Abstract
While genetics clearly influences dental caries risk, few caries genes have been discovered and validated. Recent studies have suggested differential genetic factors for primary dentition caries and permanent dentition caries, as well as for pit-and-fissure- (PF) and smooth- (SM) surface caries. We performed separate GWAS for caries in permanent-dentition PF surfaces (1,017 participants, adjusted for age, sex, and the presence of Streptococcus mutans) and SM surfaces (1,004 participants, adjusted for age, education group, and the presence of Streptococcus mutans) in self-reported whites (ages 14 to 56 yrs). Caries scores were derived based on visual assessment of each surface of each tooth; more than 1.2 million SNPs were either successfully genotyped or imputed and were tested for association. Two homologous genes were suggestively associated: BCOR (Xp11.4) in PF-surface caries (p value = 1.8E-7), and BCORL1 (Xq26.1) in SM-surface caries (p value = 1.0E-5). BCOR mutations cause oculofaciocardiodental syndrome, a Mendelian disease involving multiple dental anomalies. Associations of other plausible cariogenesis genes were also observed for PF-surface caries (e.g., INHBA, p value = 6.5E-6) and for SM-surface caries (e.g., CXCR1 and CXCR2, p value = 1.9E-6). This study supports the notion that genes differentially affect cariogenesis across the surfaces of the permanent dentition, and nominates several novel genes for investigation.
Keywords: genetics, genomics, cariogenesis, tooth decay, BCOR, BCORL1
Introduction
Dental caries is one of the most common diseases worldwide. In the US, the prevalence in adults is approximately 90% (Beltran-Aguilar et al., 2005), and in some high-risk populations, the prevalence is even higher. Treatment of dental caries consumes significant resources each year, and untreated caries lesions can lead to pain, tooth loss, and oral infection, or other co-morbidities.
As a multi-factorial process, cariogenesis is affected by a combination of environmental and behavioral factors, including dietary behaviors, bacterial flora, fluoride intake and exposures, oral hygiene, salivary composition and flow rate, tooth positional and morphological features, genetic predisposition, and gene-by-environment interactions (Hunter, 1988; Anderson, 2002). Despite the acknowledged importance of genetic factors (Townsend et al., 1998) and estimates of heritability of 30% to 50% (Bretz et al., 2005; Wang et al., 2010; Shaffer et al., 2012b), only a few caries susceptibility genes have been identified and validated thus far.
We have previously shown that both shared and unique genetic risk factors may affect dental caries of the primary dentition and permanent dentition (Wang et al., 2010). Genome-wide association studies (GWAS) performed separately for the 2 dentitions have nominated several biologically plausible genes (Shaffer et al., 2011; Wang et al., submitted), although these genes cumulatively explain only a fraction of the genetic variance of dental caries. In addition, recent work has suggested that the effects of genetic factors on dental caries may differ between pit-and-fissure (PF) and smooth (SM) tooth surfaces (Shaffer et al., 2012b). It has also been well-established that PF surfaces exhibit much greater risk of developing caries lesions than do SM surfaces (Batchelor and Sheiham, 2004; Shaffer et al., 2012a,b), and progression of decay varies between these surface types. To further study the potential differences in genetic contributions to PF- and SM-surface caries risk, we performed separate GWAS in the permanent dentition for the 2 types of surfaces.
Materials & Methods
Data Collection
Details on participant recruitment and data collection are available in the Appendix. Only self-reported whites (ages 14 to 56 yrs) with dental caries assessments were included in our study, with 1,063 individuals having data on all covariates for the PF-surface analysis, while 1,049 had data on all covariates for the SM-surface analysis. Two classes of tooth surfaces, PF and SM, were defined by similarities in morphology and risk of developing caries lesions. PF surfaces included buccal and occlusal surfaces of mandibular molars and lingual and occlusal surfaces of maxillary molars. SM surfaces included all other tooth surfaces. Two caries scores were generated, PF D1MFS and SM D1MFS, calculated as the total number of surfaces scored as pre-cavitated, decayed, missing due to decay, or filled for PF and SM surface types.
Genotyping and Statistical Analysis
Details regarding allele calling, data cleaning, and quality assurance metrics have been previously described (Laurie et al., 2010). Genotyping was performed on an Illumina platform (Illumina, Inc., San Diego, CA, USA). We successfully genotyped 1,017 individuals for the PF-surface analysis, and 1,004 for the SM-surface analysis. Genotype data on more than 548,000 single-nucleotide polymorphisms (SNPs) passed quality control filters. Un-genotyped SNPs were imputed for 996 individuals in the PF-surface analysis and 982 in the SM-surface analysis. In total, more than 1.2 million SNPs were either genotyped or imputed. Linear regression was used to model additive genetic effects. PF surface analysis was adjusted for the covariates age, sex, and the presence or absence of Streptococcus mutans. SM-surface analysis was adjusted for the covariates age, education group (i.e., up to high school, some college, four-year degree or beyond), and the presence or absence of Streptococcus mutans. Covariates were determined by a forward selection strategy. Association between PF- or SM-surface caries scores and each SNP was tested. We used a conservative threshold of α = 5E-8 to declare genome-wide statistical significance and α = 5E-5 for suggestive significance. Suggestive associations near plausible caries genes were reported among our results. Additional genotyping, covariate selection, and statistical analysis details are provided in the Appendix.
Results
Phenotypes and covariates are summarized in Table 1. The mean D1MFS scores were 9.0 for PF surfaces and 12.2 for SM surfaces. The caries prevalence was 92% for PF surfaces and 82% for SM surfaces. Female participants exhibited more PF- and SM-surface caries than male participants (9.3 vs. 8.5 and 12.4 vs. 11.9, respectively). No genome-wide significant signal was observed for either PF- or SM-surface scans. Instead, several suggestive associations near biologically plausible caries genes were observed.
Table 1.
Sample Characteristics
| Characteristics | All | Male | Female | |
|---|---|---|---|---|
| PF | Sample size | 1,063 | 408 | 655 |
| Age at examination (mean ± SD, yrs) | 31.46 ± 10.29 | 31.66 ± 11.24 | 31.34 ± 9.67 | |
| Presenting Streptococcus mutans (%) | 79.0 | 79.2 | 78.9 | |
| PF D1MFS (mean ± SD) | 8.98 ± 6.07 | 8.50 ± 5.97 | 9.28 ± 6.12 | |
| PF caries prevalence (%) | 92.0 | 91.4 | 92.4 | |
| SM | Sample size | 1,049 | 405 | 644 |
| Age at examination (mean ± SD, yrs) | 31.48 ± 10.32 | 31.72 ± 11.22 | 31.33 ± 9.72 | |
| Presenting Streptococcus mutans (%) | 79.2 | 79.3 | 79.2 | |
| Education group* | 680 / 230 / 139 | 282 / 69 / 54 | 398 / 161 / 85 | |
| SM D1MFS (mean ± SD) | 12.22 ± 15.44 | 11.93 ± 15.10 | 12.41 ± 15.66 | |
| SM caries prevalence (%) | 82.1 | 82.2 | 82.0 | |
Education group categories are: up to high school/some college or two-year degree/college or advanced degree.
PF-surface GWAS
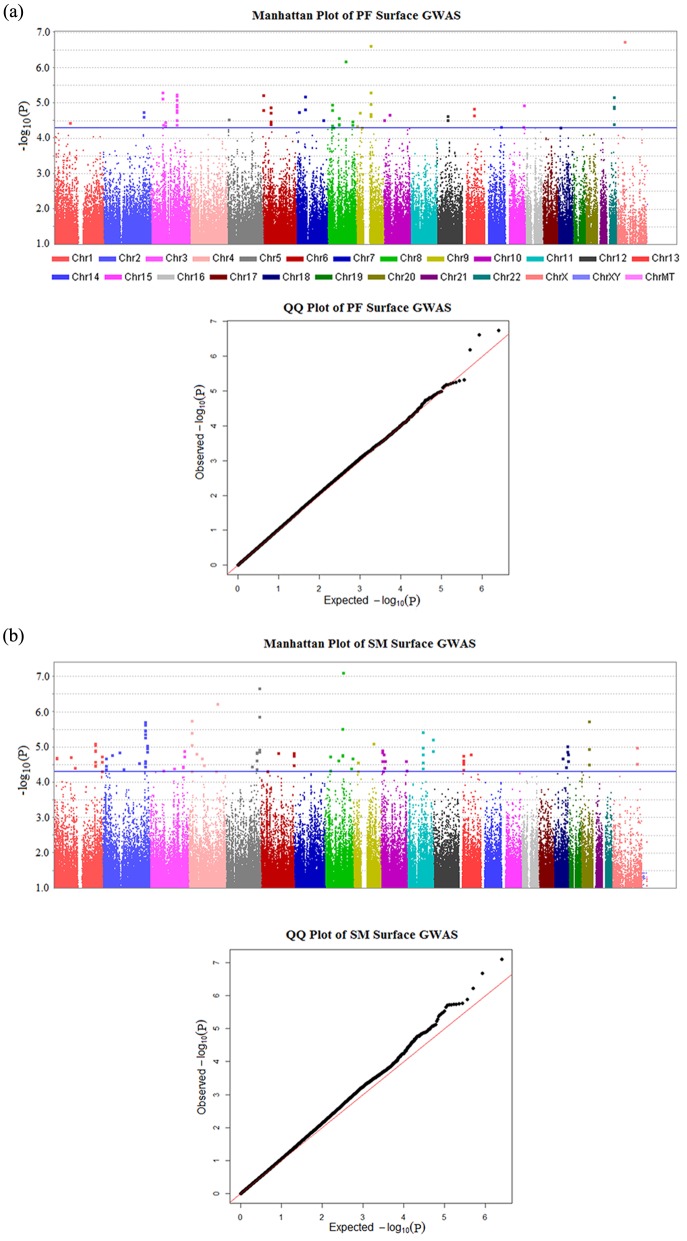
The most significant SNP was rs17145638 on chromosome Xp11.4 (p value = 1.79E-7); see Appendix Table 1 for a full list of SNPs with p values under 5E-5. The genomic inflation factor (λ) was 1.02 for PF-surface scans (Fig. 1a). This very slight inflation of p values may be due to the presence of related individuals in the sample.
Figure 1.
Manhattan plots (upper panel) and quantile-quantile plots (lower panel) for GWAS of PF- and SM-surface caries. (a) PF-surface scan; (b) SM-surface scan. Manhattan plots show negative log10-transformed p values (y-axis) across the whole genome (x-axis). The genomic inflation factors (λ) are 1.02 for PF-surface scans and 1.04 for SM-surface scans. Percentage of p values used to calculate λ was set at .95. Blue horizontal lines represent the suggestive significance threshold (p value < 5E-5). Red diagonal lines represent the expected distribution of p values under the null hypothesis of no association. Genotyped and imputed SNPs are plotted together.
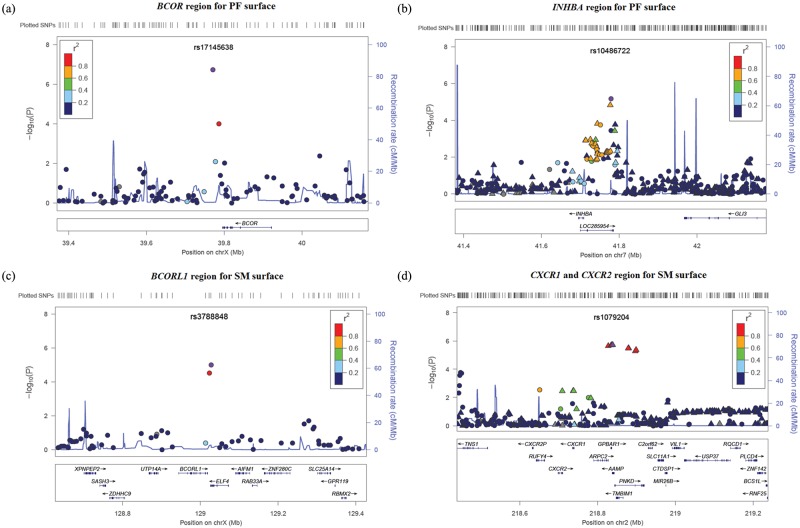
SNP rs17145638 is located in the 3′ downstream region of the BCOR gene (BCL6 co-repressor) (Fig. 2a). BCOR is a key transcription regulator during early embryonic development (Ng et al., 2004). Null mutations in BCOR are the sole cause of the X-linked dominant Mendelian disorder oculofaciocardiodental (OFCD) syndrome (Ng et al., 2004; Hilton et al., 2009), which results in dental abnormalities including radiculomegaly, hypodontia, fusion and duplication of teeth, persistent primary teeth, delayed tooth development and eruption, and defective tooth enamel, as well as other developmental defects (e.g., microphthalmia, congenital cataracts, cardiac and digital defects) (Schulze et al., 1999; Oberoi et al., 2005). Bcor expression was observed during mouse development in multiple tissues that are affected in OFCD patients (Wamstad and Bardwell, 2007), and normal Bcor expression is required for differentiation of multiple tissue lineages (Wamstad et al., 2008). Further, the osteo-dentinogenic potential was found to be increased in mesenchymal stem cells isolated from an OFCD patient with a BCOR mutation (Fan et al., 2009). A study exploring the role of Bcor in tooth development of mice found that silenced Bcor expression by RNA interference in dental tissues caused dentinogenesis defects and retardation of tooth root development (Cai et al., 2010). No direct role of BCOR in dental caries has been established. However, since null mutations in BCOR cause severe dental abnormalities, it is plausible that genetic variation within or near this gene may affect tooth development sufficiently to modify susceptibility to dental caries.
Figure 2.
LocusZoom plots for regions of interest. (a) Suggestive locus near BCOR for PF-surface GWAS; (b) suggestive locus near INHBA for PF-surface GWAS; (c) suggestive locus near BCORL1 for SM-surface GWAS; and (d) suggestive locus near CXCR1 and CXCR2 for SM-surface GWAS. Negative log10-transformed p values and physical positions for SNPs in the regions are shown. The recombination rate overlay as well as colors indicating linkage disequilibrium between the top SNP (purple) and other SNPs are based on HapMap Phase II CEU data (hg18) for chromosome X, or 1000 Genomes CEU data (hg18) for other chromosomes. The rug plot indicates regional SNP density. Gene positions and directions of transcription are annotated. Circles represent genotyped SNPs, and triangles represent imputed SNPs.
Another suggestive gene associated with PF D1MFS was INHBA (inhibin, beta A) on chromosome 7p14.1. The most significant SNP in this region was rs10486722, located in the 5′ region upstream of INHBA and also in a non-coding RNA INHBA-AS1, with a p value of 6.50E-6 (Fig. 2b). INHBA encodes a subunit of activin and inhibin, members of the TGFβ superfamily, which plays a role in reproduction and development (Mather et al., 1997). Expression of Inhba in the mesenchymal cells responsible for early tooth development is essential for tooth bud formation. Mouse knockouts for Inhba demonstrate disruption of tooth eruption of incisors and mandibular molars (Ferguson et al., 1998; Brown et al., 2000). Moreover, inhibition of activin signaling in tooth development by exogenous soluble receptors as well as mutation of activin receptors IIA and IIB, and Smad2, which are effectors in the activin signaling pathway, produced similar dental aberrations (Ferguson et al., 2001). Possibly, INHBA participates in the pathogenesis of caries by affecting tooth morphology.
SM-surface GWAS
The genomic inflation factor (λ) was 1.04 for the SM-surface scan (Fig. 1b). The most significant association for SM D1MFS was with rs2046315 on chromosome 8q21.3 (p value = 7.85E-8) (see Appendix Table 2 for a full list of SNPs with p values under 5E-5). This SNP was also suggestively associated with PF D1MFS (p value = 6.50E-7). However, no known gene is close to this SNP. The nearest gene, RIPK2 (receptor-interacting serine-threonine kinase 2), is 560 kb away. To further annotate SNP rs2046315, we searched the eQTL database (http://www.scandb.org/newinterface/about.html). No gene’s expression is predicted by rs2046315 with p value less than .0001 in CEPH (samples of northern and western European ancestry). The Mammal Conservation Score for rs2046315 is -0.128, corresponding to a p value of .745 under a null hypothesis of neutral evolution. In other words, this SNP is not conserved across species. In addition, this SNP does not reside on any genomic element identified by the ENCODE project.
A suggestive association was observed on chromosome Xq26.1 near BCORL1 (BCL6 co-repressor-like 1; rs3788848; p value = 1.01E-5) (Fig. 2c). SNP rs3788848 is located in the 3′ region downstream of BCORL1 and also in the 3′UTR region of ELF4. Of note, chromosome Xq has been reported to be linked to low caries susceptibility. In a genome-wide linkage scan, a marker in Xq27.1 yielded a non-parametric p value of 5E-5 (Vieira et al., 2008). BCORL1 is a strong transcriptional repressor, so named because of its sequence similarity with BCOR (Pagan et al., 2007). Three homologous regions between BCOR and BCORL1 proteins have been identified: a small amino-terminal region and 2 substantial regions spanning 600 amino acids within the central region and 336 amino acids at the carboxyl terminus, respectively (Pagan et al., 2007). No known function of BCORL1 directly relates to cariogenesis. However, it is the only gene similar to BCOR in the entire human genome. Furthermore, both the association signals of BCOR and BCORL1 are located on the 3′ ends of the genes. It is interesting to observe such suggestive associations near 2 closely related but physically separated genes.
Several imputed SNPs on chromosome 2q35 were associated with SM D1MFS at the suggestive significance level (e.g., rs1079204, located in an intron of AAMP; p value = 1.90E-6) near 2 chemokine receptor genes, CXCR1 and CXCR2 (Fig. 2d). These genes are major receptors of interleukin 8, a chemokine that is an essential mediator of the inflammation response. Several studies have shown potential roles for this chemokine and its receptors in the process of oral infection. Increased expression of interleukin 8 has been reported in inflamed human dental pulps (Huang et al., 1999). CXCR1 and CXCR2 mRNA expressions were found to be related to the presence of certain periodontopathic bacteria in inflamed gingival tissues (Noda et al., 2007). Also, CXCR2 was reported to be associated with periodontitis in a Brazilian population (Viana et al., 2010). These lines of evidence support the view that CXCR1 and CXCR2 contribute to cariogenesis by influencing host susceptibility to oral bacteria.
Discussion
In this study, we performed separate GWAS to detect genetic variants associated with dental caries of PF and SM surfaces in the permanent dentition. We successfully identified several potential caries genes specific for PF or SM surfaces, including BCOR and BCORL1 (2 X-linked genes with sequence similarity), INHBA, CXCR1, and CXCR2. Nominated genes were involved in a variety of possible cariogenic processes, such as tooth morphology, tooth development, and immune defense, which is consistent with the prevailing view that the genetic etiology of dental caries includes many genes acting through multiple mechanisms.
A previous family-based study reported that the genetic correlation between PF- and SM-surface caries scores did not significantly differ from 100%. Although this estimate of correlation might be inflated, the 2 surface types are likely to be influenced by the same genetic components (Shaffer et al., 2012b). However, it does not rule out the possibility that different association patterns may be observed and different genes identified for PF- and SM-surface caries, since the “detectable” associated genes might account for only a very small portion of heritability, and there could be many overlapping weak association signals across the 2 surface types that did not meet suggestive significance. Moreover, SNPs within the regions of interest mentioned in the ‘Results’ have p values under .05 for both types of surfaces (Table 2), indicating a certain level of pleiotropic effect of the “detectable” hits, which could also contribute to genetic correlation. From these points of view, our results do not conflict with the reported high genetic correlation and, furthermore, showed that the subdivision of complex phenotypes was useful for the detection of genes with sub-phenotype-specific effects.
Table 2.
SNPs of Interest Showing Genetic Associations
| SNP | CHR | BP | Description and Nominated Gene(s) | PF p value | PF beta | SM p value | SM beta | Genotyped or Imputed |
|---|---|---|---|---|---|---|---|---|
| rs2046315 | 8 | 90280216 | Top hit for SM, no gene | 6.50E-7 | 1.87 | 7.85E-8 | 5.19 | Genotyped |
| rs17145638 | X | 39770574 | Top hit for PF, BCOR | 1.79E-7 | -2.33 | .001 | -3.85 | Genotyped |
| rs10486722 | 7 | 41778433 | Suggestive hit for PF, INHBA | 6.50E-6 | 1.22 | .009 | 1.81 | Genotyped |
| rs3788848 | X | 129027493 | Suggestive hit for SM, BCORL1 | .015 | 0.82 | 1.01E-5 | 3.81 | Genotyped |
| rs1079204 | 2 | 218838758 | Suggestive hit for SM, CXCR1 and CXCR2 | .006 | 1.92 | 1.90E-6 | 8.45 | Imputed |
Associations shown in this Table are all of the same direction for PF and SM surfaces. Top hit: SNP with the smallest p value in a scan. Suggestive hit: SNP with p value less than 5E-5 in a scan.
There is consistent evidence across populations for higher caries incidence in female individuals than in male individuals. A similar sex difference was also observed in our current study. While this difference is generally attributed to environmental factors and genetic factors that work through changing individual environmental factors (Lukacs and Largaespada, 2006; Lukacs, 2010), Vieira et al. (2008) hypothesized that X-linked genetic variants may partly explain the gender differences in dental caries susceptibility. Our observation of suggestive associations between the X-linked genes BCOR and BCORL1 and caries phenotypes supports their hypothesis. We summarized caries scores by genotypes of the most significant SNPs for the 2 genes (Appendix Table 3). The risk alleles appeared to affect homozygous female participants more severely than hemizygous male participants. The effect of X-linked genes on sex differences in dental caries is an open question and deserves further investigation.
We compared our results with those of a contemporary GWAS, conducted by Wang et al. (submitted), of total dental caries (caries scores not divided by tooth-surface types) in the permanent dentition, which was based on the same sample as well as additional cohorts. Two suggestive regions in that study also showed suggestive associations in ours (p values <5E-5). One is in an intron of RPS6KA2 for SM surfaces, and the other is near PTK2B for both SM and PF surfaces. Both of these genes are involved in the p38-dependent MAPK pathway important for oral-related diseases, including dental caries.
Although this study had many strengths, including well-defined caries phenotypes, high-quality GWAS data, and important environmental covariates, a major limitation was the lack of replication. However, this issue is partly alleviated by the compelling biological evidence for the involvement of the nominated genes in mechanisms related to cariogenesis. In particular, we observed intriguing suggestive associations with BCOR, the gene for OFCD syndrome, and BCORL1, its only known homologue, for separate caries phenotypes.
In conclusion, we found different genes that may be associated with PF- and SM-surface dental caries, several of which have plausible biological functions relevant to cariogenesis. These findings contribute to our understanding of the genetic pathogenic mechanisms of cariogenesis and, we hope, will lead to more functional studies on the reported genes as well as the search for causal variants.
Supplementary Material
Footnotes
Support was provided by the NIDCR as part of the GENEVA consortium (U01-DE018903; https://www.genevastudy.org/). Genotyping was done by the Center for Inherited Disease Research (http://www.cidr.jhmi.edu/), funded by NIH contract HHSN268200782096C. Assistance with phenotype harmonization and genotype cleaning was provided by the GENEVA Coordinating Center (U01-HG004446) and by NCBI. Additional support was provided by NIDCR grants R01-DE014899 and R03-DE021425.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
References
- Anderson M. (2002). Risk assessment and epidemiology of dental caries: review of the literature. Pediatr Dent 24:377-385. [PubMed] [Google Scholar]
- Batchelor PA, Sheiham A. (2004). Grouping of tooth surfaces by susceptibility to caries: a study in 5-16 year-old children. BMC Oral Health 4:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltran-Aguilar ED, Barker LK, Canto MT, Dye BA, Gooch BF, Griffin SO, et al. (2005). Surveillance for dental caries, dental sealants, tooth retention, edentulism, and enamel fluorosis—United States, 1988-1994 and 1999-2002. MMWR Surveill Summ 54:1-43. [PubMed] [Google Scholar]
- Bretz WA, Corby PM, Schork NJ, Robinson MT, Coelho M, Costa S, et al. (2005). Longitudinal analysis of heritability for dental caries traits. J Dent Res 84:1047-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CW, Houston-Hawkins DE, Woodruff TK, Matzuk MM. (2000). Insertion of Inhbb into the Inhba locus rescues the Inhba-null phenotype and reveals new activin functions. Nat Genet 25:453-457. [DOI] [PubMed] [Google Scholar]
- Cai J, Kwak S, Lee JM, Kim EJ, Lee MJ, Park GH, et al. (2010). Function analysis of mesenchymal Bcor in tooth development by using RNA interference. Cell Tissue Res 341:251-258. [DOI] [PubMed] [Google Scholar]
- Fan Z, Yamaza T, Lee JS, Yu J, Wang S, Fan G, et al. (2009). BCOR regulates mesenchymal stem cell function by epigenetic mechanisms. Nat Cell Biol 11:1002-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson CA, Tucker AS, Christensen L, Lau AL, Matzuk MM, Sharpe PT. (1998). Activin is an essential early mesenchymal signal in tooth development that is required for patterning of the murine dentition. Genes Dev 12:2636-2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson CA, Tucker AS, Heikinheimo K, Nomura M, Oh P, Li E, et al. (2001). The role of effectors of the activin signalling pathway, activin receptors IIA and IIB, and Smad2, in patterning of tooth development. Development 128:4605-4613. [DOI] [PubMed] [Google Scholar]
- Hilton E, Johnston J, Whalen S, Okamoto N, Hatsukawa Y, Nishio J, et al. (2009). BCOR analysis in patients with OFCD and Lenz microphthalmia syndromes, mental retardation with ocular anomalies, and cardiac laterality defects. Eur J Hum Genet 17:1325-1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang GT, Potente AP, Kim JW, Chugal N, Zhang X. (1999). Increased interleukin-8 expression in inflamed human dental pulps. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 88:214-220. [DOI] [PubMed] [Google Scholar]
- Hunter PB. (1988). Risk factors in dental caries. Int Dent J 38:211-217. [PubMed] [Google Scholar]
- Laurie CC, Doheny KF, Mirel DB, Pugh EW, Bierut LJ, Bhangale T, et al. (2010). Quality control and quality assurance in genotypic data for genome-wide association studies. Genet Epidemiol 34:591-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukacs JR. (2010). Sex differences in dental caries experience: clinical evidence, complex etiology. Clin Oral Investig 15:649-656. [DOI] [PubMed] [Google Scholar]
- Lukacs JR, Largaespada LL. (2006). Explaining sex differences in dental caries prevalence: saliva, hormones, and “life-history” etiologies. Am J Hum Biol 18:540-555. [DOI] [PubMed] [Google Scholar]
- Mather JP, Moore A, Li RH. (1997). Activins, inhibins, and follistatins: further thoughts on a growing family of regulators. Proc Soc Exp Biol Med 215:209-222. [DOI] [PubMed] [Google Scholar]
- Ng D, Thakker N, Corcoran CM, Donnai D, Perveen R, Schneider A, et al. (2004). Oculofaciocardiodental and Lenz microphthalmia syndromes result from distinct classes of mutations in BCOR. Nat Genet 36:411-416. [DOI] [PubMed] [Google Scholar]
- Noda D, Hamachi T, Inoue K, Maeda K. (2007). Relationship between the presence of periodontopathic bacteria and the expression of chemokine receptor mRNA in inflamed gingival tissues. J Periodontal Res 42:566-571. [DOI] [PubMed] [Google Scholar]
- Oberoi S, Winder AE, Johnston J, Vargervik K, Slavotinek AM. (2005). Case reports of oculofaciocardiodental syndrome with unusual dental findings. Am J Med Genet A 136:275-277. [DOI] [PubMed] [Google Scholar]
- Pagan JK, Arnold J, Hanchard KJ, Kumar R, Bruno T, Jones MJ, et al. (2007). A novel corepressor, BCoR-L1, represses transcription through an interaction with CtBP. J Biol Chem 282:15248-15257. [DOI] [PubMed] [Google Scholar]
- Schulze BR, Horn D, Kobelt A, Tariverdian G, Stellzig A. (1999). Rare dental abnormalities seen in oculo-facio-cardio-dental (OFCD) syndrome: three new cases and review of nine patients. Am J Med Genet 82:429-435. [DOI] [PubMed] [Google Scholar]
- Shaffer JR, Wang X, Feingold E, Lee M, Begum F, Weeks DE, et al. (2011). Genome-wide association scan for childhood caries implicates novel genes. J Dent Res 90:1457-1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer JR, Feingold E, Wang X, Tcuenco KT, Weeks DE, DeSensi RS, et al. (2012a). Heritable patterns of tooth decay in the permanent dentition: principal components and factor analyses. BMC Oral Health 12:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer JR, Wang X, Desensi RS, Wendell S, Weyant RJ, Cuenco KT, et al. (2012b). Genetic susceptibility to dental caries on pit and fissure and smooth surfaces. Caries Res 46:38-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend GC, Aldred MJ, Bartold PM. (1998). Genetic aspects of dental disorders. Aust Dent J 43:269-286. [DOI] [PubMed] [Google Scholar]
- Viana AC, Kim YJ, Curtis KM, Renzi R, Orrico SR, Cirelli JA, et al. (2010). Association of haplotypes in the CXCR2 gene with periodontitis in a Brazilian population. DNA Cell Biol 29:191-200. [DOI] [PubMed] [Google Scholar]
- Vieira AR, Marazita ML, Goldstein-McHenry T. (2008). Genome-wide scan finds suggestive caries loci. J Dent Res 87:435-439. [DOI] [PubMed] [Google Scholar]
- Wamstad JA, Bardwell VJ. (2007). Characterization of Bcor expression in mouse development. Gene Expr Patterns 7:550-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wamstad JA, Corcoran CM, Keating AM, Bardwell VJ. (2008). Role of the transcriptional corepressor Bcor in embryonic stem cell differentiation and early embryonic development. PLoS One 3:e2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Shaffer JR, Weyant RJ, Cuenco KT, DeSensi RS, Crout R, et al. (2010). Genes and their effects on dental caries may differ between primary and permanent dentitions. Caries Res 44:277-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.