuous treatment with α-MSH for 3 hours resulted in greater increase in cAMP levels than treatment for 45 minutes (Fig. 4c), suggesting rapid receptor recycling. These results and the fact that the MC1R lacks Ser-Thr-rich clusters in its third intracellular loop and C-terminus (Reiter and Lefkowitz, 2006) suggest that MC1R belongs to class A of GPCRs that interact transiently with β-arrestins, internalize with them into clathrin coated pits, dissociate, and are rapidly recycled.
Beside GRKs, PKA might also be involved in MC1R desensitization. However, in human melanocytes, the PKA inhibitor H-89 did not block the α-MSH-induced MC1R desensitization, suggesting that PKA activation does not mediate this process (Fig. 4d). In this regard, the MC1R differs from MC2R expressed in mouse adrenal tumor cells, and MC4R expressed in mouse hypothalamic cells, both of which undergo PKA-mediated receptor desensitization and internalization, which are inhibited by treatment with H-89 (Baig et al., 2001; Shinyama et al., 2003; Kilianova et al., 2006).
Our results suggest that binding of the antagonists ASIP and HBD3 to the MC1R is an important mechanism for regulating receptor activity. Studies on heterologous cells transfected with the MC3R or MC4R showed that binding of these receptors by the synthetic inhibitor SHU-9119 resulted in retention of the antagonist-receptor complex on the cell surface, while treatment with agonist caused agonist-receptor internalization (Cai et al., 2006). We show that pretreatment with either ASIP, or HBD3, which we found to be an antagonist for MC1R, blocked the responsiveness of melanocytes to α-MSH, suggesting that these two antagonists enhance receptor internalization, inhibit its recycling, and/or have prolonged receptor occupancy.
Using Western blot analysis, we compared expression of the GRK2, 3, 5, and 6, as well as β-arrestin 1 in a panel of melanocyte strains with different melanin contents (Fig. 5). We did not investigate the expression of GRK1, 7, or 4, since the former two are predominantly expressed in the retina, while GRK4 is mainly expressed in the testis (Pitcher et al., 1998). We observed that melanocyte strains derived from different donors express the ubiquitous GRK2, 3, 5, and 6, as well as β-arrestin 1 (Fig. 5). Expression of GRK2 and β-arrestin 1 was the least variable, followed by expression of GRK3, while expression of GRK5 and 6 varied markedly among the different strains. Expression of GRKs and β-arrestin 1 was not dependent on MC1R genotype, since melanocytes expressing 2 red hair alleles that result in loss of function of MC1R (830C and 1307C) had similar levels of expression as strains with functional MC1R (e.g.1292C). Interestingly, two adult melanocyte strains with high melanin content had the least expression of GRK3, and very low levels of GRK5 and GRK6 (AHM 108B in Fig. 5, and data not shown).
We found that treatment of different human melanocyte strains (n=7) with 10 nM of the agonist α-MSH, HBD3 or ASIP for 45 minutes, 1 hour or 90 minutes had variable effects on the levels of GRK2, 3, 5, and 6 (data not shown). This variability might explain the differential responses of different melanocyte strains to continuous treatment with α-MSH, where in some, cAMP levels returned to basal levels, while in others, it was reduced but remained significantly high after 6 hours of treatment (Fig. 4c). This might possibly be due to a greater increase in GRKs levels resulting in more desensitization and/or less resensitization of MC1R in the former, compared to the latter. In future experiments, more detailed time course experiments will be conducted to determine the effects of MC1R agonist and antagonists on GRKs, and to correlate these effects with the levels of cAMP induced by α-MSH treatment .
Our results identify HBD3 as an antagonist of the MC1R that is expressed in human melanocytes, and shed new light on the regulation of MC1R expression and activity by its agonist and antagonists. That UV reduces, while α-MSH up regulates MC1R expression, underscores the significance of this agonist in up regulating the MC1R, which is critical for the proper DNA damage response of melanocytes to UV (Kadekaro et al., 2005; Kadekaro et al., 2010). Our findings demonstrate that α-MSH causes MC1R desensitization, which seems to be transient, and show that the MC1R antagonists ASIP and HBD3 inhibit MC1R activity by blocking its activation by α-MSH, and possibly causing MC1R desensitization. In addition to the known effects of different MC1R genotypes, the present findings identify additional mechanisms that modulate MC1R expression and activity, and thus affect human pigmentation and the UV response.
Materials and Methods
Primary melanocyte cultures
Human melanocyte cultures were established from neonatal foreskins or discarded surgery-derived adult skin, as previously described (Suzuki et al., 1996). The protocol for obtaining these tissues was deemed exempt by the University of Cincinnati Institutional Review Board.
Response of melanocytes to HBD3
To determine the effects of HBD3 on cAMP levels, melanocytes were treated with 1, 10, or 100 nM HBD3, in the presence or absence of 1 nM α-MSH, or alternatively with 100 nM HBD3 and 1 GM forskolin for 45 minutes, and cAMP levels were determined using radioimmunoassay kit (Perkin Elmer, Waltham, MA) as described (Suzuki et al., 1996). Included in these experiments were two groups, one treated only with 100 nM ASIP, and another treated with 100 nM ASIP and 1 nM α-MSH. The effects of HBD3 or ASIP on melanocyte proliferation and tyrosinase activity were determined following 6 days of treatment with 100 nM HBD3 or with ASIP with or without 1 nM α-MSH, with the medium and treatment replenished every other day, as described (Abdel-Malek et al., 1995). Cell numbers were determined using a Coulter Counter. Triplicate samples were included in each group and normalized to untreated control.
Quantitative reverse transcriptase PCR
To investigate the transcriptional regulation of MC1R by its agonists, antagonists, and UV, melanocytes were treated with 1 nM α-MSH,1 μM forskolin,100 nM ASIP or HBD3, or irradiated with 75 or 105 mJ/cm2 UV. Total RNA was extracted 8 hours after treatment or post UV using the RNeasy Mini Kit combined with the RNase-free DNase system (Qiagen, Inc., Valencia, CA). First-strand cDNA was synthesized using 2.5 μg of total RNA using the SuperScript VILO Synthesis Kit (Invitrogen, Carlsbad, CA), and quantitative real-time polymerase chain reaction (qRT-PCR) was performed using human MC1R and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) RT2 qPCR Primers and RT2 SYBR Green/Fluorescein PCR Master Mix (SABiosciences/Qiagen). Amplification was performed using the BIO-RAD iCycler iQ system (BioRad, Hercules, CA). The cycle threshold (CT) values were determined using the iCycle iQ system software (BioRad), and the comparative ΔΔCT method was used to calculate the relative MC1R expression levels after normalization to GAPDH (Livak and Schmittgen, 2001). Biological triplicates were processed individually for RNA isolation and analyzed in technical triplicate. Values were normalized to expression in control melanocytes.
Flow cytometry
Regulation of MC1R expression on the melanocyte cell membrane by 20, 50, 75, or 105 mJ/cm2 UV was determined 24 hours post irradiation. Regulation of MC1R by 1nM α-MSH, 1 μM forskolin, or 5 ng/ml TPA was determined 14 hours after treatment. Viable melanocytes were harvested using 5 mM EDTA at 4°C, and all staining steps were carried out on ice. Cells were blocked with normal goat serum, then incubated with rabbit anti-MC1R (H-60, Santa Cruz Biotechnology, Santa Cruz, CA) for 1 hour. Following washes, the cells were incubated with goat anti-rabbit IgG Quantum Dot 655 (Invitrogen), then post-fixed with 1% paraformaldehyde. Negative controls consisted of immunostaining with secondary antibody and no primary antibody, and rabbit IgG isotype control (Imgenex, San Diego, CA). Cells were analyzed using a BD LSRII flow cytometer (Becton-Dickinson, San Jose, CA). Ten thousand events were collected for each sample.
MC1R desensitization
For receptor desensitization, melanocytes were pretreated for 20 minutes with 1 nM α-MSH, 1nM ASIP or HBD3, washed once, then incubated for 2 hours in medium lacking α-MSH or antagonist, followed by retreatment for 45 minutes with 1 nM α-MSH. The following groups were also included: treated with 1 μM forskolin for 20 minutes, and pretreated with 100 nM ASIP or HBD3 as described above, then challenged with forskolin for 45 minutes. Melanocytes were also treated continuously for 45 minutes, 3 or 6 hours with 1 nM α-MSH. To determine the role of PKA in MC1R desensitization, melanocytes were treated concomitantly with 1 nM α-MSH and 100 nM H-89, a PKA inhibitor for 20 minutes, followed by washing and incubation in medium containing only H-89 for 2 hours, then treatment with α-MSH and H-89 for 45 minutes. Following each of these treatments, cAMP levels were determined using a radioimmunoassay. Triplicates were included in each group.
Western blot analysis for GRKs
Expression of GRK2, 3, 5, and 6 and β-arrestin 1 was compared by Western blotting using protein extracts derived from 10 different human melanocyte strains with different pigmentary phenotypes and MC1R genotypes. The antibodies used were as follows: SC-562, clone C-15 for detection of GRK2, SC-563, clone C-14 for GRK3, SC-565, clone C-20 for visualization of GRK5, and SC-566, and clone C-20 for GRK6 (Santa Cruz, CA). The antibody for β-arrestin is a rabbit antibody raised against a GST-β-arrestin fusion protein.
Statistical analysis
Statistical analysis was carried out on triplicate samples per group using a one-way ANOVA followed by Newman-Keuls Multiple Comparison test.
Acknowledgments
Supported in part by R01ES009110, R01ES017561 and P30-ES006096 for Zalfa Abdel-Malek, and R01DK064265 for Glenn Milhauser.
We thank Ms. Renny Kavanagh Starner for her technical assistance.
Abbreviations
- ASIP
agouti signaling protein
- GPCR
G protein-coupled receptor
- GRK
G protein receptor kinase
- HBD3
human β-defensin 3
- MC1R
melanocortin 1 receptor
- α-MSH
α-melanocyte stimulating hormone, α-melanocortin
- TPA
12-o-tetradecanoyl phorbol13-acetate
- UV
ultraviolet radiation
Footnotes
Conflict of Interest
The authors claim no conflict of interest.
References
- Abdel-Malek Z, Swope VB, Suzuki I, et al. Mitogenic and melanogenic stimulation of normal human melanocytes by melanotropic peptides. Proc Natl Acad Sci USA. 1995;92:1789–93. doi: 10.1073/pnas.92.5.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdel-Malek ZA, Scott MC, Furumura M, et al. The melanocortin 1 receptor is the principal mediator of the effects of agouti signaling protein on mammalian melanocytes. J Cell Sci. 2001;114:1019–24. doi: 10.1242/jcs.114.5.1019. [DOI] [PubMed] [Google Scholar]
- Baig AH, Swords FM, Noon LA, et al. Desensitization of the Y1 cell adrenocorticotropin receptor: evidence for a restricted heterologous mechanism implying a role for receptor-effector complexes. J Biol Chem. 2001;276:44792–7. doi: 10.1074/jbc.M108572200. [DOI] [PubMed] [Google Scholar]
- Beaumont KA, Newton RA, Smit DJ, et al. Altered cell surface expression of human MC1R variant receptor alleles associated with red hair and skin cancer risk. Hum Mol Genet. 2005;14:2145–54. doi: 10.1093/hmg/ddi219. [DOI] [PubMed] [Google Scholar]
- Beaumont KA, Wong SS, Ainger SA, et al. Melanocortin MC receptor in human genetics and model systems. Eur J Pharmacol. 2011;660:103–10. doi: 10.1016/j.ejphar.2010.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Box NF, Duffy DL, Chen W, et al. MC1R genotype modifies risk of melanoma in families segregating CDKN2A mutations. Am J Hum Genet. 2001;69:765–73. doi: 10.1086/323412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Box NF, Wyeth JR, O’Gorman LE, et al. Characterization of melanocyte stimulating hormone receptor variant alleles in twins with red hair. Hum Mol Gen. 1997;6:1891–7. doi: 10.1093/hmg/6.11.1891. [DOI] [PubMed] [Google Scholar]
- Bunemann M, Hosey MM. G-protein coupled receptor kinases as modulators of G-protein signalling. J Physiol. 1999;517 (Pt 1):5–23. doi: 10.1111/j.1469-7793.1999.0005z.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai M, Varga EV, Stankova M, et al. Cell signaling and trafficking of human melanocortin receptors in real time using two-photon fluorescence and confocal laser microscopy: differentiation of agonists and antagonists. Chem Biol Drug Des. 2006;68:183–93. doi: 10.1111/j.1747-0285.2006.00432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candille SI, Kaelin CB, Cattanach BM, et al. A -defensin mutation causes black coat color in domestic dogs. Science. 2007;318:1418–23. doi: 10.1126/science.1147880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donatien PD, Hunt G, Pieron C, et al. The expression of functional MSH receptors on cultured human melanocytes. Arch Dermatol Res. 1992;284:424–6. doi: 10.1007/BF00372074. [DOI] [PubMed] [Google Scholar]
- Ferguson SS. Evolving concepts in G protein-coupled receptor endocytosis: the role in receptor desensitization and signaling. Pharmacol Rev. 2001;53:1–24. [PubMed] [Google Scholar]
- Funasaka Y, Chakraborty AK, Hayashi Y, et al. Modulation of melanocyte-stimulating hormone receptor expression on normal human melanocytes: evidence for a regulatory role of ultraviolet B, interleukin-1alpha, interleukin-1beta, endothelin-1 and tumour necrosis factor-alpha. Br J Dermatol. 1998;139:216–24. doi: 10.1046/j.1365-2133.1998.02357.x. [DOI] [PubMed] [Google Scholar]
- Garcia-Borron JC, Sanchez-Laorden BL, Jimenez-Cervantes C. Melanocortin-1 receptor structure and functional regulation. Pigment Cell Res. 2005;18:393–410. doi: 10.1111/j.1600-0749.2005.00278.x. [DOI] [PubMed] [Google Scholar]
- Geschwind II, Huseby RA, Nishioka R. The effect of melanocyte-stimulating hormone on coat color in the mouse. Recent Progress Hormone Res. 1972;28:91–130. [PubMed] [Google Scholar]
- Harder J, Bartels J, Christophers E, et al. Isolation and characterization of human beta -defensin-3, a novel human inducible peptide antibiotic. J Biol Chem. 2001;276:5707–13. doi: 10.1074/jbc.M008557200. [DOI] [PubMed] [Google Scholar]
- Hauser JE, Kadekaro AL, Kavanagh RJ, et al. Melanin content and MC1R function independently affect UVR-induced DNA damage in cultured human melanocytes. Pigment Cell Res. 2006;19:303–14. doi: 10.1111/j.1600-0749.2006.00315.x. [DOI] [PubMed] [Google Scholar]
- Hennessy A, Oh C, Diffey B, et al. Eumelanin and pheomelanin concentrations in human epidermis before and after UVB irradiation. Pigment Cell Res. 2005;18:220–3. doi: 10.1111/j.1600-0749.2005.00233.x. [DOI] [PubMed] [Google Scholar]
- Hunt G, Kyne S, Wakamatsu K, et al. Nle4DPhe7 α-melanocyte-stimulating hormone increases the eumelanin:phaeomelanin ratio in cultured human melanocytes. J Invest Dermatol. 1995;104:83–5. doi: 10.1111/1523-1747.ep12613565. [DOI] [PubMed] [Google Scholar]
- Im S, Moro O, Peng F, et al. Activation of the cyclic AMP pathway by α-melanotropin mediates the response of human melanocytes to ultraviolet B radiation. Cancer Res. 1998;58:47–54. [PubMed] [Google Scholar]
- Kadekaro AL, Kavanagh R, Kanto H, et al. α-Melanocortin and endothelin-1 activate anti-apoptotic pathways and reduce DNA damage in human melanocytes. Cancer Res. 2005;65:4292–9. doi: 10.1158/0008-5472.CAN-04-4535. [DOI] [PubMed] [Google Scholar]
- Kadekaro AL, Leachman S, Kavanagh RJ, et al. Melanocortin 1 receptor genotype: an important determinant of the damage response of melanocytes to ultraviolet radiation. FASEB J. 2010;24:3850–60. doi: 10.1096/fj.10-158485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy C, ter Huurne J, Berkhout M, et al. Melanocortin 1 receptor (MC1R) gene variants are associated with an increased risk for cutaneous melanoma which is largely independent of skin type and hair color. J Invest Dermatol. 2001;117:294–300. doi: 10.1046/j.0022-202x.2001.01421.x. [DOI] [PubMed] [Google Scholar]
- Kesting MR, Stoeckelhuber M, Holzle F, et al. Expression of antimicrobial peptides in cutaneous infections after skin surgery. Br J Dermatol. 2010;163:121–7. doi: 10.1111/j.1365-2133.2010.09781.x. [DOI] [PubMed] [Google Scholar]
- Kilianova Z, Basora N, Kilian P, et al. Human melanocortin receptor 2 expression and functionality: effects of protein kinase A and protein kinase C on desensitization and internalization. Endocrinol. 2006;147:2325–37. doi: 10.1210/en.2005-0991. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- McNulty JC, Jackson PJ, Thompson DA, et al. Structures of the agouti signaling protein. J Mol Biol. 2005;346:1059–70. doi: 10.1016/j.jmb.2004.12.030. [DOI] [PubMed] [Google Scholar]
- Mountjoy KG, Robbins LS, Mortrud MT, et al. The cloning of a family of genes that encode the melanocortin receptors. Science. 1992;257:1248–51. doi: 10.1126/science.1325670. [DOI] [PubMed] [Google Scholar]
- Ollmann MM, Wilson BD, Yang Y-K, et al. Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science. 1997;278:135–8. doi: 10.1126/science.278.5335.135. [DOI] [PubMed] [Google Scholar]
- Palmer JS, Duffy DL, Box N, et al. Melanocortin-1 receptor polymorphisms and risk of melanoma: Is the association explained solely by pigmentation phenotype? Am J Hum Genet. 2000;66:176–86. doi: 10.1086/302711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitcher JA, Freedman NJ, Lefkowitz RJ. G protein-coupled receptor kinases. Annu Rev Biochem. 1998;67:653–92. doi: 10.1146/annurev.biochem.67.1.653. [DOI] [PubMed] [Google Scholar]
- Premont RT, Gainetdinov RR. Physiological roles of G protein-coupled receptor kinases and arrestins. Annu Rev Physiol. 2007;69:511–34. doi: 10.1146/annurev.physiol.69.022405.154731. [DOI] [PubMed] [Google Scholar]
- Reiter E, Lefkowitz RJ. GRKs and beta-arrestins: roles in receptor silencing, trafficking and signaling. Trends Endocrinol Metab. 2006;17:159–65. doi: 10.1016/j.tem.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Ringholm A, Klovins J, Rudzish R, et al. Pharmacological characterization of loss of function mutations of the human melanocortin 1 receptor that are associated with red hair. J Invest Dermatol. 2004;123:917–23. doi: 10.1111/j.0022-202X.2004.23444.x. [DOI] [PubMed] [Google Scholar]
- Robbins LS, Nadeau JH, Johnson KR, et al. Pigmentation phenotypes of variant extension locus alleles result from point mutations that alter MSH receptor function. Cell. 1993;72:827–34. doi: 10.1016/0092-8674(93)90572-8. [DOI] [PubMed] [Google Scholar]
- Sanchez-Laorden BL, Sanchez-Mas J, Martinez-Alonso E, et al. Dimerization of the human melanocortin 1 receptor: functional consequences and dominant-negative effects. J Invest Dermatol. 2006;126:172–81. doi: 10.1038/sj.jid.5700036. [DOI] [PubMed] [Google Scholar]
- Sanchez-Mas J, Guillo LA, Zanna P, et al. Role of G protein-coupled receptor kinases in the homologous desensitization of the human and mouse melanocortin 1 receptors. Mol Endocrinol. 2005;19:1035–48. doi: 10.1210/me.2004-0227. [DOI] [PubMed] [Google Scholar]
- Sawamura D, Goto M, Shibaki A, et al. Beta defensin-3 engineered epidermis shows highly protective effect for bacterial infection. Gene Ther. 2005;12:857–61. doi: 10.1038/sj.gt.3302472. [DOI] [PubMed] [Google Scholar]
- Scott MC, Suzuki I, Abdel-Malek ZA. Regulation of the human melanocortin 1 receptor expression in epidermal melanocytes by paracrine and endocrine factors, and by UV radiation. Pigment Cell Res. 2002;15:433–9. doi: 10.1034/j.1600-0749.2002.02051.x. [DOI] [PubMed] [Google Scholar]
- Scott MC, Wakamatsu K, Ito S, et al. Human melanocortin 1 receptor variants, receptor function and melanocyte response to UV radiation. J Cell Sci. 2002;115:2349–55. doi: 10.1242/jcs.115.11.2349. [DOI] [PubMed] [Google Scholar]
- Shinyama H, Masuzaki H, Fang H, et al. Regulation of melanocortin-4 receptor signaling: agonistmediated desensitization and internalization. Endocrinol. 2003;144:1301–14. doi: 10.1210/en.2002-220931. [DOI] [PubMed] [Google Scholar]
- Silvers WK. A Model for Mammalian Gene Action and Interaction. Springer-Verlag; New York: 1979. The Coat Colors of Mice. [Google Scholar]
- Smith R, Healy E, Siddiqui S, et al. Melanocortin 1 receptor variants in Irish population. J Invest Dermatol. 1998;111:119–22. doi: 10.1046/j.1523-1747.1998.00252.x. [DOI] [PubMed] [Google Scholar]
- Suzuki I, Cone R, Im S, et al. Binding of melanotropic hormones to the melanocortin receptor MC1R on human melanocytes stimulates proliferation and melanogenesis. Endocrinol. 1996;137:1627–33. doi: 10.1210/endo.137.5.8612494. [DOI] [PubMed] [Google Scholar]
- Suzuki I, Tada A, Ollmann MM, et al. Agouti signaling protein inhibits melanogenesis and the response of human melanocytes to α-melanotropin. J Invest Dermatol. 1997;108:838–42. doi: 10.1111/1523-1747.ep12292572. [DOI] [PubMed] [Google Scholar]
- Tamate HB, Takeuchi T. Action of the e locus of mice in the response of phaeomelanic hair follicles to α-melanocyte-stimulating hormone in vitro. Science. 1984;224:1241–2. doi: 10.1126/science.6328651. [DOI] [PubMed] [Google Scholar]
- Yen TT, Gill AM, Frigeri LG, et al. Obesity, diabetes, and neoplasia in yellow A(vy)/− mice: Ectopic expression of the agouti gene. FASEB J. 1994;8:479–88. doi: 10.1096/fasebj.8.8.8181666. [DOI] [PubMed] [Google Scholar]