Abstract
We propose the minicommunity design to estimate indirect effects of vaccination. Establishing indirect effects of vaccination in unvaccinated subpopulations could have important implications for global vaccine policies. In the minicommunity design, the household or other small transmission unit serves as the cluster in which to estimate indirect effects of vaccination, similar to studies in larger communities to estimate indirect, total, and overall effects. Examples from the literature include studies in small transmission units to estimate indirect effects of pertussis, pneumococcal, influenza, and cholera vaccines. We characterize the minicommunity design by several methodologic considerations, including the assignment mechanism, ascertainment, the role of transmission outside the transmission unit, and the relation of the size of the transmission unit to number of people vaccinated. The minicommunity study for indirect effects is contrasted with studies to estimate vaccine effects on infectiousness and protective effects under conditions of household exposure within small transmission units. The minicommunity design can be easily implemented in individually randomized studies by enrolling and following-up members of households of the randomized individuals. The methodology for the minicommunity design for estimating indirect effects of vaccination deserves much future research.
1 Introduction
Vaccination in a community can reduce transmission producing effects beyond the direct protective effects in vaccinated individuals. The population effects of vaccination are primarily due to an increase in the population level of immune protection, also known as herd immunity (Fox and Elveback, 1975; Fine, 1993). Herd immunity describes the collective immunological status of a population of hosts, as opposed to an individual host, with respect to a given pathogen. Population-level effects of vaccination include indirect, total and overall effects (Halloran and Struchiner, 1991). Indirect effects of a vaccination strategy are the effects in those individuals who were not vaccinated, or at least who were not vaccinated as part of the strategy of interest. The total effects are the combined population-level effects of the vaccination strategy and the direct protective effects of vaccination in those individuals who received the vaccine. The overall effect of a vaccination strategy is the average effect in the population in those who did and did not receive the vaccine compared to if the population had not had the vaccination strategy. In this paper we are concerned primarily with indirect effects.
The indirect effects of interest may be in subpopulations outside the target age groups, such as infants, or in whom it is believed the vaccine might not be efficacious, such as in elderly or immunocompromised people. For example, one might be interested in the effect of a strategy of vaccinating school-aged children with influenza vaccine in reducing incidence of influenza in pre-school children or in people over 65 years of age. Evidence that vaccine-induced herd immunity produces indirect protection has been demonstrated for several vaccines, including those against Haemophilius influenzae b (Adegbola et al., 2005), pneumococcal bacteria (Metlay et al., 2006; Grijalva et al., 2007), meningococcal C bacteria (Ramsay et al., 2003), among others. Establishing indirect protection of vaccination in unvaccinated subpopulations can have implications for global vaccine policies. Population effects of vaccination could also be detrimental. In areas of high transmission of malaria, maintenance of partial direct protection against disease depends on repeated boosting of immunity to exposure to natural infection. Malaria vaccination could reduce transmission, thus lower boosting, resulting in unvaccinated individuals becoming susceptible to more severe disease again (Halloran et al., 1989). Thus, interest in evaluating the indirect effects of vaccination programs has increased.
Heuristically, evaluation of the indirect effects of vaccination requires comparison of the outcomes in individuals who did not receive the vaccine in communities with the vaccination strategy and individuals who did not receive the vaccine in communities without the vaccination strategy (Halloran and Struchiner, 1991; Struchiner et al., 1990). In this situation, the indirect vaccine effect would generally be estimated as one minus the ratio of some measure of risk in unvaccinated individuals in the vaccinated communities compared to the unvaccinated individuals in the unvaccinated communities:
Ideally the communities would be randomized to receive either the vaccination strategy or a strategy using a control vaccine. Because vaccines are administered to individuals, randomization can occur at two stages, namely the group level and the individual level (Hudgens and Halloran, 2008; VanderWeele and Tchetgen Tchetgen 2011b). In a study with two levels of randomization, communities could be randomized to either receive the vaccination strategy or not, then individuals randomized to be vaccinated or not in the communities with the vaccination strategy and to receive control or not in the communities without the vaccination strategy. Then presumably those who did not receive vaccine in the vaccinated community would be comparable to those who did not receive control in the communities randomized to control. In general, assignment mechanisms that are not independent of the infection outcomes of interest could be in place at either or both of the two levels, making a study observational at either or both of the two levels. In these situations, the comparability of the unvaccinated individuals across the populations would need further examination.
One could also consider comparing two different vaccination strategies, say where one strategy vaccinated a certain proportion of the people and the other strategy vaccinated a different proportion of the people. If vaccination coverage varied over a range of values in a collection of communities, one could estimate how the indirect effect varied with the proportion of the population vaccinated.
Discussions of evaluating indirect effects in populations have generally been concerned with relatively large clusters (Halloran and Struchiner, 1991; Moulton et al., 2001; Hayes and Moulton, 2009), although Hayes and Moulton (2009) briefly mention small clusters such as households (pp. 51–52). However, in recent years, a number of studies have been conducted to assess indirect effects in smaller clusters such as households or other small transmission units. The transition from small transmission units, such as households, to units of extended families as in compounds in Niakhar, Senegal (Préziosi and Halloran, 2003) or baris in Bangladesh (Ali et al., 2005), to day care centers, to schools, and to villages is fairly continuous. In households, the indirect effect of vaccination could be estimated by
The measure of risk could be attack rates (cases per household members at risk) or incidence rates (cases per person-time at risk). The relative risk could be estimated by an odds ratio from a case-control study. Studies in smaller clusters have many similarities to studies in larger communities. However, studies in small transmission units do have differences compared to studies in larger clusters or groups. Thus, this type of study deserves its own characterization.
We propose the name of minicommunity design for studies of indirect effects in small clusters. Orenstein et al. (1988) proposed that individuals in a small transmission unit exposed to an infectious case can be thought of as a minicohort that has its own reference date for exposure to infection. When evaluating the indirect effects of vaccinating one or several individuals in a small transmission unit, the small transmission unit can be thought of as a minicommunity, or minicluster or minigroup, in which to estimate indirect effects.
In this paper, we characterize minicommunity studies to estimate indirect effects of vaccination in small clusters or transmission units. To motivate the development, we first present several published examples of what we call mini-community studies to estimate indirect effects. The examples include studies of the indirect effects of pertussis, pneumococcal, influenza, and cholera vaccination. They illustrate diverse methodological considerations which we discuss. We compare the minicommunity design with studies for indirect effects in larger communities, as well as with studies based on temporal trends.
2 Examples
2.1 Pertussis vaccination
Trollfors et al. (1998) nested a study of the indirect effects of pertussis vaccination in a double-blind, placebo controlled, individually randomized trial in 3450 infants of a vaccine containing diphtheria, tetanus and acellular pertussis toxoids (DTaP) versus a vaccine containing only diphtheria and tetanus toxoids (DT). The goal was to determine indirect protection in close contacts. Parents and siblings in households of the randomized infants were followed for two years. The original study took place in 1991–1992 in the Goteberg area of western Sweden and showed direct protection in the vaccinated compared to unvaccinated infants. Pertussis vaccination in Sweden was stopped in 1979 due to safety and efficacy considerations, and no licensed pertussis vaccine was available there between 1979 and 1995. Pertussis became endemic in Sweden again, with a high incidence. DTaP versus DT trials were possible from an ethical standpoint, in contrast to other countries where the whole-cell pertussis vaccine was still licensed. Parents of study participants had likely been vaccinated before 1979.
The indirect effects were estimated using the ratio of the incidence rates (pertussis cases divided by total time at risk) in parents, younger siblings, and older siblings of recipients of DTaP or DT:
They considered four different case definitions, the first being similar to the definition of the World Health Organization (WHO), and the second based on criteria developed by their group. They further divided the cases by ≥21 days of paroxysmal cough and cough ≥7 days. The results for parents and younger siblings are in Table 1. Depending on the case definition, the point estimates of indirect protection ranged from 38% to 60% in parents and 37% to 61% in younger siblings with several of the confidence intervals excluding 0. There was no significant difference in the rates in older siblings.
Table 1.
Number of pertussis cases in parents and younger siblings of study children and indirect protection achieved by vaccination of the study child with pertussis toxoid (from Trollfors et al., 1998)
| Pertussis Cases |
Indirect | |||
|---|---|---|---|---|
| Protection |
||||
| DTaP | DT | (%) | 95% CI | |
| Parents | ||||
| WHO definition | ||||
| ≥21 days of paroxysmal cough | 11 | 26 | 60 | 16, 82 |
| ≥7 days of cough | 23 | 35 | 38 | −9, 65 |
| Göteborg definition | ||||
| ≥21 days of paroxysmal cough | 14 | 32 | 58 | 20, 80 |
| ≥7 days of cough | 26 | 44 | 44 | 7, 67 |
| Younger siblings | ||||
| WHO definition | ||||
| ≥21 days of paroxysmal cough | 10 | 18 | 43 | −31, 76 |
| ≥7 days of cough | 11 | 10 | 37 | −40, 73 |
| Göteborg definition | ||||
| ≥21 days of paroxysmal cough | 10 | 26 | 61 | 15, 83 |
| ≥7 days of cough | 11 | 26 | 56 | 9, 81 |
2.2 Influenza vaccination
A double-blind randomized placebo-controlled trial was conducted during the 2000–2001 influenza season in Italy (Esposito et al., 2003). Similar to the pertussis study in Sweden, the goal was to evaluate the direct effectiveness in the children and the indirect effectiveness in household contacts. Children between 6 months and 14 years with a history of recurrent respiratory tract infection but no serious chronic disease were recruited through a clinic at the University of Milan. The intranasal vaccine was an inactivated, trivalent, virosome-formulated subunit influenza vaccine. The placebo was saline solution. Sixty-four children received vaccine, and 63 received placebo. All children received two doses before the influenza season began the last week of 2000. Only 6 of 176 household contacts of vaccinated children and 7 of 173 contacts of control children had received influenza vaccine.
Indirect vaccine effectiveness in household contacts was estimated as a variant based on the attack rate, namely
Household contacts of vaccinated children had significantly fewer respiratory tract infections, medical visits for respiratory tract infections, antipyretic and antibiotic prescriptions, and missed school or working days due to respiratory illnesses (partly shown in Table 2). For example, influenza vaccination of children had an indirect effect in adults, reducing loss of parental work days by 84%.
Table 2.
Indirect effectiveness for household contacts of influenza vaccinated children compared with controls (from Esposito et al., 2003).
| Event | Contacts of vaccinated children (n = 176) |
Contacts of controls (n = 173) |
Indirect effectiveness (%) |
P-value |
|---|---|---|---|---|
| No. of respiratory tract infections | 2.19 ± 0.99 (2) | 2.90 ± 1.68 (3) | 24 | <0.0001 |
| Parents | 1.77 ± 1.01 (2) | 2.26 ± 1.40 (2) | 22 | <0.0001 |
| Siblings | 3.29 ± 0.54 (3) | 4.59 ± 1.81 (6) | 29 | <0.0001 |
| No. of medical visits for respiratory illness | 1.25 ± 1.24 (1) | 2.06 ± 0.99 (2) | 39 | <0.0001 |
| Parents | 1.08 ± 1.26 (1) | 1.86 ± 0.92 (2) | 42 | <0.0001 |
| Siblings | 1.68 ± 1.10 (1) | 2.57 ± 0.99 (3) | 35 | <0.0001 |
| Loss of parental work days | 0.42 ± 0.88 (0) | 2.50 ± 2.31 (2) | 84 | <0.0001 |
| Mothers | 0.66 ± 1.88 (1) | 4.05 ± 2.34 (4) | 83 | <0.0001 |
| Fathers | 0.19 ± 0.37 (0) | 0.97 ± 2.24 (1) | 80 | <0.0001 |
| Missed school days of siblings | 1.31 ± 2.40 (1) | 2.76 ± 4.42 (3) | 53 | 0.001 |
Mean values ± S.D (median in parentheses)
Two studies of influenza vaccination of health care workers in long-term-care hospitals, one an observational study (Potter et al., 1997), the other a parallel-group, cluster randomized trial (Carman et al., 2000) were conducted in Scotland. The goal was to assess indirect effects of vaccinating health care workers on mortality and morbidity of elderly patients. In both studies, influenza vaccination of health care workers reduced mortality of elderly patients. In Carman et al. (2000), 20 long-term care hospitals ranging in size from 44-105 patients were included. One might argue these hospitals are a little large to consider this a minicommunity study.
2.3 Pneumococcal vaccination
Some bacteria, such as pneumococcal and meningococcal bacteria, can colonize the mucous membranes of the nasopharyngeal passages without causing symptoms. Such colonization, also known as carriage, is the source of transmission of the bacteria in the population. Vaccines are developed to protect against serious disease by these bacteria, such as pneumonia or meningitis. However, the newer vaccines are also expected to protect against colonization, possibly substantially reducing transmission. They could thus have important indirect effects. There are many different antigenic types of pneumococcal bacteria. The vaccines usually contain antigens of several different types, and the included types are called vaccine types.
Millar et al. (2008) examined the indirect effect of a heptavalent pneumococcal conjugate vaccine on pneumococcal colonization among unvaccinated household members. The study was conducted as a follow-up to a Phase III group-randomized study in a Native American population in the southwestern US of a pneumoccocal vacccine with meningococcal vaccine control (Moulton et al., 2001). The randomization units were existing, well-defined communities, not minicommunities (households). Children aged 6 weeks to 2 years were eligible to be enrolled in the original group-randomized study. The primary outcome in the follow-up household-based study of indirect effects was vaccine-type carriage in households with a child in the original group-randomized study. Median (range) size of households enrolled in the carriage study in communities randomized to receive pneumococcal vaccine was 6 (2–17) and in communities randomized to receive meningococcal vaccine was 5 (2–17). Based on the odds ratio (OR), adults and unvaccinated children <5 yrs living in households with a pneumococcal vaccinee were less likely to be colonized with vaccine-type pneumococci than those living in households with meningococcal vaccinees. In adults ≥18 yrs, the OR was 0.57, (95% CI 0.33–0.99), p = 0.05, and in unvaccinated children <5 yrs, the OR was 0.57, (95% CI 0.26–0.98), p = 0.04. The OR was not significant for children 5–17 yrs.
Using a case-control study, Metlay et al. (2006) evaluated the indirect protective effect of pediatric vaccination with pneumococcal conjugate vaccine on the risk of bacteremic pneumococcal pneumonia in adults. One goal of their study was to determine whether vaccination of children protects adults in the same home from bacteremic pneumococcal pneumonia. Cases from April 2002 through June 2004 were enrolled from hospitals of five counties around Philadelphia, PA, USA. Controls (609) obtained by random digit dialing were frequency matched to cases by month to control for secular trends only. They enrolled 223 of 495 eligible cases and 609 controls. Adjusted for multiple risk factors, cases with at least one child in the home (N = 40) had an 80% reduced odds of reporting that the youngest child in the home had received the pneumococcal conjugate vaccine (inability to report vaccine status classified as negative) than controls (N = 106) with at least one child in the home (OR = 0.2, 95% CI 0.1–0.8). Excluding subjects who were unable to report vaccine status of the youngest child, cases had a 70% reduced odds compared to controls, but it was not significant (OR = 0.3, 95% CI 0.1–1.4). Metlay et al. (2006) also analyzed secular trends from surveillance data. They concluded that introduction of the pneumococcal conjugate vaccine for children had reduced the population rate of adult pneumococcal bacteremia due to vaccine serotypes and is associated with a reduced risk of bacteremic pneumococcal pneumonia for adults with children in the home.
A case-control study in South Africa was conducted between September 2000 and August 2001 to estimate the indirect effect on adults living at the same address as children who had participated in a randomized controlled trial of a 9-valent pneumococcal vaccine (Albrich et al., 2007). The odds ratios for pneumococcal pneumonia and for all-cause pneumonia were near one and not significant. The brief report did not include a description of the ascertainment method for cases or controls, or the number of controls.
2.4 Cholera vaccination
Ali et al. (2005) re-analyzed an individually randomized, placebo-controlled trial of two killed oral cholera vaccines in children aged 2–15 years and women older than 15 years in Bangladesh to evaluate evidence of indirect protection from vaccination. Killed oral cholera vaccines were licensed for older children and adults but not for infants and young children. The study began in 1985, and the first year of surveillance is included in their secondary analysis. The minicommunity unit of analysis was the bari, where baris are patrilinearly-related households living in clusters. A total of 6423 baris were included in the analysis. The median number of individuals per bari who had been eligible for the trial was 17 (interquartile range 7–26). The level of coverage in a bari was defined as the number of vaccinated individuals divided by the number of people who were eligible (children and women) to participate in the trial by age and sex criteria, not the actual population of the bari. Vaccine coverage in baris ranged from 4% to 65%. Indirect protection was evaluated by comparing the incidence in placebo recipients in the trial by level of vaccine coverage. The incidence per 1000 population was 7.01 in the lowest quintile of vaccine coverage (<28%) compared to 1.47 in the highest quintile of vaccine coverage (>51%) (p<0.0001 for trend). A further analysis examined the indirect protection against cholera on children less than 2 years of age who were too young to receive the vaccine (Ali et al., 2008). The incidence per 1000 population ranged from 18.9 in the lowest quintile of vaccine coverage to 8.6 in the highest quintile (p<0.004). Both analyses concluded that killed oral cholera vaccines confer significant herd protection to unvaccinated individuals within baris.
3 Contrast to Estimating Infectiousness Effects
The studies in Section 2 have in common the goal of estimating indirect effects of vaccination in small clusters or transmission units. Another vaccine effect of interest is the effect on infectiousness of vaccinated infected people compared to unvaccinated infected people for others. It, too, is generally evaluated in small transmission units such as households. The change in secondary transmission from the vaccinated infected person to another within the household compared to if the infected person had not been vaccinated is the effect of vaccination on infectiousness. To begin, we compare and contrast estimating indirect effects with estimating vaccine effects on infectiousness in small clusters.
Estimating vaccine efficacy for infectiousness is based on contrasts between the transmission risk from infected vaccinated individuals compared with the transmission risk from infected unvaccinated individuals to susceptible individuals exposed within the small transmission units (Halloran et al., 1997). In contrast, when estimating the indirect effects of vaccination in the minicommunity design, the analysis conditions only on the vaccination status of the index individual or individuals in the household, not whether the individual is actually infected or not. To estimate the vaccine effect on infectiousness, the index individual must be infected and exposing the others in the household. Similar to the indirect effects in larger populations, indirect effects in the minicommunity design result both from vaccination preventing individuals from becoming infected at all, thus not exposing others, and the change in infectiousness of an infected vaccinated person for another.
Préziosi and Halloran (2003) estimated the effects of pertussis vaccination on infectiousness and susceptibility within transmission units from a study in Niakhar, Senegal (see also Cisse et al., 1999; Millar et al., 2008). Other theoretical aspects of estimating vaccine effects on infectiousness have been explored in several papers (Koopman and Little, 1995; Rida, 1996; Longini et al., 1996; Halloran et al., 1997; Datta et al., 1998, 1999; Becker et al., 2006). More recently, Halloran and Hudgens (2012) and VanderWeele and Tchetgen Tchetgen (2011a) developed methods for estimating vaccine efficacy for infectiousness in a causal inference framework.
The risk that a person exposed by an infected person within a household during his/her infectious period can be estimated by the secondary attack rate, e.g., the proportion of exposed people who become infected. Historically, more studies have focused on how the vaccine protects the vaccinated people under conditions of household exposure than on how vaccination affects the infectiousness of the index cases. The former has been of interest since the 1930s at least (Kendrick and Eldering, 1939). Detailed discussion of that goes beyond the scope of this paper, but can be found in Halloran et al. (2010). In contrast, the indirect effects are usually estimated by some measure of risk that does not condition on exposure to infection, such as the attack rate or incidence rate (Halloran et al., 1997).
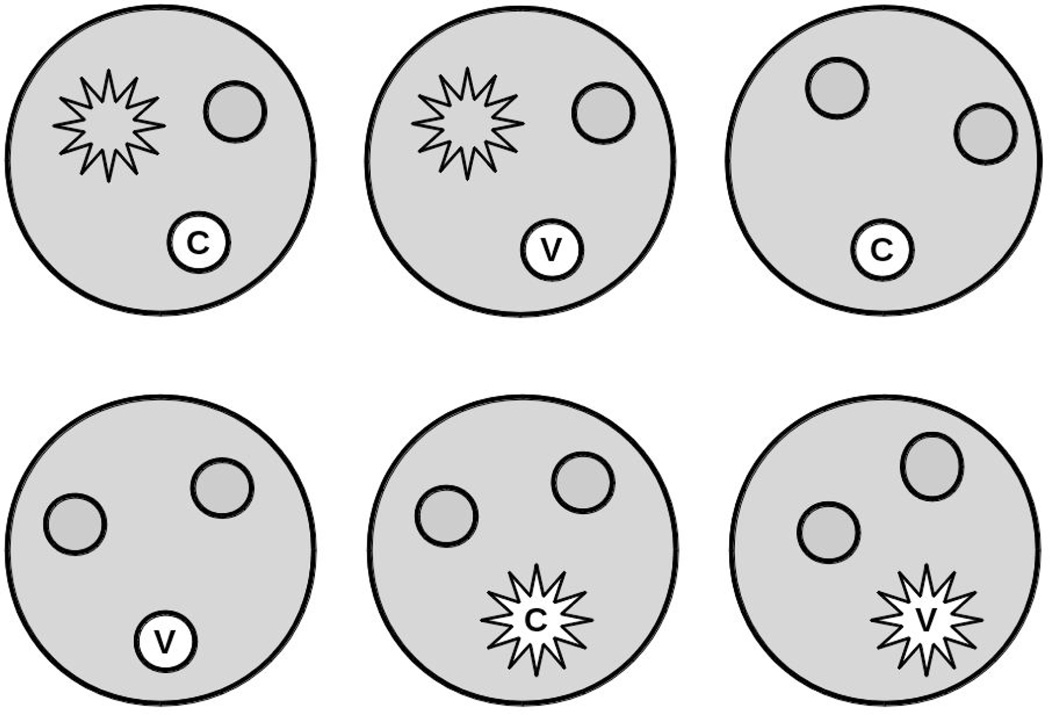
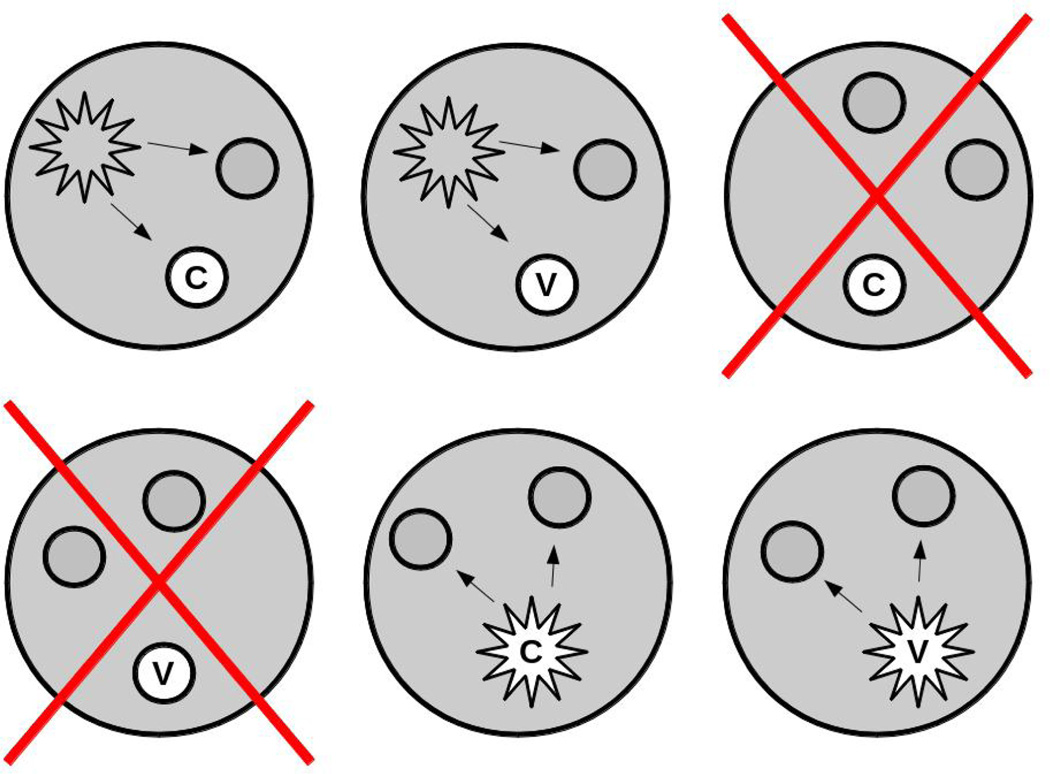
Similar to the minicommunity design, studies to evaluate vaccine effects on infectiousness or the protective effects under household exposure can be nested in individually randomized studies. To illustrate the difference between the two types of studies, consider an individually randomized trial of a vaccine such as the pertussis vaccine trial in Section 2.1. Assume that members of the households of the trial participants had been enrolled for follow-up. If our goal was to estimate the indirect effect of vaccination on household members, we would include all of the households in the analysis, regardless of whether the trial participants had been infected (Figure 1). However, to estimate the vaccine effect on infectiousness or the protective vaccine effect under conditions of household exposure, we would include only those households in which at least one person was infected (Figure 2). Thus, we would include only a subset of the households in this latter analysis. With our focus on the vaccine effect on infectiousness, we might include only households in which the first case in the household was a participant in the randomized vaccine trial.
Figure 1.
Sample of households used in the analysis in a minicommunity study. All households are included, avoiding post-randomization selection bias. The larger shaded circles represent households. Each household has three individuals in it, one of whom, indicated in white, is in an individually randomized, controlled trial of a vaccine (v) versus control (c). The smaller circles represent uninfected people, the stars represent infected people.
Figure 2.
Sample of households used to estimate vaccine efficacy for infectiousness. As in Figure 1, the larger shaded circles represent households. Each household has three individuals in it, one of whom, indicated in white, is in an individually randomized, controlled trial of a vaccine (v) versus control (c). The smaller circles represent uninfected people, the stars represent infected people. The large Xs indicate that a household is not included in the analysis. One could include only households in which the first case in the household was in the randomized trial.
The difference in the inclusion criteria of the households in the analyses results in another fundamental difference in the two designs. As illustrated in Figure 1, in the minicommunity design for indirect effects, the households in the analysis are not chosen based on the infection status of the participants in the individually randomized study. All households are included. Thus, the randomization of the households is preserved. However, conditioning on an event, such as infection, that occurs subsequent to receipt of vaccine or control, as in Figure 2, may result in selection bias. Because the set of individuals who would become infected if vaccinated is likely not identical to those who would become infected if given control, comparisons that condition on infection do not necessarily have a causal interpretation (Gilbert et al., 2003; Frangakis and Rubin, 2002). Estimating indirect vaccine effects from a minicommunity design could be used for a wide variety of infections, including those where most infections occur without symptoms, such as asymptomatic nasopharyngeal carriage of pneumococcal or meningococcal bacteria. However, evaluating the effect of vaccination on infectiousness or the protective effects under household exposure for diseases that are usually ascertained on symptomatic cases has been more useful in diseases such as measles, mumps, and pertussis.
4 Methodological Considerations
The studies in Section 2 illustrate a wide variety of complex methodological considerations. Key among them are the assignment mechanism, the size of the minicommunities, the number of people vaccinated in each minicommunity, the ascertainment method for each minicommunity, the role of transmission in the wider community, and the interrelation of these study aspects to one another. First we consider briefly studies using larger communities versus minicommunity studies, and studies of indirect effects based on temporal trends.
4.1 What is a minicommunity?
Minicommunities might be considered similar to transmission units. The general idea of a transmission unit is that individuals make contact sufficient for transmission within it. Households are the most common form of transmission units used in studies. They allow easy identification of contacts, and families are convenient units of study. Many other settings are also used as transmission units in studies. These include sexual partnerships, day care centers, workplaces, school buses, airplanes, among others. Not all transmission units are good candidates for minicommunity studies. For a minicommunity study to estimate indirect effects of vaccination, the stability of the transmission unit may be important. Thus households with nuclear families, households with extended families, school classes, sexual partnerships, and long-term care settings may all be candidates as minicommunities. It could be that stability of only the vaccinated or control individuals is crucial, for example in a study of the indirect effects of influenza vaccination of health care workers on influenza in patients. The patient population could fluctuate, while the health care workers remain in place.
4.2 Larger communities versus minicommunity studies
There is little difference in principal between indirect effects in larger communities and indirect effects in small transmission units. Both result from an increased population level of immunity. An advantage of a minicommunity study over a study in larger communities is that minicommunity studies could be much less expensive. As illustrated in the pertussis and influenza examples above, individually randomized studies can be augmented by the members of the households of the study participants to estimate indirect effects. Also, for a given number of individuals in the study, in general, a larger number of small clusters could be more powerful than a small number of larger clusters. Hayes and Moulton (2009) define the design effect of a cluster randomized study as a measure of the increase in variance that results from randomizing by cluster rather than by individual. If ρ is the intracluster correlation and m is the size of each cluster, the design effect is 1 + (m — 1)ρ. In general, the design effect would be small for smaller clusters and small intracluster correlation. However, as they point out (pp. 53–54), the intracluster correlation could be small in larger clusters, so that randomizing fewer larger clusters could be more powerful under some circumstances. (See Hayes and Moulton (2009), Chapter 7, for more details.).
Estimates of indirect effects in small clusters might differ from estimates of indirect effects in larger communities. In general, estimates of indirect effects of vaccination, whether in minicommunities or larger communities, are nearly always going to be specific to a context, with the local level of coverage, transmission intensity, and mixing patterns of the population playing a role. Demonstrating positive indirect effects in minicommunities could have important policy implications, even if the actual estimate might be context-specific.
4.3 Minicommunity studies versus pre- and post-vaccination comparisons
Sometimes indirect effects of vaccination are evaluated by studying temporal trends in disease incidence in population groups not indicated for vaccination before and after introducing a vaccine. Grivjalva et al. (2007) found a decline in pneumococcal pneumonia admissions in adults aged 18–39 years after introduction of the pneumococcal conjugate vaccine in the USA. Other examples include pertussis vaccination in Niakhar, Senegal (Préziosi et al., 2002), pneumococcal vaccination in Alaska (Hennessy et al., 2005), and meningococcal vaccination in the United Kingdom (Ramsay et al., 2003). However, if the change in an outcome, such as disease incidence rate or hospitalization rate, before and after introducing vaccination, is to be attributed to the vaccination strategy, then it needs to be assumed that the secular trends are small. The advantage of a minicommunity design over using pre- and post-vaccination comparisons for evaluating indirect effects is that the number of clusters can be large, as well as randomized, so an observed effect would less likely to have happened by chance or some reason not related to vaccination.
4.4 Assignment mechanism
A variety of assignment mechanisms occur in the minicommunity studies in Section 2, but in general, they are not formally addressed. In studies in larger communities, the assignment mechanism would ideally include two stages of randomization (Halloran and Hudgens, 2008; VanderWeele and Tchetgen Tchetgen 2011b). At the first stage, communities would be randomized to one or other of the vaccination strategies, then at the second stage, individuals would be randomized within the community to receive vaccine or not. If a mini-community study is nested in an individually randomized study in which just one person per household is in the randomized study, the transmission units are also randomized to either vaccine or control of the index individual, and no second stage randomization would be necessary. In the Swedish pertussis study (Section 2.1) and the Italian influenza study (Section 2.2), individual randomization of the children to vaccine or placebo resulted in randomization of the cluster, though this was not discussed in the papers.
In an individually randomized study, it would be possible that several people in a household would be randomized to receive vaccine or control. Then the estimates of indirect effects could be by the level of vaccine coverage. For example, in the reanalysis of the individually randomized cholera vaccine trial (Section 2.4), the indirect effects were estimated by quintiles of level of vaccine coverage of the target population in the bari. However, the individual-level randomization does not necessarily result in the level of coverage in the household being randomized. The propensity to participate in a vaccine trial could easily be associated with factors related to transmission, thus households with high levels of vaccine coverage might not be comparable with households with lower vaccine coverage. The estimates of indirect effects might, therefore, be confounded.
The assignment strategy, even if randomizing minicommunities to either vaccine or control, could depend on whether the indirect effects in individuals not in the target group for vaccination or indirect effects in the target group were of primary interest. Consider a minicommunity study to estimate the indirect effects of vaccination in individuals who are not eligible to receive the vaccine. The greatest indirect effects in individuals not eligible to receive the vaccine would be achieved by vaccinating as many of the eligible members in the minicommunities assigned to vaccine and giving control to as many of the eligible members in the other minicommunities. To estimate indirect effects in the population indicated for the vaccine, one would need enough eligible individuals to be not vaccinated to estimate the indirect effects. Likely the minicommunity design is not well-suited for this latter goal. This is one difference to the larger community studies where more individuals are available in each cluster.
In contrast, the minicommunity study is suitable for estimating total effects in the target group for the vaccination. In this case, the minicommunities would again be randomized to either vaccine or control and the goal would be to enroll all eligible individuals in each minicommunity. In the original cluster-randomized pneumoccocal vaccine study in the Native American population (Section 2.3), the primary goal was to estimate total effects, so the interest was in vaccinating every eligible child. Thus, randomization of a community to vaccine or control implied randomization of those individuals to the vaccine as a group. However, not every child consented to be vaccinated. So the issue there was one of noncompliance. In the study of indirect effects in households, only households of trial participants were included in the analysis. However, because the reasons for noncompliance across randomization units might have been comparable, the households with pneumococcal vaccine recipients and meningococcal vaccine recipients might be comparable.
Studies to estimate indirect effects in which no randomization takes place either at the minicommunity level or the individual level could be conducted. An example of such a study might be to examine the indirect effect of vaccination of school children against influenza on the other household members, such as the adults, or the very young children. In the USA, because of the recommendation of universal influenza vaccination, it would not be ethical to randomize school children to influenza vaccine and control, even an active one, though this had been recommended before the recommendation went into place (Halloran and Longini, 2006). Potential confounding would need to be taken into account.
In case-control studies, estimates of the indirect effect could be based on the reduction in the odds that cases have a vaccinated child in the home compared with the odds that controls have a vaccinated child in the home, in households that have at least one child in the home (Metlay et al., 2006). In the case-control studies, generally there would have been no randomization at either the household or the individual level. Confounding could occur at both the household and the individual level due to the study being observational at both levels.
4.5 Ascertainment
The method of ascertaining the minicommunities in a study of indirect effects is closely related to the assignment mechanism. In a study nested in an individually-randomized study, the households of individuals in the study can be recruited. In a case-control study, the households are ascertained after finding the cases and the controls. In an observational study, households might be ascertained from a roster or census, then the immunization status of the household members determined. Alternatively, lists of individuals of a certain age, such as school children, may lead to enrollment of households, and the immunization status recorded. In contrast, in observational studies based on the secondary attack rate, households are often ascertained by an index case, either at a clinic or by active surveillance.
4.6 Size of transmission unit versus number vaccinated
The relation between the size of the transmission units in a minicommunity study and the number of individuals vaccinated in each unit is important in determining whether vaccination will have indirect effects and whether it will be possible to estimate them. As the number of individuals in the transmission unit increases, vaccinating one individual may not be sufficient to observe any indirect effects of vaccination. For example, if a minicommunity study is embedded in an individually-randomized study in which one child per household is in the study, such as in Trollfors et al. (1998), then the ability to detect an indirect effect of vaccination will likely be greater in households with two or three other members, than in households with 10 or more. This could be due to more opportunities for exposure from outside the home in the larger households. Studies with negative results may simply not have randomized a sufficient portion of the individuals in the household to vaccine.
It is important to report distribution of sizes of the clusters or households, the number of individuals vaccinated, and the level of coverage achieved. The level of coverage should be reported based on the entire household membership. Although it might also be of interest to report the coverage in those eligible to participate in the vaccine study, this would generally be an overestimate of the actual vaccine coverage in the minicommunity. As an example, in Ali et al. (2005) the vaccine coverage in the bari was defined as the proportion of the women and children eligible to take part in the vaccine trial who had received vaccine.
4.7 Role of transmission in the community
Transmission outside of the households and multiple introductions into the household could swamp out indirect effects within households. Similar to larger group-randomized studies, we desire groups to be transmission dynamically separate (Halloran and Struchiner, 1991). Prior to and during the conduct of a minicommunity study, the level of transmission outside the households in relation to transmission within the households should be assessed. This information could play a role in the design of the study and in the analysis and interpretation of the results.
4.8 Implementing minicommunity studies
The question of when to implement a minicommunity study depends on whether indirect effects of vaccination are of interest. In some individually randomized, controlled vaccine trials, it may be straightforward to enroll households of trial participants for follow up, such as in the Swedish pertussis study by Trollfors et al. (1998). A similar suggestion, called the augmented study design, was made by Longini et al. (1996) and Datta et al. (1998) to estimate vaccine efficacy for infectiousness in HIV vaccine trials. Whether the study was randomized or observational, one would want to consider carefully whether vaccination of one or several individuals in a small transmission unit would reasonably be expected to reduce transmission within the unit sufficiently to be estimated and not be overwhelmed by exposure to infection outside the transmission unit.
5 Analysis
In estimating the indirect effects of vaccinating an individual or individuals in a household compared to control, the outcomes of interest are the disease or infection status of the other members of the household. Estimates of the indirect effects in the other members of the household are based on one of the unconditional risk measures, such as attack rate or cases per person-time in the other members of the household (Halloran et al., 2010). If more than one other member of the household is included in the analysis, then clustering within households can be taken into account with the use of generalized estimating equations (Liang and Zeger, 1986).
If just one person is randomized to vaccine or control, one approach to the analysis if the attack rate in the family is the measure of interest would be similar to the analysis in Halloran et al. (2003). The model would be based on the probability of the other members becoming infected. Let xi·1 denote the vaccine status of the person of interest in the vaccine study in household i, and xij2 may be a covariate, such as age group or vaccine status, of the jth other household member in household i. The vaccine status of the index child, xi·1, enters the analysis as a household-level, environmental variable. Let pij denote the probability that the jth person in the ith household becomes infected (or develops disease). The marginal model for the logit of the pij of the jth person in the ith household is
| (1) |
Coefficients can be estimated using generalized estimating equations, assuming an exchangeable working correlation matrix (Liang and Zeger, 1986). To obtain estimates of VEindirect on the attack rate scale, transform the parameters from the logistic model (1) back to the probability scale. Let ARv·, denote the attack rate in the household members with the index person of interest of vaccine status v. Then,
The indirect effect of protection in family members is then estimated by
Inference and confidence intervals can be obtained with the bootstrap (Efron and Tibshirani, 1993) using the household as the sampling unit. Alternatively, direct estimation of the relative risk in other household members could be achieved using log-binomial regression (Greenland, 2004; Spiegelman and Hertzmark 2005) with generalized estimating equations. If the estimate of VEindirect was based on the events per person-time at risk, a similar approach could be based on Poisson regression.
In Millar et al. (2008), where the household-level analysis was nested in a group-randomized study rather than in an individually randomized study, generalized estimating equations were used to control both for clustering within households and within randomization units. If a proportion of people are vaccinated in each household, the percentage of vaccine coverage in each household can be entered directly as a variable into the regression equations, as in Ali et al. (2005).
In determining the sample size for minicommunity studies, it is also necessary to take the effect of clustering into account. Approaches as in large cluster-randomized studies would be appropriate (Hayes and Moulton, 2009).
6 Discussion
In this paper, we have proposed the minicommunity design for estimating indirect effects or total effects of vaccination in small transmission units. We have presented several examples of published studies that have been conducted without the foundation of a systematic approach. The minicommunity study design could be a cost-effective method to evaluate indirect effects of vaccination. Methodological aspects such as assignment mechanism, ascertainment method, role of transmission in the community, and the relation of size versus vaccine coverage in the transmission units have been presented. The minicommunity study might be most suited for estimating indirect effects in individuals not indicated for the vaccine and for total effects. The interpretation of the indirect effect estimate from a minicommunity study depends on the particular study. To extrapolate to other settings or to generalize to larger communities might be difficult. Establishing there is a significant indirect effect can have important vaccine policy implications. On the other hand, failure of a study to detect indirect effects, such as in Albrich et al. (2007), does not mean that vaccination could not have indirect effects. Inadequate study design or a low level of coverage relative to the size of the minicommunities could explain a negative finding.
The minicommunity design for estimating indirect effects has counterparts in causal inference. The indirect causal effects of vaccination in populations are defined in the presence of interference (Cox, 1958). That is, the potential outcomes under vaccine or control in individuals depend on the treatment assignment of other individuals in the population (Halloran and Struchiner, 1995; Hudgens and Halloran, 2008; VanderWeele and Tchetgen Tchetgen, 2011b). The causal effects of vaccine on infectiousness are also defined within small transmission units (Halloran and Hudgens, 2012; VanderWeele and Tchetgen Tchetgen, 2011a). For households of size two, Halloran and Hudgens (2012) defined an intention-to-treat causal estimand of the effect of vaccination that does not condition on the infectious status of the individual randomized to vaccine or control. This estimand is the causal estimand for the indirect effects in a minicommunity design in households of size two.
The approach in this paper has been deliberately nontechnical. Much technical development remains to be done. The assignment mechanisms, ascertainment, role of transmission outside the household and the relation of household size to level of coverage in the household to the power of a study all need further exploration. All aspects of the minicommunity design deserve systematic development. We hope that the minicommunity design will be the subject of considerable future research.
Acknowledgments
This research was partially supported by National Institute of Allergy and Infectious Diseases grant R37-AI032042. We thank Nicole Basta for the literature search and Valerie Obenchain for help with the figures.
References
- Adegbola R, Secka O, Lahai G, Lloyd-Evans N, Njie A, Usen S, Oluwalana C, Obaro S, Weber M, Corrah T, Mulholland K, McAdam K, Greenwood B, Milligan P. Elimination of Haemophilus influenzae type b (Hib) disease from The Gambia after the introduction of routine immunisation with a Hib conjugate vaccine: A prospective study. Lancet. 2005;366:144–150. doi: 10.1016/S0140-6736(05)66788-8. [DOI] [PubMed] [Google Scholar]
- Albrich W, Madhi S, Lafond K, Klugman K. Herd immunity after pneumococcal conjugate vaccination. Lancet. 2007;370:218–219. doi: 10.1016/S0140-6736(07)61119-2. author reply 219–20. [DOI] [PubMed] [Google Scholar]
- Ali M, Emch M, von Seidlein M, Yunus M, Sack D, Rao M, Holmgren J, Clemens J. Herd immunity conferred by killed oral cholera vaccines in Bangladesh: A reanalysis. Lancet. 2005;366:44–49. doi: 10.1016/S0140-6736(05)66550-6. [DOI] [PubMed] [Google Scholar]
- Ali M, Emch M, Yunus M, Sack D, Lopez A, Holmgren J, Clemens J. Vaccine protection of Bangladeshi infants and young children against cholera: implications for vaccine deployment and person-to-person transmission. Pediatr Infect Dis J. 2008;27:33–37. doi: 10.1097/INF.0b013e318149dffd. [DOI] [PubMed] [Google Scholar]
- Becker N, Britton T, O’Neill P. Estimating vaccine effects from studies of outbreaks in household pairs. Statistics in Medicine. 2006;25:1079–1093. doi: 10.1002/sim.2236. [DOI] [PubMed] [Google Scholar]
- Carman W, Elder A, Wallace L, McAulay K, Walker A, Murray G, Stott D. Effects of influenza vaccination of health-care workers on mortality of elderly people in long-term care: a randomised controlled trial. Lancet. 2000;355:93–97. doi: 10.1016/S0140-6736(99)05190-9. [DOI] [PubMed] [Google Scholar]
- Cisse B, Aaby P, Simondon F, Samb B, Soumaré M, Whittle H. Role of schools in the transmission of measles in rural Senegal: Implications for measles control in developing countries. Am J Epidemiol. 1999;149:295–301. doi: 10.1093/oxfordjournals.aje.a009811. [DOI] [PubMed] [Google Scholar]
- Cox D. Planning of Experiments. New York: John Wiley and Sons, Inc.; 1958. [Google Scholar]
- Datta S, Halloran M, Longini I. Augmented HIV vaccine trial designs for estimating reduction in infectiousness and protective efficacy. Stat Med. 1998;17:185–200. doi: 10.1002/(sici)1097-0258(19980130)17:2<185::aid-sim732>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Datta S, Halloran M, Longini I. Efficiency of estimating vaccine efficacy for susceptibility and infectiousness: Randomization by individual versus household. Biometrics. 1999;55:792–798. doi: 10.1111/j.0006-341x.1999.00792.x. [DOI] [PubMed] [Google Scholar]
- Efron B, Tibshirani R. An Introduction to the Bootstrap. New York: Chapman and Hall; 1993. [Google Scholar]
- Esposito S, Marchisio P, Cavagna R, Gironi S, Bosis S, Lambertini L, Droghetti R, Principi N. Effectiveness of influenza vaccination of children with recurrent respiratory tract infections in reducing respiratory-related morbidity within the households. Vaccine. 2003;21:3162–3168. doi: 10.1016/s0264-410x(03)00253-6. [DOI] [PubMed] [Google Scholar]
- Fine P. Herd immunity. Epidemiol Rev. 1993;15:265–302. doi: 10.1093/oxfordjournals.epirev.a036121. [DOI] [PubMed] [Google Scholar]
- Fox J, Elveback L. Herd immunity: Changing concepts, chapter 16. In: Notkins A, editor. Viral Immunology and Immunopathology. New York: Academic Press; 1975. pp. 273–290. [Google Scholar]
- Frangakis C, Rubin D. Principal stratification in causal inference. Biometrics. 2002;58:21–29. doi: 10.1111/j.0006-341x.2002.00021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert P, Bosch R, Hudgens M. Sensitivity analysis for the assessment of causal vaccine effects on viral load in HIV vaccine trials. Biometrics. 2003;59:531–541. doi: 10.1111/1541-0420.00063. [DOI] [PubMed] [Google Scholar]
- Greenland S. Model-based estimation of relative risks and other epidemiologic measures in studies of common outcomes and in case-control studies. Am J Epidemiol. 2004;160:301–305. doi: 10.1093/aje/kwh221. [DOI] [PubMed] [Google Scholar]
- Grijalva C, Nuorti J, Arbogast P, Martin S, Edwards K, Griffin M. Decline in pneumonia admissions after routine childhood immunisation with pneumococcal conjugate vaccine in the USA: a time-series analysis. Lancet. 2007;369:1179–1186. doi: 10.1016/S0140-6736(07)60564-9. [DOI] [PubMed] [Google Scholar]
- Halloran M, Hudgens M. Causal vaccine effects for infectious-ness. International Journal of Bio statistics. 2012;8 Article 6, [Google Scholar]
- Halloran M, Longini I. Community studies for vaccinating schoolchildren against influenza. Science. 2006;311:615–616. doi: 10.1126/science.1122143. [DOI] [PubMed] [Google Scholar]
- Halloran M, Longini I, Struchiner C. Design and Analysis of Vaccine Studies. New York: Springer; 2010. [Google Scholar]
- Halloran M, Préziosi M, Chu H. Estimating vaccine efficacy from secondary attack rates. Journal of the American Statistical Association. 2003;98:38–46. [Google Scholar]
- Halloran M, Struchiner C. Study designs for dependent happenings. Epidemiology. 1991;2:331–338. doi: 10.1097/00001648-199109000-00004. [DOI] [PubMed] [Google Scholar]
- Halloran M, Struchiner C. Causal inference for infectious diseases. Epidemiology. 1995;6:142–151. doi: 10.1097/00001648-199503000-00010. [DOI] [PubMed] [Google Scholar]
- Halloran M, Struchiner C, Longini I. Study designs for different efficacy and effectiveness aspects of vaccination. Am J Epidemiol. 1997;146:789–803. doi: 10.1093/oxfordjournals.aje.a009196. [DOI] [PubMed] [Google Scholar]
- Halloran M, Struchiner C, Spielman A. Modeling malaria vaccines II: Population effects of stage-specific malaria vaccines dependent on natural boosting. Math Biosci. 1989;94:115–149. doi: 10.1016/0025-5564(89)90074-6. [DOI] [PubMed] [Google Scholar]
- Hayes R, Moulton L. Cluster Randomised Trials: A Practical Approach. Boca Raton, FL: Chapman and Hall/CRC; 2009. [Google Scholar]
- Hennessy T, Singleton R, Bulkow L, Bruden D, Hurlburt D, Parks D, Moore M, Parkinson A, Schuchat A, Butler J. Impact of heptavalent pneumococcal conjugate vaccine on invasive disease, antimicrobial resistance and colonization in Alaska Natives: Progress towards elimination of a health disparity. Vaccine. 2005;23:5464–5473. doi: 10.1016/j.vaccine.2005.08.100. [DOI] [PubMed] [Google Scholar]
- Hudgens M, Halloran M. Towards causal inference with interference. J Am Stat Assoc. 2008;103:832–842. doi: 10.1198/016214508000000292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendrick P, Eldering G. A study in active immunization against pertussis. Am J Hyg, Sect B. 1939;38:133. [Google Scholar]
- Koopman J, Little R. Assessing HIV vaccine effects. Am J Epidemiol. 1995;142:1113–1120. doi: 10.1093/oxfordjournals.aje.a117564. [DOI] [PubMed] [Google Scholar]
- Liang K, Zeger K. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- Longini I, Datta S, Halloran M. Measuring vaccine efficacy for both susceptibility to infection and reduction in infectiousness for prophylactic HIV-1 vaccines. J Acq Immun Def Synd. 1996;13:440–447. doi: 10.1097/00042560-199612150-00007. [DOI] [PubMed] [Google Scholar]
- Metlay JP, Fishman NO, Joffe M, Edelstein PH. Impact of pediatric vaccination with pneumococcal conjugate vaccine on the risk of bacteremic pneumococcal pneumonia in adults. Vaccine. 2006;24:468–475. doi: 10.1016/j.vaccine.2005.07.095. epub 2005 Aug 15. [DOI] [PubMed] [Google Scholar]
- Millar E, Watt J, Bronsdon M, Dallas J, Reid R, Santosham M, O’Brien K. Indirect effect of 7-valent pneumococcal conjugate vaccine on pneumococcal colonization among unvaccinated household members. Clin Infect Dis. 2008;47:989–996. doi: 10.1086/591966. [DOI] [PubMed] [Google Scholar]
- Moulton L, O’Brien K, Kohberger R, Chang I, Reid R, Weatherholtz R, Hackell J, Siber G, Santosham M. Design of a group-randomised Streptococcus pneumoniae vaccine trial. Contr Clin Trials. 2001;22:438–452. doi: 10.1016/s0197-2456(01)00132-5. [DOI] [PubMed] [Google Scholar]
- Orenstein W, Bernier R, Hinman A. Assessing vaccine efficacy in the field: Further observations. Epidemiol Rev. 1988;10:212–241. doi: 10.1093/oxfordjournals.epirev.a036023. [DOI] [PubMed] [Google Scholar]
- Potter J, Stott DJ, Roberts MA, Elder AG, O’Donnell B, Knight PV, Carman WF. Influenza vaccination of health care workers in long-term-care hospitals reduces the mortality of elderly patients. J Infect Dis. 1997;175:1–6. doi: 10.1093/infdis/175.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Préziosi M, Halloran M. Effects of pertussis vaccination on transmission: vaccine efficacy for infectiousness. Vaccine. 2003;21:1853–1861. doi: 10.1016/s0264-410x(03)00007-0. [DOI] [PubMed] [Google Scholar]
- Préziosi M, Yam A, Wassilak S, Chabirand L, Simaga A, Ndiaye M, Dia F, Dabis M, Simondon F. Epidemiology of whooping cough in a West African community before and after introduction of a widespread vaccination programme. Am J Epidemiol. 2002;155:891–896. doi: 10.1093/aje/155.10.891. [DOI] [PubMed] [Google Scholar]
- Ramsay M, Andrews N, Trotter C, Kaczmarski E, Miller E. Herd immunity from meningococcal serogroup C conjugate vaccination in England: Database analysis. Br Med J. 2003;326:365–366. doi: 10.1136/bmj.326.7385.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rida W. Assessing the effect of HIV vaccination on secondary transmission. Statistics in Medicine. 1996;15:2393–2404. doi: 10.1002/(sici)1097-0258(19961130)15:22<2393::aid-sim458>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Spiegelman D, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol. 2005;162:199–200. doi: 10.1093/aje/kwi188. [DOI] [PubMed] [Google Scholar]
- Struchiner C, Halloran M, Robins J, Spielman A. The behavior of common measures of association used to assess a vaccination program under complex disease transmission patterns - a computer simulation study of malaria vaccines. Int J Epidemiol. 1990;19:187–196. doi: 10.1093/ije/19.1.187. [DOI] [PubMed] [Google Scholar]
- Trollfors B, Taranger J, Lagergard T, Sundh V, Bryla D, Schneerson R, Robbins J. Immunization of children with pertussis toxoid decreases spread of pertussis within the family. Pediatr Infect Dis J. 1998;17:196–199. doi: 10.1097/00006454-199803000-00005. [DOI] [PubMed] [Google Scholar]
- VanderWeele T, Tchetgen Tchetgen E. Bounding the infectiousness effects in vaccine trials. Epidemiology. 2011a;22:686–693. doi: 10.1097/EDE.0b013e31822708d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanderWeele T, Tchetgen Tchetgen E. Effect partitioning under interference in two-stage randomized vaccine trials. Statistics and Probability Letters. 2011b;81:861–869. doi: 10.1016/j.spl.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]